Abstract
Introduction
Randomised controlled trials (RCTs) have compared biological and targeted systemic disease-modifying antirheumatic drugs (DMARDS) against placebo in psoriatic arthritis (PsA); few have compared them head to head.
Objectives
To compare the efficacy and safety of all evaluated DMARDs for active PsA, with a special focus on biological DMARDs (bDMARDs) licensed for PsA or psoriasis.
Methods
A systematic review identified RCTs and Bayesian network meta-analysis (NMA) compared treatments on efficacy (American College of Rheumatology (ACR) response, Psoriasis Area and Severity Index (PASI) response, resolution of enthesitis and dactylitis) and safety (patients discontinuing due to adverse events (DAE)) outcomes. Subgroup analyses explored ACR response among patients with and without prior biological therapy exposure.
Results
The NMA included 46 studies. Results indicate that some tumour necrosis factor inhibitors (anti-TNFs) may perform numerically, but not significantly, better than interleukin (IL) inhibitors on ACR response but perform worse on PASI response. Few significant differences between bDMARDs on ACR response were observed after subgrouping for prior bDMARD exposure. Guselkumab and IL-17A or IL-17RA inhibitors—brodalumab, ixekizumab, secukinumab—were best on PASI response. These IL-inhibitors and adalimumab were similarly efficacious on resolution of enthesitis and dactylitis. Infliximab with and without methotrexate, certolizumab 400 mg every 4 weeks and tildrakizumab showed the highest rates of DAE; abatacept, golimumab and the IL-inhibitors, the lowest.
Conclusions
Despite similar efficacy for ACR response, IL-17A and IL-17RA inhibitors and guselkumab offered preferential efficacy to anti-TNFs in skin manifestations, and for enthesitis and dactylitis, thereby supporting drug selection based on predominant clinical phenotype.
Keywords: arthritis, psoriatic; biological therapy; outcome assessment, health care
Key messages.
What is already known about this subject?
Increasingly, choice of psoriatic arthritis (PsA) treatments are being tailored based on a patient’s exposure to prior therapies, disease severity, comorbidities and individual manifestations of disease, including enthesitis, dactylitis and axial disease.
What does this study add?
This is a contemporary and comprehensive analysis of the efficacy and safety of systemic therapies for moderate to severe active PsA, with a focus on biological disease-modifying anti-rheumatic drugs licensed in PsA or psoriasis.
In addition to American College of Rheumatology and Psoriasis Area and Severity Index responses, we report resolution of enthesitis and dactylitis, both highly relevant and for which comparative evidence is limited.
How might this impact on clinical practice or further developments?
Faced with a multitude of therapeutic options, these study results could help clinicians tailor treatment choice according to different domains of disease and provides additional evidence for developing patient-centred treatment guidelines.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease associated with psoriasis (PsO).1 Up to 30% of patients with PsO may go on to develop PsA during their lifetime.2 Annual incidence rates of PsA are estimated at approximately six per 100 000 (0.006%) in the general population, and in those with PsO, 2.7%. Affecting males and females equally, the majority of patients with PsA develop skin symptoms first, some develop skin and joint symptoms at the same time and in 10%–15% of patients, joint symptoms develop first.1 PsA is a heterogeneous condition characterised by sore, painful and stiff joints, involving both articular and dermatological manifestations.3 4 Due to the different patterns of involvement, PsA can mimic different inflammatory arthritides.5 Delayed diagnosis has been identified as a contributor to poorer quality of life and disease outcomes in the long term.6
Traditionally PsA was treated with non-steroidal anti-inflammatory drugs, corticosteroids and conventional systemic disease-modifying antirheumatic drugs (csDMARDs).2 7 Therapy for PsA has advanced, with an improved understanding of the immunological processes underlying the pathogenesis of disease and the introduction of biological treatment (biological DMARDs, bDMARDs). Antitumour necrosis factor (TNF) agents have been shown to be successful in treating PsA across different domains of the disease.8 Newer biological agents approved by the European Medicine Agency or the US Food and Drug Administration, or both, for the treatment of PsA include the interleukin (IL)−12/IL-23 inhibitor ustekinumab,9 IL-17A inhibitors secukinumab10 and ixekizumab,11 IL-23 inhibitor guselkumab12 and the selective T-cell costimulation modulator abatacept,13 as well as non-biological treatments such as phosphodiesterase 4 inhibitor apremilast14 and the Janus kinase (JAK) inhibitors tofacitinib15 and upadacitinib.16 Interleukin-23 inhibitors tildrakizumab and risankizumab, and IL-17RA inhibitor brodalumab, all currently licensed for the treatment of PsO, have been evaluated in the treatment of PsA and shown to be efficacious8 17–25 as has the new JAK inhibitor filgotinib.26
Active PsA as defined by the American College of Rheumatology (ACR) and National Psoriasis Foundation (NPF), is disease causing symptoms at an unacceptably bothersome level as reported by the patient, and judged by the examining clinician to be due to PsA based on ≥1 of the following: swollen joints, tender joints, dactylitis, enthesitis, axial disease, active skin and/or nail involvement, and extraarticular inflammatory manifestations such as uveitis or inflammatory bowel disease.1 Current pharmacological therapy options for treating long-term active PsA vary depending on prior treatments, disease severity and comorbidities. General recommendations involve treating with anti TNF agents first, followed by IL-17A and IL-12/23 inhibitor therapies. Oral small molecule therapies apremilast and tofacitinib may also be recommended. More recently, the ACR/NPF, EULAR and GRAPPA guidelines have put greater emphasis on tailoring treatments based on the individual manifestations of PsA, including enthesitis, dactylitis and axial disease, recognising that the efficacy of bDMARDs may vary across these different domains based on their mode of action.1 7 27
The aim of this systematic literature review (SLR) and network meta-analysis (NMA) was to identify the latest evidence and compare the efficacy and safety of all evaluated systemic therapies for the treatment of active PsA.
Materials and methods
Search strategy
An initial search was performed on 11 March 2020 and updated on 18 August 2020 in Embase, MEDLINE, MEDLINE In-Process via Ovid and the Cochrane Library (online supplemental table S1). These were supplemented by searching conference abstracts and clinical trial databases for ongoing or recently completed studies (online supplemental table S2). In addition, reference lists of included studies and any relevant SLRs or NMAs identified during the screening were searched to identify further studies.
rmdopen-2021-002074supp001.pdf (5.9MB, pdf)
Study selection
Titles and abstracts were assessed for inclusion by one reviewer, with another reviewer performing a 40% check. Full-text articles were fully assessed by two independent reviewers. Randomised controlled trials (RCTs) in patients who were at least 16 years old with active PsA, with ≥50 patients randomised to at least one trial arm, were included in the review. Interventions of interest were limited to abatacept, apremilast, adalimumab, bimekizumab, brodalumab, certolizumab pegol, etanercept, filgotinib, golimumab, guselkumab, infliximab, ixekizumab, netakimab, risankizumab, secukinumab, tildrakizumab, tofacitinib, upadacitinib and ustekinumab. Treatments could be reported as monotherapy or in combination with another systemic therapy. Only articles published in English were considered eligible. A full set of inclusion and exclusion criteria can be found in online supplemental table S3.
Data extraction and quality assessment
Details of the study design, baseline patient characteristics, interventions, outcomes and results were extracted by one reviewer and quality checked by another. The methodological quality of included studies was assessed by one reviewer using the Cochrane Risk of Bias tool28 and checked by a second reviewer.
To reduce the risk of statistical heterogeneity in our analysis, we assessed clinical heterogeneity in our evidence by closely examining variability in the participants, interventions and outcomes studied. We also assessed methodological heterogeneity by looking for variability in study design and risk of bias. The aim of these assessments was to identify imbalances between trials in potential treatment effect modifiers and use this information to inform the statistical analysis plan.
Network meta-analysis
Using recommended methods for evidence synthesis,29 a Bayesian NMA compared the relative efficacy and safety of licensed and unlicensed systemic therapies for the treatment of active PsA. Efficacy endpoints included ACR response rates (ACR20, ACR50 and ACR70), Psoriasis Area and Severity Index (PASI) response rates (PASI75 and PASI90), and the resolution of enthesitis and dactylitis (measured by any scale that represents resolution with a score of zero); safety end points included the proportion patients discontinuing due to adverse events (DAE). Relative efficacy for all endpoints was based on results reported at 12–16 weeks, where available, or up to 26 weeks if the earlier time point was unavailable. Safety outcomes were evaluated at study endpoint. All outcomes were assessed in the overall population regardless of prior bDMARD exposure, while ACR response rates were also explored in bDMARD-naïve and bDMARD-experienced subgroups.
ACR and PASI responses were analysed using a multinomial likelihood model with a probit link. Resolution of enthesitis and dactylitis, and DAE were analysed using a binomial likelihood model with a logit link. Both random-effects and fixed-effects models were run, and the goodness of fit was assessed using the deviance information criterion. For efficacy outcomes, network meta-regressions to control for cross-trial variation in placebo-arm responses were also carried out on the model with the best fit.30 Adjusted and unadjusted models were then compared for fit, informed by the statistical significance of the regression coefficient. The model with the best fit was used to draw conclusions.
Inconsistency between direct and indirect estimates of effect was assessed for any loops in the evidence the network using the two-stage Bucher method.31 32 Across the networks for all outcomes, there were up to four closed loops, made up of placebo, adalimumab and either ixekizumab, secukinumab, tofacitinib or upadacitinib. No evidence of inconsistency was found.
WinBUGS V.1.433 was used to perform all statistical analyses, using non-informative priors. After an initial burn-in of at least 20 000 simulations, convergence was confirmed through visual inspection the Brook-Gelman-Rubin diagnostic and history plots. Sampled parameters were then estimated using 50 000 simulations on three chains. Results were calculated as the absolute probabilities of response for each treatment and as treatment effects for each pairwise comparison vs placebo for each endpoint. Point estimates reflecting the median value are presented, along with 95% credible intervals (95% CrI), reflecting the range of true effects with 95% probability. For results presented on a scale that requires a baseline for calculation, a meta-analysis estimate of the placebo arm effect across the placebo-controlled trials was used.34 Significance between comparators was determined from the 95% CrI of the treatment effect. Where it excludes the line of null effect, we can describe the difference as statistically significant.
