Abstract
Background
The leaves of the plant Ilex latifolia Thunb. can be made into Kuding tea, which is a drink rich in polyphenols. This study aimed to observe the effect of Ilex latifolia Thunb. polyphenols (ILTPs) on human lung cancer cell line A549 (A549 cells) by regulating the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway.
Methods
In vitro cultured cells were treated with ILTPs; the proliferation of A549 cells and BEAS-2B human normal lung epithelial cells (Beas-2B cells) was observed using the 3-(4,5-dimethylazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and the survival status of A549 cells was observed by fluorescence staining. The expression of A549 cells was observed by quantitative polymerase chain reaction (qPCR) assay and Western blot analysis, while the compound composition of ILTPs was detected using high-performance liquid chromatography (HPLC).
Results
The experimental results showed that the proliferation of Beas-2B cells was unaffected by treatment with 0–500 μg/mL of ILTPs, whereas the decreased proliferation of A549 cells was observed with the increasing concentrations of ILTPs. Additionally, ILTPs elevated the levels of lactate dehydrogenase (LDH) and reactive oxygen species (ROS) and promoted apoptosis in A549 cells. The results of qPCR experiments showed that ILTPs upregulated caspase-9 mRNA expression and downregulated phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt), mammalian target of rapamycin (mTOR), B-cell lymphoma-2 (Bcl-2), nuclear factor-κB (NF-κB), vascular endothelial growth factor (VEGF), hypoxia-inducible factor-1 alpha (HIF-1α), and cyclooxygenase-2 (COX-2) expression in A549 cells. The Western blot analysis results also showed that ILTPs could reduce the protein expression of PI3K and Akt. The HPLC results showed that the main compounds present in the ILTPs were rutin, kaempferol, isochlorogenic acid A, isochlorogenic acid B, and isochlorogenic acid C.
Conclusions
Thus, this study indicated that the polyphenols of I. latifolia act as a class of natural functional food materials that potently suppress cancer by exerting their inhibitory effects on A549 cell proliferation through five key polyphenolic compounds.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-022-03568-3.
Keywords: Lung cancer, MTT, PI3K-Akt signaling pathway, Polyphenols, Ilex latifolia Thunb
Background
The leaves of Ilex latifolia Thunb. are harvested from plants mainly distributed in southwest and south China. The leaves are dried and brewed into a tea that is consumed as a traditional natural health drink commonly known as Kuding tea in China. The Kuding tea contains more than 200 components, such as saponins, amino acids, vitamin C, polyphenols, flavonoids, caffeine, and protein [1]. I. latifolia has the functions of clearing and relieving heat, moistening the throat and relieving cough, reducing blood pressure and weight, inhibiting and preventing cancer, activating blood vessels, and exerting anti-aging effects [2]. Analysis revealed that I. kudingcha contains nearly 6% polyphenols [3]. The polyphenols in the tea can remove harmful free radicals, block lipid peroxidation, increase the activity of enzymes in the human body, exert anti-mutation and anticancer effects, and thus can be used for the prevention and auxiliary treatment of various cancers such as gastric and colon cancers [4].
Lung cancer is a common primary cancer. The incidence rate and mortality rate of lung cancer rank second and first, respectively, among those of all other cancer types [5]. The incidence rate of lung cancer has increased significantly over the past 50 years. The incidence and mortality rates of lung cancer are the highest among all cancer types, with these rates being the second highest among females [6]. Studies have shown that the polyphenols from green tea, apples, seaweed, and rice husks can help in inhibiting lung cancer in vitro or in vivo [7, 8].
The expression of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway is abnormally activated in the occurrence and development of some cancers. The two most widely discovered mechanisms of PI3K/Akt activation in human cancer are triggered by receptor tyrosine kinase and somatic mutations in specific elements of signaling pathways [9]. The stimulation and promotion of the PI3K pathway may have a negative impact on cancer treatment, and, therefore, the inhibition of PI3K may inhibit cancer development [10]. Abnormal stimulation of the PI3K/Akt pathway is related to tumor growth, angiogenesis, and survival [11], in which the loss of function of tumor suppressor gene phosphatase and tensin homolog deleted on chromosome ten (PTEN) is commonly seen in human tumors and leads to the stimulation of the PI3K/Akt pathway [12]. The key expression of the PI3K/Akt pathway, which includes PI3K, Akt, and mammalian target of rapamycin (mTOR), has been confirmed to have a clinical targeted therapeutic effect [13].
This study verified the inhibitory effect of I. latifolia polyphenols on A549 cells by regulating the PI3K/Akt signaling pathway in vitro. Moreover, I. latifolia polyphenols were analyzed to elucidate the relationship between the active components of I. latifolia polyphenols and the regulation of the PI3K/Akt signaling pathway in lung cancer.
Methods
Preparation of I. latifolia Thunb. polyphenol extract
After freeze-drying, the I. latifolia leaves (Yuqing Lvye tea processing factory, Zunyi, Guizhou, China) were crushed, 70% ethanol (v/v) was added according to the ratio of liquid to material 20:1, and the mixture was heated in a water bath at 60°C for 3 h. The ethanol extract was passed through the FL-3 macroporous resin (Shanghai Yiji Biology Co., Ltd., Shanghai, China), the filtrate was collected, and the extract was obtained by rotary distillation of the filtrate. After freeze-drying the extract, the dried product obtained was a fine powder.
Analysis of I. latifolia Thunb. polyphenols extract composition
High-performance liquid chromatography (HPLC, UltiMate3000 HPLC System, Thermo Fisher Scientific, Inc., MA, USA) was used to determine the composition of the flavonoids in I. latifolia leaves. The chromatographic conditions were as follows: C18 column (4.6 mm × 150 mm, 2.6 μm); mobile phase A—0.5% acetic acid solution, B—acetonitrile; flow rate, 0.6 mL/min; column temperature, 30°C; detector, ultraviolet visible; detection wavelength, 359 nm; and injection volume, 10 μL. The flavonoid quantitation in mg/g was calculated by the external standard method: Mx = Cr × Ax/Ar × C, where Cr (mg/mL) denotes the mass concentration of the standard, Ax the peak area of the sample, Ar the peak area of the standard, and C (1.0 mg/mL) the concentration of the sample stock solution.
