Abstract
The effects of the hematoregulatory peptide SK&F 107647 were examined in a persistently and profoundly neutropenic rabbit model of disseminated candidiasis in order to determine its potential to enhance resistance against infection and its role as an adjunct to conventional antifungal chemotherapy. In healthy animals, SK&F 107647 elicited a time-dependent increase in CD11b-positive monocytes and neutrophils. When administered to neutropenic rabbits infected with Candida albicans, no significant differences in the number of CFU per gram in any of the tissues tested compared with the number in untreated control rabbits were detected. However, when SK&F 107647 was administered in combination with low doses of amphotericin B, there was a significant reduction in organism burden in the lungs, liver, spleen, and kidneys compared with the burdens in the organs of untreated control animals and in the lungs and kidneys compared with the burdens in the lungs and kidneys of animals treated with amphotericin B alone. These data suggest a potential role for this peptide as adjunctive therapy in combination with conventional antifungal agents in the treatment of disseminated candidiasis in the setting of profound and persistent neutropenia.
Disseminated candidiasis is the most frequently occurring fungal infection in immunocompromised patients. Immunosuppression, whether as a consequence of disease or therapy, is the primary cause for the increased incidence of infections caused by Candida albicans. Fungal infections are often associated with a poor prognosis, especially in patients with prolonged periods of neutropenia (22). The administration of biological response modifiers may ameliorate immunosuppression, and such modifiers may serve as novel adjuncts to conventional antifungal chemotherapy (17, 20).
SK&F 107647, (S)-5-oxo-l-prolyl-l-α-glutamyl-l-α-aspartyl-N8(5-amino-1-carboxypentyl)-8-oxo-N7-[N-[N-(5-oxo-l-prolyl)-l-α-glutamyl]-l-α-aspartyl]-l-threo-2,7,8-triaminooctanoyl lysine, a novel synthetic dimeric pentapeptide, is closely related to the hematoregulatory peptide HP5B (11, 15). In place of a disulfide bridge, SK&F 107647 contains a nonreducible carbon bridge, lending greater stability to the molecule. It has been shown to be more potent than HP5B in stimulating endogenous hematopoietic colony-stimulating factors and has been shown to upregulate macrophage colony-stimulating factor (M-CSF) mRNA (11, 15). When administered subcutaneously, SK&F 107647 has been shown to protect rats from lethal sepsis caused by gram-negative and gram-positive bacteria (8) and to protect mice from lethal C. albicans and herpes simplex virus infections (6, 7). In order to establish an experimental basis for the rational use of this compound in granulocytopenic patients, we investigated the potential role of SK&F 107647 treatment alone and the role of SK&F 107647 as an adjunct with amphotericin B against disseminated candidiasis in profoundly and persistently granulocytopenic rabbits.
MATERIALS AND METHODS
Animals.
Pathogen-free female New Zealand White rabbits (weight, 2.0 to 3.0 kg; Hazleton, Rockville, Md.) were received at the Surgery Section of the Veterinary Resource Branch of the National Institutes of Health in Bethesda, Md. In order to provide for continued nontraumatic vascular access during the course of granulocytopenia, silastic central venous catheters were inserted into the rabbits by sterile technique and with the rabbits under general anesthesia as described previously (21). All rabbits were housed, fed, and treated according to National Institutes of Health guidelines for animal care and in fulfillment of the standards of the American Association for Accreditation of Laboratory Animal Care (5).
Immunosuppression.
Granulocytopenia (<500 granulocytes/μl) was induced and maintained by the administration of cytosine arabinoside (kindly provided by the Upjohn Company, Kalamazoo, Mich.) according to the following schedule: 440 mg/m2/day intravenously (i.v.) for days 1 to 5 for induction and then 440 mg/m2/day i.v. every other 2 days for maintenance of immunosuppression. Total granulocyte counts were monitored daily and were determined by the product of the total leukocyte (WBC) count and the percent granulocytes. Total WBC counts were determined with a Coulter Counter (Coulter Electronics, Inc., Hialeah, Fla.), and the percent granulocytes was determined by peripheral blood smear differential counts.
