Abstract
Enterococcus faecalis BM4405 was resistant to low levels of vancomycin (MIC, 16 μg/ml) and was susceptible to teicoplanin (MIC, 0.5 μg/ml). No PCR product was obtained when the total DNA of this clinical isolate was used as a template with primers specific for glycopeptide resistance genes vanA, vanB, vanC, and vanD. However, a 604-bp PCR fragment was obtained when V1 and V2 degenerate primers were used and total DNA was digested with HindIII as a template. The product was cloned and sequenced. The deduced amino acid sequence had greater identity (55%) with VanC than with VanA (45%), VanB (43%), or VanD (44%). This was consistent with the fact that BM4405 synthesized peptidoglycan precursors that terminated in d-serine residues. After induction with vancomycin, weak d,d-dipeptidase and penicillin-insensitive d,d-carboxypeptidase activities were detected in cytoplasmic extracts of BM4405, whereas a serine racemase activity was found in the membrane preparation. This new type of acquired glycopeptide resistance was named VanE.
Glycopeptide antibiotics are used for the treatment of infections caused by gram-positive bacteria. They form a complex with the C-terminal d-alanyl-d-alanine (d-Ala-d-Ala) of peptidoglycan precursors and block their incorporation into the bacterial cell wall (15).
Glycopeptide-resistant enterococci have a broad geographical distribution and are phenotypically and genotypically heterogeneous. Three types of acquired resistance to glycopeptides have been described in enterococci (3). VanA-type strains display high-level inducible resistance to both vancomycin and teicoplanin following acquisition of transposon Tn1546 or closely related elements (2). VanB-type strains have variable levels of inducible resistance to vancomycin only (3). VanD-type strains are resistant to various levels of vancomycin and teicoplanin (14). In strains of all three phenotypes, glycopeptide resistance is due to synthesis of peptidoglycan precursors that terminate in d-lactate (d-Lac) instead of d-Ala (5).
The VanA, VanB, and VanD ligases synthesize the depsipeptide d-Ala-d-Lac. In VanA- and VanB-type strains three other enzymes are required for resistance: the VanH dehydrogenase reduces pyruvate to d-Lac (6), whereas the VanX d,d-dipeptidase and the penicillin G-insensitive VanY d,d-carboxypeptidase (3) prevent synthesis of UDP-MurNAc-l-Ala-d-Glu-l-Lys-d-Ala-d-Ala (pentapeptide[Ala]). The VanA and VanB types of resistance, but not the VanD type, are generally transferable to other enterococci by conjugation.
VanC-type resistance, characterized by low-level resistance to vancomycin, is specific to Enterococcus gallinarum, Enterococcus casseliflavus, and Enterococcus flavescens (11, 13). The vanC gene product synthesizes d-Ala-d-serine (d-Ala-d-Ser), which is substituted for d-Ala-d-Ala in peptidoglycan precursors (18). Insertional inactivation of vanC in E. gallinarum BM4174 led to vancomycin susceptibility and exclusive synthesis of precursors that end in acyl-d-Ala-d-Ala, indicating that intrinsically resistant enterococci also produce a d-Ala:d-Ala ligase and that vanC is necessary for expression of vancomycin resistance (7, 18). The level of synthesis of pentapeptide[Ala] in BM4174 is low due to the presence of weak d,d-dipeptidase and d,d-carboxypeptidase activities (18). It has been shown recently (i) that production in E. gallinarum BM4174 of VanXYc, a protein that has both d,d-dipeptidase and d,d-carboxypeptidase activities, allows hydrolysis of the dipeptide d-Ala-d-Ala and removal of the ultimate d-Ala from pentapeptide[Ala] and (ii) that the presence of vanC1 and vanXYc is sufficient for resistance when d-Ser is added to the culture medium of Enterococcus faecalis JH2-2 into which the two genes had been introduced (17). A membrane-bound serine racemase, VanT, which produces d-Ser peptidoglycan synthesis in BM4174 has also been characterized (1).
