Abstract
Background
Uncomplicated urinary tract infections (UTIs) are amongst the most frequent infections presenting in the outpatient setting. A growing number of clinical trials are assessing the most effective treatment interventions for uncomplicated UTI. Due to the heterogeneity of the outcomes reported in these trials, however, comparing these outcomes is challenging.
Objectives
Identify the core outcomes that have been reported in trials and systematic reviews of interventions treating uncomplicated UTI in adults.
Methods
We conducted a systematic search for core outcomes used to evaluate treatments of UTIs. We searched the Cochrane Database of Systematic Reviews, PubMed and Embase. One researcher independently screened each article for inclusion, and the Core Outcome Set for treatment of Urinary Tract Infections (COSUTI) team acted as second reviewers. All included articles were screened by two reviewers. All outcomes were extracted verbatim, and similar outcomes were grouped into domains and subdomains.
Results
In total, 334 outcomes were reported across 41 papers, the average number of outcomes reported being 8. Outcomes were categorized across 18 domains, the majority of which were related to clinical cure outcomes. Many outcomes varied in the timepoints within which the outcome was measured and reported.
Conclusions
Comparing the outcomes of trials investigating uncomplicated UTI treatment remains challenging due to the difference in outcomes currently reported. Consistency of reporting of outcomes would be improved by developing a minimum number of consistent outcomes that should be reported in all trials.
Introduction
Urinary tract infections (UTIs) are among the most commonly presenting infections in the outpatient setting1 and account for significant morbidity and mortality at both individual and societal levels.2 A UTI is categorized as either complicated or uncomplicated. The most common causative agent in both is the Gram-negative bacteria uropathogenic Escherichia coli (UPEC); however, Gram-positive bacteria and fungi are also implicated.3
An acute, uncomplicated UTI in adults is characterized as the onset of acute cystitis occurring in otherwise healthy individuals without known structural or functional abnormalities of the urinary tract.4 A complicated UTI is associated with a structural or functional abnormality that increases the risk of treatment failure or serious complications.5 The epidemiology of UTIs varies depending on factors such as age and sex.6 The incidence of UTI is higher in females than in males. It is estimated that every woman will have at least one UTI in their lifetime7 and by the age of 24 years one in three women will have had at least one UTI diagnosed by a clinician requiring antibiotic treatment.8
In a bid to assess the most effective treatment intervention for uncomplicated UTI, clinical trials have been, and continue to be, completed internationally. Clinical trials are research studies undertaken to evaluate the safety and efficacy of a medical, surgical or behavioural intervention on human health outcomes. However, the comparison of the outcomes of these clinical trials is complicated by the variation in outcomes reported, which hampers evidence synthesis,9 limiting the reliability of evidence to guide healthcare decisions. The development and adoption of a core outcome set (COS) addresses this lack of standardization in measuring outcomes, facilitates evidence synthesis and can help reduce reporting biases.10,11 A COS is an agreed minimum set of outcomes that should be reported in, and across, clinical trials of a specific condition.12
To date, a COS on the outcomes reported in trials examining the safety and efficacy of interventions for the treatments for uncomplicated UTI in adults has not been developed. The Core Outcome Set for treatment of Urinary Tract Infections (COSUTI) study seeks to address this knowledge gap. The first step is to review the treatment outcomes currently reported within clinical trials systematically. This systematic review aims to identify a comprehensive list of outcomes currently reported in clinical trials examining the effectiveness of interventions for the treatments for uncomplicated UTI in adults. For this review, uncomplicated UTI is defined as ‘the acute onset of dysuria, frequency, or urgency in healthy male and non-pregnant women without known functional or anatomical abnormalities of the urinary tract’.13 The outcomes reported in trials will be categorized, and any variance discussed to develop a core outcome set.
Methods
The protocol for this systematic review has been published previously as part of the COSUTI study protocol14 and a summary is presented below.
Included in this review were all randomized trials and systematic reviews of randomized trials (with and without meta-analyses) comparing the effectiveness of any interventions for the treatment of uncomplicated UTI or cystitis in healthy adults. Recurrent UTIs and pyelonephritis were excluded as they were beyond the scope of this study. A combination of search terms was used to search for relevant studies between 2007 and 2017 within the Cochrane Database of Systematic Reviews (including CENTRAL, CDSR and DARES), PubMed and Embase. Search terms included: urinary tract infection; UTI; Cystitis, Randomized controlled trial; Controlled clinical trial; randomized; Placebo; randomly; trial. The full search strategy is available by accessing the COSUTI protocol.14
A 10 year period was chosen to ensure that outcomes identified reflected contemporary treatments.
Only papers available in English were screened. Two reviewers independently screened the title and abstracts and the subsequent eligible full text. Additional reviewers were consulted if there was uncertainty.
Outcomes were extracted verbatim. Similar outcomes were grouped into domains using a paired comparison process and where required were further categorized into subdomains. This categorization process allowed for similar outcomes to be grouped together under themes. Once this process was complete, each outcome and its domain categorization were verified by the COSUTI team. Patterns of outcome reporting were examined, including the number of studies and frequency with which outcomes were reported and defined, the time frame of reporting and reproducibility. The PRISMA and COMET11 guidelines will be used to report the conduct and findings of this review.
