Abstract
Recent worldwide outbreak of SARS-COV-2 pandemic has increased the thirst to discover and introduce antiviral drugs to combat it. The bioactive compounds from plant sources, especially terpenoid have protease inhibition activities so these may be much effective for the control of viral epidemics and may reduce the burden on health care system worldwide. Present study aims the use of terpenoid from selected plant source through bioinformatics tools for the inhibition of SARS-COV-2. This study is based on descriptive analysis. The Protein Data Bank and PubChem database were used for the analysis of SARS-COV-2 protease and plant source terpenoids. Molecular docking by using molegro virtual docker (MVD) software was carried out. The findings of study are based on the inhibitory actions of different plant sourced terpenoid against SARS-COV-2. As per the available resources and complementary analysis these phytochemicals have capacity to inhibit 3CLpro protease. The study reports that (3,3-dimethylally) isoflavone (Glycine max), licoleafol (Glycyrrhiza uralensis), myricitrin (Myrica cerifera), thymoquinone (Nigella sativa), bilobalide, ginkgolide A (Ginkgo biloba), Salvinorin A (Salvia divinorum), citral (Backhousia citriodora) and prephenazine (drug) showed high activity against SARS-COV-2 protease 3CLpro. The drug like and ADMET properties revealed that these compounds can safely be used as drugs. Cross structural analysis by using bioinformatics study concludes that these plant source terpenoid compounds can be effectively used as antiprotease drugs for SARS-COV-2 in future.
Keywords: Phytochemicals, Terpenoid, Interaction, Antiproteases, COVID-19, ADMET, Drug development
1. Introduction
The recent outbreak of corona virus SARS-CoV-2 and its delta variant (Omicron) has proved to be a huge burden over the healthcare system of almost all countries. The spread of virus is quick through human to human transmission and no treatment has been found yet. The viral RNA genome of corona virus infects the new host cell and like messenger, directs the host cell to produce polyproteins to make replication machinery for new corona virus. SARS-CoV-2 genome produces a papain like cysteine protease (PLPro) and another 3-chymotrypsin like cysteine protease (3CLPro). Both the enzymes are responsible for proteolytic processing of viral proteins during its maturation (Chen et al., 2020a, Chen et al., 2020b, Krichel et al., 2020). These proteases convert polyproteins into functional ones and act like thievs inside the host cell. The dimer of functional subunits unites to produce its two active sites. Folding of this protein is just like serine proteases (trypsin) however, cysteine and nearby histidine amino acids act for the stabilization of dimer as well as protein cutting for functional unit formation. Phytochemicals and peptide like inhibitor may bind at active site of the dimmer (John et al., 2015, Cui et al., 2019).
The use of bioinformatics tools has revolutionized the search of new drugs as innovative approaches in early stage drug design and effectiveness study. Molecular docking of phytochemicals has opened new era for target point determination, modification and chemical stability studies (Mukesh and Rakesh, 2011, Grinter and Zou, 2014, Hilgenfeld, 2014). The basic strategy applicable now a days is the search of natural inhibitors instead of chemical formation against viral enzymes. The drugs obtained from natural compounds may have minimal side effects and effective inhibitory actions. The most targeted natural resources for such drug development are plants and microorganisms and most likely terpenoids due to low IC-50. More than 36,000 species of plant sourced terpenoid so far identified (Augustin et al. 2011). Plant alkaloids, flavonoids and terpenoids have shown numerous medicinal activities including antibacterial, antiviral, (Nosrati and Behbahani, 2015, Farooq et al., 2020) anti-cancer (Roy and Luck, 2007, Topcu et al., 2007), anti-oxidant (Ghaffar et al. 2015) and anti-inflammatory effect (del Carmen Recio et al., 1995, Lattanzio et al., 2011).
SARS-COV-2 viral genome variation and especially its delta variant has derived the drug discovery campaign to be targeted to its proteins used in replication and polyprotein processing to inhibit other structural proteins synthesis. Phytochemicals from selected medicinal plants may bind to important structural and functional proteins and interact with amino acids in active sites of its enzymes to inhibit replication and spread of SARS-COVID-19. Some initial studies on plant phytochemical have shown promising potential to inhibit SARS-COV-2 protease (Jo et al., 2020, ul Qamar et al., 2020, Federico et al., 2021, Liu et al., 2021, Hasan et al., 2022, Shree et al., 2022). Pakistani flora, especially in northern areas of Himalayan mountains are natural gifts which may be used to combat many diseases through herbal treatment. The objectives of current study are to investigate and explore phytochemicals from local plants which may be used in anti-SARS-COV-2 drug development.
