To the Editor,
We read with great interest the article by Ane F Salinas et al. [1] concerning early immune responses after BNT162b2 immunization in a cohort of 41 SARS-CoV-2 infection-naïve or previously infected patients with common variable immune deficiencies (CVID) and 6 X-linked agammaglobulinemia (XLA) patients, as it adds valuable information about the humoral and cellular immunogenicity of the vaccine in patients with inborn errors of immunity (IEI). They evaluated the serum levels of SARS-CoV-2-specific antibodies and Spike-specific B- and T-cells before immunization and 1 week after the second dose of BNT162b2, and found antibody responses in eight of the 34 CVID patients (23.5%) and in none of the 6 XLA patients [1].
In a similar study of a cohort of 60 CVID patients (49 previously uninfected and 11 previously infected by SARS-CoV-2) and six XLA patients (only one previously infected) vaccinated with BNT162b2 or mRNA-1273, we measured the serum concentration of anti-Spike Receptor Binding Domain antibodies (total Ig; Roche Elecsys Anti-SARS-CoV-2 S reagent) before (T0) and 4 weeks after vaccination (T2). All the patients were receiving replacement therapy and tests of the infused immunoglobulin products showed no detectable levels of anti-Spike Ig.
Our CVID patients were similar to those of Salinas et al. and were vaccinated at approximately the same time of the year (see Patients and Methods as Supplemental data). We found that 35/49 patients previously uninfected by SARS-CoV-2 (71.4%) responded to the vaccine and produced anti-Spike Ig, a percentage that is three times higher than that found by Salinas et al. [1].
The difference in response rates may have been due to a difference in antibody production between the two cohorts:
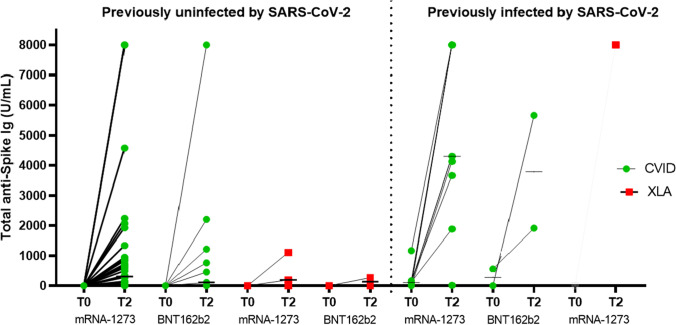
Salinas et al. evaluated early immune responses 1 week after the second dose of BNT162b2, whereas our patients were tested 4 weeks after two-dose immunization, thus giving them a longer time to produce antibodies. Thirty-seven of our previously uninfected CVID patients (76%) received mRNA-1273 vaccine, and 29 of these (78.4%) had detectable serum anti-Spike Ig concentrations with a median T2 level of 311 U/mL (range 0.4 to > 7500), whereas six of the 12 receiving BNT162b2 (50%) produced antibodies with a median T2 level of 114 U/mL (range 0.4 to > 7500). Therefore, although both studies showed that median serum Ig levels increased between T0 to T2, our findings show that the increase was greater in the patients receiving mRNA-1273 (see Fig. 1).
Fig. 1.
Total anti-Spike Ig antibodies in previously SARS-CoV-2-uninfected and previously SARS-CoV-2-infected patients with CVID (green circles) or XLA (red squares) before vaccination (T0) and 4 weeks after the second dose (T2) of mRNA-1273 or BNT162b2 vaccine. The bars show the median values. Previously uninfected patients: 49 with CVID and five with XLA. Previously infected patients: 11 with CVID and one with XLA
Moreover, comparison of antibody production between the previously infected (N = 9) and previously uninfected CVID patients (N = 37) receiving mRNA-1273 vaccine showed that total anti-Spike Ig production at T2 was higher among the former (4302 U/mL vs 311 U/mL, respectively; p = 0.026). This data was also observed in the two previously infected CVID patients who received BNT162b2 (median T2 titer 3783.5 U/mL vs 114 U/mL of the previously 12 uninfected patients) (see Fig. 1).
