Abstract
Papillary thyroid cancer (PTC) remains the most common endocrine malignancy, despite marked achieves in recent decades, and the mechanisms underlying the pathogenesis and progression for PTC are incompletely elucidated. Accumulating evidence show that γ-glutamylcyclotransferase (GGCT), an enzyme participating in glutathione homeostasis and is elevated in multiple types of tumors, represents an attractive therapeutic target. Using bioinformatics, immunohistochemistry, qRT-PCR, and Western blot assays, we found that GGCT expression was upregulated in PTC and correlated with more aggressive clinicopathological characteristics and worse prognosis. GGCT knockdown inhibited the growth and metastasis ability of PTC cells both in vitro and in vivo and reduced the expression of mesenchymal markers (N-cadherin, CD44, MMP2, and MMP9) while increasing epithelial marker (E-cadherin) in PTC cells. We confirmed binding of microRNA-205-5p (miR-205-5p) on the 3′-UTR regions of GGCT by dual-luciferase reporter assay and RNA-RNA pull-down assay. Delivery of miR-205-5p reversed the pro-malignant capacity of GGCT both in vitro and in vivo. Lastly, we found that GGCT interacted with and stabilized CD44 in PTC cells by co-immunoprecipitation and immunohistochemistry assays. Our findings illustrate a novel signaling pathway, miR-205-5p/GGCT/CD44, that involves in the carcinogenesis and progression of PTC. Development of miR-205-mimics or GGCT inhibitors as potential therapeutics for PTC may have remarkable applications.
Keywords: GGCT, miR-205-5p, PTC, CD44
Papillary thyroid cancer (PTC) represents the most commonly occurring type of differentiated thyroid carcinoma, accounting for approximately 80% to 90% of all thyroid cancer patients, and with rapidly rising incidence during the last several decades worldwide (1, 2). Despite common perceptions that PTC possesses indolent behavior and carries relatively low disease-specific mortality, locoregional relapse and distant metastases occur in up to 20% and 10% cases at 10 years, respectively, causing enormous impacts on the survival of these patients (3, 4). Based on molecular profiles, PTC can be roughly grouped into 2 distinct subclasses: BRAFV600E-like PTCs (harboring BRAFV600E mutations and showing insistent activation of MAPK signaling pathway) and RAS-like PTCs (characterizing by K/H/N-RAS mutations and activating in both the MAPK and PI3K/AKT pathways) (5). Nevertheless, with the deepening of the research, a series of novel onco-driver genes in PTC have been identified (6-8), which prompts the existence of a much more complex regulatory network in the occurrence and progression of PTC and calls for the importance of revealing the pathogenesis of this condition.
An enzyme involved in glutathione metabolism cycle, γ-glutamylcyclotransferase (GGCT) catalyzes the reaction that induces 5-oxoproline and free amino acids generation from the γ-glutamyl peptide (9). Intriguingly, growing evidence substantiates a vital role of GGCT in oncogenesis. GGCT was identified to be highly expressed in several carcinomas, including bladder cancer (10), breast cancer (11), ovarian cancer (12), and gastric cancer (13). RNA interference (RNAi)-mediated knockdown of GGCT weakened proliferation of various tumor cell lines without disturbing growth of normal cells (14). Moreover, GGCT was involved in the shift of oxidative phosphorylation to aerobic glycolysis by regulating the expression of HIF-1α in multiple tumors, which made tumor cells better adapted to the hypoxia environmental stresses (15). Additionally, GGCT was required for oncogenic K-RAS-driven lung tumor formation and the scavenge of exceeding reactive oxygen species (ROS) in mice (16). However, studies on the functions of GGCT in PTC have not been reported.
MicroRNAs are a group of endogenous small RNAs that control gene expression in various biological processes such as cell differentiation, proliferation, and apoptosis (17). MicroRNAs can prevent the translation of target mRNA or promote its degradation by binding to the 3′ untranslated regions (3′-UTR) of mRNA in a complementary manner (18). In human cancers, both carcinogenic and tumor suppressor roles of microRNA have been proposed, based on the distinct functions of substrate genes (19, 20). Of particular concern is that the initiation and progression of PTC are often accompanied by the alteration of microRNA profiles (21). MicroRNA-205-5p (miR-205-5p), a novel microRNA, has been reported to reduce angiogenesis and suppress cell proliferation via post-transcriptional manipulation of VEGFA in PTC (22). Another study found a DLEU2/miR-205-5p/TNFAIP8 signaling axis involved in the regulation of the proliferation, migration, and aerobic glycolysis processes in PTC cells (23). Interestingly, later clinical sample–based research suggested that miR-205-5p combined with TSHR mRNA could be used to distinguish benign thyroid nodules from malignant ones (24). These findings highlight the potential of miR-205-5p to serve as a candidate molecular for both diagnosis and treatment of PTC.