Results for key comparators—biological therapies at doses licensed for use in PsO or PsA—are presented below. Results for all comparators, including conventional and targeted oral systemic and unlicensed biological therapies or unlicensed doses of licensed biological therapies) are presented in online supplemental file 1 along with pairwise comparisons between key comparators and selected systemic therapies.
Results
Identification of trials
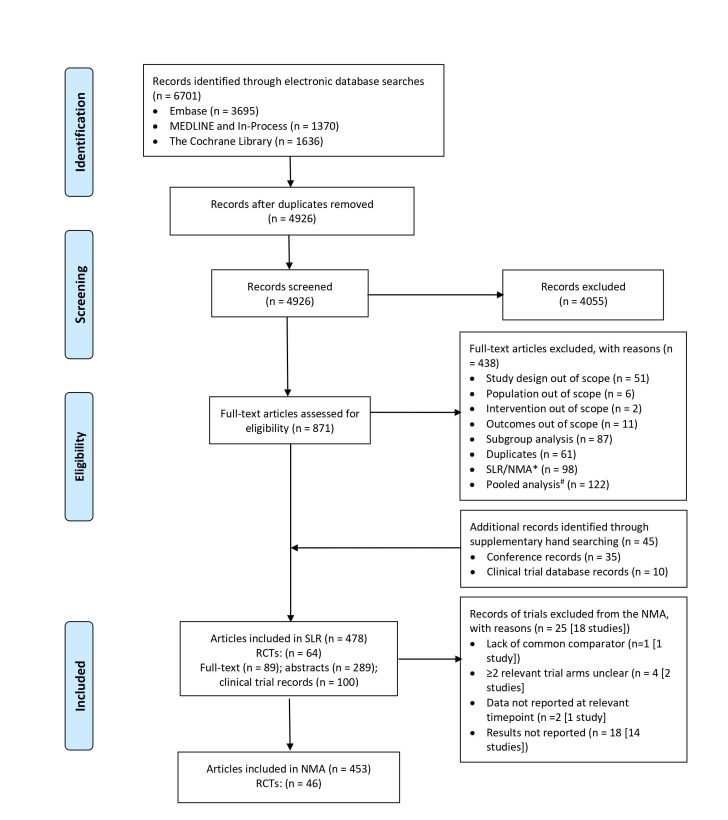
Electronic database searches identified 6701 articles. After deduplication, 4926 titles and abstracts were screened for inclusion and a subsequent 871 publication assessed for their eligibility. A further 45 articles were identified and included through searching conferences and clinical trial databases. A total of 64 RCTs reported in 478 articles were included in the SLR. Seventeen trials were not included in the NMA either because they did not report results or did not report them at a time point of interest or because the dosing regimen during the randomised treatment phase was unclear. One further trial was considered for the NMA, but due to a lack of common comparator with other studies in the network it could not be included.35 Data from 46 unique RCTs were included in at least one NMA. Details are shown in figure 1.
Figure 1.
PRISMA flow diagram. NMA, network meta-analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCTs, randomised controlled trial; SLR, systematic literature review.* Bibliographies of eligible SLR/ NMAs were reviewed to identify any RCTs that met the inclusion criteria and then excluded. # Bibliographies of eligible pooled analyses were reviewed to identify relevent RCTs that met the inclusion criteria.and included only if data were not reported in the individual RCTs.
Network meta-analysis
Study and patient characteristics
Key details of trials included in the NMA are summarised in table 1. Further details of baseline and patient characteristics from studies included in the NMA are provided in online supplemental table S4.
Table 1.
Studies included in the NMA
| Study | Treatment arms | No. patients (N) | Timepoint (weeks) | Outcomes included in NMA |
| ASTRAEA24 | Placebo | 211 | 16 | ACR–20, 50, 70; PASI 75*; EnthR*; DactR*; DAE |
| Abatacept 125 mg | 213 | |||
| ADEPT70 | Placebo | 162 | 12 | ACR–20, 50, 70; PASI–75, 90; DAE |
| Adalimumab 40 mg | 153 | |||
| Genovese 200771 | Placebo | 51 | 12 | ACR–20, 50, 70; DAE |
| Adalimumab 40 mg | 51 | |||
| Mease 201861 | Placebo | 24 | 12 | ACR–20, 50, 70; PASI–75; 90; DAE |
| Adalimumab 40 mg | 72 | |||
| Remtolumab 120 mg | 71 | |||
| Remtolumab 240 mg | 73 | |||
| ACTIVE62 | Placebo | 109 | 16 | ACR–20, 50, 70; DAE |
| Apremilast 30 mg twice daily | 110 | |||
| Schett 201236 | Placebo | 68 | 12 | ACR–20, 50, 70; DAE |
| Apremilast 20 mg twice daily | 69 | |||
| Apremilast 40 mg once daily | 67 | |||
| PALACE 137 | Placebo | 168 | 16 | ACR–20, 50, 70; PASI 75*; EnthR; DactR; DAE |
| Apremilast 20 mg twice daily | 168 | |||
| Apremilast 30 mg twice daily | 168 | |||
| PALACE 238 | Placebo | 159 | 16 | ACR–20, 50, 70; PASI 75; EnthR; DactR; DAE |
| Apremilast 20 mg twice daily | 163 | |||
| Apremilast 30 mg twice daily | 162 | |||
| PALACE 339 | Placebo | 169 | 16 | ACR–20, 50, 70; PASI 75; EnthR; DactR; DAE |
| Apremilast 20 mg twice daily | 169 | |||
| Apremilast 30 mg twice daily | 167 | |||
| Mease 201422 | Placebo | 55 | 12 | ACR–20, 50, 70; DAE |
| Brodalumab 140 mg | 57 | |||
| Brodalumab 280 mg | 56 | |||
| AMVISION 123 | Placebo | 161 | 16 | ACR–20, 50, 70; PASI–75, 90; EnthR; DactR; DAE |
| Brodalumab 140 mg | 158 | |||
| Brodalumab 210 mg | 159 | |||
| AMVISION 223 | Placebo | 161 | 16 | ACR–20, 50, 70; PASI–75, 90; EnthR; DactR; DAE |
| Brodalumab 140 mg | 160 | |||
| Brodalumab 210 mg | 163 | |||
| RAPID-PsA40 | Placebo | 136 | 12 | ACR–20, 50, 70; PASI–75, 90; DAE |
| Certolizumab 200 mg every two weeks | 138 | |||
| Certolizumab 400 mg every four weeks | 135 | |||
| Mease 200472 | Placebo | 104 | 12 | ACR–20, 50, 70; PASI 75*; DAE |
| Etanercept 25 mg twice weekly | 101 | |||
| PRESTA63 | Etanercept 50 mg once weekly | 373 | 12 | ACR–20, 50, 70; PASI 75*; DAE |
| Etanercept 50 mg twice weekly | 379 | |||
| SEAM-PsA66 | Placebo +MTX 20 mg once weekly | 284 | 16 | ACR–20, 50, 70; DAE |
| Placebo +Etanercept 50 mg once weekly | 284 | |||
| Etanercept 50 mg +MTX 20 mg once weekly | 283 | |||
| EQUATOR26 | Placebo | 66 | 16 | ACR–20, 50, 70; PASI 75; EnthR; DactR; DAE |
| Filgotinib 200 mg | 65 | |||
| GO-REVEAL41 | Placebo | 113 | 14 | ACR–20, 50, 70; PASI–75, 90; DAE |
| Golimumab 50 mg | 146 | |||
| Golimumab 100 mg | 146 | |||
| GO-VIBRANT42 | Placebo | 239 | 14 | ACR–20, 50, 70; PASI–75, 90; DAE |
| Golimumab 2 mg/kg | 241 | |||
| GO-DACT64 | Placebo +MTX 15–25 mg | 23 | 12 | DactR |
| Golimumab 50 mg +MTX 15–25 mg | 21 | |||
| DISCOVER 117 | Placebo | 126 | 16 | ACR–20, 50, 70; PASI*–75, 90; EnthR*, DactR*; DAE |
| Guselkumab 100 mg every eight weeks | 128 | |||
| Guselkumab 100 mg every four weeks | 128 | |||
| DISCOVER 218 | Placebo | 247 | 16 | ACR–20, 50, 70; PASI*–75, 90; EnthR*; DactR*; DAE |
| Guselkumab 100 mg every eight weeks | 248 | |||
| Guselkumab 100 mg every four weeks | 246 | |||
| Deodhar 201819 | Placebo | 49 | 16 | ACR–20, 50, 70; PASI*–75, 90; EnthR*; DactR*; DAE |
| Guselkumab 100 mg every eight weeks | 100 | |||
| IMPACT43 | Placebo | 52 | 16 | ACR–20, 50, 70; PASI–75, 90; DAE |
| Infliximab 5 mg/kg | 52 | |||
| IMPACT 244 | Placebo | 100 | 14 | ACR–20, 50, 70; PASI–75, 90; DAE |
| Infliximab 5 mg/kg | 100 | |||
| RESPOND65 | Infliximab 5 mg/kg+MTX 15 mg | 57 | 16 | ACR–20, 50, 70; PASI–75, 90; DAE |
| MTX 15 mg | 58 | |||
| SPIRIT H2H45 | Adalimumab 40 mg | 283 | 16 | ACR–50; PASI*–75, 90; EnthR*; DactR*; DAE |
| Ixekizumab 80 mg | 283 | |||
| SPIRIT P146 | Placebo | 106 | 12 | ACR–20, 50, 70; PASI–75, 90; EnthR†; DactR†; DAE |
| Adalimumab 40 mg | 101 | |||
| Ixekizumab 80 mg every four weeks | 107 | |||
| Ixekizumab 80 mg every two weeks | 103 | |||
| SPIRIT P247 | Placebo | 118 | 12 | ACR†–20, 50, 70; PASI–75, 90; EnthR; DactR; DAE |
| Ixekizumab 80 mg every four weeks | 122 | |||
| Ixekizumab 80 mg every two weeks | 123 | |||
| PATERA48 | Placebo | 97 | 24 | ACR–20, 50, 70 |
| Netakimab 120 mg | 97 | |||
| CHOICE49 | Placebo | 52 | 16 | ACR–20, 50, 70; PASI–75, 90; EnthR; DactR; DAE |
| Secukinumab 150 mg | 103 | |||
| Secukinumab 300 mg | 103 | |||
| FUTURE 150 | Placebo | 202 | 16 | ACR–20, 50, 70; PASI*–75, 90; EnthR*; DactR*; DAE |
| Secukinumab 75 mg (IV LD) | 202 | |||
| Secukinumab 150 mg (IV LD) | 202 | |||
| FUTURE 251 | Placebo | 98 | 16 | ACR–20, 50, 70; PASI*–75, 90; EnthR*; DactR*; DAE |
| Secukinumab 75 mg (SC LD) | 99 | |||
| Secukinumab 150 mg | 100 | |||
| Secukinumab 300 mg | 100 | |||
| FUTURE 367 | Placebo | 137 | 16 | ACR–20, 50; PASI*–75, 90; EnthR*; DactR*; DAE |
