Abstract
Diastolic dysfunction has been reported in patients with COVID-19. Due to the role of N-terminal pro-brain natriuretic peptide (NT-proBNP) in the diagnosis of heart failure, this study investigated the relationship between serum NT-proBNP levels and diastolic heart failure in patients with COVID-19. This descriptive-analytical study was performed at Ayatollah Rouhani Hospital in Babol. Fifty-two patients with confirmed COVID-19 diagnosis, who were admitted to the ICU, were included in this study. The primary outcome was about the relationship and predictive role of NT-proBNP and diastolic heart failure in patients with severe SARS-CoV-2 infection. Patients with pro BNP > 125 pg/ml underwent echocardiography, and the relationship between echocardiographic indices and NT-proBNP was assessed as the secondary outcome. Our study showed that plasma NT-proBNP levels in patients with increased diastolic dysfunction were associated with disease severity. It was also found that the cut-off point of NT-proBNP = 799 pg/ml could be a predictor of diastolic dysfunction grades two and three. In this study, patients with a serum NT-proBNP level > 799 had 37 times higher chance of having diastolic dysfunction than those with a serum NT-proBNP < 799. Patients with NT-proBNP > 556 had RV_EA > 2 in echocardiography, indicating increased right-sided filling pressures. Despite the confounding factors in the interpretation of the NT-proBNP level in COVID-19, its level can be used to estimate the presence of high-grade diastolic heart failure on the left side and the right side of the heart and the presence of high filling pressures. Lower levels of NT-proBNP are associated with right-sided diastolic failure.
Keywords: NT-proBNP, COVID-19, Diastolic dysfunction
Background
Since December 2019, the world has experienced a rapid outbreak of a newly discovered infectious disease. COVID-19 occurs primarily as an acute respiratory disease with interstitial and alveolar pneumonia, but can affect various organs such as the heart, kidneys, gastrointestinal tract, blood, and central nervous system [1]. Microangiopathy, myocarditis, myocardial infarction or even heart failure have been reported in COVID-19 [2, 3]. A meta-analysis of 25 studies that were reported on non-COVID-19 pneumonia stated that cardiac complications were observed in a quarter of patients with COVID-19 as the most common (14%) characteristic [4], but the incidence of heart failure in patients with COVID-19 and even other diseases caused by coronaviruses such as severe acute respiratory syndrome (SARS-CoV-1) and Middle East respiratory syndrome (MERS-CoV) had not received much attention.
A cohort study on 3,080 confirmed patients with COVID-19 who were followed up for 30 days showed an incidence of acute heart failure in 2.5% of these patients. The incidence and progression of heart failure in patients in this study were associated with a poorer outcome [5]. Moreover, a significant association has been declared between COVID-19 and diastolic heart failure, subclinical diastolic failure, or exacerbation of diastolic heart failure [3].
The overlap of clinical signs and radiological manifestations of COVID-19 and heart failure is an undeniable obstacle to the correct diagnosis of these conditions [6, 7]. Restrictive criteria for the use of non-invasive imaging tests such as echocardiography and the recommendation to use simple physical examinations recommended by the international scientific community make it difficult to diagnose heart failure in COVID-19 [6–9]. The role of cardiac biomarkers such as N-terminal pro-brain natriuretic peptide (NT-proBNP) has been proven in the diagnosis of acute heart failure in patients with shortness of breath and no previous history of heart failure, especially if imaging techniques are not available or limited use, likewise in COVID-19 pandemic [9]. In this regard, NT-proBNP has played a growing role in this area over the years.
NT-ProBNP is released from myocytes in response to increased cardiac wall stress and provides a strong independent prognostic value in patients with various cardiovascular diseases such as heart failure, acute coronary syndrome, aortic valve stenosis, and stable coronary involvement [10]. NT-ProBNP in COVID-19 rises unpredictably in response to the criteria we have in heart failure due to hypoxia, sepsis, and inflammatory responses [11]. Due to the limited role of echocardiography because of the close distance between the operator and the patient and the time needed to obtain more accurate parameters in echocardiography, this study aimed to examine the predictive role of NT-proBNP in diastolic heart failure and its relationship with echocardiographic parameters in patients with COVID-19.