In this study, we comprehensively evaluated GGCT expression, its relationship with clinicopathological characteristics and prognosis, and its effect on PTC cells’ biological behaviors through clinical samples and loss-of-function assays. Moreover, the role of miR-205-5p as an upstream negative regulator of GGCT has been confirmed through dual-luciferase reporter assay and RNA pull-down assay as well as genetic rescue experiments. Lastly, we found GGCT can bind to CD44 and maintain CD44 protein stability, which has been shown to increase the malignant potential of tumor cells (25). Our study shed light on a novel signaling pathway, miR-205-5p/GGCT/CD44, which plays critical roles in cell proliferation and metastasis of PTC.
Materials and Methods
Antibody Information
Antibodies against GGCT (ab198503, RRID: AB_2905580, https://antibodyregistry.org/search?q=AB_2905580), E-cadherin (ab227639, RRID: AB_2905581, https://antibodyregistry.org/search?q=AB_2905581), N-cadherin (ab76011, RRID: AB_1310479, https://antibodyregistry.org/search?q=AB_1310479), CD44 (ab189524, RRID: AB_ 2885107, https://antibodyregistry.org/search?q=AB_2885107), MMP2 (ab92536, RRID: AB_10561597, https://antibodyregistry.org/search?q=AB_10561597), MMP9 (ab76003, RRID: AB_1310463, https://antibodyregistry.org/search?q=AB_1310463) were purchased from Abcam Biotechnology Co, Ltd. (USA). Antibodies against Flag-tag (14793S, RRID: AB_2572291, https://antibodyregistry.org/search?q=AB_2572291), His-tag (12698S, RRID: AB_2744546, https://antibodyregistry.org/search?q=AB_2744546), GAPDH (5174S, RRID: AB_10622025, https://antibodyregistry.org/search?q=AB_10622025), β-actin (4970S, RRID: AB_2223172, https://antibodyregistry.org/search?q=AB_2223172) were bought from Cell Signaling Technology, Inc. (USA).
Cell Culture and Patient Specimens
Human normal thyroid cell line Nthy-ori 3-1; PTC cell lines TPC-1, BCPAP, and K1; and human embryonic kidney 293T (HEK293T) cell line were purchased from Shanghai Cell Bank of Chinese Academy of Sciences (China). Nthy-ori 3-1, TPC-1, and BCPAP were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, USA), while K1 and HEK293T were cultured in RPMI DMEM medium (Gibco, USA). All media contained 10% fetal bovine serum (FBS) (Gibco, USA) and 1% penicillin-streptomycin (Meilun Bio, China) and all cells were kept in a cell incubator at 37 °C and 5% carbon dioxide.
The samples of carcinomas (n = 178) and the adjacent normal thyroid tissues (n = 82) were obtained from surgical specimens from patients with PTC who were hospitalized at Tongji hospital (Wuhan, China) between January 2017 and October 2020. The whole blood samples were collected prior to surgery and 24 hours after surgery from each patient and serum was extracted by centrifugation. Fresh specimens were formalin-fixed and paraffin-embedded or directly frozen into liquid nitrogen for further analysis. The patients enrolled received no prior drug or radioiodine ablation therapy before the surgical intervention. An informed consent was obtained from each participant and the study protocol was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and followed the ethical guidelines of the Declaration of Helsinki.
Quantitative Real-Time Polymerase Chain Reaction
Total mRNA and MicroRNA of cells or tissues were extracted with Ultrapure RNA Kit (CWBIO, China) and miRNA Purification Kit (CWBIO, China) respectively. HiScript II 1st Strand cDNA Synthesis Kit (+gDNA wiper) (Vzayme, China) or miRNA 1st Strand cDNA Synthesis Kit (by stem-loop) (Vzayme, China) was used for reverse transcription of mRNA or MicroRNA, respectively. Subsequently, qPCR SYBR Green (YEASEN, China) was used for fluorescence quantitative analysis in the Bio-Rad CFX96 Touch real-time fluorescent quantitative PCR system (USA). The quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) program was set as follows: 95 °C for 5 minutes, followed by 40 cycles of 95 °C for 10 seconds, 60 °C for 20 seconds, and 72 °C for 20 seconds. U6 and GAPDH are used as internal reference genes for miRNA and mRNA respectively. The qPCR primers involved in this study are provided in Table S1 (26) of Supplementary Materials. The 2-ΔΔCt method was utilized to quantify gene expression.