Results
Study selection
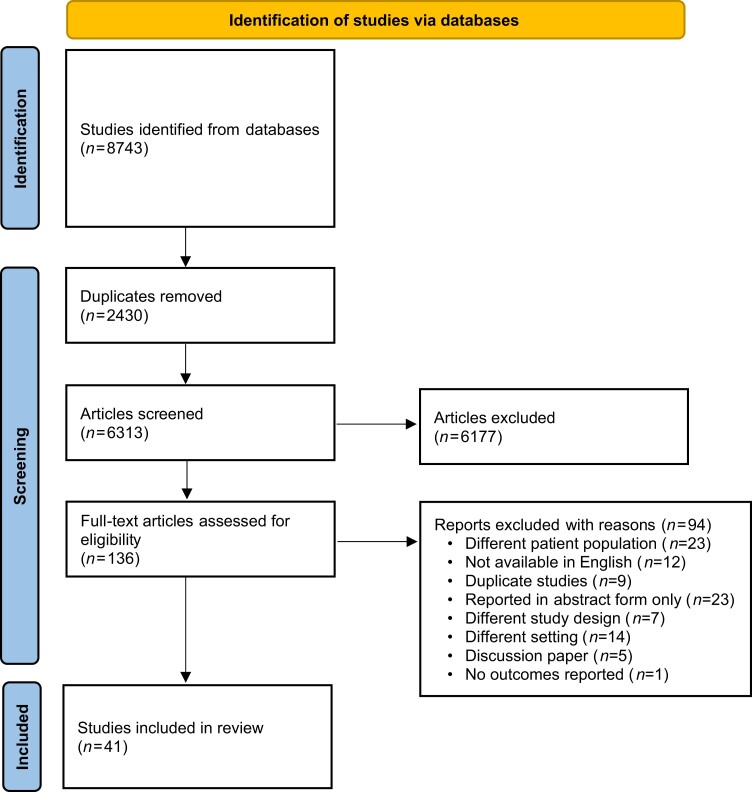
Across the four databases, we identified 8743 papers. Once duplicates were removed 6313 were eligible for title and abstract screening. A further 6177 papers were excluded during title and abstract screening. The majority of papers were excluded as they referred to treatments of complicated UTIs, recurrent UTI or children. Trials conducted on animals and other illnesses were also excluded. Once title screening was complete 136 studies were deemed eligible for full text screening. A further 94 were excluded for reasons outlined in Figure 1. Papers were excluded for various reasons including different patient population (n = 23); not available in English (n = 12); duplicate studies (n = 9); reported in abstract form only (n = 23); different study design (n = 7) or setting (n = 14); discussion paper (n = 5); and no outcomes reported (n = 1). Forty-one studies were included in the data extraction process. Figure 1 summarizes the COSUTI PRISMA.
Figure 1.
COSUTI PRISMA flow diagram.
Characteristics of studies
In total, 334 outcomes were reported across 41 papers.15–55 Table 1 summarizes the aim and interventions investigated within the studies. A definition was provided for 73 (22%) of these outcomes. The average number of outcomes reported per trial was eight. The lowest number of outcomes reported was 1, the highest being 15, which were reported within three papers.
Table 1.
Summary of aim and interventions undertaken in included studies
| First author | Title | Aim | Intervention |
|---|---|---|---|
| Bjerrum 200915 | Pivmecillinam versus sulfamethizole for short-term treatment of uncomplicated acute cystitis in general practice: a randomized controlled trial | To test whether, in women with acute uncomplicated UTI, short-term treatment with pivmecillinam was more effective than sulfamethizole | Short-term treatment with pivmecillinam was more effective than sulfamethizole in patients with acute uncomplicated UTI |
| Bleidorn 201016 | Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection? - results of a randomized controlled pilot trial | To (i) make a rough estimate of the equivalence of ibuprofen and ciprofloxacin for uncomplicated UTI with regard to symptom resolution; and (ii) demonstrate the feasibility of a double-blind, randomized controlled drug trial in German general practices | Equivalence of a 3 day treatment course of 3 × 400 mg ibuprofen compared with 2 × 250 mg ciprofloxacin in women with symptoms of uncomplicated UTI with regard to symptomatic outcome |
| Ceran 201017 | A randomized comparative study of single-dose fosfomycin and 5-day ciprofloxacin in female patients with uncomplicated lower urinary tract infections | To evaluate the clinical effectiveness of FMT compared with ciprofloxacin in females admitted to the outpatient department with uncomplicated UTIs | The 3 g single-dose FMT was administered to one-half of the study population, and ciprofloxacin was administered at a dose of 500 mg twice a day for 5 days to the other half of the patients |
| Chen 201218 | Prulifloxacin versus levofloxacin in the treatment of respiratory and urinary tract infections: a multicentre, double-blind, randomized controlled clinical trial | To evaluate the efficacy and safety of prulifloxacin, a reference fluroquinolone, for the treatment of respiratory tract infections and UTI in Chinese patients | Prulifloxacin versus levofloxacin |
| Dawson-Hahn 201719 | Short-course versus long-course oral antibiotic treatment for infections treated in outpatient settings: a review of systematic reviews | To summarize the evidence comparing the effectiveness of short and long courses of oral antibiotics for infections treated in outpatient settings | Short and long courses of oral antibiotics |
| Deepalatha 201120 | A comparative study of phenazopyridine (Pyridium) and Cystone as short-term analgesic in uncomplicated urinary tract infection | To assess the analgesic efficacy in treating burning micturition and pain during voiding of urine within 48 h of diagnosis of uncomplicated UTI | Phenazopyridine group, phenazopyridine with antibiotic and Cystone group |
| Drozdov 201321 | Procalcitonin, pyuria and proadrenomedullin in the management of urinary tract infections - ‘triple p in uti’: study protocol for a randomized controlled trial | For Intervention A, to investigate antibiotic exposure of patients treated with a protocol based on the type of UTI, procalcitonin and pyuria. Second, for Intervention B, to investigate the usefulness of the prognostic biomarker proadrenomedullin integrated into an interdisciplinary assessment bundle for site-of-care decisions | Intervention A: to analyse the efficacy and safety of a procalcitonin- and pyuria-guided antibiotic therapy in individualizing and reducing the duration of antibiotic treatment compared with the guidelines |
| Dybowski 200822 | Ciprofloxacin and furagin in acute cystitis: comparison of early immune and microbiological results | To test the hypothesis that therapy for acute cystitis with ciprofloxacin results in faster resolution of mucosal inflammation in comparison with furagin | Ciprofloxacin 250 mg twice a day for 3 days or furagin 100 mg three times a day for 7 days was prescribed alternatively |