2. Methods
The descriptive analytical approach was applied for this study and interaction of different terpenoid compounds were investigated by using bioinformatic approaches. Two and three dimensional structure of these phytochemicals, were identified by using PubChem database (https://pubchem.ncbi.nlm.nih.gov) (Lipinski CA (2004). The protease enzyme structure for said inhibition was obtained from PDB database (https://www.rcsb.org/) (Frimayanti et al., 2011, Liu et al., 2020).
2.1. Molecular docking
The molecular interaction of terpenoids used for inhibition of protease enzymes was studied by using molecular docking MVD software (https://omictools.com). The three dimensional observation facility in this software enables to observed the complete interaction upto amino acid level which may elaborate the formation of inhibitory complex. The following docking conditions for terpenoid compounds were studied here.
-
•
The number of interactions
-
•
Area of interaction
-
•
3CLpro protease
-
•
Rate of docking
-
•
Hydrogen bonding
-
•
Ester bonding
-
•
Electrostatic interactions
-
•
Bond energies
-
•
Amino acid binding energies
The interaction with 3CLpro cysteine protease active site was investigated by using all above mentioned parameters to compare the compound.
Drug likeliness characteristics of these phytochemicals were studied through Lipinski’s rule five on Molinspiration server (https://www.molinspiration.com). The phytochemical molecular structures were subjected to ADMET–SAR tool for determination of pharmaceutical and pharmacodynamic parameters as human intestinal absorption (HIA), aqueous solubility, Caco–2 permeability, blood–brain barrier penetration, cytochrome P450 inhibitory effect, renal cation transportation, fish, rat, AMES toxicity, human ether–ago–go–related gene inhibition, reproductive, mutagenic and tumorigenic risks were determined.
3. Results
The investigation of almost 1000 compounds for their inhibitory actions against 3- chymotrypsin-like cysteine protease (3CLPro) revealed nine important compounds to interact significantly with this protease. The study reports that (3,3-dimethylally) isoflavone (Glycine max), licoleafol (Glycyrrhiza uralensis), myricitrin (Myrica cerifera), thymoquinone (Nigella sativa), bilobalide, ginkgolide A (Ginkgo biloba), Salvinorin A (Salvia divinorum), citral (Backhousia citriodora) and prephenazine (drug) show high activity against flap regions of protease (Table 1). The binding energies calculated for these compounds is represented as total energy, and different bond energies including ester bond and hydrogen bonds (Table 2). Ester bond and H-bond energies demonstrated that these terpenoid compounds from different plant sources bind to 3CLpro. Plant phytochemicals showed important binding potential with several amino acids present in conserved regions of protease (Table 3). The important amino acids which interacted with these compounds included Val72, Lys73, Tyr135, Gly151, Cys144 and His-41. The interaction of these compounds with amino acids present in active site of this protease may change 3D structure to decrease catalytical activity. The binding of plant terpenoids to amino acids in viral proteins may inhibit the synthesis of its structural as well as functional proteins. These compounds also showed H-bonding with water (Table 4). The cavities that may be drug binding regions are presented in Fig. 1. The binding of plant terpenoids and protease CLpro active site is shown in Fig. 2.
Table 1.
Interaction of ligands with 3CLpro protease of SARS-COV-2.
