Abstract
The brown bear (Ursus arctos) is an iconic carnivoran species of the Northern Hemisphere. Its population history has been studied extensively using mitochondrial markers, which demonstrated signatures of multiple waves of migration, arguably connected with glaciation periods. Among Eurasian brown bears, Siberian populations remain understudied. We have sequenced complete mitochondrial genomes of four ancient (~4.5–40 kya) bears from South Siberia and 19 modern bears from South Siberia and the Russian Far East. Reconstruction of phylogenetic relationships between haplotypes and evaluation of modern population structure have demonstrated that all the studied samples belong to the most widespread Eurasian clade 3. One of the ancient haplotypes takes a basal position relative to the whole of clade 3; the second is basal to the haplogroup 3a (the most common subclade), and two others belong to clades 3a1 and 3b. Modern Siberian bears retain at least some of this diversity; apart from the most common haplogroup 3a, we demonstrate the presence of clade 3b, which was previously found mainly in mainland Eurasia and Northern Japan. Our findings highlight the importance of South Siberia as a refugium for northern Eurasian brown bears and further corroborate the hypothesis of several waves of migration in the Pleistocene.
Keywords: ancient DNA, Denisova, glacial refugium, mitochondrial genome, population structure
INTRODUCTION
The brown bear (Ursus arctos Linnaeus, 1758) is one of the largest terrestrial predators on the planet, and its range covers the mountain and forest areas of the Northern Hemisphere. Owing to significant decline of habitats and excessive hunting, only small, isolated populations have remained in Southern and Central Europe, whereas larger populations exist in Eastern and Northern Europe, where the species is protected. Declining but still contiguous populations exist in the north-west of North America. Northern Asia represents the largest uninterrupted habitat, where the brown bear occupies continuous areas from the Ural Mountains to the Russian Far East.
Many brown bear population genetics studies have been based mostly on mitochondrial markers (Taberlet & Bouvet, 1994; Saarma et al., 2007; Korsten et al., 2009; Anijalg et al., 2018), although some authors have also used nuclear genome markers (Tammeleht et al., 2010; Norman et al., 2013; Xenikoudakis et al., 2015; Benazzo et al., 2017; Barlow et al., 2018; Tumendemberel et al., 2019). The nomenclature for mitochondrial haplotypes was proposed by Leonard et al. (2000) and extended in subsequent studies (Barnes et al., 2002; Miller et al., 2006; Calvignac et al., 2008; Davison et al., 2011). According to recent analyses, mitogenomes of brown bears are presented as seven major haplogroups that are split further into subgroups. The geographical distribution of these groups in some areas putatively reflects stages of successive waves of migration (Matsuhashi et al., 1999, 2001; Korsten et al., 2009; Davison et al., 2011; Hirata et al., 2013; Anijalg et al., 2018). The most abundant Holarctic haplogroup, 3a, is widespread in Eurasia; different studies have estimated the origin of the clade from ~53 kya (Anijalg et al., 2018) to > 100 kya (Salis et al., 2021). Among other clades, clade 3b is specific to mainland Eurasia (Gus’kov et al., 2013; Hirata et al., 2013, 2014; Salomashkina et al., 2014; Salis et al., 2021), and together with clade 4, it was also found on the islands of Japan (Hirata et al., 2013, 2014). Representatives of clades 2c, 3a, 3b, 3c and 4 have been found among ancient samples of Eastern Beringia (Salis et al., 2021).
Understanding brown bear phylogeography and migration patterns in north-eastern Eurasia requires more extensive sampling, especially from Siberia. In this study, we obtained nearly complete mitochondrial genomes of four ancient brown bears (~4.5–40 kyr old) from various palaeontological and archaeological excavations in Southern Siberia and supplemented these data with complete mitochondrial genomes of 19 modern bears from Siberia and the Russian Far East. We then performed phylogenetic analysis of these data together with previously published sequences to corroborate the hypothesis that ancient brown bears from mainland Eurasia represent multiple waves of dispersal to the islands of Japan, Eastern Beringia and North America.
MATERIAL AND METHODS
Sampling
Ancient samples, representing various palaeontological and archaeological excavations in Southern Siberia, were included in the study (Table 1). The most recent sample, U16 Kol, was excavated in 2010 from a 22nd–17th century BC settlement of ancient miners and metallurgists belonging to the Elunin Culture (Early Bronze Age), located on the north-eastern shore of lake Kolyvanskoe, 3.7 km from the village Savvushka (Zmeinogorsky district, Altai Krai) (Grushin, 2015). Sample U17 DC4 was from the famous Denisova Cave, located on the river Anuy (Altai Krai). The bone was discovered in lithological layer 4, dated to ~3.5 kyr BCE (Derevyanko et al., 2003). Samples U2 Chu and U3 Chi were excavated from sediments of the rivers Chumysh (Altai Krai) and Chik (Novosibirsk Oblast), respectively. These were the oldest samples; U2 Chu was radiocarbon dated to 40 kya (Vasiliev et al., 2016: sample ID NSKA-01087), whereas U3 Chi was estimated to be ~30 kyr old based on previously dated samples from the same layer (Vasiliev et al., 2016, 2018, 2020).
Table 1.
