Abstract
Background:
Patients with medically-treated opioid overdose are at high risk for subsequent adverse outcomes, including repeat overdose. Understanding factors associated with repeat overdose can aid in optimizing post-overdose interventions.
Methods:
We conducted a longitudinal, retrospective cohort study using NJ Medicaid data from 2014 to 2019. Medicaid beneficiaries aged 12–64 with an index opioid overdose from 2015 to 2018 were followed for one year for subsequent overdose. Exposures included patient demographics; co-occurring medical, mental health, and substance use disorders; service and medication use in the 180 days preceding the index overdose; and MOUD following index overdose.
Results:
Of 4898 individuals meeting inclusion criteria, 19.6% had repeat opioid overdoses within one year. Index overdoses involving heroin/synthetic opioids were associated with higher repeat overdose risk than those involving prescription/other opioids only (HR = 1.44, 95% CI = 1.22–1.71). Risk was higher for males and those with baseline opioid use disorder diagnosis or ED visits. Only 21.7% received MOUD at any point in the year following overdose. MOUD was associated with a large decrease in repeat overdose risk among those with index overdose involving heroin/synthetic opioids (HR = 0.30, 95% CI = 0.20–0.46). Among those receiving MOUD at any point in follow-up, 10.5% (112/1065) experienced repeat overdose versus 22.1% (848/3833) for those without MOUD.
Conclusions:
Repeat overdose was common among individuals with medically-treated opioid overdose. Risk factors for repeat overdose varied by type of opioid involved in index overdose, with differential implications for intervention. MOUD following index opioid overdose involving heroin/synthetic opioids was associated with reduced repeat overdose risk.
Keywords: Opioid overdose, Opioid use disorder, Medication for opioid use disorder, Medicaid
1. Introduction
Patients treated for opioid overdose accounted for 305,623 visits to US emergency departments in 2017 (Vivolo-Kantor et al., 2020), and have represented an increasing share of ED visits since, both before and during the COVID-19 pandemic (Centers for Disease Control, 2021a). Patients experiencing these events are at high risk for subsequent adverse outcomes (Olfson et al., 2018a; Weiner et al., 2020), including repeat overdose (Karmali et al., 2020; Suffoletto and Zeigler, 2020). Assertive, systemic strategies are needed to engage overdose survivors in treatment with medications for opioid use disorder (MOUD); however, studies earlier in the opioid epidemic found low rates of post-overdose treatment initiation (Alinsky et al., 2020; Frazier et al., 2017; Larochelle et al., 2018; Macmadu et al., 2021). Intervention is complicated by inconsistent access to treatment, the very limited proportion of physicians who prescribe MOUD (Stein et al., 2021), and extensive behavioral health and medical comorbidity (Crystal et al., 2021).
Among people with opioid use disorder, it has been well-established through randomized controlled trials that MOUD reduces illicit opioid use (Fudala et al., 2003; Johnson et al., 1995; Kakko et al., 2003; Ling et al., 1998, 2010; Schwartz et al., 2006). However, most trial data are from earlier periods of the opioid overdose epidemic, preceding the more recent fentanyl-dominated phase of the epidemic. Many of the earlier studies focused on a single treatment modality, and few focused on overdose survivors. While it is well understood that MOUD confers benefits for many outcomes, there remains a gap in evidence regarding the association between MOUD and repeat overdose in the current “third wave” of the opioid overdose epidemic, with heroin and fentanyl-related overdoses increasingly predominating nationally since 2013 (Centers for Disease Control, 2021b). In an environment with increasing opioid overdoses, driven by the spread of fentanyl (Mattson et al., 2021), a better understanding of MOUD initiation and outcomes among overdose survivors is needed to inform clinical and health system interventions to reduce subsequent risk.
Understanding repeat overdose risk is particularly important for beneficiaries of Medicaid, the largest single payer of OUD treatment in the U.S. Using New Jersey Medicaid data from 2014 to 2019, this study evaluates the association of MOUD with repeat opioid overdose in a large, usual-care population, during a period of high and rising risk of overdose in a state with widespread fentanyl penetration and increasing overdose rates predominantly associated with injected, illicit drugs (Crystal et al., 2021). We: (1) identified predictors of repeat nonfatal overdose in the year following an index overdose, (2) examined differences in risk depending on the type of opioid involved in the initial overdose, and (3) assessed associations between post-overdose MOUD utilization and repeat overdose, using a time-varying covariate to capture periods of MOUD use and non-use. We hypothesized that patients whose initial overdose involved heroin or synthetic opioids would be at greater risk for repeat overdose compared to those whose overdose involved prescription opioids, and that MOUD initiated after overdose would be associated with substantially lower repeat overdose risk.
2. Methods
This longitudinal, retrospective cohort study used de-identified New Jersey Medicaid claims data for individuals treated for opioid overdose from years 2014–2019.
2.1. Cohort selection, index events and outcomes
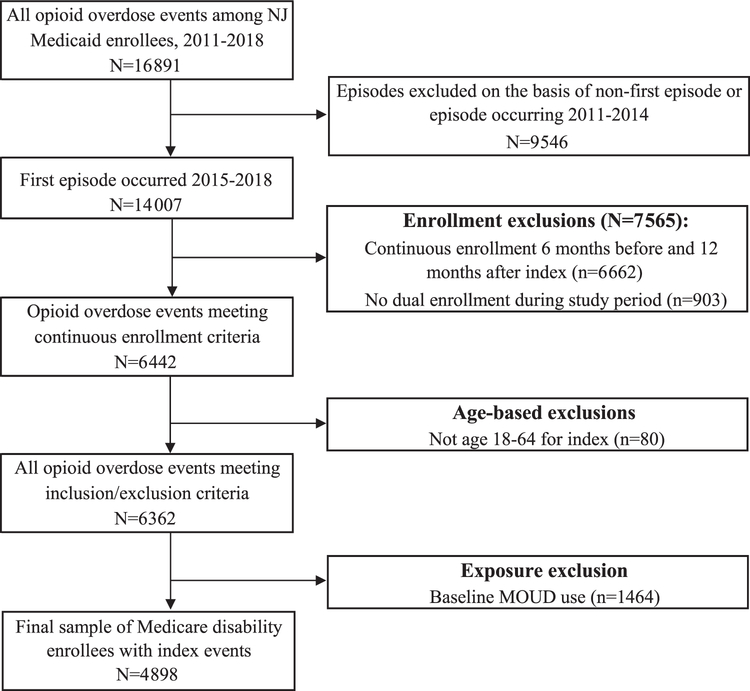
We included patients aged 12–64 years with a medically-treated opioid overdose, identified using ICD-9 and ICD-10 diagnostic codes (Supplement, eTable 1) associated with an ED or inpatient claim in 2015–2018. Validation studies using diagnosis fields in medical claims verified by chart audits have shown high sensitivity and specificity for detection of opioid poisoning using ICD codes (Green et al., 2017, 2019), including poisonings attributed specifically to heroin and other opioids (Slavova et al., 2020; Vivolo-Kantor et al., 2021). We included beneficiaries with continuous Medicaid eligibility and no eligibility for Medicare for 6 months before the index overdose (baseline period) and 12 months afterwards (follow-up period) to allow sufficient time to identify patient characteristics and observe repeat overdose. We assessed repeat overdose within 12 months based on prior literature showing extended periods of risk following index overdose (Olfson et al., 2018b; Suffoletto and Zeigler, 2020). We excluded beneficiaries with any MOUD supply in the 180 days prior to the index overdose, to focus on effects of newly-initiated MOUD. A cohort flow diagram (Vandenbroucke et al., 2007) showing exclusion restrictions is included as Fig. 1. We defined index opioid overdose as the first ED or inpatient claim indicating medically-treated overdose following a baseline period of 180 days with no observed overdose. Using diagnostic codes in claims (Supplement, eTable 1), we determined whether the index overdose involved heroin or a synthetic opioid, whether or not other opioids also contributed to the overdose. We identified benzodiazepine involvement in the index overdose and whether the overdose resulted in inpatient treatment.
