Chronic kidney disease (CKD) is associated with increased risk for coronary artery disease (CAD) and more severe clinical presentations of CAD. However, validated therapies to reduce CKD-associated CAD risk remain lacking.
Addressing residual inflammatory risk is posited to provide particular cardiovascular benefit to patients with CKD. A post hoc analysis of the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) indicated that individuals with CKD may have derived a greater cardiovascular benefit from interleukin (IL)-1β inhibition (1). Recently, results from the RESCUE trial suggested that among patients with CKD, ziltivekimab, a monoclonal antibody targeting IL-6, yielded marked reduction in C-reactive protein (CRP) (2). These foundational observations and others led to the recently announced phase 3a trial, Ziltivekimab Cardiovascular Outcomes Study (ZEUS) to test the hypothesis that IL-6 inhibition among CKD patients will reduce cardiovascular risk.
We leveraged naturally occurring genetic variation mimicking the effect of IL-6 inhibition and examined its effect on clinical cardiovascular events among CKD patients in the UK Biobank. A common non-synonymous variant (p.Asp358Ala, 40% prevalence in Europeans) in the gene encoding the IL-6 receptor (IL-6R), IL6R, is modestly but significantly associated with lower CAD odds in the general population (3), putatively through enhanced buffering capacity for circulating IL-6 through increased levels of soluble IL-6R shed from IL-6R (4). Here, we assessed the impact of genetic modification in IL-1β/IL-6 pathway on the CKD-associated CAD risk through using a Cox proportional hazards model stratified by IL6R p.Asp358Ala genotype. CKD status was modeled as a time-dependent covariate. Study population included 437,014 unrelated individuals without prevalent CAD in the UK Biobank with unrelatedness defined as less than 3rd degree relatedness. CAD was defined as self-reported, hospitalization with or death due to myocardial infarction (UK Biobank Field Code 42001), or hospitalization with Office of Population Censuses and Surveys (OPCS4) codes for coronary artery bypass grafting (K40, K41, and K45) or coronary angioplasty (K49, K50.2, and K75). CKD was defined as self-reported renal failure, nephropathy, or kidney transplant, or hospitalization or death with ICD codes for renal failure, end-stage renal disease, or all stages of CKD (ICD10 codes: I12.0, I13.1, I13.2, N18; ICD9 codes: 585, 5859), or hospitalization with OPCS4 codes for kidney transplantation (M01). These secondary data analyses were approved by the Massachusetts General Hospital Institutional Review Board.
There were 1,226 participants with CKD at baseline. During 11.1-year median follow-up, 11,150 (2.6%) participants developed incident CKD. The adjusted incidence rate of CAD was significantly higher among wild type with CKD than IL6R p.Asp358Ala carriers with CKD, while there was no difference for those without CKD. CKD showed stronger association with higher CAD risk among wild type [hazard ratio (HR): 2.33, 95% confidence interval (CI): 2.03–2.66] than IL6R p.Asp358Ala carriers (HR: 1.94, 95% CI: 1.74–2.17; Pinteraction=0.048) (Figure). There was no significant interactions between CKD and polygenic scores predisposing to lower body mass index, low-density lipoprotein cholesterol, systolic blood pressure, smoking, and heart rate, and nominally significant interaction in favor of those without CKD with genetically lower diastolic blood pressure for incident CAD.