Ancient brown bear samples and sequencing results
| Sample | Age (kya) | Location | Sample type | Total number of reads | Number of reads mapped | Percentage of mitogenome recovered | Mean coverage (number of unique reads per position) | NCBI accession number |
|---|---|---|---|---|---|---|---|---|
| U2 Chu | ~40 | Chumysh river (53.40°N, 85.74°E) | Tibia | 670 582 | 4427 | 97.4 | 21.8 | MW991397 |
| U3 Chi | ~30 | Chik river (55.00°N, 82.42°E) | Ulna | 537 611 | 1948 | 91.0 | 8.7 | MW991398 |
| U16 Kol | 4.2–3.6 | Kolyvan-1 (51.3745°N, 82.2144°E) | Tooth | 2 977 306 | 1398 | 96.7 | 6.7 | MW991399 |
| U17 DC4 | ~5.5 | Denisova Cave, layer 4 (51.3975°N, 84.6750°E) | Unidentifiable bone | 1 843 902 | 11 716 | 99.8 | 76.6 | MW991400 |
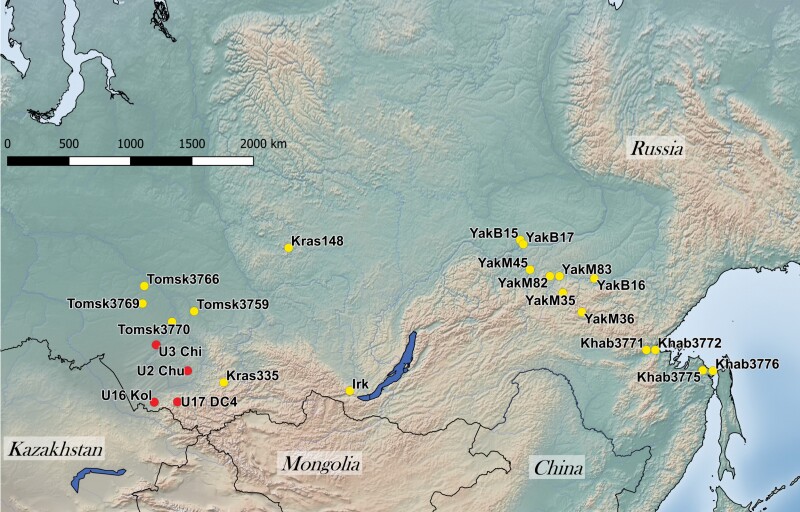
Nineteen samples of modern brown bears represented various regions of Siberia (Tomsk, Krasnoyarsk, Irkutsk and Yakutia) and the Russian Far East (Khabarovsk) (Fig. 1). The muscle samples were collected from legally hunted brown bears. A description of the modern samples is given in the Supporting Information (Table S1).
Figure 1.
Map of sample collection sites. Colour code: yellow, modern samples; red, ancient samples.
Modern DNA extraction and sequencing
DNA from modern samples was isolated using DNeasy Blood & Tissue Kit (Qiagen, The Netherlands) and the High PCR Template Preparation Kit (Roche Diagnostics, Mannheim, Germany), following the manufacturer’s instructions.
Polymerase chain reaction (PCR), Sanger sequencing and reconstruction of the complete modern brown bear mitochondrial genomes were performed as described by Keis et al. (2013) and Anijalg et al. (2018).
Ancient DNA extraction and enrichment
Ancient DNA was extracted as described by Druzhkova et al. (2013). Sequencing libraries were made using the TruSeq DNA Sample Preparation Kit (Illumina, USA), according to the manufacturer’s protocol. Libraries were enriched using hybridization with contemporary U. arctos biotinylated mitochondrial DNA immobilized on Dynabeads Streptavidin magnetic beads (Life Technologies, USA), as in the study by Maricic et al. (2010).
Biotinylated probes for enrichment were generated from the modern brown bear sample (Irk; Supporting Information, Table S1) using PCR (Vorobieva et al., 2020) with primers presented in the Supporting Information (Table S2). Enriched sequencing libraries were quantified using quantitative PCR in the presence of SYBR Green I.
Ancient DNA sequencing and mitochondrial genome reconstruction
Paired-end sequencing was performed on an Illumina MiSeq using TruSeq Reagent Kits v.2, with 500 cycles.
Reference-based recovery of ancient bear mitochondrial genomes was performed using PALEOMIX v.1.2.13.2 BAM pipeline (Schubert et al., 2014). Reads were trimmed and collapsed using AdapterRemoval v.2.1.7 (Lindgreen, 2012), then aligned to the reference brown bear mitochondrial genome (GenBank NC003427) with bwa mem v.0.7.17-r118 (Li, 2013). Only hits with mapping quality > 20 were retained. The PCR duplicates were removed with a script incorporated in PALEOMIX. The C-to-T damage profiles were estimated, and base qualities were recalibrated with MapDamage v.2.0.8 (Jónsson et al., 2013). Indel regions were realigned with GATK v.3.8-0-ge9d806836 (McKenna et al., 2010-9). Contaminant reads were removed by alignment to a human mitochondrial genome (GenBank NC012920) and mapping quality comparison using a custom Python script (https://github.com/lca-imcb/lca-ngs/blob/master/contam_filter.py). Consensus sequences were reconstructed in Geneious (https://www.geneious.com), using the 75% majority rule, a minimum coverage of three and subsequent manual refinement.
Phylogenetic analysis
Phylogenetic analysis was performed for sequences obtained in the present study, along with additional previously published bear mitochondrial sequences listed in the Supporting Information (Table S3).
Sequences were aligned using MAFFT v.7.407 (Katoh & Standley, 2013). Stretches of tandem repeats in the hypervariable region were removed from the alignment before the analysis. The optimal alignment partitioning scheme and substitution models were chosen using PartitionFinder v.2 (Lanfear et al., 2017). Phylogenies were reconstructed using Bayesian inference (BI) in MrBayes v.3.2.5 (Ronquist et al., 2012) and using maximum likelihood (ML) in RAxML v.8.2.3 (Stamatakis, 2014). For BI, four Markov chains were run for five million iterations and sampled at intervals of 2500 generations, and the first 25% of chains were discarded as burn-in. Posterior probabilities were obtained from the 50% majority-rule consensus tree. For the ML method, the GTR+G substitution model was used instead of the ones indicated by PartitionFinder. A strategy of ‘ML+thorough bootstrap’ was implemented, with 10 000 replications in ten runs. Final tree manipulations were performed in FigTree v.1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/). The median-joining network was reconstructed with with PopArt v.1.7.2 (http://popart.otago.ac.nz).
To estimate divergence times, we took a Bayesian approach implemented in BEAST v.1.8.4 (Suchard et al., 2018). The tree prior was set to a constant-size coalescent model. The molecular clock was set as strict, and for approximation we used a previously estimated mitogenomic mutation rate of 2.48 × 10−8 mutations per site per year (Anijalg et al., 2018). The molecular clock was calibrated using our ancient samples (Table 1) and the 130- to 110-kyr-old ancient polar bear sample from Svalbard, Norway (Lindqvist et al., 2010).
In order to compare our findings of clade 3b in continental Eurasia with previous studies, we obtained COXII and mitochondrial control region (CR) sequences for Central Asian brown bears from the study by Tumendemberel et al. (2019) and aligned those to our complete mitochondrial genome dataset using MAFFT. Then, we extracted 671-bp-long COXII and 263-bp-long CR alignments, concatenated those, and reconstructed the phylogenetic tree using FastTree.