Fig. 1.
Cohort flow diagram.
2.2. Explanatory variables
Diagnoses in the look-back period identified baseline OUD diagnosis and comorbidities including alcohol use disorder, benzodiazepine use disorder, cannabis use disorder, schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, personality disorder, asthma, cerebrovascular disease, chronic pain, chronic obstructive pulmonary disease (COPD), diabetes, heart failure, hepatitis C, human immunodeficiency virus (HIV), hypertension, pneumonia, and sleep apnea (Supplement, eTable 2). Measures of pre-index-overdose health services use included ED visits and psychosocial behavioral health services (Supplement, eTable 3). ED visits were determined using claim type, place of service, and provider taxonomy codes. Daily calendars of medication availability for prescribed opioids and benzodiazepines based on days’ supply in claims identified active prescriptions for opioids and benzodiazepines at the time of index overdose (i.e., the prior day) and availability in the look-back period (Supplement, eTable 4).
To examine the effect of active receipt of MOUD, we defined a time-varying covariate indicating whether MOUD treatment was active on each day. Availability of MOUD changes frequently, as disruptions in care are common (Saloner et al., 2017), and most recent availability of MOUD is likeliest to affect risk for a repeat overdose. Daily calendars for MOUD availability used days’ supply for buprenorphine and naltrexone prescriptions from pharmacy data. For MOUD dispensed or administered in a health care setting, which are captured in medical rather than pharmacy claims, we derived availability from HCPCS codes for administration (injectable naltrexone, injectable buprenorphine, and daily or weekly buprenorphine and methadone administered in an opioid treatment program). Methadone maintenance treatment visits were recorded in either daily or weekly procedure codes, allowing for a two-day grace period to account for take-home doses. For each day in the follow-up period, we examined the time-varying effect on repeat overdose of MOUD supply on the preceding day in order to exclude any MOUD that may have been prescribed on the day of a second overdose in response to the overdose itself. Our primary outcome was time (in days) to repeat overdose resulting in an inpatient hospitalization or ED visit. Patients without a repeat overdose were censored at 1 year (365 days) after the index event.
2.3. Statistical analysis
Bivariate associations between explanatory variables and repeat overdose were examined with chi-square tests; the final analysis included those with a p-value approaching significance (p < 0.2) to conserve degrees of freedom. Next, a multivariable Cox proportional hazards model was fit for time to repeat overdose. In addition to time-varying receipt of MOUD after the index overdose, models included all baseline covariates described above for demographic characteristics, medical and behavioral health diagnoses, and health services utilization. Subgroup analyses with separate models investigated differential risk factors for repeat overdose across relevant subgroups, including type of opioid implicated in the index overdose (heroin/synthetic opioids versus only prescription opioids). We performed additional sensitivity analyses to test whether findings were robust to alternate specifications. In the first, we limited the follow-up period to 3 months to reduce the continuous eligibility requirement from 18 to 9 months and increase the analytic sample size. The second sensitivity analysis included fixed effects for hospitals where index overdoses were treated, to control for variation among hospitals in efforts to initiate or link patients to treatment. Because of power constraints, we performed sensitivity analyses only for all overdoses, and not for models that stratified by type of opioid involved in the index overdose. All statistical analyses were performed in SAS Enterprise Guide 7.1.
3. Results
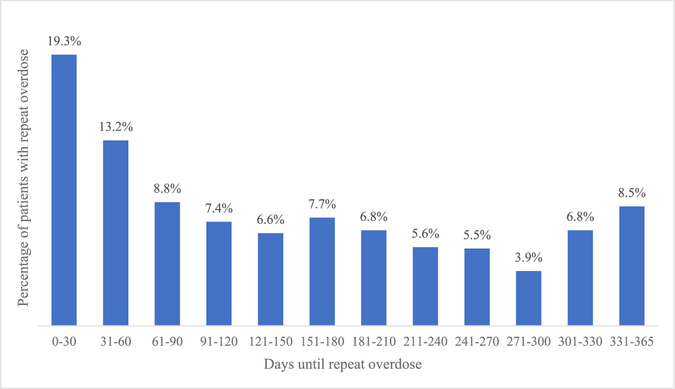
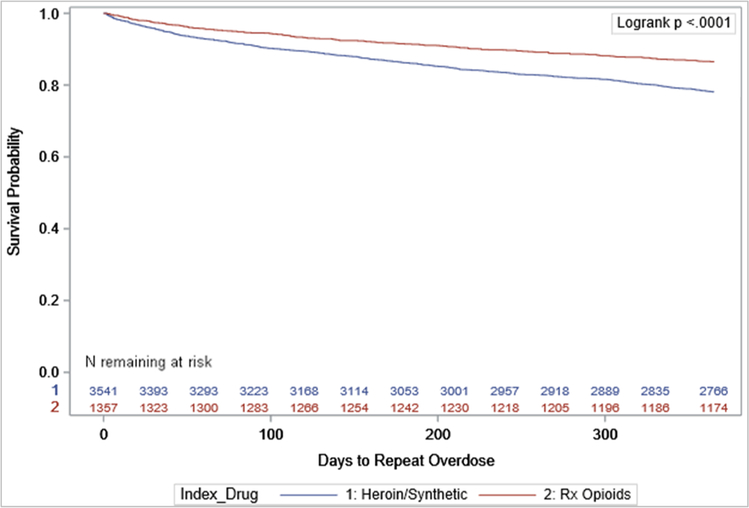
The sample included 4898 NJ Medicaid enrollees with an index opioid overdose who met eligibility criteria (Table 1). The majority were male (60.7%) and White (57.1%); modal age was 25–39 (43.0%). Most index overdoses involved heroin or synthetic opioids (72.3%). The rate of repeat overdose in the follow-up period was 19.6% (n = 960), with median days until repeat overdose of 126 (IQR:42, 237.5) and mean days of 146. Among those with a repeat overdose, 19.3% of the events occurred within 30 days (Fig. 2). Repeat overdose rates were consistently higher following index overdoses involving heroin or synthetic opioids (Fig. 3).
Table 1.
