Abstract
Background:
The most common clinical manifestation of adenovirus (AdV) infection is acute respiratory illness (ARI). Specific AdV species associated with ARI hospitalizations are not well defined in the Middle East.
Methods:
A viral surveillance study was conducted among children <2 years hospitalized in Amman, Jordan, from March 2010 to March 2013. Nasal and throat respiratory specimens were obtained from enrolled children and tested for viruses using real-time reverse-transcription quantitative polymerase chain reaction. AdV-positive specimens were typed by partial hexon gene sequencing. Demographic and clinical features were compared between AdV detected as single pathogen vs. co-detected with other respiratory viruses, and between AdV-B and AdV-C species.
Results:
AdV was detected in 475/3168 (15%) children hospitalized with ARI; of these, 216 (45%) specimens were successfully typed with AdV-C as the most common species detected (140/216; 65%). Children with AdV-single detection (88/475; 19%) had a higher frequency of fever (71% vs. 56%; p=0.015), diarrhea (18% vs. 11%; p=0.048), and/or seizures/abnormal movements (14% vs. 5%; p=0.003). Children with AdV co-detected with other viruses more likely required oxygen support (adjusted odds ratio [aOR] 1.91 [95% CI: 1.08, 3.39], p=0.027) than those with AdV-single detection. Children with AdV-C had higher odds of co-detections with other viruses compared to those with AdV-B (aOR 4.00 [95% CI: 1.91, 8.44], p<0.001).
Conclusion:
Clinical differences were identified between AdV-single and AdV co-detected with other viruses, and between AdV-B and AdV-C. Larger studies with AdV typing are needed to determine additional epidemiological and clinical differences between specific AdV species and types.
Keywords: Adenovirus, adenovirus types, adenovirus species, acute respiratory illness, viral co-detection
INTRODUCTION
Adenovirus (AdV) is a non-enveloped double-stranded DNA virus that was first detected in adenoid tissue in 1953. Human AdVs (genus Mastadenovirus) are subdivided into seven species A–G, with over 100 genotypes discovered to date.1 Currently, AdV classification is based on molecular typing using polymerase chain reaction (PCR) methods targeting the hexon gene.2 Multiple studies have reported the circulation of specific AdV species and types in countries worldwide, with some predominating in the Southern Hemisphere,3,4 and others in the Northern Hemisphere.5–7
Although AdV-associated acute respiratory illnesses (ARI) occur sporadically with mild to moderate illnesses and self-limited courses, outbreaks have been reported with severe and even fatal outcomes with the emergence of new strains worldwide.4–6,8–10 Specifically, species that are associated with ARI as the most common clinical presentation are AdV-B (types B3, B7, B11, B14, B16, and B21), AdV-C (types C1, C2, C5, and C6), and AdV-E (type E4). Globally, AdV is a common cause of ARI in children. AdV accounts for approximately 5–10% of all ARIs and 4–15% of pneumonia cases in children under five years.11–13 AdV was detected in up to 15% of respiratory specimens from children <18 years hospitalized with ARI worldwide.14–16 However, data focusing on the clinical features and outcomes associated with AdV illnesses, and specific circulating AdV species among children in the Middle East are not well described.17–19
The objective of the current study was to describe the seasonality, burden, and clinical characteristics of AdV by species in children under two years of age hospitalized at Al-Bashir Hospital in Amman, Jordan, over three respiratory seasons. In addition, given high frequency of co-detection with other respiratory viruses, we compared AdV detected as a single pathogen to AdV co-detected with other respiratory viruses.
METHODS
Active Population-Based Surveillance
We conducted a prospective respiratory viral surveillance study in young children who were hospitalized at Al-Bashir Hospital, a large government hospital in Amman, Jordan from March 15, 2010 through March 31, 2013.20,21 Active, year-round, hospital-based surveillance was conducted five days per week (Sunday-Thursday) to recruit eligible children.20,21 Children were eligible if they presented with fever (>38 degrees Celsius) and/or respiratory symptoms (e.g. cough, shortness of breath, wheezing) and had any of the following admission diagnoses within 48 hours after admission: ARI, apnea, asthma exacerbation, bronchiolitis, bronchopneumonia, croup, cystic fibrosis exacerbation, febrile seizure, fever without localizing signs, respiratory distress, pneumonia, pneumonitis, pertussis-like cough, rule-out sepsis, or febrile seizure.20,21 Exclusion criteria for this study were: age older than two years, fever and neutropenia, newborns who had never been discharged, or had been admitted for more than 48 hours.20 The institutional review boards of the University of Jordan, the Jordanian Ministry of Health, and Vanderbilt University Medical Center (VUMC) approved the study.
