Abstract
The characterization of the genetic basis of sulfonamide resistance in Campylobacter jejuni was attempted. The resistance determinant from a sulfonamide-resistant strain of C. jejuni was cloned and was found to show 42% identity with the folP gene (which codes for dihydropteroate synthase, the target of sulfonamides) of the related bacterium Helicobacter pylori. The sequences of the areas surrounding the folP gene in C. jejuni showed similarity to those of the areas surrounding the corresponding gene in H. pylori. The folP gene of C. jejuni, which mediates the resistance, was observed to show particular features when it was compared to other known folP genes. One of these features is the presence of two pairs of direct repeats (15 and 27 bp) within the coding sequence of the gene. Comparison of the C. jejuni folP genes that mediate susceptibility and resistance revealed the occurrence of mutations that changed four amino acid residues. Resistance of C. jejuni to sulfonamides could be associated with one or several of these four mutational substitutions, which all occurred in the five different resistant isolates studied. The codon for one of these changed amino acids was found to be located in the second direct repeat within the coding sequence of the gene. The change made the repeat perfect. The transformation of both the resistance and the susceptibility variants of the gene into an Escherichia coli folP knockout mutant was found to complement the dihydropteroate synthase deficiency, confirming that the characterized sulfonamide resistance determinant codes for the C. jejuni dihydropteroate synthase enzyme. Kinetic measurements established different affinities of sulfonamide for the dihydropteroate synthase enzyme isolated from the resistant and susceptible strains. In conclusion, sulfonamide resistance in C. jejuni was shown to be associated with mutational changes in the chromosomally located gene for dihydropteroate synthase, the target of sulfonamides.
Sulfonamides were once used successfully in the treatment of a variety of bacterial infections. However, the rapid emergence of sulfonamide resistance and the development of more potent drugs have limited their clinical use. The target of sulfonamides is the enzyme dihydropteroate synthase (DHPS), which catalyzes the formation of dihydropteroic acid in bacteria and some eucaryotic cells (3), but it is not present in human cells (15). This difference is the basis of the selective action of sulfonamide drugs. Sulfonamide is a structural analog of p-aminobenzoic acid (PABA), the substrate of the DHPS enzyme, and inhibits it competitively. It can also function as an alternative substrate for the production of a sulfonamide-containing pteroate analog that cannot be used in the subsequent steps of the biosynthetic pathway. The folate cofactor pool in the bacterial cell is consequently depleted (29, 37), resulting in growth inhibition and cell death (15).
Chromosomal mutations in the folP gene that result in low levels of sulfonamide resistance can be isolated in the laboratory (15, 24). On the other hand, acquired resistance to high concentrations of sulfonamides has been observed in gram-negative bacteria. This resistance is plasmid borne and is due to the presence of genes that code for alternative drug-resistant variants of the DHPS enzyme (33, 39). Two such plasmid-borne resistance genes have been found (28, 35). One of them, sul1, is found almost exclusively on integron structures carried by large conjugative plasmids (35). The other sulfonamide resistance determinant, sul2, is frequently found on small mobilizable plasmids (28).
The susceptibilities of Campylobacter jejuni strains to sulfonamides, either alone or in combination with trimethoprim, were found to be variable according to the geographical source of the isolates (9, 10, 18, 36). The genetic basis of sulfonamide resistance in C. jejuni has not previously been investigated at the molecular level, however.
In the study described here, the DHPS gene of C. jejuni was characterized. Sulfonamide resistance was shown to be associated with the mutational substitution of four amino acid residues that resulted in a reduced affinity for sulfonamide of the resistant variant of DHPS. Some information about the surrounding areas of the folP gene on the chromosome of C. jejuni are also reported here.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains and plasmids involved in this study are listed in Table 1. Sulfonamide-resistant and -susceptible clinical isolates of C. jejuni were obtained from the laboratory of clinical bacteriology in two Swedish hospitals (Department of Infectious Diseases, Uppsala University, and Department of Clinical Bacteriology, Göteborg University).
TABLE 1.
