Abstract
Clonal hematopoiesis (CH) in patients with acute myeloid leukemia (AML) may persist beyond attaining complete remission. From a consecutive cohort of 67 NPM1mut AML patients, we identified 50 who achieved NPM1 mutation clearance and had parallel multicolor flow cytometry (MFC) and next generation sequencing. Thirteen (26%) cleared all mutations, 37 (74%) had persistent CH, frequently involving DNMT3A (70%), TET2 (27%), IDH2 (19%), IDH1 (11%). A small (<1%) number of aberrant CD34+ myeloblasts but immunophenotypically different from original AML blasts (herein referred to as a pre-leukemic (PL) phenotype) was detected in 17 (49%) patients with CH but not in any patients with complete clearance of all mutations (p=0.0037). A PL phenotype was associated with higher mutation burden (p=0.005). Persistent IDH2 and SRSF2 mutations were exclusively observed in PL+CH+ cases (p=0.016). Persistent dysplasia was seen exclusively in cases with a PL+ phenotype (29% vs none; p=0.040). PL+ phenotype did not correlate with age, intensity of induction therapy or relapse-free survival. Post-remission CH in the setting of NPM1mut clearance is common and may result in immunophenotypic changes in myeloid progenitors. It is important to not misinterpret these cells as AML MRD.
Introduction
Measurable residual disease (MRD) in a patient with acute myeloid leukemia (AML) has been proven to be one of the most reliable predictors of leukemia relapse and outcome. Therefore, monitoring AML MRD has become increasingly part of standard of care for AML patients. MRD assessment can be performed using multicolor flow cytometry (MFC) immunophenotyping or by molecular methods, the latter including next generation sequencing (NGS) or ultrasensitive methods such as digital droplet PCR, single molecule molecular inversion probe capture or other sequencing methods incorporating error correction schemes based on molecular barcoding (1–9). In the recent consensus guidelines by the European LeukemiaNet MRD Working Party for AML MRD (10), MFC monitoring of MRD is recommended for all AML subtypes, using the approach of identification of classic leukemia associated immunophenotypes (LAIP) (11) and identification of immunophenotypic deviation from normal (DfN). MFC and molecular methods, when used in combination, have shown to enhance risk stratification for AML patients. (3, 8, 12, 13)
Clonal hematopoiesis (CH), defined by the acquisition of somatic gene mutations in hematopoietic stem or progenitor cells, is associated with increasing age and has been shown to confer an increased risk of developing hematopoietic malignancies (14), as well as increased susceptibility to complications arising from other non-neoplastic conditions including coronary artery disease (15, 16). The most frequently mutated genes in the setting of CH are also mutated in myeloid neoplasms including AML. These genes include DNMT3A, TET2, ASXL1, SRSF2, BCOR, TP53, and IDH1/IDH2. CH can persist beyond achieving remission in patients treated for AML and the potential impact on MRD assessment and risk of relapse remains unclear. (17–19).
In AML patients attaining morphologic remission, we routinely perform MRD assessment by MFC on surveillance BM samples and have become aware of the presence of a small number of immunophenotypically aberrant CD34+ myeloblasts in a subset of patients who attain morphological remission. These CD34+CD13+CD33+ myeloblasts are often present at very low (<1%) levels, frequently show abnormally increased CD117 and/or CD123, and decreased CD38 and/or HLA-DR, but no expression of aberrant lymphoid antigens or complete loss or gain of other antigens, reminiscent of changes observed in low-grade myelodysplastic syndromes (MDS)(20–22). This specific immunophenotype bears no resemblance to the original AML. These cells have been suspected to represent underlying MDS, or persistent CH. henceforth referred to as “pre-leukemic” (PL) cells.
To understand potential clinical importance of these immunophenotypically aberrant cells, and examine their association with persistent CH, we conducted this study on a cohort of NPM1mut AML patients. We chose this specific subtype of AML for the following reasons: 1) NPM1 mutations occur in ∼30% of AML and co-mutations in other genes including FLT3, DNMT3A, IDH1, IDH2, and TET2, are frequent (23–26). Several of these mutations have been shown to persist beyond attaining NPM1mut-negative remission (27, 28) (19); 2) NPM1 mutation is strongly associated with AML and is particularly rare in the “pre-leukemic” setting (3, 29); 3) NPM1 mutated cells are highly sensitive to therapy and the NPM1 mutation frequently becomes undetectable in patients who achieve a durable remission; and 4) Most NPM1mut AML cases show highly aberrant and characteristic immunophenotypic alterations at diagnosis, being frequently negative for CD34, with an APL-like immunophenotype or monocytic differentiation (30). This immunophenotype is very different from the above described myeloblasts with a PL+ phenotype. Therefore, NPM1mut AML provides an excellent model for characterization of these specific immunophenotypic changes and their association with CH.
Materials/Subjects and Methods:
We retrospectively reviewed 67 consecutive patients with de novo NPM1-mutated (NPM1mut) AML who were diagnosed and treated at our institution between January 1, 2017 and November 30, 2019 for whom flow cytometric immunophenotyping and molecular profiling by next generation sequencing (NGS) at baseline and at least on one remission sample were available for analysis. Patients with secondary AML and therapy-related disease were excluded from this study. The study was conducted in accord with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) at MD Anderson Cancer Center.