Data and Specimen Collection
Prior to enrollment, written informed consents in Arabic were obtained from parents and/or guardians of children by trained research staff.20,21 Parents/guardians were interviewed using a standardized questionnaire to obtain the child’s demographic, socioeconomic, and past medical and clinical history, with subsequent collection of nasal and throat respiratory specimens within 48 hours after hospitalization.20 After hospital discharge, charts were systematically abstracted for the following: antibiotic use; blood, urine, and CSF culture results; chest radiography results; oxygen use; intensive care unit admission; mechanical ventilation; length of stay in the hospital; and status at discharge.21 All data were entered into a standardized, secure Research Electronic Data Capture (REDCap) database (Vanderbilt University, Nashville, Tennessee, USA).22 Quality data checks were performed on a minimum of 10% of the charts and data were verified from all case report forms after entry.20
Laboratory Typing
Research nasal and throat respiratory specimens were obtained from enrolled subjects, collected in transport medium (M4RT, Remel, USA), and stored at −80°C. Aliquots of the original specimen and the specimen mixed with a lysis buffer were shipped on dry ice to VUMC and initially tested by real-time reverse-transcription quantitative polymerase chain reaction (RT-qPCR) for eleven respiratory viruses: respiratory syncytial virus (RSV); human metapneumovirus (HMPV); human rhinovirus (HRV); influenza A, B, and C; parainfluenza virus 1, 2, and 3 (PIV 1–3); AdV; and Middle East respiratory syndrome coronavirus.21 Specimens were subsequently tested by RT-qPCR at VUMC for common human coronaviruses (HCoVs) (HKU1, OC43, 229E, and NL63) and PIV-4,23,24 along with AdV typing using a CDC protocol for single-plex qPCR assays specific for endemic respiratory AdV types 1–7, 11, 14, 16, and 21 targeting the hexon gene.25,26 In addition, each specimen was tested for cellular ribonuclease P (RNP) as an indicator of specimen quality and internal control.
Definitions
AdV co-viral detection group is defined as the detection of AdV with at least one other respiratory virus (RSV; HMPV; HRV; PIV 1–4; influenza A, B, or C; HCoV), regardless of AdV species or type. AdV-single detection denotes the detection of AdV with no other respiratory viruses identified, regardless of AdV species or type. AdV types (1–7, 11, 14, 16, and 21) co-detection is defined as the detection of more than one AdV types in the same specimen regardless of co-detection with other respiratory viruses. AdV species B included the following types: 3, 7, 11, 14, 16, 21; C: 1, 2, 5, 6; and E: 4. AdV species co-detection is defined as the identification of more than one species in the same specimen regardless of co-detection with other respiratory viruses. Underlying medical conditions are defined as having at least one of the following: diabetes, heart disease, Down syndrome, kidney disease, sickle cell disease, cystic fibrosis, cancer, genetic/metabolic syndrome, cerebral palsy, neurological disorder, developmental delay, seizure disorder, diarrhea >2 weeks, gastroesophageal reflux disease, immunodeficiency, asthma/reactive airway disease, or liver disease.20,21
STATISTICAL ANALYSIS
For categorical variables, descriptive statistics were reported as absolute and relative frequencies and analyzed using Pearson’s χ2 test. For continuous variables, descriptive statistics were reported as mean/standard deviation (SD) using two-sample t-tests allowing unequal variances to compare continuous variables between groups. We used three multivariable logistic regression models with robust standard errors to estimate odds ratios (ORs) and 95% confidence intervals (CIs): 1) comparing the need for oxygen support between AdV-single and AdV co-viral detection; 2) comparing the need for oxygen support between AdV-B and AdV-C; and 3) comparing co-viral detections between AdV-B and AdV-C species. Adjustment variables were identified a priori and included age, sex, underlying medical conditions, number of siblings in all models. The third model evaluating the association between AdV species and oxygen support was adjusted for the aforementioned covariates and AdV co-viral detection. Statistical significance was determined to be achieved at the nominal α=0.05 level; all statistical analyses were performed in STATA/IC 15.1 (StataCorp LLC, College Station, TX).
RESULTS
Of the 3,168 children enrolled, 475 (15%) had AdV detected from a respiratory specimen, and 216 (45%) of these were successfully typed by RT-PCR (Figure 1) with a total of 234 target detections. After exclusion of specimens with AdV types co-detection (n=15) (Figure 1), AdV-2 (n=70, 35%), AdV-3 (n=48, 24%), and AdV-1 (n=40, 20%) were the most common detected types; and AdV-C (n=140, 65%) and AdV-B (n=53, 25%) were the most commonly detected species (Figure 1). More than one AdV type was identified in 15/216 (7%) of respiratory specimens. Furthermore, AdV-2 (n=8) and AdV-3 (n=9) were the most common types involved in AdV types co-detection (Supplemental Table 1).
Figure 1.