Bacterial strains and plasmids involved in this study
| Strain or plasmid | Relevant genotype or descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| C. jejuni | ||
| CJ1 | Clinical isolate, Tpr Sur | This study |
| CJ3 | Clinical isolate, Tpr Sur | This study |
| CJ4 | Clinical isolate, Tpr Apr | This study |
| CJ9 | Clinical isolate, Tpr Nar Smr Sur | This study |
| CJ14 | Clinical isolate, Tpr Apr Dcr Nar Sur | This study |
| CJ17 | Clinical isolate, Tpr Dcr Sur | This study |
| E. coli | ||
| DH5α | F−endA1 thi-1 hsdR17 supE44 relA1 gyrA 96 recA1 ΔlacU169 | 30 |
| C600ΔfolP::Kmr | thi-1 thr-1 leuB6 lacY1 tonA21 supE44 ΔfolP::Kmr | 8 |
| Plasmids | ||
| pUC19 and pUC18 | Apr cloning vectors | 42 |
| pCS1 | 2.4-kb HindIII fragment from CJ9 in HindIII-cleaved pUC19 | This study |
Tpr, Apr, Dcr, Nar, Sur, Smr, and Kmr, resistance to trimethoprim, ampicillin, doxycycline, nalidixic acid, sulfonamide, streptomycin, and kanamycin, respectively.
Sulfonamide susceptibility testing of C. jejuni strains.
Susceptibility testing was initially carried out with commercially available sulfisoxazole disks (AB Biodisk, Solna, Sweden). The procedures were as described previously (11). Zone sizes were interpreted according to the guidelines of the National Committee for Clinical Laboratory Standards (22). The MICs of sulfonamide were determined by the agar dilution method, as described previously (17).
Cloning of the sulfonamide resistance determinant from a clinical isolate of C. jejuni.
The chromosomal DNA from a sulfonamide-resistant C. jejuni strain (strain CJ9; Table 1), which was isolated from a patient with gastroenteritis, was cleaved with the HindIII (this restriction enzyme and the other enzymes mentioned below were purchased from Boehringer Mannheim, Mannheim, Germany). The HindIII chromosomal digest was ligated into a HindIII-cleaved pUC19 vector as described previously (31). Ligation was performed in 50 mM Tris hydrochloride (pH 7.5)–10 mM magnesium chloride–10 mM dithiothreitol–1 mM ATP–1 mg of bovine serum albumin per ml–2 U of T4 DNA ligase (New England BioLabs, Inc., Beverly, Mass.). The ligation mixture was incubated at 16°C for 12 h. For transformation, competent Escherichia coli DH5α was prepared as described by Dagert and Ehrlich (5). Selection of the sulfonamide-resistant transformants was performed on Iso-Sensitest agar plates (Oxoid, Basingstoke, United Kingdom) containing both ampicillin (50 μg/ml) and sulfathiazole (28 μg/ml; 0.1 mM). Ampicillin and sulfathiazole were purchased from Sigma Chemical, Co., St. Louis, Mo. The inoculated plates were incubated at 37°C for 24 h.
Amplification of the DHPS-encoding gene from both sulfonamide-resistant and -susceptible strains of C. jejuni by PCR.
Two PCR primers (see Fig. 1), SUp1 (5′-CAGGATCCGCTCTTTGATACTAGCTAC-3′) and SUp2 (5′-GCGAATTCCCTCATCCAAGAAGCAGCC-3′), were designed according to the sequence of the sulfonamide resistance gene cloned from the resistant C. jejuni strain. BamHI and EcoRI sites (underscored sequences) were added to the 5′ ends of SUp1 and SUp2, respectively, to enable subsequent cloning of the PCR amplification product for sequencing analysis. The PCRs were carried out in a total volume of 25 μl. DNA amplification was carried out in a Perkin-Elmer Thermocycler (Perkin-Elmer Cetus, Norwalk, Conn.) in a buffer composed of 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, and 0.01% gelatin. Each deoxynucleoside triphosphate at a concentration of 0.2 mM, 20 pmol of each primer, and 1 U of Taq DNA polymerase (Perkin-Elmer Cetus) were used for each 25-μl PCR mixture. The template for PCR was prepared by suspending a loopful of each isolate growing on a petri dish in 500 μl of sterile water, followed by boiling for 10 min and centrifugation (in an Eppendorf centrifuge) for 8 min. A total of 10 μl of the supernatant was used in the amplification procedure. Thirty cycles of PCR amplification were performed. Each cycle consisted of a denaturation step at 94°C for 1 min, annealing at 56°C for 1 min, and then an extension step at 72°C for 2 min. The cycles were terminated by a final 10-min extension at 72°C. A positive control and a negative control were included in each PCR run. The size of the amplified PCR product (1.2-kb) was visualized by electrophoresis on 1% agarose gels (Bio-Rad Laboratories, Richmond, Calif.). The PCR products were purified as described previously (23) and digested with EcoRI and BamHI and were then cloned into pUC19 cloning vectors as described previously (31) for further characterization.