We present in this report evidence that E. faecalis BM4405, which is resistant to low levels of vancomycin and which is susceptible to teicoplanin, is of a new glycopeptide resistance type which has similarities with the intrinsic VanC type of resistance.
MATERIALS AND METHODS
Strains.
E. faecalis BM4405 was isolated from the peritoneal dialysis fluid of a patient in a Chicago, Ill., hospital who was diagnosed with peritonitis and who had received vancomycin. E. faecalis JH2-2 is a derivative of strain JH-2, which is resistant to fusidic acid and rifampin (10).
Antibiotic susceptibility testing.
MICs were determined by the agar dilution method on Mueller-Hinton agar (Diagnostics Pasteur, Marnes-la-Coquette, France) with an inoculum of 104 CFU per spot. Plates were incubated overnight at 37°C.
Filter mating.
Transfer of vancomycin resistance from BM4405 to the recipient strain E. faecalis JH2-2 was attempted by filter mating (6) with selection on brain heart infusion (BHI) containing rifampin (20 μg/ml), fusidic acid (10 μg/ml), and spectinomycin (60 μg/ml) or vancomycin (4 μg/ml).
DNA amplification, cloning, and sequencing.
Identification of strain BM4405 to the species level was performed by PCR (8). A PCR assay with primers specific for resistance genes vanA, vanB, vanC1, vanC2, and vanD (8, 14) was used to determine the glycopeptide resistance genotype of E. faecalis BM4405. Amplifications were carried out in a final volume of 100 μl containing 40 pmol of each oligonucleotide primer, 50 nmol of each 2′-5′-triphosphate deoxynucleoside, 100 ng of template DNA, and 2 U of Taq DNA polymerase. The reactions were performed in a DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.) as described previously (8, 14). Amplification of fragments internal to genes encoding related ligases with degenerate V1 and V2 primers was performed as described previously (7). Prior to amplification, total DNA from BM4405 was digested for 2 h at 37°C with HindIII (Pharmacia LKB, Saint-Quentin-en-Yvelines, France). PCR fragments were purified on Microspin S-400 HR Columns (Pharmacia LKB), cloned into pCR2.1 (Invitrogen, Leek, The Netherlands), and sequenced by the dideoxynucleotide chain termination method (19) with T7 DNA polymerase (Pharmacia LKB) and α-35S-dATP (Amersham Radiochemical Center). Specific amplification of a 513-bp fragment internal to vanE was obtained with primers VANE1 (5′-TGTGGTATCGGAGCTGCAG-3′) and VANE2 (5′-GTCGATTCTCGCTAATCC-3′) under the following conditions: 30 s at 94°C, 30 s at 52°C, and 30 s at 72°C (30 cycles).
Sequence analysis.
The programs included in the GCG package (Genetics Computer Group, Madison, Wis.) were used for sequence editing, translation, and alignment.
Analysis of peptidoglycan precursors.
Analysis of the peptidoglycan precursors of BM4405 was performed as described previously (16). The strain was grown in BHI with or without vancomycin (8 μg/ml) to the midexponential phase (A600 = 1). Ramoplanin was added, and incubation was continued for 20 min. The bacteria were harvested by centrifugation (12,000 × g, 2 min, 4°C), and the cytoplasmic precursors which had accumulated were extracted with 8% trichloroacetic acid (15 min, 4°C), desalted, and analyzed by high-performance liquid chromatography.
d,d-Dipeptidase and d,d-carboxypeptidase activities.
The enzymatic activities were assayed in BM4405 extracts as described previously (4). The d-Ala released from d-Ala-d-Ala by d,d-peptidase and from pentapeptide terminating in D-Ala (pentapeptide[Ala]) by d,d-carboxypeptidase was determined by using d-amino acid oxidase (12). Bacteria were lysed by treatment with lysozyme (2 mg/ml) at 37°C, followed by sonication, and the membrane fraction was pelleted (100,000 × g, 45 min). Activities were measured in the supernatant and in the resuspended pellet fraction.