| Sr. no | Compound name | Source | S-score | RMSD | 2-Structrue | Residues |
|---|---|---|---|---|---|---|
| 1 | (3,3-dimethylally) isoflavone | Glycine max | −7.1573 | 3.0013 |  |
Val72,Lys73,Tyr135, Gly151,Cys-144, His-41 |
| 2 | Licoleafol |
Glycyrrhiza uralen- sis |
−12.1018 | 2.5731 |  |
Val72,Lys73,Tyr135, Gly151,Cys-144, His-41 |
| 3 | Myricitrin | Myrica cerifera | −15.9059 | 2.6515 | 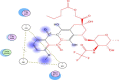 |
Val72,Lys73,Tyr135, Gly151,Cys-144, His-41 |
| 4 | Thymoquinone | Nigella sativa | −12811 | 1.4356 | 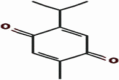 |
Asn23,Asp54, Gly151 |
| 5 | Salvinorin A | Salvia divinorum | −32181 | 3.4321 | 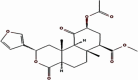 |
Lys73,Tyr135, Gly151,Cys-144, His-41 |
| 6 | Bilobalide | Ginkgo biloba | −43761 | 2.4321 | 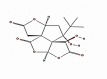 |
Val72,Lys73,Tyr135, Gly151,Cys-144, His-41 |
| 7 | Citral | Backhousia citriodora | −13421 | 1.3423 | Val72,Lys73,Tyr135, Gly151,Cys-144, His-41 | |
| 8 | Ginkgolide A | Ginkgo biloba | −65412 | 2.5412 |  |
Val72,Lys73,Tyr135, Gly151,Cys-144, His-41 |
| 9 | Prephenazine | Chemical drug | −10.8661 | 2.4656 |  |
Val72,Lys73,Tyr135, Gly151,Cys-144, His-41 |
Table 2.
Interactions of terpenoids as bond energies with 3CLpro protease.
| Sr. No | Compound name | Total Energy | Ester Bond | Hydrogen Bond | Electrostatic Bond |
|---|---|---|---|---|---|
| 1 | (3,3-dimethylally) isoflavone | −64 | −57 | −4 | 0 |
| 2 | Licoleafol | −85 | −101 | −6 | 0 |
| 3 | Myricitrin | −81 | −83 | −5 | 0 |
| 4 | Thymoquinone | −54 | −54 | −2.4 | 0 |
| 5 | Salvinorin A | −113 | −118 | −3 | 0 |
| 6 | Bilobalide | −98 | −94 | −6 | 0 |
| 7 | Citral | −85 | −98 | −5 | 0 |
| 8 | Ginkgolide A | −63 | −64 | 0 | 0 |
| 9 | Prephenazine | −67 | −60 | −1.4 | 0 |
Table 3.
The amino acid binding energies of phytochemicals with 3CLpro protease.
| AA | Arg | Asn | Asp | Asp | Cys | Cys | Gln | Gln | Ile | Ile | Lys | Phe | Phe | Phe | Pro | Ser | Thr | Thr | Val |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Residue ID | 105 | 151 | 153 | 295 | 156 | 160 | 107 | 110 | 106 | 152 | 102 | 8 | 112 | 294 | 293 | 158 | 111 | 292 | 104 |
| (3,3-dimethylally) isoflavone | −17 | −13 | −4 | −1.2 | −5.2 | −1.7 | −8 | −4.9 | −0.8 | −0.8 | |||||||||
| Licoleafol | −1.3 | −16 | −15 | −6.7 | −0.5 | −3.3 | −19 | −5 | −2 | −5.1 | –22 | −1.8 | −9.1 | 4.5 | |||||
| Myricitrin | −17 | −8.1 | −2.4 | −0.5 | −12 | 1.6 | −4.4 | −3.8 | −1 | −21 | −1.6 | −9.2 | −4.3 | −0.9 | |||||
| Thymoquinone | −17 | −14 | −0.5 | −0.7 | −5.6 | −1.2 | −6.9 | −6.2 | −0.5 | −0.9 | |||||||||
| Salvinorin A | −3.3 | −16 | −11 | −0.4 | −2.2 | −9.7 | −12 | −4.5 | −2.2 | −0.4 | −8.9 | −3.2 | 0.9 | −5.8 | |||||
| Bilobalide | −4.4 | −11 | −5.6 | −3.3 | −0.4 | −3.4 | −15 | −14 | −0.9 | −1.2 | −19 | −0.7 | −4 | −4.4 | −5.3 | −2.6 | |||
| Citral | −1 | −19 | −14 | −3.9 | −0.5 | −2.5 | −18 | −3 | −6.6 | −0.4 | −1.2 | −16 | −3.1 | −5.6 | −2.3 | −2.3 | |||
| Ginkgolide A | −15 | −13 | −0.6 | −0.4 | −2.7 | −4.4 | −2.4 | −17 | −3.3 | −0.7 | −0.8 | ||||||||
| Prephenazine | −12 | −12 | −2 | −2 | −4.8 | −2.7 | −15 | −1.2 | −6 | −0.9 | |||||||||
Table 4.