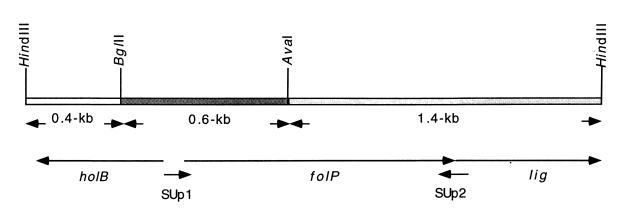
FIG. 1.
Restriction map of the DNA fragment carrying the sulfonamide resistance determinant located on plasmid pCS1 (Table 1). The sizes of the three fragments resulting from cleavage with HindIII, BglII, and AvaI are indicated. The arrows below the map show the direction of the transcription of the open reading frames located on the inserted HindIII fragment. SUp1 and SUp2 refer to a primer pair designed to amplify the folP gene of C. jejuni by PCR. Abbreviations: holB, gene coding for DNA polymerase III delta prime subunit; folP, gene coding for DHPS; and lig, gene coding for DNA ligase in the genome of H. pylori (40).
Transformation of the C. jejuni folP gene into an E. coli folP knockout mutant.
A mutant of E. coli K-12 (C600) in which the folP gene was inactivated by inserting a kanamycin resistance cassette and deleting 330 bp of the coding region of the folP gene was described earlier (8). Competent cells of this knockout mutant were prepared as described previously (5). Genes coding for the DHPS enzymes from both sulfonamide-resistant and -susceptible C. jejuni strains were transformed into the mutant. Due to the deficiency of the chromosomal DHPS enzyme, the mutant requires rich medium for growth, such as Luria-Bertani medium (2) or twofold concentrated YT medium (21). Media that are not rich enough, such as Iso-Sensitest agar, cannot support mutant growth unless a complementing folP gene is introduced by transformation. Therefore, selection of the folP transformants was carried out on Iso-Sensitest agar plates supplemented with kanamycin (40 μg/ml) and sulfathiazole (56 μg/ml; 0.2 mM) in the case of the sulfonamide resistance gene and with kanamycin only in the case of the gene not associated with resistance. The inoculated plates were incubated at 37°C for 24 h.
Determination of DHPS activity.
Host bacteria (E. coli DH5α) with folP-carrying vectors were grown at 37°C to the late exponential phase in 400 ml of Iso-Sensitest broth (Oxoid) supplemented with the appropriate antibiotic. The cells were harvested by centrifugation, washed once in 0.1 M potassium phosphate buffer (pH 7.0), and then resuspended in 3 ml of the same buffer. The cell-free extract was prepared by sonicating the cells three times, for 20 s each time, followed by centrifugation for 20 min. The DHPS activity was determined by the incorporation of 14C-labelled PABA into dihydropteroic acid as described previously (37). Enzyme kinetics were analyzed by varying the concentration of the PABA substrate with the other substrate, 2-hydroxy-4-amino-6-hydroxymethylpteridine pyrophosphate, in excess. The Km for PABA was determined in a Lineweaver-Burk diagram (19), and the Ki was determined by measuring the apparent Km in the presence of different concentrations of the inhibitor.
Other methods.
Cleavage with restriction endonucleases and agarose gel electrophoresis were carried out by standard procedures (31). Chromosomal DNA was prepared from C. jejuni strains as described by Pitcher et al. (27).