Culture of cancer cells
The A549 cells and BEAS-2B human normal lung epithelial cells (Beas-2B cells) were used in this study. The Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen, CA, USA) was added with 10% fetal bovine serum (v/v) and 1% penicillin–streptomycin (v/v). The prepared medium was used to culture A549 cells and Beas-2B cells (Shanghai Yaji Biotechnology Co., Ltd., Shanghai, China) at 37°C and under a 5% CO2 atmosphere, and the medium was changed every 2 days. The cells in the control group were not treated with Ilex latifolia Thunb. polyphenols (ILTPs), while the treated cells received 0–500 μg/mL ILTPs.
Detection of cell proliferation
The cell proliferation was detected using the 3-(4,5-dimethylazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For this, 1 × 104/mL cells were seeded into 96-well plates, 180 μL per well, and cultured for 24 h at 5% CO2 and 37°C. After the cells adhered to the plate walls, 20 μL of LLTP solution at different concentrations was added to each well. After incubation for 48 h, the medium was discarded, 200 μL of MTT reagent (5 mg/mL, Solarbio, Beijing, China) was added to each well, and the cells were cultured for 4 h. After the end of incubation, the supernatant was discarded, 200 μL of dimethyl sulfoxide was added to each well, and the plate was oscillated for 30 min. The optical density (OD) value of each well was measured at 490 nm, and the inhibition rate of cell proliferation was calculated. The proliferation inhibition rate (%) = (ODcontrol well - ODsample well)/ODcontrol well × 100 [14].
Determination of lactate dehydrogenase levels in the cell culture medium
After the cells were treated according to the method described in the previous section, the medium was collected and the lactate dehydrogenase (LDH) level in the medium was determined using a kit according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
Detection of apoptosis of cancer cells by flow cytometry
The A549 cells in the logarithmic growth phase were treated with 125, 250, and 500 μg/mL LLTPs for 48 h. Then, the cells were washed with phosphate-buffered saline (PBS) three times, suspended in annexin V–FITC binding solution, mixed with annexin V–FITC and propidium iodide staining solution (Thermo Fisher Scientific, Inc.), and cultured at 4°C in the dark for 15 min. Finally, the apoptosis of cancer cells was analyzed by flow cytometry (FACSCalibur, BD, NJ, USA) [15].
Detection of reactive oxygen species levels in cancer cells by flow cytometry
The A549 cells treated with nuciferine were collected, washed three times with PBS, resuspended in RPMI-1640 medium containing 10 μmol/L 2',7'-dichlorodihydrofluorescein diacetate at 37°C for 20 min, washed three times with PBS after incubation, resuspended in RPMI-1640 medium, and the fluorescence intensity of each test sample was assessed using a flow cytometer (FACSCalibur, BD, NJ, USA), with the mean fluorescence intensity (MFI) representing the content of reactive oxygen species (ROS) [16].
Quantitative polymerase chain reaction assay
The total RNA was extracted using a kit, and the concentration and purity of RNA were determined using a microspectrophotometer (Nano 300, All for Life Science, Hangzhou, Zhejiang, China). Reverse transcription of RNA into cDNA was performed using a Reverse Aid First Strand cDNA synthesis kit (Tiangen Biotech Co., Ltd., Beijing, China). Hieff quantitative polymerase chain reaction (qPCR) SYBR Green Master Mix (High Rox Plus), and StepOne Plus real-time PCR system (Thermo Fisher Scientific, Inc.) were used to measure the expression levels of target genes. The qPCR primers used are listed in Table 1. qPCR was carried out under the following cycle conditions: pre-denaturation at 95°C for 3 min, and then denaturation for 15 s at 95°C for 60 cycles; annealing at 55°C for 30 s; denaturation at 95°C for 30 s; and annealing at 55°C for 35 s. β-Actin was used for target gene expression, and the relative intensity of expression was calculated by the 2−ΔΔCt method [17].
Table 1.
Sequences of primers used in this study.
| Gene Name | Sequence |
|---|---|
| PI3K |
Forward: 5’-CTGCCTGCGACAGATGAGTG-3’ Reverse: 5’-TCCGATTACCAAGTGCTCTTTC-3’ |
| Akt |
Forward: 5’-AGCGACGTGGCTATTGTGAAG-3’ Reverse: 5’-GCCATCATTCTTGAGGAGGAAGT-3’ |
| mTOR |
Forward: 5’-ACAACTTTGGTATCGTGGAAGG-3’ Reverse: 5’-GCCATCACGCCACAGTTTC-3’ |
| Bcl-2 |
Forward: 5’-ATGTGTGTGGAGAGCGTCAACC-3’ Reverse: 5’-CAGAGACAGCCAGGAGAAATCAA-3’ |
| Caspase-9 |
Forward: 5’-CTCAGACCAGAGATTCGCAAAC-3’ Reverse: 5’-GCATTTCCCCTCAAACTCTCAA-3’ |
| NF-κB |
Forward: 5’-GAAGCACGAATGACAGAGGC-3’ Reverse: 5’-GCTTGGCGGATTAGCTCTTTT-3’ |
| VEGF |
Forward: 5’-TGCCCACTGAGGAGTCCAAC-3’ Reverse: 5’-TGGTTCCCGAAACGCTGAG-3’ |
| HIF-1α |
Forward: 5’-ATTCCAGCAGACTCAAATACAAGA-3’ Reverse: 5’-GACTCAAAGCGACAGATAACACG-3’ |
| COX-2 |
Forward: 5’-CTGGCGCTCAGCCATACAG-3’ Reverse: 5’-CGCACTTATACTGGTCAAATCCC-3’ |
| β-actin |
Forward: 5’-TCAAGAAGGTGGTGAAGCAGG-3’ Reverse: 5’-AGCGTCAAAGGTGGAGGAGTG-3’ |
Western blot analysis
The cells were treated in the same way as the qPCR assay. Then, 1 mL of pre-cooled lysate was added to the treatment cells, incubated on ice for 15 min, and then centrifuged at 4°C and 10,000 rpm for 15 min. After centrifugation, the supernatant was removed, and the bicinchoninic acid method was used to determine the protein content. The protein samples were separated using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Thermo Fisher Scientific), and the protein in the polyacrylamide gel was transferred to the nitrocellulose membrane. The nitrocellulose membrane was blocked with a 5% skimmed milk powder solution at room temperature for 1 h and incubated with the primary antibody (Thermo Fisher Scientific) overnight at 4°C on a shaker. Subsequently, the membrane was washed three times with PBS and Tween 20 (PBST) for 5 min each. Then, the secondary antibody (Thermo Fisher Scientific) was added, the membrane was incubated on a shaker for 2 h, and washed with PBST three times for 5 min each time to enhance the luminescence, development, and imaging effects of the chemiluminescent agent (iBright, Thermo Fisher Scientific) [17].