Methods
This cross-sectional descriptive study was performed on 52 adult patients with COVID-19 admitted to the ICU of Ayatollah Rouhani Hospital in Babol from June 21 to September 21, 2020. All steps were followed according to the instructions of the Research Ethics Committee of Babol University of Medical Sciences, Babol, Iran (IR.MUBABOL.REC.1399.321). The primary outcome was the determination of the predictive role of NT-proBNP in the diagnosis of diastolic heart failure in patients with severe SARS-COV-2 infection. Inclusion criteria included patients with severe COVID-19 disease who were admitted to ICU and whose COVID-19 infection was confirmed by polymerase chain reaction (PCR) or a combination of clinical manifestations and chest CT scan and those patients whose level NT-proBNP in their plasma was measured to be above 125 pg/ml. Echocardiography was performed for eligible patients.
Those excluded from the study were; patients under 18, pregnant women, patients without NT-proBNP results, those with malignant tumors, stroke or myocardial infarction, and patients who died during the first days of hospitalization or were transferred to other hospitals. Demographic and clinical information was reviewed and recorded via asking the patient questions and reviewing the patient file. For laboratory data, blood samples were collected and NT-proBNP was measured using fluorescence immunoassay (Triage® BNP; Alere). Patients with NT-proBNP < 125 pg/ml underwent echocardiography performed by a cardiologist. Additional laboratory test results including blood count, renal function analysis, electrolytes, CRP, IL-6, and pre-calcitonin and D-dimer oxygen saturation level were also recorded. All clinical and laboratory data were collected within 24 h after admission.
Items measured on echocardiography included: the size of the cardiac cavities in diastole, ejection fraction in systole on the right and left, right and left atrial size, systolic venous pressure, IVC diameter, pericardial effusion rate, diastolic function including E and A- wave velocity and the E/A ratio and the E/E tissue movement velocity on the right and left, separately. Estimation of the severity of diastolic heart failure was calculated using the guidelines of the American Society of Echocardiography [12]. Statistical analysis was performed by SPSS 22.0 (SPSS, Chicago, IL, USA). Data were presented as mean standard deviation and frequency (%). Independent samples t-test and chi-square test in rank variables were used for intergroup comparison in continuous variables with normal distribution. The ROC curve was used to determine the “optimal” NT-proBNP cut-off point for predicting diastolic dysfunction and the Yuden index (Ref) method. Patients were classified into two groups due to the fact that their NT-proBNP level was higher or lower than this cut-off point, and the relationship between NT-proBNP level above the cut-off point and mortality was tested using chi-square test. Bilateral p < 0.05 was considered statistically significant.
Results
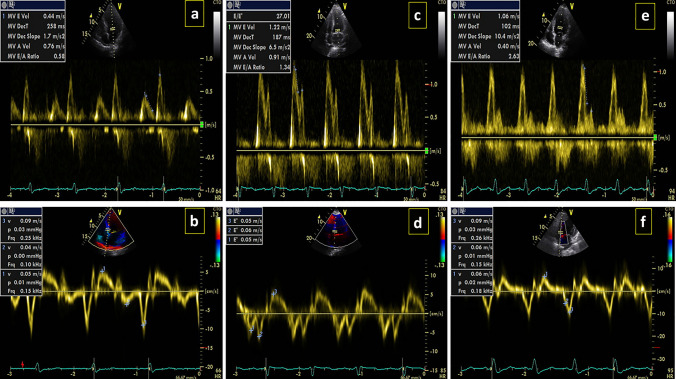
During the study period, data from 52 patients with COVID-19-confirmed infection who met the inclusion criteria were included in the present analysis. The mean age of patients was 63.4 ± 17.56 (46.2%) patients were male and 28 (53.8%) patients were female. According to echocardiographic results, 50 patients had diastolic dysfunction, of which 34 (65.4%) had grade 1 dysfunction (Fig. 1a, b), 14 (26.9%) patients had grade 2 dysfunction (Fig. 1c, d), and 2 (3.8%) patients had grade 3 dysfunction (Fig. 1e, f). The mean level of NT-proBNP in these patients was 5001.31 ± 2899.34 with the median of 1371 (24,647–473.75). The demographic and baseline characteristics of the studied patients are presented in Table 1.