Flow Cytometric Analysis
Eight-color flow cytometry immunophenotypic analysis was performed using a combination of the following 4 tubes on bone marrow (BM) aspirate samples: (1) CD7-FITC, CD33-PE, CD19 PerCP-Cy5.5, CD34-PE-Cy7, CD13-APC, CD38-BV421, CD45-V500; (2) HLADR-FITC, CD117-PE, CD4-PerCP-Cy5.5, CD34-PE-Cy7, CD123-APC, CD19-APC-eF780, CD38-BV421, CD45-V500; (3) HLA-DR-FITC, CD36 or CD71-PE, CD56-PerCP-Cy5.5, CD34-PE-Cy7, CD64-APC, CD19-APC-eF780, CD14-V450, CD45-V500; and (4) CD5 or CD52 FITC, CD2 or CD54-PE, CD22 or CD5 PerCP-Cy5.5, CD34-PE-Cy7, CD38-APC, CD19-eF780, CD15-V450, CD45-V500. All antibodies were obtained from Becton Dickinson (San Jose, CA, USA) or eBioscience (San Diego, CA, USA). Samples were acquired on FACSCanto II instruments (BD Biosciences, San Diego, CA, USA). MRD was quantified as a percentage of total leukocytes after the exclusion of most red blood cell precursors by forward scatter. At least 200,000 live events and 200 CD34+ myeloid progenitors were acquired to achieve a minimum sensitivity of 10−3-10−4 (0.1–0.01%). MRD was defined as cells with an aberrant immunophenotype similar to the original AML (LAIP), or representing a subclone of the original AML or markedly deviating from normal (such as the presence of aberrant lymphoid antigens, or completely loss or gain of certain antigens), a method previously described (11, 31–33).
Cytogenetic Analysis
Conventional karyotyping was performed on G-banded metaphase cells prepared from unstimulated 24-hour and 48-hour BM cultures as described previously.(34) The results were reported using current International System for Human Cytogenetic Nomenclature. (35) Cytogenetic risk was assigned in accord with the revised Medical Research Council classification.(36)
Molecular Analysis
Next-generation sequencing (NGS) was performed interrogating the entire exonic or hotspot regions of 81 genes that are frequently mutated in myeloid malignancies (Supplemental Table 1). This panel was validated at the CLIA-certified molecular diagnostic laboratory at our institution as described previously (19). A sequencing library was prepared using 250 ng of genomic DNA and respective sequencing libraries were subjected to the Illumina MiSeq (Illumina, Inc., San Diego, CA, USA) sequencer. NGS data were analyzed using the SureCall application (Agilent HaloPlex Target Enrichment System). The Integrative Genomics Viewer (IGV, Broad Institute) was used for variant calling. A minimum sequencing coverage of x250 (bidirectional true paired-end sequencing) was required. The analytical sensitivity was established at 1% mutant reads in a background of wild-type reads. Follow up samples were deemed NPM1 wild-type if no NPM1 p.W288fs*12 mutation was detected on manual review of IGV reads and given the specificity of the 4bp insertion even 1 read with exhibiting this change was considered positive. The average coverage of the NPM1 p.W288 locus for our NGS assay is x1323, rendering the sensitivity of NPM1 p.W288fs mutation detection with manual review of IGV reads > 0.01%.
Additionally, a multiplex fluorescent-based polymerase chain reaction (PCR) analysis followed by capillary electrophoresis was performed for detection of internal tandem duplication (ITD) and/or tyrosine kinase domain (TKD) mutations in FLT3 on DNA isolated from BM aspirate samples, as described previously (37).
Statistical analysis
Data analysis was performed using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Parametric samples were compared using the Fisher’s exact test. Non-parametric independent samples were analyzed using the Mann-Whitney U test. Relapse-free survival (RFS) analysis was performed by the Kaplan-Meier method using the log rank test. RFS was calculated from the date of confirmed diagnosis until relapse the time of last available follow up. All variables with a p value <0.05 were considered to be statistically significant. Oncoplots were generated using the Maftools and colorspace, R packages.(38)
Results
Patients
Patients included 26 (39%) men and 41 (61%) women with a median age of 64 years (range, 19–84) at time of diagnosis. The median hemoglobin concentration, white blood cell count and platelet count were 9.3 g/dL (range, 6.2 to 11.1), 5.5×109/L (range, 0.7 to 69.8×109/L) and 43×109/L (range, 9 to 184×109/L), respectively (Table 1). The median bone marrow blast count was 62% (range, 12 to 92). Patients were treated with intensive (n=35; 52%) and/or non-intensive (n=32; 48%) induction regimens (Figure 1). 35 patients received venetoclax-containing regimens. Median follow up for the study cohort was 14.2 months (range 2.1–38.3 months).
Tables 1.
Patient characteristics at baseline (n=67)
| Median | Range | |
| Age (years) | 64 | 19–84 |
| Woman (%) | 41 (61) | |
| Hemoglobin (g/dL) | 9.3 | 6.2 – 11.1 |
| White blood cells, ×109 L | 5.5 | 0.7–69.8 |
| Platelets, ×109/L | 43 | 9 –184 |
| Bone marrow blasts (%) | 62 | 12–92 |
| Number of patients | (%) | |
| Cytogenetic risk | ||
| Intermediate | 57 | 85 |
| Adverse | 3 | 4.5 |
| Insufficient analysis | 7 | 10.5 |
| Treatment | ||
| Intensive | 27 | 40 |
| Intensive+ven | 8 | 12 |
| HMA+ven | 18 | 27 |
| HMA+other | 3 | 4.5 |
| Low-dose cytarabine+other | 3 | 4.5 |
| Ven+ cladribine and low dose AraC | 8 | 12 |
| Allogeneic stem cell transplant | 32 | 48 |
| Best Response | ||
| CR | 60 | 89.5 |
| CRi | 7 | 10.5 |
Abbreviations: HMA: hypomethylating agent; ven:venetoclax
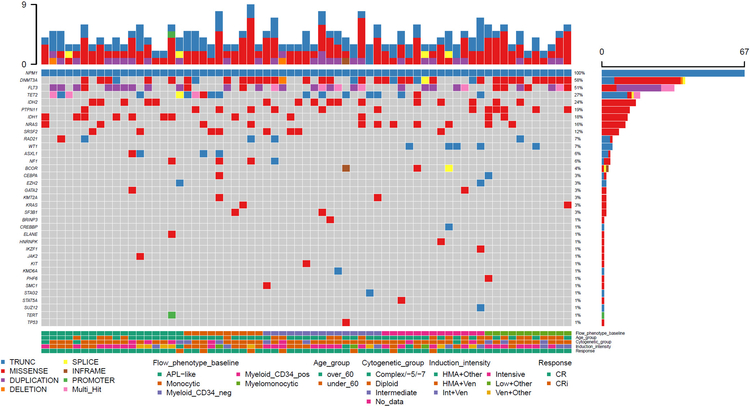
Figure 1.