Population genetic analysis
Population genetic analysis of modern brown bear mitogenomes was performed with Arlequin v.3.5 (Excoffier & Lischer, 2010) and DnaSP v.5.10 (Librado & Rozas, 2009) software. Arlequin was used to calculate molecular diversity indexes and population average pairwise differences and to perform pairwise mismatch distribution analysis, AMOVA computations and neutrality tests. For pairwise mismatch distribution, we estimated parameters of the demographic expansion using pairwise difference as a metric and 1000 bootstrap replicates. Standard AMOVA (Excoffier et al., 1992) was performed with 1000 permutations to identify differences between populations of Siberia, the Russian Far East (mainland), Kamchatka, Japan and Europe. Nucleotide diversity (π) was calculated using DnaSP with default parameters. The remaining calculations and tests were performed using Arlequin with default parameters and molecular distance set as pairwise differences.
RESULTS
Mitochondrial genomes
Mitochondrial genomes for four ancient and one modern brown bear were reconstructed from Illumina shotgun sequencing of DNA libraries enriched by hybridization capture. For ancient samples (listed in Table 1) aged 3.6–40 kyr, 0.04–0.66% reads were mapped to the reference genome, and with 0.5–3 million reads we were able to achieve 6.7- to 76.6-fold mitochondrial genome coverage. As a result, consensus sequences were successfully reconstructed almost completely (91–99.8% based on 3× coverage cut-off) for the ancient samples. We also ensured the lack of contamination with DNA from hybridization probes; we sequenced the modern sample used for probe generation (Irk) and checked manually that its characteristic variants were not present in ancient DNA data.
Complete mitochondrial genomes for 18 modern bears were reconstructed with Sanger sequencing as described by Anijalg et al. (2018). Here, we extended sampling geography by including samples ranging from Western Siberia to Khabarovsk.
Phylogenetic analysis
We performed phylogenetic analysis based on complete mitochondrial sequences of the four ancient and 19 modern bears from Siberia and the Russian Far East, together with 259 previously published mitochondrial genomes of brown and polar bears sampled worldwide (Supporting Information, Table S3). We were able to restore 91–99.8% of the ancient bear mitochondrial genomes and whole mitochondrial genomes for modern brown bear samples from Siberia (Irkutsk, Tomsk, Krasnoyarsk and Yakutia regions) and the Russian Far East (Khabarovsk region). Our results in general support the existence of clades delineated previously (Leonard et al., 2000; Davison et al., 2011; Hirata et al., 2013), hence we will adhere to the nomenclature proposed there (Fig. 2).
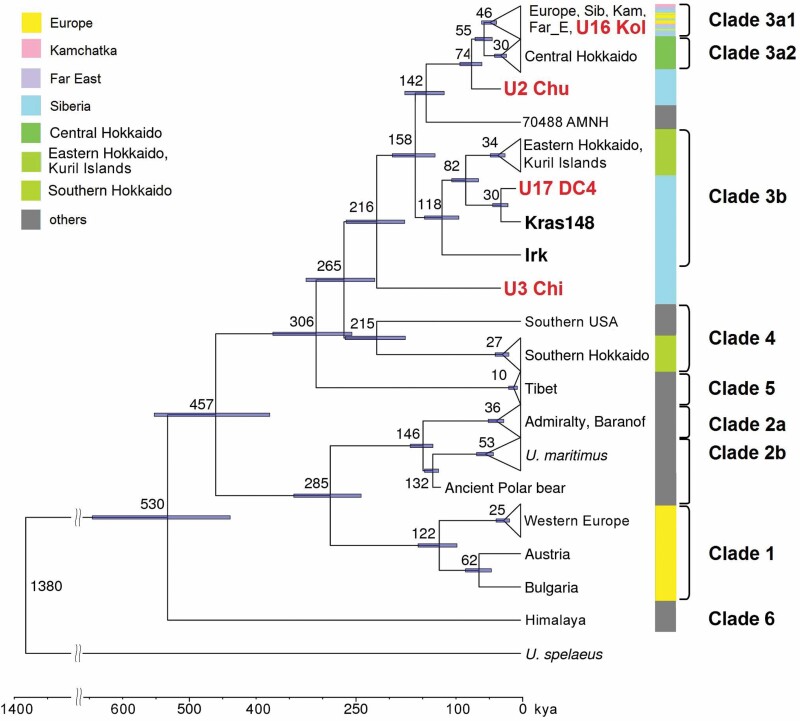
Figure 2.
BEAST phylogeny for complete mitochondrial genomes of 282 brown bears. Some subtrees have been collapsed for brevity. Red labels indicate ancient samples, and bold black labels indicate modern samples from the present study. Node numbers and blue lines are the mean and 95% confidence interval of divergence time (in thousands of years). Numbers over branches are the posterior Bayesian probabilities. Outgroups: India, Ursus thibetanus MG066704 and Ursus spelaeus NC_011112.
Both ancient and modern samples from Siberia and the Russian Far East fell within clade 3 (Fig. 2). The most ancient samples in our set, U2 Chu and U3 Chi (both estimated to be ~30–40 kyr old), were identified as outgroups for subclade 3a and the whole of clade 3, respectively. The most recent sample, U16 Kol (~4.5 kyr old), was attributed to the current clade 3a1, which is widely distributed and whose closest relatives are modern bears from Eastern and Northern European Russia. The sample U17 DC (~5.5 kyr old) was found to belong to clade 3b and, together with a modern sample from Siberia (Kras148), formed a subclade distinct from the previously described group of bears from Eastern Hokkaido and Kuril Islands.
Previously, clade 3b samples were reported from Central Asia based on partial mitochondrial sequences (Tumendemberel et al., 2019). Among these data, closely related sequences were found for each of three mitochondrial genomes of clade 3b in our study: COXII + CR sequence was identical between Irk (a modern bear from the Irkutsk region) and 60014Dor (a modern bear from Ikh Khyangan, Mongolia) belonging to clade 3b2, while Kras128 (a modern bear from the Krasnoyarsk region) and U17 (an ancient bear from Denisova Cave) were similar, but not identical to 200BU1 and 200S4 (from the Mongolian Altai), representatives of clade 3b1. In clade 3a1, samples from the study by Tumendemberel et al. (2019) (Khentii and Bogd Khan, Mongolia) clustered separately from the other samples in our study (Supporting Information, Fig. S1). No additional samples grouped together with U3, which retained its basal position relative to clade 3.