Statistical analysis
SPSS v17.0 and GraphPad Prism 7 software were used to analyze the data. The experimental results were expressed as mean ± standard deviation. One-way analysis of variance and t test were used to analyze the statistical difference at the level of P <0.05. All experiments were repeated three times.
Results
Chemical composition of ILTPs
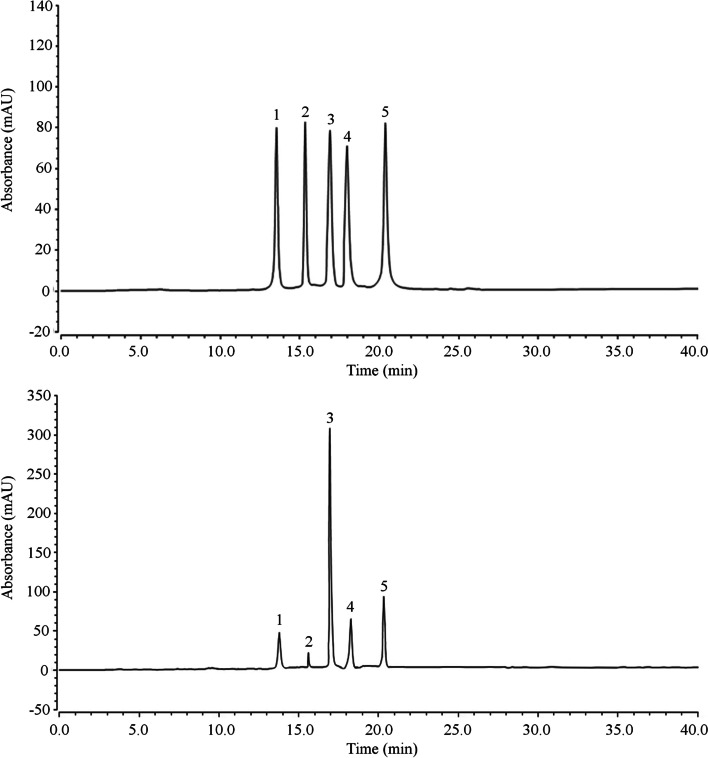
The analysis indicated that ILTPs were composed of five polyphenols, (i) rutin, (ii) kaempferol, (iii) isochlorogenic acid A, (iv) isochlorogenic acid B, and (v) isochlorogenic acid C (Fig. 1) at 216.35, 167.28, 102.52, 88.12, and 128.36 mg/g (mg/g of ILTPs extract), respectively.
Fig. 1.
Analysis of ILTPs component. (A) Standard chromatograms; (B) ILTPs chromatograms. 1: rutin, 2: kaempferol, 3: isochlorogenic acid B, 4: isochlorogenic acid A, 5: isochlorogenic acid C
Cytotoxic effect of ILTPs
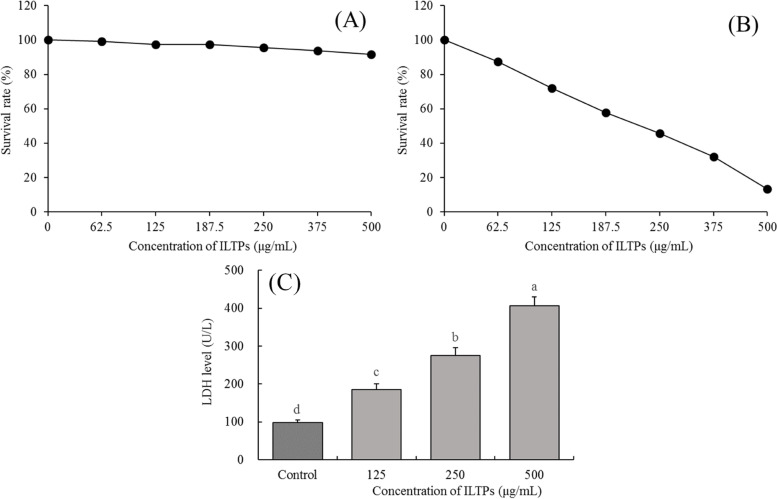
Figure 2A and B shows that at the concentration of 0–500 μg/mL, ILTPs had only a slight effect on the growth and proliferation of Beas-2B cells. However, at the same concentration, ILTPs inhibited the growth and proliferation of A549 cells, and the inhibition rate positively correlated with the concentration of ILTPs. The results showed that ILTPs had little effect on normal lung cells with no toxicity, but an inhibitory effect was observed on the proliferation of lung cancer cells. At concentrations of 125, 250, and 500 μg/mL, ILTPs inhibited the proliferation of A549 cells by 28.10%, 54.16%, and 86.74%, respectively (Table 2). Based on the aforementioned experimental results, 125, 250, and 500 μg/mL concentrations of ILTPs were used for further experiments. As shown in Fig. 2C, the LDH level in the cell culture medium of the control group was the lowest (97.82 U/L). With the increase in the concentration of ILTPs, the LDH level in the cell culture medium also increased. The LDH level in the culture medium of A549 cells treated with 125, 250, and 500 μg/mL ILTPs was 186.05, 274.88, and 406.73 U/L, respectively.