Clinical features of patients, and flow cytometric immunophenotype and mutational landscape of acute myeloid leukemia samples at baseline. Each column represents one patient/sample. HMA: hypomethylating agent; Ven: venetoclax; CR: complete remission; CRi: complete remission with incomplete hematologic recovery; APL: acute promyelocytic leukemia; neg: negative; pos: positive.
Immunophenotypic characteristics at baseline
Flow cytometric immunophenotypic characterization of the aberrant blast population was performed for all cases at baseline. We classified the aberrant blasts into 5 categories: 18 (27%) cases had acute promyelocytic-like blasts (CD34-, CD117+, HLA-DR-); 13 (19%) cases had aberrant CD34+ myeloid blasts; 15 (22%) cases had aberrant CD34-, CD117+, HLA-DR+ blasts; 11 (16%) cases had myelomonocytic blasts; and 10 (15%) cases had predominantly monocytic blasts, either without a myeloblast component or with a very minor (<10%) myeloblast component. Overall, 81% of cases had CD34- blasts. (Figure 1)
“Pre-Leukemic immunophenotype”
We identified a small number of immunophenotypically aberrant CD34+CD13+CD33+ myeloid progenitors in a subset of patients (detailed numbers described below). This population of cells did not resemble the original AML immunophenotype and did not show aberrant expression of lymphoid antigens or complete gain or loss of antigens when compared with normal myeloid progenitors, however, it showed significantly increased CD123 and/or CD117, frequently with decreased CD38 and/or HLA-DR deviating from the normal patterns of maturation. The alterations in the levels were at least 2 standard deviation beyond or below the normal mean fluorescence intensity (MFI) ranges for normal or regenerating CD34+ myeloid precursors in our laboratory. These cells often represent <1% of total nucleated events, and are referred to as “pre-leukemic” clones for the purpose of this study. Normal range MFI for our flow cytometry assay for these markers is include: CD38-APC >18000; CD117-PE <5000; CD123-APC <1100; HLA-DR-FITC >15000. The identification of a combination of any 2 of the aforementioned alterations was considered as a PL immunophenotype. This PL immunophenotype was not discernable in any baseline AML samples, likely due to the presence of overwhelming number of leukemic blasts at baseline. In most cases, these cells were present in conjunction with normal myeloid progenitors.
Cytogenetic characteristics and mutational landscape at baseline
Among 60 cases with a sufficient metaphase yield for adequate karyotypic analysis, 82% (n=49) had a diploid karyotype; overall 95% (n=57) of patients were assigned an intermediate and 5% (n=3) an adverse cytogenetic risk. All samples had NPM1 p.W288fs mutation at baseline confirmed by NGS. Other recurrent co-mutations included DNMT3A (58%), FLT3 (51%), TET2 (27%), IDH2 (24%), PTPN11 (19%), IDH1 (18%), NRAS (16%), SRSF2 (12%), RAD21 (7%), WT1 (7%), ASXL1 (6%) and NF1 (6%). Other rare co-mutations are included in detailed schematic form in Figure 1.
Post-remission clonal hematopoiesis
Sixty patients achieved complete remission (CR) and 7 achieved CR with incomplete hematologic recovery (CRi). We evaluated serial BM samples on each patient and recorded residual somatic mutations (clonal hematopoiesis) in the setting of NPM1mut clearance and correlated the results with MFC results. Among all follow up BM samples, 61 patients had at least one BM sample with adequate paired MFC and NGS data available for comparison analysis. The median time from initiation of treatment to BM sample analysis ranged from 20–630 days (median 119 days). Eleven (16%) patients had residual detectable NPM1 mutation at all analyzed time points (6 had detectable AML MRD by FCL; in 4 patients the concurrent BM aspirate was insufficient for FCM analysis; and 1 had no detectable AML MRD by FCM; however the VAF of NPM1 mutation in this patient was <0.01); these 11 patients were excluded from further analysis. Of the remaining 50 patients, 13 (26%) had no residual detectable mutations whereas 37 (74%) had persistent CH. The most common mutated genes in the setting of residual CH were DNMT3A (70%), TET2 (27%), IDH2 (19%) and IDH1 (11%); SRSF2 (8%), and ASXL1 (8%); other mutations were less frequent (Figure 2).