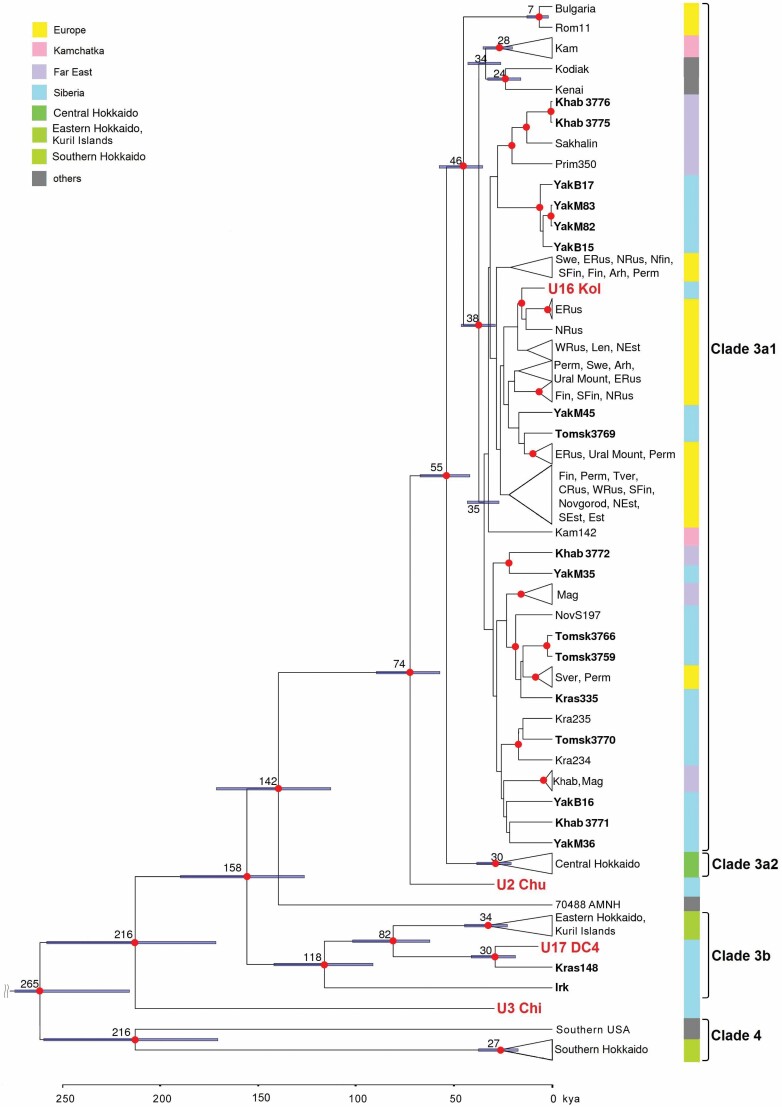
The two most ancient branches within subclade 3a1 have been described earlier and are represented by South-East European and Alaska–Kamchatka groups (Anijalg et al., 2018) (Fig. 3). The remainder of clade 3a1 is divided tentatively into two poorly resolved subgroups that diverged ~35 kya. The first subgroup can be subdivided further into a more eastern one, where modern samples from Khabarovsk and Yakutia cluster together with samples from Primorsky Krai and Sakhalin, and a more western one, consisting mainly of bears originating from Europe. However, it also included two modern samples from Tomsk and Yakutia that were close to haplotypes typical for Northern Europe. The second subgroup consists of samples scattered across the vast area from the Russian Far East to the Ural Mountains. In the present study, we enriched this subgroup with three samples from Tomsk, three from Yakutia, two from Khabarovsk and a sample from Krasnoyarsk.
Figure 3.
Expanded view of clade 3 from Figure 2 representing phylogenetic placements of modern (shown in bold) and ancient (red) samples obtained in the present study. For labels of Published samples are described in Supporting Information (Table S3).
Analysis of modern populations
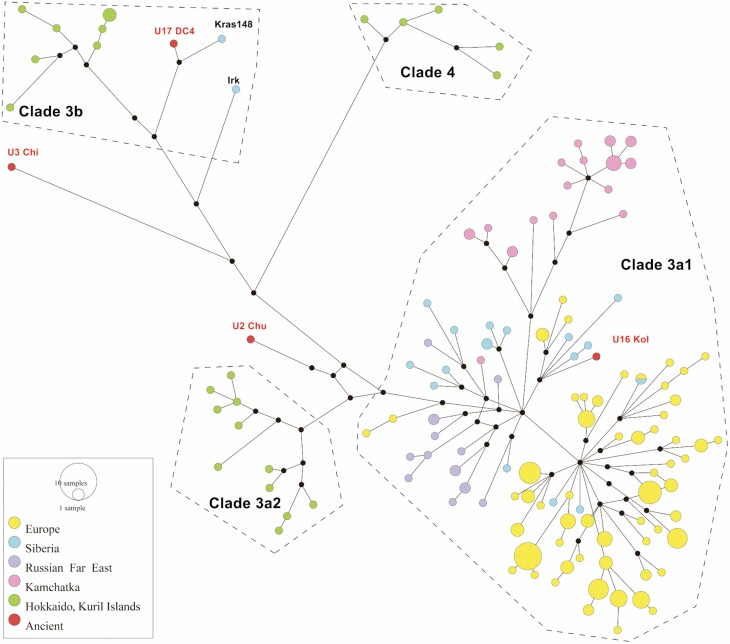
The relationships between mitochondrial haplotypes of northern Eurasian clades 3 and 4 were examined further using a median-joining network (Fig. 4). As expected, Hokkaido Island and Kuril Island samples fell into three distinct groups corresponding to clades 3a2, 3b (which also included Siberian samples Kras148 and Irk) and 4. The remaining diversity (clade 3a1) had a single origin, with a clear distinction between three groups: Kamchatka, Europe and Siberia together with the Russian Far East (mainland).
Figure 4.
Phylogenetic network of 136 modern bear mitochondrial genomes belonging to clades 3 and 4. Circle size corresponds to the number of identical samples. Colour code: yellow, Europe; blue, Siberia; green, Japan; red, Kamchatka; purple, the Russian Far East.