Fig. 2.
Survival rate of ILTPs treated (A) BEAS-2B human normal lung epithelial cells, (B) A549 lung cancer cells and (C) LDH level of A549 lung cancer cells culture medium (n=6). Duncan multiple range test showed that a-d of different letters showed significant difference in the mean value of each group (P < 0.05), the same letter indicates that there is no significant difference in the average value of each group (P > 0.05)
Table 2.
Inhibitory effect of different concentrations of ILTPs on proliferation of A549 lung cancer cells (n=6)
| Group | OD490 (Concentration of ILTPs, μg/mL) |
Cell growth inhibition rate (%) | ||||
|---|---|---|---|---|---|---|
| 125 | 250 | 500 | 125 | 250 | 500 | |
| Control | 0.445±0.005a | / | ||||
| ILTPs | 0.320±0.011b | 0.204±0.007c | 0.059±0.005d | 28.10±2.51C | 54.16±2.02B | 86.74±1.76A |
The experimental results are mean ± standard deviation. Duncan multiple range test showed that A-D and a-c of different letters showed significant difference in the mean value of each group (P < 0.05), the same letter indicates that there is no significant difference in the average value of each group (P > 0.05)
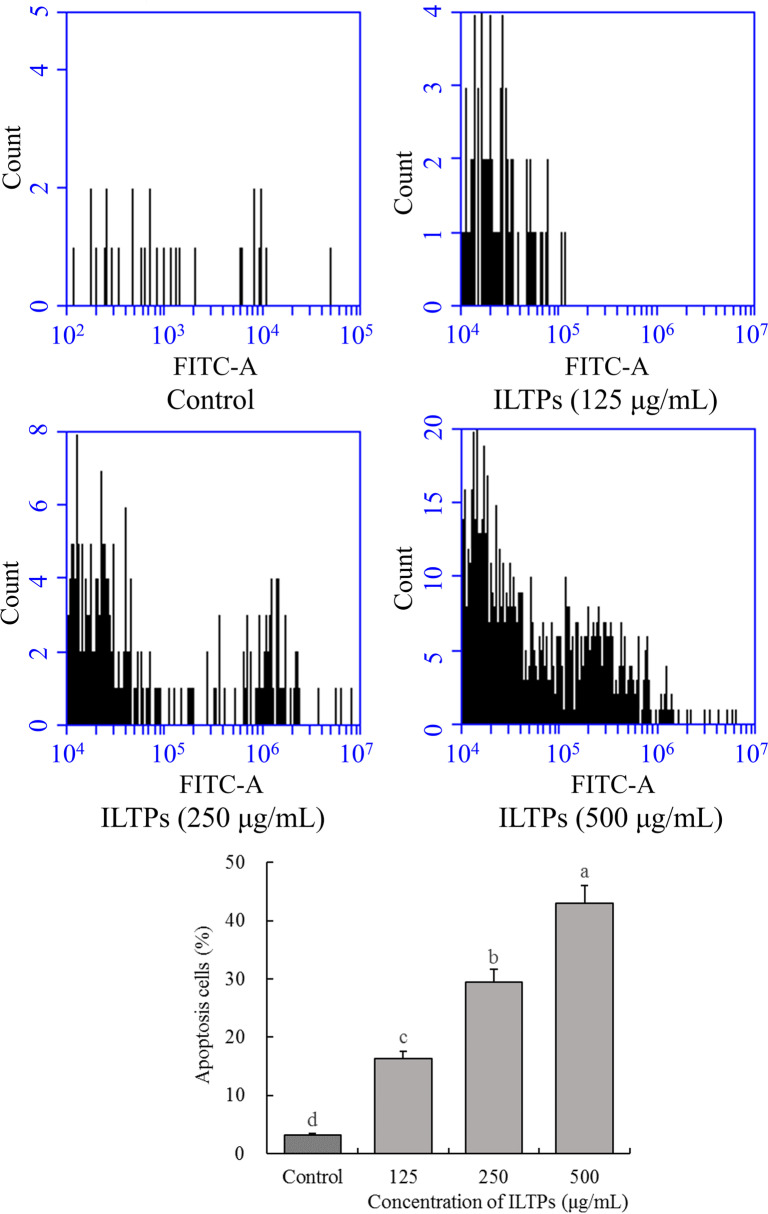
Apoptosis of A549 cells
As shown in Fig. 3, 500 μg/mL of ILTPs (42.92%) significantly induced the apoptosis of A549 cells compared with the control group. Similarly, A549 cells treated with 125 (16.35%) and 250 μg/mL (29.41%) ILTPs also showed apoptosis, and the number of apoptotic cells was higher than that in the control group, but less than that observed in the group treated with 500 μg/mL ILTPs. Also, the concentration of ILTP treatment positively correlated with the effect of apoptosis induction. The apoptosis-inducing effect of ILTPs on A549 cells positively correlated with the concentration of ILTPs.
Fig. 3.
