Dear editor,
Androgen receptor (AR) is an important transcription factor; thus, androgen and AR-associated pathways play pivotal roles in the progression of several diseases, including prostate cancer (PCa), benign prostatic hyperplasia (BPH), breast cancer, acne, and alopecia [1,2]. Anti-androgen therapy is widely used for the clinical treatment of prostatic diseases, including androgen deprivation therapy (ADT) via surgical castration, pharmacological castration,
or androgen receptor blocked therapy for PCa, and 5 alpha-reductase inhibitor (5ARI) for BPH. Influenced by the COVID-19 pandemic, the effects of SARS-CoV-2 on patients receiving anti-androgen therapy have attracted increasing attention. Montopoli et al. [3] first reported the potential impact of anti-androgen therapy on SARS-CoV-2 infection, where ADT may provide partial protection of PCa patients from SARS-CoV-2 infection. Lyon et al. [4] also demonstrated a reduction in community-acquired SARS-CoV-2 infection with long-term 5ARI administration. However, inconsistent voices were also acknowledged. Notably, several clinical trials (e.g., NCT04446429, NCT04530500, NCT04475601, NCT04354701) also tested the potential protective function of anti-androgen therapy.
In this study, we comprehensively searched publications up to March 15, 2022, in PubMed, Google Scholar, Embase, Cochrane Library and ClinicalTrials, with the following keywords: “androgen”, “anti-androgen therapy”, “androgen deprivation therapy”, “ADT”, “5 alpha-reductase inhibitor”, “5ARI”, “COVID-19″, “2019-nCoV”, “SARS-CoV-2″, “2019 novel coronavirus”, and “coronavirus disease 2019″. We also searched the reference lists of relevant reviews and studies to avoid any missing articles within the topic. The inclusion criteria were preset: (1) case control study with anti-androgen therapy group and non-therapy group; (2) infection of SARS-CoV-2 detected by reverse transcriptase–polymerase chain reaction test; (3) available data for each group and the accurate number of events; (4) study populations being at least fifteen cases. Case reports, repeated articles, review papers and preprints were eliminated. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. Detailed information on the enrolled studies is listed in Tables S1 and S2.
The “metafor” R package was employed to perform the meta-analysis. The odds ratio (OR) with 95% confidence interval (CI) generated from the total number and event number was used to evaluate the impact of anti-androgen therapy and was further integrated into the overall OR. The I 2 and Tau 2 values were calculated to quantify the heterogeneity of each subset. Cumulative meta-analysis was used to display the cumulative impact of anti-androgen therapy on events; specifically, we accumulated the studies in the order of fewer to more patients in the control group. Potential publication bias was evaluated by a funnel plot.
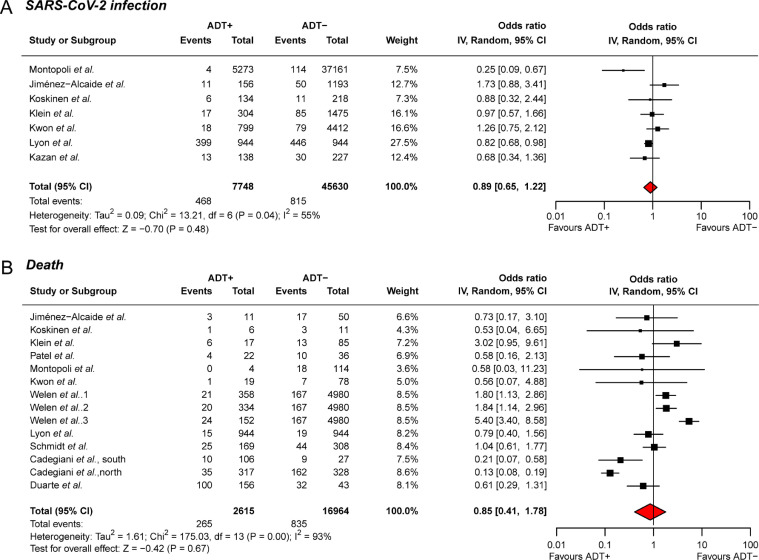
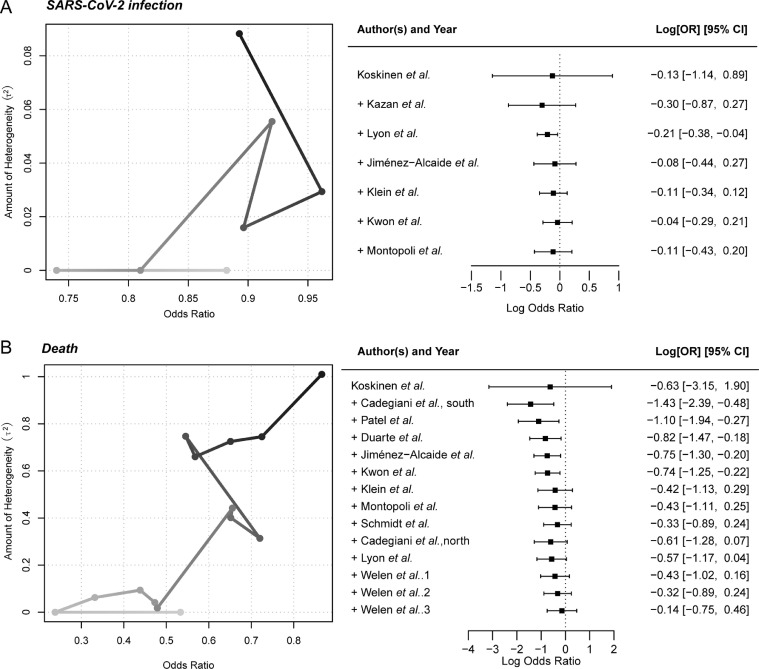
