In this issue of JEM, Diny et al. identify the aryl hydrocarbon receptor (AHR) as a key orchestrator of eosinophil tissue adaptation in the small intestine.
Abstract
In this issue of JEM, Diny et al. (2022. J. Exp. Med. https://doi.org/10.1084/jem.20210970) identify the aryl hydrocarbon receptor (AHR) as a key orchestrator of eosinophil tissue adaptation in the small intestine, controlling their lifespan, degranulation, and tissue-remodeling activities.
The last decades have seen a flowering of new reports addressing eosinophil homeostatic activities within tissues. Far from the historical view of eosinophils as being exclusively terminally differentiated effector cells—perpetrating tissue damages in the context of Th2 immunity—these granulocytes are increasingly recognized as important modulators of immune, metabolic, and tissue morphogenetic processes. Eosinophils are particularly abundant in the lamina propria of the gastrointestinal (GI) tract, where the range of their activities is only beginning to be unraveled. Recent evidence suggests eosinophils actively promote intestinal homeostasis by maintaining epithelial barrier integrity, protecting against pathogens, and regulating local immune responses (Gurtner et al., 2021). In this issue of JEM, Diny et al. (2022) expand the scope of this burgeoning field by showing that eosinophils transcriptionally adapt to the small intestinal niche and adopt a tissue residency program that is partly dependent on the ligand-activated transcription factor aryl hydrocarbon receptor (AHR). AHR controlled several cell-intrinsic properties such as degranulation and lifespan, and further promoted eosinophil interactions with extracellular matrix (ECM) components and tissue remodeling activity. Thus, Diny et al. add another stone to the mosaic of eosinophil activities by revealing their contribution to the homeostatic remodeling of the gut architecture.

Insights from Isabelle Catherine Arnold.
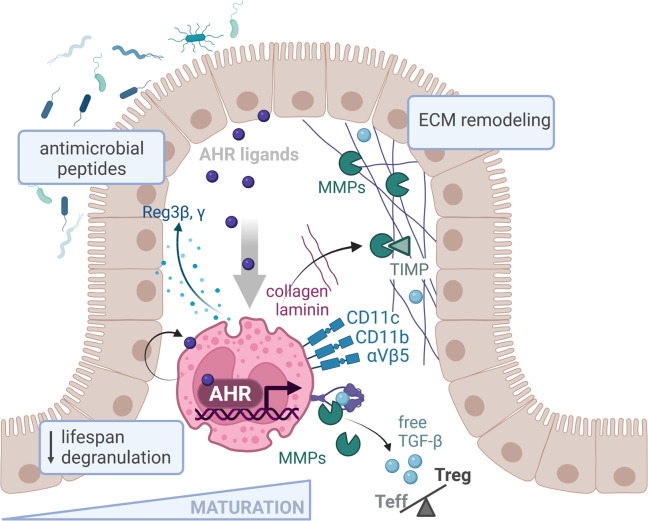
The GI tract harbors the largest number of immune cells in the body, which are separated from our microbial residents by just a single layer of epithelial cells. Like other mucosal sites, this tissue poses numerous challenges to newly recruited immune cells, as it is constantly stimulated by a variety of external agents. Rapid adaptation to this ever-changing environment is thus essential to protect against invading pathogens while tolerating the natural stimulation occurring from innocuous commensal bacteria. To maintain this homeostatic state, intestinal immune cells have developed functional programs tailored to their niche’s requirements, which are often dictated by the tissue microenvironment itself. While the notion of tissue adaptation has already been proposed for other myeloid cells such as macrophages, dendritic cells, or neutrophils (Ballesteros et al., 2020; Kang et al., 2020), the adaptability of eosinophils to specific microenvironments has so far only been suggested by their functional and surface marker heterogeneity across tissues. We learn now from Diny and co-workers that eosinophils undergo extensive transcriptional and functional adaptation upon GI residency. Even more astonishing is the extent and nature of their transcriptional reprogramming. When compared to their mature bone marrow counterparts, small intestinal eosinophils upregulated over 450 genes, suggesting they actively integrate microenvironmental cues to exert their tissue-specific functions. Within the intestinal lamina propria, eosinophils upregulated a broad range of transcripts relating to the synthesis of immune mediators and antimicrobial peptides as well as genes functioning in cell–cell communication, junction formation, and ECM remodeling. Interestingly, Ahr was among the most upregulated eosinophil transcripts in the small intestine and was required for the full expression of intestine-adapted gene programs. AHR expression was higher in long-term resident than in newly recruited eosinophils, suggesting tissue adaptation and acquisition of tissue-specific functions might be a gradual process: as AHR expression increases in response to endogenous ligands, eosinophils progress toward a more differentiated state. The feedback loop of AHR pathway activation might thus serve as a checkpoint to control the kinetics of their maturation in the small intestine. In the absence of AHR, eosinophils indeed retained a more immature phenotype, and their life span was extended. Mice lacking AHR specifically in the eosinophil compartment thus harbored higher numbers of eosinophils in the small intestine. The availability of AHR ligands might thus control their tissue accumulation by regulating the dynamics of their local differentiation. The presence of normal eosinophil numbers in the colon and other tissues of EpxCre/+ Ahrfl/fl mice further suggests that AHR is predominantly active in the small intestine, possibly promoting regional specialization.