| Falagas 200923 | P685 Antibiotics versus placebo in the treatment of women with uncomplicated cystitis: a meta-analysis of randomised controlled trials | To compare the effectiveness and safety profile of fosfomycin versus other antibiotics in patients with cystitis by performing a meta-analysis of relevant RCTs | Fosfomycin versus other antibiotics |
| Falagas 201024 | Fosfomycin versus other antibiotics for the treatment of cystitis: a meta-analysis of randomized controlled trials | To compare the effectiveness and safety profile of fosfomycin versus other antibiotics in patients with cystitis by performing a meta-analysis of relevant RCTs | Fosfomycin versus other antibiotics |
| Ferry 200725 | Clinical and bacteriological outcome of different doses and duration of pivmecillinam compared with placebo therapy of uncomplicated lower urinary tract infection in women: the LUTIW project | To analyse associations between symptoms and bacteriuria in uncomplicated lower UTI in women and to evaluate outcome of therapy with three different regimens of pivmecillinam or placebo | Patients were randomized to three different regimens of pivmecillinam (Selexid®: 200 mg × 3 × 7 days, 200 mg × 2 × 7 days or 400 mg × 2 × 3 days or placebo (i.e. all patients were given 2 + 1 + 2 identical tablets each day for 7 days) |
| Gagyor 201227 | Immediate versus conditional treatment of uncomplicated urinary tract infection - a randomized-controlled comparative effectiveness study in general practices | Investigates whether the use of antibiotics for uncomplicated UTI could be reduced by initial treatment with ibuprofen | Participating patients receive either immediate antibiotic therapy with FMT 1 × 3 g or initial symptomatic treatment with ibuprofen 3 × 400 mg for 3 days |
| Gágyor 201526 | Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial | Can treatment of the symptoms of uncomplicated UTI with ibuprofen reduce the rate of antibiotic prescriptions without a significant increase in symptoms, recurrences or complications? | A single dose of fosfomycin 3 g (n = 246; 243 analysed) or ibuprofen 3 × 400 mg (n = 248; 241 analysed) for 3 days (and the respective placebo dummies in both groups) |
| Grabein 201728 | Intravenous fosfomycin-back to the future. Systematic review and meta-analysis of the clinical literature | To summarize the clinical evidence and usage patterns of IV fosfomycin from its development to the present time | IV fosfomycin |
| Grigoryan 201413 | Diagnosis and management of urinary tract infections in the outpatient setting: a review | To define the optimal approach for treating acute cystitis in young healthy women and in women with diabetes and men and to define the optimal approach for diagnosing acute cystitis in the outpatient setting | Diagnosis and management of UTIs |
| Gupta 200729 | Short-course nitrofurantoin for the treatment of acute uncomplicated cystitis in women | To assess the efficacy and tolerance of a 5 day course of nitrofurantoin compared with a standard 3 day regimen of trimethoprim/sulfamethoxazole for the treatment of acute uncomplicated cystitis. The effects of trimethoprim/sulfamethoxazole resistance on efficacy were also assessed | Randomized to open label treatment with trimethoprim/sulfamethoxazole,1 double strength tablet twice daily for 3 days, or nitrofurantoin (Macrobid; Procter & Gamble Pharmaceuticals, Cincinnati, OH, USA), 100 mg twice daily for 5 days |
| Gutiérrez-Castrellón 201530 | Efficacy and safety of ciprofloxacin in the treatment of urinary tract infections (UTIs) in adults: a systematic review with meta-analysis | A systematic review with meta-analysis of RCTs on the efficacy and safety of ciprofloxacin in the treatment of acute or complicated UTIs in adults | Ciprofloxacin in the treatment of acute or complicated UTIs in adults |
| Haghighi 201031 | Comparison of 3-day and 7-day ciprofloxacin regimen for the treatment of uncomplicated urinary tract infection in women: a randomized double-blind clinical trial | To compare efficacy and safety of 3 day and 7 day ciprofloxacin regimen for the treatment of uncomplicated UTI in women | One group received ciprofloxacin, 250 mg twice a day for 3 days (n = 39) and the other group received ciprofloxacin 250 mg twice a day for 7 days (n = 37). |
| Hamasuna 201432 | Treatment of acute uncomplicated cystitis with faropenem for 3 days versus 7 days: multicentre, randomized, open-label, controlled trial | To assess the treatment of acute uncomplicated cystitis with faropenem for 3 days versus 7 days | 200 mg faropenem sodium tablet that was administered three times daily (600 mg/day) for 3 or 7 days |
| Hooton 201233 | Cefpodoxime vs ciprofloxacin for short-course treatment of acute uncomplicated cystitis: a randomized trial | To assess whether cefpodoxime would have clinically acceptable efficacy and tolerance compared with ciprofloxacin | A 3 day course of cefpodoxime compared with a standard 3 day regimen of ciprofloxacin |
| Huttner 201534 | Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials | To assess nitrofurantoin’s efficacy and toxicity in the treatment of lower UTI | Nitrofurantoin |