Serine racemase activity.
The assay of serine racemase was carried out as described previously (1). Cell fractions were prepared by osmotic lysis of bacteria treated with lysozyme (400 μg ml−1) and M1 muramidase (70 μg ml−1). The membrane fraction was separated from the cytoplasm by centrifugation at 100,000 × g for 45 min.
Nucleotide sequence accession number.
The sequence was submitted to GenBank and was assigned accession no. AF136925.
RESULTS AND DISCUSSION
Characterization of strain BM4405.
Clinical isolate E. faecalis BM4405 was resistant to low levels of vancomycin (MIC, 16 μg/ml) but was susceptible to teicoplanin (MIC, 0.5 μg/ml); it was also resistant to spectinomycin (MIC, 64 μg/ml). To identify BM4405 to the species level, primers specific for genes encoding d-Ala:d-Ala ligases in enterococci were used (8), and an amplification product was obtained with the primer pair specific for the E. faecalis ddl gene. To determine the glycopeptide resistance genotype of BM4405 we used primers specific for resistance genes vanA, vanB, vanC1, vanC2, and vanD (8, 14), but no PCR product was obtained. Furthermore, in Southern blots with nonstringent washing conditions, none of the probes specific for these resistance genes hybridized with total DNA from BM4405. These results indicate that BM4405 is an E. faecalis strain with a newly acquired glycopeptide resistance genotype.
Determination of the glycopeptide resistance genotype of BM4405.
The degenerate primers V1 and V2, which allow amplification of fragments internal to genes that encode related ligases (7), were used in a PCR with total DNA of BM4405 as the template. A ca. 600-bp fragment was obtained, cloned into Escherichia coli, and sequenced. The 10 clones analyzed exhibited a sequence identical to that of part of the E. faecalis ddl ligase gene. E. faecalis ddl has a HindIII restriction site which is absent from the vanA, vanB, vanC, and vanD ligase resistance genes. After digestion of total BM4405 DNA with HindIII, a PCR with V1 and V2 primers was performed and a ca. 600-bp fragment was obtained with low efficiency. This PCR product was digested with HindIII, to eliminate putative partial digests of total DNA, and a second PCR was performed under the same conditions. The reaction resulted, with high efficiency, in the production of a ca. 600-bp fragment which was cloned, and both strands were sequenced (Fig. 1). The deduced amino acid sequence was compared with those of the host d-Ala:d-Ala ligase, the d-Ala:d-Lac ligases of VanA, VanB, and VanD strains, and the VanC1 d-Ala:d-Ser ligase (Fig. 2); the percentages of identity were calculated from this alignment (Table 1). The sequence obtained had a higher degree of identity with the corresponding portion of VanC1 (55%) than with that of VanA, VanB, or VanD (from 43 to 45%). The motifs conserved in the related ligases were present, and of the four amino acids that are invariably present in the VanC-type d-Ala:d-Ser ligases (EKYQ, at positions 142 to 145 [9]; Fig. 2 numbering), three (EKY) were conserved. The 604-bp product used as a probe in a Southern blot under stringent binding and washing conditions hybridized with total DNA from BM4405 but not with total DNA from E. faecium BM4147 (VanA), E. faecalis V583 (VanB), E. gallinarum BM4174 (VanC), or E. faecium BM4339 (VanD) (data not shown). These results confirm that the 604-bp fragment is internal to a new glycopeptide resistance gene that we have called vanE. Primers specific for the new gene were synthesized and used in an attempt to amplify fragments internal to genes encoding related ligases from Enterococcus strains BM4147 (VanA), V583 (VanB), BM4174 (VanC), BM4339 (VanD), and BM4405. A PCR fragment of the expected size of 513 bp was obtained only with BM4405 DNA, and direct sequencing of this product confirmed that the oligonucleotides were specific for vanE. The 513-bp fragment hybridized only with BM4405 DNA (data not shown).