Western Blot Analysis
The RIPA Lysis Solution (Beyotime, China) was used to extract the total protein and the BCA kit (Beyotime, China) was used to quantify the protein concentration. A quantity of 30 μg of total protein was separated in 10% polyacrylamide gel, and then the target protein was transferred to a polyvinylidene fluoride (PVDF) membrane and blocked with 5% skim milk dissolved in Tris-buffered saline with Tween-20 (TBST) at room temperature for 1 hour. The primary antibody was incubated overnight at 4 °C and then washed with TBST. The secondary antibody incubation was then performed at room temperature for 1 hour. The blots were exposed digitally using an ChemiDoc Imaging System (Bio-Rad, USA) and target bands were quantified using Image Lab Software (version 6.0, Bio-Rad, USA).
Human Serum and Enzyme-Linked Immunosorbent Assays
Human serum was separated and collected from whole blood following centrifugation at 1500g for 10 minutes. The level of secreted GGCT was quantified by a human enzyme-linked immunosorbent assay (ELISA) kit (RX100080H, RRID: AB_2905582, https://antibodyregistry.org/search?q=AB_2905582, Ruixin Biotechnology Co, Ltd., China) in strict accordance with the manufacturer’s instructions. The detection range of the ELISA Kit was 125 to 4000 pg/mL (limit of quantification, 10 pg/mL). The intra-assay coefficient of variation (CV) was below 10% and the inter-assay CV was below 15%.
Plasmid Construction and Lentivirus
The GGCT 3′-UTR sequence was amplified from the human genome, and the fragment was connected to pmirGLO (Addgene, USA) to obtain the wild-type luciferase reporter plasmid (WT-pmirGLO-GGCT). Using WT-pmirGLO-GGCT as a template, the binding site of miR-205-5p and GGCT 3′-UTR was mutated to obtain a mutant luciferase reporter plasmid (MUT-pmirGLO-GGCT). The coding sequence of CD44 or GGCT was amplified using human cDNA as a template, and a 6× His-tag was added to the C-terminal of CD44, while a 3× Flag-tag was inserted to the N-terminal of GGCT. The obtained fragments were connected to pcDNA3.1 (Addgene, USA). For lentivirus-Mediated gene silencing, we designed 3 distinct short hairpin RNAs (shRNAs) oligonucleotides for the target sequence of GGCT (shRNA#1: GATTATTTGCATGGGTGCAAA, shRNA#2: GCAATAGAACCAAATGACTAT, shRNA#3: GCTGGAGTATCAAGAGAAGTT). Non-targeting control of shRNA (NC) was also synthesized. The above oligonucleotides were inserted into the pLKO.1 vector. Lentivirus vectors expressing pre-miR-205 and miR-205-NC were obtained from GeneChem (Shanghai, China). For stable GGCT overexpression, the GGCT gene was amplified and inserted into PLVX-EF1α-IRES-puro to generate recombinant pLVX-GGCT for lentivirus production.
Cell Counting Kit-8 Assay
Cell proliferation was monitored with a cell counting kit-8 (CCK8) kit (Dojindo, Japan) following the producer’s instructions. PTC cells (1 × 103 cells per well) were seeded in a 96-well plate and cultivated for the indicated times. Then, 10 μL CCK8 solution was added to each well for incubation 2 hours before analysis. The absorbance (450 nm) of each well was determined using a multifunctional microplate reader (Thermo Fisher Scientific, USA).
Wound Healing Assay
1 × 106 thyroid cancer cells were seeded into a 6-well plate. When the cell confluence reached about 90%, the cells were scratched with a 200 μL sterile pipette tip, and the medium replaced with reduced serum medium containing 0.2% FBS to maximally maintain cell survival but limit cell proliferation. Areas of the wound gap were assessed and photographed at 0, 24, or 48 hours after wound generation with an inverted microscope (Olympus, Japan), and the gap areas were quantified by using Image J software (NIH, USA). The experimental results were obtained after 3 independent repetitions.
