Abstract
Introduction
Men are missing along the HIV care continuum. However, the estimated proportions of men in sub‐Saharan Africa meeting the UNAIDS 95‐95‐95 goals vary substantially between studies. We sought to estimate proportions of men meeting each of the 95‐95‐95 goals across studies in sub‐Saharan Africa, describe heterogeneity, and summarize qualitative evidence on factors influencing care engagement.
Methods
We systematically searched PubMed and Embase for peer‐reviewed articles published between 1 January 2014 and 16 October 2020. We included studies involving men ≥15 years old, with data from 2009 onward, reporting on at least one 95‐95‐95 goal in sub‐Saharan Africa. We estimated pooled proportions of men meeting these goals using DerSimonion‐Laird random effects models, stratifying by study population (e.g. studies focusing exclusively on men who have sex with men vs. studies that did not), facility setting (healthcare vs. community site), region (eastern/southern Africa vs. western/central Africa), outcome measurement (e.g. threshold for viral load suppression), median year of data collection (before vs. during or after 2017) and quality criteria. Data from qualitative studies exploring barriers to men's HIV care engagement were summarized using meta‐synthesis.
Results and discussion
We screened 14,896 studies and included 129 studies in the meta‐analysis, compiling data over the data collection period. Forty‐seven studies reported data on knowledge of serostatus, 43 studies reported on antiretroviral therapy use and 74 studies reported on viral suppression. Approximately half of men with HIV reported not knowing their status (0.49 [95% CI, 0.41–0.58; range, 0.09–0.97]) or not being on treatment (0.58 [95% CI, 0.51–0.65; range, 0.07–0.97]), while over three‐quarters of men achieved viral suppression on treatment (0.79 [95% CI, 0.77–0.81; range, 0.39–0.97]. Heterogeneity was high, with variation in estimates across study populations, settings and outcomes. The meta‐synthesis of 40 studies identified three primary domains in which men described risks associated with engagement in HIV care: perceived social norms, health system challenges and poverty.
Conclusions
Psychosocial and systems‐level interventions that change men's perceptions of social norms, improve trust in and accessibility of the health system, and address costs of accessing care are needed to better engage men, especially in HIV testing and treatment.
Keywords: Africa South of the Sahara, continuity of patient care, HIV infections, HIV testing, men, qualitative research
1. INTRODUCTION
Men are disproportionately missing, compared with women, throughout the HIV care continuum in sub‐Saharan Africa [1, 2, 3] and have higher mortality from HIV‐related illnesses [2, 4, 5]. This gap must be bridged if UNAIDS 95‐95‐95 fast‐track goals are to be achieved by 2030 – so that 95% of persons with HIV (PWH) know their status, 95% of persons with known HIV are on antiretroviral therapy (ART) and 95% of PWH on ART are virally suppressed [6]. UNAIDS 2020 estimates show substantial heterogeneity in achieving these goals across the continent, with higher proportions of men meeting these goals in eastern and southern Africa as compared to western and central Africa [5]. However, national and regional data do not capture variability across subgroups of men, which would help target resources towards those who need it most. Moreover, while national programs provide updated yearly data on these goals, examining data over an extended period provides a more nuanced understanding of where there have been, and may continue to be, gaps over time despite some yearly gains, particularly for certain highly vulnerable populations.
Efforts to effectively engage men in HIV care must be informed not only by estimates of where and how they experience challenges along the care continuum but also by a better understanding of subgroup variation. Recent work on the UNAIDS goals has documented socio‐demographic heterogeneity among men who have sex with men (MSM) [7] and among both men and women [8], but the latter study was limited by the availability of data. Certain groups of men are at higher risk of being missed by HIV care, including older men [9] and MSM [7]. However, men are often treated as a homogenous population in assessments of progress towards the UNAIDS goals, without disaggregation by socio‐demographic factors, including education, employment and mobility [10]. There are also challenges in consistently estimating UNAIDS goals due to variation in how they are measured [9, 11]. The extent to which this variation may affect population‐level estimates of men's progression along the continuum is unknown.
Care‐seeking decisions may be contextualized within the framework of risk perception, drawn from behavioural economics, which suggests that people are highly loss averse, meaning that they generally prefer to avoid losses more than they prefer an equivalent gain [12, 13]. This framework has been used to understand HIV care engagement [14] in showing that people are highly influenced by subjective concerns (ie, “losses” or risks), including stigma and costs, which can discourage seeking care [14]. However, it is unclear which perceived risks of HIV care engagement are most salient for men across different settings in sub‐Saharan Africa. This is an important gap in the literature because such information may help guide the design of scalable interventions. While strategies have been designed to engage men in HIV care, including community‐based programs, workplace testing and comprehensive men's health services, data remain limited on their effectiveness [15, 16].
To address these gaps, we conducted a meta‐analysis to estimate the pooled proportion of men in sub‐Saharan Africa meeting the 95‐95‐95 goals and to describe heterogeneity across studies in sub‐Saharan Africa with the aim of identifying which subgroups of men may be most vulnerable throughout the continuum. We applied meta‐synthesis to qualitative studies on factors influencing men's engagement in HIV care to elucidate potential psychosocial and structural drivers of our quantitative findings and identify avenues for intervention.
2. METHODS
2.1. Search strategy and selection criteria
We systematically searched PubMed and Embase for peer‐reviewed articles published after 1 January 2014 (the year in which the UNAIDS goals were set) for consideration of the meta‐analysis or meta‐synthesis (Appendix). For the meta‐analysis, we included cross‐sectional, longitudinal, case–control or randomized trial (including only the control arm) studies conducted in sub‐Saharan Africa involving men ≥15 years of age in which at least part of the sample was enrolled on or after 1 January 2009, so as to focus on the modern HIV testing and treatment era. If studies with data after 2009 included data spanning years prior to 2009, they were included. For the meta‐synthesis, we included qualitative or mixed method studies conducted in sub‐Saharan Africa exploring factors influencing men's engagement in any stage of the continuum, enrolling participants on or after 1 January 2009. For the meta‐analysis and meta‐synthesis, we excluded mathematical modelling studies or studies lacking data disaggregated by sex. The evidence searches were conducted on 15 July 2019. We updated the searches to identify additional studies for the meta‐synthesis on 1 July 2020 and to identify additional studies for the meta‐analysis on 16 October 2020.
We imported all records into Covidence systematic review management software, automatically excluding duplicates [17]. We screened titles and abstracts and then screened the remaining full manuscripts to select studies meeting inclusion criteria for the meta‐analysis and/or meta‐synthesis. Conflicts between any two reviewers were resolved through discussion with a third reviewer.
For the meta‐analysis, we independently extracted the following primary outcomes of interest, selected a priori: the numerator and denominator of men meeting any 95‐95‐95 goal(s) reported. For studies that reported sex‐disaggregated data, we extracted the numerator and denominator of women meeting any 95‐95‐95 goal(s) reported. For the first 95‐95‐95 goal, the numerator was defined as “persons with HIV aware of their serostatus,” and the denominator was defined as “persons with HIV.” For the second 95‐95‐95 goal, the numerator was defined as “persons with HIV on antiretroviral therapy,” and the denominator was defined as “persons with HIV aware of their serostatus.” For the third 95‐95‐95 goal, the numerator was defined as “persons with HIV on antiretroviral therapy and virally suppressed,” and the denominator was defined as “persons with HIV on antiretroviral therapy” (Table A1). In publications where data were not disaggregated by sex, we emailed study authors to request sex‐specific estimates. We extracted data on study and population characteristics for each 95‐95‐95 goal. Study characteristics included: country, setting (rural vs. urban), facility (healthcare vs. community‐based), year of publication and study period year(s). Population characteristics included: employment status, occupation, migratory status, relationship status, sexual minority status (exclusively focused on MSM vs. not exclusively focused on MSM), HIV prevalence as documented in the study data and age of participants.
To assess variation in how the 95‐95‐95 goals were measured, we extracted the following data: whether knowledge of serostatus was ascertained pre‐ versus post‐testing campaign, whether ART status was measured by self‐report or blood test, and the viral load threshold and minimum follow‐up time on ART when viral suppression was ascertained.
To identify items that should be included in our quality assessment of the quantitative studies, we referenced the Newcastle‐Ottawa Quality Assessment Scale for observational studies and the Revised Cochrane risk‐of‐bias tool for randomized trials [18, 19]. To make our quality review straightforward to implement among multiple reviewers, we focused on items most relevant to our analyses of the 95‐95‐95 goals, including the sampling and recruitment process as well as setting, participant characteristics and goal measurement. Therefore, we inspected the full text of manuscripts for clear descriptions of (1) the study setting; (2) the participant selection process; (3) participant characteristics; and (4) the measurement of the 95‐95‐95 goal(s). We categorized the studies into two quality categories: “all criteria met” or, if any of the four criteria were not met, “criteria partially met.” Quality assessment for the qualitative studies was based on criteria used in prior research [20, 21], representing the key conceptual domains in the Critical Appraisal Skills Programme quality assessment tool [22]: clear descriptions of (1) the role of the researcher; (2) the sampling method; (3) the method of data collection; and (4) the method of analysis. Again, we categorized the studies into two quality categories: “all criteria met” or “criteria partially met.”
MFN, OA and CP independently conducted all stages of screening and data extraction. All data were cross‐checked and discrepancies were resolved by consensus.
2.2. Data analysis
Using Stata statistical software (version 16, StataCorp LLC, College Station, TX), we transformed proportions using the Freeman–Tukey variance‐stabilizing double arcsine transformation [23]. We computed pooled estimates of prevalence using the DerSimonian and Laird random effects model [24]. Study‐specific confidence intervals were estimated using the score method [25, 26]. We characterized the extent of heterogeneity between studies using the I 2 statistic [27]. We re‐estimated pooled prevalence stratified by available study‐level variables. The systematic review and meta‐analysis were reported in accordance with PRISMA guidelines [28].
For qualitative studies, we used the iterative process of meta‐synthesis, which stems from early methodology proposed by Noblit and Hare [29] and has come to define a collection of approaches for synthesizing qualitative research [30, 31]. Our process of meta‐synthesis is adapted from more recent interpretations, including approaches used in thematic synthesis [21, 32]. We summarized key themes from the studies, which formed the basis of second‐order constructs, defined as the study authors’ interpretations of participants’ beliefs. We resolved discrepancies through team discussion and created a codebook of second‐order constructs and first‐order constructs, that is direct quotations from study participants. We generated a summary definition for each second‐order construct, which was consolidated into a line of argument leading to a third‐order analysis. We grouped third‐order constructs into broad third‐order labels encompassing domains in which men described perceived risks of engagement in HIV care. Based on participant quotations, we identified factors that heightened men's perceived risks of engagement in care (“barriers”) and factors that lessened their perceived risk and facilitated initial engagement in care and/or reinforced ongoing engagement. While these “facilitators” of care did not address all barriers that men face, we grouped them under the third‐order labels to highlight where there may be potential in mitigating some perceived risks of engagement.
3. RESULTS AND DISCUSSION
Our initial search identified 12,946 articles for screening, of which 1341 were removed as duplicates (Figure 1). We screened titles and abstracts of the remaining 11,605 studies, excluding 10,959 records that did not meet inclusion criteria, and reviewed the full text of 646 articles. Of these, 81 studies were included in the meta‐analysis [33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113] and 29 studies were included in the meta‐synthesis [105, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141]. Our updated searches identified 48 additional studies for the meta‐analysis [142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189] and 11 additional studies for the meta‐synthesis [190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200], most published in 2020.
Figure 1.
Study selection. Search process for selected studies in meta‐analysis and meta‐synthesis.
3.1. Meta‐analysis
For the meta‐analysis, 47 studies reported data on knowledge of positive serostatus [39, 41, 44, 52, 53, 54, 55, 58, 59, 63, 64, 65, 69, 73, 77, 79, 80, 81, 83, 84, 86, 91, 94, 95, 97, 99, 101, 102, 108, 110, 111, 112, 113, 144, 146, 149, 151, 154, 157, 167, 171, 178, 185, 186, 188, 189], 43 studies reported data on ART use [35, 36, 39, 47, 51, 55, 60, 62, 67, 70, 83, 84, 85, 95, 97, 98, 99, 104, 105, 107, 108, 112, 142, 144, 145, 146, 148, 149, 150, 154, 161, 162, 166, 167, 168, 170, 171, 181, 184, 185, 186, 188, 189] and 74 studies reported data on viral suppression (Table 1 and Table A2) [33, 34, 37, 38, 40, 41, 42, 43, 45, 46, 48, 49, 50, 55, 56, 57, 61, 66, 68, 71, 72, 74, 75, 76, 78, 82, 84, 87, 88, 89, 90, 92, 93, 95, 96, 97, 100, 103, 106, 107, 108, 109, 143, 146, 147, 149, 152, 153, 154, 155, 156, 158, 159, 160, 161, 163, 164, 165, 169, 171, 172, 173, 174, 175, 176, 177, 179, 180, 182, 183, 185, 186, 187, 188]. While all studies included data collected in 2009 or later, some studies included data spanning back to 2002 and as recent as 2019, representing 1,564,019 participants in 21 countries. South Africa was the most represented country (40 [31.0%]). Three studies included representation from eastern and southern Africa as well as western and central Africa [76, 150, 180]; of the remaining studies, eastern and southern Africa was more represented (113 [89.7%]) as compared to western and central Africa (13 [10.3%]). The median number of participants was 1688 (interquartile range [IQR], 552–5666; range, 63–248,002). Studies reporting data on knowledge of positive status were most often conducted in community settings (31/47 [66.0%]), as were studies reporting data on ART status (27/43 [62.8%]). In contrast, most studies reporting data on viral suppression were conducted in healthcare facilities (55/74 [74.3%]). MSM were the focus of 14 studies [47, 53, 58, 64, 65, 67, 78, 80, 84, 91, 97, 144, 154, 186]. Nearly, half of studies (61/129 [47.3%]) only partially met quality criteria.
Table 1.
Characteristics of studies included in meta‐analysis (N = 129)
| Characteristics | Studies (n, %) |
|---|---|
| Study design | |
| Prospective cohort | 33 (25.6) |
| Retrospective cohort | 24 (18.6) |
| Cross‐sectional | 63 (48.8) |
| Case–control | 2 (1.6) |
| Randomized trial a | 7 (5.4) |
| Population focus | |
| MSM | 14 (10.9) |
| Heterosexual men or not specified | 115 (89.1) |
| Transgender women | 5 (3.9) |
| Transgender men (explicitly included) | 1 (0.8) |
| Migrant men | 1 (0.8) |
| Year of publication | |
| 2014–2016 | 31 (24.0) |
| 2017–2018 | 34 (26.4) |
| 2019–2020 | 64 (49.6) |
| Region/country b | |
| Eastern and southern Africa c , d | 113 (89.7) |
| South Africa | 39 (31.0) |
| Kenya | 19 (15.1) |
| Uganda | 18 (14.3) |
| Other | Angola (1), Botswana (4), Ethiopia (7), Lesotho (1), Malawi (5), Mozambique (3), Rwanda (6), Swaziland (2), United Republic of Tanzania (6), Zambia (9), Zimbabwe (4) |
| Western and central Africa e , f | 13 (10.3) |
| Nigeria | 7 (5.6) |
| Other | Burkina Faso (1), Cameroon (2), Ghana (1), Mali (1), Senegal (1), Togo (1) |
| Quality criteria | |
| All criteria met | 68 (52.7) |
| Criteria partially met | 61 (47.3) |
Data from randomized trials obtained from control arm.
N = 126 because three studies included compiled data from countries in both regions.
Angola, Botswana, Comoros, Eritrea, Eswatini, Ethiopia, Kenya, Lesotho, Madagascar, Malawi, Mauritius, Mozambique, Namibia, Rwanda, Seychelles, South Africa, South Sudan, Uganda, United Republic of Tanzania, Zambia and Zimbabwe.
There are nine studies representing eastern and southern Africa that include more than one country from this region.
Benin, Burkina Faso, Burundi, Cabo Verde, Cameroon, Central African Republic, Chad, Congo, Cote d'Ivoire, Democratic Republic of Congo, Equatorial Guinea, Gabon, Gambia, Ghana, Guinea, Guinea‐Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Sao Tome and Principe, Senegal, Sierra Leone and Togo.
There is one study representing western and central Africa that includes more than one country from this region.
Most studies reporting on knowledge of positive status asked participants about their status prior to testing in the study (33/47 [70.2%]), whereas some studies provided unclear details (7/47 [14.9%]) or used other methods (7/47 [14.9%]), including asking about knowledge of status after testing within the study. Most studies measured treatment status by self‐report (23/43 [53.5%]), whereas seven studies used a blood test for ART detection (16.3%), five studies used chart documentation (11.6%) and eight studies used more than one method (18.6%). Most studies reporting on viral suppression used 1000 copies/ml as the threshold detection limit of viral suppression (44 [59.5%]), but the limit ranged from 20 to 5000 copies/ml. The minimum amount of time on ART required for measuring viral load varied from 2 to 24 months.
In our analysis of data from 2009 (or prior) through 2020, the pooled prevalence of men with HIV who knew their positive status was 0.49 (95% confidence interval [CI], 0.41–0.58; range, 0.09–0.97) with evidence of high between‐study heterogeneity (I 2 = 99.68%) (Figure 2). The pooled prevalence of men with HIV on ART was 0.58 (95% CI, 0.51–0.65; range, 0.07–0.97), with evidence of high between‐study heterogeneity (I 2 = 99.59%) (Figure 3). The pooled prevalence of men with HIV on ART who achieved viral suppression was 0.79 (95% CI, 0.77–0.81; range, 0.39–0.97), with evidence of high between‐study heterogeneity (I 2 = 98.64%) (Figure 4).
Figure 2.
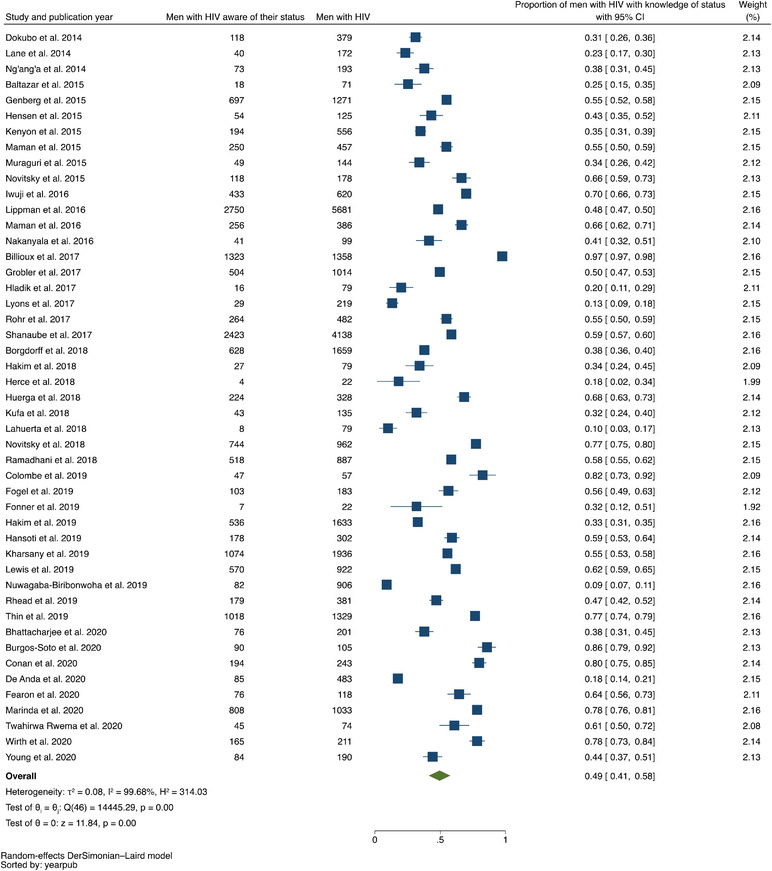
Forest plot of studies reporting data on proportion of men with HIV with knowledge of their status, listed in ascending order of year of publication.
Figure 3.
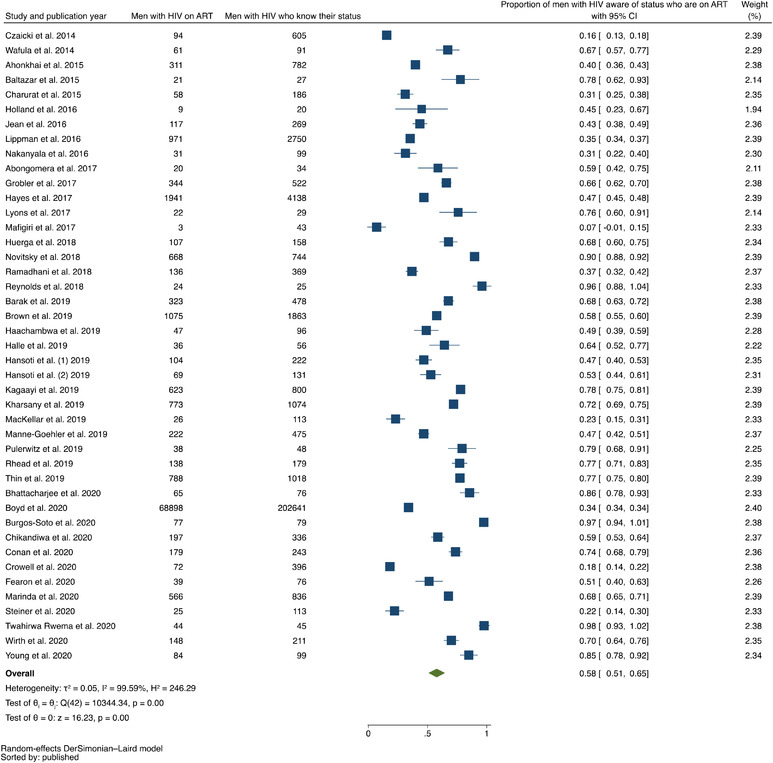
Forest plot of studies reporting data on proportion of men with HIV on ART out of all men with HIV who know their status, listed in ascending order of year of publication.
Figure 4.
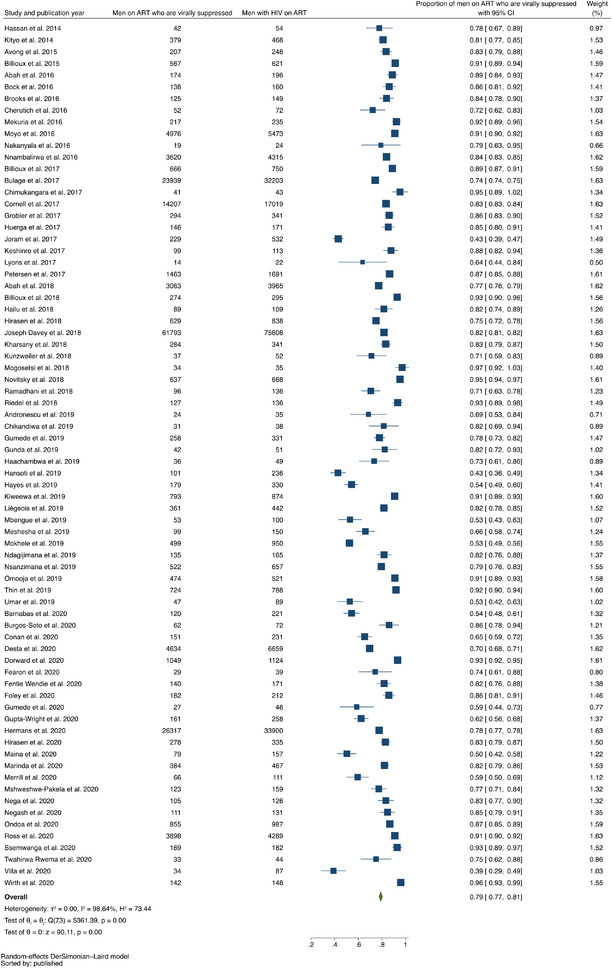
Forest plot of studies reporting data on proportion of men on ART who were virally suppressed out of all men with HIV on ART, listed in ascending order of year of publication.
