Abstract
Objective
We sought to assess the efficacy of a hydrolyzed collagen supplement (Lapi Gelatine, Empoli, Italy) for improving skin moisturization and elasticity as well as the appearance of wrinkles.
Methods
A monocentric, randomized, controlled study was performed; the study included 52 volunteers who were divided into two groups. Twenty-six subjects took the collagen supplement and the other 26 participants received maltodextrin for a period of 56 days. Skin moisturization, skin elasticity, and wrinkle depth were instrumentally evaluated. Clinical parameters, including skin softness, skin firmness, skin smoothness, and the visibility of wrinkles were subjectively evaluated by a dermatologist. Finally, the gastrointestinal tolerability was evaluated during the period of treatment.
Results
Improvements in skin moisturization, skin elasticity and wrinkle depth were measured in the participants taking the hydrolyzed collagen after 28 days. In all enrolled participants, the results of the analysis of variance showed a statistically significant effect of the interaction between product and time. The evolution over time of the investigated parameters was significantly different between the participants taking the hydrolyzed collagen and the participants taking the maltodextrin. Furthermore, it was well tolerated and no adverse effects were recorded.
Keywords: Wrinkles, skin elasticity, skin moisturization, hydrolyzed collagen
Collagen synthesis changes as we age: younger skin comprises 80-percent Type I collagen and about 15-percent Type III collagen. With age, collagen fibers become thicker and shorter; consequently, Type I collagen is lost. In addition, with advancing age there is also a loss of hyaluronic acid. These factors contribute to the appearance of skin aging, with a reduction in its elasticity and tone, giving way to the increased appearance of wrinkles, sagging eyelids, and bags under the eyes.1,2
Recently, researchers have devoted some attention to protein hydrolyzates as dietary supplements with the potential to improve signs of skin aging. Collagen peptides are the product of the enzymolysis of collagen or gelatin and are used as important active components due to their various bioactivity, high bioavailability, and good biocompatibility.3–5 Hydrolyzed collagen consists of small peptides with a low molecular weight (0.3–8 kDa). Due to its low molecular weight, hydrolyzed collagen is easily digested, absorbed, and distributed in the human body.6 The quality of the final hydrolyzed collagen depends on its average molecular size, which can vary according to the method used to extract it.6
Once administered orally, the collagen peptides and free amino acids are then distributed into the dermis, where they can remain for up to 14 days.7 In the dermis, hydrolyzed collagen exerts a dual mechanism of action: 1) free amino acids contribute to the formation of collagen and elastin fibers; and 2) collagen oligopetides stimulate the production of new collagen, elastin and hyaluronic acid.6
The United States Food and Drug Administration (FDA) has classified gelatin, from which collagen peptides are prepared, as a safe substance. Furthermore, based on the results of international research,6,8 both the World Health Organization (WHO) and the European Commission for Health and Consumer Protection have stated that hydrolyzed collagen is safe. Minor side effects, such as nausea, flatulence, or dyspepsia can occur in some people ingesting collagen peptides.
Many studies have already been performed to evaluate the effect of hydrolyzed collagen in improving the appearance and properties of the skin,9,10 but more clinical studies are needed to better investigate the benefits of hydrolyzed collagen on the skin. Here, we report results from a monocentric, randomized, controlled study evaluating the efficacy of a hydrolyzed collagen supplement (Lapi Gelatine, Empoli, Italy) for the improvement of hydration, elasticity, and the appearance of wrinkles.
METHODS
A randomized controlled study to evaluate the efficacy of a hydrolyzed collagen supplement was conducted in 2020 at Dermo-Cosmetic and Medical R&D Center of Bio Basic Europe in Milan, Italy. All subjects provided a written informed consent prior to enrollment into the study. The trial was run in accordance with the Declaration of Helsinki and with principles of Good Clinical Practices; it was conducted with a double-blind procedure: the volunteers, the dermatologist, and the operators involved in the conduction of the study were unaware of the type of products (hydrolyzed collagen or maltodextrin), which were identical in packaging, labels, appearance, and method of use.
Healthy female subjects (N=52), aged 40 to 60 years, were selected for the investigation and divided into two groups: 26 subjects took the hydrolyzed collagen supplement and 26 took the control treatment, maltodextrin, for a period of 56 days. Following a baseline clinical visit, patients with low skin elasticity and with face wrinkles were included into the study. Volunteers were excluded from the study in instances of known allergies to collagen or maltodextrin. Pregnant and/or breastfeeding volunteers were also excluded. Participants were required to not change their usual daily routine.