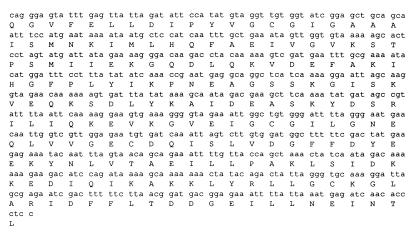
FIG. 1.
Sequence of the 604-bp PCR product internal to the vanE gene of E. faecalis BM4405 and the corresponding amino acid sequence.
FIG. 2.
Alignment of the deduced partial amino acid sequence of VanE and of the corresponding regions of VanA, VanB, VanC, VanD, and Efa, the d-Ala:d-Ala ligase of E. faecalis. The amino acids conserved in all the sequences are printed in boldface. Dots indicate gaps introduced to optimize sequence similarity. The conserved motif EKY(Q/N) present in VanC-type and VanE d-Ala:d-Ser ligases is underlined. GenBank accession numbers are as follows: X56895 for VanA, U35369 for VanB, M75132 for VanC, AF130997 for VanD, AF136925 for VanE, and U00457 for the d-Ala:d-Ala ligase for E. faecalis.
TABLE 1.
Sequence identity between the deduced amino acid sequence of VanE and related ligases
| Sequence compared | % Sequence identity
|
||||
|---|---|---|---|---|---|
| VanE | VanC | VanA | VanB | VanD | |
| VanC | 55 | ||||
| VanA | 45 | 43 | |||
| VanB | 43 | 39 | 76 | ||
| VanD | 44 | 43 | 69 | 69 | |
| Efaa | 32 | 35 | 34 | 36 | 38 |
Efa, d-Ala:d-Ala ligase of E. faecalis.
Transfer of vancomycin resistance.
Attempts to transfer vancomycin resistance from BM4405 to E. faecalis JH2-2 by filter mating were unsuccessful. Only transfer of spectinomycin resistance, which is not associated with vancomycin resistance, was obtained.
Characterization of peptidoglycan precursors of BM4405.
To analyze the cytoplasmic peptidoglycan precursors, cultures of E. faecalis BM4405, grown with or without vancomycin (8 μg/ml), were incubated in the presence of ramoplanin to inhibit cell wall synthesis after formation of the precursors. The results obtained indicated that, in the absence of vancomycin, UDP-MurNAc-pentapeptide was the unique precursor synthesized, whereas after incubation with vancomycin, UDP-MurNAc-pentapeptide[Ser] and UDP-MurNAc-tetrapeptide were the main compounds produced (Table 2). These data indicate that resistance in BM4405 is inducible by vancomycin and are consistent with the finding that vanE is more closely related to vanC than to other ligase genes (vanA, vanB, and vanD). Incubation with vancomycin did not induce resistance to teicoplanin.
TABLE 2.
Peptidoglycan precursors synthesized by E. faecalis BM4405a
| Culture | % Synthesis
|
||
|---|---|---|---|
| UDP-MurNAc-tetrapeptide | UDP-MurNAc-pentapeptide[Ala] | UDP-MurNAc-pentapeptide[Ser] | |
| Uninduced | <1 | 100 | <1 |
| Induced (Vmb, 4 μg/ml) | 10 | <1 | 90 |
Cultures were incubated with ramoplanin to inhibit peptidoglycan synthesis for 20 min. Cell extracts were prepared, and precursors were analyzed by high-performance liquid chromatography (4). The individual precursors were characterized by amino acid analysis and mass spectroscopy (19).