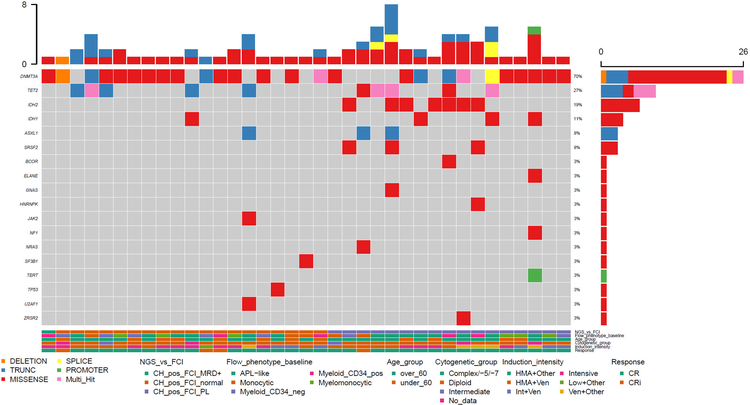
Figure 2.
Clinical features of patients and flow cytometric immunophenotype and mutational landscape of samples with post-remission clonal hematopoiesis after NPM1 mutation clearance. Each column represents one patient/sample. FC: flow cytometry; NGS: next generation sequencing; CH: clonal hematopoiesis; MRD: measurable residual disease; PL: preleukemic immunophenotype; HMA: hypomethylating agent; Ven: venetoclax; CR: complete remission; CRi: complete remission with incomplete hematologic recovery; APL: acute promyelocytic leukemia; neg: negative; pos: positive.
We did not observe a PL immunophenotype in any of the patients with complete clearance of all mutations (n= 12; p=0.0037). Among 37 patients with residual CH, 19 (51%) had no immunophenotypic alterations detected by MFC whereas 17 (49%) had myeloblasts with a “pre-leukemic” (PL+) phenotype; in one case, the original AML immunophenotype had some overlap with the PL+ phenotype and the case was initially thought to be AML MRD positive by flow although no residual NPM1 mutation was detected. The original blasts had an APL-like immunophenotype in this case with partial CD7 expression. The MRD-like PL cells had typical PL like features however, a small subset <10% showed dim CD7 expression which is not an aberrant feature when present on CD13-negative myeloid progenitors in the setting of regeneration; however, due to the presence of CD7 on the original leukemia, this was interpreted as MRD, although other similarities (loss of CD34 with concurrent expression CD7) were not present on the PL cells. This case was not included in the subsequent analyses for this study, although in retrospect, it is likely represented a PL phenotype as well. One patient had very low-level detectable AML MRD (0.01%) by MFC, but no residual detectable mutation by NGS, likely attributable to the higher sensitivity of MFC vs NGS in this study.
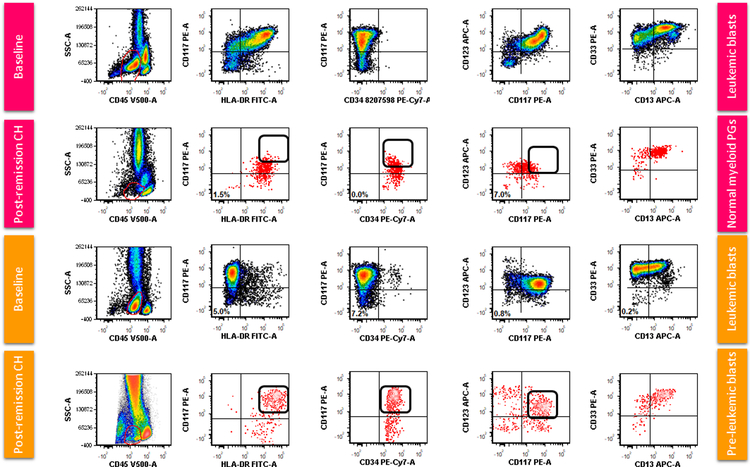
Among cases with persistent CH, we compared the group harboring myeloid progenitors showing a PL immunophenotype with those showing re-emergence of myeloid progenitors with a completely normal immunophenotype. Figure 3 shows examples of MFC dot plots at baseline and at the time of post-remission CH for each of these two groups, respectively. The CD34+ myeloid precursors (hematogones were excluded) start to show diverse differentiation, some towards plasmcytoid dentirtic cell differentiation (CD123bright+), and others towards erythroid differentiation (CD123-). The cells committed to myeloid differentiation are the ones exhibiting the abnormal phenotype with high CD117 and CD123. We also compared hematogones between the PL+ and PL- groups and observed no significant difference between % hematogones as a fraction of CD34-positive progenitors between the PL+ and PL- groups in patients with residual CH (p=0.45).
Figure 3.
Flow cytometric dot plots comparing a case with aberrant myeloid blasts (CD34-, CD117+, HLA-DR+) at presentation and normalization of myeloid progenitors (top half of panel) with a case with acute promyelocytic-like leukemia blasts (CD34-, CD1117+, HLA-DR+) and persistent preleukemic (PL) cells (bottom half of panel), both in the setting of post-remission clonal hematopoiesis. Note the brighter expression of CD13, CD117 and CD123 on PL CD34+ myeloid progenitors (red events) in comparison with normal myeloid progenitors (PGs). The arrows highlight the increased expression of CD117 and CD123 in the CD34+ myeloid progenitors in the case with PL phenotype compared with the case showing normal regeneration.