| Secukinumab 150 mg | 138 | |||
| Secukinumab 300 mg | 139 | |||
| FUTURE 468 | Placebo | 114 | 16 | ACR–20, 50, 70; PASI–75, 90; EnthR; DactR |
| Secukinumab 150 mg (no LD) | 113 | |||
| Secukinumab 150 mg | 114 | |||
| FUTURE 552 | Placebo | 332 | 16 | ACR–20, 50, 70; PASI–75, 90; EnthR; DactR; DAE |
| Secukinumab 150 mg (no LD) | 222 | |||
| Secukinumab 150 mg | 220 | |||
| Secukinumab 300 mg | 222 | |||
| EXCEED53 | Adalimumab 40 mg | 427 | 16 | ACR–20, 50, 70; PASI–75, 90; EnthR; DactR; DAE |
| Secukinumab 300 mg | 426 | |||
| MAXIMISE69 | Placebo | 166 | 12 | ACR 20 |
| Secukinumab 150 mg | 165 | |||
| Secukinumab 300 mg | 167 | |||
| Gottlieb 201920 | Placebo | 79 | 16 | ACR–20, 50, 70; PASI–75, 90; DAE |
| Tildrakizumab 20 mg | 78 | |||
| Tildrakizumab 100 mg | 77 | |||
| Tildrakizumab 200 mg | 79 | |||
| Opal Beyond54 | Placebo | 131 | 13 | ACR–20, 50, 70; PASI 75; EnthR; DactR; DAE |
| Tofacitinib 5 mg twice daily | 131 | |||
| Tofacitinib 10 mg twice daily | 132 | |||
| Opal Broaden55 | Placebo | 105 | 13 | ACR–20, 50, 70; PASI 75; EnthR; DactR; DAE |
| Adalimumab 40 mg | 106 | |||
| Tofacitinib 5 mg twice daily | 107 | |||
| Tofacitinib 10 mg twice daily | 104 | |||
| SELECT-PsA 156 | Placebo | 423 | 16 | ACR‡–20, 50, 70; PASI 75; EnthR1; DactR1; DAE |
| Upadacitinib 15 mg once daily | 429 | |||
| Upadacitinib 30 mg once daily | 423 | |||
| Adalimumab 40 mg | 429 | |||
| SELECT-PsA 257 | Placebo | 212 | 16 | ACR‡–20, 50, 70; PASI 75; EnthR; DactR; DAE |
| Upadacitinib 15 mg once daily | 211 | |||
| Upadacitinib 30 mg once daily | 218 | |||
| Gottlieb 200958 | Placebo | 70 | 12 | ACR–20, 50, 70; PASI–75, 90; DAE |
| Ustekinumab 90 mg | 76 | |||
| PSUMMIT 159 | Placebo | 206 | 16 | ACR–20; PASI 75*; EnthR*; DactR*; DAE |
| Ustekinumab 45 mg | 205 | |||
| Ustekinumab 90 mg | 204 | |||
| PSUMMIT 260 | Placebo | 104 | 16 | ACR–20; PASI 75*; EnthR*; DactR*; DAE |
| Ustekinumab 45 mg | 103 | |||
| Ustekinumab 90 mg | 105 |
*Data reported at week 24.
†Data reported at week 16.
‡Data reported at week 12.
ACR, American College of Rheumatology; DactR, resolution of dactylitis; DAE, withdrawal due to adverse events; EnthR, resolution of enthesitis; IV, intravenous; LD, loading dose; MTX, methotrexate; NMA, network meta-analysis; PASI, Psoriasis Area and Severity Index; SC, subcutaneous.
Patient eligibility criteria across the included studies were largely consistent. Thirty-eight trials required patients to have been diagnosed with PsA for a minimum of 3 months,17–20 22 23 26 36–65 and 36 trials used CASPAR as PsA diagnostic criteria.17–20 22 24 26 35 37–40 42 45–57 60–62 64 66–69 All trials except for GO-DACT64 and MAXIMISE69 reported the number of swollen and tender joints required at baseline.
Patients mean age at baseline was reasonably consistent across the included studies, ranging from 41.265 to 53.4 years.57 More variable characteristics were the duration of PsA (ranging from 3.266 to 11.4 years),43 and the percentage of females (ranging from 37.1%63 and 64.6%22). The number of patients with prior exposure to biological therapies varied across trials from 100% exposure in three trials,47 54 57 to no exposure in twenty.18 35 41–46 49 53 59 62–66 69–72 Twenty further RCTs reported the percentage of patients previously exposed to biologics, ranging from 3.2%55 to 61.1%.24 Ten trials included 100% of patients with prior exposure to csDMARDs.24 26 35 43 45 47 53 55 56 71 Twelve further RCTs reported the percentage of patients previously exposed to csDMARDs, ranging from 13.1%66 to 88.1%.19
A summary of the risk of bias of included studies, as measured by the Cochrane risk of bias tool, is presented in online supplemental figures S1 and S2. Statistics used to assess goodness of model fit for each network of evidence are presented in online supplemental table S5.
ACR response
The ACR network for the overall population is presented in figure 2 and included 45 studies17–20 22–24 26 36–47 49–62 65–68 70 71 of 19 treatments broken down across 44 unique treatment regimens. Figures for the evidence networks broken down by bDMARD-exposure subgroup are presented in online supplemental figures S3 and S4.
Figure 2.
Network diagram for ACR response note that the node size denotes total number of patients randomised to that treatment; edge line thickness denotes total number of studies informing that comparison. ACR, American College of Rheumatology; BID, twice daily; BIW, twice weekly; IV, intravenous; LD, loading dose; MTX, methotrexate; QD, once daily; QW, weekly; Q2W, every two weeks; Q4W, every four weeks; Q8W, every eight weeks; Q12W, every twelve weeks.
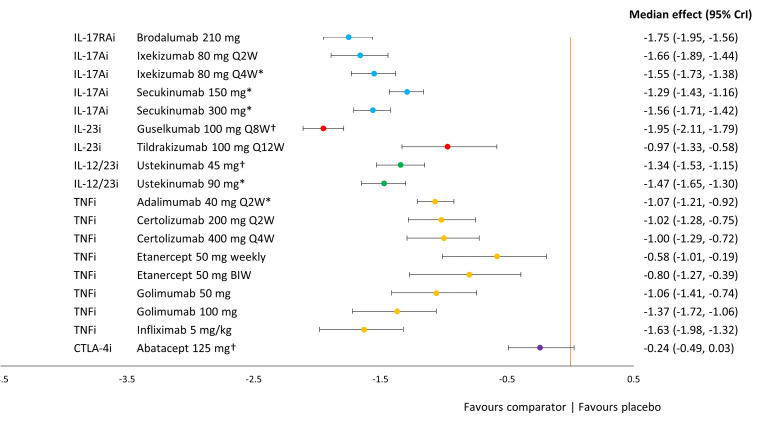
Figure 3 presents the ACR treatment effects of each key comparator vs placebo on the probit scale, where 0 represents no difference and negative values indicate higher response rates associated with treatment. All key comparators were more efficacious than placebo. Infliximab 5 mg in combination with or without methotrexate showed the greatest effect, followed by all regimens of etanercept: 50 mg QW, 50 mg two times in a week and 50 mg in combination with methotrexate. The poorest performing licensed biological therapies were ustekinumab (45 mg and 90 mg) and abatacept, which tended to be significantly less effective than TNFi therapies and a subset of IL-17A and IL-23 inhibitor therapies (online supplemental table S6).
Figure 3.
Forest plot of treatment effects for key comparators versus placebo on ACR response. ACR overall treatment effect was based on a random-effects model with placebo adjustment. median treatment effects and 95% credible intervals are plotted on the probit scale. Key comparators include bDMARDs at doses licensed for use in PSA or PSO. ACR, American College of Rheumatology; bDMARD, biological disease modifying anti-rheumatic drug; BIW, twice weekly; MTX, methotrexate; PSA, psoriatic arthritis; PSO, psoriasis; QD, once daily; QW, weekly; Q2W, every two weeks; Q4W, every four weeks; Q8W, every eight weeks; Q12W, every twelve weeks.
Treatment effects for other comparators included in the NMA are reported in online supplemental figure S5. Of these comparators, the licensed oral therapies—upadacitinib, tofacitinib and apremilast—performed similarly to golimumab, IL-23 inhibitors and ustekinumab, respectively. Unlicensed comparators, such as filgotinib and remtolumab showed a strong response, though the evidence for each come from small phase 2 studies.
Treatment effects from subgroup analyses of ACR response based on prior bDMARD exposure are presented in online supplemental figures S6 and S7. Expected probabilities of ACR 20, 50 and 70 response for the overall population as well as bDMARD-naïve and bDMARD-experienced patients are presented together in table 2 for key comparators and in online supplemental table S7 for all evaluated interventions.
Table 2.