We estimated levels of genetic differentiation between populations of brown bears of Northern Europe (we excluded samples from Central and Southern Europe from this analysis), Siberia, the Russian Far East (mainland), Kamchatka and Hokkaido for a subset of completely recovered mitochondrial genomes that were available for 218 modern samples (Table 2). The genetic differentiation between the populations of Hokkaido and the other four is high (the fixation index [FST] between 0.2 and 0.5). European populations are also fairly differentiated from the Asian ones (FST between 0.15 and 0.38). Kamchatka is significantly differentiated from both Europe and the Russian Far East (mainland) (FST > 0.37 for both), and less so from Siberia (FST = 0.29). Differentiation between the Russian Far East (mainland) and Siberia is very low. In terms of within-population nucleotide diversity (Supporting Information, Table S4), populations from Hokkaido (π = 0.007) and Siberia (π = 0.0023) are much more diverse compared with Europe, the Russian Far East (mainland) and Kamchatka (π = 0.001), which reflects the presence of remnants from two (Siberia) or three (Japan) waves of migration.
Table 2.
Brown bear population differentiation
| F ST P-value | ||||||
|---|---|---|---|---|---|---|
| Hokkaido | Northern Europe | Siberia | Russian Far East (mainland) | Kamchatka | ||
| F ST | Hokkaido | – | 0.00 ± 0.00 | 0.00 ± 0.000 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Northern Europe | 0.496 | – | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Siberia | 0.208 | 0.149 | – | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Russian Far East (mainland) | 0.266 | 0.164 | 0.057 | – | 0.00 ± 0.00 | |
| Kamchatka | 0.341 | 0.378 | 0.29 | 0.401 | – |
Values below the diagonal are the population pairwise FST and those above the diagonal are FSTP-values.
DISCUSSION
Until recently, it was believed that a group of brown bears (U. arctos) from northern Eurasia expanded through the territory of modern continental Eurasia and to North America in the Late Pleistocene (37–29 kya) (Korsten et al., 2009; Anijalg et al., 2018). Here, we provide additional evidence supporting the hypothesis of Eurasian origin for the entire clade 3 based on the analysis of full mitochondrial DNA. Although mitochondrial lineages represent only maternal dispersal, our data demonstrate the presence of basal haplotypes among ancient pre-Last Glacial Maximum samples from Siberia.
Most of the modern samples from northern Eurasia belong to clade 3a1, and our data on Siberian and Far Eastern samples is concordant with widespread dispersion of this clade. This clade corresponds to the latest wave of brown bear migration (Anijalg et al., 2018). Two samples from Siberia belonged to clade 3b, which was previously found in Hokkaido and the Kuril islands, based on complete mitochondrial DNA analysis (Hirata et al., 2013), and additional samples from Northern Caucasus, Siberia and the Russian Far East were attributed to clade 3b based on mitochondrial sequences of cytB, CR and amplified product length polymorphism analysis (Gus’kov et al., 2013; Hirata et al., 2014; Salomashkina et al., 2014; Tumendemberel et al., 2019). Finding modern brown bears from South Siberia (Irk) from both clades 3b1 and 3b2 further confirms the continental origin of clade 3 proposed by Hirata et al. (2013).
Clade 4 includes modern brown bears from Hokkaido (Hirata et al., 2013) and North America (Barnes et al., 2002; Delisle & Strobeck, 2002). Based on the presence of three genetic lineages in Hokkaido, Matsuhashi et al. (2001) proposed that the island was invaded by bears in three distinct waves similar to those in North America. Descendants from the most recent wave inhabit the northern and central parts of the island, whereas descendants from the first wave inhabit the south-western part (Hirata et al., 2013). Similar phylogeographical structures in Hokkaido and North America indicate their simultaneous migration from Eurasia through Beringia to Alaska and through Sakhalin to Japan during glacial periods of lowered sea levels. This model of migration to Japan through Sakhalin was also described for other species, such as the Siberian flying squirrel, Pteromys volans (Oshida et al., 2005), Siberian chipmunk, Tamias sibiricus (Lee et al., 2008), and sable, Martes zibellina (Kinoshita et al., 2015).
Phylogenetic analysis revealed that the analysed ancient samples are very diverse and contain haplotypes characteristic for different migration waves. For example, the youngest ancient bear, U16 Kol (~4.5 kyr old), from the foothills of the Altai belongs to the widespread Eurasian haplogroup 3a1, being highly similar to modern bears from the European part of Russia and Estonia. A sample (U2 Chu) from the Chumysh River (~40 kyr old) takes a basal position within the entire clade 3a, which includes the Eurasian population and the population of central Hokkaido. Clade 3a is the most widespread modern clade, and it is believed that it spread widely after the Last Glacial Maximum. The ancient bear U17 DC from Denisova Cave (~5.5 kyr old) belongs to subclade 3b, which includes modern bears from Hokkaido and the Kuril islands, Siberia, Northern Caucasus and the continental Russian Far East. The phylogenetic analysis suggests that the representatives of clade 3b from Japan and Siberia had a most recent common ancestor ~82 kya (Fig. 2), which roughly corresponds to estimates by Salis et al. (2021). Sample U3 Chi from Chik river (~30 kyr old) is basal to the entire clade 3, with a divergence time estimated as 216 kya. This result might be somewhat confounded by the fact that this genome has the lowest completeness (91%, as opposed to > 96% in other ancient samples). However, similar observations of a basal position of ancient brown bears from Siberia relative to modern Eurasian populations were reported recently (Rey-Iglesia et al., 2019).
In this study, we have shown that most of modern brown bears from Siberia and the Russian Far East belong to the main Eurasian haplogroup 3. High genetic diversity among ancient brown bears indicates the shift that occurred in haplotype diversity and the presence of a common ancestral population for modern bears of the continental Russian Far East and Siberia. The isolation of the West European group might have resulted from human activities, which limited the free movement of surviving populations. Siberia and the Russian Far East are less susceptible to the impact of anthropogenic factors, and the habitat remains intact, which could have contributed to the wide distribution of clade 3a1. The data obtained on the representation of haplogroups, in combination with reconstruction of possible waves of migration of brown bears through Eurasia, highlight the importance of a southern Siberian refugium for brown bear populations not only during the Last Glacial Maximum, but also in earlier periods for at least the last 200 kyr.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Table S1. Description of modern samples of brown bears, for which complete sequences of mitochondrial genomes were obtained. Colours represent regions as follows: blue, Siberia; purple, Russian Far East.