Vm, vancomycin.
d,d-Dipeptidase and d,d-carboxypeptidase activities of E. faecalis BM4405.
d,d-Dipeptidase (VanX) and d,d-carboxypeptidase (VanY) hydrolyze the dipeptide d-Ala-d-Ala and remove the terminal d-Ala residue of precursors ending in acyl-d-Ala-d-Ala, respectively (3). After centrifugation at 100,000 × g the d,d-dipeptidase activity in the supernatant of lysed BM4405 that had been grown in the absence or in the presence (8 μg/ml) of vancomycin was assayed (Table 3). Cytoplasmic extracts from vancomycin-induced E. faecalis BM4405 possessed weak d,d-dipeptidase activity, comparable to that found in E. gallinarum BM4174 (VanC) (16), and no activity was detected in extracts from uninduced bacteria. No d,d-carboxypeptidase activity was found in membrane preparations of induced or uninduced cells, even in the absence of penicillin G in the assay (Table 3). However, weak d,d-carboxypeptidase activity was detected in cytoplasmic extracts of induced BM4405 cells. This activity, which was insensitive to penicillin G, could account for the presence of tetrapeptide peptidoglycan precursors. Interestingly, the localization of d,d-carboxypeptidase activity in the cytoplasm is similar to that in the VanC-type strain E. gallinarum BM4174 (18) but different from that of VanA- and VanB-type strains, in which VanY is predominantly membrane bound. It is possible that the d,d-peptidase and d,d-carboxypeptidase activities of BM4405 are encoded by a single gene, as in BM4174 (17).
TABLE 3.
Enzymatic activities in extracts from E. faecalis BM4405
| Vancomycin concn (μg/ml) | Activity (nmol min−1 mg of protein−1)
|
|||||
|---|---|---|---|---|---|---|
|
d,d-Dipeptidase
|
d,d-Carboxypeptidase
|
Serine racemase
|
||||
| Membrane fraction | Cytoplasmic extract | Membrane fraction | Cytoplasmic extract | Membrane fraction | Cytoplasmic extract | |
| 0 | <1 | <1 | <1 | <1 | 1 | <1 |
| 8 | 1 | 16 | 1 | 9 | 60 | <1 |
Serine racemase activity.
VanC-type resistance requires three proteins: VanC and VanXYc, which catalyze synthesis of d-Ala-d-Ser (18) and which eliminate precursors ending in d-Ala-d-Ala (17), and VanT, a membrane-bound serine racemase for production of d-Ser (1). BM4405 (VanE) synthesizes peptidoglycan precursors that end in d-Ala-d-Ser and therefore also requires a source of d-Ser. Serine racemase activity was present in the membrane fraction of BM4405 (Table 3), and the enzyme was inducible by vancomycin. The serine racemase activity was ca. 10-fold greater than that of BM4174, which could account for the higher ratio of pentapeptide[Ser]:tetrapeptide (9:1) present in cytoplasmic extracts of ramoplanin-inhibited BM4405 than in BM4174, for which the ratio was between 0.5:1 and 1:1 (data not shown). All the serine racemase activity was membrane bound, whereas alanine racemase activity was present almost exclusively in the cytoplasm (data not shown). The distribution of the alanine and serine racemases between the cytoplasm and the membrane fractions was identical in BM4405 (VanE) and BM4174 (VanC).
In conclusion, VanE-type glycopeptide resistance in E. faecalis BM4405 is due to synthesis of late peptidoglycan precursors ending in d-Ala-d-Ser. The VanC and VanE types of resistance are biochemically and phenotypically similar. The vanE gene cluster in BM4405 is under study.
ACKNOWLEDGMENT
This work was supported in part by a Bristol-Myers Squibb Unrestricted Biomedical Research Grant in Infectious Diseases.