Fig. 1.
Grading of diastolic dysfunction. a, b Grade 1, c, d Grade 2, e, f Grade 3
Table 1.
Baseline characteristics of total and different degrees of NT-proBNP
| Variable | WithoutDD (n = 2) | GradeDD1 (n = 34) | GradeDD2 (n = 14) | gradeDD3 (n = 2 | N = 52 | |
|---|---|---|---|---|---|---|
| DD | ||||||
| Male/female (n) | 1/1 | 16/18 | 6/8 | 1/1 | 24/28 | |
| Age (years) | 34 | 62 ± 17.92 | 68.5 ± 14.29 | 76 ± 14.14 | 63.4 ± 17.56 | |
| History of HTN n (%) | 0 | 12 (42.9) | 6 (60) | 1 (50) | 19 (36.5) | |
| History of DM n (%) | 0 | 9 (31) | 4 (40) | 1 (50) | 14 (26.9) | |
| History of cardiovascular disease n (%) | 0 | 12 (38.7) | 6 (46.2) | 2 (100) | 20 (38.46) | |
| Serum NT-proBNP pg/Ml | Mean ± SD | 963.5 ± 942.57 | 1344.96 ± 1375.5 | 5391.57 ± 7031.89 | 13,814 ± 12,436.59 | 2899.34 ± 5001.31 |
| Median (IQR) | 963.5 (297–963.5) | 724.5 (354–1911.25) | 2236 (1094.5–7540.5) | 13,814 (5020–13,814) | 1371.5 (473.75–2464) | |
| Creatinine (mg/dl) | Mean ± SD | 5.3 ± 6.5 | 1.74 ± 2.5 | 3.64 ± 3.42 | 4.4 ± 2.96 | 2.48 ± 3.02 |
| Median (IQR) | 5.3 (0.7–5.3) | 0.9 (0.72–1.32) | 2.3 (0.9–6.2) | 4.4 (2.3–4.4) | 1 (0.8–2.3) | |
| WBC (109 /l) | Mean ± SD | 13,500 ± 5091.16 | 8091.52 ± 3442.31 | 8600 ± 3776.24 | 9650 ± 1202.08 | 8606.40 ± 3597.78 |
| Median (IQR) | 13,500 (9900–13,500) | 7500 (5900–11,800) | 8400 (5500–11,800) | 9650 (8800–9650) | 8250 (5975–11,475) | |
| LYM (109/l) | Mean ± SD | 11.5 ± 2.82 | 10.31 ± 8.03 | 8.81 ± 8.76 | 4.15 ± 0.07 | 9.74 ± 7.69 |
| Median (IQR) | 11.5 (9.51–11.5) | 9.9 (0.9–15.4) | 8.05 (0.32–15.37) | 4.15 (4.10–4.15) | 9.3 (2.5–13.8) | |
| Neu | Mean ± SD | 82.32 ± 0.59 | 49.35 ± 38.41 | 38.85 ± 42.01 | 45.26 ± 62.69 | 49.19 ± 38.77 |
| Median (IQR) | 82.32 (81.9–82.32) | 68.4 (0.85–83.12) | 33.51 (0.87–82) | 45.26 (0.93–45.26) | 68.4 (0.88–82.7) | |
| CRP (mg/l) | Mean ± SD | – | 75.28 ± 70.3 | 114 ± 96.16 | 29 | 76.84 ± 70.12 |
| Median (IQR) | – | 74 (2.8–126) | 114 (64–114) | 29 | 68 (9.5–127) | |
| Hb | Mean ± SD | 14.3 ± 3.81 | 11.24 ± 1.82 | 10.42 ± 1.54 | 8.8 | 11.15 ± 2.05 |
| Median (IQR) | 14.3 (11.6–14.3) | 11.4 (9.9–12.2) | 10 (9.25–11.8) | 8.80 | 11.15 (9.62–12.17) | |