Effects of ILTPs on apoptosis of A549 lung cancer cells (n=3). Duncan multiple range test showed that a-d of different letters showed significant difference in the mean value of each group (P < 0.05), the same letter indicates that there is no significant difference in the average value of each group (P > 0.05)
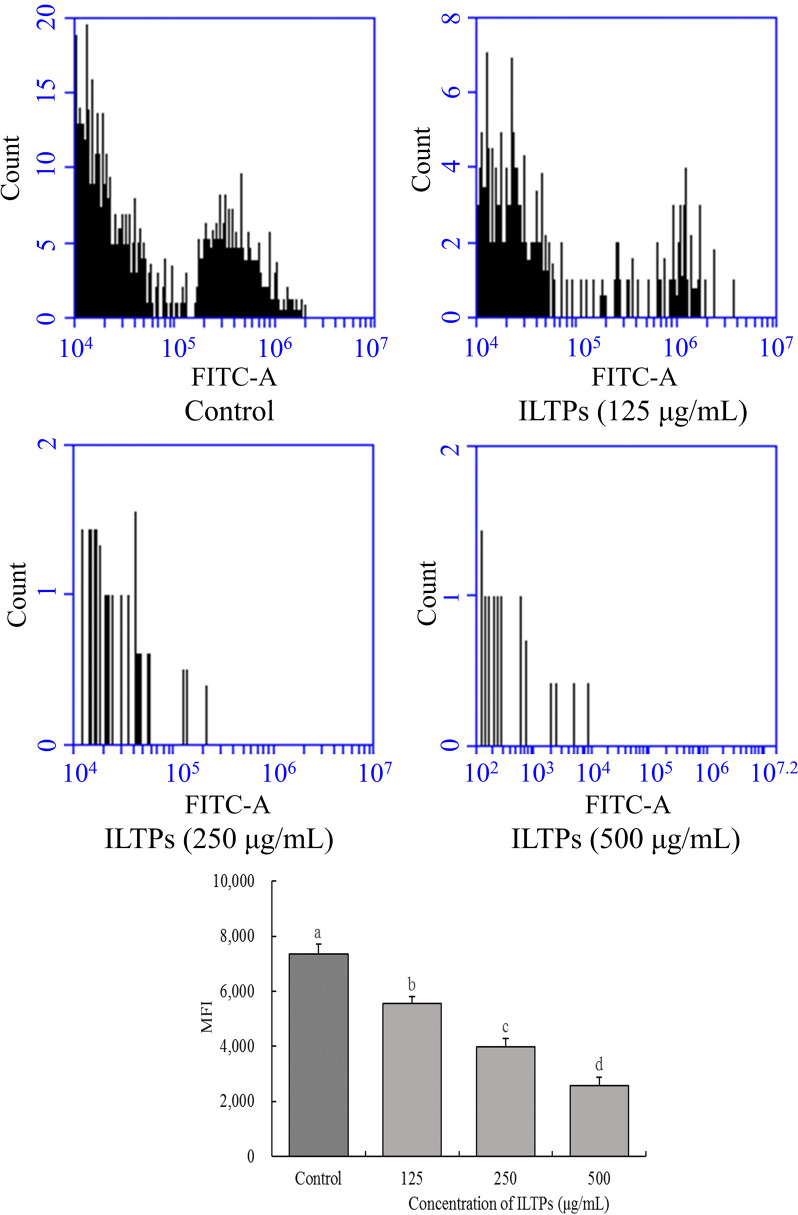
ROS levels in A549 cells
The flow cytometry results shown in Fig. 4 revealed that the MFI of the 125, 250, and 500 μg/mL ILTP groups was significantly lower than that of the control group (P < 0.05). The higher the ILTP concentration, the lower the fluorescence intensity, indicating a lower ROS level.
Fig. 4.
Effects of ILTPs on ROS level in A549 lung cancer cells (n=3). Duncan multiple range test showed that a-d of different letters showed significant difference in the mean value of each group (P < 0.05), the same letter indicates that there is no significant difference in the average value of each group (P > 0.05)
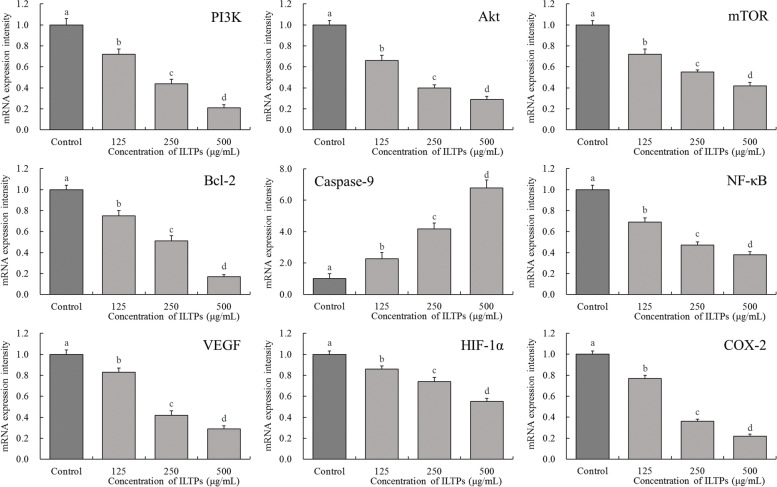
Expression of PI3K, Akt, mTOR, Bcl-2, caspase-9, NF-κB, VEGF, HIF-1α, and COX-2 mRNA in A549 cells
As shown in Fig. 5, A549 cells in the control group exhibited the weakest mRNA expression of caspase-9, while the strongest mRNA expression of PI3K, Akt, mTOR, B-cell lymphoma-2 (Bcl-2), nuclear factor-κB (NF-κB), vascular endothelial growth factor (VEGF), hypoxia-inducible factor-1 alpha (HIF-1α), and cyclooxygenase-2 (COX-2). Moreover, ILTPs was able to downregulate the expression of PI3K, Akt, mTOR, Bcl-2, NF-κB, VEGF, HIF-1α, and COX-2 and upregulate the expression caspase-9 in A549 cells. Compared with the control group, the expression of caspase-9 was stronger, while that of PI3K, Akt, mTOR, Bcl-2, NF-κB, VEGF, HIF-1α, and COX-2 decreased as the concentration of ILTPs increased.
Fig. 5.
Effect of ILTPs on mRNA expression of A549 lung cancer cells (n=3). Duncan multiple range test showed that a-d of different letters showed significant difference in the mean value of each group (P < 0.05), the same letter indicates that there is no significant difference in the average value of each group (P > 0.05)
Expression of PI3K and Akt protein in A549 cells
As shown in Fig. 6, the A549 cells in the control group showed the strongest protein expression of PI3K and Akt . After treatment with ILTPs, the protein expression of PI3K and Akt decreased, and with the increase in the ILTP concentration, the protein expression of PI3K and Akt became weaker.