In studies that enrolled both men and women with HIV, the proportions of men at each stage of the continuum were lower than those for women. A lower pooled proportion of men knew their HIV‐positive serostatus (0.53 [95% CI, 0.44–0.63; range, 0.09–0.97] among men vs. 0.66 [95% CI, 0.59–0.73; range, 0.13–0.98] among women; p = 0.04). A lower pooled proportion of men were on ART (0.54 [95% CI, 0.47–0.62; range, 0.07–0.97] among men vs. 0.62 [95% CI, 0.57–0.67; range, 0.17–0.99] among women; p = 0.09). A lower pooled proportion of men were virally suppressed (0.79 [95% CI, 0.77–0.81; range, 0.39–0.97] among men vs. 0.82 [95% CI, 0.80–0.83; range, 0.44–0.97] among women; p = 0.01) (Appendix).
Population, study setting and outcome measurement varied between studies (Appendix). The pooled proportion of men with HIV who knew their serostatus was lower in studies that focused exclusively on MSM compared with studies that did not exclusively focus on MSM (0.36 [95% CI, 0.23–0.49; range, 0.09–0.97; I 2 = 97.55] among MSM vs. 0.53 [95% CI, 0.44–0.62; range, 0.13–0.64; I 2 = 99.73] in mixed samples, p = 0.04). Similarly, the pooled proportion of men on ART who achieved viral suppression was lower in studies that focused exclusively on MSM compared with studies that did not (0.71 [95% CI, 0.66–0.77; range, 0.39–0.97; I 2<0.001] among MSM vs. 0.79 [95% CI, 0.78–0.81; range, 0.64–0.75; I 2 = 98.73] in mixed samples, p<0.001).
Other differences were noted (Appendix). Comparing data by time period, the pooled proportion of men on ART who were virally suppressed was higher in studies in which the median year of data collection was 2009–2016 versus in studies in which the median year of data collection was 2017–2020 (0.81 [95% CI, 0.79–0.83; range, 0.43–1.03; I 2 = 98.58] for 2009–2016 vs. 0.74 [95% CI, 0.68–0.79; range, 0.39–0.93; I 2 = 98.71] for 2017–2020, p = 0.02). The pooled proportion of men with HIV who knew their status was lower among studies in which knowledge was measured by self‐report prior to study testing versus studies in which knowledge was measured by other methods (0.46 [95% CI, 0.39–0.52; range, 0.09–0.86; I 2 = 99.07] based on self‐report prior to testing vs. 0.51 [95% CI, 0.42–0.60; range, 0.44–0.97; I 2 = 99.47] based on other methods, p<0.001). Finally, the pooled proportion of men with HIV on ART was lower in studies in which ART status was ascertained in a healthcare facility versus studies in which ART status was ascertained in a community setting (0.43 [95% CI, 0.34–0.51; range, 0.07–0.76; I 2 = 98.99] in healthcare facility‐based samples vs. 0.67 [95% CI, 0.58–0.75; range, 0.23–0.97; I 2 = 99.20] in community samples, p<0.001). We found no significant differences comparing by the other variables extracted, including study period, publication year and study quality.
The proportions of men at each stage of the continuum were generally lower in western and central Africa, although the only significant difference was found in comparing the pooled proportions of men on ART, which was higher in eastern and southern Africa in comparison to western and central Africa (0.60 [95% CI, 0.52–0.68; range, 0.07–0.98; I 2 = 99.66] in eastern and southern Africa vs. 0.47 [95% CI, 0.38–0.56; range, 0.31–0.76; I 2 = 88.44] in western and central Africa, p = 0.02). These comparisons likely were limited by the far fewer number of studies from western and central Africa.
3.2. Meta‐synthesis
The meta‐synthesis included 40 studies, representing views from 2683 participants in 10 countries (Table 2). The median number of participants was 38 (IQR, 25–93; range, 15–230). Participants ranged in age from 15 to 80 years.
Table 2.
Qualitative studies of men's engagement in HIV care in sub‐Saharan Africa meeting inclusion criteria for meta‐synthesis (N = 40)
| Population | Country | Dates | Sample size | Male sample size | Component of HIV care continuum | Quality criteria | |
|---|---|---|---|---|---|---|---|
| Adams et al. (2017) | Men with and without HIV and men with unknown status | Swaziland | 2013–2014 | 76 | 76 | Testing and treatment linkage, including Test and Start | All criteria met |
| Adeabgo et al. (2019) | People ages 18–79 | South Africa | 2017–2018 | 32 | 32 | Testing and treatment linkage | Criteria partially met |
| Brown et al. (2019) | Men with HIV | Kenya and Uganda | 2015–2016 | 190 | 190 | Retention in care, including intervention strategies | All criteria met |
| Camlin et al. (2016) | Men with and without HIV and men with unknown status | Kenya and Uganda | 2014 | 111 | 111 | Testing, including barriers and intervention strategies | All criteria met |
| Chikovore et al. (2016) | Men with and without HIV and men with unknown status ages 17–70 | South Africa | 2013 | 20 | 10 | Treatment as prevention | All criteria met |
| Conserve et al. (2019) | Men with unknown status ages 20–51 | Tanzania | 2015 | 146 | 23 | Testing, including barriers and intervention strategies | All criteria met |
| Daniels et al. (2019) | Men who have sex with men (MSM) with HIV | South Africa | 2013, 2017 and 2018 | 20 | 16 | Antiretroviral therapy (ART) adherence | Criteria partially met |
| DiCarlo et al. (2014) | Men with known and unknown HIV status ages 24–57 | Lesotho | 2011 | 230 | 30 | Testing, including barriers and intervention strategies | Criteria partially met |
| Fleming et al. (2016) | People who participated in gender and health equality‐based intervention ages 17–75 | South Africa | 2010 | 60 | 60 | Overall HIV care engagement, including testing | All criteria met |
| Graham et al. (2018) | MSM with HIV ages 19–51 | Kenya | 2013–2014 | 30 | 30 | Overall care engagement, including ART adherence | Criteria partially met |
| Hendrickson et al. (2019) | People with unknown status, with various levels of treatment engagement ages 25–49 | Côte d'Ivoire | 2016 | 227 | 227 | ART use | Criteria partially met |
| Hill et al. (2018) | People with and without HIV ages 18–49 | South Africa | 2012–2014 | 25 | 25 | Testing and treatment | All criteria met |
| Jennings et al. (2017) | Men who socialize at “camps” ages 20–51 | Tanzania | 2015 | 23 | 23 | Self‐testing | All criteria met |
| Krakowiak et al. (2020) | Heterosexual couples with a median age of 28 years for men | Kenya | 2015 | 42 | 21 | Home‐based couple testing | All criteria met |
| Lavender et al. (2019) | Pregnant or postpartum women and male partners ages 20–48 | Malawi and Kenya | 2016–2017 | 76 | 36 | Testing for antenatal partner | All criteria met |
| Mak et al. (2016) | Household community members age 15–49 | Swaziland | 2011–2012 | 33 | 33 | Utilization of HIV services, including testing | All criteria met |
| Mantell et al. (2019) | Men who are actively participating in clinic‐based community ART refill groups age 18+ | Zimbabwe | 2017 | 147 | 118 | ART refill groups | Criteria partially met |
| Martinez Perez et al. (2016) | People who denied HIV counselling and testing, couples who received counselling and testing, and HIV‐caregivers age 20–41 | South Africa | 2014–2015 | 20 | 9 | Home self‐testing | All criteria met |
| Mburu et al. (2014) | People with HIV, their household members and healthcare providers age 30–64 | Uganda | 2010 | 65 | 40 | Overall HIV care engagement and stigma | All criteria met |
| Micheni et al. (2017) | MSM age 18+ with a mean of 39 | Kenya | 2013–2014 | 29 | 14 | ART adherence | All criteria met |
| Mooney et al. (2017) | Men with and without HIV with various levels of care engagement age 18–49 | South Africa | 2015 | 25 | 25 | Treatment as prevention | All criteria met |
| Naugle et al. (2019) | Men with HIV and men with unknown HIV status age 25–49 | Côte d'Ivoire | 2016 | 227 | 227 | Testing and treatment | Criteria partially met |
| Ndyabakira et al. (2019) | Men living in rural areas age 18–45+ | Uganda | 2016 | 60 | 60 | Testing | All criteria met |
| Ogunbajo et al. (2018) | MSM with HIV age 18+ | Ghana | 2015 | 30 | 30 | Overall HIV care engagement | All criteria met |
| Okal et al. (2020) | Men with HIV and health counsellors age 20–54 | Kenya | 2018 | 38 | 30 | Testing | Criteria partially met |
| Orr et al. (2017) | Men age 18–44 | South Africa | .. | 97 | 97 | Testing and treatment initiation | Criteria partially met |
| Osingada et al. (2019) | Male football fans age 19–71 | Uganda | 2018 | 50 | 50 | Testing | Criteria partially met |
| Rankin‐Williams et al. (2017) | Married subsistence farmers ages 23–50 | Malawi | 2014–2015 | 50 | 50 | Testing | All criteria met |
| Rosen et al. (2020) | Fisherman with HIV ages 29–46 | Uganda | 2017–2018 | 25 | 15 | ART sharing | All criteria met |
| Russell et al. (2019) | Low‐income people ages 30–74, some with HIV | Uganda | 2011–2012 | 38 | 18 | Treatment adherence | All criteria met |
| Sandfort et al. (2015) | MSM age 20–39, some with HIV | South Africa | 2014 | 81 | 81 | Testing | All criteria met |
| Schatz et al. (2018) | People with HIV ages 50–80 | South Africa | 2016–2017 | 21 | 11 | Testing | All criteria met |
| Sileo et al. (Qualitative…) (2019a) | Fisherfolk with HIV on ART ages 20–50 | Uganda | 2016–2017 | 30 | 30 | Treatment adherence | All criteria met |
| Sileo et al. (Masculinity…) (2019b) | Fisherfolk with HIV on ART ages 20–50 | Uganda | 2016–2017 | 30 | 30 | Overall HIV care engagement | All criteria met |
| Skovdal et al. (2019) | Family members of men who died from HIV |
Kenya, Malawi, South Africa, Tanzania, Uganda and Zimbabwe |
2015–2016 | 26 | 26 | HIV treatment engagement | All criteria met |
| Tibbels et al. (2019) | Men with HIV and men with unknown status age 25–49 | Cote d'Ivoire | 2016 | 227 | 227 | Overall HIV care engagement | All criteria met |
| Tsang et al. (2019) | Male sex workers and MSM ages 19–38 | Zimbabwe | 2016–2017 | 15 | 15 | MSM testing | All criteria met |
| Van Heerden et al. (2015) | Men ages 18–37 with unknown HIV status | South Africa | 2011–2012 | 20 | 10 | Testing | All criteria met |
| Wamoyi et al. (2017) | Men with HIV with various levels of care engagement | South Africa | 2015–2016 | 107 | 55 | Overall HIV care engagement | Criteria partially met |
| Zissette et al. (2016) | Men ages 24–80 with HIV on ART | South Africa | 2014 | 21 | 21 | Overall HIV care engagement | All criteria met |
Our detailed review of the qualitative manuscripts identified 24 second‐order constructs, 11 third‐order constructs and three third‐order labels. Each third‐order label encompassed barriers to men's care engagement, as well as supportive factors, which allowed some men to engage in care despite these barriers (Table A3).
3.3. Theme 1: Perceived social norms
Most studies described how men believed that engaging in HIV care threatened their sense of social norms. Men may feel uncomfortable in health facilities perceived to be feminine spaces or that are staffed by women because, as one South African man explained, “men are not comfortable discussing their issues with women” [119] (p. 7). Moreover, HIV testing was felt to be a woman's responsibility because “men perceive their partners to be the ones that brought infection in the family” [118] (p. 9). In addition, participants described that HIV threatened men's ideals of strength, sexuality, livelihood, social standing and a fun lifestyle. HIV was “the end of your fun, the end of your joy,” imposing limitations on men's sexual choices because women will “run away” from a man who has HIV [192] (p. 6). Therefore, it was better not to know one's status. Participants also shared how engaging in care would compete with men's ability to work – something that many participants in Cote d'Ivoire described as being what “defines a man,” giving “social freedom…social status…and respect” [192] (p. 7). Men worried that engaging in HIV care would take away from time socializing with other men and “men activities,” leading them to feel “left behind, weak and incapable of fully being a man” [119] (p. 7).
However, many studies identified how some men were able to draw on positive coping skills to facilitate engagement in HIV care while still prioritizing their social roles. For example, a man in Uganda reported that knowing his positive status motivated him to “fight for my life” and “save money” in order to provide for his children [132] (p. 1204). Drawing on social support from other men was another coping strategy used by some participants. One man who was frequently ill shared how “my friends would advise me that why don't you go to a health facility such that you can be checked” [136] (p. 781). Participants also coped by seeing themselves as courageous and strong in the face of an HIV diagnosis. One man described, “[I have] ARVs as treatment and therefore I have no reason to be afraid.” [132] (p. 1207). Emphasizing his strong appearance, a fisherman in Uganda said, “I tell the people around that I am HIV infected…I show off because I look good” [132] (p. 1203).
3.4. Theme 2: Health system challenges
Numerous structural and social challenges related to the health system were described as barriers to accessing care. Social challenges included the experience or anticipation of poor treatment from providers with stigma towards people with HIV. One man shared his experience that “when [hospital staff] discover it is HIV, they give you a weird look….the staff laughs” [137] (p. 5). MSM described experiencing or anticipating stigma regardless of what their serostatus might be; as one MSM participant in Kenya said, “If I went to a health facility the moment I meet you I can tell how homophobic you are” [120] (p. 100). Other disincentives to seeking care were that participants doubted their HIV test results (“sometimes the person who does the test can be wrong” [137] (p. 6) ) or believed that there is no effective treatment for HIV. Structural challenges included men's concern about lacking privacy due to clinics’ physical layouts and procedures, such as a bench reserved for patients with HIV [137] (p. 7). Participants were also disincentivized to seek care at under‐resourced clinics experiencing clinician shortages or medication or test kit stockouts. A man with HIV in Côte d'Ivoire described that “…when there's no medication…I am discouraged” [137] (p. 8).
On the other hand, men described how strategies to mitigate these challenges did help them to access care. Convenient access to health facilities helped accommodate men's work schedules, such as one man's suggestion for facilities that “operate 24 hours” [194] (p. 14). Self‐testing and home‐testing were identified as quick and confidential ways for men to avoid having to return for follow‐up visits if their testing returned negative. One man in Tanzania described how self‐testing allowed him to avoid stigma because “none sees me while I test” [122] (p. 5). In addition to strategies promoting initial care engagement, personal support from providers and personally experiencing the effectiveness of ART helped to facilitate ongoing engagement in care. A man with HIV in South Africa described, “I believe that this treatment is good because…I look healthy and my body has recovered compared to last year” [128] (p. 279). One Ghanaian MSM participant related how a nurse “called me often and even when I am unable to go to the clinic, she'd get my medication for me and then I'll go collect it at her house” [129] (p. 834).
3.5. Theme 3: Poverty
Men explained how “the illness finds us in poverty” [136] (p. 780), making it challenging to overcome economic challenges associated with transport costs and medical expenses. A man in Côte d'Ivoire shared that men may opt for traditional healers because they “if they go to the hospital, the costs will be exorbitant” [137] (p. 8). Participants described the opportunity costs of engaging in HIV care, because such activities compete with the substantial time and energy needed for seeking employment and food [136] (p. 780).
However, strategies that made care more affordable helped offset these economic challenges. Specifically, home‐testing and self‐testing allowed men to avoid travelling and waiting in line. Some men also perceived self‐testing kits to be less expensive because “in private hospitals, you must pay to be tested” [122] (p. 5).
4. DISCUSSION
In this systematic review of 168 studies conducted in a wide range of settings across sub‐Saharan Africa, we found that health and social welfare systems have failed to achieve the UNAIDS 95‐95‐95 goals for men. Our meta‐analysis, combining data from 2009 (or prior) to 2020, showed that in aggregate over this time period, men have been behind in testing and treatment. Studies including only MSM found lower proportions in their knowledge of status and viral suppression as compared to the proportions for these goals in studies including all men. In studies comparing men and women, we found that men have had lower knowledge of HIV status and rates on ART, and slightly lower rates of viral suppression.
Our finding of lower proportions of men earlier in the care continuum contrasts with 2020 UNAIDS estimates from eastern and southern Africa [5], despite the fact that most studies in our meta‐analysis are from this region. It is more consistent with UNAIDS estimates from western and central Africa, showing that men have fallen behind especially in knowledge of status. We observed a lower rate of being on ART in western and central Africa as compared to eastern and southern Africa, whereas 2020 UNAIDS estimates found these rates to be comparable. These discrepancies may be explained by the fact that our meta‐analysis includes data over an extended time period of time, in contrast to a yearly estimate. They also may be explained by the marked heterogeneity in our studies. Lastly, the lack of statistically significant differences by region for knowledge of status and viral suppression, as predicted by current UNAIDS estimates, may be due to our small number of studies from western and central Africa.
Our findings regarding MSM support research showing that health systems in sub‐Saharan Africa inadequately engage MSM in achieving the 95‐95‐95 goals [7, 201]. A recent meta‐analysis on HIV testing and treatment among MSM in sub‐Saharan Africa similarly found that only 19% MSM with HIV knew their status, 60% of those MSM were on ART and 76% of those on ART achieved viral suppression – lower rates compared with the general population of all men [7]. There is an urgent need to better reach MSM, particularly as MSM are estimated to have a three‐fold greater prevalence of HIV compared with heterosexual men in sub‐Saharan Africa [202]. Complementing these findings, our meta‐synthesis identified unique barriers to care engagement for MSM. Intersecting stigmas attached to HIV and sexual minority status [127], consistent with prior research [203, 204], remain major challenges. Despite efforts to better reach MSM [205], there is an ongoing need for structural interventions to address large‐scale social forces beyond health systems.
In addition, we found that study setting, facility, age, employment status and migration status vary significantly among studies. More research is needed to focus on certain sub‐populations of men to understand where resources may be best utilized. For example, studies have noted the difficulty of engaging men in communities with substantial mobility [163, 206, 207]. At the same time, our meta‐synthesis revealed important areas of overlap among factors influencing engagement in HIV care for all men, suggesting opportunities for scalable interventions. Testing at venues telecasting football games [195], incentive‐based testing [193], self‐ and home testing [15], and outreach at bars and churches [124, 195] may help address the need for more men with HIV to know their status by incentivizing testing and bringing it to where men are in the community. Men‐only ART refill groups [191], expanded clinic opening hours [115], and social and livelihood interventions [208, 209] may address common concerns about stigma and the inconveniences and costs of care, helping more men with HIV to be on treatment. Gender‐transformative initiatives may also have an important role in helping men to reframe limiting norms and improve their testing and treatment outcomes [119].
Figure 5 depicts the conceptual model emerging from an integration of our quantitative and qualitative findings, drawing upon the framework of risk perception. For some men, the perceived risks of engaging in HIV care are substantial and influenced by perceived threats to their social role and economic wellbeing, as well as perceived threats within the health system. For other men – or for the same men at different points in time – these threats were mitigated by supportive factors, allowing the benefits of engaging in care to outweigh perceived risks. Supportive factors could facilitate initial care engagement (e.g. existing coping skills and social support, affordable and accessible care) as well as reinforce ongoing care engagement (e.g. strengthened coping skills and social support, positive experiences and trust in the health system). Coping is the process by which individuals manage their response to stressors [210, 211], and it encompasses both emotional coping strategies, such as feeling resilient, and problem‐focused coping strategies, such as turning to others for support [212, 213]. Positive coping strategies, both emotional and problem‐focused, have been shown to promote treatment decisions for persons with HIV [14, 214]. It is also possible that some individuals already in care may be encouraged to stay in care by experiencing or observing its benefits, such as a man who described recovering his physical health after being on treatment [128] (p. 279) or one who received invaluable treatment support from a nurse [129] (p. 834). Lastly, research has identified subgroups of patients with personal characteristics (e.g. younger men) that predispose them to progress more successfully through the care continuum [215, 216].
Figure 5.
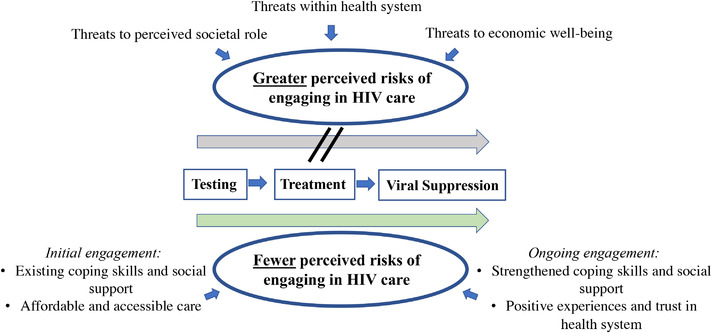
Conceptual model of men's engagement in the HIV care continuum.
Our findings should be interpreted in light of some limitations. First, our meta‐analysis combined data over time from research studies rather than presenting annual data from programmatic surveillance. Therefore, our aggregate results are not directly comparable to annually updated surveillance data. Additionally, they may mask changes over this time period, in which there have been advances in HIV care. However, there may be advantages to our approach in that we included only research data rather than also estimates from modelling. It is also possible that our inclusion of some smaller studies may have captured important gaps throughout these regions that may be missed by larger population‐scale surveillance. Second, we found that the pooled estimates were characterized by a high degree of heterogeneity. However, this finding was not unexpected (and was consistent with heterogeneity estimates obtained in other recently published meta‐analyses), given that we had purposefully included a wide range of studies conducted in different settings. Third, we noted variation in measurement of the 95‐95‐95 goals, including in viral load thresholds. While the majority of studies used 1000 copies/ml as the threshold, the use of thresholds as high as 5000 copies/ml (or as low as 20 copies/ml) may over‐estimate (or under‐estimate) viral suppression in those studies. In addition, self‐report bias could have affected our pooled estimates of the first and second goals. Variation in outcome measurement will continue to hamper efforts to generate reliable estimates of men's engagement in the HIV care continuum and, therefore, efforts to develop scalable interventions to enhance HIV‐related outcomes in this population. We have found limited discussion of these issues in the literature [9, 217], and our finding highlights the need for harmonization of measurements across settings. Fourth, a majority of the screened studies did not meet our inclusion criteria. We found that the most common reasons for exclusion related to a lack of sex disaggregated data or outcomes that differed from the UNAIDS 95‐95‐95 goals, for example linkage to care but not being on ART. Despite our efforts to contact authors for additional data where possible, our results cannot account for data not included in the original manuscripts. Fifth, while we did not restrict inclusion to studies of cisgender men, there were very few studies that contained explicit specification. One included study explicitly included transgender men and one explicitly excluded transgender men. Thus, our review identifies this important gap in the literature. Lastly, we limited our search to PubMed and Embase for the meta‐synthesis given we anticipated that most qualitative studies would be in these biomedical and public health databases; however, it is possible that these searches missed relevant literature outside of these fields.