| BUN | Mean ± SD | 16 | 29.34 ± 20.11 | 46.71 ± 36.97 | 75 ± 38.18 | 35.39 ± 27.27 |
| Median (IQR) | 16 | 22 (19–32) | 30 (22–95) | 75 (48–75) | 25 (19–42) | |
| Na | Mean ± SD | 135 | 134.6 ± 3.4 | 136.5 ± 5.35 | 133.5 ± 7.77 | 134.9 ± 3.93 |
| Median (IQR) | 135 | 135 (132–137) | 137 (130–141) | 133 (128–133) | 135 (131–137) | |
| K | Mean ± SD | 3.7 | 4.18 ± 0.67 | 4.4 ± 0.61 | 5.3 ± 1.97 | 4.28 ± 0.76 |
| Median (IQR) | 3.7 | 4.2 (3.9–4.6) | 4.5 (3.8–4.8) | 5.3 (3.9–5.3) | 4.20 (3.85–4.65) | |
| IL-6 | Mean ± SD | 10.4 ± 3.95 | 78.48 ± 143.91 | 15.8 ± 8.9 | – | 64.71 ± 129.87 |
| Median (IQR) | 10.40 (7.60–10.40) | 17.8 (7.19–51) | 15.8 (9.5–15.8) | – | 17.50 (7.60–48.50) | |
| D-dimer | Mean ± SD | 1249 ± 345.06 | 1046.82 ± 1249.70 | 481.75 ± 680.56 | – | 1010.53 ± 1140.23 |
| Median (IQR) | 1249 (1005–1249) | 486.5 (229.25–1400.02) | 481.75 (0.50–481.75) | – | 661.50 (229.25–1400.02) | |
| Procalcitonin (PCT) | Mean ± SD | 0.16 | 0.18 ± 0.17 | 0.46 ± 0.4 | 44.4 ± 57.41 | 4.88 ± 19.41 |
| Median (IQR) | 0.16 | 0.10 (0.08–0.25) | 0.40 (0.10–0.40) | 44.40 (3.80–44.40) | 0.16 (0.10–0.40) | |
| O2sat | Mean ± SD | 94.5 ± 0.7 | 94.41 ± 3.44 | 90.35 ± 4.18 | 86.5 ± 2.12 | 93.01 ± 4.15 |
| Median (IQR) | 94.5 (94–94.5) | 95.5 (92–97) | 90.5 (85.75–94.25) | 86.5 (85–86.5) | 94 (90–96) | |
| In-hospital death n (%) | 0 | 2(5.9) | 3(21.4) | 2(100) | 7 (13.5) |
Mean serum NT-proBNP levels increased with increasing grade of diastolic dysfunction. Table 2 compares the mean serum level of NT-proBNP in terms of the diastolic dysfunction grade. There was a correlation between NT-proBNP level and some echocardiographic parameters and the relationship between serum NT-proBNP level and these indices was positive. Among the echocardiographic parameters with a positive correlation with NT-proBNP, the severity of the correlation was related to RA_size (r = 0.61, p = 0.0001), LVED (r = 0.45, p = 0.0008), LV_ E/e′(r = 0.27, p = 0.05), LV_E (r = 0.35, p = 0.01), respectively. Among the echocardiographic parameters, left ventricular ejection fraction (LVEF) (r = − 0.43, p = 0.0012) had a significant and negative correlation with serum NT-proBNP level.
Table 2.