Nucleotide sequencing.
The dideoxynucleotide chain termination method of Sanger et al. (32) was used. Double-stranded templates were prepared by cloning the sequences into pUC19 or pUC18 as described previously (31). The commercially available universal 17-nucleotide primer and the reverse 16-nucleotide primer specific for the pUC cloning vector were used for sequencing. Other oligonucleotide primers derived from the sequence of the cloned folP gene that was determined were designed and were also used for sequence walking. These primers include 5′-GGAGCTGAGCTTATAACACAC-3′ (nucleotides 175 to 195), 5′-GGCCTTAAATCCCGAGTATATTG-3′ (nucleotides 450 to 472), and 5′-GGAAAGAGTGCGGGGCATAATATG-3′ (nucleotides 907 to 930). The nucleotide sequences of both strands of the susceptible or the resistant variant of the folP gene were determined for two different clones. Double-stranded templates were prepared for sequencing by the method of Wong et al. (41). The modified T7 DNA polymerase (Amersham, Cleveland, Ohio) was used for elongation. [α-35S]dATP from New England Nuclear, Dreieich, Germany, was the labelling component.
Data bank analyses.
For analysis of the sequencing data, software from the University of Wisconsin Genetics Computer Group (7) was used. Nucleotide sequences were compared to those in the EMBL and GenBank databases by using the FASTA algorithm (25), and the derived protein sequences were analyzed for similarity to protein sequences in the databases by using the BLAST algorithm (1).
Nucleotide sequence accession number.
The nucleotide sequence of the folP gene and a part of the lig gene, reported here, has been deposited in the EMBL database under accession no. AJ242727.
RESULTS AND DISCUSSION
Sulfonamide susceptibility of C. jejuni.
Sulfonamide-resistant C. jejuni CJ9 (Table 1), which was resistant to 256 μg of sulfathiazole per ml, was used for cloning of the sulfonamide resistance determinant. The chromosomal folP gene was further amplified by PCR from four other sulfonamide-resistant strains, strains CJ1, CJ3, CJ14, and CJ17 (Table 1). They were resistant to 128 to 256 μg of sulfathiazole per ml. For comparison, one susceptible C. jejuni strain (strain CJ4; Table 1) for which the sulfathiazole MIC was 16 μg/ml was investigated.
Characterization of the sulfonamide resistance trait of a clinical isolate of C. jejuni.
Cloning of the sulfonamide resistance determinant from a sulfonamide-resistant isolate of C. jejuni (isolate CJ9 [Table 1]; see the Materials and Methods section) resulted in four sulfonamide-resistant clones. The four sulfonamide-resistant transformants, for which MICs were >560 μg/ml, were found to contain a HindIII fragment of about 2.4 kb. A restriction map of the inserted fragment carried on plasmid pCS1 (Table 1) was made with different restriction enzymes and is shown in Fig. 1.
For further characterization of the resistance determinant, the cloned fragment was cleaved with HindIII, AvaI, and BglII into three smaller fragments (Fig. 1). Subcloning of each of the resulting fragments into the pUC19 cloning vector and transformation into E. coli DH5α resulted in the loss of the sulfonamide resistance phenotype. The nucleotide sequence of each of the subcloned fragments was determined in order to further localize the sulfonamide resistance determinant within the 2.4-kb HindIII fragment and to characterize its surroundings.
Analysis of the resulting sequence data suggested that the 2.4-kb HindIII fragment carries a gene that codes for the DHPS enzyme. The gene was found to show 54% (at the nucleotide level) and 42% (at the amino acid level) identities when it was compared to the corresponding folP gene of the stomach bacterium Helicobacter pylori, which is known to be closely related to C. jejuni (Fig. 2A). The major part of the folP open reading frame was found to be located on the 1.4-kb HindIII-AvaI fragment, while the first 450 bp of the folP gene was detected on the BglII-AvaI fragment (Fig. 1).
FIG. 2.