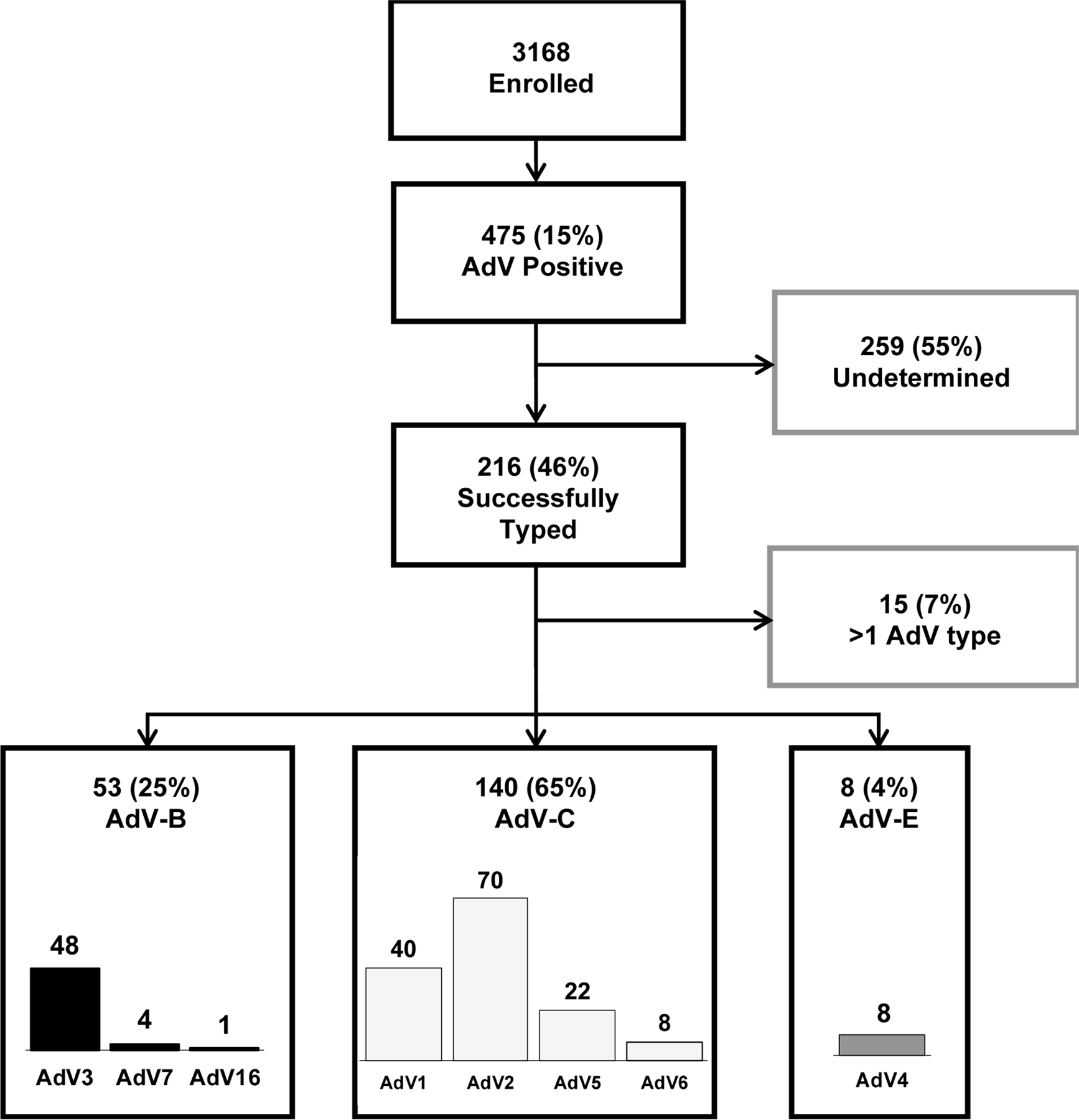
Consort Diagram of Children Hospitalized with ARI in Amman, Jordan Over Three Respiratory Seasons, 2010–2013
Abbreviations: AdV, human adenovirus
AdV-positive vs. AdV-negative
Compared to AdV-negative children, AdV-positive children had a slightly higher mean age (7 vs. 6 months, p<0.001), higher frequency of antibiotic use prior to hospitalization (46% [217/475] vs. 40% [1,069/2,693], p=0.048), and more often presented with seizure or abnormal movements upon admission (7% [31/475] vs. 3% [94/2,693], p=0.002).
AdV Co-viral Detection vs. AdV-single
AdV co-viral detection was noted in 387/475 (81%), and the most common co-detected respiratory pathogens were RSV (102/387; 26%) and HRV (99/387; 26%), followed by PIV (16/387; 4%), HMPV (12/387; 3%), HCoVs (13/387; 3%), and influenza (5/387; 1%). One-third of children with co-viral detections (140/387, 36%) had AdV plus two or more co-detected other respiratory viruses in various combinations. Children with AdV-single detection had a higher frequency of fever, diarrhea, or seizures/abnormal movements, but less frequently presented with cough or shortness of breath and required less oxygen support compared to those with AdV co-viral detections (Table 1). Notably, three children with detected AdV died. One 5-month-old male patient with an underlying cardiac condition was diagnosed with bronchopneumonia requiring oxygen support and had AdV-B (AdV-3) co-detected with HRV. The two other children had unidentified AdV type: a 46-day old female, born preterm at 35 weeks, with osteogenesis imperfecta had co-viral detection with HRV and influenza, and a 22-day old male with no underlying medical condition had AdV-single detection.
Table 1.