In order to prevent morbidity and mortality from bacterial infections, supportive treatment with parenteral antibiotics was begun on day 4 of chemotherapy and was continued throughout the course of granulocytopenia. Ceftazidime (150 mg/kg of body weight/day), vancomycin (15 mg/kg/day), and gentamicin (5 mg/kg every other day) were administered i.v.
Antifungal chemotherapy.
Amphotericin B desoxycholate (Bristol Myers-Squibb, Princeton, N.J.) was reconstituted with distilled water and was then diluted with 5% glucose to a 1-mg/ml concentration immediately prior to use. In rabbits receiving amphotericin B alone or amphotericin B in combination with SK&F 107647, treatment with a dosage of 0.1 mg/kg/day was begun 24 h postinoculation. Rabbits also received sterile normal saline for hydration.
A low dose of amphotericin B was used in these studies in order to determine whether the combination with SK&F 107647 would have an amphotericin B-sparing effect; i.e., the combination would permit the use of a lower dose of amphotericin B while maintaining efficiency. Previous studies using amphotericin B at 1 mg/kg/day resulted in marked reductions in the concentrations of C. albicans in tissue (23). Thus, such dosages would not permit assessment of the effects of combined immunomodulatory therapy.
Preparation of inoculum.
C. albicans NIH-86-21, originally isolated from a granulocytopenic patient with autopsy-proven disseminated candidiasis, was used for all experiments. Stock cultures of the isolate were maintained at −70°C in a skim milk suspension.
The C. albicans isolate was cultured for 24 h on Sabouraud dextrose agar and was then suspended into 50 ml of Sabouraud dextrose broth (National Institutes of Health Media Center) in a 250-ml Erlenmeyer flask. Flasks were incubated in a gyratory water bath at 37°C for 18 h. The inoculum suspension was centrifuged at 4,500 × g for 10 min, and the pellet was resuspended in sterile normal saline. Organisms were centrifuged and resuspended two more times. The concentration of the inoculum was adjusted by hemacytometer counts and was confirmed by quantitative cultures of 10-fold serial dilutions. An inoculum of 0.5 × 104 to 1 × 104 CFU was administered to each rabbit by i.v. infusion in 1 ml of sterile normal saline over 1 min.
Administration of SK&F 107647.
A biodegradable microsphere formulation of SK&F 107647 was used for these studies. The lyophilized peptide was reconstituted in phosphate-buffered saline and was administered subcutaneously to rabbits under light anesthesia. Each rabbit received a single subcutaneous dose of 80 μg/kg at 4 to 5 days preinoculation. This formulation has been shown to release drug slowly over an extended period (8). Control rabbits were given a subcutaneous injection of a similar volume of phosphate-buffered saline.
Assessment of SK&F 107647 immunoregulatory activity in rabbits.
Increases in positively staining CD11b monocytes and neutrophils were measured to confirm the activity of the compound in rabbits. The microsphere formulation of SK&F 107647 was administered as described above, and whole blood was collected on days 1, 4, and 7 following administration. WBCs were lightly fixed with 0.4% paraformaldehyde, followed by erythrocyte lysis with 0.83% ammonium chloride–0.01 M Tris HCl (pH 7.4). Cells were washed two times and were then incubated with 1 μg of fluorescein isothiocyanate-labelled rabbit anti-CD11b monoclonal antibodies (Spring Valley Labs, Sykesville, Md.) or isotype-matched control antibodies (mouse immunoglobulin G1; Immunotech, Miami, Fla.) for 30 min at 4°C in the dark. The cells were washed twice and were then resuspended in 0.5 ml of paraformaldehyde (2%) and analyzed with a fluorescence-activated cell sorter (FACScan; Becton-Dickinson) equipped with a 15-mW argon laser set at 488 nm.
Assessment of therapy with SK&F 107647. (i) Survival.