(A) Comparison of the deduced amino acid sequence of the folP gene of sulfonamide-resistant C. jejuni CJ9 (Table 1) with that of the corresponding gene of H. pylori. Data are taken from the complete genome sequence of H. pylori (40). Dots within the sequence indicate gaps that give optimal similarity. Vertical lines between two amino acids indicate identical residues. The two pairs of direct repeats that were detected within the folP sequence of C. jejuni are shown in boldface type and are underlined. An asterisk denotes the stop codon. (B) Comparison of the deduced amino acid sequence of a part of the sulfonamide-susceptible variant of the C. jejuni folP gene and the corresponding sequence of the resistant variant. The four amino acid residues associated with sulfonamide resistance in C. jejuni are shown in boldface type and are underlined. Some of the consensus amino acid residues derived from the folP genes of other bacteria and shown to be involved in the binding of hydroxymethylpteridine pyrophosphate (13) are shown below the sequence and underlined.
Inspection of the DNA sequence immediately 5′ of the folP gene revealed a potential ribosome-binding site (RBS) 4 nucleotides upstream of the start codon (ATG) of the gene. The RBS that was detected differs from the conserved sequence by only one nucleotide (AGGAGG [12]). Promoter sequences (starting at positions −20 and −44 of the start codon, respectively) were detected upstream of the RBS. They match five and four of the six nucleotides of the consensus −10 and −35 sequences of E. coli recognized by the ς70 factor (TATAAT and TTGACA [14]), respectively. The existence of the proposed promoter sequences could be consistent with a low level of expression of the DHPS. Alternatively, other −10 and −35 promoter sequences, starting at positions −44 and −70 of the start codon of the gene, respectively, could also be detected. The latter promoter regions are less close to the consensus sequence, and their presence may therefore also be consistent with low level expression.
It should be mentioned that the folP gene of C. jejuni has special characteristics in comparison to the corresponding genes of other bacterial species. First, it is the largest folP gene characterized so far. Its product consists of 390 amino acid residues, compared to the 380 residues of the corresponding DHPS of the related organism H. pylori. The other, previously characterized folP genes of E. coli (6), Staphylococcus aureus (13), Streptococcus pneumoniae (20), and Bacillus subtilis (34) are relatively smaller. Second, the coding sequence of the C. jejuni folP gene was found to have two relatively long direct repeats (Fig. 2A).
The rest of the sequence of the BglII-AvaI fragment upstream of the characterized folP gene and the sequence of the HindIII-BglII fragment (Fig. 1) were also analyzed. These sequences are similar to another gene in H. pylori, known as the holB gene, which codes for the DNA polymerase III delta prime subunit (Fig. 1) (40).
Downstream of the coding sequence of the folP gene, the existence of a starting codon of a new open reading frame was observed (Fig. 1). Analysis of this sequence revealed that it is similar to a part of a gene (lig) that codes for DNA ligase in H. pylori (40); these genes exhibited 59.2 and 51.8% similarity and identity, respectively. The complete genome sequence of H. pylori (40) showed that the lig gene codes for 656 amino acid residues. In this study, only 245 amino acid residues of the lig gene coding sequence were detected up to the HindIII end of the chromosomal insert in pCS1 (Fig. 1).
The areas surrounding the folP gene on the chromosome of C. jejuni were thus found to show similarity to the corresponding parts of the chromosome of H. pylori. The gene coding for the DNA polymerase III delta prime subunit, detected upstream of the folP gene, was found to have the same location in both the C. jejuni and the H. pylori genomes (40). The two organisms differ, however, in the type of gene located downstream of the folP gene. In C. jejuni, a gene that codes for DNA ligase was detected at this position, while in H. pylori, the gene located downstream of the folP gene was defined as an unknown gene (40). The gene that codes for DNA ligase was detected at another location in the H. pylori genome (40). Data from the available sequencing database for C. jejuni at the Sanger Center (16) confirmed our results regarding the location of the lig and the holB genes flanking the folP gene.
Detection of the folP gene from a sulfonamide-susceptible and other sulfonamide-resistant isolates of C. jejuni by PCR.