Relationship between NT-proBNP levels and echocardiographic parameters
| Echo parameters (left) | Pearson correlation coefficient | p value |
|---|---|---|
| LVED | 0.45 | 0.0006 |
| RVEDd | 0.02 | 0.84 |
| LAsize | 0.1 | 0.47 |
| TAPSE | − 0.13 | 0.34 |
| LVEF | − 0.43 | 0.0012 |
| SPAP | − 0.44 | 0. 12 |
| LV_E | 0.35 | 0.01 |
| LV_A | 0.01 | 0.89 |
| LV_ E/A | − 0.03 | 0.82 |
| LV_ E/e′ | 0.27 | 0.05 |
| RV_E | 0.07 | 0.58 |
| RV_A | − 0.05 | 0.73 |
| RV_ E/A | 0.01 | 0.93 |
| RV_ E/e′ | − 0.16 | 0.25 |
| RA_size | 0.61 | 0.0001 |
| IVC_size | 0.18 | 0.46 |
The cut-off point as a predictor of grade 2 and 3 diastolic dysfunction for NT-proBNP level was 799 pg/ml with 100% sensitivity and 52.78% specificity (Fig. 2a). The area under the curve for this cut-off point was (AUC = 0.81). Among the echocardiographic parameters, LV_E > 50 cm at the cut-off point NT-proBNP = 1479 pg/ml, had an acceptable area under curve and a favorable predictive value (AUC = 0.66, PPV = 100) (Fig. 2b) as well as the echocardiographic parameter RV_E/A > 2 at the cut-off point NT-proBNP = 556 pg/ml, had an acceptable area under curve and a good predictive value (AUC = 0.71, PPV = 97.2) (Fig. 2c, Table 3).
Fig. 2.
a ROC curve for predicting cutoff value of NT-proBNP for diastolic dysfunction. Area under the curve was 0.81%. b ROC curve for predicting cutoff value of LV_E > 50. Area under the curve was 0.67%. c ROC curve for predicting cutoff value of RV_EA < 2. Area under the curve was 0.71%
Table 3.
AUC, sensitivity, specificity, and positive and negative predictive values by NT-proBNP at a cut off-value
| Parametera | Cut-off value NT-proBNP | AUC (95% CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Diastolic dysfunction | 799 | 0.81 (0.7–0.93) | 100 | 52.78 | 48.5 | 100 |
| Grade2,3 | ||||||
| LVEDD | 1736 | 0.60 (0.4–0.81) | 63.64 | 65.85 | 33.3 | 87.1 |
| LA size | 587 | 0.63 (0.39–0.88) | 100 | 34.7 | 16.7 | 100 |
| TAPSE < 1/7 | 1140 | 0.77 (0.60–0.94) | 100 | 52.17 | 21.4 | 100 |
| TAPSE < 2/4 | 805 | 0.50 (0.32–0.68) | 81.82 | 43.9 | 28.1 | 90 |
| SPAP | 1497 | 0.68 (0.52–0.84) | 65 | 70 | 59.1 | 75 |
| LV_E | 1479 | 0.66 (0.5–0.83) | 53.33 | 100 | 100 | 25 |
| LV_EA > 0/8 | 1630 | 0.55 (0.39–0.71) | 51/72 | 71/43 | 71.4 | 51.7 |
| LV_EA > 2 | 955 | 0.62 (0.42–0.82) | 80 | 47.5 | 27.6 | 90.5 |
| LV_E/e′ | 150 | 0.64 (0.48–0.80) | 57.89 | 72.73 | 75 | 56.6 |
| RV_E/A > 0_0.8 | 1850 | 0.53 (0.35–0.70) | 52.17 | 75 | 63.2 | 65.6 |
| RV_E/A > 2 | 556 | 0.71 (0.43–0.99) | 74.47 | 75 | 97.2 | 20 |
| > 4 RV_E/e′ | 556 | 0.51 (0.34–0.69) | 76.67 | 38.1 | 63.9 | 53.3 |
| RV_E/e > 6 | 556 | 0.49 (0.30–0.68) | 81.82 | 32.5 | 25 | 86.7 |
| Pericardial_effusion | 488 | 0.46 (0.12–0.81) | 100 | 27.08 | 10.3 | 100 |
aAbnormal reng: LVEDD > 5.3(f) or > 5.9(m)—RVEDd > 4.1 mm—LA size > 4 cm—SPAP≥ 30 mmHg—LV_E > 50 cm/s- LV_E e/ > 14—RA_size > 19 cm
After multivariate logistic regression analysis, those with a serum pro BNP level equal and above 799 had a 37 times higher chance of diastolic dysfunction than those with a serum pro BNP level below 799 E (OR 37.16, p = 0.04). All echocardiographic parameters were evaluated by logistic regression model and only SPAP and LV_E were compared to other indices so the chance of diastolic dysfunction increases by 22% (OR 1.22, p = 0.01) that for every unit of increase in SPAP. For each unit of increase in LV_E, the chance of diastolic dysfunction increases by 16% (OR 1.16, p = 0.01, Table 4).