Demographic and Clinical Characteristics of Children Hospitalized with Adenovirus in Amman, Jordan Over Three Respiratory Seasons, 2010–2013
| Characteristics | AdV-positive n=475 N (%) | AdV-single detection n=88 N (%) | AdV co-viral detection n=387 N (%) | p-value* |
|---|---|---|---|---|
| Age, months (mean [SD]) | 7 (6) | 8 (7) | 7 (6) | 0.250 |
| Sex, male | 298 (63) | 59 (67) | 239 (62) | 0.354 |
| Cesarean section | 140 (29) | 28 (32) | 1112 (29) | 0.593 |
| Premature, <37 weeks | 81 (17) | 12 (14) | 69 (18) | 0.345 |
| Gestational age, weeks (mean [SD]) | 39 (3) | 39 (3) | 38 (3) | 0.228 |
| Birth weight, kg (mean [SD]) | 3 (0.7) | 3 (0.7) | 3 (0.7) | 0.991 |
| Underlying medical condition | 62 (13) | 12 (14) | 50 (13) | 0.857 |
| Breastfeeding Hx. | 390 (82) | 76 (86) | 314 (81) | 0.248 |
| No. days reported sick (mean [SD]) | 4a (5) | 4b (7) | 4 (4) | 0.852 |
| No. of household members (mean [SD]) | 6 (2) | 5 (3) | 6 (2) | 0.655 |
| Siblings | 394 (83) | 65 (74) | 329 (85) | 0.012 |
| Number of siblings (mean [SD]) | 2 (2) | 2 (2) | 2 (2) | 0.265 |
| Childcare attendance | 9 (2) | 2 (3) | 7 (2) | 0.773 |
| Smoke exposure, nargila or cigarette | 370 (78) | 68 (77) | 302 (78) | 0.876 |
| Antibiotics prior to hospitalization | 217 (46) | 40 (45) | 177 (46) | 0.962 |
| Antibiotics during hospitalization | 432 (91) | 79 (90) | 353 (91) | 0.181 |
|
| ||||
| Symptoms | ||||
| Fever | 280 (59) | 62 (71) | 218 (56) | 0.015 |
| Cough | 358 (75) | 46 (52) | 312 (81) | <0.001 |
| Congestion | 3 (1) | 0 (0) | 3 (1) | |
| Runny nose | 5 (1) | 2 (2) | 3 (1) | |
| Vomiting | 86 (18) | 21 (24) | 65 (17) | 0.120 |
| Diarrhea | 57 (12) | 16 (18) | 41 (11) | 0.048 |
| Shortness of breath | 268 (56) | 32 (36) | 236 (61) | <0.001 |
| Wheezing† | 264 (56) | 43 (49) | 221 (57) | 0.160 |
| Seizures/abnormal movements | 31 (7) | 12 (14) | 19 (5) | 0.003 |
|
| ||||
| Severity | ||||
| Length of stay, days (mean [SD]) | 5c (4) | 6d (6) | 5e (3) | 0.195 |
| Admission to ICU | 32 (7) | 3 (3) | 29 (7) | 0.168 |
| Oxygen | 152f (32) | 18g (21) | 134e (35) | 0.010 |
| Oxygen duration, days (mean [SD]) | 4h (4) | 5i (6) | 3j (3) | 0.200 |
| Mechanical ventilation | 14k (3) | 3d (3) | 11e (3) | 0.778 |
| Death | 3 (1) | 1b (1) | 2l (1) | 0.434 |
p-values were calculated using two-sample t-tests allowing unequal variances for continuous variables and Pearson χ2 test for categorical variables.
Data are in n(%), unless otherwise indicated.
denotes collected on physical exam.
n=474;
n=87;
n=469;
n=87;
n=382;
n=469;
n=87;
n=152;
n=18;
n=134;
n=469;
n=381.
Abbreviations: AdV, human adenovirus; Hx, history; ICU, intensive care unit.
Children with AdV co-viral detection more likely required oxygen support compared to those with AdV-single detection (adjusted odds ratio [aOR] 1.91 [95% CI: 1.08, 3.39], p=0.027). Further, children with AdV co-detected with RSV specifically had a 3.38-fold (95% CI: 1.74, 6.57) higher odds of oxygen requirement than children with AdV-single detection (Table 2).
Table 2.
Association Between Adenovirus Co-viral Detection and Oxygen Requirement*
| Adjusted OR* | 95% CI | P-value | |
|---|---|---|---|
| AdV-single (N=88) | Ref | Ref | Ref |
| AdV + RSV (N=102) | 3.38 | 1.74, 6.57 | <0.001 |
| AdV + HMPV (N=12) | 0.86 | 0.15, 4.87 | 0.867 |
| AdV + HRV (N=99) | 1.12 | 0.55, 2.30 | 0.750 |
| AdV + Influenza (N=5) | 3.02 | 0.42, 21.87 | 0.273 |
| AdV + Coronavirus (N=13) | 0.32 | 0.04, 2.45 | 0.276 |
| AdV + PIV (N=16) | 0.99 | 0.27, 3.64 | 0.994 |
| AdV + 2 or more co-pathogens detected (N=140) | 2.19 | 1.15, 4.19 | 0.017 |
| Age, months | 0.92 | 0.89, 0.96 | <0.001 |
| Sex, male | 0.68 | 0.45, 1.04 | 0.073 |
| Siblings in family | 1.20 | 0.67, 2.10 | 0.525 |
| Underlying medical condition | 2.83 | 1.46, 5.49 | 0.002 |
Model adjusted for age, sex, number of siblings, and underlying medical condition.
Abbreviations: AdV, human adenovirus; RSV, respiratory syncytial virus; HMPV, human metapneumovirus; HRV, human rhinovirus; PIV, parainfluenza virus.
Seasonal Distribution of AdV Detections
Year-round detection of AdV was documented, but with the higher frequency of detection seen primarily during the winter months (i.e., December-February). The frequency varied by respiratory season with a higher frequency of AdV detection noted during the last year of surveillance (Supplemental Figure 3).