By using the sequence obtained for the folP gene, which mediates sulfonamide resistance, and by PCR amplification (see the Materials and Methods section), the folP genes from a sulfonamide-susceptible C. jejuni strain (strain CJ4; Table 1) and four other sulfonamide-resistant strains of C. jejuni (strains CJ1, CJ3, CJ14, and CJ17; Table 1) were amplified. The PCR products were then cloned into pUC19 and were transformed into E. coli DH5α. For transformants carrying folP genes from resistant isolates, MICs were >560 μg/ml, while MICs of 14 μg/ml were found for the corresponding susceptible transformants. The sequences of the amplified folP genes from the sulfonamide-resistant isolates were found to be identical to that of the corresponding gene cloned from isolate CJ9. The sequence of the folP gene amplified from the susceptible strain, however, showed differences in four amino acid residues compared to the sequences of the corresponding resistance genes from all five isolates studied. These differences involve (susceptibility to resistance) L186F, D238N, N245K, and F246Y (Fig. 2B). The mutation of Leu to Phe (amino acid residue 186) was found to participate in the formation of the 27-bp perfect direct repeats within the coding sequence of the resistant folP gene (Fig. 2A). The amino acid substitutions detected in the present study do not coincide with the mutations in the folP genes from other sulfonamide-resistant organisms described previously (15). A parallel case was observed in S. aureus, in which in sulfonamide-resistant clinical isolates as many as 14 residues were found to be involved in the development of resistance (13). In this case, the changed residues were distributed over the DHPS protein and in some cases do not coincide with the previously characterized mutations in the folP genes from other sulfonamide-resistant organisms (13). In our study, three of the amino acid substitutions associated with resistance were observed to be located close to two conserved amino acid residues known to be involved in the binding of hydroxymethylpteridine pyrophosphate (Fig. 2B) (13).
Determination of DHPS enzyme kinetics.
To test whether the amino acid differences between the susceptible and the resistant variants of the DHPS enzyme are reflected in the kinetic parameters of the enzyme, the inhibitory constant for sulfonamide was determined. A dramatic difference in the Ki for sulfathiazole was detected (500 μM for the resistant enzyme and 0.5 μM for the susceptible one), indicating that the amino acid substitutions detected in the present study have resulted in a reduced binding affinity of the resistant DHPS enzyme for sulfonamides. A difference in the Km for PABA was also apparent (0.85 μM for the resistant enzyme and 0.25 μM for the susceptible one), indicating that the amino acid changes in the resistant enzyme have also resulted in a less efficient enzyme. This is in parallel to the situation in Neisseria meningitidis (8) and Streptococcus pyogenes (38), in which similar differences in Km values for PABA for susceptible and resistant variants of the enzyme were observed. In all these cases, sulfonamide resistance seems to persist, even though the use of this drug has all but ceased in clinical practice in Sweden (cf. reference 26 though). This is in contradiction to the expected selection pressure against the less efficient enzyme.
Transformation of the C. jejuni folP gene into an E. coli folP knockout mutant.
The transformation of the cloned folP gene from C. jejuni strains (susceptible or resistant variant of the gene) was found to complement the DHPS deficiency so that the mutant could grow on Iso-Sensitest agar plates (see the Materials and Methods section). This led to the conclusion that the cloned sulfonamide resistance determinant as well as the amplified folP gene from the sulfonamide-susceptible strain are coding for the chromosomal DHPS enzyme of C. jejuni. The G+C content of the folP gene that was detected (about 30%) is also in agreement with the low G+C content of Campylobacter species. The promoter sequences (−35 and −10 sequences) detected upstream of the folP gene were also found to closely resemble the sequence of the ς70 promoter, which is known to be the main sigma factor involved in the transcription of the housekeeping genes (14, 30). It seems likely that ς70 promoters of the housekeeping genes of C. jejuni will function in E. coli, as indicated previously (4).