An intriguing observation of Diny et al. is the higher extent of eosinophil degranulation and activation state in the absence of AHR. AHR may thus raise the activation threshold of eosinophils in the small intestine to prevent cytotoxic tissue damage that could occur from excessive microbial responsiveness. This observation is in line with the general refractory state observed in other myeloid cells populating the lamina propria. Both intestinal macrophages, and to a lesser extent dendritic cells, are hyporesponsive to TLR stimulation, with IL-10 and TGF-β emerging as key drivers of this adaptation process (Cerovic et al., 2009; Smythies et al., 2010; Ueda et al., 2010). While the adaptation of intestinal myeloid cells under homeostatic condition is often characterized by the acquisition of an anti-inflammatory phenotype (Zigmond et al., 2014), favoring the terminal differentiation and maintenance of FoxP3+ regulatory T cells, the transcriptional profile of intestinal eosinophils is more nuanced, with upregulation of both pro- and anti-inflammatory cytokine genes such as Il1b, Csf1, Il4, Il6, and Tgfb2. It is thus possible that eosinophil function in the lamina propria requires a certain degree of inflammatory activity (i.e., similar to intestinal macrophages that constitutively express both IL-10 and TNF-α). Further mechanistic studies will be required to understand in which context these individual cytokines are released, and how they synergize with local immune pathways to maintain tissue homeostasis.
Upon migration to the GI tract, eosinophils undergo extensive transcriptional reprogramming, including upregulation of AHR. The availability of AHR ligands dictates the expression of a regional specialization program, which promotes eosinophil maturation, reduces their lifespan, and raises their activation threshold in the small intestine. Tissue-adapted eosinophils respond to AHR through the expression of antimicrobial peptides and ECM components as well as matrix-degrading enzymes and their inhibitors (MMPs and TIMPs). MMPs also cleave latent TGF-β into its active form, which affects the balance between effector (Teff) and regulatory (Treg) T cells. Created with Biorender.com.