Transwell Invasion Assay
The bottom of the transwell chamber (8 μm, Corning, USA) used in the experiment was covered with Matrigel (1:50 dilution, BD, USA), and the cells were serum-deprived for 12 hours, and then 3 × 104 cells were inoculated into the upper chamber of the transwell with 5% FBS in 150 μL medium. Then 600 μL medium containing 15% FBS was added to the lower chamber. After incubation for 24 hours, noninvading cells were wiped off with a cotton swab and invading cells were stained using 0.1% crystal violet and 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 minutes. An inverted microscope (Olympus, Japan) was used to visualize the invaded cells, and 5 different fields with a 10× objective were counted from each invasion chamber independently. The experimental results obtained were repeated 3 times independently.
Dual-Luciferase Reporter Assay
WT-pmirGLO-GGCT or MUT-pmirGLO-GGCT and miR-205 mimics were co-transfected into HEK293T cells, and the cells were harvested 24 hours. According to the instructions of the Luciferase Reporter Gene Detection Kit (Meilun, China), add cell lysis buffer to the cells and lyse for 10 minutes on ice. After centrifugation at 12 000g for 5 minutes, 20 μL of supernatant was added to 100 μL Firefly Luciferase Reaction Buffer and Renilla Luciferase in sequence; Firefly Luciferase and Renilla Luciferase reporter gene activities were read in the microplate reader (Bio-Rad, USA) respectively. The experimental data was obtained after 3 independent repetitions.
Biotin RNA-RNA Pull-Down Assay
K1 cell lysates were prepared and incubated with biotinylated double-stranded RNA of wild-type (WT) miR-205-5p (Bio-miR-205-5p-WT) or mutant miR-205-5p (Bio-miR-205-5p-MUT) or control random RNA (Biotin-NC) for 2 hours at 4 °C to form RNA-RNA complexes. The coupled complexes were captured by magnetic beads conjugated with streptavidin (Dynabeads MyOne Streptavidin C1, Thermo Fisher Scientific, USA). Finally, the complexes of miRNA/mRNA were eluted and the mRNA expression of GGCT was assessed by qRT-PCR.
Endogenous and Exogenous Co-Immunoprecipitation (Co-IP) Assays
For endogenous co-immunoprecipitation (Co-IP) assays, K1 cells were lysed in 1 mL Cell Lysis Buffer for IP (RM00022, ABclonal, China) at 4 °C for 20 minutes. The input group contains 10 μL protein supernatant mixed with an equal volume of 2× loading buffer. The remaining protein supernatant was divided into 2 equal parts and either Mouse Control IgG (A19711, RRID: AB_2905583, https://antibodyregistry.org/search?q=AB_2905583, ABclonal, China) or Anti-GGCT (ab198503, RRID: AB_2905580, Abcam) was added, respectively, and the mixer inverted at 4 °C for 2 hours. The rProtein A/G Plus MaqPoly Beads (RM09008, ABclonal, China) were washed twice with Cell Lysis Buffer for IP and blocked with 3% BSA at 4 °C Celsius for 1 hour. The antibody-antigen binding complex was then mixed with the ready-for-use magnetic beads and reacted at 4 °C for 2 hours. We put the above-mentioned magnetic bead-antibody-antigen complex on a magnetic separator for separation and washed twice, then added 30 μL 2× SDS-PAGE Loading Buffer to it, mixed and heated at 95 °C for 15 minutes. It was then placed on a magnetic separator for magnetic separation, and the supernatant collected for SDS-PAGE detection.
For exogenous detection, the HEK293T cell group was divided into 3 groups that overexpress Flag-GGCT or His-CD44 or co-transfected with Flag-GGCT and His-CD44. After the cells were lysed in the cell lysis buffer, a portion of the supernatant was used for the input group, and the remaining protein supernatant was respectively incubated with Anti-Flag (14793S, RRID: AB_2572291, CST) or Anti-His (12698S, RRID: AB_2744546, CST) for antigen-antibody incubation. After the magnetic beads-antibody-antigen complex incubation, SDS-PAGE detection was performed.
Animal Experiment
Four-week-old nude mice were purchased from Model Animal Research Center of Nanjing University (Nanjing, China). All nude mice were kept in a specified-pathogen-free (SPF) environment, and all experiments in animals were approved by the Animal Ethics Committee of Wuhan University of Science and Technology.