| Jamil 201635 | Role of symptomatic treatment in comparison to antibiotics in uncomplicated urinary tract infections | To compare potassium citrate plus flurbiprofen versus ciprofloxacin in patients with uncomplicated UTI | Potassium citrate plus flurbiprofen versus ciprofloxacin |
| Jansåker 201636 | The efficacy of pivmecillinam: 3 days or 5 days t.i.d against community acquired uncomplicated lower urinary tract infections - a randomized, double-blinded, placebo-controlled clinical trial study protocol | To identify and to compare the efficacy of pivmecillinam 400 mg three times a day in a 3 day or 5 day regimen, for community-acquired uncomplicated LUTI, i.e. in women at the age of 18–70 years | 3 and 5 day regimen of pivmecillinam 400 mg |
| Knottnerus 201237 | Comparative effectiveness of antibiotics for uncomplicated urinary tract infections: network meta-analysis of randomized trials | To compare the efficacies and adverse effects of all relevant antibiotics for UTI treatment simultaneously by performing a network meta-analysis using direct and indirect treatment comparisons | All relevant antibiotic treatments for UTI |
| Kronenberg 201738 | Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial | To investigate whether symptomatic treatment with NSAIDs is non-inferior to antibiotics in the treatment of uncomplicated lower UTI in women | Women allocated to diclofenac received capsules containing 75 mg diclofenac retard for 3 days (Olfen-75 duo release; Mepha Pharma, Basel, Switzerland) and women allocated to norfloxacin received capsules containing 400 mg norfloxacin for 3 days |
| Letelier 201739 | BG126® phytodrug improves urinary tract infection treatment with nitrofurantoin in adult women in a double-blind randomized clinical trial | To evaluate the effect of Buddleja globosa Hope standardized extract (BG126) exhibiting a high content of antioxidant molecules, upon gastrointestinal adverse effects exerted by nitrofurantoin | Recruited patients were simply randomly distributed to nitrofurantoin + placebo or nitrofurantoin + BG126 |
| Little 201040 | Effectiveness of five different approaches in management of urinary tract infection: randomised controlled trial | To assess the effectiveness of management using dipstick or clinical algorithms compared with the alternative management strategies (empirical antibiotic treatment, delayed prescribing, and targeted prescribing based on midstream urine results) | Patients were randomized to five management approaches: empirical antibiotics; empirical delayed (by 48 h) antibiotics; or targeted antibiotics based on a symptom score (two or more of urine cloudiness, urine smell, nocturia or dysuria), a dipstick result (nitrite or both leucocytes and blood) or a positive result on midstream urine analysis. Self-help advice was controlled in each group |
| Lutters 200841 | Antibiotic duration for treating uncomplicated, symptomatic lower urinary tract infections in elderly women | To determine the optimal duration of antibiotic treatment for uncomplicated symptomatic lower UTI in elderly women | Optimal duration of antibiotic treatment for uncomplicated symptomatic lower UTI in elderly women |
| Masson 200942 | Meta-analyses in prevention and treatment of urinary tract infections | To determine the current evidence for the prevention and treatment of UTI in adults and children from meta-analyses | Treatments for UTI |
| Maurya 201443 | Clinical efficacy of Moringa oleifera Lam. stems bark in urinary tract infections | To evaluate the value of Moringa oleifera Lam. stem bark as a potential medicine for UTIs | Shigru bark versus modern medicines |
| Naber 201144 | Antibiotic treatment of uncomplicated urinary tract infection in premenopausal women | After a systematic literature search, recommendations for empirical treatment of acute uncomplicated cystitis and acute uncomplicated pyelonephritis and for follow-up strategies were developed | Antibiotic treatment |
| O’Kane 201646 | Urinary alkalisation for uncomplicated urinary tract infection | To assess the benefits and harms of the use of urinary alkalizers for the treatment of uncomplicated UTIs in adult women | Urinary alkalizers for the treatment of uncomplicated UTIs in adult women |
| Palou 201347 | Randomized comparative study for the assessment of a new therapeutic schedule of fosfomycin trometamol in postmenopausal women with uncomplicated lower urinary tract infection | The assessment of a new therapeutic schedule of FMT in postmenopausal women with uncomplicated lower UTI | FMT 3 g, 2 doses separated by 72 h and ciprofloxacin 250 mg every 12 h for 3 days |
| Pinart 201748 | Optimal dosage and duration of pivmecillinam treatment for uncomplicated lower urinary tract infections: a systematic review and meta-analysis | To compare the efficacy and safety of different pivmecillinam regimens for uncomplicated lower UTIs | Pivmecillinam regimens for uncomplicated lower UTIs |
| Sadahira 201749 | Efficacy and safety of 3 day versus 7 day cefditoren pivoxil regimens for acute uncomplicated cystitis: multicentre, randomized, open-label trial | To evaluate the clinical and microbiological efficacies of cefditoren pivoxil against acute uncomplicated cystitis and to determine the optimal duration of cefditoren pivoxil treatment | A 3 or 7 day regimen of cefditoren pivoxil for acute uncomplicated cystitis was examined in a randomized study, by evaluating the clinical and microbiological efficacies |