How does AHR-related transcriptional program impact eosinophil function in the small intestine? It seems now from Diny et al. (2022) that a fundamental task of these tissue resident eosinophils relates to the homeostatic remodeling of the ECM. The ECM is a complex and dynamic network of proteins, which not only provides a scaffold for the tissues but also closely interacts with them to modulate their phenotypes and functions. The ECM continuously undergoes controlled renewal and remodeling through the coordinated action of ECM-producing cells, degrading enzymes and their specific inhibitors. This activity may be particularly relevant within the intestinal tissue, since the high cellular turnover rate and the constant mechanical stress during bowel movements necessitates timely regeneration. Diny et al. show that eosinophils upregulate multiple transcripts related to ECM components as well as proteins functioning in ECM remodeling upon small intestinal residency, including several laminins, collagens, and matrix metalloproteinases (MMPs). Interestingly, many of these genes were also dependent on AHR. In a series of functional assays, the authors showed that AHR-deficient eosinophils were functionally impaired in their adhesion to ECM components and were less capable of degrading collagen derivatives. Eosinophils are indeed well equipped to communicate with the ECM, as they express multiple integrins capable of interacting with its components, such as CD11c, CD11b, and β 5 integrin. Of note, their high CD11c expression in the small intestine relative to other GI compartments such as the colon, cecum, or stomach (Chu et al., 2014) suggests eosinophil–ECM interaction might be particularly important at this site. Besides the expression of matrix degrading MMPs and their inhibitory enzymes (TIMPs), intestinal eosinophils may also promote homeostatic ECM turnover indirectly. Eosinophils are a prominent source of TGF-β, and eosinophil-derived TGF-β stimulates the synthesis of ECM components by fibroblast (Gomes et al., 2005). Although TGF-β gene expression was not reported here to directly depend on AHR, the cleavage of latent TGF-β into its active form is promoted by MMP9 (Kobayashi et al., 2014), itself regulated by AHR. The release of active TGF-β might thus also rely on this pathway, at least to some extent. As eosinophil-derived TGF-β has recently been shown to drive the expansion of a peripherally induced regulatory T cell subset in the lamina propria (Fallegger et al., 2022), a connection between AHR activation and the release of TGF-β could provide an explanation for the reduced regulatory T cell frequency observed in EpxCre/+ Ahrfl/fl mice.
Already in 2010, a provocative hypothesis of Lee et al. (2010) stipulated that eosinophils are intrinsically homeostatic cells that regulate local immunity and/or remodeling/repair (LIAR hypothesis), generally associated with sites of high cell turnover and/or stem cell activities. It further proposes that the mechanisms by which these cells propagate pathophysiological responses may be the same as those employed in their residential tissues to maintain homeostasis. It is indeed interesting to note that eosinophil mostly reside in tissues experiencing intense homeostatic remodeling, cellular turnover, or mechanical stress (i.e., lung, intestine, thymus, uterus) and are further recruited to site experiencing injury or inflammation. Following the LIAR postulate, eosinophil-related pathological conditions may thus arise from the failure of eosinophils to perform their tissue-specific tasks rather than resulting from purely detrimental effector activities. A better understanding of the microenvironmental signals driving eosinophil tissue adaptation and how they may translate into a functional response in health and disease is therefore essential to develop refined eosinophil targeting strategies. The fantastic work of Diny et al. is certainly a milestone in that direction. Whether AHR-expressing eosinophils might also promote tissue regenerative responses in other organs or might attenuate fibrosis in inflammatory settings are open questions that deserve further attention.
References
- Ballesteros, I., et al. 2020. Cell. 10.1016/j.cell.2020.10.003 [DOI] [Google Scholar]
- Cerovic, V., et al. 2009. J. Immunol. 10.4049/jimmunol.0802318 [DOI] [Google Scholar]
- Chu, D.K., et al. 2014. J. Exp. Med. 10.1084/jem.20131800 [DOI] [Google Scholar]
- Diny, N.L., et al. 2022. J. Exp. Med. 10.1084/jem.20210970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallegger, A., et al. 2022. Mucosal Immunol. 10.1038/s41385-022-00484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, I., et al. 2005. J. Allergy Clin. Immunol. 10.1016/j.jaci.2005.06.031 [DOI] [PubMed] [Google Scholar]
- Gurtner, A., et al. 2021. Semin. Immunopathol. 10.1007/s00281-021-00856-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, B., et al. 2020. Mucosal Immunol. 10.1038/s41385-019-0228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, T., et al. 2014. Am. J. Physiol. Lung Cell Mol Physiol. 10.1152/ajplung.00015.2014 [DOI] [Google Scholar]
- Lee, J.J., et al. 2010. Clin. Exp. Allergy. 10.1111/j.1365-2222.2010.03484.x [DOI] [Google Scholar]
- Smythies, L.E., et al. 2010. J. Biol. Chem. 10.1074/jbc.M109.069955 [DOI] [Google Scholar]
- Ueda, Y., et al. 2010. Int. Immunol. 10.1093/intimm/dxq449 [DOI] [Google Scholar]
- Zigmond, E., et al. 2014. Immunity. 10.1016/j.immuni.2014.03.012 [DOI] [Google Scholar]