The duration of survival (in days postinoculation) was recorded for each rabbit. The surviving rabbits were euthanatized on day 7 postinoculation by i.v. pentobarbital anesthesia prior to autopsies.
(ii) Fungal cultures.
Representative sections of lung, liver, spleen, kidney, cerebrum, cerebellum, choroid, and vitreous were weighed and were then homogenized in sterile reinforced polyethylene bags (Tekmar Corp., Cincinnati, Ohio) with a Tekmar Stomacher 80 as described previously (24). Each tissue homogenate was serially diluted in sterile normal saline. A 0.1-ml aliquot of undiluted homogenate and of each dilution was plated separately onto Sabouraud dextrose agar containing chloramphenicol and gentamicin. Culture plates were incubated at 37°C for 24 h, after which the numbers of CFU were counted. The number of CFU per gram of tissue was calculated for each organ. The method was sensitive for detection of ≥10 CFU/g.
(iii) Renal and hepatic function studies.
Serial samples of blood were drawn from all animals and the serum was collected in order to monitor renal and hepatic functions. Chemical analyses included determination of serum urea nitrogen, creatinine, potassium, aspartate aminotransferase, and alkaline phosphatase levels (AniLytics, Gaithersburg, Md.).
Statistical analysis.
Survival curves were estimated by the Kaplan-Meier method, and differences were analyzed by the Mantel-Haenszel chi-square test. Comparisons of numerical variables between means were performed by the Mann-Whitney test and by Student’s t test. All analyses were two-sided, and a P value of ≤0.05 was considered significant. All values are expressed as means ± standard errors of the means.
RESULTS
Effects of SK&F 107647 on CD11b-positive WBCs.
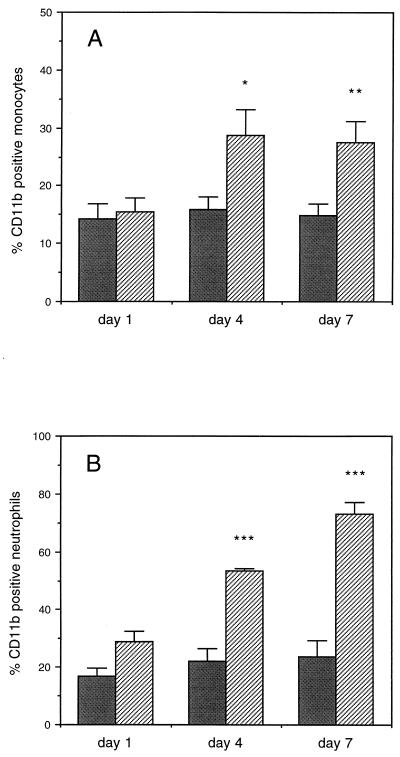
In order to determine if SK&F 107647 was active in the rabbit model, changes in the percentage of CD11b-positive cells were monitored following subcutaneous administration of the peptide. As shown in Fig. 1, there was an increase in the percentage of CD11b-positive monocytes that approached significance by day 4 (P = 0.06) and that reached significance by day 7 (P = 0.03). There was a significant increase in CD11b-positive neutrophils by day 4 after administration of the peptide (P < 0.01).
FIG. 1.
Effects of SK&F 107647 treatment on the percentage of CD11b-positive WBCs. The percentage of CD11b-positive cells was determined with monocytes (A) and neutrophils (B) isolated from blood collected on days 1, 4, and 7 by using fluorescein isothiocyanate-labelled anti-CD11b monoclonal antibodies. ∗, P = 0.06; ∗∗, P = 0.03; ∗∗∗, P < 0.01.  , control; ▨, SK&F 107647.
, control; ▨, SK&F 107647.
Effects of SK&F 107647 administration alone and in combination with amphotericin B.