Association of beneficiary characteristics with repeat overdose in year following medically-treated opioid overdose occurring 2015 – 2018.
| Characteristics | Beneficiaries with Index Overdosea No. (%) (N = 4898) |
Repeat Overdose No. (%) (N = 960) |
No Repeat Overdose No. (%) (N = 3938) |
p-valueb |
|---|---|---|---|---|
|
| ||||
| Overall | ||||
| Index Overdose Characteristics | ||||
| Involved Heroin/Synthetic Opioids | 3541 (72.3) | 776 (80.8) | 2765 (70.2) | < 0.0001 |
| Involved Benzodiazepines | 287 (5.9) | 42 (4.4) | 245 (6.2) | 0.029 |
| Resulted in Inpatient Hospitalization | 1092 (22.3) | 160 (16.7) | 932 (23.7) | < 0.0001 |
| Age | ||||
| 12–24 | 495 (10.1) | 100 (10.4) | 395 (10) | 0.0151 |
| 25–39 | 2108 (43) | 454 (47.3) | 1654 (42) | |
| 40–55 | 1681 (34.3) | 298 (31) | 1383 (35.1) | |
| 56–64 | 614 (12.5) | 108 (11.3) | 506 (12.8) | |
| Racial/Ethnic Group | ||||
| White | 2796 (57.1) | 579 (60.3) | 2217 (56.3) | 0.1596 |
| Black | 1272 (26) | 228 (23.8) | 1044 (26.5) | |
| Hispanic | 421 (8.6) | 77 (8) | 344 (8.7) | |
| Other/Unknown | 409 (8.4) | 76 (7.9) | 333 (8.5) | |
| Gender | ||||
| Female | 1924 (39.3) | 325 (33.9) | 1599 (40.6) | 0.0001 |
| Male | 2974 (60.7) | 635 (66.1) | 2339 (59.4) | |
| Year of Index Overdose 2015 | 869 (17.7) | 146 (15.2) | 723 (18.4) | 0.0866 |
| 2016 | 1254 (25.6) | 249 (25.9) | 1005 (25.5) | |
| 2017 | 1447 (29.5) | 306 (31.9) | 1141 (29) | |
| 2018 | 1328 (27.1) | 259 (27) | 1069 (27.1) | |
| SUD Comorbidities | ||||
| Alcohol Use Disorder | 984 (20.1) | 231 (24.1) | 753 (19.1) | 0.0006 |
| Benzodiazepine Use Disorder | 314 (6.4) | 82 (8.5) | 232 (5.9) | 0.0026 |
| Cannabis Use Disorder | 514 (10.5) | 124 (12.9) | 390 (9.9) | 0.0063 |
| Opioid Use Disorder | 1869 (38.2) | 482 (50.2) | 1387 (35.2) | < 0.0001 |
| Psychiatric Comorbidities | ||||
| Schizophrenia | 314 (6.4) | 72 (7.5) | 242 (6.1) | 0.1244 |
| Bipolar Disorder | 808 (16.5) | 176 (18.3) | 632 (16) | 0.0872 |
| Major Depressive Disorder | 1262 (25.8) | 287 (29.9) | 975 (24.8) | 0.0011 |
| Anxiety Disorder | 1640 (33.5) | 350 (36.5) | 1290 (32.8) | 0.0294 |
| Personality Disorder | 206 (4.2) | 50 (5.2) | 156 (4) | 0.0844 |
| Medical Comorbidities | ||||
| Asthma | 408 (8.3) | 84 (8.8) | 324 (8.2) | 0.5994 |
| Cerebrovascular Disease | 174 (3.6) | 42 (4.4) | 132 (3.4) | 0.1247 |
| Chronic Pain | 2574 (52.6) | 519 (54.1) | 2055 (52.2) | 0.2959 |
| COPD | 361 (7.4) | 64 (6.7) | 297 (7.5) | 0.3521 |
| Diabetes | 661 (13.5) | 127 (13.2) | 534 (13.6) | 0.7878 |
| Heart Failure | 170 (3.5) | 28 (2.9) | 142 (3.6) | 0.2955 |
| Hepatitis C | 700 (14.3) | 180 (18.8) | 520 (13.2) | < 0.0001 |
| HIV | 155 (3.2) | 38 (4) | 117 (3) | 0.1171 |
| Hypertension | 1343 (27.4) | 272 (28.3) | 1071 (27.2) | 0.479 |
| Pneumonia | 264 (5.4) | 64 (6.7) | 200 (5.1) | 0.0507 |
| Sleep Apnea | 120 (2.4) | 20 (2.1) | 100 (2.5) | 0.4125 |
| Prescriptions Before Overdose | ||||
| Benzodiazepines (BZ) | ||||
| No BZ before index | 3581 (73.1) | 721 (75.1) | 2860 (72.6) | 0.2714 |
| BZ in 6 months before index | 479 (9.8) | 90 (9.4) | 389 (9.9) | |
| BZ at time of index | 838 (17.1) | 149 (15.5) | 689 (17.5) | |
| Prescription Opioids | ||||
| No opioids before index | 2944 (60.1) | 620 (64.6) | 2324 (59) | 0.0001 |
| Opioids in 6 months before index | 1086 (22.2) | 214 (22.3) | 872 (22.1) | |
| Opioids at time of index | 868 (17.7) | 126 (13.1) | 742 (18.8) | |
| Health Service Utilization | ||||
| ED visit in prior 6 months | 2668 (54.5) | 600 (62.5) | 2068 (52.5) | < 0.0001 |
| Any psychosocial service in prior 6 months | 1482 (30.3) | 312 (32.5) | 1170 (29.7) | 0.0916 |
Note: Unless otherwise noted, all covariates were measured in the 6 months before index overdose.
Includes NJ Medicaid beneficiaries meeting study inclusion criteria.
Chi-square tests were used in bivariate analyses.
Fig. 2.
Distribution of time from index overdose to repeat overdose (N = 960).
Fig. 3.
Survival probability of repeated opioid overdose during the 365 days after index opioid overdose.
Fewer than one-quarter (21.7%) of those with an index overdose received MOUD in the follow-up period, increasing moderately from 18.9% in 2015–2016–23.9% in 2017–2018 (p < 0.001). Only 9.9% had MOUD availability at the end of their follow-up period (day preceding a repeat overdose or last day of the 12-month follow-up). Only 2.9% had active MOUD on the day preceding the repeat overdose.
Results of Cox proportional hazards analysis (Table 2) indicate that repeat overdose risk was higher among those whose index overdose involved heroin or synthetic opioids (HR = 1.44, 95% CI = 1.22–1.71); males (HR = 1.25, 95% CI = 1.08–1.43); and those with an ED visit in the baseline period (HR = 1.31, 95% CI = 1.13–1.52). Baseline diagnosis of OUD was also associated with higher repeat overdose risk (HR = 1.53, 95% CI = 1.32–1.77). Other medical, psychiatric, and SUD diagnoses were not significantly associated with repeat overdose.
Table 2.