Table S2. Primers for Ursus arctos full mitochondrial DNA probe (length of fragments ~4 kb, primer melting temperature (Tm) ~68 ℃).
Table S3. Samples used for phylogenetic analysis; a total of 259 mitochondrial sequences of modern bears. Colours represent geographical regions as follows: green, Hokkaido and Kuril islands; yellow, Europe; blue, Siberia; purple, Russian Far East (mainland); red, Kamchatka.
Table S4. Within-population nucleotide diversity calculated for samples indicated by colour in the Supporting Information (Tables1 and S3; Western and Southern European samples are excluded from the analysis).
Figure S1. Phylogeny for COXII and mitochondrial control region (CR) sequences for Central Asian brown bears from the study by Tumendemberel et al. (2019) and complete mitochondrial genomes from this study.
ACKNOWLEDGEMENTS
The study is supported by grant Russian Foundation of Basic Research (RFBR) #20-04-00213 and by research funding (grant numbers IUT20-32 and PRG1209) from the Estonian Ministry of Education and Research. S.K.V. and M.V.S. were supported by RFBR # 20-29-01011. The work by G.G.B. was conducted within the framework of state assignment of the Diamond and Precious Metals Geology Institute, Siberian Branch of the Russian Academy of Sciences. A.I.M. was supported by the Wellcome Trust (grant number 206194). We thank three anonymous reviewers for their insightful comments and suggestions. The authors have no conflicts of interest to declare.
Contributor Information
Anna S Molodtseva, Institute of Molecular and Cellular Biology, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
Alexey I Makunin, Institute of Molecular and Cellular Biology, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia; Wellcome Sanger Institute, Hinxton, Cambridge, UK.
Valentina V Salomashkina, A. N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow, Russia.
Ilya G Kichigin, Institute of Molecular and Cellular Biology, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
Nadezhda V Vorobieva, Institute of Molecular and Cellular Biology, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
Sergey K Vasiliev, Institute of Archaeology and Ethnography, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
Mikhail V Shunkov, Institute of Archaeology and Ethnography, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
Alexey A Tishkin, Altai State University, Barnaul, Russia.
Sergey P Grushin, Altai State University, Barnaul, Russia.
Peeter Anijalg, Department of Zoology, Institute of Ecology and Earth Sciences, University of Tartu, Tartu, Estonia.
Egle Tammeleht, Department of Zoology, Institute of Ecology and Earth Sciences, University of Tartu, Tartu, Estonia.
Marju Keis, Department of Zoology, Institute of Ecology and Earth Sciences, University of Tartu, Tartu, Estonia.
Gennady G Boeskorov, Geological Museum, Institute of Diamond and Precious Metals Geology, Siberian Branch of the Russian Academy of Sciences, Yakutsk, Russia.
Nikolai Mamaev, Institute for Biological Problems of Cryolithozone, Siberian Branch of the Russian Academy of Sciences, Yakutsk, Russia.
Innokenty M Okhlopkov, Institute for Biological Problems of Cryolithozone, Siberian Branch of the Russian Academy of Sciences, Yakutsk, Russia.
Alexey P Kryukov, Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far Eastern Branch of the Russian Academy of Sciences, Vladivostok, Russia.
Elena A Lyapunova, N. K. Koltzov Institute of Developmental Biology, Russian Academy of Sciences, Moscow, Russia.
Marina V Kholodova, A. N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow, Russia.
Ivan V Seryodkin, Pacific Institute of Geography, Far East Branch, Russian Academy of Sciences, Vladivostok, Russia.
Urmas Saarma, Department of Zoology, Institute of Ecology and Earth Sciences, University of Tartu, Tartu, Estonia.
Vladimir A Trifonov, Institute of Molecular and Cellular Biology, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
Alexander S Graphodatsky, Institute of Molecular and Cellular Biology, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
AUTHOR CONTRIBUTIONS
A.S.G., N.V.V. and U.S. conceived the original idea; all authors collected the data; A.S.M., A.I.M., I.G.K. and N.V.V. analysed the data; A.S.M. and A.I.M. wrote the first draft of the manuscript; and all authors contributed to writing the final version.
DATA AVAILABILITY
Mitochondrial genomes generated in this study are available at NCBI GenBank under accession numbers MW991379–MW991401.
REFERENCES
- Anijalg P, Ho SYW, Davison J, Keis M, Tammeleht E, Bobowik K, Tumanov IL, Saveljev AP, Lyapunova EA, Vorobiev AA, Markov NI, Kryukov AP, Kojola I, Swenson JE, Hagen SB, Eiken HG, Paule L, Saarma U. 2018. Large-scale migrations of brown bears in Eurasia and to North America during the Late Pleistocene. Journal of Biogeography 45: 394–405. [Google Scholar]
- Barlow A, Cahill JA, Hartmann S, Theunert C, Xenikoudakis G, Fortes GG, Paijmans JLA, Rabeder G, Frischauf C, Grandal-d’Anglade A, García-Vázquez A, Murtskhvaladze M, Saarma U, Anijalg P, Skrbinšek T, Bertorelle G, Gasparian B, Bar-Oz G, Pinhasi R, Slatkin M, Dalén L, Shapiro B, Hofreiter M. 2018. Partial genomic survival of cave bears in living brown bears. Nature Ecology & Evolution 2: 1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes I, Matheus P, Shapiro B, Jensen D, Cooper A. 2002. Dynamics of Pleistocene population extinctions in Beringian brown bears. Science 295: 2267–2270. [DOI] [PubMed] [Google Scholar]