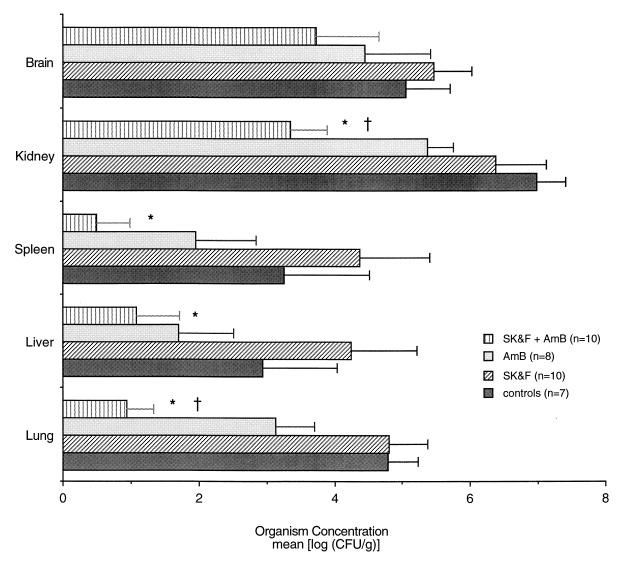
As shown in Fig. 2, there was a significant decrease in organism burdens in the kidneys, spleen, liver, and lungs of animals receiving combination therapy with SK&F 107647 and amphotericin B compared with the burdens in the tissues of untreated control animals (P ≤ 0.01). Additionally, there was a significant reduction in organism burdens in the kidneys and lungs of animals receiving the combination therapy compared with those in the kidneys and lungs of animals receiving amphotericin B alone (P ≤ 0.01). These data were generated from three separate experiments with a total of 7 to 10 rabbits in each group. All animals treated with amphotericin B alone or in combination with SK&F 107647 survived, while control animals began dying on day 2 postinoculation and animals treated with SK&F 107647 alone began dying on day 4 postinoculation. Although there was an increase in the rate of survival in the initial 5 days of infection for the rabbits receiving SK&F 107647 alone compared with the rate for control animals, animals receiving SK&F 107647 alone along with control animals were all dead by day 7 postinoculation. Throughout these experiments, circulating granulocyte counts were monitored with a Coulter Counter, and differential counts were determined by examining peripheral blood smears. Increases in the absolute neutrophil counts and the total WBC counts were observed in the SK&F 107647-treated rabbits (those treated with SK&F 107647 alone and in combination with amphotericin B), although these trends did not reach statistical significance (data not shown).
FIG. 2.
Effects of combination therapy with SK&F 107647 (SK&F) and amphotericin B (AmB) on organism burden in tissues of animals infected with C. albicans. Tissues were harvested from animals at the time of killing, and the mean log CFU per gram of tissue was calculated. Data were collected from three separate experiments with a total of 7 to 10 animals per group. There was a significant reduction in organism burden in the kidneys, spleen, liver, and lungs of animals receiving combination therapy with SK&F 107647 plus amphotericin B compared with the burdens in the tissues of untreated control animals. ∗, P ≤ 0.01. In addition, there was a significant reduction in organism burden in the kidneys and lungs of animals receiving combination therapy compared with the burden in the kidneys and lungs of those receiving amphotericin B alone. †, P ≤ 0.01.
In addition to the outcome variables described above, the overall well-being of the SK&F 107647-treated animals appeared to be enhanced, as measured most notably by increased activity. In order to determine if this difference was due to changes in renal or hepatic function, serum urea nitrogen, creatinine, potassium, alkaline phosphatase, and aspartate aminotransferase levels were obtained with sera collected at the baseline, 2 to 3 days postinfection, and 5 to 6 days postinfection. There were no significant differences in the number of animals with elevated levels or the degree of elevation between the SK&F 107647-treated rabbits and untreated control rabbits (data not shown).
DISCUSSION
We examined the efficacy of the immunomodulatory peptide SK&F 107647, alone and in combination with amphotericin B, in our rabbit model of disseminated candidiasis. When administered alone, we found a trend toward increased survival early in infection, although this trend was not statistically significant. However, while all animals treated with amphotericin B survived, combination therapy with low doses of amphotericin B and SK&F 107647 significantly reduced the organism burdens in the lungs and kidneys over those seen after treatment with amphotericin B alone.