Significantly higher antibody production after mRNA-1273 vaccination has been demonstrated in a recent study that directly compared humoral responses to BNT162b2 and mRNA-1273 in a cohort of healthcare workers in Belgium [2]. Greater responses to mRNA-1273 were observed in previously infected and previously uninfected workers of all ages, and the authors speculated that this may have been due to the higher mRNA content in mRNA-1273 and the longer time interval between priming and boosting (4 vs 3 weeks).
Regardless of the vaccine received, we also found that the humoral response of CVID patients was less than that of healthy subjects or 13 patients with hypogammaglobulinemia who had responded to vaccinations against pneumococcus and tetanus, defined as having unclassified primary antibody deficiency (UnPAD). Comparison of T2 anti-Spike Ig production between the 37 previously uninfected CVID patients and the nine previously uninfected UnPAD patients, receiving mRNA-1273 vaccine showed that the CVID patients had lower median levels (311 U/mL vs 2904 U/mL, respectively).
We also compared the previously uninfected CVID patients’ humoral response with those of 2968 previously uninfected healthcare workers at our hospital. Four weeks after their second dose of BNT162b2 vaccine, six of the workers (0.2%) were non-responders, 200 (7.4%) had serum anti-Spike Ig level > 7500 U/mL [3], and the median serum anti-Spike Ig level was 1374 U/mL (range 0.4 to > 7500), which is more than ten times higher than that observed in the CVID cohort. Our findings are similar to those described by Arroyo‑Sánchez et al. in 17 CVID patients.
Seven of the 11 CVID patients previously infected by SARS-CoV-2 (64%) had detectable anti-Spike Ig levels before receiving their first vaccine dose, a proportion that is similar to the 57% found by Salinas et al. (4/7) and confirms the hypothesis that antibodies may persist after primary infection in some CVID patients. Our patients received their first vaccine dose 3–5 months after testing negative for the infection. Like Salinas et al., we observed an increase in Ig levels before the second dose (T1), thus showing that subsequent immunization with BNT162b2 (2 patients) or mRNA-1273 (9 patients) had a boosting effect: all of the patients showed a detectable response, and four had anti-Spike Ig levels of > 7500 U/mL (see Fig. 1).
Anti-SARS-CoV-2 vaccine responses were also analyzed in six XLA patients belonging to the Italian XLA cohort of the IPINet registry [4], all of whom were receiving replacement therapy. In four of six patients, antibodies were detected after two vaccine doses. The supplementary table shows their genetic data at the time of diagnosis and their Ig levels before the first dose. Four of the six patients presented with atypical XLA clinical and laboratory findings, including normal IgM, IgG, or IgA levels and fewer than 2% of peripheral B cells. As BTK protein was detectable, no BTK gene analysis was initially made but a later NGS target panel analysis for hypogammaglobulinemia (including the BTK gene) showed missense mutations: one in the pleckstrin homology (PH) domain and three in the Src-homology-2 (SH2) domain. This genotype has been associated with a delayed diagnosis and an atypical XLA phenotype [5]. These patients had detectable responses to tetanus toxoid vaccine and failed to respond to pneumococcal vaccine.
Among the five previously uninfected XLA patients, two with the classical XLA phenotype showed no response even after the second vaccine dose (0.2 U/mL), whereas the three carrying BTK SH2- or PH-domain mutations responded with an increase in anti-Spike Ig levels at T2 (median titer 272 U/mL, range 193 to 1110).
The only previously infected XLA patient (a carrier of a BTK SH2-domain mutation) contracted the infection 5 months before being vaccinated. He developed severe COVID-19 with lung involvement, required intubation for 6 weeks, and continued to have detectable SARS-CoV-2 RNA in respiratory specimens for 3 months after the onset of the infection. His total anti-Nucleocapside Ig level before the first dose of mRNA-1273 was 40.6 ICO and his T2 anti-Spike Ig level was > 7500 U/mL (his anti-Spike Ig level was not tested at T0).