Hydrogen bond energies of phytochemical with water.
| Sr. No | HOH 408 | HOH 417 | HOH 440 | HOH 456 | HOH 463 | HOH 479 | |
|---|---|---|---|---|---|---|---|
| Residue ID | 7 | 16 | 39 | 55 | 62 | 78 | |
| 1 | (3,3-dimethylally) isoflavone | −4.2 | −3.8 | −1.8 | |||
| 2 | Licoleafol | −2.5 | −2.4 | 6.8 | −0.78 | −0.4 | −2 |
| 3 | Myricitrin | −1.6 | −1.5 | 7 | −0.4 | −5 | |
| 4 | Thymoquinone | −1.5 | −1.7 | ||||
| 5 | Salvinorin A | −7.2 | −7.3 | −3.8 | −0.7 | ||
| 6 | Bilobalide | −0.5 | −2.9 | −0.5 | 2.8 | −3 | −0.7 |
| 7 | Citral | −1.2 | −3.9 | −2.7 | −0.5 | −4.5 | −1.2 |
| 8 | Ginkgolide A | −1.9 | −1.6 | −1.1 | −1.6 | ||
| 9 | Prephenazine | −3.2 | −0.9 | 1.7 | −0.4 |
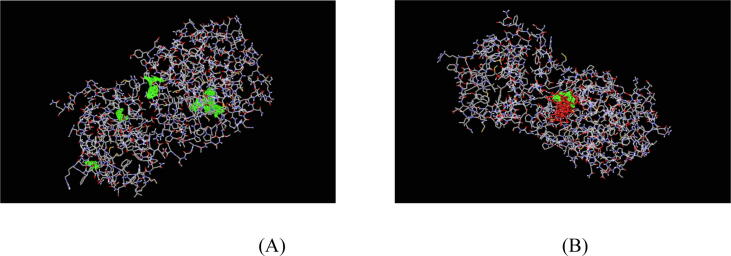
Fig. 1.
(A) Drug binding cavities of SARS-COV-2 CLpro protease. (B) Interactions among phytochemicals from selected plants with active site amino acids of SARS-COV-2 CLpro protease.
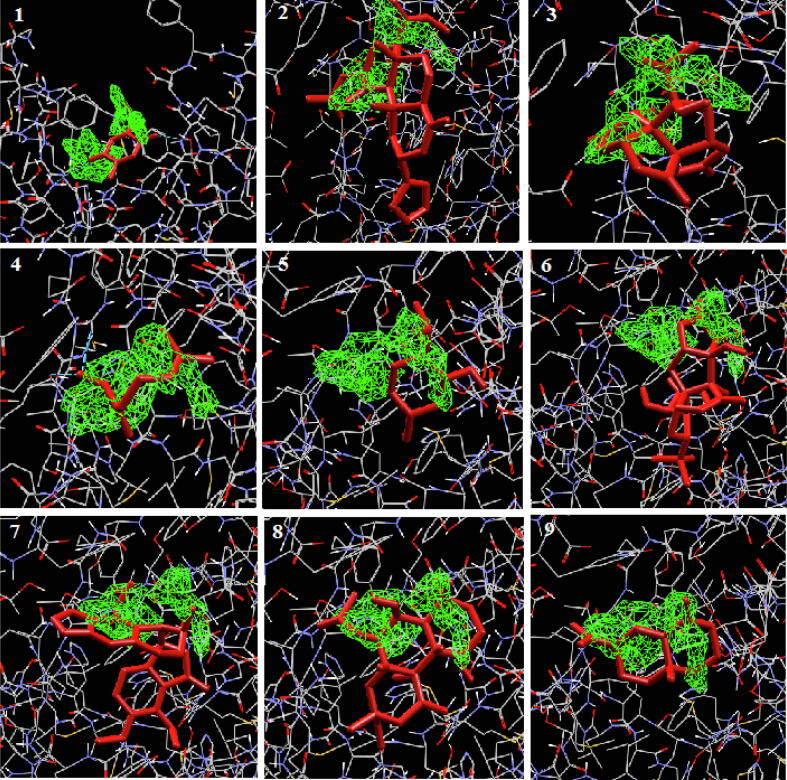
Fig. 2.