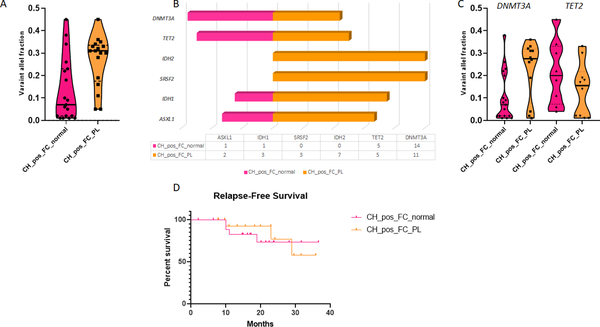
Mutation VAF ≥0.05 was significantly more common (p=0.008) in cases with MFC PL+ (100%) vs cases with a normal MFC phenotype (63%). The median VAF of the most dominant mutation in the group with a PL phenotype was 0.31 (range 0.05—0.45) vs 0.07 (range 0.01–0.045) for the group with normal immunophenotype (p=0.005; Figure 4A). Cases with a PL phenotype tended to have more mutations compared with the group with a normal MFC immunophenotype (median 1 mutation for MFC normal vs 2 mutations for MFC PL), but the difference did not attain statistical significance (p=0.06). IDH2 and SRSF2 mutations were exclusively observed in PL+CH+ cases, with the former being statistically significant when compared with the MFC-normal group (p=0.016; Figure 4B). The distribution of other mutations was not different among the two groups; however, the median VAF for DNMT3A mutations was significantly higher in the group with a PL phenotype compared with the group with normal myeloid progenitors (0.28 vs 0.07, respectively; p=0.02, Figure 4C).
Figure 4.
(A) Comparison of variant allelic fraction (VAF) of mutations in the setting of post-remission clonal hematopoiesis (CH) in the group with normalization of myeloid progenitors by multicolor flow cytometry (MFC), compared with those with preleukemic (PL) CD34+ cells. The median VAF of the most dominant mutation in the group with a PL immunophenotype was 0.31 (range 0.05—0.45) vs 0.07 (range 0.01–0.045) in the group with myeloid progenitors with normal immunophenotype (p=0.005). (B) The distribution of the most common mutations in the setting of post-remission CH between the MFC normal and MFC PL groups: SRSF2 and IDH2 mutations were exclusively seen in the PL group. (C) Comparison of VAF of DNMT3A and TET2 mutations between the PL and MFC normal groups. The VAF for DNMT3A mutations was significantly higher in the group with PL immunophenotype compared with the group with normal myeloid progenitors (0.28 vs 0.07, respectively; p=0.02) (D) There was no difference in relapse free survival between the FC normal and FC PL groups.
We compared CBC indices for patients with residual CH and a PL immunophenotype by MFC and those with complete normalization of myeloid progenitors by MFC at a time point closest to day 90 from therapy at which data was available for comparison (median 119 days, range 42–262 days). There was no significant difference in hemoglobin level (median 9.4 g/dl for FC normal vs 10.3 g/dl for FC PL; p=0.16), platelet count (median 74 × 109/L for FC normal vs 83 × 109/L for FC PL; p=0.56), or absolute neutrophil count (median 800 × 109/L for FC normal vs 550 × 109/L for FC PL; p=0.99).
Multiple follow up BM specimens were assessed morphologically for sustained morphologic evidence of dysplasia using the criteria proposed by Della Porta et al (39), blinded to mutation and MFC information. Two cases had persistent BM aplasia and were excluded from this analysis. In the remaining 34 patients, dysplasia was seen exclusively in cases with a MFC PL phenotype (5/17; 29%); none of the cases with a normal MFC immunophenotype (n=17) had morphologic evidence of dysplasia (p=0.04).
There was no association between PL immunophenotype and age (median 64 years for patients with complete normalization of CD34+ myeloid blasts vs 67 years for patients with a PL immunophenotype; p=0.2) or the intensity of induction therapy (58% intensive and 42% non-intensive in FC normal group vs 41% intensive and 59% non-intensive in the FC PL group; p=0.5). Presence of a PL phenotype was not associated with a shorter relapse-free survival (RFS; median not reached for both groups; p=0.75, Figure 4D). Relapse occurred in 3/17 patients with PL phenotype (1 treated with intensive induction regimen and 2 with non-intensive) and 4/19 patients showing normal FCM phenotype (2 treated with intensive induction regimen and 2 with non-intensive).
Discussion
Data derived from this large cohort of NPM1mut AML proved this neoplasm to be an excellent model to study clonal hematopoiesis (CH) and associated MFC findings in AML remission and MRD negative BM.
In the timeframe of approximately 3 years, we identified 67 adult NPM1mut AML patients who had paired MFC and NGS data at initial diagnosis. As expected for NPM1mut AML, 82% of these neoplasms had a diploid karyotype and carried other co-mutations. The frequency and distribution of these mutations is similar to what has been reported in NPM1mut AML (23–26, 40). Approximately half of the patients received intensive chemotherapy with or without venetoclax, and another half received hypomethylating agents (HMA) (27%) or low-dose chemotherapy, either with venetoclax or other small molecule inhibitors. All patients achieved morphological remission, but in 16% of patients the NPM1 mutation never cleared either prior to stem cell transplant, or at the time of last follow up.