Fig. 6.
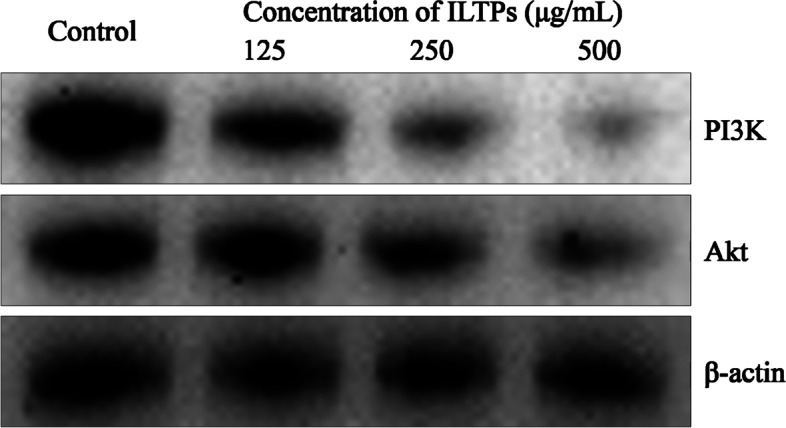
Effect of ILTPs on protein expression of A549 lung cancer cells (n=3)
Discussion
The main causes of cell death are apoptosis and cytotoxicity. ILTPs can induce apoptosis and death of cancer cells, but they have low toxicity and little effect on normal cell proliferation [18]. The present study demonstrated that ILTPs significantly inhibited only the proliferation of lung cancer cells, and did not affect normal lung tissue cells. Thus, ILTPs have the potential to be used as an active substance to inhibit lung cancer.
Under normal physiological conditions, only small amounts of LDH are present in body fluid, and intracellular LDH is released only after cell membranes are damaged. When the cancer cells are destroyed and die down, the LDH level in the culture medium significantly increases [19]. In this study, the LDH levels in the cell culture medium increased after lung cancer cells were treated with ILTPs, which showed that the ILTPs inhibited the proliferation of cancer cells and destroyed them. ROS can destroy normal tissues and DNA, leading to tissue lesions, which may be a contributing factor to cancer. In addition, a previous study showed that the ROS levels in cancer cells were higher than those of normal cells, and high levels of ROS might promote the growth and proliferation of cancer cells [20]. ILTPs have antioxidant and free-radical scavenging activity to protect the body [1]. The present study also showed similar results, where the ROS level of A549 cells was high, and after treatment with ILTPs, they might exert the ability to inhibit the high level of ROS in cancer cells. The ROS level of cancer cells decreased, subsequently inhibiting their negative effects.
The PI3K/Akt signaling pathway is an important cancer pathway because it can promote the growth and survival of cancer cells [21]. mTOR is a key kinase downstream of PI3K/Akt, and it plays a role in regulating the growth, proliferation, survival, and even metastasis of cancer cells [22]. The expression of PI3K, Akt, and mTOR inhibits pro-apoptotic factors and activates anti-apoptotic factors to promote the occurrence and development of cancer. PI3K/Akt-mTOR inhibits the activity of pro-apoptotic members and activates anti-apoptotic members through phosphorylation [23–25]. In addition, some studies showed that oxidative stress could activate PI3K/Akt [26, 27]. In this study, ILTPs effectively inhibited the expression of PI3K, Akt, and mTOR in the PI3K/Akt pathway. They also inhibited the PI3K/Akt pathway activated by oxidative stress, subsequently inhibiting the growth and proliferation of A549 cells.
In PI3K/Akt signaling activation, Akt activation is related to apoptosis, which, in turn, is related to the Bcl-2 family. The BAD of the Bcl-2 family can form dimers with Bcl-xL and promote cell apoptosis through signal transduction. Activated Akt can phosphorylate the ser136 site on BAD, thus preventing the apoptotic signal transmitted by BAD. After PI3K inhibitor transplantation, Akt-induced BAD phosphorylation is blocked [28]. Akt can also promote cell growth and inhibit cell apoptosis after the phosphorylation of NF-κB and activation of NF-κB in the nucleus [29]. When the cell is damaged, secondary damage to the mitochondrial membrane releases caspase and cytochrome c, and activates the cell death process through signal transmission. Activated Akt not only inhibits the release of apoptotic factors and cytochrome c but also inactivates caspase-9 by phosphorylating ser 196, and thus blocking its pro-apoptotic pathway [30]. After affecting the PI3K/Akt pathways, ILTPs also influence Bcl-2, NF-κB, and caspase-9 expression, thereby promoting cancer cell apoptosis.
PI3K joined the vascular endothelial signaling pathway of VEGF action via the activation of the PI3K/Akt pathway by forming a complex with E-cadherin, β-catenin, and VEGFR-12. Hypoxia and other factors, such as growth factors and insulin, can act on membrane surface receptors to induce the expression of HIF-1α, which prompts the translational expression of downstream angiogenic genes such as VEGF [31]. The activation of the PI3K/Akt pathway, which upregulates HIF-1α through multiple pathways, drives the expression of VEGF to enable the migration of endothelial cells to form a neovasculature network, so as to promote the growth and metastasis of cancer cells [32, 33]. VEGF, a major regulatory factor involved in angiogenesis, binds to endothelial cells in a targeted manner to promote vascular endothelial cell growth, increase their permeability, and then generate new blood vessels [33]. COX-2 is able to influence endothelial cell movement and the generation of new blood vessels, and the activation of the PI3K/Akt signaling pathway can upregulate COX-2, which in turn is involved in tumor angiogenesis [34]. On the contrary, ILTPs function to control cancer cell metastasis and proliferation by regulating the expression of VEGF, HIF-1α, and COX-2 under the influence of angiogenesis.