Expected probabilities of ACR response by bDMARD exposure subgroup for key comparators
| Intervention* | Probability of response, median (95% credible interval) | ||||||||
| All patients | bDMARD-naïve | bDMARD-exposed | |||||||
| ACR 20 | ACR 50 | ACR 70 | ACR 20 | ACR 50 | ACR 70 | ACR 20 | ACR 50 | ACR 70 | |
| Brodalumab 210 mg | 48.6% (37.6, 59.7) |
24.6% (16.7, 34.3) |
9.8% (16.7, 34.3) |
53.1% (40.4, 65.3) |
28.5% (18.7, 40) |
13% (18.7, 40) |
32.3% (15.9, 53.1) |
14.7% (5.6, 30.6) |
5.4% (5.6, 30.6) |
| Ixekizumab 80 mg every two weeks | 56% (44.5, 66.6) |
30.9% (21.5, 41.3) |
13.4% (21.5, 41.3) |
60.2% (45.1, 74.2) |
34.9% (22.1, 50.1) |
17.3% (22.1, 50.1) |
47.2% (28.1, 67) |
25.5% (12.1, 44.1) |
11.2% (12.1, 44.1) |
| Ixekizumab 80 mg every four weeks | 55.1% (44.5, 64.8) |
30% (21.5, 39.3) |
12.9% (21.5, 39.3) |
55.5% (43.1, 67.7) |
30.6% (20.6, 42.5) |
14.4% (20.6, 42.5) |
49.4% (29.9, 69) |
27.3% (13.2, 46.4) |
12.2% (13.2, 46.4) |
| Secukinumab 150 mg | 51.5% (42.7, 60.4) |
27% (20.2, 35) |
11.1% (20.2, 35) |
54.2% (45.4, 63.3) † |
29.4% (22.2, 38) † |
13.6% (22.2, 38) † |
40.7% (23.7, 59.7) † |
20.5% (9.6, 36.6) † |
8.3% (9.6, 36.6) † |
| Secukinumab 300 mg | 56.3% (47.4, 64.8) |
31.2% (23.7, 39.4) |
13.6% (23.7, 39.4) |
57.2% (48.2, 66.3)† |
32.1% (24.4, 41.1)† |
15.4% (24.4, 41.1)† |
51% (32.2, 69.4) † |
28.6% (14.7, 46.9)† |
13.1% (14.7, 46.9)† |
| Guselkumab 100 mg every eight weeks | 51.6% (40.5, 61) |
27.1% (18.6, 35.5) |
11.1% (18.6, 35.5) |
52.6% (41, 63.4)† |
28.1% (19.1, 38.1)† |
12.8% (19.1, 38.1)† |
63% (36.2, 84.8)‡ |
39.8% (17.3, 67)‡ |
20.7% (17.3, 67)‡ |
| Tildrakizumab 100 mg every twelve weeks | 48.9% (32.9, 64.3) |
24.9% (13.7, 38.8) |
9.9% (13.7, 38.8) |
– | – | – | – | – | – |
| Ustekinumab 45 mg | 34.2% (24.6, 45.9) |
14.5% (9.1, 22.5) |
4.8% (9.1, 22.5) |
41.8% (29.7, 55.1) |
19.6% (11.9, 30.2) |
7.9% (11.9, 30.2) |
32.9% (13.6, 59) |
15.1% (4.6, 36) |
5.6% (4.6, 36) |
| Ustekinumab 90 mg | 41.6% (32, 52.7) |
19.4% (13.2, 28) |
7.1% (13.2, 28) |
46% (33.5, 59) |
22.8% (14.1, 33.8) |
9.6% (14.1, 33.8) |
31.9% (13, 57.9) |
14.5% (4.3, 34.9) |
5.3% (4.3, 34.9) |
| Adalimumab 40 mg every two weeks | 55.8% (46.5, 63.6) |
30.7% (23, 38.1) |
13.3% (23, 38.1) |
55.5% (46.3, 64.8) |
30.6% (22.9, 39.4) |
14.4% (22.9, 39.4) |
– | – | – |
| Certolizumab 200 mg every two weeks | 61.3% (47.1, 73.8) |
35.8% (23.5, 49.4) |
16.6% (23.5, 49.4) |
55.8% (41, 70.3) |
30.9% (19.1, 45.4) |
14.6% (19.1, 45.4) |
65.3% (36.4, 88.1) |
42.2% (17.5, 72.3) |
22.5% (17.5, 72.3) |
| Certolizumab 400 mg every four weeks | 52.2% (37.9, 65.7) |
27.6% (16.9, 40.3) |
11.5% (16.9, 40.3) |
||||||
| Etanercept 50 mg once weekly | 61.8% (47.2, 75.6) |
36.3% (23.6, 51.7) |
16.9% (23.6, 51.7) |
60.3% (42.8, 75.1) |
35% (20.3, 51.3) |
17.3% (20.3, 51.3) |
– | – | – |
| Etanercept 50 mg twice weekly | 64.8% (46.7, 80.4) |
39.4% (23.2, 58.1) |
19% (23.2, 58.1) |
63.3% (40.8, 80.4) |
38% (19, 58.3) |
19.5% (19, 58.3) |
– | – | – |
| Etanercept 50 mg and Methotrexate 20 mg once weekly | 63% (44.4, 79.4) |
37.5% (21.5, 56.7) |
17.7% (21.5, 56.7) |
61.6% (38.7, 79.3) |
36.2% (17.5, 56.7) |
18.2% (17.5, 56.7) |
– | – | – |
| Golimumab 50 mg | 58.4% (43.3, 73.9) |
33% (20.6, 49.6) |
14.7% (20.6, 49.6) |
55.9% (35.3, 73.3) |
30.9% (15.3, 48.9) |
14.6% (15.3, 48.9) |
– | – | – |
| Golimumab 100 mg | 57.2% (42, 72.9) |
32% (19.7, 48.3) |
14.1% (19.7, 48.3) |
55% (34.6, 72.5) |
30.1% (14.8, 48) |
14.1% (14.8, 48) |
– | – | – |
| Infliximab 5 mg/kg | 68.4% (56.7, 79.9) |
43.2% (31.5, 57.5) |
21.8% (31.5, 57.5) |
66.6% (51.6, 79.3) |
41.4% (27.2, 56.8) |
22% (27.2, 56.8) |
– | – | – |
| Infliximab 5 mg/kg and methotrexate 15 mg | 76.5% (52, 92.1) |
52.9% (27.4, 77.7) |
29.6% (27.4, 77.7) |
75.3% (45, 92.2) |
51.5% (22, 77.9) |
30.2% (22, 77.9) |
– | – | – |
| Abatacept 125 mg | 38.4% (25.7, 51.6) |
17.2% (9.6, 27.1) |
6% (9.6, 27.1) |
45.9% (29.1, 63.8) |
22.7% (11.5, 38.4) |
9.6% (11.5, 38.4) |
26.4% (12.6, 45.1) |
11.1% (4.1, 23.9) |
3.8% (4.1, 23.9) |
| Placebo | 20.7% (9.5, 37.3) |
7.1% (2.5, 16.5) |
1.9% (2.5, 16.5) |
20.9% (10.2, 36.4)† |
7.2% (2.7, 16)† |
2.2% (2.7, 16)† |
18.3% (9.6, 30.7)† |
6.8% (2.9, 13.8)† |
2% (2.9, 13.8)† |
*Key comparators include bDMARDs at doses licensed for use in PsA or PsO.
†Based on a combination of studies reporting outcomes at week 12 or 16 and week 24.
‡Based on studies reporting outcomes at week 24 only.
ACR, American College of Rheumatology; bDMARD, biological disease-modifying antirheumatic drug; PsA, psoriatic arthritis; PsO, psoriasis.
Thirty-six studies17–19 23 24 26 37–46 49–53 55 59 60 62 63 65–72 assessing 33 unique treatment regimens were included in the bDMARD-naïve network and findings were consistent with the overall analysis: all key comparators were more effective than placebo. Similarly, infliximab showed the greatest efficacy, followed by etanercept. Most key comparators performed the same or slightly better among bDMARD-naive patients than in the overall analysis; only the efficacy of guselkumab appeared to decrease slightly. Ustekinumab, abatacept and apremilast remained the least efficacious licensed therapies in this subgroup.
For the subgroup of bDMARD-experienced patients, 20 studies17 19 22–24 26 37–40 47 50–52 54 57 60 67 68 evaluating 25 unique treatment regimens were included. All key comparators, except ustekinumab, were more effective than placebo. Certolizumab performed best, followed by ixekizumab and secukinumab. The treatment effect of abatacept showed a slight improvement and brodalumab, a slight deterioration compared with the overall population analysis.
PASI response
Twenty-two studies reported PASI 75 and/or PASI 90 at 12–16 weeks and a further 14 studies reported PASI outcomes at 24 weeks, allowing for the evaluation of 37 treatment regimens (see online supplementary figure S8 for network diagram).17–20 23 24 26 37–55 58–61 63 67 68 70 72 Figure 4 presents the PASI treatment effects of each key comparator versus placebo on the probit scale and table 3 presents the expected probabilities of PASI 75 and 90 response. Treatment effects for other comparators included in the NMA are reported in online supplementary figure S9 and online supplemental table S8.
Figure 4.
Forest plot of treatment effects for key comparators versus placebo on PASI response. *Based on a combination of studies reporting outcomes at week 12 or 16 and week 24; †Based on studies reporting outcomes at week 24 only note that PASI treatment effect was based on a fixed-effects model with placebo adjustment. Median treatment effects and 95% credible intervals are plotted on the probit scale. Key comparators include bDMARDs at doses licensed for use in PSA or PSO. bDMARD, biological disease modifying antirheumatic drug; BIW, twice weekly; PASI, Psoriasis Area and Severity Index; PSA, psoriatic arthritis; PSO, psoriasis; Q2W, every two weeks; Q4W, every four weeks; Q8W, every eight weeks; Q12W, every twelve weeks.
Table 3.