For subcutaneous tumor xenograft model, stable knockdown (sh-Control and sh-GGCT) and overexpression (Lenti-PLVX, Lenti-GGCT, Lenti-miR-205, Lenti-GGCT + miR-205) PTC cell lines were generated via the lentiviral system as described above. A total of 5 × 106 PTC cells resuspended in 100 μL PBS were injected into the right axillary fossa of nude mice. The length and width of the subcutaneous tumor was measured with a vernier caliper every 7 days after inoculation. The formula for detecting the tumor volume was: volume (mm3) = 0.5 × width2 × length. At 28 days after the inoculation, the subcutaneous tumors were harvested and the tumor weights were measured. The subcutaneous tumor tissues were subjected to hematoxylin and eosin (H&E) staining, immunohistochemistry, and Western blot analysis.
For tail vein lung metastatic model, K1 cells were stably transfected with firefly luciferase (fLuc) gene by lentivirus. Mice were then injected with 1 × 106 fLuc-labeled K1 cells via the tail vein. Growth of pulmonary K1-fLuc metastases was monitored by bioluminescence imaging (BLI) every 7 days. Mice were anesthetized with 2% to 3% isoflurane and intraperitoneally injected with 150 mg/kg D-luciferin potassium salt (Yeason, Shanghai, China). Tumors exhibiting fLuc expression were imaged 10 minutes postinjection with a Xenogen IVIS Lumina system (Caliper Life Sciences). On the twenty-first day after the initial inoculation, the mice were euthanized and the visceral organs were gently removed and used for ex vivo bioluminescence imaging.
Hematoxylin and Eosin Staining and Immunohistochemistry
For human and mouse histological examination, tissues were fixed in 10% neutral buffered formalin for 24 hours. All simples were paraffin-embedded and were sliced to 4 μm. H&E staining was performed by Servicebio Biotechnology Co. Ltd. (Wuhan, China) according to standard protocols. For immunohistochemistry (IHC), the sections were deparaffinized and rehydrated, followed by antigen repaired with citrate antigen repair buffer (pH 6.0), and then subjected to endogenous peroxidase inactivation with 3% hydrogen peroxide for 15 minutes at room temperature. After washing, unspecific antigen binding was blocked with 10% normal goat serum for 30 minutes at 37 °C and probed with indicated primary antibodies overnight at 4 °C before incubation with HRP-conjugated secondary antibodies for 45 minutes at room temperature. Chromogen detection was conducted with the 3,3′-diaminobenzidine (DAB) chromogen kit (Servicebio, Wuhan, China). Nuclei were counterstained with hematoxylin. The IHC results were evaluated using following criteria: the intensity score was judged by a scale of 0 to 3 points: 0 for no staining, 1 for weak staining, 2 for moderately positive staining, and 3 for strongly positive staining; the percentage of positive areas was judged by a scale of 0 to 4 points: 0% (0), <10% (1), 10-30% (2), 31-70% (3), and 71-100% (4). The final IHC score was determined through multiplying the intensity score by the percentage score to obtain a maximum of 12. In addition, high-GGCT expression was defined as IHC score ≥ 6, whereas low-GGCT expression was defined as IHC score < 6.
Survival Analysis
Follow-up and outcome data of 178 PTC patients in our cohort were collected and used for the assessment of the impact of GGCT expression on disease-free survival (DFS). Patients were dichotomized into a GGCT-high or GGCT-low group according to the IHC score as described above. Disease progression was defined as recurrent, metastatic, or persistent disease as confirmed by imaging modalities, thyroglobulin-positive serology and/or pathologic diagnosis. The follow-up duration ranged from 4 to 60 months (median, 39 months). At the last follow-up assessment, a total of 34 patients showed disease progression. In addition, 4 patients were lost to follow-up and were censored for survival as of the last visit. The survival curve was generated using the Kaplan–Meier method with the log-rank test.
DFS for CD44 was conducted by retrieving the publicly available online database Kaplan–Meier Plotter (http://kmplot.com/analysis/). Patients were dichotomized by the median value of CD44. The follow-up threshold was set to 240 months. Survival difference was evaluated using log-rank test.