Hazards of repeat opioid overdose, stratified by drugs involved in index overdose.
| Full Sample, no MOUD before Index Overdose (n = 4898) | Index Overdose Involved Heroin and/or Synthetic Opioidsa (n = 3541) | Index Overdose Involved Prescription Opioids Onlyb (n = 1357) | |
|---|---|---|---|
|
| |||
| Covariate | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| MOUD After Overdose | 0.35 (0.24, 0.51)* | 0.30 (0.20, 0.46)* | 0.71 (0.31, 1.62) |
| Index Overdose Characteristics | |||
| Involved Heroin/Synthetic Opioids | 1.44 (1.22, 1.71)* | – | – |
| Involved Benzodiazepines | 0.97 (0.70, 1.35) | 1.06 (0.68, 1.64) | 0.95 (0.57, 1.58) |
| Resulted in Inpatient Hospitalization | 0.75 (0.63, 0.9)* | 0.78 (0.64, 0.96)* | 0.70 (0.49, 1.00)* |
| Age | |||
| 12–24 | 1.26 (0.93, 1.71) | 1.25 (0.89, 1.76) | 1.08 (0.53, 2.19) |
| 25–39 | 1.24 (0.97, 1.57) | 1.12 (0.85, 1.47) | 1.57 (0.95, 2.58) |
| 40–55 | 1.02 (0.81, 1.27) | 0.92 (0.70, 1.19) | 1.36 (0.86, 2.12) |
| 56–64 | REF | REF | REF |
| Racial/Ethnic Group | |||
| White | REF | REF | REF |
| Black | 0.88 (0.74, 1.04) | 0.81 (0.67, 0.99) | 1.14 (0.80, 1.62) |
| Hispanic | 0.87 (0.68, 1.10) | 0.77 (0.58, 1.02) | 1.22 (0.74, 1.99) |
| Other/Unknown | 0.92 (0.72, 1.17) | 0.99 (0.76, 1.28) | 0.69 (0.37, 1.30) |
| Gender | |||
| Female | REF | REF | REF |
| Male | 1.25 (1.08, 1.43)* | 1.25 (1.07, 1.47) | 1.19 (0.87, 1.62) |
| Year of index overdose | |||
| 2015 | REF | REF | REF |
| 2016 | 1.11 (0.9, 1.37) | 1.06 (0.84, 1.34) | 1.33 (0.83, 2.11) |
| 2017 | 1.16 (0.95, 1.42) | 1.09 (0.87, 1.36) | 1.43 (0.91, 2.25) |
| 2018 | 1.08 (0.88, 1.33) | 1.04 (0.82, 1.31) | 1.26 (0.77, 2.05) |
| SUD Comorbiditiesc | |||
| Alcohol Use Disorder | 1.03 (0.88, 1.21) | 0.99 (0.83, 1.19) | 1.3 (0.90, 1.90) |
| Benzodiazepine Use Disorder | 1.05 (0.82, 1.35) | 1.12 (0.85, 1.46) | 0.76 (0.41, 1.43) |
| Cannabis Use Disorder | 0.96 (0.78, 1.18) | 1.04 (0.83, 1.29) | 0.6 (0.32, 1.12) |
| Opioid Use Disorder | 1.53 (1.32, 1.77)* | 1.46 (1.24, 1.73) | 1.71 (1.22, 2.39)* |
| Psychiatric Comorbiditiesc | |||
| Bipolar Disorder | 0.97 (0.8, 1.17) | 0.95 (0.77, 1.17) | 1.17 (0.76, 1.80) |
| Major Depressive Disorder | 1.11 (0.94, 1.31) | 1.08 (0.90, 1.31) | 1.16 (0.81, 1.67) |
| Anxiety Disorder | 1.00 (0.85, 1.19) | 1.00 (0.83, 1.21) | 1.05 (0.71, 1.54) |
| Personality Disorder | 1.13 (0.84, 1.54) | 1.32 (0.95, 1.82) | 0.52 (0.20, 1.31) |
| Schizophrenia | 1.04 (0.8, 1.36) | 1.02 (0.76, 1.38) | 1.09 (0.60, 1.98) |
| Medical Comorbiditiesc | |||
| Chronic Pain | 1.09 (0.94, 1.27) | 1.07 (0.91, 1.26) | 1.26 (0.87, 1.83) |
| Heart Failure | 0.83 (0.56, 1.23) | 0.71 (0.42, 1.20) | 1.01 (0.53, 1.96) |
| Hepatitis C | 1.16 (0.97, 1.38) | 1.09 (0.90, 1.32) | 1.58 (1.06, 2.37)* |
| HIV | 1.26 (0.90, 1.77) | 1.19 (0.80, 1.77) | 1.30 (0.66, 2.58) |
| Hypertension | 1.14 (0.96, 1.34) | 1.16 (0.96, 1.41) | 1.00 (0.70, 1.44) |
| Pneumonia | 1.22 (0.93, 1.59) | 1.24 (0.91, 1.69) | 1.16 (0.67, 2.03) |
| Prescriptions Before Overdose | |||
| Benzodiazepines (BZ) | |||
| BZ in 6-month baseline | 0.96 (0.76, 1.22) | 1.04 (0.80, 1.35) | 0.73 (0.41, 1.31) |
| BZ at index overdose | 1.10 (0.89, 1.36) | 0.93 (0.72, 1.20) | 1.57 (1.04, 2.36)* |
| Prescription Opioids | |||
| Opioids in 6-month baseline | 0.88 (0.74, 1.04) | 0.96 (0.79, 1.15) | 0.57 (0.37, 0.89)* |
| Opioids at index overdose | 0.68 (0.54, 0.86)* | 0.70 (0.53, 0.93)* | 0.59 (0.39, 0.90)* |
| Health Service Utilization | |||
| ED visit in 6-month baseline | 1.31 (1.13, 1.52)* | 1.33 (1.13, 1.57)* | 1.20 (0.85, 1.68) |
| Any psychosocial service in 6-month baseline | 0.84 (0.71, 0.99)* | 0.87 (0.73, 1.05) | 0.66 (0.45, 0.97)* |
Note. CI = confidence interval; SUD = substance use disorder.
Statistically significant at p < 0.05.
Includes opioid poisonings with ICD-9 or ICD-10 codes for heroin or synthetic opioid poisoning, even if codes for methadone or other natural/semi-synthetic opioid poisoning were also present.
Includes opioid poisonings without ICD-9 or ICD-10 codes for heroin or synthetic opioid poisoning.
Reference for each comorbid condition is the absence of the condition.
Beneficiaries with MOUD supply were at much lower risk of repeat overdose (HR = 0.35, 95% CI = 0.24–0.51). Repeat overdose risk was also lower among those with prescription opioid availability at the time of the index overdose (HR=0.68 95% CI=0.54–0.86), those whose index overdose resulted in an inpatient hospitalization (HR = 0.75, 95% CI = 0.63–0.90), and those who received any psychosocial services in the 6-month baseline period (HR = 0.84, 95% CI = 0.71–0.99).
In sensitivity analyses, the inclusion of fixed effects for hospitals in which patients were treated produced near-identical results (Supplement, eTable 5), but minor differences were present in a model limiting follow-up to 3 months. In this model, overdoses occurring in years 2017 and 2018 and diagnoses of HIV and hypertension were associated with greater risk of repeat overdose, and receipt of any psychosocial service in the baseline period was not associated with lower risk (Supplement, eTable 6).
In proportional hazards analyses stratified by type of opioid involved in the index overdose, male sex was associated with increased risk of repeat overdose for beneficiaries whose index overdose involved heroin or synthetic opioids (HR = 1.25, 95% CI = 1.07–1.47) but not for those whose index overdose involved only prescription opioids. An ED visit during the baseline period was similarly associated with higher risk of repeat overdose only among those whose index overdose involved heroin or synthetic opioids (HR = 1.33, 95% CI = 1.13–1.57). Compared to non-Hispanic Whites, non-Hispanic Black enrollees whose index overdose involved heroin or synthetic opioids had lower risk of repeat overdose (HR = 0.81, 95% CI = 0.67–0.99).
Notably, among those whose index overdose involved heroin or synthetic opioids, time-varying receipt of MOUD after the index overdose was associated with a more than two-thirds reduction in the hazard of repeat overdose (HR = 0.30, 95% CI = 0.20–0.46). For those whose index overdose involved prescription opioids only, this association did not reach significance (HR = 0.71, 95% CI = 0.31–1.62).