Administration protocol. For the first month of the study period, the intake of either the collagen or the maltodextrin (in granular format) occurred once a day in the morning, during or immediately after breakfast, respecting the following proportion: 1g per 10kg of body weight, dissolved in water; for the second month, the dose to be taken was 5g in total, dissolved in water.
Instrumental evaluation of skin parameters. At the beginning and throughout the entire study period at selected timepoints (after 28 days and 56 days of treatment), instrumental evaluations of skin moisturization, skin elasticity, and wrinkle depth, as well as clinical evaluation of gastrointestinal tolerability, skin softness, skin firmness, skin smoothness, and visibility of wrinkles were performed.
Skin moisturization. Skin moisturization was measured using CORNEOMETER® CM 825 (Courage and Khazaka, Koln, Germany). Corneometer measures the electric capacity of the skin surface that is related to skin moisture. Electric capacity and the conductance of biological tissue change according to the water content, and they increase as the water content increases. This instrument translates the electrical parameters in moisturization units (scale: 0÷130).
Skin elasticity. Skin elasticity was measured using CUTOMETER® MPA 580 (Courage and Khazaka, Koln, Germany). The Cutometer measures elasticity of the upper skin layer using negative pressure, which deforms the skin mechanically. The measuring principle is based on the suction method. Negative pressure is created in the device and the skin is drawn into the aperture of the probe, and after a defined time, the skin is released again. Inside the probe, the penetration depth is determined by a non-contact optical measuring system. This optical measuring system consists of a light source and a light receptor, as well as two prisms facing each other, which project the light from transmitter to receptor. The light intensity varies due to the penetration depth of the skin. The resistance of the skin to the negative pressure (firmness) and its ability to return into its original position (elasticity) are displayed as curves (penetration depth in mm/time) in real time during the measurement. As showed in Figure 1, in the first part of the suction phase (red arrow), skin enters the probe immediately and straight (green arrow). This is the immediate elastic deformation and in the literature is described as Ue. In the second part of the suction phase, skin “creeps” into the probe (purple arrow). This part represents the viscoelastic suction part Uv. The more elastic a material, the smaller Uv. The maximum penetration after the suction time can be seen with black arrow (Uf). The second part is the relaxation phase (light green arrow). With a viscoelastic material, such as skin, the complete relaxation (Ua, blue arrow) can again be divided into two parts: the immediate elastic return Ur (orange arrow) and the flat, visco-elastic part Ua–Ur. Uf–Ua shows the overall ability of the skin of returning into its original shape.
FIGURE 1.
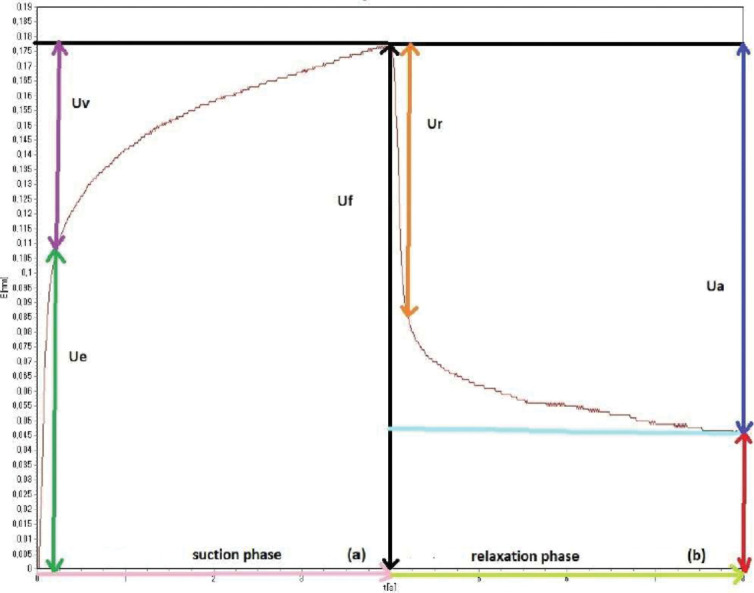
The measuring mechanism used to obtain information on the elastic and mechanical properties of the skin surface in participants
Elasticity=R2=Ua/Uf = portion between the max amplitude and the ability of returning to the original position (blue distance/black distance - gross elasticity). The closer the value is to 1 (100 %) the more elastic the curve.
Wrinkle depth. Wrinkle depth measurements were taken by using Antera 3D® (Miravex Limited, Dublin, Ireland). Images of areas of the face of study participants were taken with Antera 3D® and analysis were performed using the software Antera 3D®. The Antera 3D software measures a set of parameters related to lines expression, wrinkles, and folds (characteristics of the skin which are deep-set in comparison to the normal skin surface). Depth measures are the average depth of the selected wrinkle and are expressed in millimeters.