Among those with a negative MRD status who had parallel MFC and NGS follow-up BM samples, persistent co-mutations, or persistent CH was detected in 74% patients. This frequency appears to be higher than that reported in 430 unselected AML patients by others (12); the differences are likely attributed to frequent co-mutations in NPM1mut AML and a larger NGS panel used in the current study. Among persistent co-mutations, those involved in the signaling pathways, including FLT3, NRAS and PTPN11 mutations, all became undetectable; these observations are in keeping with MRD negativity in these BM samples. The most frequently detected mutations involved DNMT3A (70%), followed by TET2 (27%), IDH2 (19%), IDH1 (11%), SRSF2 (8%) and ASXL1 (8%). Others have reported that DTA (DNMT3A, TET2, ASXL1) mutations are the most common persistent mutations in CH; with DNMT3A 78.7%, TET2 54.2%, and ASXL1 51.6% among previously mentioned 430 unselected AML (12), In the current cohort of NPM1mut AML patients, DNMT3A and TET2 were the top two persistent co-mutations, but ASXL1 was detected in only 8% of cases, likely reflecting the uncommon association of ASXL1 with NPM1mut AML. There was a trend for shorter RFS in patients with persistent CH compared with those with complete clearance of all mutations among patients who achieved MRD negativity; however the difference did not reach statistical significance during this short follow up time (median survival not reached for both groups; p=0.11, Supplemental Figure 1). The clinical implications of CH in the setting of post-AML remission have been controversial. Some studies have shown that persistent DTA mutations appear to bear no impact on the risk of relapse in patients with AML (12, 41) whereas other studies with longer term follow up have contradicted these results (42). There is also evidence to suggest that the persistence of other mutations such as IDH1/IDH2 does confer an increased risk of relapse in patients despite achieving flow cytometric MRD-negative CR. (19) It is noteworthy that in a European LeukemiaNet MRD Working Party for AML MRD consensus statement (10), molecular MRD is limited to acute promyelocytic leukemia, core binding factor AML, and NPM1mut AML by real time quantitative PCR. These guidelines do not include monitoring mutations in FLT3 (ITD or TKD), NRAS, KRAS, IDH1, IDH2, or MLL-partial tandem duplications, as single markers of MRD. Recently the term clonal hematopoiesis is proposed to denote the presence of any non-AML-related mutations or cytogenetic alteration in patients achieving morphologic CR after treatment for AML. (43)
Immunophenotypically, in about 80% of NPM1mut AML cases in this study the blasts were negative for CD34, showing a frequent APL-like immunophenotype or monocytic differentiation, with a LAIP distinctly different from the “preleukemic cell immunophenotype”. In only one case, the baseline AML immunophenotype shared a close resemblance to the PL-immunophenotype. The CD34+CD13+CD33+ cells with a PL-immunophenotype, represented <1% of total nucleated events in all cases, were detected in around half of the patients with persistent CH, and were not detected in patients without persistent co-mutations. A further comparison among patients with persistent CH with or without a PL+ cells, the VAFs of detected co-mutations were significant higher in BM specimens with PL+ CD34+ myeloblasts than those without, either by median VAF or by a cutoff VAF ≥5%. The BM specimens with PL+ cells also showed a tendency to have more co-mutations. Interestingly, IDH2 and SRSF2 mutations were exclusively observed in PL+CH+ cases. Both IDH2 and SRSF2 mutations are among those that appear to persist in the setting of CR in patients with AML. Whereas SRSF2 is not associated with relapse (12, 44), persistence of IDH2 mutations in CR showed increased risk of AML relapse, including in NPM1mut AML. (45, 46) The exclusive association of IDH2 and SRSF2 mutations with a residual PL phenotype in the setting of CH warrants further investigation. In this cohort, the presence of PL phenotype cells was not associated with age, intensity of induction, or relapse-free survival. Regarding the latter, we acknowledge that the analysis is limited due to a short follow-up time and that the median relapse-free survival was not reached in either group.
Since these PL cells closely resemble CD34+ myeloblasts in low-grade MDS, described by others and confirmed in our own laboratory (20–22), we sought to determine whether BM samples with PL cells had morphologic evidence of underlying MDS. CBC indices were collected and compared; however we did not find any significant difference between patients with or without PL cells. We note that most of these patients were still receiving active therapy and therefore myelosuppression and cytopenia(s) were common. Additionally, the therapeutic modalities and time intervals from initial diagnosis varied significantly when CBC indices were collected. On the other hand, morphologic evidence of dysplasia in BM (evaluated blind to MFC and NGS data) beyond mild changes was observed and persistent in 5 patients, all of whom had PL cells (29%). However, unlike some cases of MDS, the aberrant PL blasts do not show aberrant expression of lymphoid antigens (CD2, CD5, CD7) or complete loss or aberrant gain of normally present antigens. Although the presence of these PL cells, and CH may not be associated with AML relapse, the effect on the recovery of normal hematopoiesis in patients who achieved long term remission remains to be determined and longer follow up is necessary to determine whether the presence of PL cells is associated with increased risk of relapse. We acknowledge that the wide range of time for which the analysis on follow up samples was done is a limitation of this study; however, the retrospective nature of the study unfortunately makes a more consistent analysis with a narrow time frame of follow up and analysis across all patients impossible. Another limitation is the fact that our AML MRD assay is not designed to analyze maturing myelomonocytic cells or erythroid precursors; instead, we focused on the analysis of CD34+ myeloid progenitors that give rise to other mature myeloid cells. The flow cytometric changes observed in post-remission CH are in some ways reminiscent of cases of low grade MDS where CD34+ myeloblasts constitute a small proportion of BM cells, exhibiting phenotypic abnormalities, whereas mutations are detected in high VAF (total BM cells) because NGS is performed on bulk BM cells whereas flow cytometry assessment for AML MRD and MDS focuses mainly on immature cells..
In summary, we describe a pre-leukemic cell immunophenotype (PL) in AML patients with persistent co-mutations/clonal hematopoiesis (CH), using NPM1mut AML as our model. The PL-phenotype is characterized by a small number (<1%) of aberrant CD34+CD13+CD33+ myeloid precursors, showing altered levels of several normally expressed antigens beyond reactive/regenerative changes, but different from the original AML. In the detection of AML MRD by MFC, these cells should not be interpreted as AML MRD because of their difference from normal. On the other hand, a PL+ phenotype is associated with persistent underlying co-mutations/CH with a high mutation burden.. Although patients may not have an associated risk for AML relapse, the long term significance of these PL cells and associated CH remain to be determined. Additional studies in other AML subtypes are warranted for further characterization of cells with a PL immunophenotype.
Supplementary Material
Footnotes
Conflict of Interest: None of the authors have declared any relevant conflicts of interest.