Active benzodiazepine prescription at the time of the index overdose was associated with increased repeat overdose risk (HR 1.57, CI = 1.04–2.36) following index overdoses involving prescription opioids only, but not among those whose index overdose involved heroin or synthetic opioids. Also, among those whose index overdose involved prescription opioids only, risk of repeat overdose was lower for those who received prescription opioid analgesics during the baseline period (HR = 0.57, 95% CI = 0.37–0.89) and at the time of the index overdose (HR = 0.59, 95% CI = 0.39–0.90), as well as those who received any psychosocial service in the 6-month baseline period (HR = 0.66, 95% CI = 0.45–0.97).
4. Discussion
Results identify the high risk of repeat overdose faced by this population, continuing through the one-year follow-up period, which highlights the importance not only of initiating treatment soon after the event but also sustaining treatment over time, as brief episodes of MOUD do not confer substantial longer-term post-treatment overdose risk reduction (Samples et al., 2020; Williams et al., 2020). Repeat overdose risk is particularly high for those experiencing an index overdose involving non-prescribed opioids, underscoring the importance of intervention in this population (Compton et al., 2016; Moore et al., 2007). The high rates of medical and behavioral health comorbidity identified in this population (Table 1) highlight the need for integrated care models that can address multiple treatment needs in a comprehensive fashion. The greatly lowered risk of overdose associated with current MOUD receipt following the index overdose is consistent with prior studies of MOUD effectiveness and extends these findings to the post-overdose population in a high-fentanyl environment. In this study, risk of repeat overdose was 65% lower for patients receiving MOUD (before stratification by substance involved in the index overdose), consistent with similarly large effects found in studies on the association between MOUD and overdose mortality risk (Ma et al., 2019; Santo et al., 2021). Those who received MOUD at any point in follow-up had an overdose rate of 10.5% (112/1065) versus 22.1% (848/3833) for those with no MOUD receipt, suggesting that if rates of MOUD treatment following overdose could be significantly improved, substantial reductions in repeat overdose would be possible. Although we did not conduct a rigorous simulation study of the potential reduction in overdoses if MOUD were initiated in all such patients following the index overdose, our model results imply that if all individuals in the sample received MOUD during their follow-up, approximately 53% (515) of observed overdoses might have been prevented. This calculation does not take into account the time-varying nature of MOUD in this analysis and as a result may under-estimate the number of repeat overdoses that could be averted with MOUD retention.
The high overall risk, and the reduction in risk associated with MOUD, indicate the importance of intensified initiatives to reduce repeat overdose risk, including assertive outreach and case management to engage patients in MOUD treatment. Strategies identified as promising in prior studies include initiation of MOUD with buprenorphine in the emergency department (ED; D’Onofrio et al., 2015); naloxone prescribing and distribution (Samuels et al., 2018); education on overdose risk and prevention and safe injection practices (Samuels et al., 2016); and, as appropriate, referral to community-based harm reduction and syringe access programs, which decrease risk of repeat overdose and mortality (Strayer et al., 2020). These and other strategies are included in consensus recommendations on ED-based OUD treatment issued by the American College of Emergency Physicians (Hawk et al., 2021), but widespread adoption has lagged because of barriers such as limited referral options for continuing care, provider inexperience in treating OUD, and others (Hawk et al., 2020). Payer strategies that incentivize uptake of such practices may hold promise. For example, Pennsylvania’s Hospital Quality Improvement Program OUD ED Initiative created financial incentives for hospitals that implemented pathways to address OUD in the ED, and additional incentives for hospitals that improved rates of post-discharge treatment follow-up (Kilaru et al., 2021).
In addition to initiatives to support prompt initiation of MOUD, sustained and assertive support following overdose is important to support continuation of MOUD, given the high risk of treatment dropout. Further research is needed to identify the roles of multiple potential barriers, such as access to providers, system fragmentation, failure to assertively follow up with patients after the index episode, untreated mental health comorbidity, homelessness, and patient readiness, that may be contributing to the low rate of treatment engagement and continuation, and to test assertive strategies to overcome them. Such strategies could borrow from assertive community treatment models for people with severe and persistent mental illness that have shown promise in overcoming barriers to treatment engagement resulting from complex and fragmented service systems (Dore-Gauthier et al., 2020; Thorning and Dixon, 2020; Trane et al., 2021), which despite their promise have seldom been deployed by MOUD providers. Care models that integrate assertive case management strategies, direct provision of MOUD, and primary medical care may have promise in improving outcomes for this at-risk population.
Those whose index overdose did not lead to hospitalization were at higher risk than those experiencing hospitalization. While engagement of these individuals may be more difficult, it is particularly important for reducing repeat overdose risk. Although unobserved differences in those who were or were not hospitalized could account for the difference, it may also be that hospitalization provides a protective effect, which would support broadening inpatient admissions criteria for this highrisk population. Receipt of any psychosocial service in the 6-month baseline period was also associated with reduced likelihood of repeat overdose, but only among those whose index overdose involved prescription opioids only. Although prior research has shown some psychosocial interventions to be effective (Dutra et al., 2008), patients receiving higher intensity services may have higher baseline risk of repeat overdose, offsetting the potential benefits of psychosocial services among those whose index overdose involved heroin/synthetic opioids.
Those whose index overdose did not involve heroin or synthetic opioids represent another distinct at-risk group, although they accounted for a minority (27.7%) of index overdoses. Within this group, OUD diagnosis was associated with risk of repeat overdose, as expected; these individuals had prior contact with the health system related to their OUD diagnosis, potentially indicating missed opportunities to initiate treatment. Those with benzodiazepine prescriptions at the time of index overdose were also more likely to experience a repeat overdose, potentially reflecting the increased risk associated with use of multiple medications that depress respiration. Receipt of prescribed opioids was associated with reduced risk of repeat overdose. This could suggest that people who used opioids under physician supervision were less vulnerable to experiencing a repeated overdose. Many states, including NJ, have implemented opioid prescribing limits in an effort to reduce risk related to prescribed opioids. Findings regarding outcomes of such policies are mixed, and some studies suggest policies have unintended consequences if not carefully designed and implemented (Davis et al., 2020; Sacks et al., 2021). Prior studies have found that rapid discontinuation of opioids may increase risk of overdose and can lead patients to seek out more dangerous illicit opioids (Alpert et al., 2018; Mark and Parish, 2019; Oliva et al., 2020). Efforts to manage prescribing (e.g., prescribing limits policies, pharmacy utilization management) may be important components of an overall strategy for reducing overdoses, but must be carefully designed to avoid unintended consequences, and implemented alongside robust prevention, treatment, and harm reduction strategies.
Only a minority of those with an index overdose received MOUD during the follow-up period – 18.9% in 2015–2016 and 23.9% in 2017–2018. These findings are similar to low rates of post-overdose MOUD found in studies in Medicaid populations in Pennsylvania, where 15% of Medicaid beneficiaries with prescription drug overdose and 33% with heroin overdose received MOUD in the six months after nonfatal overdose (Frazier et al., 2017), and in Massachusetts where 30% of individuals with medically-treated opioid overdose received MOUD within 12 months (Larochelle et al., 2018).