| Stange 201750 | Results of a randomized, prospective, double-dummy, double-blind trial to compare efficacy and safety of a herbal combination containing Tropaeoli majoris herba and Armoraciae rusticanae radix with co-trimoxazole in patients with acute and uncomplicated cystitis | To demonstrate non-inferiority of a herbal combination (horseradish root and nasturtium herb) to an antibiotic (co-trimoxazole) in acute uncomplicated cystitis | Patients received the herbal combination (five tablets, four times per day) or the antibiotic (two tablets daily) for a period of 7 or 3 days, respectively, followed by 21 days without drug treatment. Placebos ensured blinding |
| Trill 201751 | Uva-ursi extract and ibuprofen as alternative treatments of adult female urinary tract infection (ATAFUTI): study protocol for a randomised controlled trial | To investigate in adult women with suspected UTI who accepted the delayed prescription strategy whether NSAIDs or uva-ursi (a herbal product) provide relief from urinary symptoms and reduce antibiotic use | Group 1: uva-ursi + advice to take ibuprofen Group 2: placebo + advice to take ibuprofen Group 3: uva-ursi + no advice to take ibuprofen Group 4: placebo + no advice to take ibuprofen |
| Vachhani 201552 | Effectiveness and tolerability of short course co-trimoxazole, norfloxacin and levofloxacin in bacteriological cure of uncomplicated urinary tract infection in outpatient setting. An open label, parallel group, randomized controlled trial | To compare the bacteriological cure rate of short-course (3 day) treatment of uncomplicated UTI using co-trimoxazole, norfloxacin and levofloxacin | Patients with uncomplicated UTI were randomized to receive either co-trimoxazole (960 mg) twice a day or norfloxacin (400 mg) twice a day or levofloxacin (250 mg) once a day for 3 days |
| Vidal 200753 | Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials | To compare the efficacy and adverse effects of any aminoglycoside as a single antibiotic with other antibiotics for the treatment of patients with infection | Aminoglycoside antibiotics as single treatment for patients with infection |
| Vik 201454 | Ibuprofen versus mecillinam for uncomplicated cystitis - a randomized controlled trial study protocol | To evaluate ibuprofen versus mecillinam in the treatment of uncomplicated cystitis in healthy, adult, non-pregnant women | Ibuprofen versus mecillinam in the treatment of uncomplicated cystitis in healthy, adult, non-pregnant women |
| Zalmanovici Trestioreanu 201055 | Antimicrobial agents for treating uncomplicated urinary tract infection in women | To compare the efficacy, resistance development and safety of different antimicrobial treatments for acute uncomplicated lower UTI | Different antimicrobial treatments for acute uncomplicated lower UTI |
FMT, fosfomycin trometamol; NSAIDs, non-steroidal anti-inflammatory drugs; RCTs, randomized controlled trials.
Once duplicate outcomes were removed, 124 outcomes that demonstrated similar characteristics were categorized under 18 domains, as illustrated in Table 2 and Table S1 (available as Supplementary data at JAC-AMR Online).
Table 2.
Summary of number of outcomes reported
| Domain | Sub domain | No. of outcomes |
|---|---|---|
| Antibiotic use | Antibiotic | 2 |
| Treatment duration | 1 | |
| Clinical cure | Clinical cure | 7 |
| Symptom resolution | Symptom resolution | 12 |
| Symptoms | Duration | 3 |
| Symptom severity | 2 | |
| Overall symptoms | 5 | |
| Complications | Complications | 17 |
| Adverse treatment reactions | Adverse treatment reactions | 25 |
| Relapse | Relapse | 16 |
| Failure | Failure | 4 |
| Re-consultation | 4 | |
| Secondary antibiotics | 1 | |
| Pyuria | Pyuria | 1 |
| Discontinuation | Discontinuation | 1 |
| Tolerance | Adherence | 1 |
| Quality of life | Quality of life | 7 |
| Patient satisfaction | Satisfaction | 2 |
| Antibiotic resistance | Antibiotic resistance | 1 |
| Bacterial cure | Bacterial cure | 3 |
| Microbiological cure | Microbiological cure | 1 |
| Microbiological relapse | Microbiological relapse | 3 |
| Microbiological failure | Microbiological failure | 5 |
The variety of domains reflects the difference across the outcome measures that have been reported in UTI treatment trials. The majority of outcomes were categorized under the clinical cure domain, with outcomes further categorized into eight related subdomains (clinical cure, symptom resolution, symptoms, complications, adverse treatment reactions, relapse and failure). Microbiological cure was also identified as a domain, with outcomes further categorized into four subdomains.
Other domains investigated the patients’ quality of life, satisfaction with treatment, discontinuance, tolerance and antibiotic resistance. Outcomes related to antibiotic use included the use of antibiotics and the duration of treatment.
Only 3 out of the 18 domains (antibiotic use, symptoms and failure) were further classified into subdomains, which reflected nuances in what the outcome was measuring.