- Benazzo A, Trucchi E, Cahill JA, Maisano Delser P, Mona S, Fumagalli M, Bunnefeld L, Cornetti L, Ghirotto S, Girardi M, Ometto L, Panziera A, Rota-Stabelli O, Zanetti E, Karamanlidis A, Groff C, Paule L, Gentile L, Vilà C, Vicario S, Boitani L, Orlando L, Fuselli S, Vernesi C, Shapiro B, Ciucci P, Bertorelle G. 2017. Survival and divergence in a small group: the extraordinary genomic history of the endangered Apennine brown bear stragglers. Proceedings of the National Academy of Sciences of the United States of America 114: E9589–E9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvignac S, Hughes S, Tougard C, Michaux J, Thevenot M, Philippe M, Hamdine W, Hänni C. 2008. Ancient DNA evidence for the loss of a highly divergent brown bear clade during historical times. Molecular Ecology 17: 1962–1970. [DOI] [PubMed] [Google Scholar]
- Davison J, Ho SYW, Bray SC, Korsten M, Tammeleht E, Hindrikson M, Østbye K, Østbye E, Lauritzen SE, Austin J, Cooper A, Saarma U. 2011. Late-Quaternary biogeographic scenarios for the brown bear (Ursus arctos), a wild mammal model species. Quaternary Science Reviews 30: 418–430. [Google Scholar]
- Delisle I, Strobeck C. 2002. Conserved primers for rapid sequencing of the complete mitochondrial genome from carnivores, applied to three species of bears. Molecular Biology and Evolution 19: 357–361. [DOI] [PubMed] [Google Scholar]
- Derevyanko AP, Shunkov MV, Agadzhanyan AK. 2003. The natural environment and man in the Paleolithic of Gorny Altai. Living conditions in the vicinity of the Denisova Cave. Novosibirsk: Publishing house of the Institute of Archeology and Ethnography of the Siberian Branch of the Russian Academy of Sciences. [Google Scholar]
- Druzhkova AS, Thalmann O, Trifonov VA, Leonard JA, Vorobieva NV, Ovodov ND, Graphodatsky AS, Wayne RK. 2013. Ancient DNA analysis affirms the canid from Altai as a primitive dog. PLoS One 8: e57754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10: 564–567. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131: 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grushin SP. 2015. Itogi i perspektivy issledovaniya poseleniya Kolyvanskoe-I v Rudnom Altae [Results and prospects of the research of the Kolyvan-I Settlement in Mining Altai]. Teoriya I Praktika Arkheologicheskikh Issledovaniy [Theory and Practice of Archaeological Research] 2: 12. [Google Scholar]
- Gus’kov VY, Sheremet’eva IN, Seredkin IV, Kryukov AP. 2013. Mitochondrial cytochrome b gene variation in brown bear (Ursus arctos Linnaeus, 1758) from southern part of Russian Far East. Russian Journal of Genetics 49: 1213–1218. [PubMed] [Google Scholar]
- Hirata D, Abramov AV, Baryshnikov GF, Masuda R. 2014. Mitochondrial DNA haplogrouping of the brown bear, Ursus arctos (Carnivora: Ursidae) in Asia, based on a newly developed APLP analysis. Biological Journal of the Linnean Society 111: 627–635. [Google Scholar]
- Hirata D, Mano T, Abramov AV, Baryshnikov GF, Kosintsev PA, Vorobiev AA, Raichev EG, Tsunoda H, Kaneko Y, Murata K, Fukui D, Masuda R. 2013. Molecular phylogeography of the brown bear (Ursus arctos) in Northeastern Asia based on analyses of complete mitochondrial DNA sequences. Molecular Biology and Evolution 30: 1644–1652. [DOI] [PubMed] [Google Scholar]
- Jónsson H, Ginolhac A, Schubert M, Johnson PLF, Orlando L. 2013. mapDamage2.0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics 29: 1682–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keis M, Remm J, Ho SYW, Davison J, Tammeleht E, Tumanov IL, Saveljev AP, Männil P, Kojola I, Abramov AV, Margus T, Saarma U. 2013. Complete mitochondrial genomes and a novel spatial genetic method reveal cryptic phylogeographical structure and migration patterns among brown bears in north-western Eurasia. Journal of Biogeography 40: 915–927. [Google Scholar]
- Kinoshita G, Sato JJ, Meschersky IG, Pishchulina SL, Simakin LV, Rozhnov VV, Malyarchuk BA, Derenko MV, Denisova GA, Frisman LV, Kryukov AP, Hosoda T, Suzuki H. 2015. Colonization history of the sable Martes zibellina (Mammalia, Carnivora) on the marginal peninsula and islands of northeastern Eurasia. Journal of Mammalogy 96: 172–184. [Google Scholar]
- Korsten M, Ho SYW, Davison J, Pähn B, Vulla E, Roht M, Tumanov IL, Kojola I, Andersone-Lilley Z, Ozolins J, Pilot M, Mertzanis Y, Giannakopoulos A, Vorobiev AA, Markov NI, Saveljev AP, Lyapunova EA, Abramov AV, Männil P, Valdmann H, Pazetnov SV, Pazetnov VS, Rõkov AM, Saarma U. 2009. Sudden expansion of a single brown bear maternal lineage across northern continental Eurasia after the last ice age: a general demographic model for mammals? Molecular Ecology 18: 1963–1979. [DOI] [PubMed] [Google Scholar]
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2017. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34: 772–773. [DOI] [PubMed] [Google Scholar]
- Lee M-Y, Lissovsky AA, Park S-K, Obolenskaya EV, Dokuchaev NE, Zhang Y-P, Yu L, Kim Y-J, Voloshina I, Myslenkov A, Choi T-Y, Min M-S, Lee H. 2008. Mitochondrial cytochrome b sequence variations and population structure of Siberian chipmunk (Tamias sibiricus) in Northeastern Asia and population substructure in South Korea. Molecules and Cells 26: 566–575. [PubMed] [Google Scholar]
- Leonard JA, Wayne RK, Cooper A. 2000. Population genetics of Ice Age brown bears. Proceedings of the National Academy of Sciences of the United States of America 97: 1651–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv. Preprint arXiv:1303.3997. Available at: http://arxiv.org/abs/1303.3997
- Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. [DOI] [PubMed] [Google Scholar]
- Lindgreen S. 2012. AdapterRemoval: easy cleaning of next-generation sequencing reads. BMC Research Notes 5: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist C, Schuster SC, Sun Y, Talbot SL, Qi J, Ratan A, Tomsho LP, Kasson L, Zeyl E, Aars J, Miller W, Ingólfsson O, Bachmann L, Wiig O. 2010. Complete mitochondrial genome of a Pleistocene jawbone unveils the origin of polar bear. Proceedings of the National Academy of Sciences of the United States of America 107: 5053–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricic T, Whitten M, Pääbo S. 2010. Multiplexed DNA sequence capture of mitochondrial genomes using PCR products. PLoS One 5: e14004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi T, Masuda R, Mano T, Murata K, Aiurzaniin A. 2001. Phylogenetic relationships among worldwide populations of the brown bear Ursus arctos. Zoological Science 18: 1137–1143. [Google Scholar]