REFERENCES
- 1.Arias C A, Martin-Martinez M, Blundell T L, Arthur M, Courvalin P, Reynolds P E. Characterization and modelling of VanT: a novel, membrane-bound, serine racemase from vancomycin-resistant Enterococcus gallinarum BM4174. Mol Microbiol. 1999;31:1653–1664. doi: 10.1046/j.1365-2958.1999.01294.x. [DOI] [PubMed] [Google Scholar]
- 2.Arthur M, Molinas C, Depardieu F, Courvalin P. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1993;175:117–127. doi: 10.1128/jb.175.1.117-127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur M, Reynolds P E, Courvalin P. Glycopeptide resistance in enterococci. Trends Microbiol. 1996;4:401–407. doi: 10.1016/0966-842X(96)10063-9. [DOI] [PubMed] [Google Scholar]
- 4.Arthur M, Depardieu F, Reynolds P, Courvalin P. Quantitative analysis of the metabolism of soluble cytoplasmic peptidoglycan precursors of glycopeptide-resistant enterococci. Mol Microbiol. 1996;21:33–44. doi: 10.1046/j.1365-2958.1996.00617.x. [DOI] [PubMed] [Google Scholar]
- 5.Bugg T D H, Wright G D, Dutka-Malen S, Arthur M, Courvalin P, Walsh C T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry. 1991;30:10408–10415. doi: 10.1021/bi00107a007. [DOI] [PubMed] [Google Scholar]
- 6.Dutka-Malen S, Leclercq R, Coutant V, Duval J, Courvalin P. Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in gram-positive bacteria. Antimicrob Agents Chemother. 1990;34:1875–1879. doi: 10.1128/aac.34.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutka-Malen S, Molinas C, Arthur M, Courvalin P. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a d-alanine:d-alanine ligase-related protein necessary for vancomycin resistance. Gene. 1992;112:53–58. doi: 10.1016/0378-1119(92)90302-6. [DOI] [PubMed] [Google Scholar]
- 8.Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;30:1621–1624. doi: 10.1128/jcm.33.5.1434-1434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evers S, Casadewall B, Charles M, Dutka-Malen S, Galimand M, Courvalin P. Evolution of structure and substrate specificity in d-alanine:d-alanine ligases and related enzymes. J Mol Evol. 1996;42:706–712. doi: 10.1007/BF02338803. [DOI] [PubMed] [Google Scholar]
- 10.Jacob A E, Hobbs S J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974;117:360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leclercq R, Dutka-Malen S, Duval J, Courvalin P. Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob Agents Chemother. 1992;36:2005–2008. doi: 10.1128/aac.36.9.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messer J, Reynolds P E. Modified peptidoglycan precursors produced by glycopeptide-resistant enterococci. FEMS Microbiol Lett. 1992;94:195–200. doi: 10.1016/0378-1097(92)90608-q. [DOI] [PubMed] [Google Scholar]
- 13.Navarro F, Courvalin P. Analysis of genes encoding d-alanine:d-alanine ligase-related enzymes in Enterococcus casseliflavus and Enterococcus flavescens. Antimicrob Agents Chemother. 1994;38:1788–1793. doi: 10.1128/aac.38.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Périchon B, Reynolds P, Courvalin P. VanD-type glycopeptide-resistant Enterococcus faecium BM4339. Antimicrob Agents Chemother. 1997;41:2016–2018. doi: 10.1128/aac.41.9.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds P E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1989;8:943–950. doi: 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds P E, Depardieu F, Dutka-Malen S, Arthur M, Courvalin P. Glycopeptide resistance mediated by enterococcal transposon Tn1546 requires production of VanX for hydrolysis of d-alanyl-d-alanine. Mol Microbiol. 1994;13:1065–1070. doi: 10.1111/j.1365-2958.1994.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds, P. E., C. A. Arias, and P. Courvalin. Gene vanXYc encodes d,d-dipeptidase (VanX) and d,d-carboxypeptidase (VanY) activities in vancomycin-resistant Enterococcus gallinarum BM4174. Mol. Microbiol., in press. [DOI] [PubMed]
- 18.Reynolds P E, Snaith H A, Maguire A J, Dutka-Malen S, Courvalin P. Analysis of peptidoglycan precursors in vancomycin-resistant Enterococcus gallinarum BM4174. Biochem J. 1994;301:5–8. doi: 10.1042/bj3010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]