Rutin, kaempferol, and isochlorogenic acids A, B, and C are all polyphenolic antioxidant substances, and studies have shown that these compounds also exert some anticancer effects [35–39]. Rutin and kaempferol, as the main effective chemicals contained in some food products, also showed some interventional effects on lung cancer [40, 41]. The combined effect of these five compounds was responsible for the inhibitory effect by ILTPs on A549 cells in vitro, including the interventional effect on the pathway.
Conclusions
This study found that ILTPs had little effect on normal lung tissue cells cultured in vitro but could inhibit cancer cell proliferation and cause A549 cells to undergo apoptotic death. ILTPs also played a role in regulating lung cancer cell apoptosis at the molecular level by regulating the expression of the PI3K/Akt signaling pathway. Five important polyphenol compounds contained in ILTPs contributed to these effects. The present study only preliminarily verified the effect and mechanism of ILTPs on lung cancer through in vitro experiments, and the effects and mechanisms that play a role in animals need to be explored in further studies.
Supplementary Information
Additional file 1. Original strip of PI3K.
Additional file 2. Original strip of Akt.
Additional file 3. Original strip of β-actin.
Acknowledgements
Not applicable.
Abbreviations
- ILTPs
Ilex latifolia Thunb. polyphenols
- PI3K
phosphatidylinositol 3-kinase
- Akt
protein kinase B
- MTT
3-(4,5-dimethylazol-2-yl)-2,5-diphenyltetrazolium bromide
- qPCR
quantitative polymerase chain reaction
- HPLC
high-performance liquid chromatography
- LDH
lactate dehydrogenase
- ROS
reactive oxygen species
- mTOR
mammalian target of rapamycin
- Bcl-2
B-cell lymphoma-2
- NF-κB
nuclear factor-κB
- VEGF
vascular endothelial growth factor
- HIF-1α
hypoxia-inducible factor-1 alpha
- COX-2
cyclooxygenase-2.
Authors’ contributions
JC and YD performed the majority of the experiments and wrote the manuscript; YL, DT, MH, YJ and YW contributed to the data analysis; DY designed and supervised the study, and checked the final manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by Chongqing Science and Technology Commission Technology Innovation and Application Demonstration Project (cstc2018jscx-msybX0153), China.
Availability of data and materials
The western blot original strip in the supplementary material (Additional files 1, 2 and 3).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Chen and Yesheng Du contributed equally.
References
- 1.Fan J, Wu Z, Zhao T, Sun Y, Ye H, Xu R, Zeng X. Characterization, antioxidant and hepatoprotective activities of polysaccharides from Ilex latifolia Thunb. Carbohydr Polym. 2014;101:990–997. doi: 10.1016/j.carbpol.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Xu LJ, Ma GZ, Dong YM, Peng Y, Xiao PG. The large-leaved Kudingcha (Ilex latifolia Thunb and Ilex kudingcha C.J. Tseng): a traditional Chinese tea with plentiful secondary metabolites and potential biological activities. J Nat Med. 2013;67:425–437. doi: 10.1007/s11418-013-0758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Sun Y, Laura T, Liang X, Ye H, Zeng X. Determination of polyphenolic content and antioxidant activity of kudingcha made from Ilex kudingcha C.J. Tseng. Food Chem. 2009;112:35–41. doi: 10.1016/j.foodchem.2008.05.038. [DOI] [Google Scholar]
- 4.Kuroda Y, Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res. 1999;436:69–97. doi: 10.1016/s1383-5742(98)00019-2. [DOI] [PubMed] [Google Scholar]
- 5.Global Burden of Disease. Cancer Collaboration (2021) Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019 A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol Published online. 2019. 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed]
- 6.Zhao J, Kim JE, Reed E, Li OQ. Molecular mechanism of antitumor activity of taxanes in lung cancer (Review) Int J Oncol. 2005;27:247–256. [PubMed] [Google Scholar]
- 7.Fabiani R, Minelli L, Rosignoli P. Apple intake and cancer risk: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2016;19:2603–2617. doi: 10.1017/S136898001600032X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haider S, Li Z, Lin H, Jamil K. Optimization of preparative separation and purification of total polyphenols from Sargassum tenerrimum by column chromatography. J Ocean Univ China. 2009;8:425. doi: 10.1007/s11802-009-0425-x. [DOI] [Google Scholar]
- 9.Vara JAF, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene. 2008;27:5511–5526. doi: 10.1038/onc.2008.246. [DOI] [PubMed] [Google Scholar]
- 12.Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim HJ, Crowe P, Yang JL. Current clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment of human cancer. J Cancer Res Clin Oncol. 2015;141:671–689. doi: 10.1007/s00432-014-1803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu T, Park ES, Song GJ, Zhao X, Yi RK, Park KY. Kimchi markedly induces apoptosis in HT-29 human colon carcinoma cells. J Food Biochem. 2021;45:e13532. doi: 10.1111/jfbc.13532. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Tan F, Liu X, Yi R, Zhao X. Exploring the antioxidant effects and periodic regulation of cancer cells by polyphenols produced by the fermentation of grape skin by Lactobacillus plantarum KFY02. Biomolecules. 2019;9:575. doi: 10.3390/biom9100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Xu D, Zhang X, Zhao H. Protective effect of lemon peel polyphenols on oxidative stress-induced damage to human keratinocyte HaCaT cells through activation of the Nrf2/HO-1 signaling pathway. Front Nutr. 2021;7:606776. doi: 10.3389/fnut.2020.606776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi R, Deng L, Mu J, Li C, Tan F, Zhao X. The impact of Antarctic ice microalgae polysaccharides on D-galactose-induced oxidative damage in mice. Front Nutr. 2021;8:651088. doi: 10.3389/fnut.2021.651088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Wu X, Jin X, Wang J, Togo Y, Suzuki T, Hashimoto T, Yamada Y, Nakanishi Y, Kanematsu A, Nojima M, Kakehi Y, Yamamoto S. Enhancement of death receptor 4-mediated apoptosis and cytotoxicity in renal cell carcinoma cells by anisomycin. Anti-Cancer Drugs. 2017;28:180–186. doi: 10.1097/CAD.0000000000000450. [DOI] [PubMed] [Google Scholar]