AdV-B vs. AdV-C
Children with AdV-C were more often born prematurely (<37 weeks of gestation) than those with AdV-B (Table 3).
Table 3.
Demographic and Clinical Characteristics of Adenovirus Species B and Adenovirus Species C in Children Hospitalized with Acute Respiratory Illness in Amman, Jordan Over Three Respiratory Seasons, 2010–2013
| Characteristics | All Species€ n=201 N (%) | AdV-B n=53 N (%) | AdV-CΩ n=140 N (%) | p-value* |
|---|---|---|---|---|
| Age, months (mean [SD]) | 9 (6) | 9 (7) | 9 (5) | 0.515 |
| Sex, male | 130 (65) | 32 (60) | 93 (66) | 0.432 |
| Cesarean section | 62 (31) | 13 (25) | 47 (34) | 0.226 |
| Premature, <37 weeks | 35 (17) | 4 (8) | 31 (22) | 0.019 |
| Gestational age, weeks (mean [SD]) | 39 (3) | 39 (2) | 38 (3) | 0.010 |
| Birth weight, kg (mean [SD]) | 3 (0.7) | 3 (0.6) | 3 (0.7) | 0.032 |
| Underlying medical condition | 27 (13) | 8 (15) | 17 (12) | 0.586 |
| Breastfeeding Hx. | 165 (82) | 47 (89) | 111 (79) | 0.131 |
| No. days reported sick (mean [SD]) | 4 (7) | 4 (8) | 4 (3) | 0.682 |
| No. of household members (mean [SD]) | 5 (2) | 6 (2) | 5 (2) | 0.302 |
| Siblings | 161 (80) | 42 (79) | 114 (81) | 0.731 |
| Number of siblings (mean [SD]) | 2 (2) | 2 (2) | 2 (2) | 0.114 |
| Childcare attendance | 1 (0.5) | 0 (0) | 1 (0.7) | 0.537 |
| Smoke exposure, nargila or cigarette | 167 (83) | 44 (83) | 117 (84) | 0.927 |
| Antibiotics prior to hospitalization | 107 (53) | 32 (60) | 72 (51) | 0.266 |
| Antibiotics during hospitalization | 187 (93) | 51 (96) | 128 (91) | 0.251 |
|
| ||||
| Symptoms | ||||
| Fever | 142 (71) | 39 (74) | 96 (69) | 0.498 |
| Cough | 150 (75) | 39 (74) | 107 (76) | 0.681 |
| Congestion | 1 (0.5) | 0 (0) | 1 (0.5) | 0.537 |
| Runny nose | 3 (1) | 1 (2) | 2 (1) | 0.818 |
| Vomiting | 35 (17) | 8 (15) | 24 (17) | 0.733 |
| Diarrhea | 29 (14) | 11 (21) | 17 (12) | 0.129 |
| Shortness of breath | 113 (56) | 27 (51) | 84 (60) | 0.256 |
| Wheezing† | 120 (60) | 27 (51) | 88 (63) | 0.132 |
| Seizures/ab. movement | 22 (11) | 4 (8) | 16 (11) | 0.430 |
|
| ||||
| Severity | ||||
| Length of stay, days (mean [SD]) | 5 (4) | 5 (3) | 5 (4) | 0.366 |
| Admission to ICU | 11 (5) | 1 (2) | 9 (6) | 0.204 |
| Admin. oxygen | 46a (23) | 11 (21) | 34b (25) | 0.571 |
| Oxygen duration, days (mean [SD]) | 4 (3) | 4 (4) | 3 (3) | 0.489 |
| Mechanical ventilation | 7c (3) | 2 (4) | 5d (4) | 0.960 |
| Death | 1e (0.5) | 1f (2) | 0 (0) | 0.102 |
p-values were calculated using two-sample t-tests allowing unequal variances for continuous variables and Pearson χ2 test for categorical variables to compare AdV-B and AdV-C species.
Includes AdV-B, AdV-C and AdV-E; does not include specimens with AdV types co-detection (see Supplemental Table 1.)
Does not include specimens with AdV types co-detection (see Supplemental Table 1.)
Data are in n (%), unless otherwise indicated.
denotes collected on physical exam.
n=199;
n=138;
n=199;
n=138;
n=198;
n=52.
Abbreviations: AdV, human adenovirus; Hx, history; ICU, intensive care unit.
No significant difference in oxygen requirement was found between children with detected AdV-B and AdV-C (aOR 1.42 [95% CI: 0.65, 3.11], p=0.380). However, children with AdV-C had 4 times higher odds of co-viral detections with other respiratory viruses than those with AdV-B (aOR 4.00 [95% CI: 1.91, 8.44], p<0.001).
DISCUSSION
AdV was detected in 15% of young, hospitalized children in our study conducted in Amman, Jordan. In addition, over three-quarters of the children had AdV co-viral detection. Although AdV ARI characteristics in children continue to be reported worldwide,27–29 this study helps fill a knowledge gap about AdV ARI in the Middle East region.
The frequency of AdV detection in hospitalized children with ARI in our study is lower compared to studies from Jordan30 and Egypt31 which reported AdV detection by RT-qPCR in 37% hospitalized and 18% seen in the emergency department (ED), respectively. In contrast, a study in Saudi Arabia by Fagbo et al. showed that only 6% of children seen in the ED with ARI symptoms had AdV detected by RT-qPCR.32 Differences between previously reported AdV detection frequencies and findings from our study can be explained by the inclusion of children older than two years, the exclusion of hospitalized children in two studies, and small sample sizes. Additional surveillance studies in the Middle East are needed to precisely document the burden of pediatric AdV ARI in different age groups and medical settings.