- Matsuhashi T, Masuda R, Mano T, Yoshida MC. 1999. Microevolution of the mitochondrial DNA control region in the Japanese brown bear (Ursus arctos) population. Molecular Biology and Evolution 16: 676–84. [DOI] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. 2010-9. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research 20: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CR, Waits LP, Joyce P. 2006. Phylogeography and mitochondrial diversity of extirpated brown bear (Ursus arctos) populations in the contiguous United States and Mexico. Molecular Ecology 15: 4477–4485. [DOI] [PubMed] [Google Scholar]
- Norman AJ, Street NR, Spong G. 2013. De novo SNP discovery in the Scandinavian brown bear (Ursus arctos). PloS One 8: e81012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshida T, Abramov A, Yanagawa H, Masuda R. 2005. Phylogeography of the Russian flying squirrel (Pteromys volans): implication of refugia theory in arboreal small mammal of Eurasia. Molecular Ecology 14: 1191–1196. [DOI] [PubMed] [Google Scholar]
- Rey-Iglesia A, García-Vázquez A, Treadaway EC, van der Plicht J, Baryshnikov GF, Szpak P, Bocherens H, Boeskorov GG, Lorenzen ED. 2019. Evolutionary history and palaeoecology of brown bear in North-East Siberia re-examined using ancient DNA and stable isotopes from skeletal remains. Scientific Reports 9: 4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarma U, Ho SYW, Pybus OG, Kaljuste M, Tumanov IL, Kojola I, Vorobiev AA, Markov NI, Saveljev AP, Valdmann H, Lyapunova EA, Abramov AV, Männil P, Korsten M, Vulla E, Pazetnov SV, Pazetnov VS, Putchkovskiy SV, Rõkov AM. 2007. Mitogenetic structure of brown bears (Ursus arctos L.) in northeastern Europe and a new time frame for the formation of European brown bear lineages. Molecular Ecology 16: 401–413. [DOI] [PubMed] [Google Scholar]
- Salis AT, Bray SCE, Lee MSY, Heiniger H, Barnett R, Burns JA, Doronichev V, Fedje D, Golovanova L, Harington CR, Hockett B, Kosintsev P, Lai X, Mackie Q, Vasiliev S, Weinstock J, Yamaguchi N, Meachen JA, Cooper A, Mitchell KJ. 2021. Lions and brown bears colonized North America in multiple synchronous waves of dispersal across the Bering Land Bridge. Molecular Ecology. doi: 10.1111/mec.16267. [DOI] [PubMed] [Google Scholar]
- Salomashkina VV, Kholodova MV, Tuten’kov OY, Moskvitina NS, Erokhin NG. 2014. New data on the phylogeography and genetic diversity of the brown bear Ursus arctos Linnaeus, 1758 of Northeastern Eurasia (mtDNA control region polymorphism analysis). Biology Bulletin 41: 38–46. [PubMed] [Google Scholar]
- Schubert M, Ermini L, Der Sarkissian C, Jónsson H, Ginolhac A, Schaefer R, Martin MD, Fernández R, Kircher M, McCue M, Willerslev E, Orlando L. 2014. Characterization of ancient and modern genomes by SNP detection and phylogenomic and metagenomic analysis using PALEOMIX. Nature Protocols 9: 1056–1082. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A. 2018. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evolution 4: vey016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taberlet P, Bouvet J. 1994. Mitochondrial DNA polymorphism, phylogeography, and conservation genetics of the brown bear Ursus arctos in Europe. Proceedings of the Royal Society B: Biological Sciences 255: 195–200. [DOI] [PubMed] [Google Scholar]
- Tammeleht E, Remm J, Korsten M, Davison J, Tumanov I, Saveljev A, Männil P, Kojola I, Saarma U. 2010. Genetic structure in large, continuous mammal populations: the example of brown bears in northwestern Eurasia. Molecular Ecology 19: 5359–5370. [DOI] [PubMed] [Google Scholar]
- Tumendemberel O, Zedrosser A, Proctor MF, Reynolds HV, Adams JR, Sullivan JM, Jacobs SJ, Khorloojav T, Tserenbataa T, Batmunkh M, Swenson JE, Waits LP. 2019. Phylogeography, genetic diversity, and connectivity of brown bear populations in Central Asia. PLoS One 14: e0220746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliev SK, Parkhomchuk EV, Serednyov MA, Milutin KI, Kuzmin YV, Kalinkin PN, Rastigeev SA, Milutin VS. 2018. Radiocarbon dating of the remains of rare Pleistocene megafauna species in southern Siberia. Problems of Archaeology, Ethnography, Anthropology of Siberia and Neighboring Territories 24: 42–46. [Google Scholar]
- Vasiliev SK, Parkhomchuk EV, Serednyov MA, Milutin KI, Rastigeev SA, Parkhomchuk VV. 2020. Late Pleistocene Megafauna from the South of Western and Central Siberia: new data on radiocarbon dating and new finds from alluvial sites in 2020. Problems of Archaeology, Ethnography, Anthropology of Siberia and Neighboring Territories 26: 43–50. [Google Scholar]
- Vasiliev SK, Serednyov MA, Milutin KI, Panov VS. 2016. Collecting of the theriofaunal materials at the rivers Chumysh (Altai region), Chick and Ob near Bibikha village (Novosibirsk region) in 2016. Problems of Archaeology, Ethnography, Anthropology of Siberia and Neighboring Territories 22: 23–28. [Google Scholar]
- Vorobieva NV, Makunin AI, Druzhkova AS, Kusliy MA, Trifonov VA, Popova KO, Polosmak NV, Molodin VI, Vasiliev SK, Shunkov MV, Graphodatsky AS. 2020. High genetic diversity of ancient horses from the Ukok Plateau. PLoS One 15: e0241997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenikoudakis G, Ersmark E, Tison JL, Waits L, Kindberg J, Swenson JE, Dalén L. 2015. Consequences of a demographic bottleneck on genetic structure and variation in the Scandinavian brown bear. Molecular Ecology 24: 3441–3454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mitochondrial genomes generated in this study are available at NCBI GenBank under accession numbers MW991379–MW991401.