Adverse treatment reactions and complications
The domains adverse treatment reactions (n = 25 outcomes) and complications (n = 17 outcomes) had the most reported outcomes. A range of outcomes were documented within these domains. There were inconsistencies in how adverse treatment reactions and complications were reported in trials, which demonstrates the difficulty in reporting these outcomes. In some cases, each adverse treatment reaction or complication related to treatment was reported as a separate trial outcome.
Quality of life
Quality of life outcomes were not measured across the majority of trials. Five studies included quality of life outcomes (e.g. patient reported quality of life and mental health status), and an additional three included absenteeism from work as outcomes.
Timepoints
Eight of the 18 domains included similar outcomes measured or reported at different points in time. The variety of timepoints reported within outcomes has resulted in an overall increase in the number of outcomes reported. For example, the relapse domain included outcomes related to relapse and recurrence after the initial resolution of UTI symptoms. Thirteen of 16 outcomes included within this domain reported a timepoint. Reported timepoints ranged from short- (14 days) to long-term (1 year) periods. The outcomes that were not time-bound were temporally broad, e.g. relapse after initial resolution of symptoms, and related to measuring when a relapse happened.
Seven outcomes were categorized as clinical cure (Table 3). Temporally, these outcomes related to clinical cure generally, clinical cure after initiation of treatment, on completion of treatment and at follow-up. This illustrates the need for trialists to evaluate outcomes of treatment at different timepoints. However, the inconsistency and overlap of timepoints related to initiation and completion of treatment may also contribute to difficulties in synthesizing trial findings.
Table 3.
Clinical cure domain
| Clinical cure |
|---|
| Clinical cure |
| Clinical cure by Day 4 from initiation of treatment |
| Clinical cure by Day 7 from initiation of treatment |
| Clinical cure 5–9 days after completion of the treatment |
| Clinical cure 3 weeks after completion of the treatment |
| Clinical cure 4–6 weeks after completion of the treatment |
| Clinical cure by the 30 day follow-up visit |
Discussion
This systematic review of clinical trials for the treatment of uncomplicated UTIs demonstrates the substantial differences in how outcomes are measured, with 124 outcomes reported across 18 domains. The acute nature of UTI episodes contributes to the ambiguity around key domains such as ‘clinical cure’, which in turn also contributes to the inconsistency in associated timepoints of outcome measurement. The optimal time to report many of the outcomes remains unclear due to a lack of definition or justification for the choice of timepoint. There is a need to develop a core outcome set to improve reporting of trial evidence and strengthen evidence synthesis capability in this area. This would also help minimize the measurement and collection of outcomes of less relevance to stakeholders.
The descriptive nature of this review aligns with its purpose to describe outcomes reported currently rather than compare the results of the included trials. The definition of uncomplicated UTI described in this paper differs from the FDA guidance for developing drugs for treatment of uncomplicated UTI as well as the IDSA/ESCMID treatment guidelines for uncomplicated UTI by including male members of the population; this decision was taken as some of the trials investigating treatments of UTI specified adult populations and not exclusively female populations.
Conclusions
As the second most common infection presenting in primary care, UTI treatment contributes significantly to the prescription of antibiotics in primary care, accounting for approximately 15%–20% of antibiotic prescriptions.56 As the fight against antibiotic resistance intensifies, it is important that the search continues for effective, safe alternative treatments to antibiotics for UTI.
The impact of the trials investigating the most effective treatments for UTI that have been completed or are ongoing in this area is limited due to the volume and variation of outcomes currently reported. This systematic review highlights the inconsistencies around which outcomes are most important or at what timepoints they should be reported. This hampers evidence synthesis in this area.
To improve consistency, a standardized set of outcomes should be developed to report trials investigating effective treatments for UTI and evidence synthesis. The next phase of COSUTI will focus on the development of a core outcome set to address this research gap.
Supplementary Material
Acknowledgements
The HRB-Trials Methodology Network thank the Health Research Board for funding this study through the HRB Primary Care Clinical Trials Network.
Funding
This research was funded by the HRB Primary Care Clinical Trials Network (grant reference number CTN-2014-011).
Transparency declarations
None to declare.
Author contributions
D.D. conceived the study. All authors contributed to the systematic review study protocol development, implementation and analysis. S.D. and D.D. coordinated the review and S.D., C.B. and D.D. drafted the manuscript. All authors commented on drafts and approved the manuscript for submission.