AdV-C (predominantly AdV-2 and −1) was the most commonly detected species in our study (70%), compared to AdV-B (22%). This finding is similar to a study in Egypt in which 75% and 22% of children under two years of age had AdV-C and AdV-B detected, respectively,33 but in contrast to studies from China that reported AdV-B as the predominant species detected in hospitalized children <14 years with febrile respiratory infection symptoms34 or hospitalized with pneumonia (92.9%)28 and from Argentina that described 50% AdV-B detection followed by 40% AdV-C prevalence among patients seen in the ED or hospitalized.35 These differences might be related to AdV regional circulation, different age groups, and/or species/types shifting over time. Therefore, further surveillance in the Middle East is warranted to understand the circulation of AdV and the role of AdV species and types in pediatric ARI.
In our study we did not observe differences in oxygen requirement as a proxy for illness severity between AdV-B and AdV-C, the most frequently reported AdV species worldwide associated with ARI, after adjusting for confounders including co-viral detections. However, a recently reported cross-sectional study of children with ARI in Buenos Aires, Argentina, demonstrated that AdV-B was associated with worse outcomes with long-term sequelae or even death compared to AdV-C.35 In our study, we reported one death of a child who had AdV-B and co-viral HRV detection; this patient was diagnosed with bronchopneumonia and required oxygen support. Given a single case of death in our study population, it is not possible to draw conclusions about the role of specific types in illness severity. Therefore, it is important to continue the research of AdV types and species associated with severe illness to build a platform for prevention initiatives in the pediatric population.
A major strength of our study is that we conducted prospective three-year viral surveillance, with large pediatric sample size. During the study period, we were able to capture approximately 50–60% of ARI hospitalizations among children in Amman admitted at Al-Bashir Hospital.20 Additionally, all specimens collected from enrolled children were tested for AdV and other respiratory viruses using sensitive and specific RT-qPCR. However, we also had some limitations. First, we were unable to determine types for all AdV-positive specimens, which hindered our ability to compare outcomes across the full range of assayed types. This might be explained by specimen degradation during storage; higher cycle threshold values, indicating lower viral loads, were associated with untypeable specimens (mean 36 vs. 31, p<0.01). In addition, testing did not capture all possible respiratory targets. Second, our surveillance efforts were limited to one hospital in Amman, Jordan; thus, these results may not be generalizable to all children under two years in Jordan. Third, we performed PCR typing based on hexon gene only instead of combined hexon, penton, and fiber genes or the direct sequencing method, which could change the types distribution and identify new AdV types in our study. Moreover, we acknowledge that AdV may be incidentally detected due to prolonged shedding following previous ARI,36 and AdV might not contribute to ARI in all positive subjects, especially in children with co-viral detections. The exact role of AdV in ARI among children with co-viral detections is an important question for future studies. Lastly, our study enrolled children five days per week, potentially underestimating the true AdV burden in Amman, Jordan.
Summary
Data from this study showed that AdV is detected in nearly 1/6 of children under two years hospitalized with ARI in Amman Jordan. AdV-C was the most frequently detected species and frequently associated with co-viral detections. This knowledge will help catalyze further investigation into the epidemiology and clinical features of AdV species and types associated with pediatric ARI in the Middle East.
Supplementary Material
Supplemental Table 1. Hospitalized Children with More Than One Adenovirus Type, Including Adenovirus Species Co-detection and Adenovirus Co-viral Detection
Supplemental Figure 1. Distribution by Admission Diagnoses of Adenovirus-single/Co-viral Detection (A) and Adenovirus Species (B) Among Children Hospitalized with ARI in Amman, Jordan Over Three Respiratory Seasons, 2010–2013
Supplemental Figure 2. Distribution by Age Group of Adenovirus-single/Co-viral Detection (A) and Adenovirus Species (B) Among Children Hospitalized with ARI in Amman, Jordan Over Three Respiratory Seasons, 2010–2013
Supplemental Figure 3. Seasonal Distribution of Adenovirus Detection Among Children Hospitalized with ARI in Amman, Jordan Over Three Respiratory Seasons, 2010–2013
ACKNOWLEDGMENTS
We would like to thank Al-Bashir Hospital and Jordan University for their collaboration in this surveillance project; our research recruiters, Hanan Amin, Amani Altaber, Hana’a Khalaf, Isra’aKharbat, Darin Yasin, Shireen Issa, and Nurse Sabah Gharbli; and the doctors at Al-Bashir Hospital.
Funding support:
This work was supported by UBS Optimus Foundation, National Institutes of Health [grant number R01AI085062], and the National Center for Advancing Translational Sciences [grant number UL1TR000445]. Varvara Probst is supported by Vanderbilt Institute for Clinical and Translational Research (VICTR) [grant number VR54023] and Conducting Child Health Care Research in Vulnerable Populations [grant number 5T32HD060554–10. Training Grant 2019–2020]. Danielle A. Rankin is supported by the National Institutes of Health [award numbers TL1TR002244, DAR]. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Institutes of Health, National Center for Advancing Translational Sciences, nor Vanderbilt Institute for Clinical and Translational Research.