The immunosuppressive regimen used in the animal model described here consistently results in persistent and profound neutropenia (<500 granulocytes/μl) that lasts for 2 to 3 weeks. This degree of immunosuppression provides a useful model for the study of potential therapeutic modalities for the treatment of infections in the neutropenic host (23). These models have especially been predictive of the clinical outcomes of treatments with antifungal agents, such as fluconazole and lipid formulations of amphotericin B, in neutropenic hosts.
In a murine model of acute systemic candidiasis, treatment with SK&F 107647 resulted in a decreased organism burden in the kidneys and a prolonged survival time (7). In our rabbit model, there was a trend toward increased survival that was evident only early in the course of infection. The lack of significant enhancement in survival may be due to the profound level of immunosuppression in the rabbit model or to the different routes of administration of the peptide in these two studies. Furthermore, the level of immunosuppression in the rabbits may negate any subtle immunologic enhancement obtained in a less compromised host.
Several mechanisms may contribute to the subtle anti-Candida effects of SK&F 107647. This peptide has been shown to upregulate production of cytokines such as M-CSF (15), which not only may increase hematopoiesis but which also has been shown to enhance the antifungal activity of mature effector cells (16). In addition, the increase in WBC counts in the SK&F 107647-treated rabbits may be involved in the enhanced organism clearance observed in these animals.
Patients receiving intensive cytotoxic chemotherapy for acute leukemia, lymphoma, or bone marrow transplantation are at high risk for the development of invasive fungal infections. In some institutions, these patients receive low dosages (0.1 to 0.3 mg/kg/day) of amphotericin B prophylactically for the prevention of such infections (14). Thus, we considered it clinically relevant to examine the combined effect of low-dose amphotericin B and SK&F 107647 in the rabbit model of disseminated candidiasis. While all animals treated with amphotericin B survived, there was a significant decrease in the organism burden in those animals treated with amphotericin B plus SK&F 107647.
Data from several laboratories have demonstrated an additive or synergistic interaction between conventional antifungal agents and recombinant cytokines (2, 4, 9, 10, 12, 13, 19, 20). In a murine model of disseminated candidiasis, Graybill et al. (10) showed improved survival and reduced organism burdens in the kidneys of animals treated with a combination of granulocyte colony-stimulating factor and fluconazole above those seen in animals treated with the antifungal agent alone. Interleukin-12 (4) and granulocyte colony-stimulating factor (9) have been shown to be efficacious in reducing the organism burdens in the brains when they are administered alone and in combination with fluconazole in experimental murine models of cryptococcosis. In a neutropenic mouse model, combination therapy with fluconazole and interleukin-1 was shown to significantly reduce the numbers of C. albicans organisms in the kidneys and spleens in comparison with those in the kidneys and spleens of animals treated with either agent alone (12). This is very similar to our current findings, in which combination therapy with amphotericin B and SK&F 107647 significantly reduced the number of C. albicans organisms in the kidneys and lungs in comparison with those in the kidneys and lungs of animals treated with either agent alone. In a neutropenic rabbit model of pulmonary aspergillosis, we have previously shown that M-CSF administered in combination with low doses of amphotericin B significantly improved the rate of survival over that observed following treatment with either agent alone (13).
The precise mechanisms involved in the enhancement of the collaborative effort between antifungal agents and effector cells are not clear. When effector cells are treated with cytokines their antifungal effects are enhanced (3). Pulmonary alveolar macrophages, Kupffer cells, splenic macrophages, and renal mesangial cells serve as potential effector cells in our persistently neutropenic rabbit model of disseminated candidiasis. In addition, exposure to amphotericin B is known to induce the release of tumor necrosis factor alpha and interleukin-6, which may interact synergistically with the cytokines induced by SK&F 107647 to alter the functions of these effector cells. Furthermore, immunostimulation may enhance the accumulation of the antifungal agent in effector cells, as has been demonstrated with antibacterial agents (1). Finally, it has been shown in vitro that C. albicans isolates with drug-induced alterations in their cell membranes are more susceptible to killing by cellular oxidative metabolites (18).