In examining MOUD receipt in a time-varying framework, we found that only 2.9% of repeat opioid overdoses took place at a time when MOUD treatment was active. Rates of repeat overdose were reduced by more than two-thirds on days when MOUD treatment was active, among those whose index overdoses involving heroin or synthetic opioids. No risk reduction was detected for MOUD utilization after overdose among those whose index overdose did not involve heroin or synthetic opioids, possibly because of power limitations for this smaller group. Some of these index overdoses may have been related to accidental misuse of prescribed opioids, drug interactions, or effects of underlying health conditions (Banerjee et al., 2016; Centers for Disease Control and Prevention, 2012; Garg et al., 2017). MOUD may not be indicated after such events, and repeat overdose is less likely among those with prescription overdose.
In New Jersey, additional initiatives have been implemented since the study period, many of which were not fully implemented until after 2018; continuing assessment of the impact of such initiatives on repeat overdose warrants further research. These initiatives include expansion of initiatives for ED-initiated buprenorphine; reimbursement for navigation services as part of MOUD, as implemented recently in New Jersey’s Office-Based Addiction Treatment (OBAT) program; ED-initiated hospital-based addiction medicine consultation (Englander et al., 2019); facilitated referrals (i.e., “warm handoffs”) to treatment (Duber et al., 2018); and peer-based interventions (McGuire et al., 2020). The Opioid Overdose Recovery Program, launched in 2016 and available statewide in 2018, uses teams of peer recovery support specialists and patient navigators to intervene with patients following opioid overdose and provide linkages to treatment and recovery supports (New Jersey Department of Human Services, 2021). Hospital systems, including those affiliated with New Jersey’s two medication-assisted treatment (MAT) Centers of Excellence, have widely adopted and promoted ED buprenorphine initiation programs (Carroll et al., 2020; Rutgers New Jersey Medical School, 2020). New Jersey Medicaid’s OBAT program aims to increase the pool of community buprenorphine providers by providing enhanced reimbursement to providers meeting certain criteria, including availability of patient navigation services, now reimbursed by Medicaid (New Jersey Division of Medical Assistance and Health Services, 2020). Other interventions with promise for increasing treatment engagement among those at highest risk include low-barrier models of MOUD implemented as a result of the COVID-19 epidemic, including increased use of telemedicine; longer days’ supply on MOUD prescriptions; telephone-based initiation of MOUD; reduced requirements for toxicology testing; and longer take-home supplies in methadone maintenance treatment. Further research is needed to examine the impacts of these promising interventions on large, usual-care Medicaid populations, particularly over long-term periods.
4.1. Limitations
Findings should be interpreted with several limitations in mind. First, although Medicaid covers a high proportion of individuals at risk of overdose (Orgera and Tolbert, 2019), these findings may not generalize to uninsured individuals or those covered by other insurance types. Data are from a single U.S. state and may not be generalizable to other states. Although we controlled for a range of clinical and demographic characteristics, unmeasured confounding could affect the results, including such factors as criminal justice involvement, social support, and transitioning to use of different opioid types than those implicated in the index overdose. Individuals in the sample had to be Medicaid-eligible six months before and 12 months after the index overdose to assure consistent measurement of explanatory variables and outcomes, limiting generalizability to individuals who may be only episodically eligible for Medicaid; however, a sensitivity analysis limiting follow-up to 3 months produced substantively similar results. Poisonings with fentanyl or other synthetic opioids could have been misclassified as other opioid poisonings before the switch to ICD-10-CM in late 2015, though fentanyl poisonings were uncommon before that time (Ciccarone, 2019), and likely classified as heroin poisonings. This study included only overdose events that resulted in ED and inpatient hospital visits. Our analysis focused on the association of current MOUD use with overdose risk; it cannot be assumed that such associations extend beyond the termination of treatment; early termination remains a challenge for achieving the potential risk-reduction benefits of MOUD. Finally, since most overdoses in the study population during these years involved heroin or synthetic opioids, confidence intervals for the smaller group with overdoses involving opioid analgesics were wider, and the fact that the association between MOUD and overdose risk for this group fell short of significance should not be interpreted as a clear demonstration that MOUD may not have a protective effect in this population.
5. Conclusions
This study identified predictors of repeat opioid overdose that can inform efforts to reduce risk following the index event. Although only a minority received MOUD during follow-up, periods of treatment following overdoses involving heroin or synthetic opioids were associated with risk of repeat overdose more than two-thirds lower than other periods. Findings highlight the importance of initiating MOUD in the ED after overdose, assertive follow-up interventions, expanding community availability of MOUD, and improving integration of acute and general medical care to increase treatment utilization and eliminate gaps in care. Increasing access to MOUD during patients’ first ED/hospital visit is critically important in preventing subsequent overdoses.
Supplementary Material
Acknowledgements
We acknowledge the New Jersey Department of Human Services for providing access to data and for their review of this work.
Funding
This work was supported by the National Institute on Drug Abuse (grants 1R01 DA047347-01, K23 DA044342-01, & K01 DA049950); the Foundation for Opioid Response Efforts; and by NIH’s National Center for Advancing Translational Sciences (grant UL1TR003017). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
Dr. Samples has received consulting fees from the American Society of Addiction Medicine. Dr. Williams receives consulting fees and equity from Ophelia Health, Inc. a telehealth buprenorphine provider. No other authors have conflicts to declare.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.drugalcdep.2022.109269.
References
- Alinsky RH, Zima BT, Rodean J, Matson PA, Larochelle MR, Adger H, Bagley SM, Hadland SE, 2020. Receipt of addiction treatment after opioid overdose among Medicaid-enrolled adolescents and young adults. JAMA Pediatr. 174 (3), e195183 10.1001/jamapediatrics.2019.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert A, Powell D, Pacula RL, 2018. Supply-side drug policy in the presence of substitutes: evidence from the introduction of abuse-deterrent opioids. Am. Econ. J. Econ. Policy 10 (4), 1–35. <https://www.jstor.org/stable/26529052>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee G, Edelman EJ, Barry DT, Becker WC, Cerda M, Crystal C, Gaither JR, Gordon AJ, Gordon KS, Kerns RD, Martins SS, Fiellin DA, Marshal BDL, 2016. Non-medical use of prescription opioids is associated with heroin initiation among US veterans: a prospective cohort study. Addiction 111 (11), 2021–2031. 10.1111/add.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll GG, Wasserman DD, Shah AA, Salzman MS, Baston KE, Rohrbach RA, Jones IL, Haroz R, 2020. Buprenorphine field initiation of rescue treatment by emergency medical services (Bupe FIRST EMS): a case series. Prehosp. Emerg. Care 1–5. 10.1080/10903127.2020.1747579. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2012. CDC grand rounds: prescription drug overdoses-a US epidemic. MMWR Morb. Mortal. Wkly. Rep. 61, 10–13. <https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6101a3.htm>. [PubMed] [Google Scholar]