Clinical evaluation. Clinical evaluations were performed through visual assessment by a dermatologist and the results were recorded according to a specific scoring system for each clinic parameter. Parameters used to evaluate gastrointestinal tolerability, skin softness, skin firmness, skin smoothness, and visibility of wrinkles and their scoring system are reported in Table 1.
TABLE 1.
Clinical evaluation parameters
| Gastrointestinal adverse effects | Score |
| Absent | 0 |
| Very slight | 1 |
| Slight | 2 |
| Moderate | 3 |
| Evident | 4 |
| Skin softness/firmness/smoothness | Score |
| Insufficient | 1 |
| Sufficient | 2 |
| Fairly good | 3 |
| Good | 4 |
| Very good | 5 |
| Visibility of wrinkles | Score |
| Absent | 1 |
| Slight | 2 |
| Moderate | 3 |
| Evident | 4 |
| Very evident | 5 |
Statistical evaluation. The sample data of the quantitative endpoints were described using the usual measures of position and dispersion: mean and standard deviation. The normality of the quantitative endpoint variables was verified with the Shapiro–Wilk test. The homogeneity of the variances of the endpoint variables in the comparison groups was assessed with the Levene test. In addition, the independence of errors was verified. For each quantitative endpoint a mixed model of analysis of variance with a parametric approach was then applied with a factor component with two categories (product / placebo) and a repeated component (time), in order to compare the products under study / placebo and its effect over time. In the presence of a violation of the sphericity assumption for the repeated component of the model, the correction according to Greenhouse-Geisser was applied. In the presence of a significant interaction effect between the variables product and time, the results of the two main effects (product and time) must not be taken into account. The established significance level was α=0.05.
The sample data of the qualitative endpoints were described using the usual measures of position and dispersion: median and interquartile range. For each qualitative endpoint variable, Wilcoxon's non-parametric rank-based sign test was applied to pairwise data. In case of insufficient or doubtful confirmation of the assumption regarding the symmetry of the distribution of the differences between the pairs of evaluations, the test of the signs was applied to verify the result obtained with the Wilcoxon test. The established significance level was α= 0.05. All analyses were performed using RStudio Version 1.1.456 (RStudio, Inc, Boston, Massachusetts).
RESULTS
The study included 52 subjects with low skin elasticity and presence of face wrinkles and all participants who were enrolled successfully completed the study. Improvements in skin moisturization, skin elasticity, and wrinkle depth were measured in the participants taking the hydrolyzed collagen after 28 days. In all participants, the results of the analysis of variance showed a statistically significant effect of the interaction between product and time. The evolution over time of the investigated parameters was significantly different between the participants taking the hydrolyzed collagen and the participants taking the maltodextrin.
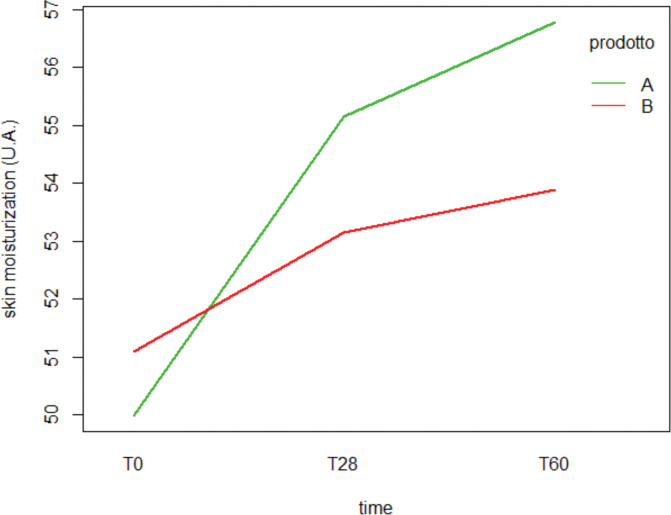
Moisturization measurements. The hydration index of the participants taking the hydrolyzed collagen increased from T28 until the end of the treatment. The mean skin moisturization index was 50.0±8.7 at T0 and increased to 55.1±7.8 and 56.8±8.2 following 28 and 56 days of treatment (p<0.01). The skin moisturization progress observed during the study is shown in Figure 2.
FIGURE 2.
Long-term skin moisturization evaluated by Corneometer, reported as mean measured throughout the study, from T0 to T56. (Two-way mixed-design ANOVA).