5. CONCLUSIONS
Men in sub‐Saharan Africa are behind in HIV testing and treatment, and MSM remain particularly vulnerable throughout the care continuum. Interventions that address men's perceived risks of care engagement by positively reframing living with HIV, providing social support, improving trust in and accessibility of the health system, and providing affordable care are needed to meet UNAIDS goals across sub‐Saharan Africa.
COMPETING INTERESTS
ACT reports receiving a financial stipend from Elsevier, Inc. for his work as Co‐Editor in Chief of the journal SSM‐Mental Health. All other authors declare no competing interests.
AUTHORS’ CONTRIBUTORS
MFN, ACT and ITK conceptualized this review and planned the analyses. MFN did the searches. MFN, OA and CP independently did all stages of screening and data extraction, and all data were checked by more than one author. MFN conducted all analyses with input from ACT and ITK. MFN interpreted the results and wrote the first draft of the manuscript, with contributions from OA and CP in creating the tables. ACT, ITK, BK, LL, SP and CGM made substantial intellectual contributions to the interpretation of the results and edited the manuscript. All authors read and approved the final version of the manuscript.
FUNDING
This publication was made possible by the U.S. National Institutes of Health (NIH) T32AI007433 (MFN), K01MH119923 (LL) and R01MH113494 (ACT).
DISCLAIMER
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The funder had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
ACKNOWLEDGEMENTS
We thank Michael Stoto, PhD, for his guidance on meta‐analysis methodology, and we thank the following students for their valuable contributions: Anna Madden, Sabrina Lin, Julian Oluwole, Julia Ralph, Danielle Reed, Lovemore Nyaumwe, Imauchechim Agba, Sonja Lazovic, Melissa Brown and Sophia Pomeroy.
Appendices A.
A.1. SEARCH TERMS USED IN PUBMED AND EMBASE
PubMed
(“HIV Infections”[Mesh] OR HIV[tiab])
AND
(“HIV testing”[tiab] OR “diagnosis” [MeSH Major Topic] OR “hiv status”[tiab] OR antiretroviral[tiab] OR HAART[tiab] OR ART[tiab] OR CART[tiab]) OR “HIV treatment” [tiab] OR “linkage to care”[tiab] OR “access to care”[tiab] OR “treatment access”[tiab]) OR adherence[tiab] OR “loss to follow up”[tiab] OR “viral suppression”[tiab] OR undetectable[tiab] OR “viral load”[tiab] OR “Viral Load”[Mesh] OR “treatment failure”[tiab] OR “virologic failure”[tiab] OR “90 90 90”[tiab] OR “care cascade”[tiab] OR “cascade of care”[tiab] OR “care continuum”[tiab] OR “continuum of care”[tiab] OR “fast track”[tiab])
AND
(“Africa South of the Sahara”[Mesh] OR africa[tiab] OR Cameroon[tiab] OR Central African Republic[tiab] OR Chad[tiab] OR Congo[tiab] OR Equatorial Guinea[tiab] OR Gabon[tiab] OR Burundi[tiab] OR Djibouti[tiab] OR Eritrea[tiab] OR Ethiopia[tiab] OR Kenya[tiab] OR Rwanda[tiab] OR Somalia[tiab] OR Sudan[tiab] OR Tanzania[tiab] OR Uganda[tiab] OR Angola[tiab] OR Botswana[tiab] OR Lesotho[tiab] OR Malawi[tiab] OR Mozambique[tiab] OR Namibia[tiab] OR South Africa[tiab] OR Swaziland[tiab] OR Zambia[tiab] OR Zimbabwe[tiab] OR Benin[tiab] OR Burkina Faso[tiab] OR Cape Verde[tiab] OR Cote d'Ivoire[tiab] OR Ivory Coast[tiab] OR Gambia[tiab] OR Ghana[tiab] OR Guinea[tiab] OR Guinea‐Bissau[tiab] OR Liberia[tiab] OR Mali[tiab] OR Mauritania[tiab] OR Niger[tiab] OR Nigeria[tiab] OR Senegal[tiab] OR Sierra Leone[tiab] OR Togo[tiab])
AND
(“2014/01/01”[PDAT] : “3000/12/31”[PDAT])
NOT
(Letter[pt] OR Editorial[pt] OR Review[pt] OR News[pt] OR Meta‐Analysis[pt] OR Guideline[pt])
EMBASE
(‘human immunodeficiency virus infection’/exp OR ‘human immunodeficiency virus infection’:ti,ab OR ‘human immunodeficiency virus infection’/mj)
AND
(‘HIV testing’:ti,ab OR ‘diagnosis’/mj OR ‘hiv status’:ti,ab OR antiretroviral:ti,ab OR haart:ti,ab OR art:ti,ab OR cart:ti,ab OR ‘hiv treatment’:ti,ab OR ‘linkage to care’:ti,ab OR ‘access to care’:ti,ab OR ‘treatment access’:ti,ab OR ‘adherence’:ti,ab OR ‘loss to follow up’:ti,ab OR ‘viral suppression’:ti,ab OR ‘undetectable’:ti,ab OR ‘viral load’:ti,ab OR ‘viral load’/mj OR ‘treatment failure’:ti,ab OR ‘virologic failure’:ti,ab OR ‘90‐90‐90’:ti,ab OR ‘care cascade’:ti,ab OR ‘cascade of care’:ti,ab OR ‘care continuum’:ti,ab OR ‘continuum of care’:ti,ab OR ‘fast track’:ti,ab)
AND
(‘africa’/exp OR ‘africa’:ti,ab)
NOT
(‘letter’:it,pt OR ‘editorial’:it,pt OR ‘review’:it,pt OR ‘meta analysis’:it,pt OR ‘news’:it,pt OR ‘guideline’:it,pt)
AND
[embase]/lim NOT ([embase]/lim AND [medline]/lim)
AND
(2014:py OR 2015:py OR 2016:py OR 2017:py OR 2018:py OR 2019:py)
A.2. DEFINITIONS OF THE UNAIDS 95‐95‐95 GOALS
Table A1.
Definition of numerator and denominator of each UNAIDS 95‐95‐95 goal
| Numerator | Denominator | |
|---|---|---|
| First 95‐95‐95 goal | Persons with HIV aware of their serostatus | Persons with HIV |
| Second 95‐95‐95 goal | Persons with HIV on antiretroviral therapy | Persons with HIV aware of their serostatus |
| Third 95‐95‐95 goal | Persons with HIV on antiretroviral therapy and virally suppressed | Persons with HIV on antiretroviral therapy |
A.3. TABLE OF INCLUDED STUDIES IN META‐ANALYSIS
Table A2.
Studies reporting on 95‐95‐95 goal(s) meeting inclusion criteria for meta‐analysis (N=129)
| Study population | Study type | Dates | Country | Total number of study participants | 95‐95‐95 Goal reported | Proportion of men meeting 95‐95‐95 goal(s) | Quality criteria | |
|---|---|---|---|---|---|---|---|---|
| Abah et al. (2016) | Individuals living with HIV initiated on NNRTI‐based ART, median age 34 years (interquartile range [IQR] 29–41 years) | Retrospective cohort study | 2009–2010 | Nigeria | 588 | Third | 0.89 | All criteria met |
| Abah et al. (2019) | Individuals living with HIV on first‐line ART, median age 34 years (IQR 29–41 years) | Retrospective cohort study | 2004–2012 | Nigeria | 12,115 | Third | 0.77 | All criteria met |
| Abongomera et al. (2017) | Individuals reporting HIV testing | Cross‐sectional study | 2015 | Uganda | 2124 | Second | 0.59 | Criteria partially met |
| Ahonkhai et al. (2015) | Individuals living with HIV on ART, median age 32 years (IQR 27–39 years) | Retrospective cohort study | 2009–2011 | Nigeria | 2496 | Second | 0.40 | All criteria met |
| Andronescu et al. (2019) | Individuals living with HIV initiating third‐line ART, median age 40 years (IQR 18–49 years) | Retrospective cohort study | 2012–2015 | Zambia | 80 | Third | 0.69 | Criteria partially met |
| Avong et al. (2015) | Individuals living with HIV on ART, age 21–60 years | Cross‐sectional study | 2004–2010 | Nigeria | 502 | Third | 0.83 | All criteria met |
| Baltazar et al. (2015) | Men working in mines, age 23–68 years | Cross‐sectional study | 2012 | South Africa and Mozambique | 432 | First and second | 0.25 and 0.78 | Criteria partially met |
| Barak et al. (2019) | Individuals with median age 51 years (IQR 34–71 years) | Prospective review cohort study | 2015–2017 | Botswana | 1969 | Second | 0.68 | All criteria met |
| Barnabas et al. (2020) | Individuals living with HIV, age ≥18 years | Household‐randomized unblinded trial | 2016–2019 | South Africa and Uganda | 1315 | Third | 0.54 | All criteria met |
| Bhattacharjee et al. (2020) | Men who have sex with men (MSM), age ≥15 years | Cross‐sectional bio‐behavioural survey | 2019 | Kenya | 1200 | First and second | 0.38 and 0.86 | All criteria met |
| Billioux et al. (2015) | Individuals living with HIV initiated on first‐line ART, median age 33 years (IQR 28–40 years) | Prospective cohort study | 2005–2011 | Uganda | 1841 | Third | 0.91 | Criteria partially met |
| Billioux et al. (2017) | Individuals living with HIV enrolled in care, age 15–49 years | Retrospective longitudinal cohort study | 2013–2015 | Uganda | 3666 | First and third | 0.97 and 0.89 | Criteria partially met |
| Billioux et al. (2018) | Individuals living with HIV enrolled in care, age 15–49 years | Census surveillance cohort study | 2015–2016 | Uganda | 1554 | Third | 0.93 | Criteria partially met |
| Bock et al. (2016) | Individuals living with HIV, age 18–49 years | Cross‐sectional study | 2012 | Swaziland | 927 | Third | 0.86 | All criteria met |
| Borgdorff et al. (2018) | Individuals aged 15–64 years | Population‐based cross‐sectional survey study | 2011–2012 and 2016 | Kenya | 28,486 | First | 0.38 | Criteria partially met |
| Boyd et al. (2020) | Individuals living with HIV, age ≥15 years | Cross‐sectional report | 2018–2019 | Zambia | 248,002 | Second | 0.34 | Criteria partially met |
| Brooks et al. (2016) | Individuals living with HIV initiated on first‐line ART, median age 41 years (IQR 23–82 years) | Cross‐sectional study | 2012–2013 | Kenya | 333 | Third | 0.84 | Criteria partially met |
| Brown et al. (2019) | Individuals living with HIV enrolled in care, age ≥15 years | Prospective cohort study | 2014–2015 | Kenya and Uganda | 5683 | Second | 0.58 | All criteria met |
| Bulage et al. (2017) | Individuals living with HIV on ART, mostly aged 35+ years | Cross‐sectional study | 2014–2015 | Uganda | 100,678 | Third | 0.74 | All criteria met |
| Burgos‐Soto et al. (2020) | Individuals aged 15–69 years | Household‐based cross‐sectional survey study | 2016 | Uganda | 1738 | First, second and third | 0.86, 0.97 and 0.86 | All criteria met |
| Charurat et al. (2015) | MSM living with HIV, age ≥16 years | Prospective cohort study | 2013–2014 | Nigeria | 186 | Second | 0.31 | All criteria met |
| Cherutich et al. (2016) | Individuals living with HIV, age 15–64 years | Cross‐sectional study | 2012–2013 | Kenya | 617 | Third | 0.72 | All criteria met |
| Chikandiwa et al. (2019) | Men living with HIV, age ≥18 years | Prospective cohort study | 2011–2012 | South Africa | 304 | Third | 0.82 | Criteria partially met |
| Chikandiwa et al. (2020) | Men living with HIV, age ≥18 years | Prospective cohort study | 2012–2013 | South Africa | 304 | Second | 0.59 | All criteria met |
| Chimukangara et al. (2017) | Individuals living with HIV on ART, median age 43 years (95% CI 39–44 years) | Cross‐sectional study | 2014 | Zimbabwe | 143 | Third | 0.95 | Criteria partially met |
| Colombe et al. (2020) | Individuals living with HIV, median age 36 years (IQR 27–46 years) | Community‐based prospective cohort study | 2006–2016 | Tanzania | 175 | First | 0.82 | Criteria partially met |
| Conan et al. (2020) | Individuals age ≥15 years old | Population‐based cross‐sectional survey study | 2016 | Zimbabwe | 4979 | First, second and third | 0.80, 0.74 and 0.65 | Criteria partially met |
| Cornell et al. (2017) | Individuals living with HIV | Prospective cohort study | 2004–2015 | South Africa | 72,812 | Third | 0.83 | Criteria partially met |
| Crowell et al. (2020) | Individuals living with HIV, median age 35.7 years (IQR 29.7–42.7) years | Retrospective cohort study | 2013–2019 | Uganda, Kenya, Tanzania and Nigeria | 972 | Second | 0.18 | Criteria partially met |
| Czaicki et al. (2014) | Individuals age ≥16 years | Prospective cohort study | 2011–2012 | Zambia | 21,612 | Second | 0.16 | All criteria met |
| De Anda et al. (2020) | Individuals living with HIV, age ≥18 years old | Cross‐sectional study | 2015–2016 | Kenya | 1136 | First | 0.18 | Criteria partially met |
| Desta et al. (2020) | Individuals living with HIV, age ≥15 years | Retrospective cross‐sectional study | 2015–2019 | Ethiopia | 19,525 | Third | 0.7 | All criteria met |
| Dokubo et al. (2014) | Individuals living with HIV, age 15–49 years | Cross‐sectional study | 2009 | Mozambique | 1182 | First | 0.31 | All criteria met |
| Dorward et al. (2020) | Individuals living with HIV on ART, age >15 years | Retrospective cohort study | 2016–2019 | South Africa | 4952 | Third | 0.93 | All criteria met |
| Fearon et al. (2020) | MSM and transgender individuals, age ≥18 years | Cross‐sectional study | 2017 | South Africa | 301 | First, second and third | 0.64, 0.51 and 0.74 | Criteria partially met |
| Fentie Wendie et al. (2020) | Individuals living with HIV on ART, age ≥15 years old | Retrospective cohort study | 2018–2019 | Ethiopia | 384 | Third | 0.82 | All criteria met |
| Fogel et al. (2019) | MSM and transgender women (TGW) who have sex with men, age 18–44 years | Prospective cohort study | 2015–2016 | Kenya, Malawi and South Africa | 183 | First | 0.56 | All criteria met |
| Foley et al. (2020) | Individuals living with HIV on ART, median age 32 years (IQR 26–40) years | Prospective cohort study | – | Uganda | 657 | Third | 0.85 | Criteria partially met |
| Fonner et al. (2019) | Individuals age ≥18 years old | Cross‐sectional study | 2006 | Tanzania | 644 | First | 0.32 | All criteria met |
| Genberg et al. (2015) | Individuals living with HIV enrolled in care, median age 36 years (IQR 30–45 years) | Retrospective cohort study | 2004–2014 | Kenya | 3482 | First | 0.54 | All criteria met |
| Grobler et al. (2017) | Individuals aged 15–49 years | Household‐based cross‐sectional survey study | 2014–2015 | South Africa | 9812 | First, second and third | 0.50, 0.66 and 0.86 | All criteria met |
| Gumede et al. (2019) | Individuals living with HIV on second‐line ART mostly 25+ years | Prospective cohort study | 2014–2015 | South Africa | 825 | Third | 0.78 | Criteria partially met |
| Gumede et al. (2020) | Individuals living with HIV on second‐line ART, median age 42 years (IQR 36–47 years) | Cross‐sectional study | 2018 | South Africa | 149 | Third | 0.59 | All criteria met |
| Gunda et al. (2019) | Individuals living with HIV on second‐line ART, median age 48 years (IQR 41–54 years) | Unmatched case–control study | 2017–2018 | Tanzania | 197 | Third | 0.82 | Criteria partially met |
| Gupta‐Wright et al. (2020) | Individuals living with HIV on ART, age ≥18 years | Observational cohort study | 2015–2017 | Malawi | 1316 | Third | 0.62 | Criteria partially met |
| Haachambwa et al. (2019) | Individuals living with HIV, age ≥18 years | Prospective cohort study | 2017–2018 | Zambia | 239 | Second and third | 0.49 and 0.74 | Criteria partially met |
| Hailu et al. (2018) | Individuals living with HIV on ART, age 10–63 years | Retrospective cohort study | 2008–2016 | Ethiopia | 260 | Third | 0.82 | Criteria partially met |
| Hakim et al. (2018) | MSM and TGW, age ≥18 years | Cross‐sectional study | 2014–2015 | Mali | 552 | First | 0.34 | Criteria partially met |
| Hakim et al. (2019) | Individuals age ≥13 years | Cross‐sectional study | 2011–2013 | Uganda | 12,233 | First | 0.33 | All criteria met |
| Halle et al. (2019) | Individuals living with HIV aged 22–82 years | Retrospective cohort study | 2007–2013 | Cameroon | 156 | Second | 0.64 | All criteria met |
| Hansoti et al. (2019) | Individuals mostly aged 20+ years | Cross‐sectional study | 2017–2018 | South Africa | 2901 | First, second and third | 0.59, 0.58 and 0.43 | Criteria partially met |
| Hansoti et al. (2019) | Individuals mostly aged 20+ years | Cross‐sectional serosurvey study | 2016 | South Africa | 2100 | Second | 0.55 | Criteria partially met |
| Hassan et al. (2014) | Individuals living with HIV on ART, median age 38.5 years (IQR 32.2–44.8 years) | Cross‐sectional study | 2008–2011 | Kenya | 232 | Third | 0.78 | All criteria met |
| Hayes et al. (2017) | Individuals age ≥18 years | Cluster randomized trial | 2013–2015 | Zambia | 121,130 | Second | 0.47 | Criteria partially met |
| Hayes et al. (2019) | Individuals aged 18–44 years | Community‐randomized trial | 2013–2018 | Zambia and South Africa | 48,301 | Third | 0.54 | All criteria met |
| Hensen et al. (2015) | Men aged 15–60 years | Cluster randomized stepped‐wedge trial | 2011–2012 | Zambia | 2828 | First | 0.43 | All criteria met |
| Herce et al. (2018) | MSM, TGW, and female sex workers (FSW), mostly >25 years | Cross‐sectional study | 2016–2017 | Malawi and Angola | 1924 | First | 0.18 | All criteria met |
| Hermans et al. (2020) | Individuals living with HIV on first‐line ART, median age 35.7 years (IQR 29.9–43.0 years) | Retrospective cohort study | 2007–2018 | South Africa | 104,719 | Third | 0.78 | All criteria met |
| Hirasen et al. (2018) | Individuals living with HIV initiated on first‐line ART, median age 37.8 years (IQR 31.7–45.0 years) | Retrospective cohort study | 2011–2015 | South Africa | 3151 | Third | 0.75 | Criteria partially met |
| Hirasen et al. (2020) | Individuals living with HIV on ART, age ≥18 years | Prospective cohort study | 2014–2017 | South Africa | 2410 | Third | 0.83 | Criteria partially met |
| Hladik et al. (2017) | MSM with median age 23 years (IQR 21–26 years) | Cross‐sectional study | 2012–2013 | Uganda | 608 | First | 0.2 | Criteria partially met |
| Holland et al. (2016) | MSM and FSW, age ≥18 years | Prospective cohort study | 2013 | Burkina Faso and Togo | 2738 | Second | 0.45 | Criteria partially met |
| Huerga et al. (2017) | Individuals aged 15–59 years | Cross‐sectional survey study | 2013 | South Africa | 5649 | Third | 0.85 | All criteria met |
| Huerga et al. (2018) | Individuals aged 15–59 years | Cross‐sectional study | 2013 | South Africa | 5649 | First and second | 0.68 and 0.68 | Criteria partially met |
| Iwuji et al. (2016) | Individuals aged ≥16 years | Cluster‐randomized trial | 2012–2014 | South Africa | 9927 | First | 0.7 | Criteria partially met |
| Jean et al. (2016) | Individuals aged 18–49 years | Cross‐sectional survey study | 2012 | South Africa | 6766 | Second | 0.43 | All criteria met |
| Joram et al. (2017) | Individuals living with HIV on ART, age 2–80 years | Retrospective cohort study | 2013–2014 | Kenya | 1859 | Third | 0.43 | All criteria met |
| Joseph Davey et al. (2018) | Individuals living with HIV initiated on first‐line ART, median age 37.8 years (IQR 31.7–45.0 years) | Retrospective cohort study | 2004–2016 | South Africa | 244,370 | Third | 0.82 | Criteria partially met |
| Kagaayi et al. (2019) | Individuals aged 15–49 years | Population‐based prospective cohort study | 2016–2017 | Uganda | 8942 | Second | 0.78 | Criteria partially met |
| Kenyon et al. (2015) | Individuals living with HIV, age 15–59 years | Cross‐sectional study | 2011 | Uganda | 1495 | First | 0.35 | All criteria met |
| Keshinro et al. (2017) | Individuals living with HIV on first‐line ART, age ≥18 years | Cross‐sectional study | 2012 | Nigeria | 325 | Third | 0.88 | All criteria met |
| Kharsany et al. (2018) | Individuals aged 15–49 years | Cross‐sectional survey study | 2014–2015 | South Africa | 9812 | Third | 0.83 | Criteria partially met |
| Kharsany et al. (2019) | Individuals aged 15–49 years | Community‐based longitudinal study | 2014–2015 | South Africa | 20,048 | First and second | 0.55 and 0.72 | All criteria met |
| Kityo et al. (2014) | Individuals living with HIV on ART, mostly aged 30+ years | Open randomized trial | 2003–2009 | Uganda and Zimbabwe | 1896 | Third | 0.81 | Criteria partially met |
| Kiweewa et al. (2019) | Individuals living with HIV, age ≥18 years | Prospective cohort study | 2013–2017 | Nigeria, Uganda, Kenya and Tanzania | 2054 | Third | 0.91 | Criteria partially met |
| Kufa et al. (2018) | Individuals, median age 26 years (IQR 23–32 years) | Cross‐sectional study | 2017–2018 | South Africa | 1054 | First | 0.32 | Criteria partially met |
| Kunzweiler et al. (2018) | MSM living with HIV, median age 27 years (IQR 22–32 years) | Prospective cohort study | 2015–2016 | Kenya | 63 | Third | 0.71 | Criteria partially met |
| Lahuerta et al. (2018) | MSM, age ≥18 years | Cross‐sectional study | 2014–2015 | Kenya, Mozambique, Rwanda and Tanzania | 552 | First | 0.1 | All criteria met |
| Lane et al. (2014) | MSM, age ≥18 years | Cross‐sectional study | 2012–2013 | South Africa | 605 | First | 0.23 | All criteria met |
| Lewis et al. (2019) | Individuals aged 15–49 years | Cross‐sectional study | 2015–2016 | South Africa | 10,236 | First | 0.62 | Criteria partially met |
| Liégeois et al. (2019) | Individuals living with HIV on ART, median age 41 years (IQR 35–49 years) | Cross‐sectional study | 2014 | Cameroon | 1700 | Third | 0.82 | All criteria met |
| Lippman et al. (2016) | Individuals aged 18–49 years | Population‐based household cross‐sectional survey study | 2014 | South Africa | 1044 | First and second | 0.48 and 0.33 | All criteria met |
| Lyons et al. (2017) | MSM and FSW, mostly aged 25+ years | Prospective cohort study | 2013–2016 | Senegal | 1482 | First, second and third | 0.13, 0.76 and 0.64 | Criteria partially met |
| MacKellar et al. (2019) | Individuals aged 18–49 years | Prospective longitudinal cohort study | 2014–2017 | Tanzania | 5067 | Second | 0.23 | All criteria met |
| Mafigiri et al. (2017) | Individuals aged 15–24 years | Cross‐sectional study | 2013–2014 | Uganda | 792 | Second | 0.07 | Criteria partially met |
| Maina et al. (2020) | Individuals living with HIV on ART, age ≥18 years | Retrospective cohort study | 2017–2019 | Kenya | 549 | Third | 0.5 | All criteria met |
| Maman et al. (2015) | Individuals aged 15–59 years | Population‐based cross‐sectional study | 2012 | Kenya | 6076 | First | 0.55 | All criteria met |
| Maman et al. (2016) | Individuals aged 15–59 years | Retrospective nested cohort study | 2013 | Malawi | 7270 | First | 0.66 | All criteria met |
| Manne‐Goehler et al. (2019) | Individuals age ≥40 years | Prospective longitudinal cohort study | 2014–2015 | South Africa | 4560 | Second | 0.47 | Criteria partially met |
| Marinda et al. (2020) | Individuals age ≥15 years | Population‐based cross‐sectional survey study | 2017–2018 | South Africa | 36,627 | First, second and third | 0.78, 0.68 and 0.82 | Criteria partially met |
| Mbengue et al. (2019) | Individuals living with HIV on ART, age ≥18 years | Prospective cohort study | 2012–2014 | South Africa | 353 | Third | 0.53 | All criteria met |