Footnotes
Competing Interests
Natasha Halasa, MD, MPH receives grant support from Sanofi and Quidel and speaker compensation from an education grant supported by Genentech. Sanofi also donated vaccines and influenza antibody testing for an influenza vaccine trial. John Williams, MD is on the Scientific Advisory Board for Quidel, serves on an Independent Data Monitoring Committee for GlaxoSmithKline, and serves on the Scientific Advisory Board for ID Connect. All other authors declare no competing interests.
REFERENCES
- 1.Chodosh JRBJ, Curiel DT, Heim A, et al. Human Adenovirus Working Group (http://hadvwg.gmu.edu/).
- 2.Madisch I, Wolfel R, Harste G, Pommer H, Heim A. Molecular identification of adenovirus sequences: a rapid scheme for early typing of human adenoviruses in diagnostic samples of immunocompetent and immunodeficient patients. Journal of medical virology 2006;78(9):1210–7. DOI: 10.1002/jmv.20683. [DOI] [PubMed] [Google Scholar]
- 3.Colom AJ, Teper AM. Post-infectious bronchiolitis obliterans. Pediatr Pulmonol 2018. DOI: 10.1002/ppul.24221. [DOI] [PubMed] [Google Scholar]
- 4.Kajon AE, Mistchenko AS, Videla C, Hortal M, Wadell G, Avendano LF. Molecular epidemiology of adenovirus acute lower respiratory infections of children in the south cone of South America (1991–1994). Journal of medical virology 1996;48(2):151–6. DOI: . [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Choi EH, Lee HJ. Comprehensive serotyping and epidemiology of human adenovirus isolated from the respiratory tract of Korean children over 17 consecutive years (1991–2007). Journal of medical virology 2010;82(4):624–31. DOI: 10.1002/jmv.21701. [DOI] [PubMed] [Google Scholar]
- 6.Lin KH, Lin YC, Chen HL, et al. A two decade survey of respiratory adenovirus in Taiwan: the reemergence of adenovirus types 7 and 4. Journal of medical virology 2004;73(2):274–9. DOI: 10.1002/jmv.20087. [DOI] [PubMed] [Google Scholar]
- 7.Adhikary AK. Genomic diversity of human adenovirus type 3 isolated in Fukui, Japan over a 24-year period. J Med Microbiol 2017;66(11):1616–1622. DOI: 10.1099/jmm.0.000625. [DOI] [PubMed] [Google Scholar]
- 8.Gray GC, McCarthy T, Lebeck MG, et al. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004–2006. Clin Infect Dis 2007;45(9):1120–31. DOI: 10.1086/522188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binder AM, Biggs HM, Haynes AK, et al. Human Adenovirus Surveillance - United States, 2003–2016. MMWR Morb Mortal Wkly Rep 2017;66(39):1039–1042. DOI: 10.15585/mmwr.mm6639a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung R, Eshaghi A, Lombos E, et al. Characterization of culture-positive adenovirus serotypes from respiratory specimens in Toronto, Ontario, Canada: September 2007-June 2008. Virol J 2009;6:11. DOI: 10.1186/1743-422X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selvaraju SB, Kovac M, Dickson LM, Kajon AE, Selvarangan R. Molecular epidemiology and clinical presentation of human adenovirus infections in Kansas City children. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 2011;51(2):126–31. DOI: 10.1016/j.jcv.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. The New England journal of medicine 2015;372(9):835–45. (In eng). DOI: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhedin S, Lindstrand A, Hjelmgren A, et al. Respiratory viruses associated with community-acquired pneumonia in children: matched case-control study. Thorax 2015;70(9):847–53. DOI: 10.1136/thoraxjnl-2015-206933. [DOI] [PubMed] [Google Scholar]
- 14.Finianos M, Issa R, Curran MD, et al. Etiology, seasonality, and clinical characterization of viral respiratory infections among hospitalized children in Beirut, Lebanon. Journal of medical virology 2016;88(11):1874–81. DOI: 10.1002/jmv.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wansaula Z, Olsen SJ, Casal MG, et al. Surveillance for severe acute respiratory infections in Southern Arizona, 2010–2014. Influenza Other Respir Viruses 2016;10(3):161–9. DOI: 10.1111/irv.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie L, Zhang B, Xiao N, et al. Epidemiology of human adenovirus infection in children hospitalized with lower respiratory tract infections in Hunan, China. Journal of medical virology 2019;91(3):392–400. DOI: 10.1002/jmv.25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chehadeh W, Al-Adwani A, John SE, et al. Adenovirus types associated with severe respiratory diseases: A retrospective 4-year study in Kuwait. Journal of medical virology 2018;90(6):1033–1039. DOI: 10.1002/jmv.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandelboim M, Dror P, Azar R, Bromberg M, Mendelson E. Adenovirus infections in hospitalized patients in Israel: epidemiology and molecular characterization. J Clin Microbiol 2011;49(2):597–601. DOI: 10.1128/JCM.00979-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shachor-Meyouhas Y, Hadash A, Kra-Oz Z, Shafran E, Szwarcwort-Cohen M, Kassis I. Adenovirus Respiratory Infection among Immunocompetent Patients in a Pediatric Intensive Care Unit During 10-year period: Co-morbidity is common. Isr Med Assoc J 2019;21(9):595–598. (https://www.ncbi.nlm.nih.gov/pubmed/31542903). [PubMed] [Google Scholar]