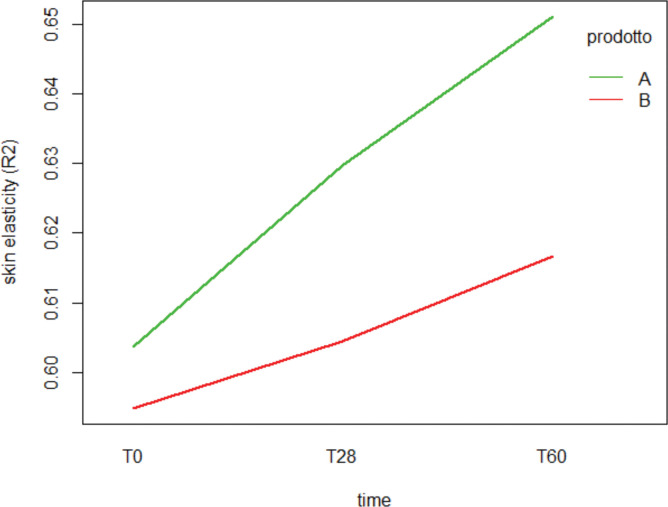
Elasticity measurements. The elasticity index of the participants taking the hydrolyzed collagen increased from T28 until the end of the treatment. The mean skin elasticity index was 0.604±0.1 at T0 and increased to 0.630±0.1 and 0.651±0.1 following 28 and 56 days of treatment (p<0.01). The skin elasticity progress observed during the study is shown Figure 3.
FIGURE 3.
Long-term skin elasticity evaluated by Corneometer, reported as mean measured throughout the study, from T0 to T56. (Two-way mixed-design ANOVA).
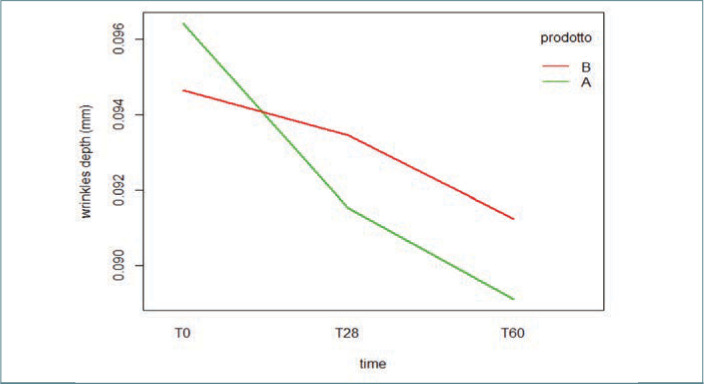
Wrinkle-depth measurements. A progressive decrease in wrinkle depth was noted throughout the treatment in patients taking the hydrolyzed collagen, reaching a difference in comparison with T0 following 28 and 56 days of treatment. As reported in Figure 4, the mean wrinkle depth observed at T0 was 0.096±0.01; at T28 and T56, the mean wrinkle depth was 0.092±0.02 and 0.089±0.02, respectively (p<0.01).
FIGURE 4.
Long-term wrinkle depth evaluated by Antera 3D®, reported as mean measured throughout the study, from T0 to T56. (Two-way mixed-design ANOVA).
Clinical evaluations. Clinical evaluations of skin softness, skin firmness, skin smoothness, and visibility of wrinkles supported the beneficial effect of the hydrolyzed collagen in improving these skin properties, estimated according to the five-point score shown in Table 1. Statistical evaluation performed by Wilcoxon signed-rank to compare the variations among the different time points underlined a statistically significant difference compared to baseline (T0) following 28 and 56 days of treatment.
Skin softness statistically improved in 35 percent and 54 percent of participants in the collagen group after 28 and 56 days of treatment, respectively. Skin smoothness statistically improved in 27 percent and 46 percent of participants in the collagen group after 28 and 56 days of treatment, respectively. Skin firmness statistically improved in 27 percent and 58 percent of participants in the collagen group after 28 and 56 days of treatment, respectively. Wrinkle visibility was reduced in the 38 percent of participants in the collagen group after 56 days of treatment. Clinical evaluation results are summarized in Table 2.
TABLE 2.