- 19.Jurisic V. Estimation of cell membrane alteration after drug treatment by LDH release. Blood. 2003;101:2894. doi: 10.1182/blood-2002-08-2397. [DOI] [PubMed] [Google Scholar]
- 20.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 22.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC, Donia M, Fagone P, Malaponte G, Nicoletti F, Libra M, Milella M, Tafuri A, Bonati A, Bäsecke J, Cocco L, Evangelisti C, Martelli AM, Montalto G, Cervello M, McCubrey JA. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging. 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yothaisong S, Dokduang H, Techasen A, Namwat N, Yongvanit P, Bhudhisawasdi V, Puapairoj A, Riggins GJ, Loilome W. Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumour Biol. 2013;34:3637–3648. doi: 10.1007/s13277-013-0945-2. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka T, Yashiro M. The role of PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers. 2014;6:1441–1463. doi: 10.3390/cancers6031441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umoh NA, Walker RK, Millis RM, Al-Rubaiee M, Gangula PR, Haddad GE. Calcitonin gene-related peptide regulates cardiomyocyte survival through regulation of oxidative stress by PI3K/Akt and MAPK signaling pathways. Ann Clin Exp Hypertens. 2014;2(1):1007. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B, Zhao Z, Meng X, Chen H, Fu G, Xie K. Hydrogen ameliorates oxidative stress via PI3K-Akt signaling pathway in UVB-induced HaCaT cells. Int J Mol Med. 2018;41:3653–3661. doi: 10.3892/ijmm.2018.3550. [DOI] [PubMed] [Google Scholar]
- 28.Zeng KW, Wang XM, Ko H, Kwon HC, Cha JW, Yang HO. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid β-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur J Pharmacol. 2011;672:45–55. doi: 10.1016/j.ejphar.2011.09.177. [DOI] [PubMed] [Google Scholar]
- 29.Badr G, Saad H, Waly H, Hassan K, Abdel-Tawab H, Alhazza IM, Ahmed EA. Type I interferon (IFN-alpha/beta) rescues B-lymphocytes from apoptosis via PI3Kdelta/Akt, Rho-A, NFkappaB and Bcl-2/Bcl(XL) Cell Immunol. 2010;263:31–40. doi: 10.1016/j.cellimm.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Yuan J, Deng Y, Zhang Y, Gan X, Gao S, Hu H, Hu S, Hu J, Liu H, Li L, Wang J. Bmp4 inhibits goose granulosa cell apoptosis via PI3K/AKT/Caspase-9 signaling pathway. Anim Reprod Sci. 2019;200:86–95. doi: 10.1016/j.anireprosci.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Tsai JL, Lee YM, Pan CY, Lee AYL. The novel VEGF121-VEGF165 fusion attenuates angiogenesis and drug resistance via targeting VEGFR2-HIF-1α-VEGF165/Lon signaling through PI3K-AKT-mTOR pathway. Curr Cancer Drug Targets. 2016;16:275–286. doi: 10.2174/156800961603160206125352. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Xiong Z, Wei T, Li Q, Tan Y, Ling L, Feng X. Nuclear factor 90 promotes angiogenesis by regulating HIF-1α/VEGF-A expression through the PI3K/Akt signaling pathway in human cervical cancer. Cell Death Dis. 2018;9:276. doi: 10.1038/s41419-018-0334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nillesen ST, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123–1131. doi: 10.1016/j.biomaterials.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 34.Xia S, Zhao Y, Yu S, Zhang M. Activated PI3K/Akt/COX-2 pathway induces resistance to radiation in human cervical cancer HeLa cells. Cancer Biother Radiopharm. 2010;25:317–323. doi: 10.1089/cbr.2009.0707. [DOI] [PubMed] [Google Scholar]
- 35.Dixit S. Anticancer Effect of rutin isolated from the methanolic extract of Triticum aestivum straw in mice. Med Sci. 2014;2:153–160. doi: 10.3390/medsci2040153. [DOI] [Google Scholar]
- 36.Yang J, Xiao P, Sun J, Guo L. Anticancer effects of kaempferol in A375 human malignant melanoma cells are mediated via induction of apoptosis, cell cycle arrest, inhibition of cell migration and downregulation of m-TOR/PI3K/AKT pathway. J Buon. 2018;23:218–223. [PubMed] [Google Scholar]
- 37.Wang Q, Xiao L. Isochlorogenic acid A attenuates acute lung injury induced by LPS via Nf-κB/NLRP3 signaling pathway. Am J Transl Res. 2019;11:7018–7026. [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Huang K, Niu Z, Mei D, Zhang B. Protective effect of isochlorogenic acid B on liver fibrosis in non-alcoholic steatohepatitis of mice. Basic Clin Pharmacol Toxicol. 2019;124:144–153. doi: 10.1111/bcpt.13122. [DOI] [PubMed] [Google Scholar]
- 39.Yu JK, Yue CH, Pan YR, Chiu YW, Liu JY, Lin KI, Lee CJ. Isochlorogenic Acid C reverses epithelial-mesenchymal transition via down-regulation of EGFR Pathway in MDA-MB-231 cells. Anticancer Res. 2018;38:2127–2135. doi: 10.21873/anticanres.12453. [DOI] [PubMed] [Google Scholar]
- 40.Wu F, Chen J, Fan LM, Liu K, Zhang N, Li SW, Zhu H, Gao HC. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Exp Ther Med. 2017;14:127–130. doi: 10.3892/etm.2017.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen TTT, Tran E, Ong CK, Lee SK, Do PT, Huynh TT, Nguyen TH, Lee JJ, Tan Y, Ong CS, Huynh H. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol. 2003;197:110–121. doi: 10.1002/jcp.10340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Original strip of PI3K.
Additional file 2. Original strip of Akt.
Additional file 3. Original strip of β-actin.
Data Availability Statement
The western blot original strip in the supplementary material (Additional files 1, 2 and 3).