Supplementary data
Table S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. Wagenlehner FME, Hoyme U, Kaase Met al. Uncomplicated urinary tract infections. Dtsch Arztebl Int 2011; 108: 415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suskind AM, Saigal CS, Hanley JMet al. Incidence and management of uncomplicated recurrent urinary tract infections in a national sample of women in the United States. Urology 2016; 90: 50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flores-Mireles AL, Walker JN, Caparon Met al. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015; 13: 269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta K, Hooton TM, Naber KGet al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52: e103–20. [DOI] [PubMed] [Google Scholar]
- 5. Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am 1997; 11: 551–81. [DOI] [PubMed] [Google Scholar]
- 6. Nicolle LE. Epidemiology of urinary tract infections. Clin Microbiol Newsl 2002; 24: 135–40. [Google Scholar]
- 7. Al-Badr A, Al-Shaikh G. Recurrent urinary tract infections management in women: a review. Sultan Qaboos Univ Med J 2013; 13: 359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foxman B, Barlow R, D’Arcy Het al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 2000; 10: 509–15. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JPT, Thompson SG, Deeks JJet al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williamson P, Altman D, Blazeby Jet al. Driving up the quality and relevance of research through the use of agreed core outcomes. J Health Serv Res Policy 2012; 17: 1–2. [DOI] [PubMed] [Google Scholar]
- 11. Williamson PR, Altman DG, Bagley Het al. The COMET Handbook: version 1.0. Trials 2017; 18Suppl 3: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007; 8: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grigoryan L, Trautner BW, Gupta K. Diagnosis and management of urinary tract infections in the outpatient setting: a review. JAMA 2014; 312: 1677–84. [DOI] [PubMed] [Google Scholar]
- 14. Duane S, Vellinga A, Murphy AWet al. COSUTI: a protocol for the development of a core outcome set (COS) for interventions for the treatment of uncomplicated urinary tract infection (UTI) in adults. Trials 2019; 20: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bjerrum L, Gahrn-Hansen B, Grinsted P. Pivmecillinam versus sulfamethizole for short-term treatment of uncomplicated acute cystitis in general practice: a randomized controlled trial. Scand J Prim Health Care 2009; 27: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bleidorn J, Gágyor I, Kochen MMet al. Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection? - results of a randomized controlled pilot trial. BMC Med 2010; 8: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ceran N, Mert D, Kocdogan FYet al. A randomized comparative study of single-dose fosfomycin and 5-day ciprofloxacin in female patients with uncomplicated lower urinary tract infections. J Infect Chemother 2010; 16: 424–30. [DOI] [PubMed] [Google Scholar]
- 18. Chen Y, Yang H, Lu Get al. Prulifloxacin versus levofloxacin in the treatment of respiratory and urinary tract infections: a multicentre, double-blind, randomized controlled clinical trial. Chemotherapy 2012; 58: 249–56. [DOI] [PubMed] [Google Scholar]
- 19. Dawson-Hahn EE, Mickan S, Onakpoya Iet al. Short-course versus long-course oral antibiotic treatment for infections treated in outpatient settings: a review of systematic reviews. Fam Pract 2017; 34: 511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deepalatha C, Deshpande N. A comparative study of phenazopyridine (Pyridium) and Cystone as short-term analgesic in uncomplicated urinary tract infection. Int J Pharm Pharm Sci 2011; 3Suppl 2: 224–6. [Google Scholar]
- 21. Drozdov D, Thomer A, Meili Met al. Procalcitonin, pyuria and proadrenomedullin in the management of urinary tract infections - ‘triple p in uti’: study protocol for a randomized controlled trial. Trials 2013; 14: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dybowski B, Jabłońska O, Radziszewski Pet al. Ciprofloxacin and furagin in acute cystitis: comparison of early immune and microbiological results. Int J Antimicrob Agents 2008; 31: 130–4. [DOI] [PubMed] [Google Scholar]
- 23. Falagas M, Kotsantis IK, Vouloumanou EKet al. P685 Antibiotics versus placebo in the treatment of women with uncomplicated cystitis: a meta-analysis of randomised controlled trials. Clin Microbiol Infect 2009; 15Suppl 4: S163–4. [DOI] [PubMed] [Google Scholar]
- 24. Falagas ME, Vouloumanou EK, Togias AGet al. Fosfomycin versus other antibiotics for the treatment of cystitis: a meta-analysis of randomized controlled trials. J Antimicrob Chemother 2010; 65: 1862–77. [DOI] [PubMed] [Google Scholar]
- 25. Ferry SA, Holm SE, Stenlund Het al. Clinical and bacteriological outcome of different doses and duration of pivmecillinam compared with placebo therapy of uncomplicated lower urinary tract infection in women: the LUTIW project. Scand J Prim Health Care 2007; 25: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gágyor I, Bleidorn J, Kochen MMet al. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ 2015; 351: h6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gagyor I, Hummers-Pradier E, Kochen MMet al. Immediate versus conditional treatment of uncomplicated urinary tract infection - a randomized-controlled comparative effectiveness study in general practices. BMC Infect Dis 2012; 28: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grabein B, Graninger W, Rodríguez Baño Jet al. Intravenous fosfomycin-back to the future. Systematic review and meta-analysis of the clinical literature. Clin Microbiol Infect 2017; 23: 363–72. [DOI] [PubMed] [Google Scholar]
- 29. Gupta K, Hooton TM, Roberts PLet al. Short-course nitrofurantoin for the treatment of acute uncomplicated cystitis in women. Arch Intern Med 2007; 167: 2207–12. [DOI] [PubMed] [Google Scholar]
- 30. Gutiérrez-Castrellón P, Díaz-García L, de Colsa-Ranero Aet al. [Efficacy and safety of ciprofloxacin in the treatment of urinary tract infections (UTIs) in adults: a systematic review with meta-analysis. Gac Med Mex 2015; 151: 225–44. [PubMed] [Google Scholar]