| Mekuria et al. (2016) | Individuals living with HIV on ART, mean age 37.7 years (SD 9.3 years) | Prospective cohort study | 2009–2013 | Ethiopia | 642 | Third | 0.92 | All criteria met |
| Merrill et al. (2020) | Individuals living with HIV on ART, age 15–24 years | Cross‐sectional study | 2017–2018 | Zambia | 272 | Third | 0.59 | Criteria partially met |
| Meshesha et al. (2020) | Individuals living with HIV on first‐line ART, age of cases and controls was 31.6 years (SD ±10.72) and 36.6 years (SD±9.48), respectively | Unmatched case–control study | 2016–2018 | Ethiopia | 389 | Third | 0.66 | All criteria met |
| Mogosetsi et al. (2018) | Individuals living with HIV on ART, age ≥21 years | Prospective cohort study | 2012–2013 | South Africa | 98 | Third | 0.97 | All criteria met |
| Mokhele et al. (2019) | Individuals living with HIV on ART, age ≥18 years | Retrospective cohort study | 2004–2014 | South Africa | 3685 | Third | 0.53 | Criteria partially met |
| Moyo et al. (2016) | Individuals living with HIV on ART, age ≥18 years | Retrospective cohort study | 2007–2012 | South Africa | 13,475 | Third | 0.91 | All criteria met |
| Mshweshwe‐Pakela et al. (2020) | Individuals living with HIV, mostly age ≥30 years | Retrospective clinical review | 2017 | South Africa | 826 | Third | 0.77 | Criteria partially met |
| Muraguri et al. (2015) | MSM, age ≥18 years | Cross‐sectional study | 2010 | Kenya | 563 | First | 0.34 | Criteria partially met |
| Nakanyala et al. (2016) | Individuals, age ≥15 years | Cross‐sectional study | 2014–2015 | Namibia | 2163 | First, second and third | 0.41, 0.31 and 0.79 | Criteria partially met |
| Ndagijimana et al. (2019) | Individuals living with HIV on ART, median age 34 years (IQR 27–41 years) | Retrospective cohort study | 2012–2015 | Rwanda | 775 | Third | 0.82 | All criteria met |
| Nega et al. (2020) | Individuals living with HIV on first‐line ART, age ≥10 years | Hospital‐based cross‐sectional study | 2018–2019 | Ethiopia | 295 | Third | 0.83 | All criteria met |
| Negash et al. (2020) | Individuals living with HIV on ART, age 5–78 years | Hospital‐based cross‐sectional study | 2019 | Ethiopia | 393 | Third | 0.85 | Criteria partially met |
| Ng'ang'a et al. (2014) | Individuals aged 15–64 years | Population‐based cross‐sectional study | 2012–2013 | Kenya | 13,720 | First | 0.38 | All criteria met |
| Nnambalirwa et al. (2016) | Individuals living with HIV on first‐line ART, median age 36.7 years (IQR 31.5–43.3 years) | Retrospective cohort study | 2004–2011 | South Africa | 11,724 | Third | 0.84 | All criteria met |
| Novitsky et al. (2015) | Individuals aged 16–64 years | Community‐based open prospective cohort study | 2010–2013 | Botswana | 6238 | First | 0.66 | Criteria partially met |
| Novitsky et al. (2018) | Individuals living with HIV aged 16–29 years | Population‐based cross‐sectional study | 2013–2015 | Botswana | 552 | First, second and third | 0.87, 0.90 and 0.95 | Criteria partially met |
| Nsanzimana et al. (2019) | Individuals living with HIV on second‐line ART, median age 41 years (IQR 33–49 years) | Retrospective observational cohort study | 2004–2016 | Rwanda | 1688 | Third | 0.79 | All criteria met |
| Nuwagaba‐Biribonwoha et al. (2019) | Individuals living with HIV, age ≥18 years | Cross‐sectional study | 2013–2014 | South Africa | 2196 | First | 0.09 | Criteria partially met |
| Omooja et al. (2019) | Individuals living with HIV on ART, median age 36 years (IQR 30–44 years) | Cross‐sectional study | 2016–2017 | Uganda | 1169 | Third | 0.91 | Criteria partially met |
| Ondoa et al. (2020) | Individuals living with HIV on ART, mostly age ≥25 years | Prospective cohort study | 2008–2015 | Kenya, South Africa, Zambia, Nigeria, Zimbabwe and Uganda | 2420 | Third | 0.87 | Criteria partially met |
| Petersen et al. (2017) | Individuals living with HIV on ART, age ≥15 years | Cross‐sectional study | 2013–2014 to 2015–2016 | Kenya and Uganda | 77,774 | Third | 0.87 | All criteria met |
| Pulerwitz et al. (2019) | Individuals aged 18–49 years | Population‐based cross‐sectional survey study | 2014 | South Africa | 2019 | Second | 0.79 | All criteria met |
| Ramadhani et al. (2018) | MSM, age ≥16 years | Community‐based prospective cohort study | 2013–2017 | Nigeria | 1506 | First, second and third | 0.58, 0.37 and 0.71 | Criteria partially met |
| Reynolds et al. (2018) | Men aged 20–34 years | Cross‐sectional study | 2016–2017 | Swaziland | 568 | Second | 0.96 | All criteria met |
| Rhead et al. (2019) | Men aged 15–54 years | Cross‐sectional survey study | 2012–2013 | Zimbabwe | 3116 | First and second | 0.47 and 0.77 | Criteria partially met |
| Riedel et al. (2018) | Individuals living with HIV on ART, age 14–86 years | Retrospective cohort study | 2008–2010 | Rwanda | 531 | Third | 0.93 | All criteria met |
| Rohr et al. (2017) | Individuals age ≥40 years | Open cohort general‐population survey study | 2014–2015 | South Africa | 4560 | First | 0.55 | All criteria met |
| Ross et al. (2020) | Individuals living with HIV on ART, age ≥15 years | Cross‐sectional study | 2018 | Rwanda | 12,238 | Third | 0.91 | All criteria met |
| Shanaube et al. (2017) | Individuals age ≥18 years | Community‐randomized trial | 2013–2015 | Zambia | 101,102 | First | 0.59 | All criteria met |
| Ssemwanga et al. (2020) | Individuals living with HIV on first‐line ART | Clinic‐based cross‐sectional survey study | 2017 | Uganda | 1611 | Third | 0.93 | All criteria met |
| Steiner et al. (2020) | Individuals age 18–49 years | Population‐based pre‐post cross‐sectional survey study | 2013–2014 | Tanzania | 5067 | Second | 0.22 | All criteria met |
| Thin et al. (2019) | Individuals age 15–59 years | Household‐based cross‐sectional survey study | 2016–2017 | Lesotho | 11,682 | First, second and third | 0.77, 0.77 and 0.92 | Criteria partially met |
| Twahirwa Rwema et al. (2020) | MSM and TGW age ≥ 18 years | Cross‐sectional bio‐behavioural survey study | 2018 | Rwanda | 736 | First, second and third | 0.61, 0.98 and 0.75 | All criteria met |
| Umar et al. (2019) | Adolescents and young adults living with HIV on ART, aged 13–24 years | Cross‐sectional study | 2016 | Malawi | 209 | Third | 0.53 | All criteria met |
| Villa et al. (2020) | Individuals living with HIV on ART, median age 48 years (IQR 42–54 years) | Prospective cohort study | 2018 | Ghana | 333 | Third | 0.39 | All criteria met |
| Wafula et al. (2014) | Individuals living with HIV, age 15–64 years | Population‐based household cross‐sectional survey study | 2012–2013 | Kenya | 363 | Second | 0.67 | All criteria met |
| Wirth et al. (2020) | Individuals age 16–64 years | Prospective longitudinal cohort study | 2013–2018 | Botswana | 10,791 | First, second and third | 0.78, 0.70 and 0.96 | Criteria partially met |
| Young et al. (2020) | Individuals living with HIV, age 15–64 years | Household‐based cross‐sectional survey study | 2012 | Kenya | 648 | First and second | 0.44 and 0.85 | Criteria partially met |
A.4. TABLE OF META‐SYNTHESIS CONSTRUCTS
Table A3.
Constructs from meta‐synthesis of qualitative studies on men's engagement in HIV care
| Third‐order constructs | Second‐order constructs | Summary definition | First‐order constructs | Source(s) | ||
|---|---|---|---|---|---|---|
| Perceived social norms | Barriers | Femininity of healthcare and HIV | Health facilities as feminine spaces | Perception that healthcare facilities are primarily oriented towards addressing the needs of women and children. Also, the staff at clinics are mostly women, which makes it harder for men to feel comfortable discussing their concerns. | It is difficult because the other problem is that virtually all the nurses and counsellors at the clinic are women and thus men are not comfortable discussing their issues with women. We men prefer talking to other men if we have health problems and thus it is hard to go to the clinic for help. [Fleming, p. 7] | Adams 2017, Adeagbo 2019, Camlin 2016, Chikovore 2016, Fleming 2016, Lavender 2019, Mak 2016, Martínez Pérez 2016, Mburu 2014, Orr 2017, Osingada 2019, Rankin‐Williams 2017, Tibbels 2019, Zissette 2016 |
| HIV as a feminine issue | Perception that women are responsible for “managing” HIV in a relationship. This includes testing so that men can know their status by proxy, which, therefore, makes it unnecessary for men to test if their female partner already has tested. | What [men] like to say is that once I have tested, he had already tested too. (Woman in focus group) [DiCarlo, p. 15] | Camlin 2016, DiCarlo 2014, Lavender 2019, Mak 2016 | |||
| HIV as a threat to social norms | Health, strength and sexuality | Men fear being seen as weak if seen involved in HIV care. They also fear that HIV and HIV treatment could lead to sexual dysfunction and/or take away from a strong and attractive physical appearance. | From the culture, [to be a man] means to be strong, to have a family. To have your things. Cattles. To get a house. The problem about this, it's never been discussed health issues about men. The only health issue they know is going to the bush [circumcision ceremony] and they come out as man… That they are HIV, it's still a taboo, they hear it in the radio, they see in the TV… [Martínez Pérez, p. 6] | Adams 2017, Chikovore 2016, DiCarlo 2014, Fleming 2016, Hendrickson 2019, Jennings 2017, Mak 2016, Martínez Pérez 2016, Mooney 2017, Naugle 2019, Ndyabakira 2019, Okal 2020, Orr 2017, Osingada 2019, Rankin‐Williams 2017, Russell 2019, Sileo 2019b, Skovdal 2019, Wamoyi 2017 | ||
| Livelihood | Belief that HIV as well as engaging in HIV care takes away from men's ability to earn a livelihood and support their family. | I had spent a long time without testing because I am always busy looking for money so one would not get time even to go to [nearest health center] get tested. [Ndyabakira, p. 3] | Chikovore 2016, Naugle 2019, Ndyabakira 2019 | |||
| Social standing | Fear that HIV and being seen engaging in HIV care will take away from a man's social and family standing. | When you suffer from some of these illnesses [HIV], you find that you do not spend enough time with other men as you have to constantly go to the hospital. Your absence from other men may make you feel less masculine… (South Africa, Age 62) [Fleming, p. 7] | Adams 2017, Camlin 2016, Chikovore 2016, DiCarlo 2014, Fleming 2016, Lavender 2019, Mantell 2019, Martínez Pérez 2016, Mburu 2014, Naugle 2019, Russell 2019, Sileo 2019b, Wamoyi 2017, Zissette 2016 | |||
| Lifestyle | Fear that HIV and engagement in HIV care will make life less fun for men because of HIV stigma in the community and the need to take treatment. | For us, men, HIV is the end of your fun, the end of your joy… it's like you are condemned. When you do not have AIDS, you go to bars, you drink your beer, you find a girl, you go and enjoy…But once you have it, in your neighborhood, if people say you have it, they will point fingers at you. (Man in focus group, age 25–34) [Naugle, p. 6] | Chikovore 2016, Conserve 2019, DiCarlo 2014, Hendrickson 2019, Naugle 2019, Ndyabakira 2019, Orr 2017, Rankin‐Williams 2017, Sileo 2019b, Zissette 2016 | |||
| Facilitators | Coping skills | Appearance of health and strength | Belief that engaging in HIV care is a way to maintain and enhance physical appearance, health, strength and sexuality. | In the past there was so much fear [about HIV] …[but now] I drink my beer and I tell the people around that I am HIV infected, and I am proud…I show off because I look good. (Man with HIV) [Russell, p. 1203] | Brown 2019, Camlin 2016, Graham 2018, Hendrickson 2019, Russell 2019, Sandfort 2015, Sileo 2019b | |
| Responsibility for family | HIV can serve as an impetus to help men realize the importance of taking care of themselves so that they are able to take care of their family. Men also described testing in order to start a serious relationship or get married. | My children are a reason to fight for my life so I can take care of them…[before HIV] I did not know how to save money or even budget, and used [money] for things that did not matter, but ever since I was told that I am HIV positive, I realized that I had to plan… (Man with HIV) [Russell, p. 1204] | Brown 2019, Camlin 2016, Hendrickson 2019, Mak 2016, Okal 2020, Russell 2019, Sandfort 2015, Schatz 2018, Sileo 2019a, Sileo 2019b, Wamoyi 2017 | |||
| Power over HIV | A sense of control over HIV and a desire to fight the diagnosis by engaging in care. | They gave me ARVs as treatment and therefore I have no reason to be afraid. When someone gives you an instrument like a shield to fight with in a war, do you say that I am afraid? You have to fight. (Man with HIV) [Russell, p. 1207] | Hendrickson 2019, Osingada 2019, Russell 2019, Sileo 2019a, Sileo 2019b | |||
| New social support | Men with HIV serve as friends and role models for other men with HIV, helping them to see how HIV and masculinity can be compatible. Men also benefit from support from other friends, family and community members. | Before being diagnosed with HIV, I used to fall sick all the time, yet I know of friends who have already initiated on ART therapy. So, my friends would advise me that why don't you go to a health facility such that you can be checked. (Fisherman, age 23) [Sileo, p. 781] | Daniels 2019, Graham 2018, Hill 2018, Mburu 2014, Mooney 2017, Osingada 2019, Rosen 2020, Russell 2019, Sileo 2019b, Wamoyi 2017, Zissette 2016 | |||
| Health system challenges | Barriers | Low‐resourced clinics | Lack of materials, medications and/or staff | Frustration that health facilities have long waiting times, unavailable providers, and unavailable testing kits and/or ART. | When I come, [the provider gives me advice, he tells me to take my medication. I tell him yes I will take the medication, but often when I come there is no medication. So when there's no medication like that, I am discouraged. [Tibbels, p. 8] | Adams 2017, Adeabgo 2019, Daniels 2019, Krakowiak 2020, Lavender 2019, Mak 2016, Ndyabakira 2019, Ogunbajo 2018, Okal 2020, Tibbels 2019, Tsang 2019, Zissette 2016 |
| Mistrust and misinformation about HIV | Doubt accuracy of test results | Mistrust that testing results are valid, as well as the ability or motivation of providers to correctly interpret and communicate results. | I do not doubt the reliability of the test, the test we all know that it is reliable, but sometimes the person who does the test can be wrong. (Man in focus group, age 35–49) [Tibbels, p. 6] |
Graham 2018, Jennings 2017, Martínez Pérez 2016, Ogunbajo 2018, Okal 2020, Osingada 2019, Sandfort 2015, Tibbels 2019 |
||
| Misinformation about HIV | False beliefs including that it is impossible to survive with HIV, that HIV is man‐made or that one's personal risk is low despite high‐risk activities. | Some [men] get involved [in HIV care] while others fear because they think if I am found positive, I would die quickly, so they better go when they are already bedridden. [Ndyabakira, p. 6] | Adams 2017, Camlin 2016, DiCarlo 2014, Jennings 2017, Mak 2016, Martínez Pérez 2016, Mooney 2017, Ndyabakira 2019, Ogunbajo 2018, Rankin‐Williams 2017, Russell 2019 | |||
| Unwanted disclosure | Lack of confidentiality given clinic layout and procedures | Concern that the lack of confidential spaces and procedures within the clinical setting leads to unwanted disclosure. | … At the hospital there is a bench for those with HIV. When you sit there, you wait for medication, people know that you have HIV. The person has so much fear of this, so much shame, that the person will not go there. [Tibbels, p. 7] | Adams 2017, Adeagbo 2019, Fleming 2016, Hendrickson 2019, Mak 2016, Mantell 2019, Martínez Pérez 2016, Ogunbajo 2018, Okal 2020, Orr 2017, Rankin‐Williams 2017, Rosen 2020, Sandfort 2015, Tibbels 2019, Van Heerden 2015, Zissette 2016 | ||
| Anticipated and enacted stigma | Enacted and anticipated stigma towards persons with HIV | Experienced of expected judgement from healthcare staff and others towards people with HIV. | When you get to the hospital, you feel as if you have failed, being sick…When they discover it is HIV, they give you a weird look. When your back is turned, the staff laughs…. I lived it yesterday and it hurt me. (Man with HIV, age 25–34) [Tibbels, p. 5] | Adams 2017, Adeagbo 2019, Chikovore 2016, Daniels 2019, DiCarlo 2014, Hendrickson 2019, Mak 2016, Mantell 2019, Micheni 2017, Mooney 2017, Naugle 2019, Ndyabakira 2019, Ogunbajo 2018, Okal 2020, Orr 2017, Osingada 2019, Rankin‐Williams 2017, Rosen 2020, Russell 2019, Sandfort 2015, Skovdal 2019, Tibbels 2019, Tsang 2019, Zissette 2016 | ||
| Enacted and anticipated stigma towards MSM, regardless of HIV status | Experienced or expected judgement from healthcare staff and others towards MSM, which intersects with stigma of HIV | If I went to a health facility the moment I meet you I can tell how homophobic you are or how friendly you are … I cannot access health care where there is stigma or a place where they are not sensitive to sexuality issues. (MSM age 22 years, ART‐naïve) [Graham, p. 100] | Daniels 2019, Graham 2018, Mak 2016, Micheni 2017, Sandfort 2015, Tsang 2019 | |||
| Facilitators | Convenient access to healthcare | Home testing | Home testing allows for a comfortable, private environment for testing and is enhanced by health counsellors. | I think men definitely would [home]‐test … because there is no place like home. It is where I know I can get all the support. [DiCarlo, p. 877] |
DiCarlo 2014, Krakowaik 2020, Martínez Pérez 2016, Ndyabakira 2019, Rankin‐Williams 2017, Van Heerden 2015 [118,125,131,139,190,193, 127,134,140,148,199,202, 128,135,141,149,200,203, 125,201,202, 127,134,140,148,199,202, 126,133,139,147,198,201, 124,131,137,145,196,199, 122,129,135,143,194,197, 121,128,134,142,193,196, 119,126,132,140,191,194, 120,127,133,141,192,195, 119,126,132,140,191,194, 118,125,131,139,190,193, 115,122,128,136,187,190, 113,120,126,134,185,188, 112,119,125,133,184,187, 111,118,124,132,183,186] |
|
| Self‐testing | Self‐testing gives men a sense of control, privacy and convenience. | Yeah, like none sees me while I test. And once I am done, I throw it to the dustbin. (Man, age 26) [Jennings, p. 5] | Adeagbo 2019, Jennings 2017, Osingada 2019 | |||
| Flexible clinic opening hours | Flexible facility hours help to accommodate busy work schedules. | …they should make these hospitals operate 24 hours because this one is a big hospital…. The reason why I'm saying that is because there are some people who work in daytime up to very late and when they come here they don't get services because the doctors and the nurses are gone. (Man with HIV, age 40) [Okal, p. 14] | Okal 2020 | |||
| Trust in health system | Belief in effectiveness of ART | Belief in ART effectiveness through clinical guidance, public advertisements and personal experience. | So I believe that this treatment is good because my skin was black, and I also lost weight, but I have recovered. People, they used to ask me what I am eating nowadays because I look healthy and my body has recovered, compared to last year. So I have started to realize that ART is helpful, and it's true. (Man with HIV, age 45–50) [Mooney, p. 279] | Brown 2019, DiCarlo 2014, Hendrickson 2019, Mooney 2017, Ogunbajo 2018, Okal 2020, Rankin‐Williams 2017, Russell 2019, Schatz 2018, Skovdal 2019 | ||
| Positive experiences with healthcare staff | Experiences in which healthcare staff have been especially helpful to men engaging in care. | When I went to the hospital, I didn't tell my parents and I did not have any money. The nurse that counseled me, she paid for my labs. I needed labs before they could put me on the medicine. The nurse I went to see paid for my labs and she is the one who made everything easier for me. [Ogunbajo, p. 836] | Brown 2019, Graham 2018, Mak 2016, Ogunbajo 2018 | |||
| Poverty | Barriers | Poverty | Direct unaffordability of seeking HIV care | Economic challenges, including transport costs, non‐subsidized medical expenses and costs for medical visits. The informal health system is perceived as more affordable. | Think that if they go to the hospital, the costs will be exorbitant. So they prefer to stay in their corners, do their traditional treatment. (Man in focus group, age 25–34) [Tibbels, p. 8] | Jennings 2017, Mak 2016, Micheni 2017, Ndyabakira 2019, Ogunbajo 2018, Schatz 2018, Sileo 2019b, Tibbels 2019 |
| Opportunity costs of care | HIV care takes time and money away from needing to seek employment, food and other needs. | You see the challenge that most of us have faced is that we are poor; the illness finds us in poverty. So, you have to strive hard to look for money and that involves use of a lot of energy, which is a very big challenge. (Boat operator, age 32) [Sileo, p. 780] | Adeabgo 2019, Camlin 2016, Jennings 2017, Krakowiak 2020, Mak 2016, Micheni 2017, Ndyabakira 2019, Ogunbajo 2018, Sileo 2019a, Tibbels 2019 | |||
| Facilitators | Affordability of care | Testing alternatives | Home and self‐testing perceived as more affordable because they cost less and they are quicker, so men lose less productive time. Home testing is preferred because men do not have to spend money to travel to clinics or hospitals. | The advantage [of HIV self‐testing kits] is there is no need to go to the hospital to take the test. And also, you save money because…in private hospitals you must pay to be tested. (Man, age 22) [Jennings, p. 5] | Adeagbo 2019, Camlin 2016, Jennings 2017, Krakowiak 2020, Mak 2016, Micheni 2017, Ndyabakira 2019, Ogunbajo 2018, Sileo 2019a, Tibbels 2019 |
A.5. FOREST PLOTS COMPARING MEN AND WOMEN FOR EACH OF THE UNAIDS 95‐95‐95 GOALS
Figures A1, A2, A3, A4, A5, A6, A7, A8, A9, A10, A11, A12, A13, A14, A15, A16
Figure A1.