Figure. CAD event incidence stratified by IL-6 signaling and CKD status.
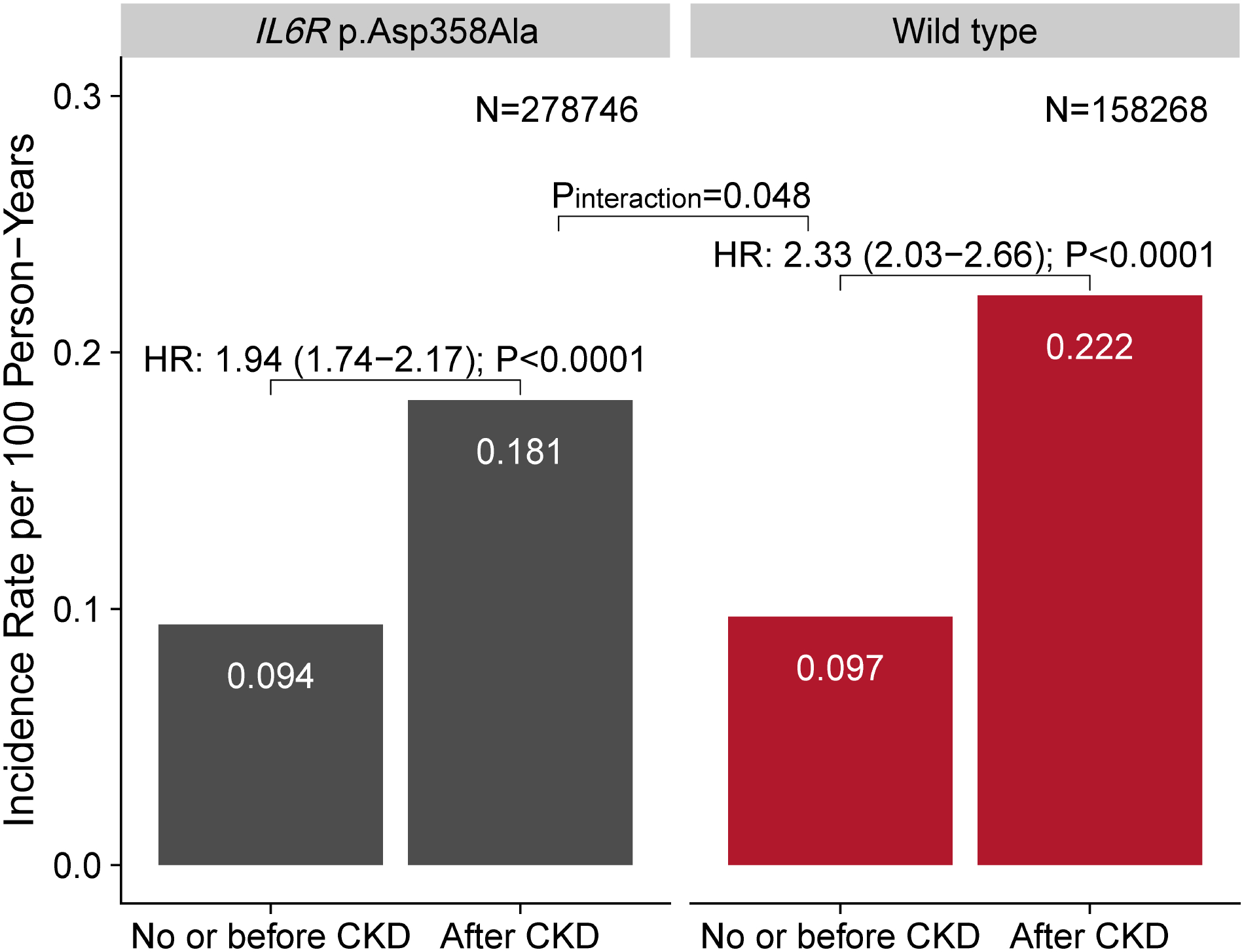
Incidence rates for CAD were adjusted for age categories, sex, race, baseline smoking status, and prevalent type 2 diabetes mellitus (T2D). CKD was associated with greater CAD risk among wild type [hazard ratio (HR): 2.33, 95% confidence interval (CI): 2.03 – 2.66] than IL6R p.Asp358Ala genotype (HR: 1.94, 95% CI: 1.74 – 2.17; Pinteraction = 0.048). HRs (95% CIs) of CKD were calculated from Cox proportional hazards models stratified by IL6R p.Asp358Ala carrier status. CKD status was modeled as a time-dependent covariate. Cox model was adjusted for age, sex, race, first 10 genetic principal components, baseline smoking status, prevalent T2D, and genotyping array. CAD: coronary artery disease; CKD: chronic kidney disease; IL-6: interleukin-6.
Our observations support the role of inflammation in CKD-associated CAD risk and the hypothesis that IL-6 inhibition will help to prevent clinical cardiovascular events among CKD patients. Our results warrant large-scale trials assessing selective IL-6 blockade for the reduction of CKD-associated CAD risk (5).
Funding:
P.N. is supported by grants from the National Heart, Lung, and Blood Institute (R01HL142711, R01HL148050, R01HL151283, R01HL148565, R01HL135242, R01HL151152), Fondation Leducq (TNE-18CVD04), and Massachusetts General Hospital (Paul and Phyllis Fireman Endowed Chair in Vascular Medicine).
Competing interests:
P.N. reports investigator-initiated grants from Amgen, Apple, Boston Scientific, and AstraZeneca, personal fees from Apple, Blackstone Life Sciences, Foresite Labs, Genentech, and Novartis, and spousal employment at Vertex, all unrelated to the present work.
Abbreviations:
- CAD
coronary artery disease
- CANTOS
Canakinumab Anti-inflammatory Thrombosis Outcome Study
- CI
confidence interval
- CKD
chronic kidney disease
- HR
hazard ratio
- ICD
International Classification of Diseases
- IL
interleukin
- IL-6R
IL-6 receptor
- OPSC4
Office of Population Censuses and Surveys
- ZEUS
Ziltivekimab Cardiovascular Outcomes Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The remaining authors have nothing to disclose.
References
- 1.Ridker PM, MacFadyen JG, Glynn RJ et al. Inhibition of Interleukin-1β by Canakinumab and Cardiovascular Outcomes in Patients With Chronic Kidney Disease. J Am Coll Cardiol 2018;71:2405–2414. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Devalaraja M, Baeres FMM et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. The Lancet. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow DI, Holmes MV, Kuchenbaecker KB et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012;379:1214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov 2018;17:395–412. [DOI] [PubMed] [Google Scholar]
- 5.George MJ, Jasmin NH, Cummings VT et al. Selective Interleukin-6 Trans-Signaling Blockade Is More Effective Than Panantagonism in Reperfused Myocardial Infarction. JACC Basic Transl Sci 2021;6:431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]