Binding of plant terpenoids with SARS-COV-2 CLpro protease. The compounds (3,3 dimethylally) isoflavone, licoleafol, myricitrin, prephenazine, thymoquinone, salvinorin a, bilobalide, citral, and ginkgolide A were docked with CLpro and the interactions are shown from 1 to 9.
Studies revealed that phytochemicals passed the Lipinski’s rule five without any violations (Table 5). The ADMET properties showed that these compound exhibit admissible properties for HIA, BBB penetration, Caco2 permeability, p-glycoprotein inhibitor and subcellular localization (Table 6). These findings have much importance in SARS-COV-2 drug development.
Table 5.
Lipinski’s rule five for some compounds of medicinal plants against COVID-19 3CLpro protease protein.
| Sr. No | Compound name | Molecular weight(g/mol) | MLogP | Number of HBA | Number of HBD |
|---|---|---|---|---|---|
| Lipinski’s rule five | <500 | <5 | <10 | <5 | |
| 1 | (3,3-dimethylally) isoflavone | 78.41 | 1 | 6 | 1 |
| 2 | Licoleafol | 290.24 | 2.68 | 6.25 | 4 |
| 3 | Myricitrin | 136.10 | 1.19 | 5.24 | 2.6 |
| 4 | Thymoquinone | 286.21 | 1.97 | 8.24 | 3.13 |
| 5 | Salvinorin A | 372.21 | 2.33 | 3 | 3.02 |
| 6 | Bilobalide | 100.03 | −1.20 | 7 | |
| 7 | Citral | 354.12 | 0.88 | 5.42 | 2.31 |
| 8 | Ginkgolide A | 372.72 | 2.12 | 6 | 4 |
| 9 | Prephenazine | 258.33 | 2.25 | 7 | 1 |
Table 6.
ADMET prediction profile of selected phytochemicals.
| Absorption |
Distribution |
||||||
|---|---|---|---|---|---|---|---|
| Sr. No | Compound name | P-glycoprotein inhibitor | P- glycoprotein substrate | Blood-brain barrier | Caco2 permeability | Human intestinal absorption | Subcellular localization |
| 1 | (3,3-dimethylally) isoflavone | Ni | S | BBB+ | Caco2+ | HIA+ | Mitochondria |
| 2 | Licoleafol | Ni | NS | BBB+ | Caco2+ | HIA+ | Mitochondria |
| 3 | Myricitrin | Ni | NS | BBB+ | Caco2+ | HIA+ | Mitochondria |
| 4 | Thymoquinone | Ni | NS | BBB+ | Caco2+ | HIA+ | Mitochondria |
| 5 | Salvinorin A | Ni | NS | BBB+ | Caco2+ | HIA+ | Mitochondria |
| 6 | Bilobalide | Ni | S | BBB+ | Caco2+ | HIA+ | Mitochondria |
| 7 | Citral | Ni | NS | BBB+ | Caco2+ | HIA+ | Mitochondria |
| 8 | Ginkgolide A | Ni | NS | BBB+ | Caco2+ | HIA+ | Mitochondria |
| 9 | Prephenazine | Ni | S | BBB+ | Caco2+ | HIA+ | Mitochondria |
| Metabolism | Excretion | ||||||
| Sr. No | Compound name | CYP450 3A4 inhibitor | CYP450 3A4 substrate | CYP450 2D6 inhibitor | CYP450 2D6 substrate | CYP450 2C9 inhibitor | ROCT |
| 1 | (3,3-dimethylally) isoflavone | Ni | NS | Ni | NS | NS | Ni |
| 2 | Licoleafol | Ni | NS | Ni | NS | NS | Ni |
| 3 | Myricitrin | Ni | NS | Ni | NS | NS | Ni |
| 4 | Thymoquinone | Ni | NS | I | NS | NS | Ni |
| 5 | Salvinorin A | Ni | NS | Ni | NS | NS | Ni |
| 6 | Bilobalide | Ni | NS | Ni | NS | NS | Ni |
| 7 | Citral | Ni | NS | Ni | NS | NS | Ni |
| 8 | Ginkgolide A | NI | NS | Ni | NS | NS | Ni |
| 9 | Prephenazine | Ni | NS | Ni | NS | NS | Ni |
| Toxicity | |||||||
| Sr. No | Compound name | Acute oral toxicity | Fish toxicity | Honeybee toxicity | AMES toxicity | Carcinogens | |
| 1 | (3,3-dimethylally) isoflavone | ɪɪ | HFHMT | HHBT | NT | NC | |
| 2 | Licoleafol | ɪ | HFHMT | HHBT | NT | NC | |
| 3 | Myricitrin | ɪɪ | HFHMT | HHBT | NT | NC | |
| 4 | Thymoquinone | ɪ | HFHMT | HHBT | NT | NC | |
| 5 | Salvinorin A | ɪ | HFHMT | HHBT | NT | NC | |
| 6 | Bilobalide | ɪɪ | HFHMT | HHBT | NT | NC | |
| 7 | Citral | ɪ | HFHMT | HHBT | NT | NC | |