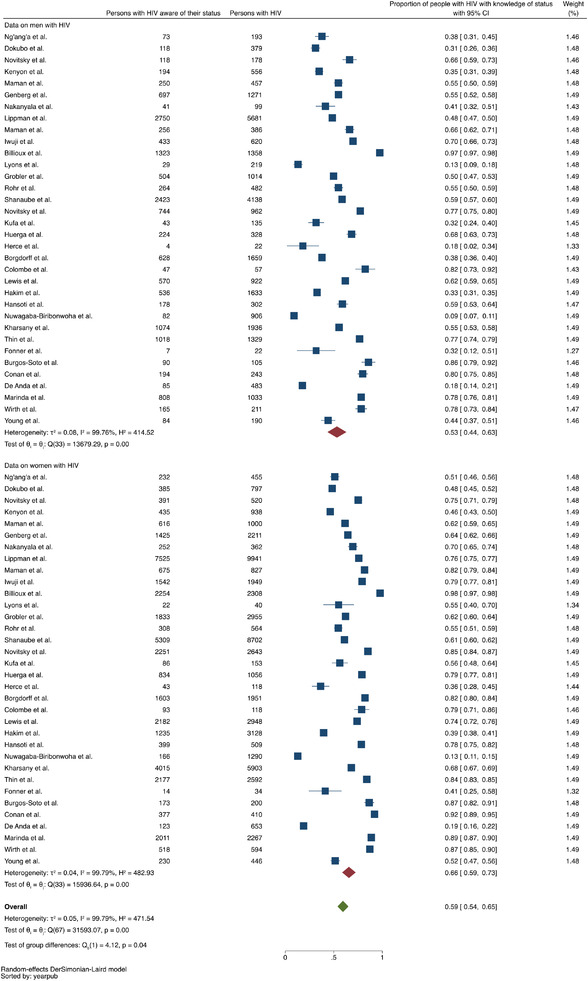
Forest plot comparing proportions of men and women with awareness of positive HIV status, including only studies reporting these data for both men and women (N=34 studies).
Figure A2.
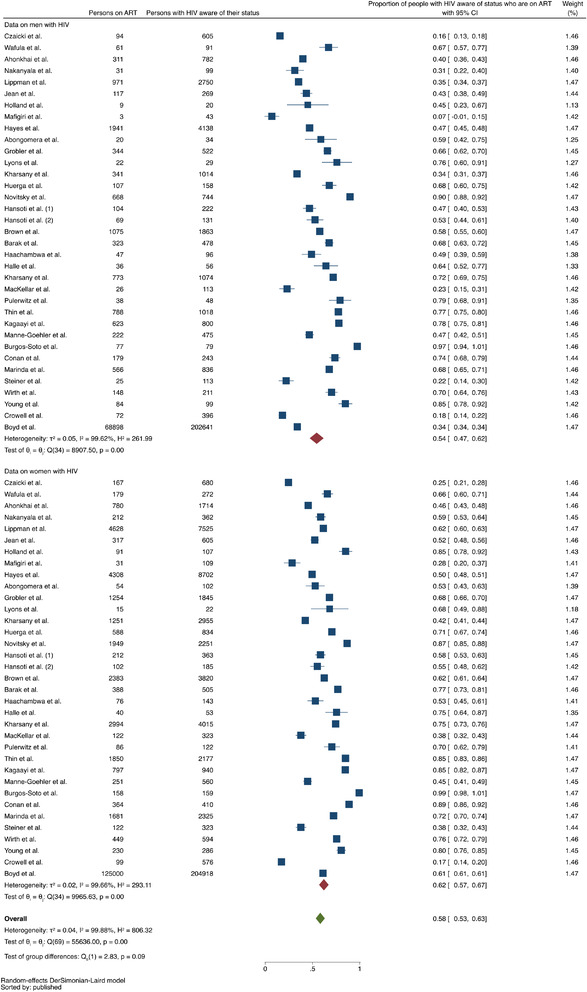
Forest plot comparing proportions of men and women on ART of those who are aware of their positive status, including only studies reporting these data for both men and women (N=35 studies).
Figure A3.
(a) Forest plot comparing proportions of men and women who are virally suppressed of those on ART, including only studies reporting these data for both men and women (N=70 studies). Data for men shown here and data for women with overall comparisons shown in Figure 3b. (b) Forest plot comparing proportions of men and women who are virally suppressed of those on ART, including only studies reporting these data for both men and women (N=70 studies). Data for women and overall comparisons shown here and data for men shown in Figure 3a.
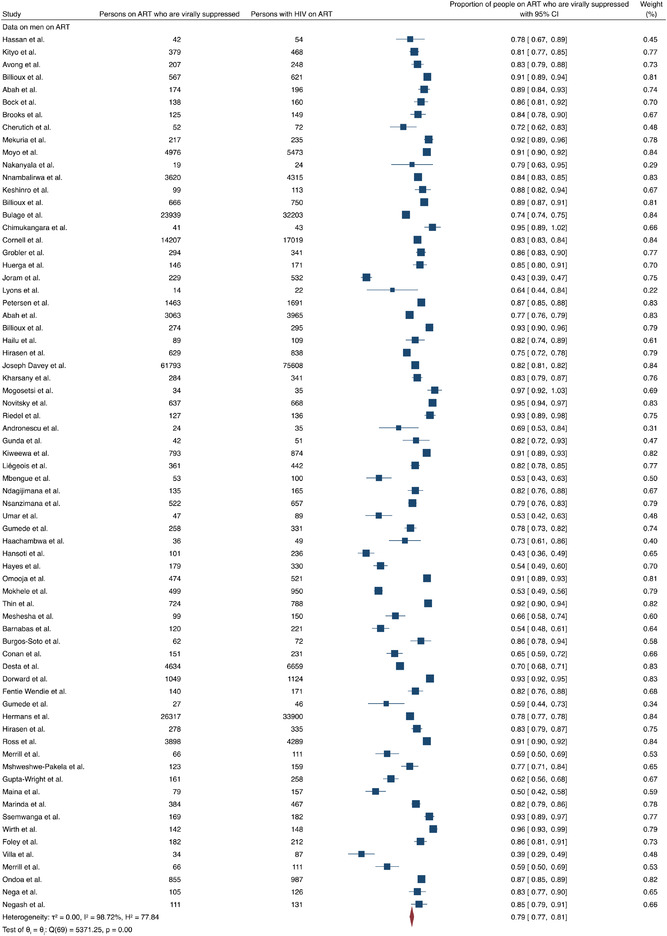
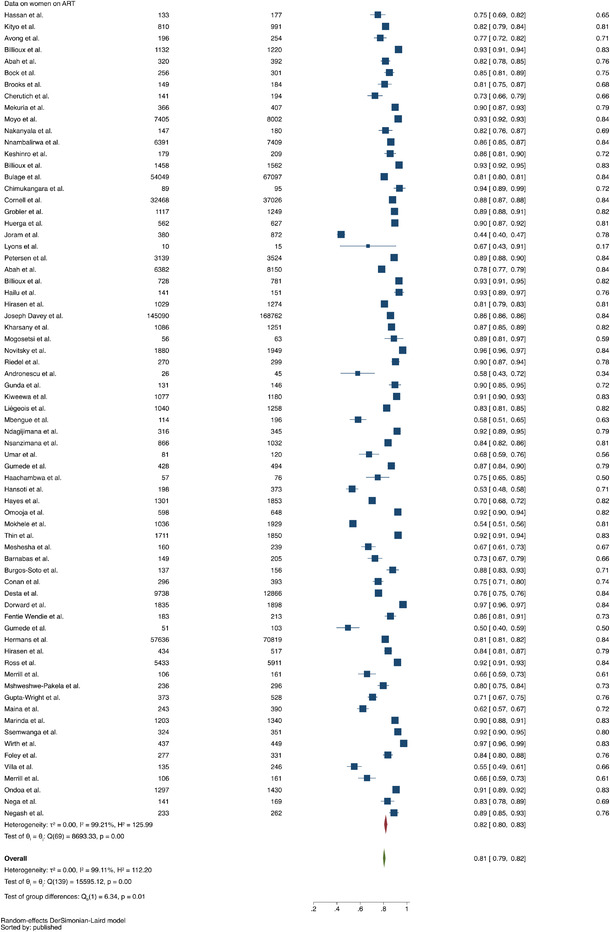
A.6. FOREST PLOTS BY SUBGROUP FOR MEN FOR THE UNAIDS 95‐95‐95 GOALS
Figure A4.
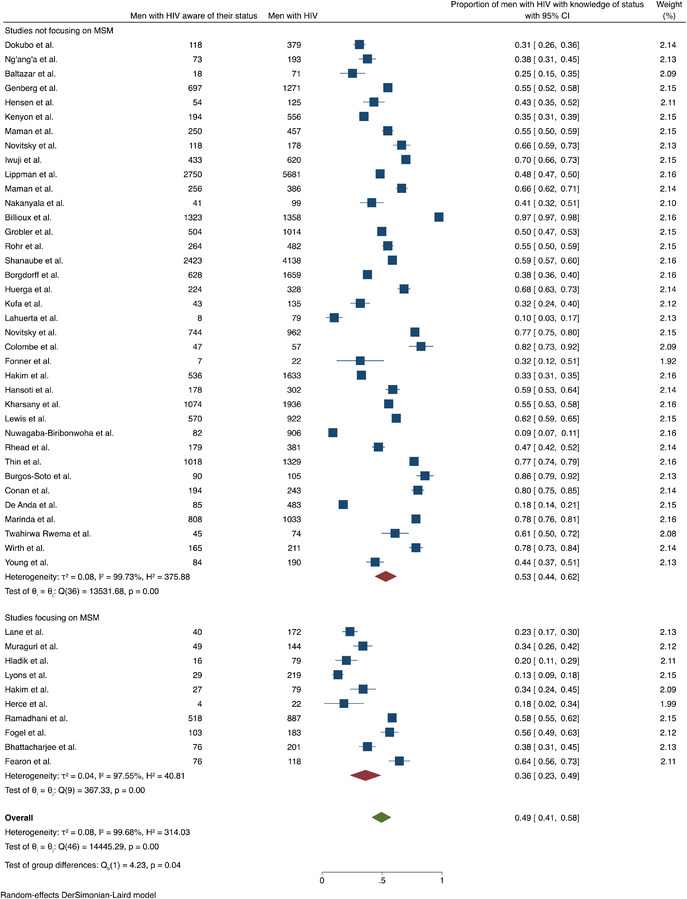
Forest plot comparing proportions of men with awareness of positive HIV status in studies focusing exclusively on MSM (N=10 studies) versus studies that did not focus exclusively on MSM (N=37 studies).
Figure A5.
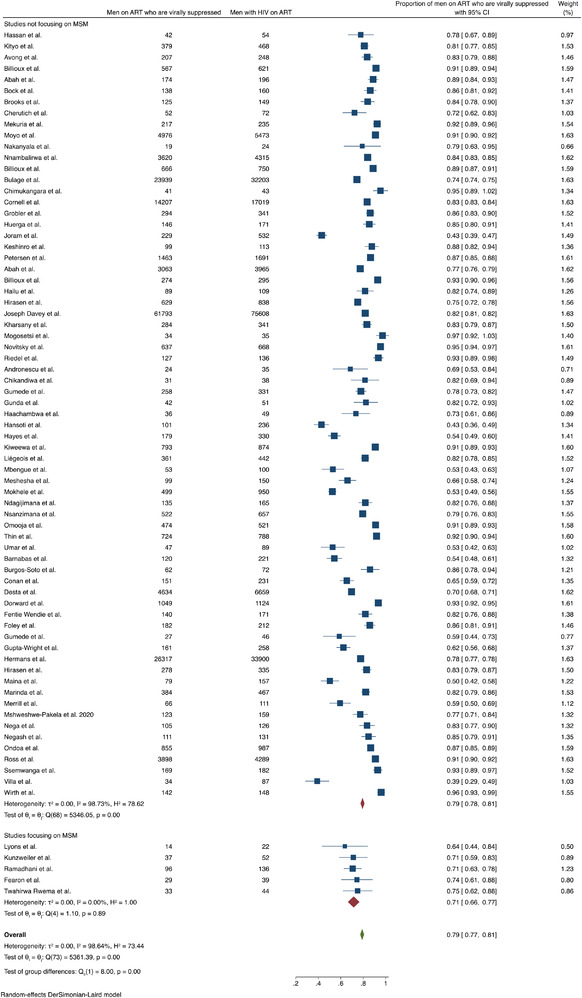
Forest plot comparing proportions of men on ART who are virally suppressed in studies focusing exclusively on MSM (N=5 studies) versus studies that did not focus exclusively on MSM (N=69 studies).
Figure A6.
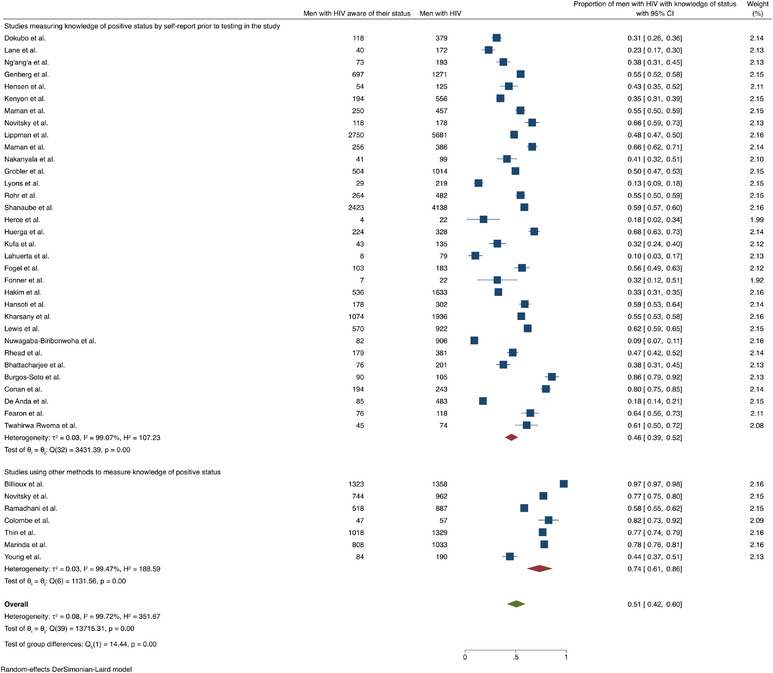
Forest plot comparing proportions of men with awareness of positive HIV status in studies in which knowledge of positive HIV status was measured by self‐report prior to testing (N=33 studies) versus studies in which knowledge was measured by other methods, such as awareness after testing in the study (N=7 studies).
Figure A7.
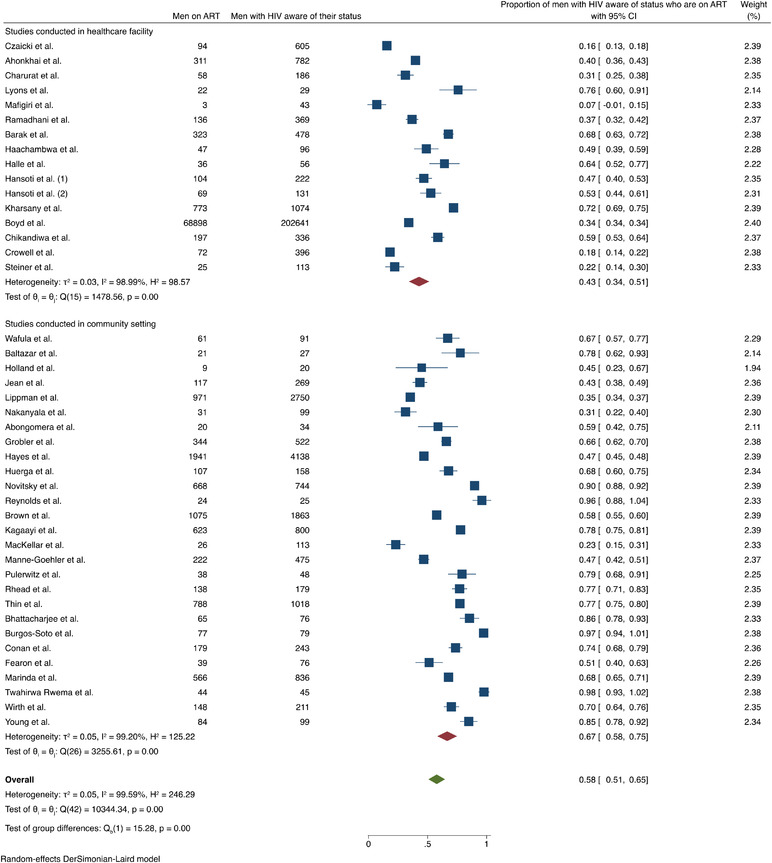
Forest plot comparing proportions of men with known HIV on ART in studies in which ART status was ascertained in a healthcare facility (N=16 studies) versus studies in which ART status was ascertained in a community setting (N=27 studies).
Figure A8.
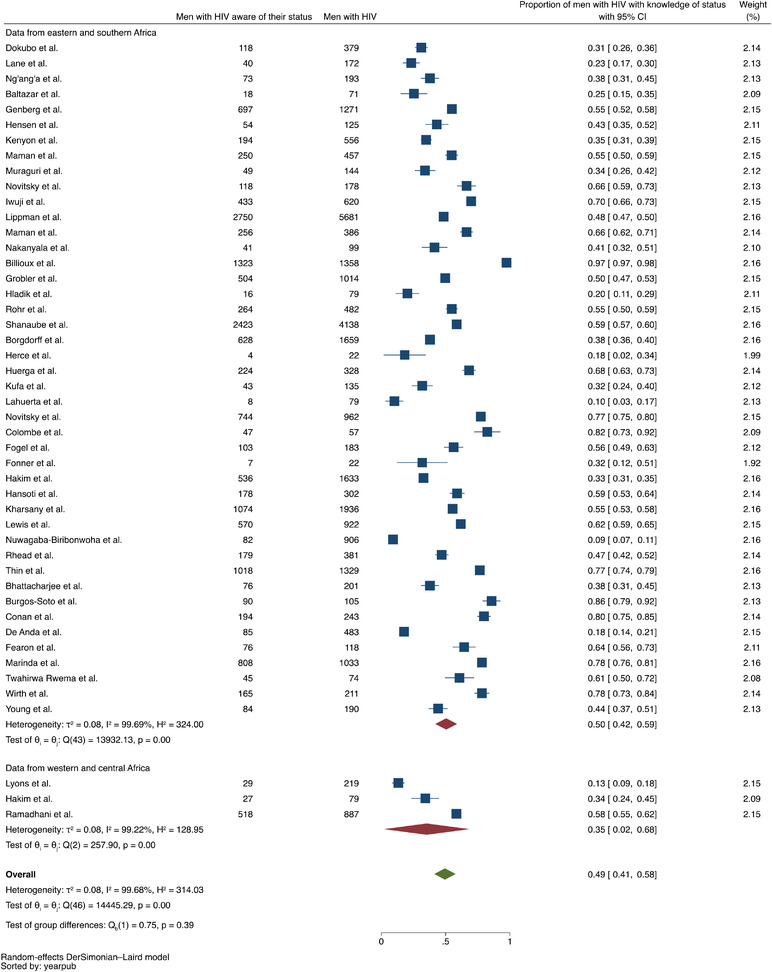
Forest plot comparing proportions of men with awareness of positive HIV status in studies conducted in eastern and southern Africa (N=44 studies) versus studies conducted in western and central Africa (N=3 studies).
Figure A9.
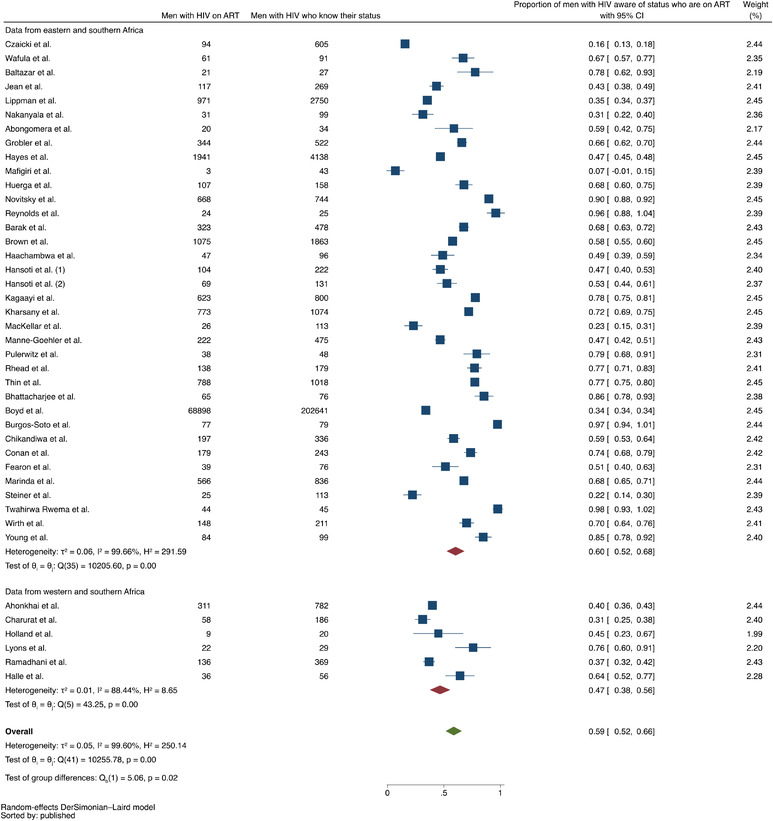
Forest plot comparing proportions of men with known HIV on ART in studies conducted in eastern and southern Africa (N=36 studies) versus studies conducted in western and central Africa (N=6 studies).
Figure A10.