Immunomodulatory proteins do not have the same potency as antifungal agents. However, data that support the potential utility of adjunctive therapy with immunomodulators for the treatment of infectious complications in immunocompromised hosts continue to accumulate. Administration of a small-molecular-weight synthetic compound such as SK&F 107647, which may elicit the effects of multiple cytokines, offers a potential alternative to administration of a single cytokine. Our data suggest that this peptide, in conjunction with conventional antifungal therapy, may have an important role in augmenting the immune response to Candida infections in immunocompromised hosts.
ACKNOWLEDGMENTS
We thank Peter DeMarsh and Carrie Frey for support and helpful suggestions.
REFERENCES
- 1.Bermudez L E, Inderlied C, Young L S. Stimulation with cytokines enhances penetration of azithromycin into human macrophages. Antimicrob Agents Chemother. 1991;35:2625–2629. doi: 10.1128/aac.35.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodey G P, Anaissie E, Gutterman J, Vadhan-Raj S. Role of granulocyte-macrophage colony-stimulating factor as adjuvant therapy for fungal infection in patients with cancer. Clin Infect Dis. 1993;17:705–707. doi: 10.1093/clinids/17.4.705. [DOI] [PubMed] [Google Scholar]
- 3.Cenci E, Perito S, Enssle K H, Mosci P, Latge J P, Romani L, Bistoni F. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect Immun. 1997;65:564–570. doi: 10.1128/iai.65.2.564-570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemens K V, Brummer E, Stevens D A. Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob Agents Chemother. 1994;38:460–464. doi: 10.1128/aac.38.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 6.DeMarsh P L, Frey C L, Sucoloski S K, Henne S L, Barney S, Bhatnagar P K, Petteway S R. Program and abstracts of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1993. Efficacy of the hematoregulatory peptide SK&F 107647 in experimental herpes simplex type II infection, abstr. 395; p. 191. [Google Scholar]
- 7.DeMarsh P L, Sucoloski S K, Frey C L, Bhatnagar P K, Koltin Y, Actor P, Petteway S R. Efficacy of the hematoregulatory peptide SK&F 107647 in experimental systemic Candida albicans infections in normal and immunosuppressed mice. Immunopharmacology. 1994;27:199–206. doi: 10.1016/0162-3109(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 8.DeMarsh P L, Wells G I, Lewandowski T F, Frey C L, Bhatnagar P K, Ostovic E J. Treatment of experimental gram-negative and gram-positive bacterial sepsis with the hematoregulatory peptide SK&F 107647. J Infect Dis. 1996;173:203–211. doi: 10.1093/infdis/173.1.203. [DOI] [PubMed] [Google Scholar]
- 9.Graybill J R, Bocanegra R, Lambros C, Luther M F. Granulocyte colony stimulating factor therapy of experimental cryptococcal meningitis. J Med Vet Mycol. 1997;35:243–247. doi: 10.1080/02681219780001221. [DOI] [PubMed] [Google Scholar]
- 10.Graybill J R, Bocanegra R, Luther M. Antifungal combination therapy with granulocyte colony-stimulating factor and fluconazole in experimental disseminated candidiasis. Eur J Clin Microbiol Infect Dis. 1995;14:700–703. doi: 10.1007/BF01690878. [DOI] [PubMed] [Google Scholar]
- 11.King A G, Bhatnager P, Balcarek J, Pelus L M. Modulation of bone marrow stromal cell production of colony stimulation activity by the synthetic peptide, SK&F 107647. Abstr. Exp Hematol. 1991;19:481. [Google Scholar]