| 8 | Ginkgolide A | ɪ | HFHMT | HHBT | NT | NC | |
| 9 | Prephenazine | ɪ | HFHMT | HHBT | NT | NC | |
4. Discussion
The SARS-COV-2 virus and its protease (3-chymotrypsin-like cysteine protease 3CLpro) structure were used in this study along with the inhibitory plant sourced terpenoid compounds. This viral enzyme plays pivotal role in its replication and protein processing as cysteine protease. The bond energy of hydrogen and easter bonding of these compounds to viral protease showed that it can effectively inhibit the enzyme through structural changes. Previous studies on phytochemical showed −8.7, −8.9, −8.9 KJ/mol binding energy of the selected ligands emodin 1-O-beta-D-glucoside, flemichin and delta-oleanolic acid, respectively (Mahmud et al. 2021). Cyanin as a phytochemical from Zingiber officinale showed wide range of potential to inhibit SARS-CoV-2 and MERS-CoV Mpro having binding energies of (−) 8.3 kcal/mol and (−) 7.7 kcal/mol, respectively (Nallusamy et al., 2021). Similar binding potential of withanoside V and somniferine has also been reported against 3CLpro and Mpro in another study (Shree et al., 2022). The present study shows five protected regions in enzymatic flap from which two flaps may be inhibited with these identified compounds.
The amino acids in protease and three active site amino acids showed binding with terpenoids from selected plants. These catalytical regions of enzyme were highly conserved as well so, by using these identified plant source drugs these amino acids could be handled. These compounds showed promising results for effective inhibition of amino acids in protease backbone as well as catalytic process of some amino acids present at active site. Previous studies have elaborated the potential of Rheum palmatum L. root and rhizome extracts to inhibit 3CLpro cysteine protease activity of SARS-COV-2 (Luo et al. 2009). Nine neutral bioactive compounds namely citral, bilobalide, salvinorin A, menthol, ginkgolide A, forscolin, thymoquinone, noscapine and ß-selinene were recognized as low risk inhibitors of COVID-19 protease activity (Andersen et al. 2010). In-vitro studies on Scutellaria baicalensis Georgi, a traditional Chinese medicinal plant has shown anti-SARS-CoV-2-3CLpro activity at 0.74 μg/ml EC50 value (Liu et al. 2020). Present study showed that selected phytochemical compounds bind to Val72, Lys73, Tyr135, Gly151, Cys144 and His-41 amino acids in 3CLpro protease of SARS-CoV-2. Three amino acids of protease active site bound to these phytochemicals effectively. Mahmud et al. (2021) reported that emodin 1-O- ß-D-glucoside, flemichin and delta-oleanolic acid bind to Mpro main protease active groove of SARS-CoV-2 at amino acids Cys145, Glu166, His41, Met49, Pro168, Met165 and Gln189. Phytochemicals of Malva neglecta Wallr had been evaluated through molecular docking to explore their potential for drug development against COVID-19 (Irfan et al., 2021). Active plant phytoconstituents Withania somnifera, Tinospora cordifolia and Ocimum sanctum have been predicted interact Mpro or 3CLpro proteaseof SARS-CoV-2. The ADMET studies through molecular docking of these phytochemicals were found to be safe (Shree et al., 2022). This suggests that the plant source compounds may efficiently be used to equip strategies for COVID-19 management as they have less side effects as well and have functional ability.