Since the completion of this letter, the CVID and XLA patients enrolled in the study have received the third dose of an mRNA vaccine and, in the period of 6–8 months between the second and third dose, none of them developed SARS-CoV-2 infection.
The limitations of this study include the lack of information about B- and T-cell immunity. Data concerning mRNA-1273 vaccine-induced B-cell response would be interesting and would complement the BNT162b2 data provided by Salinas et al.
However, T-cell immunity against SARS-CoV-2 infection seems to be more relevant in patients with primary antibody deficiency, and may play a pivotal role in their immunization.
Moreover, there have been recent reports of discordance between virus-specific antibody levels and antibody neutralization capacity in subjects with immune disorders. The production of anti-S antibodies is not clearly associated with protection against SARS-CoV-2.
In brief, our findings indicate that the humoral response to anti-SARS-CoV-2 vaccines of CVID patients and our cohort of “atypical” XLA patients is less than the rate of seroconversion in UnPAD patients and healthy subjects. They also suggest that the timing of the measurement of the response affects the way in which the results should be interpreted because, like previous studies, we recorded a higher response rate among CVID patients than that reported by Salinas et al.
We found that the mRNA-1273 vaccine seems to induce greater antibody production than BNT162b2, as has recently been described in healthy subjects. More studies are needed to confirm the interesting hypothesis of different vaccine responses in CVID patients.
Patients with primary antibody deficiency are more likely to be unable to clear infection, can suffer severe infection, and, when infected, experience prolonged viral replication that increase the risk of the emergence of viral variants.
There are therefore still a number of questions concerning the use of anti-COVID vaccines in CVID or XLA patients.
What is the duration of protection after immunization in those showing a humoral response? Which is the most appropriate vaccine? How many booster doses should be administered? What are the longer-term effects on T-cell responses and dysregulated B-cells?
Given that our study and that of Salinas et al. both show that 30 to 70% of CVID patients have a deficient humoral response after two doses of anti-SARS-CoV-2 vaccine, consideration should be given to the preventive administration of monoclonal antibodies against SARS-COV-2 infection in order to prevent severe disease in both XLA and CVID patients and to prevent the emergence of new viral variants.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
GF and MC conceived of and designed the study; FC, LB, MC, and DC prepared the study material, collected, and analyzed the data. The first draft of the manuscript was written by MC and GF, and subsequent versions were reviewed by all the authors. All of the authors read and approved the final manuscript.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Salinas AF, Mortari EP, Terreri S, Quintarelli C, Pulvirenti F, Di Cecca S, et al. SARS-CoV-2 vaccine induced atypical immune responses in antibody defects: everybody does their best. J Clin Immunol. 2021 Nov;41(8):1709–1722. 10.1007/s10875-021-01133-0. [DOI] [PMC free article] [PubMed]
- 2.Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326(15):1533–1535. doi: 10.1001/jama.2021.15125.PMID:34459863;PMCID:PMC8406205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lombardi A, Consonni D, Oggioni M, Bono P, Uceda Renteria S, Piatti A, et al. SARS-CoV-2 anti-spike antibody titres after vaccination with BNT162b2 in naïve and previously infected individuals. J Infect Public Health. 2021 Aug;14(8):1120–1122. 10.1016/j.jiph.2021.07.005. [DOI] [PMC free article] [PubMed]
- 4.Lougaris V, Soresina A, Baronio M, Montin D, Martino S, Signa S, et al. Long-term follow-up of 168 patients with X-linked agammaglobulinemia reveals increased morbidity and mortality. J Allergy Clin Immunol. 2020;146(2):429–437. doi: 10.1016/j.jaci.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Carrillo-Tapia E, García-García E, Herrera-González NE, Yamazaki-Nakashimada MA, Staines-Boone AT, Segura-Mendez NH, et al. Delayed diagnosis in X-linked agammaglobulinemia and its relationship to the occurrence of mutations in BTK non-kinase domains. Expert Rev Clin Immunol. 2018;14(1):83–93. doi: 10.1080/1744666X.2018.1413349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.