Table 4.
Multivariable logistic regression for evaluating the ability of NT-proBNP to identify diastolic dysfunction when compared with other indicators
| Indicator | OR | p value | 95% CI |
|---|---|---|---|
| NT_proBNP | 37.16 | 0.04 | 1.12–1231.48 |
| ≥ 799 | |||
| SPAP | 1.22 | 0.01 | 1.03–1.45 |
| LV_E | 1.16 | 0.01 | 1.02–1.32 |
Discussion
The study aimed to evaluate the relationship between NT-proBNP levels and left ventricular diastolic dysfunction in patients with COVID-19. Our study showed that plasma NT-proBNP levels in patients with increased diastolic dysfunction were related to the severity of the disease so that with increasing severity of diastolic dysfunction, NT-proBNP levels also increase. It was also found that the cut-off point of NT-proBNP = 799 pg/ml could be used as a predictor of diastolic dysfunction grades 2 and 3. A person with COVID-19 who has high-grade diastolic dysfunction is 100% likely to have a serum NT-proBNP level above 799 pg/ml. In this study, patients with a serum NT-proBNP level above 799 had a 37 times higher chance of having diastolic dysfunction than those with a serum NT-proBNP level below this amount. There are studies that, similar to our study, found significantly higher NT-proBNP levels in patients with advanced diastolic dysfunction.
In one study, this level was 286 ± 31 pg/ml [13], in another study, the NT-proBNP level was 46 to 48 pg/ml above normal [14] and in another study, it was 14 ± 13 pmol/l [15]. Although these late peptides have been shown to be associated with severe diastolic dysfunction, their role in the diagnosis of mild diastolic heart dysfunction is uncertain [16]. In a study on 396 patients that showed a strong association between plasma NT-proBNP levels and the risk of death in patients with COVID-19, the best median NT-proBNP for predicting mortality at 53 days of follow-up was 847.5 pg/ml. According to this study, NT-proBNP was associated with mortality both in the entire study population and after the exclusion of patients with heart failure. NT-proBNP above this level was associated with a higher risk of mortality in these patients due to cardiac complications raised by complex interactions between previous conditions, ischemia, systemic inflammation, and direct pathogen damage to the cardiovascular system [17]. This amount was lower than the cut-off of our study which predicted grades 2 and 3 diastolic dysfunctions as a poor outcome.
According to an article published in the European Heart Association in 2016, a cut-off point of 125, along with other anatomical and functional signs, was used to diagnose diastolic heart dysfunction. In patients with COVID-19, pro-BNP levels cannot be satisfied with the previous standard figures due to the possibility of high levels of infection, inflammation and hypoxia. It seems that a different cut-off point should be considered. Based on the results of this study, NT-proBNP levels above 799 pg/ml were obtained to diagnose high-grade diastolic dysfunction in patients with severe COVID-19 [18]. The sensitivity of NT-proBNP for the diagnosis of diastolic dysfunction in our study was 100% and it was an accurate screening test that in Lu Bien’s study [13] had a sensitivity of 85% for the diagnosis of diastolic dysfunction in non-COVID patients, this value in another study was reported to be 69% and NT-proBNP was not recommended for screening diastolic dysfunction. However, this study was performed on people over 45 living in the community years before the COVID pandemic who were randomly included in the study [19].