For the evaluation of anti-androgen therapy for SARS-CoV-2 infection, seven studies with a total of 53,378 samples were enrolled for the meta-analysis; six studies received ADT treatment, and one study received 5ARI treatment. Five studies showed a protective trend of anti-androgen therapy to avoid SARS-CoV-2 infection, with ORs less than one, while the remaining two studies demonstrated a risky role with ORs higher than one. Additionally, the integrative meta-analysis provided the protective trend of anti-androgen therapy (OR = 0.89, 95% CI: 0.65–1.22, Fig. 1 A). To further understand the impact of anti-androgen therapy to avoid SARS-CoV-2 infection, cumulative analysis was further conducted. The studies were accumulated in the order of fewer to more patients in control groups to avoid sparse data bias. We observed that the ORs remained less than one in each step after sample accumulation, which strongly supported the findings that anti-androgen therapy may prevent SARS-CoV-2 infection (Fig. 2 A).
Fig. 1.
Forest plot showing the odds ratio of SARS-CoV-2 infection and horrible end among the enrolled studies. (A) Association between anti-androgen therapy and SARS-CoV-2 infection; (B) Association between anti-androgen therapy and SARS-CoV-2-induced death.
Fig. 2.
Cumulative meta-analysis showing the protective trend of anti-androgen therapy against SARS-CoV-2 infection and horrible end. (A) Cumulative line chart and forest plot showing the protective trend of anti-androgen therapy against SARS-CoV-2 infection. (B) Cumulative line chart and forest plot showing the protective trend of anti-androgen therapy against death. Light gray at the beginning, dark gray at the end.
Regarding the association assessment between anti-androgen therapy and mortality of COVID-19, a total of 9,619 SARS-CoV-2-infected patients from 14 studies were collected. Out of these patients, 2,615 patients received anti-androgen therapy, and 7,004 patients were treatment free. For the 14 studies, eight collected PCa patients received ADT treatment, including three of enzalutamide; two studies recruited patients (> 18 years old) detected with COVID-19, no matter man or woman, and given proxalutamide treatment; one study applied 5ARI treatment among men older than 20 years and without PCa. A total of 64.29% (9/14) of the studies reported a protective trend of anti-androgen therapy to avoid COVID-19-associated mortality, while the other studies demonstrated the risk of death. Integrative meta-analysis revealed an overall OR of 0.85 (95% CI: 0.41–1.78; Fig. 1B). Of note, the cumulative meta-analysis reported that the ORs remained less than one after the accumulation of each study, which again indicated the protective trend of anti-androgen therapy to prevent COVID-19-associated mortality (Fig. 2B). The funnel plots were also performed among the SARS-CoV-2 infection subset and COVID-19-caused death subset (Fig. S1). The symmetrical results and Egger's test results (Infection subset: Z = −0.78, P = 0.44; Death subset: Z = −0.54, P = 0.59) indicated no potential publication bias.