- Centers for Disease Control, 2021a. Data from CDC’s Drug Overdose Surveillance and Epidemiology (DOSE) system. <https://www.cdc.gov/drugoverdose/nonfatal/all-opioids.html>. (Accessed 15 July 2021). [Google Scholar]
- Centers for Disease Control, 2021b. Three Waves of Opioid Overdose Deaths. <https://www.cdc.gov/opioids/basics/epidemic.html>. (Accessed 8 August 2021). [Google Scholar]
- Ciccarone D, 2019. The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. Int. J. Drug Policy 71, 183–188. 10.1016/j.drugpo.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT, 2016. Relationship between nonmedical prescription-opioid use and heroin use. N. Engl. J. Med. 374, 154–163. 10.1056/NEJMra1508490. [DOI] [PubMed] [Google Scholar]
- Crystal S, Nowels M, Olfson M, Samples H, Williams AR, Treitler P, 2021. Medically treated opioid overdoses among New Jersey Medicaid beneficiaries: rapid growth and complex comorbidity amid growing fentanyl penetration, 108546 J. Subst. Abus. Treat 131, 108546. 10.1016/j.jsat.2021.108546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio G, O’Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, Bernstein SL, Fiellin DA, 2015. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA 313, 1636–1644. 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CS, Piper BJ, Gertner AK, Rotter JS, 2020. Opioid prescribing laws are not associated with short-term declines in prescription opioid distribution. Pain Med. 21 (3), 532–537. 10.1093/pm/pnz159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore-Gauthier V, Miron JP, Jutras-Aswad D, Ouellet-Plamondon C, Abdel-Baki A, 2020. Specialized assertive community treatment intervention for homeless youth with first episode psychosis and substance use disorder: a 2-year follow-up study. Early Interv. Psychiatry 14 (2), 203–210. 10.1111/eip.12846. [DOI] [PubMed] [Google Scholar]
- Duber HC, Barata IA, Cioé-Peńa E, Liang SY, Kecham E, Macias-Konstantopoulos W, Ryan SA, Stavros M, Whiteside LK, 2018. Identification, management, and transition of care for patients with opioid use disorder in the emergency department. Ann. Emerg. Med. 72 (4), 420–431. 10.1016/j.annemergmed.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW, 2008. A meta-analytic review of psychosocial interventions for substance use disorders. Am. J. Psychiatry 165 (2), 179–187. 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Englander H, Dobbertin K, Lind BK, Nicolaidis C, Graven P, Dorfman C, Korthuis T, 2019. Inpatient addiction medicine consultation and post-hospital substance use disorder treatment engagement: a propensity-matched analysis. J. Gen. Intern. Med. 34 (12), 2796–2803. 10.1007/s11606-019-05251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W, Cochran G, Lo-Ciganic WH, Gellad WF, Gordon AJ, Chang CH, Donohue JM, 2017. Medication-assisted treatment and opioid use before and after overdose in Pennsylvania Medicaid. JAMA 318 (8), 750–752. 10.1001/jama.2017.7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, Collins J, Raisch D, Casadonte P, Goldsmith RJ, Ling W, Malkerneker U, McNicholas L, Renner J, Stine S, Tusel D, 2003. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N. Engl. J. Med. 349, 949–958. 10.1056/nejmoa022164. [DOI] [PubMed] [Google Scholar]
- Garg RK, Fulton-Kehoe D, Franklin GM, 2017. Patterns of opioid use and risk of opioid overdose death among Medicaid patients. Med. Care 55 (7), 661–668. 10.1097/mlr.0000000000000738. [DOI] [PubMed] [Google Scholar]
- Green CA, Perrin NA, Janoff SL, Campbell CI, Chilcoat HD, Coplan PM, 2017. Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol. Drug Saf. 26 (5), 509–517. 10.1002/pds.4157. [DOI] [PubMed] [Google Scholar]
- Green CA, Perrin NA, Hazlehurst B, Janoff SL, DeVeaugh-Geiss A, Carrell DS, Grijalva CG, Liang C, Enger CL, Coplan PM, 2019. Identifying and classifying opioid-related overdoses: a validation study. Pharmacoepidemiol. Drug Saf. 28 (8), 1127–1137. 10.1002/pds.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk K, D’Onofrio G, Chawarski M, O’Connor P, Cowan E, Lyons M, Richardson L, Rothman R, Whiteside L, Owens PH, Martel S, Coupet E Jr., Pantalon M, Curry L, Fiellin D, Edelamn J, 2020. Barriers and facilitators to clinician readiness to provide emergency department-initiated buprenorphine: results of a survey and qualitative analysis. JAMA Netw. Open 3 (5), e204561. 10.1001/jamanetworkopen.2020.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk K, Hoppe J, Ketcham E, LaPietra A, Moulin A, Nelson L, Schwarz E, Shahid S, Stader D, Wilson MP, D’Onofrio G, 2021. Consensus recommendations on the treatment of opioid use disorder in the emergency department. Ann. Emerg. Med. 78 (3), 434–442. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE, 1995. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend. 40 (1), 17–25. 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- Kakko J, Svanborg KD, Kreek MJ, Heilig M, 2003. 1-Year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in SWEDEN: a randomised, placebo-controlled trial. Lancet 361, 662–668. 10.1016/s0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- Karmali RN, Ray GT, Rubinstein AL, Sterling SA, Weisner CM, Campbell CI, 2020. The role of substance use disorders in experiencing a repeat opioid overdose, and substance use treatment patterns among patients with a non-fatal opioid overdose, 107923 Drug Alcohol Depend. 209, 107923. 10.1016/j.drugalcdep.2020.107923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilaru AS, Lubitz SF, Davis J, Eriksen W, Siegel S, Kelley D, Perrone J, Meisel ZF, 2021. A state financial incentive policy to improve emergency department treatment for opioid use disorder: a qualitative study. Psychiatr. Serv. 72 (9), 1048–1056. 10.1176/appi.ps.202000501. [DOI] [PubMed] [Google Scholar]
- Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, Bagley SM, Liebschutz JM, Walley AY, 2018. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann. Intern. Med. 169 (3), 137–145. 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Casadonte P, Bigelow G, Kampman KM, Patkar A, Bailey G, Rosenthal R, Beebe KL, 2010. Buprenorphine implants for treatment of opioid dependence: a randomized controlled trial. JAMA 304 (14), 1576–1583. 10.1001/jama.2010.1427. [DOI] [PubMed] [Google Scholar]
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS Jr., Kintaudi P, Wesson DR, Mcnicholas L, Tusel DJ, Malkerneker U, Renner JA, Santos E, Casadonte P, Fye C, Stine S, Wang RIH, Segal D, 1998. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction 93 (4), 475–486. 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- Ma J, Bao YP, Wang RJ, Su MF, Liu MX, Li JQ, Degenhardt L, Farrell M, Blow FC, Ilgen M, Shi J, Lu L, 2019. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol. Psychiatry 24 (12), 1868–1883. 10.1038/s41380-018-0094-5. [DOI] [PubMed] [Google Scholar]
- Macmadu A, Paull K, Youssef R, Batthala S, Wilson KH, Samuels EA, Yedinak JL, Marshall BDL, 2021. Predictors of enrollment in opioid agonist therapy after opioid overdose or diagnosis with opioid use disorder: a cohort study. Drug Alcohol Depend. 219, 108435 10.1016/j.drugalcdep.2020.108435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark TL, Parish W, 2019. Opioid medication discontinuation and risk of adverse opioid-related health care events. J. Subst. Abus. Treat. 103, 58–63. 10.1016/j.jsat.2019.05.001. [DOI] [PubMed] [Google Scholar]
- Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL, 2021. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013–2019. MMWR Morb. Mortal. Wkly. Rep. 70 (6), 202–207. 10.15585/mmwr.mm7006a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AB, Powell KG, Treitler PC, Wagner KD, Smith KP, Cooperman N, Robinson L, Carter J, Ray B, Watson DP, 2020. Emergency department-based peer support for opioid use disorder: emergent functions and forms. J. Subst. Abus. Treat. 108, 82–87. 10.1016/j.jsat.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, Schottenfeld RS, 2007. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J. Gen. Intern. Med. 22 (4), 527–530. 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New Jersey Department of Human Services, 2021. Division of Mental Health and Addiction Services. DMHAS opioid overdose recovery program. <https://www.state.nj.us/health/integratedhealth/documents/OORP_Providers.pdf>. (Accessed 9 September 2021). [Google Scholar]
- New Jersey Division of Medical Assistance and Health Services, 2020. Office-based addiction treatment (OBAT) update – enrollment of OBAT navigators as servicing providers. Published 2020. <https://www.njmmis.com/documentDownload.aspx?document=30-03.pdf>. (Accessed 15 December 2020). [Google Scholar]
- Olfson M, Crystal S, Wall M, Wang S, Liu S-M, Blanco C, 2018a. Causes of death after nonfatal opioid overdose. JAMA Psychiatry 75, 820–827. 10.1001/jamapsychiatry.2018.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Wall M, Wang S, Crystal S, Blanco C, 2018b. Risks of fatal opioid overdose during the first year following nonfatal overdose. Drug Alcohol Depend. 190, 112–119. 10.1016/j.drugalcdep.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva EM, Bowe T, Manhapra A, Kertesz S, Hah JM, Henderson P, Robinson A, Paik M, Sandbrink F, Gordon AJ, Trafton JA, 2020. Associations between stopping prescriptions for opioids, length of opioid treatment, and overdose or suicide deaths in US veterans: observational evaluation. BMJ 368, m283. 10.1136/bmj.m283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgera K, Tolbert J, 2019. The opioid epidemic and Medicaid’s role in facilitating access to treatment. Kaiser Family Foundation. <https://www.kff.org/medicaid/issue-brief/the-opioid-epidemic-and-medicaids-role-in-facilitating-access-to-treatment>. (Accessed 15 December 2020). [Google Scholar]
- Rutgers New Jersey Medical School, 2020. Department of Psychiatry. Northern New Jersey MAT Center of Excellence mission. Published 2020. <http://njms.rutgers.edu/departments/psychiatry/nc_mission.cfm>. (Accessed 15 December 2020). [Google Scholar]
- Sacks DW, Hollingsworth A, Nguyen T, Simon K, 2021. Can policy affect initiation of addictive substance use? Evidence from opioid prescribing. J. Health Econ. 76, 102397 10.1016/j.jhealeco.2020.102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner B, Daubresse M, Alexander CG, 2017. Patterns of buprenorphine-naloxone treatment for opioid use disorder in a multistate population. Med. Care 55 (7), 669–676. 10.1097/MLR.0000000000000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples H, Williams AR, Crystal S, Olfson M, 2020. Impact of long-term buprenorphine treatment on adverse health care outcomes in medicaid. Health Aff. 39 (5), 747–755. 10.1377/hlthaff.2019.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels EA, Bernstein SL, Marshall BDL, Krieger M, Baird J, Mello MJ, 2018. Peer navigation and take-home naloxone for opioid overdose emergency department patients: preliminary patient outcomes. J. Subst. Abus. Treat. 94, 29–34. 10.1016/J.JSAT.2018.07.013. [DOI] [PubMed] [Google Scholar]
- Samuels EA, Dwyer K, Mello MJ, Baird J, Kellogg AR, Bernstein E, 2016. Emergency department-based opioid harm reduction: moving physicians from willing to doing. Acad. Emerg. Med. 23 (4), 455–465. 10.1111/acem.12910. [DOI] [PubMed] [Google Scholar]
- Santo T Jr, Clark B, Hickman M, Grebely J, Campbell G, Sordo L, Chen A, Tran LT, Bharat C, Padmanathan P, Cousins G, Dupouy J, Kelty E, Muga R, Nosyk B, Min J, Pavarin R, Farrell M, Degenhardt L, 2021. Association of opioid agonist treatment with all-cause mortality and specific causes of death among people with opioid dependence: a systematic review and meta-analysis. JAMA Psychiatry 78 (9), 979–993. 10.1001/jamapsychiatry.2021.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RP, Highfield DA, Jaffe JH, Brady JV, Butler CB, Rouse CO, Callaman JM, O’Grady KE, Koudstaal PJ, Breteler MMB, 2006. A randomized controlled trial of interim methadone maintenance. Arch. Gen. Psychiatry 63 (1), 102–109. 10.1001/archpsyc.63.1.102. [DOI] [PubMed] [Google Scholar]
- Slavova S, Quesinberry D, Costich JF, Pasalic E, Martinez P, Martin J, Eustice S, Akpunonu P, Bunn TL, 2020. ICD-10-CM-based definitions for emergency department opioid poisoning surveillance: electronic health record case confirmation study. Public Health Rep. 135 (2), 262–269. 10.1177/0033354920904087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Saloner B, Schuler MS, Gurvey J, Sorbero M, Gordon AJ, 2021. Concentration of patient care among buprenorphine-prescribing clinicians in the US. JAMA 325 (21), 2206–2208. 10.1001/jama.2021.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer RJ, Hawk K, Hayes BD, Herring AA, Ketcham E, LaPietra AM, Lynch JJ, Motov S, Repanshek Z, Weiner SG, Nelson LS, 2020. Management of opioid use disorder in the emergency department: a white paper prepared for the American Academy of Emergency Medicine. J. Emerg. Med. 58 (3), 522–546. 10.1016/j.jemermed.2019.12.034. [DOI] [PubMed] [Google Scholar]
- Suffoletto B, Zeigler A, 2020. Risk and protective factors for repeated overdose after opioid overdose survival. Drug Alcohol Depend. 209, 107890 10.1016/j.drugalcdep.2020.107890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorning H, Dixon L, 2020. Forty-five years later: the challenge of optimizing assertive community treatment. Curr. Opin. Psychiatry 33 (4), 397–406. 10.1097/YCO.0000000000000615. [DOI] [PubMed] [Google Scholar]
- Trane K, Aasbrenn K, Ronningen M, Odden S, Lexen A, Landheim A, 2021. Flexible assertive community treatment teams can change complex and fragmented service systems: experiences of service providers. Int. J. Ment. Health Syst. 15 (1), 38. 10.1186/s13033-021-00463-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, STROBE Initiative, 2007. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology 18 (6), 805–835. 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- Vivolo-Kantor AM, Hoots BE, Scholl L, Pickens C, Roehler DR, Board A, Mustaquim D, Smith H, Snodgrass S, Liu S, 2020. Nonfatal drug overdoses treated in emergency departments — United States, 2016–2017. MMWR Morb. Mortal. Wkly. Rep. 69, 371–376. 10.15585/mmwr.mm6913a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivolo-Kantor A, Pasalic E, Liu S, Martinez PD, Gladden RM, 2021. Defining indicators for drug overdose emergency department visits and hospitalisations in ICD-10-CM coded discharge data. Inj. Prev. 27 (S1), i56–i61. 10.1136/injuryprev-2019-043521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner SG, Baker O, Bernson D, Schuur JD, 2020. One-year mortality of patients after emergency department treatment for nonfatal opioid overdose. Ann. Emerg. Med. 75 (1), 13–17. 10.1016/j.annemergmed.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Samples H, Crystal S, Olfson M, 2020. Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. Am. J. Psychiatry 177 (2), 117–124. 10.1176/appi.ajp.2019.19060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.