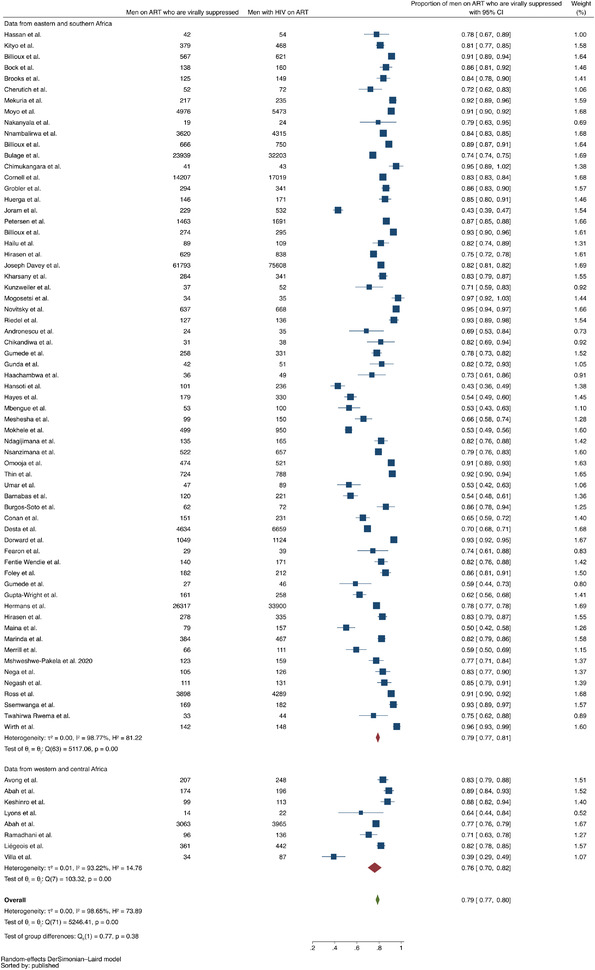
Forest plot comparing proportions of men on ART who are virally suppressed in studies conducted in eastern and southern Africa (N=64 studies) versus studies conducted in western and central Africa (N=8 studies).
Figure A11.
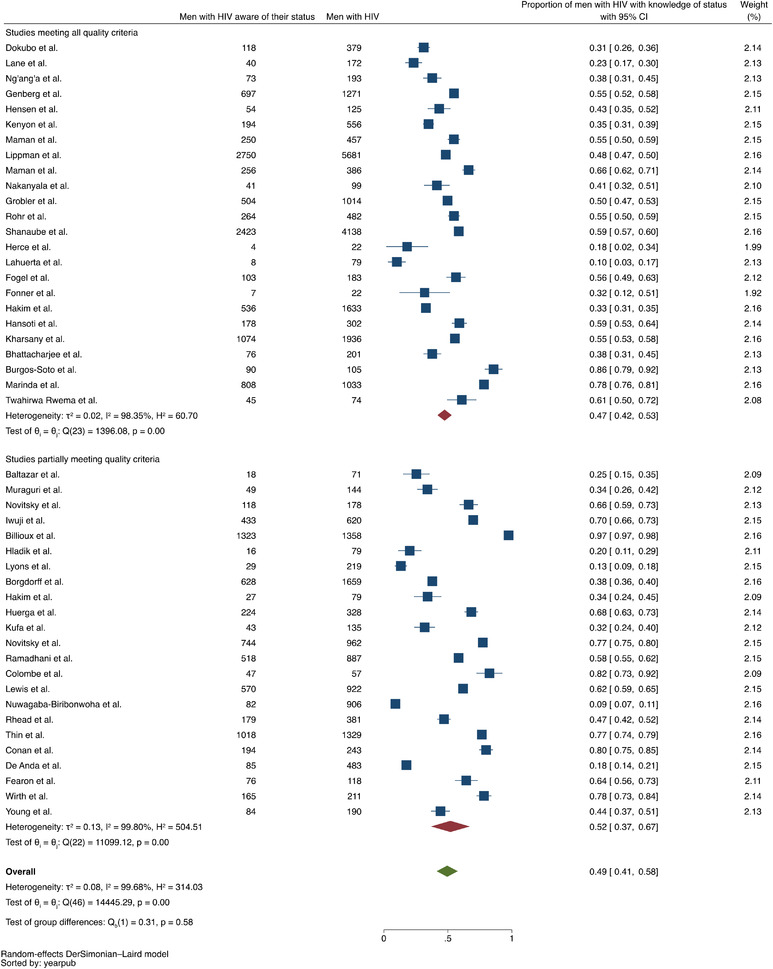
Forest plot comparing proportions of men with awareness of positive HIV status in studies in which all quality criteria are met (N=24 studies) versus studies in which quality criteria are partially met (N=23 studies).
Figure A12.
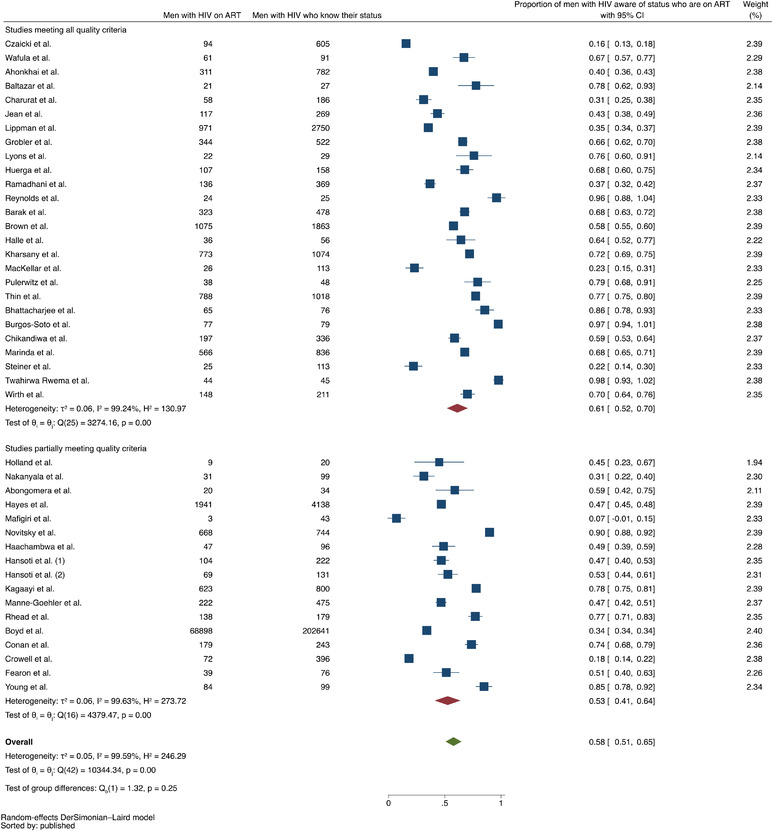
Forest plot comparing proportions of men with HIV on ART in studies in which all quality criteria are met (N=26 studies) versus studies in which quality criteria are partially met (N=17 studies).
Figure A13.
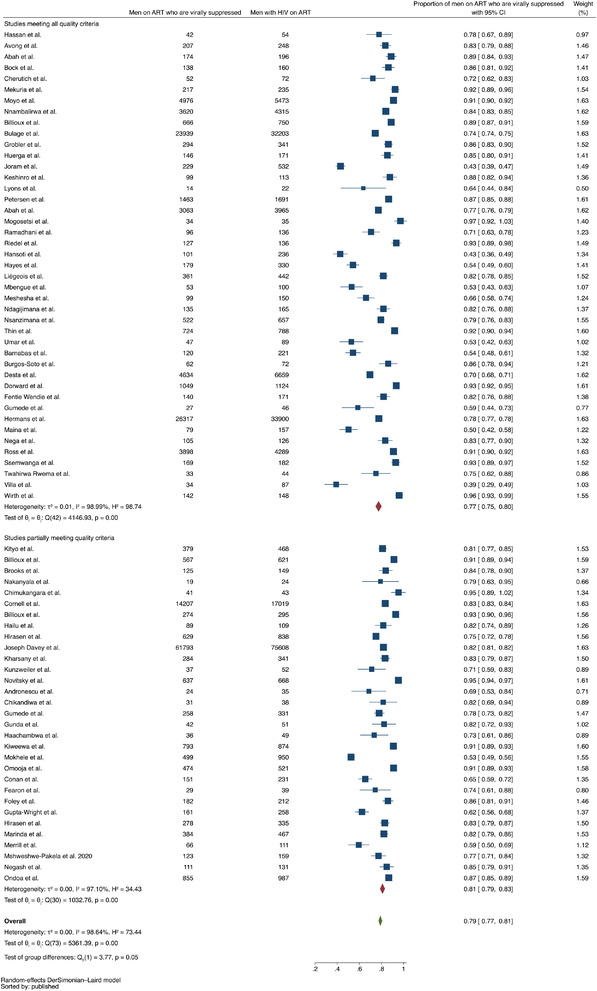
Forest plot comparing proportions of men on ART who are virally suppressed in studies in which all quality criteria are met (N=43 studies) versus studies in which quality criteria are partially met (N=31 studies).
Figure A14.
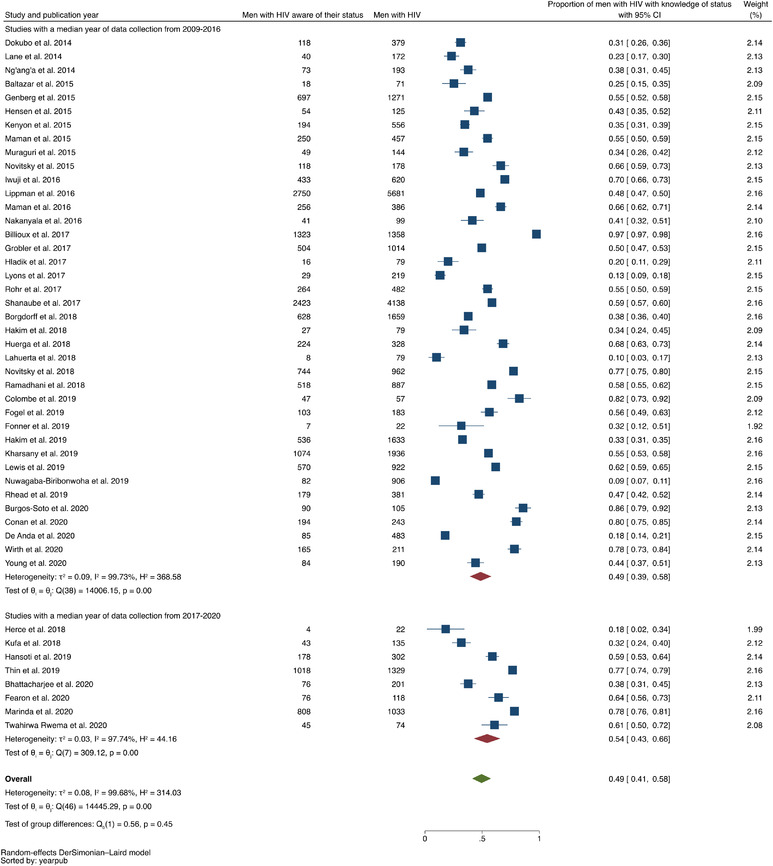
Forest plot comparing proportions of men with awareness of positive HIV status in studies in which the median year of data collection was between 2009 and 2016 (N=39 studies) versus in studies in which the median year of data collection was between 2017 and 2019 (N=8 studies).
Figure A15.
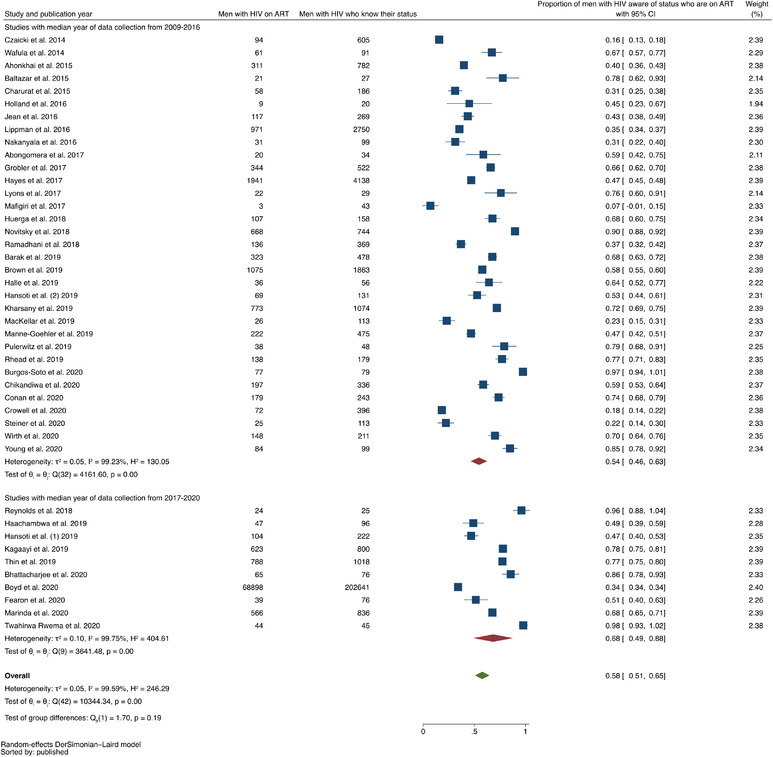
Forest plot comparing proportions of men with HIV on ART in studies in which the median year of data collection was between 2009 and 2016 (N=33 studies) versus studies in which the median year of data collection was between 2017 and 2020 (N=10 studies).
Figure A16.
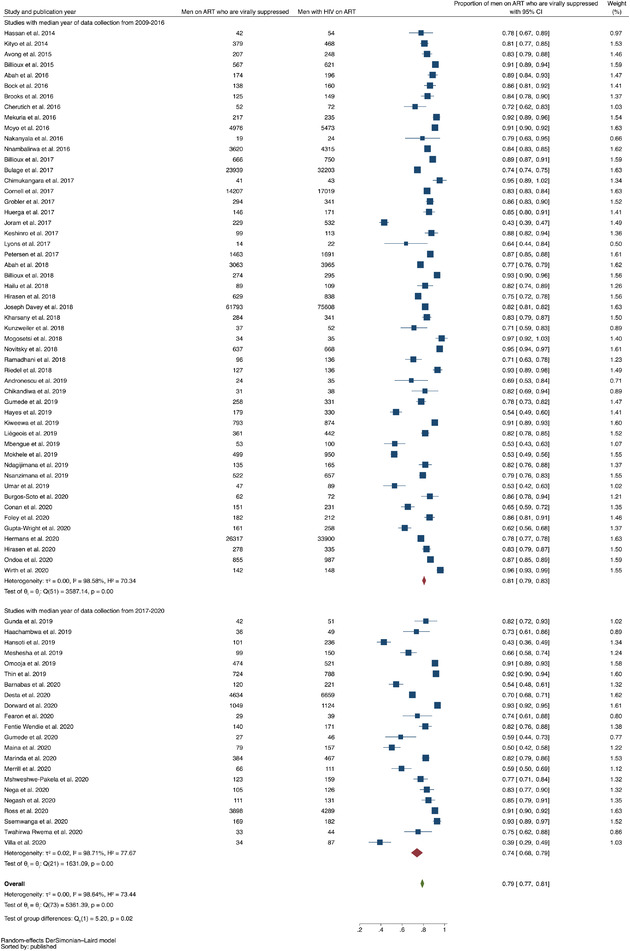
Forest plot comparing proportions of men on ART who are virally suppressed in studies in which the median year of data collection was between 2009 and 2016 (N=52 studies) versus studies in which the median year of data collection was between 2017 and 2020 (N=22 studies).
DATA AVAILABILITY STATEMENT
All relevant data are within the manuscript and its Supporting Information files. No primary data were obtained for this study.
REFERENCES
- 1. Geng EH, Bwana MB, Muyindike W, Glidden DV, Bangsberg DR, Neilands TB, et al. Failure to initiate antiretroviral therapy, loss to follow‐up and mortality among HIV‐infected patients during the pre‐ART period in Uganda. J Acquir Immune Defic Syndr. 2013;63(2):e64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osler M, Cornell M, Ford N, Hilderbrand K, Goemaere E, Boulle A. Population‐wide differentials in HIV service access and outcomes in the Western Cape for men as compared to women, South Africa: 2008 to 2018: a cohort analysis. J Int AIDS Soc. 2020;23(S2):e25530. 10.1002/jia2.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takuva S, Brown AE, Pillay Y, Delpech V, Puren AJ. The continuum of HIV care in South Africa: implications for achieving the second and third UNAIDS 90‐90‐90 targets. AIDS. 2017;31(4):545–52. [DOI] [PubMed] [Google Scholar]
- 4. Druyts E, Dybul M, Kanters S, Nachega J, Birungi J, Ford N, et al. Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta‐analysis. AIDS. 2013;27(3):417–25. [DOI] [PubMed] [Google Scholar]
- 5. Byanyima W. UNAIDS Data. 2020.. [cited 2021 Oct 14]. Available from: https://www.unaids.org/sites/default/files/media_asset/2020_aids‐data‐book_en.pdf
- 6. UNAIDS Fast track: ending the AIDS epidemic by 2030. Available from: https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf. Accessed 12 January 2022.