ACKNOWLEDGMENTS
We are grateful to Carl Påhlson and Eva Sjögren for kindly providing the clinical isolates of C. jejuni used in this study. We also thank students Leena Sahlström and Monica Johansson for help with the sequence determinations, Maria Öhagen for transforming the folP gene into the folP knockout mutant, and Tina Olsson and Rikard Pehrson for carrying out enzyme assays.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown G M. The biosynthesis of folic acid. II. Inhibition by sulfonamides. J Biol Chem. 1962;237:536–540. [PubMed] [Google Scholar]
- 4.Chan V L, Bingham H L. Complete sequence of the Campylobacter jejuni glyA gene encoding serine hydroxymethyltransferase. Gene. 1991;101:51–58. doi: 10.1016/0378-1119(91)90223-x. [DOI] [PubMed] [Google Scholar]
- 5.Dagert M, Ehrlich S D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979;6:23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- 6.Dallas W S, Gowen J E, Ray P H, Cox M J, Dev I K. Cloning, sequencing and enhanced expression of the dihydropteroate synthase gene from Escherichia coli MC4100. J Bacteriol. 1992;174:5961–5970. doi: 10.1128/jb.174.18.5961-5970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fermér C, Swedberg G. Adaptation to sulfonamide resistance in Neisseria meningitidis may have required compensatory changes to retain enzyme function: kinetic analysis of dihydropteroate synthase from N. meningitidis expressed in a knockout mutant of Escherichia coli. J Bacteriol. 1997;179:831–837. doi: 10.1128/jb.179.3.831-837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fliegelman R M, Petrak R M, Goodman L J, Segreti J, Trenholme G M, Kaplan R L. Comparative in vitro activities of twelve antimicrobial agents against Campylobacter species. Antimicrob Agents Chemother. 1985;27:429–430. doi: 10.1128/aac.27.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia M M, Lior H, Stewart R B, Ruckerbauer G M, Trudel J R, Skljarevski A. Isolation, characterization and serotyping of Campylobacter jejuni and Campylobacter coli from slaughter cattle. Appl Environ Microbiol. 1985;49:667–672. doi: 10.1128/aem.49.3.667-672.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibreel A, Sköld O. High-level resistance to trimethoprim in clinical isolates of Campylobacter jejuni by acquisition of foreign genes (dfr1 and dfr9) expressing drug-insensitive dihydrofolate reductases. Antimicrob Agents Chemother. 1998;42:3059–3064. doi: 10.1128/aac.42.12.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold L. Post-transcriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- 13.Hampele I C, D’Arcy A, Dale G E, Kostrewa D, Nielsen J, Oefner C, Page M G P, Schönfeld H-J, Stüber D, Then R L. Structure and function of the dihydropteroate synthase from Staphylococcus aureus. J Mol Biol. 1997;268:21–30. doi: 10.1006/jmbi.1997.0944. [DOI] [PubMed] [Google Scholar]
- 14.Helman J D, Chamberlin M J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 15.Huovinen P, Sundström L, Swedberg G, Sköld O. Trimethoprim and sulfonamide resistance. Antimicrob Agents Chemother. 1995;39:279–289. doi: 10.1128/aac.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlyshev A V, Henderson J, Ketley J M, Wren B W. An improved physical and genetic map of Campylobacter jejuni NCTC 11168 (UA580) Microbiology. 1998;144:503–508. doi: 10.1099/00221287-144-2-503. [DOI] [PubMed] [Google Scholar]
- 17.Karmali M A, de Grandis S, Fleming P C. Antimicrobial susceptibility of Campylobacter jejuni with special reference to resistance patterns of Canadian isolates. Antimicrob Agents Chemother. 1981;19:593–597. doi: 10.1128/aac.19.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larivière L A, Gaudreau C L, Turgeon F F. Susceptibility of clinical isolates of Campylobacter jejuni to twenty-five antimicrobial agents. J Antimicrob Chemother. 1986;18:681–685. doi: 10.1093/jac/18.6.681. [DOI] [PubMed] [Google Scholar]
- 19.Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658. [Google Scholar]