Angiotensin converting enzyme 2 (ACE2) is the receptor of the SARS-CoV-2 coronavirus S1 domain of the spike protein; the complex further received proteolytic cleavage by the transmembrane protease serine 2 (TMPRSS2), and then the virus entered the host cell [5]. The increased morbidity and mortality in men implicates a sex disparity of the male hormone in SARS-CoV-2 infection and host response. Recently, Leach et al. [6] reported that the anti-androgen enzalutamide can reduce TMPRSS2 levels in the lung cells of humans and mice and can also reduce SARS-CoV-2 entry and infection into lung cells. Deng et al. [7]. revealed the AR-binding sites located in the transcription start sites of the TMPRSS2 and ACE2 genes and confirmed that androgen deprivation had an effect on the decreased expression of TMPRSS2 and ACE2, particularly in lung tissues. These biological findings support the clinical results that anti-androgen therapy might prevent humans from the infection and the horrible end caused by SARS-CoV-2.
With the pandemic of the SARS-CoV-2 omicron variant and the clinical trials that have been widely applied to human beings with anti-androgen therapy, it is fortunate that the anti-androgen therapy has not caused greater harm from SARS-CoV-2 to patients. Although Welén et al. [8] reported disappointing results among enzalutamide failure to prevent SARS-CoV-2, we still hold a favorable expectation of anti-androgen therapy with the protective trend from the meta-analysis and the biological findings; further studies that enable uncovering the association between anti-androgen therapy and COVID-19 are highly warranted.
Data sharing statement
All the data and materials mentioned in the manuscript are available.
Funding
This work was supported by the National Natural Science Foundation of China [grant number: 82,170,787, 81,972,539, 81,972,539 and U1732157]; Scientific Research Foundation of the Institute for Translational Medicine of Anhui Province [grant number: 2017ZHYX02]; and The Key Project of Provincial Natural Science Research Project of Anhui Colleges [grant number: KJ2019A0278].
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Acknowledgment
We would like to acknowledge Dr. Vaibhav Patel from Mount Sinai University for his great attention to preparing supporting data for their study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.03.020.
Appendix. Supplementary materials
References
- 1.Shukla G.C., Plaga A.R., Shankar E., Gupta S. Androgen receptor-related diseases: what do we know? Andrology. 2016;4(3):366–381. doi: 10.1111/andr.12167. [DOI] [PubMed] [Google Scholar]
- 2.Marks D.H., Prasad S., De Souza B., Burns L.J., Senna M.M. Topical antiandrogen therapies for androgenetic alopecia and acne vulgaris. Am J Clin Dermatol. 2020;21(2):245–254. doi: 10.1007/s40257-019-00493-z. [DOI] [PubMed] [Google Scholar]
- 3.Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V., et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyon M., Li J., Cullen J., Milinovich A., Kattan M., Jehi L., et al. 5alpha-reductase inhibitors are associated with reduced risk of SARS-CoV-2 infection: a matched-pair, registry-based analysis. J Urol. 2022;207(1):183–189. doi: 10.1097/JU.0000000000002180. [DOI] [PubMed] [Google Scholar]
- 5.Qiao Y., Wang X.M., Mannan R., Pitchiaya S., Zhang Y., Wotring J.W., et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc Natl Acad Sci U S A. 2020 doi: 10.1073/pnas.2021450118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach D.A., Mohr A., Giotis E.S., Cil E., Isac A.M., Yates L.L., et al. The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells. Nat Commun. 2021;12(1):4068. doi: 10.1038/s41467-021-24342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng Q., Rasool R.U., Russell R.M., Natesan R., Asangani I.A. Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19. iScience. 2021;24(3) doi: 10.1016/j.isci.2021.102254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welen K., Rosendal E., Gisslen M., Lenman A., Freyhult E., Fonseca-Rodriguez O., et al. A phase 2 trial of the effect of antiandrogen therapy on COVID-19 Outcome: No evidence of benefit, supported by epidemiology and in vitro data. Eur Urol. 2022;81(3):285–293. doi: 10.1016/j.eururo.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.