- 20.Halasa N, Williams J, Faouri S, et al. Natural history and epidemiology of respiratory syncytial virus infection in the Middle East: Hospital surveillance for children under age two in Jordan. Vaccine 2015;33(47):6479–87. DOI: 10.1016/j.vaccine.2015.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khuri-Bulos N, Lawrence L, Piya B, et al. Severe outcomes associated with respiratory viruses in newborns and infants: a prospective viral surveillance study in Jordan. BMJ Open 2018;8(5):e021898. DOI: 10.1136/bmjopen-2018-021898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. (In eng). DOI: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haddadin Z, Chappell J, McHenry R, et al. Coronavirus Surveillance in a Pediatric Population in Jordan From 2010 to 2013: A Prospective Viral Surveillance Study. Pediatr Infect Dis J 2021;40(1):e12–e17. DOI: 10.1097/INF.0000000000002965. [DOI] [PubMed] [Google Scholar]
- 24.Howard LM, Rankin DA, Spieker AJ, et al. Clinical features of parainfluenza infections among young children hospitalized for acute respiratory illness in Amman, Jordan. BMC Infect Dis 2021;21(1):323. DOI: 10.1186/s12879-021-06001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Trujillo-Lopez E, Lott L, Erdman DD. Quantitative real-time PCR assay panel for detection and type-specific identification of epidemic respiratory human adenoviruses. J Clin Microbiol 2013;51(4):1089–93. DOI: 10.1128/JCM.03297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu X, Erdman DD. Quantitative real-time PCR assays for detection and type-specific identification of the endemic species C human adenoviruses. J Virol Methods 2016;237:174–178. DOI: 10.1016/j.jviromet.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakir J, Juarez MDV, Lucion MF, et al. Clinical and epidemiological study of acute lower respiratory tract infections caused by adenovirus in hospitalized children. Nineteen years of active epidemiological surveillance. Arch Argent Pediatr 2020;118(3):193–201. DOI: 10.5546/aap.2020.eng.193. [DOI] [PubMed] [Google Scholar]
- 28.Zou L, Yi L, Yu J, et al. Adenovirus infection in children hospitalized with pneumonia in Guangzhou, China. Influenza Other Respir Viruses 2021;15(1):27–33. DOI: 10.1111/irv.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pscheidt VM, Gregianini TS, Martins LG, Veiga A. Epidemiology of human adenovirus associated with respiratory infection in southern Brazil. Rev Med Virol 2020:e2189. DOI: 10.1002/rmv.2189. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan NM, Dove W, Abd-Eldayem SA, Abu-Zeid AF, Shamoon HE, Hart CA. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J Med Virol 2008;80(1):168–74. DOI: 10.1002/jmv.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafik CF, Mohareb EW, Yassin AS, et al. Viral etiologies of lower respiratory tract infections among Egyptian children under five years of age. BMC Infect Dis 2012;12:350. DOI: 10.1186/1471-2334-12-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fagbo SF, Garbati MA, Hasan R, et al. Acute viral respiratory infections among children in MERS-endemic Riyadh, Saudi Arabia, 2012–2013. J Med Virol 2017;89(2):195–201. DOI: 10.1002/jmv.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demian PN, Horton KC, Kajon A, et al. Molecular identification of adenoviruses associated with respiratory infection in Egypt from 2003 to 2010. BMC Infect Dis 2014;14:50. DOI: 10.1186/1471-2334-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai H, Xi H, Huang L, et al. Molecular Epidemiology and Clinical Features Analysis of Respiratory Adenovirus Infections Reveals Correlations between Genotype, Inflammatory Biomarkers, and Disease Severity. Biomed Res Int 2020;2020:4357910. (In eng). DOI: 10.1155/2020/4357910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcone DN, Culasso ACA, Reyes N, et al. Genotypes and phylogenetic analysis of adenovirus in children with respiratory infection in Buenos Aires, Argentina (2000–2018). PLoS One 2021;16(3):e0248191. DOI: 10.1371/journal.pone.0248191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song E, Wang H, Kajon AE, et al. Diagnosis of Pediatric Acute Adenovirus Infections: Is a Positive PCR Sufficient? Pediatr Infect Dis J 2016;35(8):827–34. DOI: 10.1097/INF.0000000000001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Hospitalized Children with More Than One Adenovirus Type, Including Adenovirus Species Co-detection and Adenovirus Co-viral Detection
Supplemental Figure 1. Distribution by Admission Diagnoses of Adenovirus-single/Co-viral Detection (A) and Adenovirus Species (B) Among Children Hospitalized with ARI in Amman, Jordan Over Three Respiratory Seasons, 2010–2013
Supplemental Figure 2. Distribution by Age Group of Adenovirus-single/Co-viral Detection (A) and Adenovirus Species (B) Among Children Hospitalized with ARI in Amman, Jordan Over Three Respiratory Seasons, 2010–2013
Supplemental Figure 3. Seasonal Distribution of Adenovirus Detection Among Children Hospitalized with ARI in Amman, Jordan Over Three Respiratory Seasons, 2010–2013