- 7. Stannah J, Dale E, Elmes J, Staunton R, Beyrer C, Mitchell KM, et al. HIV testing and engagement with the HIV treatment cascade among men who have sex with men in Africa: a systematic review and meta‐analysis. Lancet HIV. 2019;6(11):e769–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Green D, Tordoff DM, Kharono B, Akullian A, Bershteyn A, Morrison M, et al. Evidence of sociodemographic heterogeneity across the HIV treatment cascade and progress towards 90‐90‐90 in sub‐Saharan Africa – a systematic review and meta‐analysis. J Int AIDS Soc. 2020;23(3).e25470. 10.1002/jia2.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giguère K, Eaton JW, Marsh K, Johnson LF, Johnson CC, Ehui E, et al. Trends in knowledge of HIV status and efficiency of HIV testing services in sub‐Saharan Africa, 2000–20: a modelling study using survey and HIV testing programme data. Lancet HIV. 2021;8(5):e284–e293. 10.1016/S2352-3018(20)30315-5. Epub 2021 Mar 2. PMID: 33667411; PMCID: PMC8097636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Makusha T, Rooyen H, Cornell M. Reframing the approach to heterosexual men in the HIV epidemic in sub‐Saharan Africa. J Int AIDS Soc. 2020;23(S2).e25510. 10.1002/jia2.25510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ellman TM, Alemayehu B, Abrams EJ, Arpadi S, Howard AA, El‐Sadr WM. Selecting a viral load threshold for routine monitoring in resource‐limited settings: optimizing individual health and population impact. J Int AIDS Soc. 2017;20:e25007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warren EA, Paterson P, Schulz WS, Lees S, Eakle R, Stadler J, et al. Risk perception and the influence on uptake and use of biomedical prevention interventions for HIV in sub‐Saharan Africa: a systematic literature review. PLoS One. 2018;13(6):e0198680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tverskey A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;. 185:1124–31. [DOI] [PubMed] [Google Scholar]
- 14. Katz IT, Dietrich J, Tshabalala G, Essien T, Rough K, Wright AA, et al. Understanding treatment refusal among adults presenting for HIV‐testing in Soweto, South Africa: a qualitative study. AIDS Behav. 2015;19(4):704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma M, Barnabas RV, Celum C. Community‐based strategies to strengthen men's engagement in the HIV care cascade in sub‐Saharan Africa. PLoS Med. 2017;. 14(4):e1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grimsrud A, Ameyan W, Ayieko J, Shewchuk T. Shifting the narrative: from “the missing men” to “we are missing the men.” J Int AIDS Soc. 2020;23(S2).e25526. 10.1002/jia2.25526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Covidence systematic review software. Melbourne: Veritas Health Innovation. [Google Scholar]
- 18. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. The Ottawa Hospital Research Institute. [cited 2021. Oct 26]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 19. Higgins JPT, Savovic J, Page MJ, Sterne JA. Revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2). 2019. [cited 2021 Oct 26]. Available from: https://drive.google.com/file/d/19R9savfPdCHC8XLz2iiMvL_71lPJERWK/view
- 20. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32‐item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57. [DOI] [PubMed] [Google Scholar]
- 21. Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, et al. Impact of HIV‐related stigma on treatment adherence: systematic review and meta‐synthesis. J Int AIDS Soc. 2013;16:18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National CASP Collaboration for Qualitative Methodologies . 10 questions to help you make sense of qualitative research. Milton Keynes Primary Care Trust; 2006. [Google Scholar]
- 23. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;. 21(4):607–11. [Google Scholar]
- 24. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;. 7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 25. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;. 22(158):209–12. [Google Scholar]
- 26. Newcombe RG. Two‐sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;. 17:857–72. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;. 21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 28. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noblit G, Hare R. Meta‐ethnography: synthesizing qualitative studies. Newbury Park: Sage; 1988. [Google Scholar]
- 30. Thorne S, Jensen L, Kearney MH, Noblit G, Sandelowski M. Qualitative metasynthesis: reflections on methodological orientation and ideological agenda. Qual Health Res. 2004;14(10):1342–65. [DOI] [PubMed] [Google Scholar]
- 31. Sandelowsi M, Barroso J. Handbook for synthesising qualitative research. New York: Springer; 2007. [Google Scholar]
- 32. Lachal J, Revah‐Levy A, Orri M, Moro MR. Metasynthesis: an original method to synthesize qualitative literature in psychiatry. Front Psychiatry. 2017;8:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abah IO, Ojeh VB, Musa J, Ugoagwu P, Agaba PA, Agbaji O, et al. Clinical utility of pharmacy‐based adherence measurement in predicting virologic outcomes in an adult HIV‐infected cohort in Jos, North Central Nigeria. J Int Assoc Provid AIDS Care. 2016;15(1):77–83. [DOI] [PubMed] [Google Scholar]
- 34. Abah IO, Ncube NBQ, Bradley HA, AgbaJi OO, Kanki P. Antiretroviral therapy‐associated adverse drug reactions and their effects on virologic failure—a retrospective cohort study in Nigeria. Curr HIV Res. 2019;16(6):436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abongomera G, Kiwuwa‐Muyingo S, Revill P, Chiwaula L, Mabugu T, Phillips AN, et al. Impact of decentralisation of antiretroviral therapy services on HIV testing and care at a population level in Agago District in rural Northern Uganda: results from the Lablite population surveys. Int Health. 2017;9(2):91–9. [DOI] [PubMed] [Google Scholar]
- 36. Ahonkhai AA, Banigbe B, Adeola J, Onwuatuelo I, Bassett IV, Losina E, et al. High rates of unplanned interruptions from HIV care early after antiretroviral therapy initiation in Nigeria. BMC Infect Dis. 2015;15(1):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andronescu L, Zulu PM, Jackson SS, Hachaambwa L, Claassen CW, Stafford KA. The association between gender and HIV viral suppression on third‐line therapy in Zambia: a retrospective cohort study. Int J STD AIDS. 2019;30(5):453–9. [DOI] [PubMed] [Google Scholar]
- 38. Avong Y, Wyk B, Njab J, Abimiku A, Ndembi N, Okuma J, et al. Adherence to anti‐retroviral therapy in North Central Nigeria. Curr HIV Res. 2015;13(4):268–78. [DOI] [PubMed] [Google Scholar]
- 39. Baltazar CS, Horth R, Inguane C, Sathane I, César F, Ricardo H, et al. HIV prevalence and risk behaviors among Mozambicans working in South African mines. AIDS Behav. 2015;19(S1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Billioux A, Nakigozi G, Newell K, Chang LW, Quinn TC, Gray RH, et al. Durable suppression of HIV‐1 after virologic monitoring‐based antiretroviral adherence counseling in Rakai, Uganda. PLoS One. 2015;10(5):e0127235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Billioux VG, Chang LW, Reynolds SJ, Nakigozi G, Ssekasanvu J, Grabowski MK, et al. Human immunodeficiency virus care cascade among sub‐populations in Rakai, Uganda: an observational study. J Int AIDS Soc. 2017;. 20(1):21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Billioux VG, Grabowski MK, Ssekasanvu J, Reynolds SJ, Berman A, Bazaale J, et al. HIV viral suppression and geospatial patterns of HIV antiretroviral therapy treatment facility use in Rakai, Uganda. AIDS. 2018;32(6):819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bock NN, Emerson RC, Reed JB, Nkambule R, Donnell DJ, Bicego GT, et al. Changing antiretroviral eligibility criteria: impact on the number and proportion of adults requiring treatment in Swaziland. J Acquir Immune Defic Syndr. 2016;71(3):338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Borgdorff MW. HIV incidence in western Kenya during scale‐up of antiretroviral therapy and voluntary medical male circumcision: a population‐based cohort analysis. Lancet HIV. 2018;. 5:e241–9. [DOI] [PubMed] [Google Scholar]
- 45. Brooks K, Diero L, DeLong A, Balamane M, Reitsma M, Kemboi E, et al. Treatment failure and drug resistance in HIV‐positive patients on tenofovir‐based first‐line antiretroviral therapy in western Kenya. J Int AIDS Soc. 2016;19(1):20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bulage L, Ssewanyana I, Nankabirwa V, Nsubuga F, Kihembo C, Pande G, et al. Factors associated with virological non‐suppression among HIV‐positive patients on antiretroviral therapy in Uganda, August 2014–July 2015. BMC Infect Dis. 2017;17(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Charurat ME, Emmanuel B, Akolo C, Keshinro B, Nowak RG, Kennedy S, et al. Uptake of treatment as prevention for HIV and continuum of care among HIV‐positive men who have sex with men in Nigeria. J Acquir Immune Defic Syndr. 2015;68:S114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cherutich P, Kim AA, Kellogg TA, Sherr K, Waruru A, De Cock KM, et al. Detectable HIV viral load in Kenya: data from a population‐based survey. PLoS One. 2016;11(5):e0154318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chimukangara B, Manasa J, Mitchell R, Nyabadza G, Katzenstein D, Masimirembwa C. Community based antiretroviral treatment in rural Zimbabwe. AIDS Res Hum Retroviruses. 2017;33(12):1185–91. [DOI] [PubMed] [Google Scholar]
- 50. Cornell M, Johnson LF, Wood R, Tanser F, Fox MP, Prozesky H, et al. Twelve‐year mortality in adults initiating antiretroviral therapy in South Africa. J Int AIDS Soc. 2017;. 20(1):21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Czaicki NL, Davitte J, Siangonya B, Kastner R, Ahmed N, Khu NH, et al. Predictors of first follow‐up HIV testing for couples’ voluntary HIV counseling and testing in Ndola, Zambia. J Acquir Immune Defic Syndr. 2014;66(1):e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dokubo EK, Shiraishi RW, Young PW, Neal JJ, Aberle‐Grasse J, Honwana N, et al. Awareness of HIV status, prevention knowledge and condom use among people living with HIV in Mozambique. PLoS One. 2014;9(9):e106760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fogel JM, Sandfort T, Zhang Y, Guo X, Clarke W, Breaud A, et al. Accuracy of self‐reported HIV status among African men and transgender women who have sex with men who were screened for participation in a research study: HPTN 075. AIDS Behav. 2019;23(1):289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Genberg BL, Naanyu V, Wachira J, Hogan JW, Sang E, Nyambura M, et al. Linkage to and engagement in HIV care in western Kenya: an observational study using population‐based estimates from home‐based counselling and testing. Lancet HIV. 2015;2(1):e20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grobler A, Cawood C, Khanyile D, Puren A, Kharsany ABM. Progress of UNAIDS 90‐90‐90 targets in a district in KwaZulu‐Natal, South Africa, with high HIV burden, in the HIPSS study: a household‐based complex multilevel community survey. Lancet HIV. 2017;4(11):e505–13. [DOI] [PubMed] [Google Scholar]
- 56. Gunda DW, Kilonzo SB, Mtaki T, Bernard DM, Kalluvya SE, Shao ER. Magnitude and correlates of virological failure among adult HIV patients receiving PI based second line ART regimens in north western Tanzania; a case control study. BMC Infect Dis. 2019;19(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hailu GG, Hagos DG, Hagos AK, Wasihun AG, Dejene TA. Virological and immunological failure of HAART and associated risk factors among adults and adolescents in the Tigray region of Northern Ethiopia. PLoS One. 2018;13(5):e0196259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hakim AJ, Coy K, Patnaik P, Telly N, Ballo T, Traore B, et al. An urgent need for HIV testing among men who have sex with men and transgender women in Bamako, Mali: low awareness of HIV infection and viral suppression among those living with HIV. PLoS One. 2018;13(11):e0207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hakim AJ, Mukasa B, Hundley L, Odiit M, Ogwal M, Sendagala S, et al. Correlates of undiagnosed HIV infection and retesting among voluntary HIV testing clients at Mildmay Clinic, Uganda. AIDS Behav. 2019;23(4):820–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hansoti B, Stead D, Eisenberg A, Mvandaba N, Mwinnyaa G, Patel EU, et al. A window into the HIV epidemic from a South African emergency department. AIDS Res Hum Retroviruses. 2019;35(2):139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hassan AS, Nabwera HM, Mwaringa SM, Obonyo CA, Sanders EJ, Rinke de Wit TF, et al. HIV‐1 virologic failure and acquired drug resistance among first‐line antiretroviral experienced adults at a rural HIV clinic in coastal Kenya: a cross‐sectional study. AIDS Res Ther. 2014;. 11(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hayes R, Floyd S, Schaap A, Shanaube K, Bock P, Sabapathy K, et al. A universal testing and treatment intervention to improve HIV control: one‐year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster‐randomised trial. PLoS Med. 2017;14(5):e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hensen B, Lewis JJ, Schaap A, Tembo M, Mutale W, Weiss HA, et al. Factors associated with HIV‐testing and acceptance of an offer of home‐based testing by men in rural Zambia. AIDS Behav. 2015;19(3):492–504. [DOI] [PubMed] [Google Scholar]
- 64. Herce ME, Miller WM, Bula A, Edwards JK, Sapalalo P, Lancaster KE, et al. Achieving the first 90 for key populations in sub‐Saharan Africa through venue‐based outreach: challenges and opportunities for HIV prevention based on PLACE study findings from Malawi and Angola. J Int AIDS Soc. 2018;21:e25132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hladik W, Sande E, Berry M, Ganafa S, Kiyingi H, Kusiima J, et al. Men who have sex with men in Kampala, Uganda: results from a bio‐behavioral respondent driven sampling survey. AIDS Behav. 2017;21(5):1478–90. [DOI] [PubMed] [Google Scholar]
- 66. Hirasen K, Evans D, Maskew M, Sanne I, Shearer K, Govathson C, et al. The right combination — treatment outcomes among HIV‐positive patients initiating first‐line fixed‐dose antiretroviral therapy in a public sector HIV clinic in Johannesburg, South Africa. Clin Epidemiol. 2017;10:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Holland CE, Kouanda S, Lougué M, Pitche VP, Schwartz S, Anato S, et al. Using population‐size estimation and cross‐sectional survey methods to evaluate HIV service coverage among key populations in Burkina Faso and Togo. Public Health Rep. 2016;131(6):773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huerga H, Shiferie F, Grebe E, Giuliani R, Farhat JB, Van‐Cutsem G, et al. A comparison of self‐report and antiretroviral detection to inform estimates of antiretroviral therapy coverage, viral load suppression and HIV incidence in Kwazulu‐Natal, South Africa. BMC Infect Dis. 2017;17(1):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Iwuji CC, Orne‐Gliemann J, Larmarange J, Okesola N, Tanser F, Thiebaut R, et al. Uptake of home‐based HIV testing, linkage to care, and community attitudes about ART in rural KwaZulu‐Natal, South Africa: descriptive results from the first phase of the ANRS 12249 TasP Cluster‐Randomised Trial. PLoS Med. 2016;13(8):e1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jean K, Puren A, Cutler E, Singh B, Bouscaillou J, Rain‐Taljaard R, et al. Level of viral suppression and the cascade of HIV care in a South African semi‐urban setting in 2012. AIDS. 2016;30(13):2107–16. [DOI] [PubMed] [Google Scholar]
- 71. Joram SL, Paul G, Moses K, Stanley B, Isaac M, Allan G, et al. Misdiagnosis of HIV treatment failure based on clinical and immunological criteria in Eastern and Central Kenya. BMC Infect Dis. 2017;17(1):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Joseph Davey D, Abrahams Z, Feinberg M, Prins M, Serrao C, Medeossi B, et al. Factors associated with recent unsuppressed viral load in HIV‐1‐infected patients in care on first‐line antiretroviral therapy in South Africa. Int J STD AIDS. 2018;29(6):603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kenyon CR, Kirungi W, Kaharuza F, Buyze J, Bunnell R. Who knows their partner's HIV status? Results from a nationally representative survey in Uganda. J Acquir Immune Defic Syndr. 2015;69(1):92–7. [DOI] [PubMed] [Google Scholar]
- 74. Kharsany ABM, Cawood C, Khanyile D, Lewis L, Grobler A, Puren A, et al. Community‐based HIV prevalence in KwaZulu‐Natal, South Africa: results of a cross‐sectional household survey. Lancet HIV. 2018;5(8):e427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kityo C, Gibb DM, Gilks CF, Goodall RL, Mambule I, Kaleebu P, et al. High level of viral suppression and low switch rate to second‐line antiretroviral therapy among HIV‐infected adult patients followed over five years: retrospective analysis of the DART trial. PLoS One. 2014;9(3):e90772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kiweewa F, Esber A, Musingye E, Reed D, Crowell TA, Cham F, et al. HIV virologic failure and its predictors among HIV‐infected adults on antiretroviral therapy in the African Cohort Study. PLoS One. 2019;14(2):e0211344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kufa T, Maseko VD, Nhlapo D, Radebe F, Puren A, Kularatne RS. Knowledge of HIV status and antiretroviral therapy use among sexually transmitted infections service attendees and the case for improving the integration of services in South Africa: a cross sectional study. Medicine (Baltimore). 2018;97(39):e12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kunzweiler CP, Bailey RC, Mehta SD, Okall DO, Obondi E, Djomand G, et al. Factors associated with viral suppression among HIV‐positive Kenyan gay and bisexual men who have sex with men. AIDS Care. 2018;30(sup5):S76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lahuerta M, Patnaik P, Ballo T, Telly N, Knox J, Traore B, et al. HIV prevalence and related risk factors in men who have sex with men in Bamako, Mali: findings from a bio‐behavioral survey using respondent‐driven sampling. AIDS Behav. 2018;22(7):2079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lane T, Osmand T, Marr A, Shade SB, Dunkle K, Sandfort T, et al. The Mpumalanga Men's Study (MPMS): results of a baseline biological and behavioral HIV surveillance survey in two MSM communities in South Africa. PLoS One. 2014;9(11):e111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lewis L, Maughan‐Brown B, Grobler A, Cawood C, Khanyile D, Glenshaw M, et al. Impact of home‐based HIV testing services on progress toward the UNAIDS 90‐90‐90 targets in a hyperendemic area of South Africa. J Acquir Immune Defic Syndr. 2019;80(2):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liégeois F, Eymard‐Duvernay S, Boyer S, Maradan G, Kouanfack C, Domyeum J, et al. Heterogeneity of virological suppression in the national antiretroviral programme of Cameroon (ANRS 12288 EVOLCAM). HIV Med. 2019;20(1):38–46. [DOI] [PubMed] [Google Scholar]
- 83. Lippman SA, Shade SB, El Ayadi AM, Gilvydis JM, Grignon JS, Liegler T, et al. Attrition and opportunities along the HIV care continuum: findings from a population‐based sample, North West Province, South Africa. J Acquir Immune Defic Syndr. 2016;73(1):91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lyons CE, Ketende S, Diouf D, Drame FM, Liestman B, Coly K, et al. Potential impact of integrated stigma mitigation interventions in improving HIV/AIDS service delivery and uptake for key populations in Senegal. J Acquir Immune Defic Syndr. 2017;74(1):S52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mafigiri R, Matovu JKB, Makumbi FE, Ndyanabo A, Nabukalu D, Sakor M, et al. HIV prevalence and uptake of HIV/AIDS services among youths (15–24 years) in fishing and neighboring communities of Kasensero, Rakai District, South Western Uganda. BMC Public Health. 2017;17(1):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Maman D, Ben‐Farhat J, Chilima B, Masiku C, Salumu L, Ford N, et al. Factors associated with HIV status awareness and linkage to care following home based testing in rural Malawi. Trop Med Int Health. 2016;21(11):1442–51. [DOI] [PubMed] [Google Scholar]
- 87. Mbengue MAS, Chasela C, Onoya D, Mboup S, Fox MP, Evans D. Clinical predictor score to identify patients at risk of poor viral load suppression at six months on antiretroviral therapy: results from a prospective cohort study in Johannesburg, South Africa. Clin Epidemiol. 2019;11:359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mekuria LA, Nieuwkerk PT, Yalew AW, Sprangers MA, Prins JM. High level of virological suppression among HIV‐infected adults receiving combination antiretroviral therapy in Addis Ababa, Ethiopia.Antivir Ther. 2016;21(5):385–96. [DOI] [PubMed] [Google Scholar]
- 89. Mogosetsi NJ, Mabuza LH, Ogunbanjo GA. The prevalence of HIV load suppression and related factors among patients on ART at Phedisong 4 Clinic, Pretoria, South Africa. Open Public Health J. 2018;11(1):135–46. [Google Scholar]
- 90. Moyo F, Evans D, Long L, Ebrahim O, Chasela C, Sanne I, et al. Treatment outcomes of HIV‐positive patients on first‐line antiretroviral therapy in private versus public HIV clinics in Johannesburg, South Africa. Clin Epidemiol. 2016;8:37–47. 10.2147/CLEP.S93014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Muraguri N, Tun W, Okal J, Broz D, Raymond HF, Kellogg T, et al. HIV and STI prevalence and risk factors among male sex workers and other men who have sex with men in Nairobi, Kenya. J Acquir Immune Defic Syndr. 2015;68(1):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. de D Ndagijimana Ntwali J, Decroo T, Ribakare M, Kiromera A, Mugwaneza P, Nsanzimana S, et al. Viral load detection and management on first line ART in rural Rwanda. BMC Infect Dis. 2019;19(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nnambalirwa M, Govathson C, Evans D, McNamara L, Maskew M, Nyasulu P. Markers of poor adherence among adults with HIV attending Themba Lethu HIV Clinic, Helen Joseph Hospital, Johannesburg, South Africa. Trans R Soc Trop Med Hyg. 2016;110(12):696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Novitsky V, Bussmann H, Okui L, Logan A, Moyo S, van Widenfelt E, et al. Estimated age and gender profile of individuals missed by a home‐based HIV testing and counselling campaign in a Botswana community. J Int AIDS Soc. 2015;18(1):19918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Novitsky V, Gaolathe T, Mmalane M, Moyo S, Chakalisa U, Yankinda EK, et al. Lack of virological suppression among young HIV‐positive adults in Botswana. J Acquir Immune Defic Syndr. 2018;78(5):557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nsanzimana S, Semakula M, Ndahindwa V, Remera E, Sebuhoro D, Uwizihiwe JP, et al. Retention in care and virological failure among adult HIV+ patients on second‐line ART in Rwanda: a national representative study. BMC Infect Dis. 2019;19(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ramadhani HO, Ndembi N, Nowak RG, Ononaku U, Gwamna J, Orazulike I, et al. Individual and network factors associated with HIV care continuum outcomes among Nigerian MSM accessing health care services. J Acquir Immune Defic Syndr. 2018;79(1):e7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Reynolds Z, Gottert A, Luben E, Mamba B, Shabangu P, Dlamini N, et al. Who are the male partners of adolescent girls and young women in Swaziland? Analysis of survey data from community venues across 19 DREAMS districts. PLoS One. 2018;13(9):e0203208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rhead R, Skovdal M, Takaruza A, Maswera R, Nyamukapa C, Gregson S. The multidimensionality of masculine norms in east Zimbabwe: implications for HIV prevention, testing and treatment. AIDS. 2019;33(3):537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Riedel DJ, Stafford KA, Memiah P, Coker M, Baribwira C, Sebeza J, et al. Patient‐level outcomes and virologic suppression rates in HIV‐infected patients receiving antiretroviral therapy in Rwanda. Int J STD AIDS. 2018;29(9):861–72. [DOI] [PubMed] [Google Scholar]
- 101. Rohr JK, Xavier Gómez‐Olivé F, Rosenberg M, Manne‐Goehler J, Geldsetzer P, Wagner RG, et al. Performance of self‐reported HIV status in determining true HIV status among older adults in rural South Africa: a validation study. J Int AIDS Soc. 2017;. 20(1):21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shanaube K, Schaap A, Floyd S, Phiri M, Griffith S, Chaila J, et al. What works – reaching universal HIV testing: lessons from HPTN 071 (PopART) trial in Zambia. AIDS. 2017;31(11):1555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Umar E, Levy JA, Bailey RC, Donenberg G, Hershow RC, Mackesy‐Amiti ME. Virological non‐suppression and its correlates among adolescents and young people living with HIV in Southern Malawi. AIDS Behav. 2019;23(2):513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wafula R, Masyuko S, Ng L, Kim AA, Gichangi A, Mukui I, et al. Engagement in HIV care among Kenyan adults and adolescents: results from a national population‐based survey. J Acquir Immune Defic Syndr. 2014;. 66:S98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Brown LB, Getahun M, Ayieko J, Kwarisiima D, Owaraganise A, Atukunda M, et al. Factors predictive of successful retention in care among HIV‐infected men in a universal test‐and‐treat setting in Uganda and Kenya: a mixed methods analysis. PLoS One. 2019;14(1):e0210126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Keshinro B, Ayemoba O, Terfa K, Ake J, Crowell TA, Adamu Y, et al. Virological suppression and patterns of resistance amongst patients on antiretroviral therapy at 4 Nigerian military hospitals. Curr HIV Res. 2017;15(2):146–151. 10.2174/1570162X15666170517103704. [DOI] [PubMed] [Google Scholar]
- 107. Nakanyala T, Patel SV, Sawadogo S, Maher AD, Banda KM, Wolkon A, et al. How close to 90‐90‐90? Measuring undiagnosed HIV infection, ART use, and viral suppression in a community‐based sample from Namibia's highest prevalence region. 2016.