5. Conclusion
The docking studies of selected phytochemicals from medicinal plants have potential as anti-SARS-COV-2 activity for their interaction and binding to amino acids present in 3CLpro protease enzyme of the virus. These compounds are present in local plant species in appreciable amounts can be economically used for drug evelopment. Natural plant sources can also meet the need of bulk production through cheap source and the potential compounds may further be derivatized for more effective viral protein binding and crystalized to make effective oral drugs. It will boost local economy and save money which will be utilized against corona vaccines.
Funding
The authors of study acknowledge the Institute of Research and Consulting Studies at King Khalid University to provide financial support as research funding grant number 2-N-20/22. The authors also acknowledge Research Center for Advanced Materials Science, Saudi Arabia for research support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Andersen N.H., Christensen N.J., Lassen P.R., Freedman T.B., Nafie L.A., Strømgaard K., Hemmingsen L. Structure and absolute configuration of ginkgolide B characterized by IR-and VCD spectroscopy. Chirality: The Pharmacol. Biol. Chem Conseq. Mol. Asymmetr. 2010;22(2):217–223. doi: 10.1002/chir.20730. [DOI] [PubMed] [Google Scholar]
- Augustin J.M., Kuzina V., Andersen S.B., Bak S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry. 2011;72(6):435–457. doi: 10.1016/j.phytochem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.W., Yiu, C.P. B., Wong, K.Y., 2020b. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL pro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research. 9(129), 129. [DOI] [PMC free article] [PubMed]
- Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nature Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Carmen Recio M., Giner R.M., Máñez S., Ríos J.L. Structural requirements for the anti-inflammatory activity of natural triterpenoids. Planta Med. 1995;61(02):182–185. doi: 10.1055/s-2006-958045. [DOI] [PubMed] [Google Scholar]
- Farooq M.U., Munir B., Naeem S., Yameen M., Iqbal S.Z., Ahmad A., Mustaan M.A., Noor M.W., Nadeem A., Ghaffar A. Exploration of Carica papaya bioactive compounds as potential inhibitors of dengue NS2B, NS3 and NS5 protease. Pak. J. Pharm. Sci. 2020;33(Suppl 1):355–360. [PubMed] [Google Scholar]
- Federico L.B., Silva G.M., da Silva Hage-Melim L.I., Gomes S.Q., Barcelos M.P., Galindo Francischini I.A., de Paula T., da Silva C.H. Identification of known drugs as potential SARS-CoV-2 Mpro inhibitors using ligand-and structure-based virtual screening. Future Med. Chem. 2021;13(16):1353–1366. doi: 10.4155/fmc-2021-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimayanti N., Chee C.F., Zain S., Rahman N.A. Design of new competitive dengue Ns2b/Ns3 protease inhibitors-a computational approach. Int. J. Mol. Sci. 2011;12(2):1089–1100. doi: 10.3390/ijms12021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffar A., Yameen M., Kiran S., Kamal S., Jalal F., Munir B., Saleem S., Rafiq N., Ahmad A., Saba I., Jabbar A. Chemical composition and in-vitro evaluation of the antimicrobial and antioxidant activities of essential oils extracted from seven Eucalyptus species. Molecules. 2015;20(11):20487–20498. doi: 10.3390/molecules201119706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinter S.Z., Zou X. Challenges, applications, and recent advances of protein-ligand docking in structure-based drug design. Molecules. 2014;19(7):10150–10176. doi: 10.3390/molecules190710150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A., Biswas P., Bondhon T.A., Jannat K., Paul T.K., Paul A.K., Jahan R., Nissapatorn V., Mahboob T., Wilairatana P., Hasan M.N. Can Artemisia herba-alba be useful for managing COVID-19 and comorbidities? Molecules. 2022;27(2):492. doi: 10.3390/molecules27020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281(18):4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfan A., Imran M., Khalid N., Hussain R., Basra M.A.R., Khaliq T., Shahzad M., Hussien M., Shah A.T., Qayyum M.A., Al-Sehemi A.G. Isolation of phytochemicals from Malva neglecta Wallr and their quantum chemical, molecular docking exploration as active drugs against COVID-19. J. Saudi Chem. Soc. 2021;25(12) [Google Scholar]