- 12.Kullberg B, Vant Wout J W, Poell R J, van Furth R. Combined effect of fluconazole and recombinant human interleukin-1 on systemic candidiasis in neutropenic mice. Antimicrob Agents Chemother. 1992;36:1225–1229. doi: 10.1128/aac.36.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyman C A, Gonzalez C, Lee S, DelGuercio C, Gehrt A, Peter J, Sein T, Schreurs Y, Pizzo P A, Walsh T J. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Combination therapy with human recombinant macrophage colony stimulating factor (M-CSF) and amphotericin B desoxycholate augments host defense against Aspergillus fumigatus, abstr. G70; p. 143. [Google Scholar]
- 14.O’Donnell M R, Schmidt G M, Tegtmeier B R, Faucett C, Fahey J L, Ito J, Nademanee A, Niland J, Parker P, Smith E P. Prediction of systemic fungal infection in allogeneic marrow recipients: impact of amphotericin prophylaxis in high-risk patients. J Clin Oncol. 1994;12:827–834. doi: 10.1200/JCO.1994.12.4.827. [DOI] [PubMed] [Google Scholar]
- 15.Pelus L M, King A G, Broxmeyer H E, DeMarsh P L, Petteway S R, Bhatnagar P K. In vivo modulation of hematopoiesis by a novel hematoregulatory peptide. Exp Hematol. 1994;22:239–247. [PubMed] [Google Scholar]
- 16.Roilides E, Lyman C A, Mertins S D, Cole D J, Venzon D, Pizzo P A, Chanock S J, Walsh T J. Ex vivo effects of macrophage colony-stimulating factor on human monocyte activity against fungal and bacterial pathogens. Cytokine. 1996;8:42–48. doi: 10.1006/cyto.1996.0006. [DOI] [PubMed] [Google Scholar]
- 17.Roilides E, Pizzo P A. Modulation of host defenses by cytokines: evolving adjuncts in prevention and treatment of serious infections in immunocompromised hosts. Clin Infect Dis. 1992;15:508–524. doi: 10.1093/clind/15.3.508. [DOI] [PubMed] [Google Scholar]
- 18.Shimokawa O, Nakayama H. Increased sensitivity of Candida albicans cells accumulating 14α-methylated sterols to active oxygen: possible relevance to in vivo efficacies of azole antifungal agents. Antimicrob Agents Chemother. 1992;36:1626–1629. doi: 10.1128/aac.36.8.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spielberger R T, Falleroni M J, Coene A J, Larson R A. Concomitant amphotericin B therapy, granulocyte transfusion, and GM-CSF administration for disseminated infection with Fusarium in a granulocytopenic patient. Clin Infect Dis. 1992;16:528–530. doi: 10.1093/clind/16.4.528. [DOI] [PubMed] [Google Scholar]
- 20.Stevens D A. Combination immunotherapy and antifungal chemotherapy. Clin Infect Dis. 1998;26:1266–1269. doi: 10.1086/516362. [DOI] [PubMed] [Google Scholar]
- 21.Walsh T J, Bacher J, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab Anim Sci. 1988;38:467–471. [PubMed] [Google Scholar]
- 22.Walsh T J, Hiemenz J W, Anaissie E. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect Dis Clin N Am. 1996;10:365–400. doi: 10.1016/s0891-5520(05)70303-2. [DOI] [PubMed] [Google Scholar]
- 23.Walsh T J, Lee J W, Roilides E, Francis P, Bacher J, Lyman C A, Pizzo P A. Experimental antifungal chemotherapy in granulocytopenic animal models of disseminated candidiasis: approaches to understanding investigational antifungal compounds for patients with neoplastic diseases. Clin Infect Dis. 1992;14(Suppl 1):S139–S147. doi: 10.1093/clinids/14.supplement_1.s139. [DOI] [PubMed] [Google Scholar]
- 24.Walsh T J, McEntee C, Dixon D M. Tissue homogenization for quantitative cultures of Candida albicans using sterile reinforced polyethylene bags. J Clin Microbiol. 1987;25:931–932. doi: 10.1128/jcm.25.5.931-932.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]