- 108. Hansoti B, Mwinnyaa G, Hahn E, Rao A, Black J, Chen V, et al. Targeting the HIV epidemic in South Africa: the need for testing and linkage to care in emergency departments. EClinicalMedicine. 2019;15:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Petersen M, Balzer L, Kwarsiima D, Sang N, Chamie G, Ayieko J, et al. Association of implementation of a universal testing and treatment intervention with HIV diagnosis, receipt of antiretroviral therapy, and viral suppression in East Africa. JAMA. 2017;317:2196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ng'ang'a A, Ssempijja V, Gachuki T, Njoroge I, Kimanga DO, Maina WK, et al. The status of HIV testing and counseling in Kenya: results from a nationally representative population‐based survey. J Acquir Immune Defic Syndr. 2014;. 66:S27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Colombe S, Machemba R, Mtenga B, Lutonja P, Safari W, Beard J, et al. Cascade of care for HIV‐seroconverters in rural Tanzania: a longitudinal study. AIDS Care. 2020;32(5):666–71. [DOI] [PubMed] [Google Scholar]
- 112. Huerga H, Van Cutsem G, Ben Farhat J, Puren A, Bouhenia M, Wiesner L, et al. Progress towards the UNAIDS 90‐90‐90 goals by age and gender in a rural area of KwaZulu‐Natal, South Africa: a household‐based community cross‐sectional survey. BMC Public Health. 2018;18(1):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Maman D, Zeh C, Mukui I, Kirubi B, Masson S, Opolo V, et al. Cascade of HIV care and population viral suppression in a high‐burden region of Kenya. AIDS. 2015;29(12):1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Adams AK, Zamberia AM. “I will take ARVs once my body deteriorates”: an analysis of Swazi men's perceptions and acceptability of Test and Start. Afr J AIDS Res. 2017;16(4):295–303. [DOI] [PubMed] [Google Scholar]
- 115. Camlin CS, Ssemmondo E, Chamie G, El Ayadi AM, Kwarisiima D, Sang N, et al. Men “missing” from population‐based HIV testing: insights from qualitative research. AIDS Care. 2016;28(sup3):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chikovore J, Gillespie N, McGrath N, Orne‐Gliemann J, Zuma T, on behalf of the ANRS 12249 TasP Study Group. Men, masculinity, and engagement with treatment as prevention in KwaZulu‐Natal, South Africa. AIDS Care. 2016;28(sup3):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Conserve DF, Issango J, Kilale AM, Njau B, Nhigula P, Memiah P, et al. Developing national strategies for reaching men with HIV testing services in Tanzania: results from the male catch‐up plan. BMC Health Serv Res. 2019;19(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. DiCarlo AL, Mantell JE, Remien RH, Zerbe A, Morris D, Pitt B, et al. ‘Men usually say that HIV testing is for women’: gender dynamics and perceptions of HIV testing in Lesotho. Cult Health Sex. 2014;16(8):867–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fleming PJ, Colvin C, Peacock D, Dworkin SL. What role can gender‐transformative programming for men play in increasing men's HIV testing and engagement in HIV care and treatment in South Africa? Cult Health Sex. 2016;18(11):1251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Graham SM, Micheni M, Secor A, van der Elst EM, Kombo B, Operario D, et al. HIV care engagement and ART adherence among Kenyan gay, bisexual, and other men who have sex with men: a multi‐level model informed by qualitative research. AIDS Care. 2018;30(sup5):S97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hill LM, Gottert A, MacPhail C, Rebombo D, Twine R, Kahn K, et al. Understanding men's networks and perceptions of leadership to promote HIV testing and treatment in Agincourt, South Africa. Glob Public Health. 2018;13(9):1296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jennings L, Conserve DF, Merrill J, Kajula L, Iwelunmor J, Linnemayr S, et al. Perceived cost advantages and disadvantages of purchasing HIV self‐testing kits among urban Tanzanian men: an inductive content analysis. J AIDS Clin Res. 2017;8(8):725. 10.4172/2155-6113.1000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lavender T, Wakasiaka S, Chimwaza A, Wood R, Omoni G, Mukhwana R, et al. A qualitative study of partner engagement in HIV testing in Malawi and Kenya. Cult Health Sex. 2019;21(10):1131–45. [DOI] [PubMed] [Google Scholar]
- 124. Mak J, Mayhew SH, von Maercker A, Integra Research Team , Colombini M. Men's use of sexual health and HIV services in Swaziland: a mixed methods study. Sex Health. 2016;. 13(3):265–74. [DOI] [PubMed] [Google Scholar]
- 125. Martínez Pérez G, Cox V, Ellman T, Moore A, Patten G, Shroufi A, et al. ‘I know that I do have HIV but nobody saw me’: oral HIV self‐testing in an informal settlement in South Africa. PLoS One. 2016;11(4):e0152653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mburu G, Ram M, Siu G, Bitira D, Skovdal M, Holland P. Intersectionality of HIV stigma and masculinity in eastern Uganda: implications for involving men in HIV programmes. BMC Public Health. 2014;14(1):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Micheni M, Kombo BK, Secor A, Simoni JM, Operario D, van der Elst EM, et al. Health provider views on improving antiretroviral therapy adherence among men who have sex with men in Coastal Kenya. AIDS Patient Care STDs. 2017;31(3):113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mooney AC, Gottert A, Khoza N, Rebombo D, Hove J, Suárez AJ, et al. Men's perceptions of treatment as prevention in South Africa: implications for engagement in HIV care and treatment. AIDS Educ Prev. 2017;29(3):274–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ogunbajo A, Kershaw T, Kushwaha S, Boakye F, Wallace‐Atiapah N‐D, Nelson LE. Barriers, motivators, and facilitators to engagement in HIV care among HIV‐infected Ghanaian men who have sex with men (MSM). AIDS Behav. 2018;22(3):829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Orr N, Hajiyiannis H, Myers L, Makhubele MB, Matekane T, Delate R, et al. Development of a national campaign addressing South African men's fears about HIV counseling and testing and antiretroviral treatment. J Acquir Immune Defic Syndr. 2017;. 74:S69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rankin‐Williams AC, Geoffroy EM, Schell ES, Mguntha AM. How can male rates of HIV testing be increased? Recommendations from a mixed methods study in southern Malawi. Int Health. 2017;9(6):367–73. [DOI] [PubMed] [Google Scholar]
- 132. Russell S. Men's refashioning of masculine identities in Uganda and their self‐management of HIV treatment. Qual Health Res. 2019;29(8):1199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sandfort TGM, Knox J, Collier KL, Lane T, Reddy V. HIV testing practices of South African township MSM in the era of expanded access to ART. AIDS Behav. 2015;19(3):561–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Schatz E, Knight L. “I was referred from the other side”: gender and HIV testing among older South Africans living with HIV. PLoS One. 2018;13(4):e0196158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sileo KM, Kizito W, Wanyenze RK, Chemusto H, Musoke W, Mukasa B, et al. A qualitative study on alcohol consumption and HIV treatment adherence among men living with HIV in Ugandan fishing communities. AIDS Care. 2019;31(1):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sileo KM, Reed E, Kizito W, Wagman JA, Stockman JK, Wanyenze RK, et al. Masculinity and engagement in HIV care among male fisherfolk on HIV treatment in Uganda. Cult Health Sex. 2019;21(7):774–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Tibbels NJ, Hendrickson ZM, Naugle DA, Dosso A, Van Lith L, Mallalieu EC, et al. Men's perceptions of HIV care engagement at the facility‐ and provider‐levels: experiences in Cote d'Ivoire. PLoS One. 2019;14(3):e0211385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Tsang EY, Qiao S, Wilkinson JS, Fung AL, Lipeleke F, Li X. Multilayered stigma and vulnerabilities for HIV infection and transmission: a qualitative study on male sex workers in Zimbabwe. Am J Mens Health. 2019;13(1).353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Van Heerden A, Msweli S, Van Rooyen H. “Men don't want things to be seen or known about them”: a mixed‐methods study to locate men in a home based counselling and testing programme in KwaZulu‐Natal, South Africa. Afr J AIDS Res. 2015;14(4):353–9. [Google Scholar]
- 140. Wamoyi J, Renju J, Moshabela M, McLean E, Nyato D, Mbata D, et al. Understanding the relationship between couple dynamics and engagement with HIV care services: insights from a qualitative study in Eastern and Southern Africa. Sex Transm Infect. 2017;93(3):e052976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zissette S, Watt MH, Prose NS, Mntambo N, Moshabela M. “If you don't take a stand for your life, who will help you?”: men's engagement in HIV care in Kwazulu‐Natal, South Africa. Psychol Men Masculinity. 2016;17(3):265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Barak T, Neo DT, Tapela N, Mophuthegi P, Zash R, Kalenga K, et al. HIV‐associated morbidity and mortality in a setting of high ART coverage: prospective surveillance results from a district hospital in Botswana. J Int AIDS Soc. 2019;. 22(12):e25428. 10.1002/jia2.25428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Barnabas RV. Community‐based antiretroviral therapy versus standard clinic‐based services for HIV in South Africa and Uganda (DO ART): a randomised trial. Randomized Controlled Trial. 2020;. 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Bhattacharjee P, Isac S, Musyoki H, Emmanuel F, Olango K, Kuria S, et al. HIV prevalence, testing and treatment among men who have sex with men through engagement in virtual sexual networks in Kenya: a cross‐sectional bio‐behavioural study. J Int AIDS Soc. 2020;23(S2):e25516. 10.1002/jia2.25516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Boyd MA, Shah M, Barradas DT, Herce M, Mulenga LB, Lumpa M, et al. Increase in antiretroviral therapy enrollment among persons with HIV infection during the Lusaka HIV Treatment Surge — Lusaka Province, Zambia, January 2018–June 2019. MMWR Morb Mortal Wkly Rep. 2020;69(31):1039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Burgos‐Soto J, Ben Farhat J, Alley I, Ojuka P, Mulogo E, Kise‐Sete T, et al. HIV epidemic and cascade of care in 12 east African rural fishing communities: results from a population‐based survey in Uganda. BMC Public Health. 2020;20(1):970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chikandiwa A, Pisa Pedro T, Tamalet C, Muller Etienne E, Michelow P, Chersich Matthew F, et al. Prevalent, persistent anal HPV infection and squamous intraepithelial lesions: findings from a cohort of men living with HIV in South Africa. PLoS One. 2019;14(12):e0225571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chikandiwa A, Faust H, Chersich MF, Mayaud P, Dillner J, Delany‐Moretlwe S. Human papillomavirus seroprevalence and seroconversion among men living with HIV: cohort study in South Africa. J Acquir Immune Defic Syndr. 2020;. 84(2):141–8. [DOI] [PubMed] [Google Scholar]
- 149. Conan N, Coulborn RM, Simons E, Mapfumo A, Apollo T, Garone DB, et al. Successes and gaps in the HIV cascade of care of a high HIV prevalence setting in Zimbabwe: a population‐based survey. J Int AIDS Soc. 2020;23(9):e25613. 10.1002/jia2.25613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Crowell TA, Danboise B, Parikh A, Esber A, Dear N, Coakley P, et al. Pretreatment and acquired antiretroviral drug resistance among persons living with HIV in four African countries. Clin Infect Dis. 2021.73(7):e2311–e2322. 10.1093/cid/ciaa1161. PMID: 32785695; PMCID: PMC8492117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. De Anda S, Njoroge A, Njuguna I, Dunbar MD, Abuna F, Macharia P, et al. Predictors of first‐time and repeat HIV testing among HIV‐positive individuals in Kenya. J Acquir Immune Defic Syndr. 2020;85(4):399–407. [DOI] [PubMed] [Google Scholar]
- 152. Desta AA, Woldearegay TW, Futwi N, Gebrehiwot GT, Gebru GG, Berhe AA, et al. HIV virological non‐suppression and factors associated with non‐suppression among adolescents and adults on antiretroviral therapy in northern Ethiopia: a retrospective study. BMC Infect Dis. 2020;20(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Dorward J, Sookrajh Y, Gate K, Khubone T, Mtshaka N, Mlisana K, et al. HIV treatment outcomes among people with initiation CD4 counts >500 cells/μL after implementation of Treat All in South African public clinics: a retrospective cohort study. J Int AIDS Soc. 2020;23(4):e25479. 10.1002/jia2.25479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Fearon E, Tenza S, Mokoena C, Moodley K, Smith AD, Bourne A, et al. HIV testing, care and viral suppression among men who have sex with men and transgender individuals in Johannesburg, South Africa. PLoS One. 2020;15(6):e0234384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Fentie Wendie T, Workneh BD. Prevalence and predictors of virological failure among adults living with HIV in South Wollo Zone, Northeast Ethiopia: a retrospective cohort study. HIV AIDS. 2020;12:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Foley JD, Sheinfil A, Woolf‐King SE, Fatch R, Emenyonu NI, Muyindike WR, et al. Assessing the interaction between depressive symptoms and alcohol use prior to antiretroviral therapy on viral suppression among people living with HIV in rural Uganda. AIDS Care. 2020;32(10):1251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Fonner VA, Mbwambo JK, Kennedy CE, Sweat MD. The gendered experience of HIV testing: factors associated with prior testing differ among men and women in rural Tanzania. Int J STD AIDS. 2019;30(9):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gumede SB, Fischer A, Venter WDF, Lalla‐Edward ST. Descriptive analysis of World Health Organization‐recommended second‐line antiretroviral treatment: a retrospective cohort data analysis. S Afr Med J. 2019;109(12):919–26. [DOI] [PubMed] [Google Scholar]
- 159. Gumede SB, Venter WDF, Lalla‐Edward ST. Understanding adherence in virally suppressed and unsuppressed human immunodeficiency virus‐positive urban patients on second‐line antiretroviral treatment. South Afr J HIV Med. 2020;21(1).1107. 10.4102/sajhivmed.v21i1.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gupta‐Wright A, Fielding K, van Oosterhout JJ, Alufandika M, Grint DJ, Chimbayo E, et al. Virological failure, HIV‐1 drug resistance, and early mortality in adults admitted to hospital in Malawi: an observational cohort study. Lancet HIV. 2020;7(9):e620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Haachambwa L, Kandiwo N, Zulu PM, Rutagwera D, Geng E, Holmes CB, et al. Care continuum and postdischarge outcomes among HIV‐infected adults admitted to the hospital in Zambia. Open Forum Infect Dis. 2019;6(10):ofz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Halle MP, Essomba N, Djantio H, Tsele G, Fouda H, Luma NH, et al. Clinical characteristics and outcome of HIV infected patients with chronic kidney disease in sub Saharan Africa: an example from Cameroon. BMC Nephrol. 2019;20:253. 10.1186/s12882-019-1446-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hayes RJ, Donnell D, Floyd S, Mandla N, Bwalya J, Sabapathy K, et al. Effect of universal testing and treatment on HIV incidence — HPTN 071 (PopART). N Engl J Med. 2019;381(3):207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hermans LE, Carmona S, Nijhuis M, Tempelman HA, Richman DD, Moorhouse M, et al. Virological suppression and clinical management in response to viremia in South African HIV treatment program: a multicenter cohort study. PLoS Med. 2020;17(2):e1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hirasen K, Fox MP, Hendrickson CJ, Sineke T, Onoya D. HIV treatment outcomes among patients initiated on antiretroviral therapy pre and post‐universal test and treat guidelines in South Africa. Ther Clin Risk Manag. 2020;16:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kagaayi J. Impact of combination HIV interventions on HIV incidence in hyperendemic fishing communities in Uganda: a prospective cohort study. Lancet HIV. 2019;. 6:e680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Kharsany ABM, Cawood C, Lewis L, Yende‐Zuma N, Khanyile D, Puren A, et al. Trends in HIV prevention, treatment, and incidence in a hyperendemic area of KwaZulu‐Natal, South Africa. JAMA Netw Open. 2019;2(11):e1914378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. MacKellar D, Steiner C, Rwabiyago OE, Cham HJ, Pals S, Maruyama H, et al. Threefold increases in population HIV viral load suppression among men and young adults — Bukoba Municipal Council, Tanzania, 2014–2017. MMWR Morb Mortal Wkly Rep. 2019;68(30):658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Maina EK, Mureithi H, Adan AA, Muriuki J, Lwembe RM, Bukusi EA. Incidences and factors associated with viral suppression or rebound among HIV patients on combination antiretroviral therapy from three counties in Kenya. Int J Infect Dis. 2020;97:151–8. [DOI] [PubMed] [Google Scholar]
- 170. Manne‐Goehler J, Rohr J, Montana L, Siedner M, Harling G, Xavier Gómez‐Olivé F, et al. ART denial: results of a home‐based study to validate self‐reported antiretroviral use in rural South Africa. AIDS Behav. 2019;23(8):2072–2078. 10.1007/s10461-018-2351-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Marinda E, Simbayi L, Zuma K, Zungu N, Moyo S, Kondlo L, et al. Towards achieving the 90–90–90 HIV targets: results from the South African 2017 National HIV Survey. BMC Public Health. 2020;20(1):1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Merrill KG, Campbell JC, Decker MR, McGready J, Burke VM, Mwansa JK, et al. Past‐year violence victimization is associated with viral load failure among HIV‐positive adolescents and young adults. AIDS Behav. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Meshesha HM, Nigussie ZM, Asrat A, Mulatu K. Determinants of virological failure. BMJ Open. 2020;. 10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Mokhele I, Mashamaite S, Majuba P, Xulu T, Long L, Onoya D. Effective public–private partnerships for sustainable antiretroviral therapy: outcomes of the Right to Care health services GP down‐referral program. BMC Public Health. 2019;19(1):1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mshweshwe‐Pakela N, Hansoti B, Mabuto T, Kerrigan D, Kubeka G, Hahn E, et al. Feasibility of implementing same‐day antiretroviral therapy initiation during routine care in Ekurhuleni District, South Africa: retention and viral load suppression. South Afr J HIV Med. 2020;21(1).1085. 10.4102/sajhivmed.v21i1.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Nega J, Taye S, Million Y, Rodrigo C, Eshetie S. Antiretroviral treatment failure and associated factors among HIV patients on first‐line antiretroviral treatment in Sekota, northeast Ethiopia. AIDS Res Ther. 2020;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Negash H, Welay M, Legese H, Adhanom G, Mardu F, Tesfay K, et al. Increased virological failure and determinants among HIV patients on highly active retroviral therapy in Adigrat General Hospital, Northern Ethiopia, 2019: hospital‐based cross‐sectional study. Infect Drug Resist. 2020;13:1863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Nuwagaba‐Biribonwoha H, Wu Y, Gachuhi AB, McNairy ML, Madau V, Lamb M, et al. Low rates of prior HIV testing among HIV‐positive adults accessing outpatient services in Eswatini. AIDS Res Ther. 2019;16(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Omooja J, Nannyonjo M, Sanyu G, Nabirye SE, Nassolo F, Lunkuse S, et al. Rates of HIV‐1 virological suppression and patterns of acquired drug resistance among fisherfolk on first‐line antiretroviral therapy in Uganda. J Antimicrob Chemother. 2019;74(10):3021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ondoa P, Kim AA, Boender TS, Zhang G, Kroeze S, Wiener J, et al. Access to HIV viral load testing and antiretroviral therapy switch practices: a multicountry prospective cohort study in sub‐Saharan Africa. AIDS Res Hum Retroviruses. 2020;36(11):918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Pulerwitz J, Gottert A, Kahn K, Haberland N, Julien A, Selin A, et al. Gender norms and HIV testing/treatment uptake: evidence from a large population‐based sample in South Africa. AIDS Behav. 2019;23(S2):162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ross J, Ribakare M, Remera E, Murenzi G, Munyaneza A, Hoover DR, et al. High levels of viral load monitoring and viral suppression under Treat All in Rwanda – a cross‐sectional study. J Int AIDS Soc. 2020;23(6):e25543. 10.1002/jia2.25543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ssemwanga D, Asio J, Watera C, Nannyonjo M, Nassolo F, Lunkuse S, et al. Prevalence of viral load suppression, predictors of virological failure and patterns of HIV drug resistance after 12 and 48 months on first‐line antiretroviral therapy: a national cross‐sectional survey in Uganda. J Antimicrob Chemother. 2020;75(5):1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Steiner C, MacKellar D, Cham HJ, Rwabiyago OE, Maruyama H, Msumi O, et al. Community‐wide HIV testing, linkage case management, and defaulter tracing in Bukoba, Tanzania: pre‐intervention and post‐intervention, population‐based survey evaluation. Lancet HIV. 2020;7(10):e699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Thin K, Frederix K, McCracken S, Letsie M, Low A, Patel H, et al. Progress toward HIV epidemic control in Lesotho. AIDS. 2019;33(15):2393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Twahirwa Rwema JO, Lyons CE, Herbst S, Liestman B, Nyombayire J, Ketende S, et al. HIV infection and engagement in HIV care cascade among men who have sex with men and transgender women in Kigali, Rwanda: a cross‐sectional study. J Int AIDS Soc. 2020;23(S6):e25604. 10.1002/jia2.25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Villa G, Abdullahi A, Owusu D, Smith C, Azumah M, Sayeed L, et al. Determining virological suppression and resuppression by point‐of‐care viral load testing in a HIV care setting in sub‐Saharan Africa. EClinicalMedicine. 2020;18:100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Wirth KE, Gaolathe T, Pretorius Holme M, Mmalane M, Kadima E, Chakalisa U, et al. Population uptake of HIV testing, treatment, viral suppression, and male circumcision following a community‐based intervention in Botswana (Ya Tsie/BCPP): a cluster‐randomised trial. Lancet HIV. 2020;7(6):e422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Young PW, Zielinski‐Gutierrez E, Wamicwe J, Mukui I, Kim AA, Waruru A, et al. Use of viral load to improve survey estimates of known HIV‐positive status and antiretroviral treatment coverage. AIDS. 2020;34(4):631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Krakowiak D, Makabong'o P, Goyette M, Kinuthia J, Osoti AO, Asila V, et al. Reaching hard‐to‐reach men through home‐based couple HIV testing among pregnant women and their male partners in western Kenya: a qualitative study. BMC Public Health. 2020;20(1):724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Mantell JE, Masvawure TB, Mapingure M, Apollo T, Gwanzura C, Block L, et al. Engaging men in HIV programmes: a qualitative study of male engagement in community‐based antiretroviral refill groups in Zimbabwe. J Int AIDS Soc. 2019;22(10):e25403. 10.1002/jia2.25403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Naugle DA, Tibbels NJ, Hendrickson ZM, Dosso A, Van Lith L, Mallalieu EC, et al. Bringing fear into focus: the intersections of HIV and masculine gender norms in Côte d'Ivoire. PLoS One. 2019;14(10):e0223414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ndyabakira A, Getahun M, Byamukama A, Emperador D, Kabageni S, Marson K, et al. Leveraging incentives to increase HIV testing uptake among men: qualitative insights from rural Uganda. BMC Public Health. 2019;19(1):1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Okal J, Lango D, Matheka J, Obare F, Ngunu‐Gituathi C, Mugambi M, et al. “It is always better for a man to know his HIV status” – a qualitative study exploring the context, barriers and facilitators of HIV testing among men in Nairobi, Kenya. PLoS One. 2020;15(4):e0231645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Osingada CP, Siu G, Amollo M, Muwanguzi P, Sewankambo N, Kiwanuka N. Acceptability of HIV testing for men attending televised football venues in Uganda. BMC Public Health. 2019;19(1):1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Rosen JG, Nakyanjo N, Isabirye D, Wawer MJ, Nalugoda F, Reynolds SJ, et al. Antiretroviral treatment sharing among highly mobile Ugandan fisherfolk living with HIV: a qualitative study. AIDS Care. 2020;32(7):912–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Skovdal M, Ssekubugu R, Nyamukapa C, Seeley J, Renju J, Wamoyi J, et al. The rebellious man: next‐of‐kin accounts of the death of a male relative on antiretroviral therapy in sub‐Saharan Africa. Glob Public Health. 2019;14(9):1252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Adeagbo O, Herbst C, Blandford A, McKendry R, Estcourt C, Seeley J, et al. Exploring people's candidacy for mobile health–supported HIV testing and care services in rural KwaZulu‐Natal, South Africa: qualitative study. J Med Internet Res. 2019;21(11):e15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Daniels J, Struthers H, Maleke K, Lane T, McIntyre J, Coates T. ‘My tablets are on top of the fridge’: the roles of relationship desire and medical mistrust in ART adherence for HIV‐positive MSM and transgender women living in rural South Africa. AIDS Behav. 2019;23(10):2849–58. [DOI] [PubMed] [Google Scholar]
- 200. Hendrickson ZM, Naugle DA, Tibbels N, Dosso A, Van Lith LM, Mallalieu EC, et al. “You take medications, you live normally”: the role of antiretroviral therapy in mitigating men's perceived threats of HIV in Côte d'Ivoire. AIDS Behav. 2019;23(9):2600–9. [DOI] [PubMed] [Google Scholar]
- 201. Bigna JJ, Nansseu JR. Men who have sex with men: a key population in Africa. Lancet HIV. 2019;6(11):e728–9. [DOI] [PubMed] [Google Scholar]
- 202. Rebe K, Hoosen N, McIntyre JA. Strategies to improve access for MSM in low‐income and middle‐income countries. Curr Opin HIV AIDS. 2019;14(5):387–92. [DOI] [PubMed] [Google Scholar]
- 203. Maleke K, Daniels J, Lane T, Struthers H, McIntyre J, Coates T. How social stigma sustains the HIV treatment gap for MSM in Mpumalanga, South Africa. Glob Health Promot. 2019;26(4):6–13. [DOI] [PubMed] [Google Scholar]
- 204. Shangani S, Naanyu V, Operario D, Genberg B. Stigma and healthcare‐seeking practices of men who have sex with men in western Kenya: a mixed‐methods approach for scale validation. AIDS Patient Care STDs. 2018;32(11):477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Expanding effective HIV prevention, care, and treatment for men who have sex with men in South Africa. PEPFAR, ICAP Columbia University; 2016. [Google Scholar]
- 206. Camlin CS, Charlebois ED. Mobility and its effects on HIV acquisition and treatment engagement: recent theoretical and empirical advances. Curr HIV/AIDS Rep. 2019;16(4):314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Vearey J. Moving forward: why responding to migration, mobility and HIV in South(ern) Africa is a public health priority. J Int AIDS Soc. 2018;21:e25137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Tsai AC, Hatcher AM, Bukusi EA, Weke E, Lemus Hufstedler L, Dworkin SL, et al. A livelihood intervention to reduce the stigma of HIV in rural Kenya: longitudinal qualitative study. AIDS Behav. 2017;21(1):248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Tsai AC, Bangsberg DR, Weiser SD. Harnessing poverty alleviation to reduce the stigma of HIV in sub‐Saharan Africa. PLoS Med. 2013;10(11):e1001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer Pub. Co; 1984. [Google Scholar]
- 211. Folkman S, Lazarus RS. An analysis of coping in a middle‐aged community sample. J Health Soc Behav. 1980;21(3):219–39. [PubMed] [Google Scholar]
- 212. Baker JP, Berenbaum H. Emotional approach and problem‐focused coping: a comparison of potentially adaptive strategies. Cogn Emot. 2007;21(1):95–118. [Google Scholar]
- 213. Parker JDA, Endler NS. Coping with coping assessment: a critical review. Eur J Personal. 1992;6(5):321–44. [Google Scholar]
- 214. Earnshaw VA, Bogart LM, Courtney I, Zanoni H, Bangsberg DR, Orrell C, et al. Exploring treatment needs and expectations for people living with HIV in South Africa: a qualitative study. AIDS Behav. 2018;22(8):2543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Mody A, Eshun‐Wilson I, Sikombe K, Schwartz SR, Beres LK, Simbeza S, et al. Longitudinal engagement trajectories and risk of death among new ART starters in Zambia: a group‐based multi‐trajectory analysis. PLoS Med. 2019;16(10):e1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Bell J, Sharma S, Malone S, Levy M, Reast J, Ciecieląg J, et al. Targeting interventions for HIV testing and treatment uptake: an attitudinal and behavioural segmentation of men aged 20–34 in KwaZulu‐Natal and Mpumalanga, South Africa. PLoS One. 2021;16(3):e0247483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Staveteig S, Croft TN, Kampa KT, Head SK. Reaching the ‘first 90’: gaps in coverage of HIV testing among people living with HIV in 16 African countries. PLoS One. 2017;12(10):e0186316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files. No primary data were obtained for this study.