Expected probabilities of response by outcome for key comparators
| Intervention* | Probability of response, median (95% credible interval) | ||||
| PASI 75 | PASI 90 | Resolution of enthesitis | Resolution of dactylitis | Discontinuation due to AEs | |
| Brodalumab 210 mg | 70.3% (62.3, 77.4) | 52.7% (44, 61.3) | 38% (24.9, 52.6) | 53.6% (38.5, 70.6) | 1.4% (0.2, 7.2) |
| Ixekizumab 80 mg every two weeks | 67% (57.8, 75.5) | 49% (39.4, 58.9) | 40.5% (26.7, 56.3) | 59.8% (36.3, 77.4) | 3.9% (1, 14.5) |
| Ixekizumab 80 mg every four weeks† | 63.1% (55.4, 70.3) | 44.9% (37.1, 52.7) | 33.1% (21.5, 46.8) | 63.2% (41.7, 78.2) | 2.1% (0.6, 7.8) |
| Secukinumab 150 mg† | 53.2% (45.7, 59.5) | 35% (28.3, 41.1) | 41.6% (29.6, 53.7) | 50.5% (35.8, 67.2) | 2.3% (0.6, 8.2) |
| Secukinumab 300 mg† | 63.7% (56.3, 69.5) | 45.4% (37.9, 51.9) | 44.4% (32, 56.7) | 62.4% (47.4, 77.1) | 1.9% (0.6, 6.5) |
| Guselkumab 100 mg every eight weeks‡ | 76.6% (68.5, 82.2) | 60.3% (50.7, 67.5) | 42.3% (28.1, 56.3) | 60.4% (42.4, 73.8) | 1.9% (0.4, 8.4) |
| Tildrakizumab 100 mg every twelve weeks | 40.2% (25.5, 55.1) | 23.7% (13, 36.8) | – | – | 11.8% (0.4, 98.3) |
| Ustekinumab 45 mg‡ | 60% (52.8, 67.8) | 41.6% (34.6, 49.9) | 32.9% (21.1, 49.9) | 43.9% (29.5, 62.2) | 0.6% (0.1, 2.9) |
| Ustekinumab 90 mg | 54.8% (46.6, 63)* | 36.5% (29.1, 44.8)* | 40.4% (27.2, 57.9)† | 46.8% (32, 65)† | 0.7% (0.1, 3.2) |
| Adalimumab 40 mg every two weeks† | 44.2% (36.9, 50.7) | 27% (21.2, 32.8) | 39.7% (27.6, 52.6) | 51.3% (33.3, 68.1) | 3.8% (1.2, 11.8) |
| Certolizumab 200 mg every two weeks | 42.2% (31, 53) | 25.4% (16.8, 34.8) | – | – | 5.2% (0.7, 36.8) |
| Certolizumab 400 mg every four weeks | 41.5% (30, 53.3) | 24.8% (16.1, 35.2) | – | – | 8.2% (1.2, 47.4) |
| Etanercept 50 mg once weekly | 26.1% (15.4, 42.2) | 13.5% (6.8, 25.4) | – | – | 2.6% (0.1, 48.7) |
| Etanercept 50 mg +MTX 20 mg once weekly | – | – | – | – | 3.3% (0.1, 56.7) |
| Etanercept 50 mg twice weekly | 33.9% (20.1, 52.5) | 18.9% (9.6, 34.3) | – | – | 4.2% (0.1, 63.1) |
| Golimumab 50 mg | 43.5% (32.1, 58.4) | 26.5% (17.6, 40) | – | – | 0.8% (0.1, 6.4) |
| Golimumab 100 mg | 55.7% (44, 69.6) | 37.4% (26.9, 52) | – | – | 0.8% (0.1, 6.4) |
| Infliximab 5 mg/kg | 65.8% (54.7, 78) | 47.7% (36.4, 62) | – | – | 8.2% (1.3, 47) |
| Infliximab 5 mg/kg+MTX 15 mg | – | – | – | – | 12.4% (0.2, 89.9) |
| Abatacept 125 mg‡ | 16.3% (10.4, 23.9) | 7.4% (4.2, 12) | 33.9% (20, 51.8) | 43.1% (25.2, 62.3) | 0.6% (0, 7.2) |
| Placebo† | 9.7% (3.4, 22) | 3.9% (1.1, 10.8) | 23% (12.7, 37.8) | 31.1% (11, 62.3) | 2.4% (0.8, 7.1) |
*Key comparators include bDMARDs at doses licensed for use in PsA or PsO.
†Based on a combination of studies reporting outcomes at week 12 or 16 and week 24.
‡Based on studies reporting outcomes at week 24 only bDMARD.
AE, adverse event; bDMARD, biological disease-modifying antirheumatic drug; MTX, methotrexate; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, psoriasis.
All key comparators were more efficacious than placebo. Guselkumab 100 mg Q8W was associated with the largest treatment effect versus placebo, followed by brodalumab 210 mg, an IL-17RA inhibitor and the other IL-17A inhibitors—ixekizumab 80 mg (Q2W and Q4W) and secukinumab 300 mg—and infliximab. Differences between guselkumab, brodalumab and infliximab were not found to be statistically significantly different, nor were differences between brodalumab and infliximab and the other IL-17A inhibitors. Brodalumab and guselkumab were shown to be more efficacious than ustekinumab (45 and 90 mg); the 300 mg dose of secukinumab and the fortnightly dose of ixekizumab were also more efficacious than the 45 mg dose of ustekinumab. Brodalumab, ixekizumab and secukinumab 300 mg along with guselkumab, ustekinumab and infliximab were shown to be significantly more efficacious than adalimumab, certolizumab (200 mg and 400 mg), etanercept (50 mg weekly or two times in a week), golimumab 50 mg, abatacept, secukinumab 150 mg and tildrakizumab (online supplemental table S9).
Of the other comparators included in the NMA, the licensed oral therapies—tofacitinib and apremilast—were associated with smaller effect sizes than most biological therapies. Unlicensed comparators, such as filgotinib and remtolumab showed a similar level of PASI response to the licensed comparators, though the evidence for each come from small phase 2 studies.
Resolution of enthesitis and dactylitis
Fourteen RCTs reported evidence on the resolution of enthesitis and 12 RCTs reported evidence on the resolution of dactylitis at 12–16 weeks and a further 10 RCTs reported evidence for both outcomes at 24 weeks, allowing for the evaluation of 22 treatment regimens in each network (online supplemental figures S10 and S11).17–19 23 24 26 37–39 45–47 50–55 59 60 62 67 68
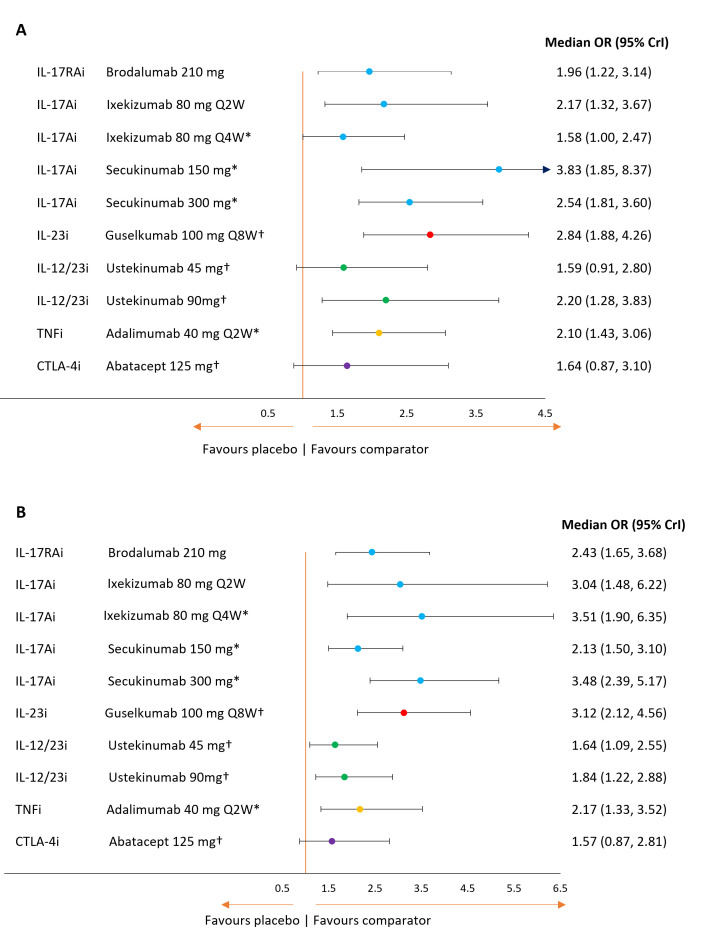
Figure 5A, B presents the ORs for each key comparator vs placebo for resolution of enthesitis and dactylitis, respectively, and table 3 presents the expected probabilities of achieving each endpoint.
Figure 5.
Forest plot of treatment effects for key comparators versus placebo on resolution of enthesitis (A) and dactylitis (B). *Based on a combination of studies reporting outcomes at week 12 or 16 and week 24; †Based on studies reporting outcomes at week 24 only. Note that treatment effect on resolution of enthesitis was based on a random-effects model with placebo adjustment. Treatment effect on resolution of dactylitis was based on a fixed-effects model with placebo adjustment. Key comparators include bDMARDs at doses licensed for use in PSA or PSO. bDMARD, biological disease modifying antirheumatic drug; CrI, credible interval; PSA, psoriatic arthritis; PSO, psoriasis; Q2W, every two weeks; Q4W, every four weeks; Q8W, every eight weeks.
All treatments were more efficacious than placebo in terms of the proportion of patients achieving a resolution of enthesitis, though the effects were not statistically significant for ustekinumab 45 mg and abatacept. Among the key comparators, there was little differentiation between adalimumab, ustekinumab 90 mg, secukinumab (150 or 300 mg), ixekizumab every 2 weeks, brodalumab 210 mg or guselkumab 100 mg every 8 weeks (online supplemental table 9).
For the resolution of dactylitis, all key interventions except abatacept were statistically superior to placebo. Based on the median effects, the IL-17A, IL-17RA and IL-23 inhibitors ranked best followed by adalimumab and then ustekinumab. Statistically significant differences between key comparators were limited to secukinumab 300 mg, which was more efficacious than both ustekinumab and abatacept (online supplemental table 9).
Among the other licensed therapies included in the resolution of enthesitis and dactylitis analyses, tofacitinib 10 mg was significantly more effective than placebo on the outcome of dactylitis, but neither tofacitinib 5 mg nor apremilast were significantly more efficacious on either outcome (online supplemental figures 1213 and online supplemental table 9). Results showed filgotinib, an unlicensed therapy, to be significantly more efficacious than placebo on the outcome of enthesitis, but not dactylitis.
Discontinuation due to adverse events
Discontinuations due to AEs were reported in two ways: discontinuation from study drug, which was used in the analysis where available, or discontinuation from study. The analysis relied on DAE reported at the end of study follow-up, howsoever defined by study authors. The DAE network included 43 studies17–20 23 24 26 36–47 49–63 65–68 70–72 2 of which were pooled23 and 43 unique treatment regimens (online supplementary figure S14). Table 3 presents the probability of patient discontinuation due to AEs for key interventions.