In this study, we compared NT-proBNP for the diagnosis of diastolic dysfunction in hospitalized patients with COVID-19 with echocardiographic indices as a routine non-invasive procedure. However, the standard for assessing diastolic heart function is to measure the pressure–volume relationship with a catheter, which is an invasive procedure. In our study, there was a positive correlation between NT-proBNP levels and some echocardiographic parameters including RA_size, LVED, LV_Ee, and LV_E, and this indicates that as NT-proBNP levels increase, these indices also increase. Significant negative correlation between NT-proBNP level and one of the echocardiographic parameters including LVEF increases with decreasing LVEF serum NT-proBNP level number. According to this study, NT-proBNP above 556 pg/ml in severe patients with COVID-19 has a predictive value of 97.2% for the presence of RV_EA > 2 and also NT-proBNP above 556 pg/ml in these patients has a predictive value of 63.9% for the presence of LV_Ee > 4 in echocardiography. These two echocardiographic parameters indicate an increase in right heart filling pressure/s and due to the relationship between right heart pressure and left heart filling pressure/s and its relationship with pulmonary pressure, hypoxia and lung pressures, it seems that diastolic dysfunction of the right side of the heart begins at lower levels of NT-proBNP.
According to other results from this study, if a person has an NT-proBNP above 1479 pg/ml, LV_E would be greater than 50 cm for 100% (E wave velocity above 50). Given that the E-wave velocity showed a compression gradient between the atrium and the ventricle and depended on the left ventricular complication and left ventricular pressure, it could be said that NT-proBNP above 1479 pg/ml should avoid volumetric and compressive overload. In this study, for every unit increase in SPAP, the chance of diastolic dysfunction increases by 22%. SPAP levels are associated with inflammation and hypoxia of the lungs and are also associated with increased and limited left-filling pressures. The limitations of this study included if it was an observational, single-center study with the inherent limitations of this type of design. The number of samples and the possibility of performing echocardiography were limited due to policies focused on the prevention of SARS-CoV-2 transmission.
Conclusion
In brief, these data suggested that elevated NT-proBNP levels were associated with grade 2 or higher grade of diastolic dysfunction in patients with COVID-19, and this result suggested that this method could be used as a predictor of diastolic dysfunction in these patients, and a better understanding of the possibility of increased right heart filling pressure/s. Following this result, by estimating the probability of increased left and right filling pressures of the heart by reaching the level of NT-proBNP above a certain number, excessive flow of fluids without considering the need and urine output and calculating the estimated filling pressures of the heart can be prevented. In addition, the results of this study indicated a new direction for further research on the use of NT-proBNP as a non-invasive method of diagnosing diastolic dysfunction in patients with COVID-19. It was recommended to investigate the levels of NT-proBNP associated with diastolic dysfunction in higher sample size and multicenter studies. Of course, the follow-up of these patients is valuable in terms of the persistence of diastolic dysfunction and pulmonary pressure.
Acknowledgements
The authors thank the Clinical Research Development Unit of Ayatollah Rouhani Hospital and finally to Farhad Seif for his kind contribution to the final revision of the manuscript.
Abbreviations
- COVID-19
Coronavirus disease 2019
- CT
Computed tomography
- LVEF
Left ventricular ejection fraction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- NT-proBNP
N-terminal pro-brain natriuretic peptide
Author contributions
SS coordinated the study; KE, SGZ, MC, PAM, and RP were responsible for data collection. NZ analyzed and interpreted the data. All authors provided comments on the manuscript at various stages of development. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
Please contact the corresponding author for data requests.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent to publish
Written informed consent was obtained from the patients. We assure that a copy of the consent form is available for review by the journal upon request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zalpoor H, Akbari A, Samei A, Forghaniesfidvajani R, Kamali M, Afzalnia A, et al. (2022) The roles of Eph receptors, neuropilin-1, P2X7, and CD147 in COVID-19-associated neurodegenerative diseases: inflammasome and JaK inhibitors as potential promising therapies. Cell Mol Biol Lett 27(1):1-21 [DOI] [PMC free article] [PubMed]