- 20.Lopez P, Espinosa M, Greenberg B, Lacks S A. Sulfonamide resistance in Streptococcus pneumoniae: DNA sequence of the gene encoding dihydropteroate synthase and characterization of the enzyme. J Bacteriol. 1987;169:4320–4326. doi: 10.1128/jb.169.9.4320-4326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller-Hill B, Crapo L, Gilbert W. Mutants that make more lac repressor. Proc Natl Acad Sci USA. 1968;59:1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobic disk susceptibility tests. Vol. 1. 1981. pp. 141–156. . Approved standard. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 23.Öfverstedt L G, Hammarström K, Balgobin N, Hjertén S, Pettersson U, Chattopadyaya J. Rapid and quantitative recovery of DNA fragments from gels by displacement electrophoresis (isotachophoresis) Biochim Biophys Acta. 1984;782:120–126. doi: 10.1016/0167-4781(84)90014-9. [DOI] [PubMed] [Google Scholar]
- 24.Pato M L, Brown G M. Mechanisms of resistance of Escherichia coli to sulfonamides. Arch Biochem Biophys. 1963;103:443–448. doi: 10.1016/0003-9861(63)90435-1. [DOI] [PubMed] [Google Scholar]
- 25.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piddock L J. Does the use of antimicrobial agents in veterinary medicine and animal husbandry select antibiotic-resistant bacteria that infect man and compromise antimicrobial therapy? J Antimicrob Chemother. 1996;38:1–3. doi: 10.1093/jac/38.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 28.Rådström P, Swedberg G. RSF1010 and a conjugative plasmid contain sulII, one of two known genes for plasmid-borne sulfonamide resistance dihydropteroate synthase. Antimicrob Agents Chemother. 1988;32:1684–1692. doi: 10.1128/aac.32.11.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roland S, Ferone R, Harvey R J, Styles V L, Morrison R W. The characteristics and significance of sulfonamides as substrates for Escherichia coli dihydropteroate synthase. J Biol Chem. 1979;254:10337–10345. [PubMed] [Google Scholar]
- 30.Rosenberg M, Court D. Regulatory sequences involved in the promotion and termination of transcriptions. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sköld O. R factor-mediated resistance to sulfonamides by a plasmid-borne, drug-resistant dihydropteroate synthase. Antimicrob Agents Chemother. 1976;9:49–54. doi: 10.1128/aac.9.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slock J, Stahly D P, Han C-Y, Six E W, Crawford I P. An apparent Bacillus subtilis folic acid biosynthetic operon containing pab, an amphibolic trpG gene, a third gene required for the synthesis of para-aminobenzoic acid, and the dihydropteroate synthase gene. J Bacteriol. 1990;172:7211–7226. doi: 10.1128/jb.172.12.7211-7226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundström L, Rådström P, Swedberg G, Sköld O. Site-specific recombination promotes linkage between trimethoprim and sulphonamide resistance genes. Sequence characterization of dhfrV and sulI and a recombination active locus of Tn21. Mol Gen Genet. 1988;213:191–201. doi: 10.1007/BF00339581. [DOI] [PubMed] [Google Scholar]
- 36.Svedhem Å, Kaijser B, Sjögren E. Antimicrobial susceptibility of Campylobacter jejuni isolated from humans with diarrhoea and from healthy chickens. J Antimicrob Chemother. 1981;7:301–305. doi: 10.1093/jac/7.3.301. [DOI] [PubMed] [Google Scholar]
- 37.Swedberg G, Castensson S, Sköld O. Characterization of mutationally altered dihydropteroate synthase and its ability to form a sulfonamide-containing dihydrofolate analog. J Bacteriol. 1979;137:129–136. doi: 10.1128/jb.137.1.129-136.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swedberg G, Ringertz S, Sköld O. Sulfonamide resistance in Streptococcus pyogenes is associated with differences in the amino acid sequence of its chromosomal dihydropteroate synthase. Antimicrob Agents Chemother. 1998;42:1062–1067. doi: 10.1128/aac.42.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swedberg G, Sköld O. Characterization of different plasmid-borne dihydropteroate synthases mediating bacterial resistance to sulfonamides. J Bacteriol. 1980;142:1–7. doi: 10.1128/jb.142.1.1-7.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomb J F, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 41.Wong W M, Au D M Y, Lam V M S, Tam J W O, Cheng L Y L. A simplified and improved method for the efficient double-stranded sequencing of mini-prep plasmid DNA. Nucleic Acids Res. 1990;18:5573. doi: 10.1093/nar/18.18.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]