Withdrawal was least likely for patients on abatacept 125 mg (0.6%) and ustekinumab 45 mg (0.6%) and 90 mg (0.7%). The analysis showed that patients receiving 50 mg or 100 mg golimumab or brodalumab 210 mg would have a low probability of DAE (0.8% and 1.4%, respectively). Treatments with the greatest risk of DAE were infliximab in combination with (12.4%) and without (8.2%) MTX, tildrakizumab 100 mg every 12 weeks (11.8%) and certolizumab 400 mg every 4 weeks (8.2%) and 200 mg every 2 weeks (5.2%). All other treatments were associated with a risk between 1.9% (guselkumab every 8 weeks and secukinumab 300 mg) to 4.2% (etanercept 100 mg two times weekly). Only the differences between ustekinumab and placebo and adalimumab and placebo reached statistical significance.
Probabilities of DAE for other comparators included in the NMA are reported along with their relative effects versus placebo in in online supplemental figures S15 and S16. Due to the low frequency of DAEs overall, there was substantial uncertainty. Although filgotinib and tildrakizumab were associated with relatively high absolute risks of DAE, there was insufficient evidence to conclude a difference. Only apremilast 30 mg and upadacitinib 30 mg were found to have a statistically significantly greater risk of DAE than placebo.
Discussion
Our SLR identified 46 RCTs for inclusion in an NMA evaluating the efficacy and safety of systemic therapies for the treatment of patients with active PsA. Data for at least one outcome of interest were available for a total of 19 638 patients receiving one of 19 treatments, divided out into up to 43 unique doses or dosing regimens. We compared these treatments on the outcomes of ACR response, PASI response and resolution of enthesitis and dactylitis as well as discontinuation due to AEs and showed that most therapies licensed for active PsA or moderate to severe PsO were better than placebo and similar to one another.
In the last several years, numerous NMAs have been published comparing the efficacy of various treatments in active PsA.73–83 This largely reflects the rapid evolution of the treatment landscape, in terms of new treatments and new clinical trials and the demand for up-to-date, rigorous comparative analyses from health technology assessment agencies. That said, none of the currently published NMAs include all evaluated treatments in this patient population. The results presented here are not dissimilar from those reported by other authors but extends the possible comparisons by taking a more inclusive approach to the available evidence.
To our knowledge, this NMA provides the most recent and comprehensive comparison of treatments evaluated for active PsA. Specifically, it is the first to include the recently published clinical trial data from EXCEED, AMVISION-1 and −2, SELECT-PsA 1 and 2 and GO-DACT.23 53 56 57 64 Though several of the most recent clinical trials have included a head-to-head comparison of targeted therapies, such direct comparisons are still a rarity and many comparisons remain unobserved.45 46 53 55 56 61 In their absence, the network meta-analytical approach gives the most reliable estimate of comparative efficacy and safety. Indeed, where it is possible to compare the results from a head-to-head RCT with those generated in our NMA, they are in broad agreement. For example, EXCEED53 directly compared secukinumab and adalimumab and reported a risk ratio of 1.05 (95% CI 0.89 to 1.26) for the outcome of ACR50. The risk ratio generated from the NMA for the same comparison was 1.02 (95% CrI: 0.85 to 1.21). Similarly, SPIRIT H2H45 and SPIRIT P146 both compared ixekizumab and adalimumab and ACR 50 outcomes at week 16 result in risk ratios of 0.96 (95% CI 0.69 to 1.33) and 1.57 (0.88 to 2.8), respectively. Meta-analysing these results in a pairwise fashion gives rise to a pooled treatment effect of 1.05 (0.89 to 1.23) which is comparable to the effect of 1.01 (95% CrI 0.76 to 1.3) generated in the NMA.
This NMA was based on a systematic review of RCTs evaluating a range of treatments, licensed and unlicensed. We followed a protocol designed for the systematic review; however, this was not registered online. English-language publications were searched, which has the potential to introduce bias; however, we believe this was unlikely to have had a substantial impact on our findings.
We intentionally took a broader approach to the comparators of interest than other recent NMAs, which restricted their inclusion criteria to licensed therapies only. We were interested in comparing any drug, at any dose, that has been evaluated in an RCT among patients with active PsA. This approach meant we could include evidence for recently approved drugs or those in late-stage development, but not yet approved. It also meant that we could assess drugs or doses of drugs that are licensed for the treatment of PsO and see how they performed in patients with PsA, on both dermatological and rheumatological outcomes. Results from our analysis of PASI response among patients with PsA show a similar pattern to those from other NMAs among patients with PsO, which indicate the highest levels of response among IL-17A, IL-17RA and IL-23 inhibitor therapies.84–90
We opted to evaluate unique doses and dosing regimens of different treatments rather than pooling across treatments in order to ensure the usefulness of the data to clinical decision-making. Although pooling all doses and dosing regimens for a given treatment would simplify the networks and streamline the analysis, the results could be misleading. Combining any evaluated dose of a drug included in phase 2 dose-finding studies with ultimately licensed doses could introduce bias by failing to take account of potential dose-response relationships and heterogeneity. The sheer volume of strategies evaluated in RCTs of PsA are reflected in our networks and are presented in full in online supplemental materials, but for the easier interpretation of results, we focused on a subset of 20 key comparators—biological therapies licensed for the treatment of PsA or PsO—that would be most relevant to clinicians.
The SLR identified phase 2, dose-finding studies for risankizumab and bimekizumab, but their small sample size failed to meet the inclusion criteria for further consideration.21 91 Results from the two KEEPsAKE phase 3 studies for risankizumab are anticipated, with preliminary results suggesting that risankizumab is associated with strong ACR and PASI responses. Phase 3 studies of bimekizumab in PsA are ongoing (NCT03895203, NCT03896581) however, completed studies in moderate to severe PsO suggest that bimekizumab will at least have a significant effect on PASI outcomes.92 93 Future updates of these NMAs should include these studies and assess the comparative efficacy and safety of both new therapies.
We focused on commonly investigated outcomes in PsA, including ACR and PASI response rates for efficacy and DAE for safety and tolerability. In addition, we presented results for the resolution of enthesitis and dactylitis, symptoms experienced by more than half of people with PsA and which contribute to disability and quality of life impairment.94 Both outcomes are highly relevant for PsA, for which comparisons in the literature are limited.79–82 Looking at this suite of outcomes helps to contextualise the evidence across disease markers relevant to the patient, including intra-articular, extraarticular and dermatological manifestations of PsA as well as the tolerability of the drugs. Outcomes such as Psoriatic Arthritis Response Criteria (PsARC) and the Health Assessment Questionnaire (HAQ) are also well reported secondary endpoints across the RCT evidence base and have been compared in other NMAs.78 80 81 95 One recent NMA also compared therapies in terms of their efficacy against structural damage using the van der Heijde-Sharp score.83 Outcomes such as PsARC and HAQ have been associated with issues of reliability, in that the former is not fully validated and may be easily achieved, as evidenced by high placebo response rates96 and the latter may be influenced by other factors, including co-morbidities and duration of disease.97
Wherever possible, outcomes reported at 12–16 weeks were synthesised. In the few cases where data were not available at this time point, outcomes reported at week 24 were extracted and included in the analysis. For the outcome of ACR response among the full study populations, all RCTs reported outcomes at the earlier time point; however, there was more diversity for the subgroup analyses on ACR response and for the secondary outcomes of PASI and resolution of enthesitis or dactylitis. For example, the DISCOVER studies17 18 of guselkumab, the ASTRAEA study24 of abatacept and the PSUMMIT studies59 60 of ustekinumab reported PASI, dactylitis and enthesitis outcomes at week 24 only. Similarly, the only PASI response evidence for etanercept was reported at week 24.63 72 For therapies such as adalimumab, ixekizumab and secukinumab, evidence was available for each from studies of varied follow-up, such that the effect sizes reflect a pooling of evidence at week 12 or 16 and week 24. These effects were synthesised with the evidence for drugs evaluated in studies reporting outcomes at week 12 or 16.22 23 26 40–44 54–58 65
Pooling across a narrower time range may have been more appropriate as relative efficacy, particularly for outcomes defined by larger percentage improvements from baseline, continues to increase between 4 and 6 months. This trend is particularly marked for PASI 75, PASI 90 and PASI 100 outcomes, as illustrated in the trends over time from studies such as RAPID-PsA, GO-REVEAL, SPIRIT P1, SPIRIT P2 and Gottlieb et al.20 40 41 46 47 The pooling of evidence across time points has the potential to introduce an important source of heterogeneity leading to bias against drugs studied over a shorter period, such as brodalumab, tildrakizumab, certolizumab, golimumab, infliximab and tofacitinib. This limitation had to be weighed against the fact that excluding evidence beyond 16 weeks would have limited the ability to make comparisons between some of the most relevant comparators. Authors of a recent NMA chose to synthesise all outcomes reported at the study defined endpoint, anywhere from 12 to 26 weeks, regardless of the potential heterogeneity introduced.83 Their results were broadly aligned with those presented here and not dissimilar from the results of evaluations in patients with moderate to severe PsO.84–88
Finally, the focus of these comparative analyses has been on outcomes reported between 3 and 6 months because this is the minimum duration of most RCTs in PsA. PsA is a chronic, lifelong condition, therefore, a better understanding of the biological drugs and targeted therapies beyond this short induction period would be worthwhile. Future work could explore the feasibility and appropriateness of comparisons of longer-term outcomes and possibly the synthesis of real-world registry studies. Indeed, an extension to other types of evidence, may be helpful to assess the durability of response and to detect rarer safety endpoints that may only emerge with long-term treatment.
Conclusions
Results of this NMA confirm the efficacy and acceptability of bDMARDs in patients with active PsA. The anti-TNF therapies infliximab, etanercept, golimumab and certolizumab were among the most effective therapies for ACR response, though the differences between them and other key interventions in these networks including adalimumab, the interleukin-17 inhibitors and guselkumab were small and not statistically significant. Results were consistent across subgroups of patients with and without prior exposure to bDMARDs. Interleukin-17 inhibitors—brodalumab, ixekizumab and secukinumab—along with guselkumab, were the most effective therapies on the outcome of PASI response, followed closely by infliximab and then golimumab, ustekinumab and adalimumab. Although data on the outcomes of enthesitis and dactylitis resolution were comparatively sparse, the analysis showed that adalimumab, guselkumab and IL-17 inhibitors were broadly similar. Tolerability was similar across drugs, though infliximab, certolizumab and tildrakizumab were associated with higher levels of discontinuation due to adverse events.