References
- 1.Ritterhouse LL, Parilla M, Zhen CJ, Wurst MN, Puranik R, Henderson CM, et al. Clinical Validation and Implementation of a Measurable Residual Disease Assay for NPM1 in Acute Myeloid Leukemia by Error-Corrected Next-Generation Sequencing. Molecular diagnosis & therapy. 2019;23(6):791–802. [DOI] [PubMed] [Google Scholar]
- 2.Forghieri F, Comoli P, Marasca R, Potenza L, Luppi M. Minimal/Measurable Residual Disease Monitoring in NPM1-Mutated Acute Myeloid Leukemia: A Clinical Viewpoint and Perspectives. International journal of molecular sciences. 2018;19(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Othus M, Walter RB, Estey EH, Wu D, Wood BL. Deep NPM1 Sequencing Following Allogeneic Hematopoietic Cell Transplantation Improves Risk Assessment in Adults with NPM1-Mutated AML. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2018;24(8):1615–20. [DOI] [PubMed] [Google Scholar]
- 4.Patkar N, Kodgule R, Kakirde C, Raval G, Bhanshe P, Joshi S, et al. Clinical impact of measurable residual disease monitoring by ultradeep next generation sequencing in NPM1 mutated acute myeloid leukemia. Oncotarget. 2018;9(93):36613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman SD, Hills RK, Virgo P, Khan N, Couzens S, Dillon R, et al. Measurable Residual Disease at Induction Redefines Partial Response in Acute Myeloid Leukemia and Stratifies Outcomes in Patients at Standard Risk Without NPM1 Mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(15):1486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz NM, Mencia-Trinchant N, Hassane DC, Guzman ML. Minimal residual disease in acute myelogenous leukemia. International journal of laboratory hematology. 2017;39 Suppl 1(Suppl 1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lussana F, Caprioli C, Stefanoni P, Pavoni C, Spinelli O, Buklijas K, et al. Molecular Detection of Minimal Residual Disease before Allogeneic Stem Cell Transplantation Predicts a High Incidence of Early Relapse in Adult Patients with NPM1 Positive Acute Myeloid Leukemia. Cancers. 2019;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Getta BM, Devlin SM, Levine RL, Arcila ME, Mohanty AS, Zehir A, et al. Multicolor Flow Cytometry and Multigene Next-Generation Sequencing Are Complementary and Highly Predictive for Relapse in Acute Myeloid Leukemia after Allogeneic Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2017;23(7):1064–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delsing Malmberg E, Johansson Alm S, Nicklasson M, Lazarevic V, Ståhlman S, Samuelsson T, et al. Minimal residual disease assessed with deep sequencing of NPM1 mutations predicts relapse after allogeneic stem cell transplant in AML. Leukemia & lymphoma. 2019;60(2):409–17. [DOI] [PubMed] [Google Scholar]
- 10.Schuurhuis GJ, Heuser M, Freeman S, Béné MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131(12):1275–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaso JM, Wang SA, Jorgensen JL, Lin P. Multi-color flow cytometric immunophenotyping for detection of minimal residual disease in AML: past, present and future. Bone Marrow Transplant. 2014;49(9):1129–38. [DOI] [PubMed] [Google Scholar]
- 12.Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. N Engl J Med. 2018;378(13):1189–99. [DOI] [PubMed] [Google Scholar]
- 13.Venditti A, Piciocchi A, Candoni A, Melillo L, Calafiore V, Cairoli R, et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood. 2019;134(12):935–45. [DOI] [PubMed] [Google Scholar]
- 14.Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood advances. 2018;2(22):3404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimm J, Bill M, Jentzsch M, Beinicke S, Häntschel J, Goldmann K, et al. Clinical impact of clonal hematopoiesis in acute myeloid leukemia patients receiving allogeneic transplantation. Bone Marrow Transplant. 2019;54(8):1189–97. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann L, Metzeler KH. Clonal hematopoiesis and preleukemia-Genetics, biology, and clinical implications. Genes, chromosomes & cancer. 2019;58(12):828–38. [DOI] [PubMed] [Google Scholar]
- 19.Ok CY, Loghavi S, Sui D, Wei P, Kanagal-Shamanna R, Yin CC, et al. Persistent IDH1/2 mutations in remission can predict relapse in patients with acute myeloid leukemia. Haematologica. 2019;104(2):305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westers TM, Ireland R, Kern W, Alhan C, Balleisen JS, Bettelheim P, et al. Standardization of flow cytometry in myelodysplastic syndromes: a report from an international consortium and the European LeukemiaNet Working Group. Leukemia. 2012;26(7):1730–41. [DOI] [PubMed] [Google Scholar]
- 21.Ogata K, Nakamura K, Yokose N, Tamura H, Tachibana M, Taniguchi O, et al. Clinical significance of phenotypic features of blasts in patients with myelodysplastic syndrome. Blood. 2002;100(12):3887–96. [DOI] [PubMed] [Google Scholar]
- 22.Tang G, Jorgensen LJ, Zhou Y, Hu Y, Kersh M, Garcia-Manero G, et al. Multi-color CD34(+) progenitor-focused flow cytometric assay in evaluation of myelodysplastic syndromes in patients with post cancer therapy cytopenia. Leuk Res. 2012;36(8):974–81. [DOI] [PubMed] [Google Scholar]