Results of clinical evaluation of skin softness, skin firmness, skin smoothness, and visibility of wrinkles in the group with hydrolyzed gelatin treatment: statistically significant percentages of improved subjects from T0 to subsequent times, T28 and T56.
| CLINICAL EVALUATION | PERCENT OF IMPROVED SUBJECTS | |
|---|---|---|
| T28, % (n) | T56, % (n) | |
| Skin softness | 35% (9) | 54% (14) |
| Skin firmness | 27% (7) | 58% (15) |
| Skin smoothness | 27% (7) | 46% (12) |
| Wrinkles visibility | - | 38% (10) |
DISCUSSION
This study evaluated the clinical effect of a hydrolyzed collagen supplement taken to improve some factors that contribute to the main signs of skin aging.
Skin aging can be of two types: extrinsic, that is caused by smoking, alcohol, stress, lack of sleep and chronic exposure to the sun, and intrinsic, that is, caused by increasing age. Aging causes a change in the appearance and function of the skin, generating the formation of wrinkles, the appearance of brown spots and the thickening of the skin.1
Collagen is the main connective tissue protein in animals and is the most abundant protein in mammals, accounting for approximately 25 percent of the total protein content. It is a long, fibrous structural protein with functions other than those performed by globular proteins, such as enzymes. Hard bundles of collagen, called collagen fibers, are an important component of the extracellular matrix that supports most tissues and supports cell structure from the outside. Collagen can be used as an anchor for cells in cell cultures and due to its high biocompatibility and biodegradability, it is widely used for the production of vascular prostheses, subcutaneous injection microparticles, for the construction of scaffolds that promote tissue regeneration, and as a raw material for the production of gelatin, glues and cosmetics. Collagen is also used as a food supplement, for the production of hydrolyzates to be administered orally.11
CONCLUSION
Our results suggest that the studied hydrolyzed collagen supplement might improve some skin properties, including hydration, elasticity, and the visibility of wrinkles. In addition, the study product appeared to significantly improve skin softness, skin firmness, and skin smoothness. The investigational food product clinical effect was demonstrated by both instrumental and clinical evaluation. The supplement was well tolerated and no adverse effects were recorded. Larger studies with a longer follow-up period are necessary to confirm our results.
REFERENCES
- Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011;69(3):249–56. doi: 10.1016/j.maturitas.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Sara Sibilla, Martin Godfrey, Sarah Brewer, Anil Budh-Raja, Licia Genovese. An Overview of the Beneficial Effects of Hydrolyzed Collagen as a Nutraceutical on Skin Properties: Scientific Background and Clinical Studies. The Open Nutraceuticals Journal. 2015;8:29–42. [Google Scholar]
- Zhuang Y, Hou H, Zhao X, Zhang Z, Li B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J Food Sci. 2009;74:H183–H188. doi: 10.1111/j.1750-3841.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- Zague V. A new view concerning the effects of collagen hydrolysate intake on skin properties. Arch Dermatol Res. 2008;300:479–483. doi: 10.1007/s00403-008-0888-4. [DOI] [PubMed] [Google Scholar]
- Oesser S, Adam M, Babel W, Seifert J. Oral administration of 14C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J Nutr. 1999;129(10):1891–1895. doi: 10.1093/jn/129.10.1891. [DOI] [PubMed] [Google Scholar]
- Figueres Juher T, Basés Pérez E. [An overview of the beneficial effects of hydrolysed collagen intake on joint and bone health and on skin aging]. Nutr Hosp. 2015;32(1):62–66. doi: 10.3305/nh.2015.32.sup1.9482. Jul 18. Article in Spanish. [DOI] [PubMed] [Google Scholar]
- Watanabe-Kamiyama M, Shimizu M, Kamiyama S et al. Absorption and Effectiveness of Orally Administered Low Molecular Weight Collagen Hydrolysate in Rats. Journal of Agricultural and Food Chemistry. 2010;58(2):835–841. doi: 10.1021/jf9031487. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Igawa K, Sugimoto K et al. Biological safety of fish (tilapia) collagen. Biomed Res Int. 2014;2014:630757. doi: 10.1155/2014/630757. Epub 2014 Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Koyama Y, Nomura Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci Biotechnol Biochem. 2009;73(4):930–2. doi: 10.1271/bbb.80649. Apr 23. Epub 2009 Apr 7. [DOI] [PubMed] [Google Scholar]
- Proksch E, Segger D, Degwert J, Schunck M, Zague V, Oesser S. Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2014;27(1):47–55. doi: 10.1159/000351376. Epub 2013 Aug 14. [DOI] [PubMed] [Google Scholar]
- Avila Rodríguez MI, Rodríguez Barroso LG, Sánchez ML. Collagen: A review on its sources and potential cosmetic applications. J Cosmet Dermatol. 2018;17(1):20–26. doi: 10.1111/jocd.12450. Feb. [DOI] [PubMed] [Google Scholar]