Acknowledgments
The authors would like to thank Emma Borg, Rikke Kongerslev and Bryony Langford for project management support and technical advice during the systematic review and Michala Mangor Bandier and Samuel Haftel for editorial assistance in writing up the research.
Footnotes
Contributors: All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, contributed substantially to the conception and design (IBM, LMS, KM, CML, CS-G and PH), acquisition of data (LMS, KM and CS-G), analysis of the data (LMS and CML), interpretation of data and drafting of the manuscript (IBM, LMS, KM, CML, CS-G and PH). All authors revised the manuscript critically for important intellectual content, approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. On behalf of all authors, LMS acts as the guarantor of the work.
Funding: This study was funded by LEO Pharma A/S.
Competing interests: IBM has received consulting fees and research funding from Astra Zeneca, Abbvie, Amgen, Boehringer Ingleheim, BMS, Cabaletta, Compugen, Causeway Therapeutics, Eli-Lilly, Evelo, Gilead, Janssen, Novartis, Pfizer, Sanofi, UCB. LMS is an employee of Symmetron Limited and CML was contracted by Symmetron Limited, which received funding from LEO Pharma for this research. CS-G and KM were employed by Symmetron Limited at the time the review was undertaken and the manuscript was written. PH received consulting fees (Eli Lilly) and fees for educational services (Abbvie, Amgen, Pfizer, Novartis, Janssen).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res 2019;71:2–29. 10.1002/acr.23789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogdie A, Coates LC, Gladman DD. Treatment guidelines in psoriatic arthritis. Rheumatology 2020;59:i37–46. 10.1093/rheumatology/kez383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am 2015;41:545–68. 10.1016/j.rdc.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tillett W, Merola JF, Thaçi D, et al. Disease characteristics and the burden of joint and skin involvement amongst people with psoriatic arthritis: a population survey. Rheumatol Ther 2020;7:617–37. 10.1007/s40744-020-00221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med 2017;17:65. 10.7861/clinmedicine.17-1-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis 2015;74:1045–50. 10.1136/annrheumdis-2013-204858 [DOI] [PubMed] [Google Scholar]
- 7.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang EJ, Kavanaugh A. Psoriatic arthritis: latest treatments and their place in therapy. Ther Adv Chronic Dis 2015;6:194–203. 10.1177/2040622315582354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EMA. Stelara . Summary of product characteristics.: European medicines Agency, 2018. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000958/WC500058513.pdf [Accessed 10 Dec 2020].
- 10.EMA. Cosentyx . Summary of product characteristics.: European medicines Agency, 2018. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003729/WC500183129.pdf [Accessed 10 Dec 2020].
- 11.EMA. Taltz . Summary of product characteristics.: European medicines Agency, 2008. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003943/WC500205804.pdf [Accessed 10 Dec 2020].
- 12.EMA. Tremfya . Summary of product characteristics.: European medicines Agency, 2021. Available: https://www.ema.europa.eu/en/documents/product-information/tremfya-epar-product-information_en.pdf [Accessed 22 Jan 2021].
- 13.EMA. Orencia . Summary of product characteristics: European medicines Agency, 2007. Available: https://www.ema.europa.eu/en/documents/product-information/orencia-epar-product-information_en.pdf [Accessed 10 Dec 2020].
- 14.EMA. Otezla . Summary of product characteristics.: European medicines Agency, 2017. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003746/WC500182627.pdf [Accessed 10 Dec 2020].
- 15.EMA. Xeljanz . Summary of product characteristics: European medicines Agency, 2017. Available: https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf [Accessed 10 Dec 2020].
- 16.EMA. Rinvoq . Summary of product characteristics.: European medicines Agency, 2021. Available: https://www.ema.europa.eu/en/documents/product-information/rinvoq-epar-product-information_en.pdf [Accessed 03 Feb 2021].
- 17.Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1115–25. 10.1016/S0140-6736(20)30265-8 [DOI] [PubMed] [Google Scholar]
- 18.Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1126–36. 10.1016/S0140-6736(20)30263-4 [DOI] [PubMed] [Google Scholar]
- 19.Deodhar A, Gottlieb AB, Boehncke W-H, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet 2018;391:2213–24. 10.1016/S0140-6736(18)30952-8 [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb AOA, Ballerini R, Chou R, et al. Tildrakizumab Efficacy on Psoriasis in Patients with Psoriatic Arthritis—An Analysis from a Phase 2 Study [abstract]. Arthritis Rheumatol 2019;71. [Google Scholar]
- 21.Mease PJ, Kellner H, Morita A. OP0307 efficacy and safety of risankizumab, a selective IL-23p19 inhibitor, in patients with active psoriatic arthritis over 24 weeks: results from a phase 2 trial. Annals of the Rheumatic Diseases 2018;77:200–1. [Google Scholar]
- 22.Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014;370:2295–306. 10.1056/NEJMoa1315231 [DOI] [PubMed] [Google Scholar]
- 23.Mease PJ, Helliwell PS, Hjuler KF, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis 2021;80:185–93. 10.1136/annrheumdis-2019-216835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis 2017;76:1550–8. 10.1136/annrheumdis-2016-210724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mease P. A short history of biological therapy for psoriatic arthritis. Clin Exp Rheumatol 2015;33:S104–8. [PubMed] [Google Scholar]
- 26.Mease P, Coates LC, Helliwell PS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:2367–77. 10.1016/S0140-6736(18)32483-8 [DOI] [PubMed] [Google Scholar]
- 27.Coates LC, Orbai A-M, Morita A, et al. Achieving minimal disease activity in psoriatic arthritis predicts meaningful improvements in patients' health-related quality of life and productivity. BMC Rheumatol 2018;2:24. 10.1186/s41927-018-0030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607–17. 10.1177/0272989X12458724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias S, Sutton AJ, Welton NJ, et al. Evidence synthesis for decision making 3: heterogeneity--subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making 2013;33:618–40. 10.1177/0272989X13485157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dias S, Welton NJ, Sutton AJ, et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013;33:641–56. 10.1177/0272989X12455847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683–91. 10.1016/s0895-4356(97)00049-8 [DOI] [PubMed] [Google Scholar]
- 33.Unit MB . WinBUGS. Available: https://www.mrc-bsu.cam.ac.uk/software/bugs/the-bugs-project-winbugs/[Accessed 08 Dec 2020].
- 34.Dias S, Welton NJ, Sutton AJ, et al. Evidence synthesis for decision making 5: the baseline natural history model. Med Decis Making 2013;33:657–70. 10.1177/0272989X13485155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coates LC, Tillett W, D’agostino MA, et al. OP0050 ADALIMUMAB INTRODUCTION VERSUS METHOTREXATE DOSE ESCALATION IN PATIENTS WITH INADEQUATELY CONTROLLED PSORIATIC ARTHRITIS: RESULTS FROM RANDOMIZED PHASE 4 CONTROL STUDY. Ann Rheum Dis 2020;79:33.2–33. 10.1136/annrheumdis-2020-eular.2393 [DOI] [PubMed] [Google Scholar]
- 36.Schett G, Wollenhaupt J, Papp K, et al. Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2012;64:3156–67. 10.1002/art.34627 [DOI] [PubMed] [Google Scholar]
- 37.Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 2014;73:1020–6. 10.1136/annrheumdis-2013-205056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol 2016;43:1724–34. 10.3899/jrheum.151376 [DOI] [PubMed] [Google Scholar]
- 39.Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis 2016;75:1065–73. 10.1136/annrheumdis-2015-207963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. 10.1136/annrheumdis-2013-203696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976–86. 10.1002/art.24403 [DOI] [PubMed] [Google Scholar]
- 42.Kavanaugh A, Husni ME, Harrison DD, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty-four of the GO-VIBRANT study. Arthritis Rheumatol 2017;69:2151–61. 10.1002/art.40226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (impact). Arthritis Rheum 2005;52:1227–36. 10.1002/art.20967 [DOI] [PubMed] [Google Scholar]
- 44.Antoni C, Krueger GG, de Vlam K, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the impact 2 trial. Ann Rheum Dis 2005;64:1150–7. 10.1136/ard.2004.032268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis 2020;79:123–31. 10.1136/annrheumdis-2019-215386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mease PJ, van der Heijde D, Ritchlin CT, et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. 10.1136/annrheumdis-2016-209709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirkham B, Okada M, Rahman P. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. The Lancet 2017;389:2317–27. [DOI] [PubMed] [Google Scholar]
- 48.Korotaeva T, Gaydukova I, Mazurov V, et al. OP0226 NETAKIMAB DECREASES DISEASE ACTIVITY IN PATIENTS WITH PSORIATIC ARTHRITIS: RESULTS FROM A RANDOMIZED DOUBLE-BLIND PHASE 3 CLINICAL TRIAL (PATERA). Ann Rheum Dis 2020;79:141–2. 10.1136/annrheumdis-2020-eular.346931537547 [DOI] [Google Scholar]
- 49.Nguyen TCM, Levin R, Valenzuela G. A Randomized, Placebo-Controlled Study Evaluating the Safety and Efficacy of Secukinumab in US Biologic-Naive Patients with Active Psoriatic Arthritis and Psoriatic Skin Lesions [abstract]. Arthritis Rheumatol 2019;71. [Google Scholar]
- 50.Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med Overseas Ed 2015;373:1329–39. 10.1056/NEJMoa1412679 [DOI] [PubMed] [Google Scholar]
- 51.McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (future 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. 10.1016/S0140-6736(15)61134-5 [DOI] [PubMed] [Google Scholar]
- 52.Mease P, van der Heijde D, Landewé R, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III future 5 study. Ann Rheum Dis 2018;77:890–7. 10.1136/annrheumdis-2017-212687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (exceed): a double-blind, parallel-group, randomised, active-controlled, phase 3B trial. Lancet 2020;395:1496–505. 10.1016/S0140-6736(20)30564-X [DOI] [PubMed] [Google Scholar]