- 23.Jain P, Kantarjian H, Patel K, Faderl S, Garcia-Manero G, Benjamini O, et al. Mutated NPM1 in patients with acute myeloid leukemia in remission and relapse. Leukemia & lymphoma. 2014;55(6):1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loghavi S, Zuo Z, Ravandi F, Kantarjian HM, Bueso-Ramos C, Zhang L, et al. Clinical features of de novo acute myeloid leukemia with concurrent DNMT3A, FLT3 and NPM1 mutations. Journal of hematology & oncology. 2014;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bataller A, Oñate G, Diaz-Beyá M, Guijarro F, Garrido A, Vives S, et al. Acute myeloid leukemia with NPM1 mutation and favorable European LeukemiaNet category: outcome after preemptive intervention based on measurable residual disease. British journal of haematology. 2020. [DOI] [PubMed] [Google Scholar]
- 26.Patel SS, Kluk MJ, Weinberg OK. NPM1 Biology in Myeloid Neoplasia. Current hematologic malignancy reports. 2020;15(4):350–9. [DOI] [PubMed] [Google Scholar]
- 27.DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachowiez CA, Loghavi S, Kadia TM, Daver N, Borthakur G, Pemmaraju N, et al. Outcomes of older patients with NPM1-mutated AML: current treatments and the promise of venetoclax-based regimens. Blood advances. 2020;4(7):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou WC, Tang JL, Lin LI, Yao M, Tsay W, Chen CY, et al. Nucleophosmin mutations in de novo acute myeloid leukemia: the age-dependent incidences and the stability during disease evolution. Cancer Res. 2006;66(6):3310–6. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Moon A, Hoyle E, Fromm JR, Chen X, Soma L, et al. Pattern associated leukemia immunophenotypes and measurable disease detection in acute myeloid leukemia or myelodysplastic syndrome with mutated NPM1. Cytometry Part B, Clinical cytometry. 2019;96(1):67–72. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Jorgensen JL, Wang SA. How Do We Use Multicolor Flow Cytometry to Detect Minimal Residual Disease in Acute Myeloid Leukemia? Clinics in laboratory medicine. 2017;37(4):787–802. [DOI] [PubMed] [Google Scholar]
- 32.Ouyang J, Goswami M, Peng J, Zuo Z, Daver N, Borthakur G, et al. Comparison of Multiparameter Flow Cytometry Immunophenotypic Analysis and Quantitative RT-PCR for the Detection of Minimal Residual Disease of Core Binding Factor Acute Myeloid Leukemia. Am J Clin Pathol. 2016;145(6):769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah MV, Jorgensen JL, Saliba RM, Wang SA, Alousi AM, Andersson BS, et al. Early Post-Transplant Minimal Residual Disease Assessment Improves Risk Stratification in Acute Myeloid Leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2018;24(7):1514–20. [DOI] [PubMed] [Google Scholar]
- 34.Tang Z, Medeiros LJ, Yin CC, Wang W, Lu X, Young KH, et al. Sex chromosome loss after allogeneic hematopoietic stem cell transplant in patients with hematologic neoplasms: a diagnostic dilemma for clinical cytogeneticists. Mol Cytogenet. 2016;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.An International System for Human Cytogenomic Nomenclature (2016). Basel, Switzerland: Karger; 2016. [Google Scholar]
- 36.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–65. [DOI] [PubMed] [Google Scholar]
- 37.Warren M, Luthra R, Yin CC, Ravandi F, Cortes JE, Kantarjian HM, et al. Clinical impact of change of FLT3 mutation status in acute myeloid leukemia patients. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25(10):1405–12. [DOI] [PubMed] [Google Scholar]
- 38.Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28(11):1747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Della Porta MG, Travaglino E, Boveri E, Ponzoni M, Malcovati L, Papaemmanuil E, et al. Minimal morphological criteria for defining bone marrow dysplasia: a basis for clinical implementation of WHO classification of myelodysplastic syndromes. Leukemia. 2015;29(1):66–75. [DOI] [PubMed] [Google Scholar]
- 40.Boddu PC, Kadia TM, Garcia-Manero G, Cortes J, Alfayez M, Borthakur G, et al. Validation of the 2017 European LeukemiaNet classification for acute myeloid leukemia with NPM1 and FLT3-internal tandem duplication genotypes. Cancer. 2019;125(7):1091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klco JM, Miller CA, Griffith M, Petti A, Spencer DH, Ketkar-Kulkarni S, et al. Association Between Mutation Clearance After Induction Therapy and Outcomes in Acute Myeloid Leukemia. JAMA. 2015;314(8):811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yilmaz M, Wang F, Loghavi S, Bueso-Ramos C, Gumbs C, Little L, et al. Late relapse in acute myeloid leukemia (AML): clonal evolution or therapy-related leukemia? Blood cancer journal. 2019;9(2):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasserjian RP, Steensma DP, Graubert TA, Ebert BL. Clonal hematopoiesis and measurable residual disease assessment in acute myeloid leukemia. Blood. 2020;135(20):1729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Press RD, Eickelberg G, Froman A, Yang F, Stentz A, Flatley EM, et al. Next-generation sequencing-defined minimal residual disease before stem cell transplantation predicts acute myeloid leukemia relapse. American journal of hematology. 2019;94(8):902–12. [DOI] [PubMed] [Google Scholar]
- 45.Debarri H, Lebon D, Roumier C, Cheok M, Marceau-Renaut A, Nibourel O, et al. IDH1/2 but not DNMT3A mutations are suitable targets for minimal residual disease monitoring in acute myeloid leukemia patients: a study by the Acute Leukemia French Association. Oncotarget. 2015;6(39):42345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferret Y, Boissel N, Helevaut N, Madic J, Nibourel O, Marceau-Renaut A, et al. Clinical relevance of IDH1/2 mutant allele burden during follow-up in acute myeloid leukemia. A study by the French ALFA group. Haematologica. 2018;103(5):822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.