- 2.Jalali F, Hatami F, Saravi M, Jafaripour I, Hedayati MT, Amin K, et al. (2021) Characteristics and outcomes of hospitalized patients with cardiovascular complications of COVID-19. J Cardiovasc Thorac Res 13(4):355 [DOI] [PMC free article] [PubMed]
- 3.Seif F, Aazami H, Khoshmirsafa M, Kamali M, Mohsenzadegan M, Pornour M, et al (2020) JAK inhibition as a new treatment strategy for patients with COVID-19. Int arch aller immunol 181(6):467-475 [DOI] [PMC free article] [PubMed]
- 4.Corrales-Medina VF, Suh KN, Rose G, Chirinos JA, Doucette S, Cameron DW, et al. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 2011;8(6):e1001048. doi: 10.1371/journal.pmed.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freaney PM, Shah SJ, Khan SS. COVID-19 and heart dysfunction with preserved ejection fraction. JAMA. 2020;324(15):1499–1500. doi: 10.1001/jama.2020.17445. [DOI] [PubMed] [Google Scholar]
- 6.Cardiology E (2020) ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic
- 7.Skulstad H, Cosyns B, Popescu BA, Galderisi M, Salvo GD, Donal E, et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J-Cardiovasc Imaging. 2020;21(6):592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Coats AJ, Zheng Z, Adamo M, Ambrosio G, Anker SD, et al. Management of heart dysfunction patients with COVID-19: a joint position paper of the Chinese Heart Dysfunction Association & National Heart Dysfunction Committee and the Heart Dysfunction Association of the European Society of Cardiology. Eur J Heart Dysfunct. 2020;22(6):941–956. doi: 10.1002/ejhf.1915. [DOI] [PubMed] [Google Scholar]
- 9.Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JG, Kozhuharov N, et al. Heart Dysfunction Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Dysfunct. 2019;21(6):715–731. doi: 10.1002/ejhf.1494. [DOI] [PubMed] [Google Scholar]
- 10.Chehrazi M, Yavarpour H, Jalali F, Saravi M, Jafaripour I, Hedayati MT, et al. Optimal cut points of N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) in patients with COVID-19. The Egyptian Heart Journal. 2022;74(1):1-10. doi: 10.1186/s43044-022-00253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpe M, Rubattu S, Burnett J., Jr Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35(7):419–425. doi: 10.1093/eurheartj/eht466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chitsazan M, Amin A, Chitsazan M, Ziaie N, Amri Maleh P, Pouraliakbar H, et al. Heart dysfunction with preserved ejection fraction in coronavirus disease 2019 patients: the promising role of diuretic therapy in critically ill patients. ESC Heart Dysfunct. 2021;8(2):1610–1614. doi: 10.1002/ehf2.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubien E, DeMaria A, Krishnaswamy P, Clopton P, Koon J, Kazanegra R, et al. Utility of B-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings. Circulation. 2002;105(5):595–601. doi: 10.1161/hc0502.103010. [DOI] [PubMed] [Google Scholar]
- 14.Mottram PM, Leano R, Marwick TH. Usefulness of B-type natriuretic peptide in hypertensive patients with exertional dyspnea and normal left ventricular ejection fraction and correlation with new echocardiographic indexes of systolic and diastolic function. Am J Cardiol. 2003;92(12):1434–1438. doi: 10.1016/j.amjcard.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 15.Alehagen U, Eriksson H, Nylander E, Dahlström U. Heart dysfunction in the elderly: characteristics of a Swedish primary health care population. Heart Drug. 2002;2(5):211–220. doi: 10.1159/000067723. [DOI] [Google Scholar]
- 16.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. Is B-type natriuretic peptide a useful screening test for systolic or diastolic dysfunction in patients with coronary disease? Data from the Heart and Soul Study. Am J Med. 2004;116(8):509–516. doi: 10.1016/j.amjmed.2003.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caro-Codón J, Rey JR, Buño A, Iniesta AM, Rosillo SO, Castrejon-Castrejon S, et al. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur J Heart Dysfunct. 2021;23(3):456–464. doi: 10.1002/ejhf.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMurray J, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Dysfunction 2012 of the European Society of Cardiology; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart dysfunction 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Dysfunction 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Dysfunction Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 19.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation. 2004;109(25):3176–3181. doi: 10.1161/01.CIR.0000130845.38133.8F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the corresponding author for data requests.