- 54.Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017;377:1525–36. 10.1056/NEJMoa1615977 [DOI] [PubMed] [Google Scholar]
- 55.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med Overseas Ed 2017;377:1537–50. 10.1056/NEJMoa1615975 [DOI] [PubMed] [Google Scholar]
- 56.Mcinnes I, Anderson J, Magrey M, et al. LB0001 EFFICACY AND SAFETY OF UPADACITINIB VERSUS PLACEBO AND ADALIMUMAB IN PATIENTS WITH ACTIVE PSORIATIC ARTHRITIS AND INADEQUATE RESPONSE TO NON-BIOLOGIC DISEASE-MODIFYING ANTI-RHEUMATIC DRUGS (SELECT-PsA-1): A DOUBLE-BLIND, RANDOMIZED CONTROLLED PHASE 3 TRIAL. Ann Rheum Dis 2020;79:16.2–17. 10.1136/annrheumdis-2020-eular.6727 [DOI] [Google Scholar]
- 57.Genovese MC, Lertratanakul A, Anderson J, et al. OP0223 EFFICACY AND SAFETY OF UPADACITINIB IN PATIENTS WITH ACTIVE PSORIATIC ARTHRITIS AND INADEQUATE RESPONSE TO BIOLOGIC DISEASE-MODIFYING ANTI-RHEUMATIC DRUGS (SELECT-PSA-2): A DOUBLE-BLIND, RANDOMIZED CONTROLLED PHASE 3 TRIAL. Ann Rheum Dis 2020;79:139–39. 10.1136/annrheumdis-2020-eular.1229 [DOI] [Google Scholar]
- 58.Gottlieb A, Menter A, Mendelsohn A, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 2009;373:633–40. 10.1016/S0140-6736(09)60140-9 [DOI] [PubMed] [Google Scholar]
- 59.McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013;382:780–9. 10.1016/S0140-6736(13)60594-2 [DOI] [PubMed] [Google Scholar]
- 60.Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990–9. 10.1136/annrheumdis-2013-204655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mease PJ, Genovese MC, Weinblatt ME, et al. Phase II study of ABT-122, a tumor necrosis factor- and Interleukin-17A-Targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol 2018;70:1778–89. 10.1002/art.40579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nash P, Ohson K, Walsh J, et al. Early and sustained efficacy with apremilast monotherapy in biological-naïve patients with psoriatic arthritis: a phase IIIB, randomised controlled trial (active). Ann Rheum Dis 2018;77:690–8. 10.1136/annrheumdis-2017-211568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sterry W, Ortonne J-P, Kirkham B, et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ 2010;340:c147. 10.1136/bmj.c147 [DOI] [PubMed] [Google Scholar]
- 64.Vieira-Sousa E, Alves P, Rodrigues AM, et al. GO-DACT: a phase 3B randomised, double-blind, placebo-controlled trial of golimumab plus methotrexate (MTX) versus placebo plus MTX in improving DACTylitis in MTX-naive patients with psoriatic arthritis. Ann Rheum Dis 2020;79:490–8. 10.1136/annrheumdis-2019-216500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baranauskaite A, Raffayová H, Kungurov NV, et al. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the respond study. Ann Rheum Dis 2012;71:541–8. 10.1136/ard.2011.152223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mease PJ, Gladman DD, Collier DH, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol 2019;71:1112–24. 10.1002/art.40851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (future 3). Arthritis Res Ther 2018;20:47. 10.1186/s13075-018-1551-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kivitz AJ, Nash P, Tahir H, et al. Efficacy and Safety of Subcutaneous Secukinumab 150 mg with or Without Loading Regimen in Psoriatic Arthritis: Results from the FUTURE 4 Study. Rheumatol Ther 2019;6:393–407. 10.1007/s40744-019-0163-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baraliakos XCL, Gossec L, Jeka S, et al. Secukinumab Improves Axial Manifestations in Patients with Psoriatic Arthritis and Inadequate Response to NSAIDs: Primary Analysis of Phase 3 Trial [abstract]. Arthritis Rheumatol 2019;71. [Google Scholar]
- 70.Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89. 10.1002/art.21306 [DOI] [PubMed] [Google Scholar]
- 71.Genovese MC, Mease PJ, Thomson GTD, et al. Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J Rheumatol 2007;34:1040–50. [PubMed] [Google Scholar]
- 72.Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72. 10.1002/art.20335 [DOI] [PubMed] [Google Scholar]
- 73.McInnes IB, Nash P, Ritchlin C, et al. Secukinumab for psoriatic arthritis: comparative effectiveness versus licensed biologics/apremilast: a network meta-analysis. J Comp Eff Res 2018;7:1107–23. 10.2217/cer-2018-0075 [DOI] [PubMed] [Google Scholar]
- 74.Wu D, Yue J, Tam L-S. Efficacy and safety of biologics targeting interleukin-6, -12/23 and -17 pathways for peripheral psoriatic arthritis: a network meta-analysis. Rheumatology 2018;57:563–71. 10.1093/rheumatology/kex452 [DOI] [PubMed] [Google Scholar]
- 75.Bilal J, Riaz IB, Kamal MU, et al. A systematic review and meta-analysis of efficacy and safety of novel interleukin inhibitors in the management of psoriatic arthritis. J Clin Rheumatol 2018;24:6–13. 10.1097/RHU.0000000000000583 [DOI] [PubMed] [Google Scholar]
- 76.Corbett M, Chehadah F, Biswas M, et al. Certolizumab pegol and secukinumab for treating active psoriatic arthritis following inadequate response to disease-modifying antirheumatic drugs: a systematic review and economic evaluation. Health Technol Assess 2017;21:1–326. 10.3310/hta21560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song GG, Lee YH. Relative efficacy and safety of apremilast, secukinumab, and ustekinumab for the treatment of psoriatic arthritis. Z Rheumatol 2018;77:613–20. 10.1007/s00393-017-0355-8 [DOI] [PubMed] [Google Scholar]
- 78.Ruyssen-Witrand A, Perry R, Watkins C, et al. Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis. RMD Open 2020;6:e001117. 10.1136/rmdopen-2019-001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gladman DD, Orbai A-M, Gomez-Reino J, et al. Network meta-analysis of tofacitinib, biologic disease-modifying antirheumatic drugs, and apremilast for the treatment of psoriatic arthritis. Curr Ther Res Clin Exp 2020;93:100601. 10.1016/j.curtheres.2020.100601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simons N, Degboé Y, Barnetche T, et al. Biological DMARD efficacy in psoriatic arthritis: a systematic literature review and meta-analysis on articular, enthesitis, dactylitis, skin and functional outcomes. Clin Exp Rheumatol 2020;38:508–15. [PubMed] [Google Scholar]
- 81.Mcinnes I, Mease PJ, Eaton K, et al. AB0820 COMPARATIVE EFFICACY OF GUSELKUMAB IN PATIENTS WITH PSORIATIC ARTHRITIS: RESULTS FROM SYSTEMATIC LITERATURE REVIEW AND NETWORK META-ANALYSIS. Ann Rheum Dis 2020;79:1713–4. 10.1136/annrheumdis-2020-eular.6013 [DOI] [Google Scholar]
- 82.Mourad A, Gniadecki R. Treatment of Dactylitis and Enthesitis in psoriatic arthritis with biologic agents: a systematic review and Metaanalysis. J Rheumatol 2020;47:59–65. 10.3899/jrheum.180797 [DOI] [PubMed] [Google Scholar]
- 83.Mease PJ, McInnes IB, Tam L-S, et al. Comparative effectiveness of guselkumab in psoriatic arthritis: results from systematic literature review and network meta-analysis. Rheumatology 2021;60:2109–21. 10.1093/rheumatology/keab119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mahil SK, Ezejimofor MC, Exton LS, et al. Comparing the efficacy and tolerability of biologic therapies in psoriasis: an updated network meta-analysis. Br J Dermatol 2020;183:638–49. 10.1111/bjd.19325 [DOI] [PubMed] [Google Scholar]
- 85.Sbidian E, Chaimani A, Afach S, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev 2020;1:CD011535. 10.1002/14651858.CD011535.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cameron C, Druchok C, Hutton B. Guselkumab for the treatment of moderate-to-severe plaque psoriasis during induction phase: a systematic review and network meta-analysis. Journal of Psoriasis and Psoriatic Arthritis 2018. [Google Scholar]
- 87.Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol 2020;156:258–69. 10.1001/jamadermatol.2019.4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One 2019;14:e0220868. 10.1371/journal.pone.0220868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yasmeen N, Sawyer LM, Malottki K, et al. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. J Dermatolog Treat 2022;33:1–15. 10.1080/09546634.2020.1743811 [DOI] [PubMed] [Google Scholar]
- 90.Wright E, Yasmeen N, Malottki K, et al. Assessing the quality and coherence of network meta-analyses of biologics in plaque psoriasis: what does all this evidence synthesis tell us? Dermatol Ther 2021;11:181–220. 10.1007/s13555-020-00463-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ritchlin CT, Kavanaugh A, Merola JF, et al. Bimekizumab in patients with active psoriatic arthritis: results from a 48-week, randomised, double-blind, placebo-controlled, dose-ranging phase 2B trial. Lancet 2020;395:427–40. 10.1016/S0140-6736(19)33161-7 [DOI] [PubMed] [Google Scholar]
- 92.Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (be vivid): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021;397:487–98. 10.1016/S0140-6736(21)00125-2 [DOI] [PubMed] [Google Scholar]
- 93.Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (be ready): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet 2021;397:475–86. 10.1016/S0140-6736(21)00126-4 [DOI] [PubMed] [Google Scholar]
- 94.Helliwell PS. Established psoriatic arthritis: clinical aspects. J Rheumatol Suppl 2009;83:21–3. 10.3899/jrheum.090215 [DOI] [PubMed] [Google Scholar]
- 95.Ruyssen-Witrand A, Sapin C, Hartz S. Effects of biologic DMARDs on physical function in patients with active psoriatic arthritis: results of network meta-analyses. Annals of the Rheumatic Diseases 2018;77:363–4. [Google Scholar]
- 96.Coates LC, Garrood T, Gullick N, et al. P174 Upadacitinib response rates in patients with psoriatic arthritis enrolled in the SELECT-PsA-1 and SELECT-PsA-2 trials assessed according to modified PsARC. Rheumatology 2021;60. 10.1093/rheumatology/keab247.169 [DOI] [PubMed] [Google Scholar]
- 97.Conaghan PG, Alten R, Deodhar A, et al. Relationship of pain and fatigue with health-related quality of life and work in patients with psoriatic arthritis on TNFi: results of a multi-national real-world study. RMD Open 2020;6. 10.1136/rmdopen-2020-001240 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002074supp001.pdf (5.9MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.