- Jo S., Kim S., Shin D.H., Kim M.S., Kim M. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S.E.S., Tomar S., Stauffer S.R., Mesecar A.D. Targeting zoonotic viruses: Structure-based inhibition of the 3C-like protease from bat coronavirus HKU4-The likely reservoir host to the human coronavirus that causes Middle East Respiratory Syndrome (MERS) Bioorgan. Med. Chem. 2015;23(17):6036–6048. doi: 10.1016/j.bmc.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichel B., Falke S., Hilgenfeld R., Redecke L., Uetrecht C. Processing of the SARS-CoV pp1a/ab nsp7-10 region. Biochem. J. 2020;477:1009–1019. doi: 10.1042/BCJ20200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio F., Greco E., Carretta D., Cervellati R., Govoni P., Speroni E. In vivo anti-inflammatory effect of Rosa canina L. extract. J. Ethnopharmacol. 2011;137(1):880–885. doi: 10.1016/j.jep.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Lipinski C.A. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discov. Today: Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Liu H., Ye F., Sun Q., Liang H., Li C., Li S., Lai L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzym. Inhib. Med. Chem. 2021;36(1):497–503. doi: 10.1080/14756366.2021.1873977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Zhang, B., Jin, Z., Yang, H., Rao, Z., 2020. The crytal structure of 2019-NCOV main protease in complex with an inhibitor N3. RCSB Protein Data Bank.
- Luo W., Su X., Gong S., Qin Y., Liu W., Li J. Anti-SARS coronavirus 3C-like protease effects of Rheum palmatum L. extracts. Biosci. Trends. 2009;3:124–126. [PubMed] [Google Scholar]
- Mahmud S., Mita M.A., Biswas S., Paul G.K., Promi M.M., Afrose S., Saleh A. Molecular docking and dynamics study to explore phytochemical ligand molecules against the main protease of SARS-CoV-2 from extensive phytochemical datasets. Expert. Rev. Clin. Pharmacol. 2021;14(10):1305–1315. doi: 10.1080/17512433.2021.1959318. [DOI] [PubMed] [Google Scholar]
- Mukesh B., Rakesh K. Review on molecular docking. Int. J. Res. Ayurveda Pharm. 2011;2(6):1746–1751. [Google Scholar]
- Nosrati M., Behbahani M. Molecular docking study of HIV-1 protease with triterpenoides compounds from plants and mushroom. J. Arak Univ. Med. Sci. 2015;18(3):67–79. [Google Scholar]
- Nallusamy S., Mannu J., Ravikumar C., Angamuthu K., Nathan B., Nachimuthu K., Ramasamy G., Muthurajan R., Subbarayalu M., Neelakandan K. Exploring phytochemicals of traditional medicinal plants exhibiting inhibitory activity against main protease, Spike glycoprotein, RNA-dependent RNA polymerase and non-structural proteins of SARS-CoV-2 through virtual screening. Front. Pharmacol. 2021;12:1704. doi: 10.3389/fphar.2021.667704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy U., Luck L.A. Molecular modeling of estrogen receptor using molecular operating environment. Biochem. Mol. Biol. Edu. 2007;35:238–243. doi: 10.1002/bmb.65. [DOI] [PubMed] [Google Scholar]
- Shree P., Mishra P., Selvaraj C., Singh S.K., Chaube R., Garg N., Tripathi Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)–a molecular docking study. J. Biomol. Struct. Dyn. 2022;40(1):190–203. doi: 10.1080/07391102.2020.1810778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topcu G., Ertas A., Kolak U., Ozturk M., Ulubelen A. Antioxidant activity tests on novel triterpenoids from Salvia macrochlamys. Arkivoc. 2007;7:195–208. [Google Scholar]
- ul Qamar, M. T., Alqahtani, S. M., Alamri, M. A., Chen, L. L., 2020. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 10(4), 313-319. [DOI] [PMC free article] [PubMed]