- 31. Haghighi B, Oskuilar H, Nejadi Oet al. Comparison of 3-day and 7-day ciprofloxacin regimen for the treatment of uncomplicated urinary tract infection in women: a randomized double-blind clinical trial. Iran J Clin Infect Dis 2010; 5: 70–4. [Google Scholar]
- 32. Hamasuna R, Tanaka K, Hayami Het al. Treatment of acute uncomplicated cystitis with faropenem for 3 days versus 7 days: multicentre, randomized, open-label, controlled trial. J Antimicrob Chemother 2014; 69: 1675–80. [DOI] [PubMed] [Google Scholar]
- 33. Hooton TM, Roberts PL, Stapleton AE. Cefpodoxime vs ciprofloxacin for short-course treatment of acute uncomplicated cystitis: a randomized trial. JAMA 2012; 307: 583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huttner A, Verhaegh EM, Harbarth Set al. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015; 70: 2456–64. [DOI] [PubMed] [Google Scholar]
- 35. Jamil MN, Farooq U, Sultan Bet al. Role of symptomatic treatment in comparison to antibiotics in uncomplicated urinary tract infections. J Ayub Med Coll Abbottabad 2016; 28: 734–7. [PubMed] [Google Scholar]
- 36. Jansåker F, Frimodt-Møller N, Bjerrum Let al. The efficacy of pivmecillinam: 3 days or 5 days t.i.d against community acquired uncomplicated lower urinary tract infections - a randomized, double-blinded, placebo-controlled clinical trial study protocol. BMC Infect Dis 2016; 16: 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Knottnerus BJ, Grigoryan L, Geerlings SEet al. Comparative effectiveness of antibiotics for uncomplicated urinary tract infections: network meta-analysis of randomized trials. Fam Pract 2012; 29: 659–70. [DOI] [PubMed] [Google Scholar]
- 38. Kronenberg A, Bütikofer L, Odutayo Aet al. Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial. BMJ 2017; 359: j4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Letelier ME, Hidalgo-Castro F, López-Valladares Met al. BG126® phytodrug improves urinary tract infection treatment with nitrofurantoin in adult women in a double-blind randomized clinical trial. J Herb Med 2017; 9: 60–7. [Google Scholar]
- 40. Little P, Moore MV, Turner Set al. Effectiveness of five different approaches in management of urinary tract infection: randomised controlled trial. BMJ 2010; 340: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lutters M, Vogt-Ferrier NB. Antibiotic duration for treating uncomplicated, symptomatic lower urinary tract infections in elderly women. Cochrane Database Syst Rev 2008: CD001535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Masson P, Matheson S, Webster ACet al. Meta-analyses in prevention and treatment of urinary tract infections. Infect Dis Clin North Am 2009; 23: 355–85. [DOI] [PubMed] [Google Scholar]
- 43. Maurya SK, Singh AK. Clinical efficacy of Moringa oleifera Lam. stems bark in urinary tract infections. Int Sch Res Notices 2014; 2014: 906843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Naber KG, Wullt B, Wagenlehner FME. Antibiotic treatment of uncomplicated urinary tract infection in premenopausal women. Int J Antimicrob Agents 2011; 38: 21–35. [DOI] [PubMed] [Google Scholar]
- 45. O’Kane DB, Dave SK, Gore Net al. Urinary alkalisation for uncomplicated urinary tract infection. Cochrane Database Syst Rev 2013: CD010745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O’Kane DB, Dave SK, Gore Net al. Urinary alkalisation for symptomatic uncomplicated urinary tract infection in women. Cochrane Database Syst Rev 2016: CD010745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Palou J, Angulo JC, Ramón de Fata Fet al. [Randomized comparative study for the assessment of a new therapeutic schedule of fosfomycin trometamol in postmenopausal women with uncomplicated lower urinary tract infection.] Actas Urol Esp 2013; 37: 147–55. [DOI] [PubMed] [Google Scholar]
- 48. Pinart M, Kranz J, Jensen Ket al. Optimal dosage and duration of pivmecillinam treatment for uncomplicated lower urinary tract infections: a systematic review and meta-analysis. Int J Infect Dis 2017; 58: 96–109. [DOI] [PubMed] [Google Scholar]
- 49. Sadahira T, Wada K, Araki Met al. Efficacy and safety of 3 day versus 7 day cefditoren pivoxil regimens for acute uncomplicated cystitis: multicentre, randomized, open-label trial. J Antimicrob Chemother 2017; 72: 529–34. [DOI] [PubMed] [Google Scholar]
- 50. Stange R, Schneider B, Albrecht Uet al. Results of a randomized, prospective, double-dummy, double-blind trial to compare efficacy and safety of a herbal combination containing Tropaeoli majoris herba and Armoraciae rusticanae radix with co-trimoxazole in patients with acute and uncomplicated cystitis. Res Rep Urol 2017; 9: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trill J, Simpson C, Webley Fet al. Uva-ursi extract and ibuprofen as alternative treatments of adult female urinary tract infection (ATAFUTI): study protocol for a randomised controlled trial. Trials 2017; 18: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vachhani AV, Barvaliya M, Naik Vet al. Effectiveness and tolerability of short course co-trimoxazole, norfloxacin and levofloxacin in bacteriological cure of uncomplicated urinary tract infection in outpatient setting. An open label, parallel group, randomized controlled trial. Infez Med 2015; 23: 155–60. [PubMed] [Google Scholar]
- 53. Vidal L, Gafter-Gvili A, Borok Set al. Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother 2007; 60: 247–57. [DOI] [PubMed] [Google Scholar]
- 54. Vik I, Bollestad M, Grude Net al. Ibuprofen versus mecillinam for uncomplicated cystitis - a randomized controlled trial study protocol. BMC Infect Dis 2014; 14: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trestioreanu A Z, Green H, Paul Met al. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database Syst Rev 2010: CD007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Butler CC, Francis NA, Thomas-Jones Eet al. Point-of-care urine culture for managing urinary tract infection in primary care: a randomised controlled trial of clinical and cost-effectiveness. Br J Gen Pract 2018; 68: e268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.