Statistical Analysis
All statistical analyses were performed using R software (R Core Team, Version 4.0.2) or GraphPad Prism (version 7.0). Data were present as mean ± SD unless otherwise noted. Normality of data distribution was assessed using Kolmogorov-Smirnov test and homogeneity of variances using Levene test. Data were log-transformed to obtain the normality of distribution and/or to meet the assumptions of equal variances. Unpaired or paired t tests were utilized to analyze the statistical significance of the differences between 2 groups. One-way analysis of variance (ANOVA), with Tukey’s HSD post hoc test was applied for multi-group comparisons. Fisher exact tests and Chi-square tests were used to determine the correlation between GGCT and the clinicopathological parameters. Survival was analyzed by Kaplan–Meier methods and presented as Kaplan–Meier curves. Linear regression analysis was used for gene correlation analysis, and a Pearson correlation coefficient (R) was calculated. Significance between groups was represented by *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
GGCT Is Overexpressed in PTC Tissues and Cell Lines
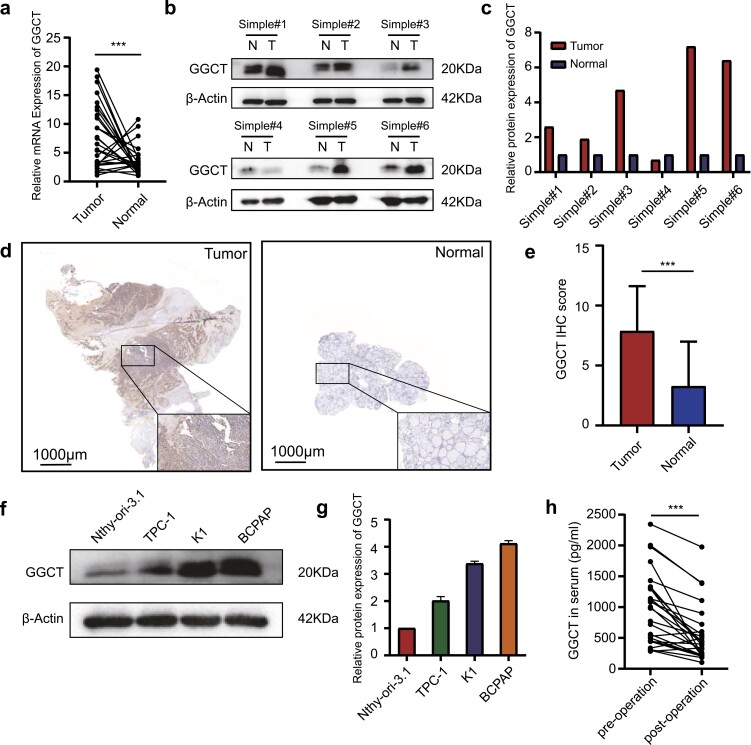
To explore the expression pattern of GGCT in PTC, we first detected its expression in 27 pairs of PTC tissues and paracancerous tissues by RT-qPCR. As illustrated in Fig. 1A, PTC tissues exhibited prominently elevated GGCT mRNA expression as compared to the corresponding paracancerous tissues. These data were also confirmed in The Cancer Genome Atlas (TCGA) database, where GGCT was significantly higher in tumor tissues than in normal ones for both unpaired (Supplementary Fig. S1a (26)) and paired (Supplementary Fig. S1b (26)) simple comparisons. Additionally, we further assessed GGCT protein level in 6 pairs of PTC and noncancerous tissues using Western blot analysis, which showed that most PTC tissues possessed greater GGCT expression than the normal controls (Fig. 1B and 1C). Similar findings were also obtained with paraffin-embedded sections including 178 PTC and 82 normal thyroid tissues by IHC. As shown in Fig. 1D and 1E, higher expression of GGCT was observed in the PTC tissues compared with the normal counterparts. (P < 0.05). Furthermore, we measured a range of thyroid-derived cell lines for GGCT expression, and as expected, high levels of GGCT expression were visible in all 3 PTC cell lines but not in the normal thyroid follicular epithelial cell line (Nthy-ori-3-1) (Fig. 1F and 1G). As GGCT has been estimated to have an elimination half-life in serum of up to 30 hours (https://web.expasy.org/cgi-bin/protparam/), we compared the blood content of GGCT preoperative and 24 hours postoperative from the same patient (n = 26), and the results clearly indicated the serum GGCT level was noticeably reduced after surgical intervention (Fig. 1H). Together these results strongly suggest that GGCT is upregulated in the PTC tissues and cell lines.
Figure 1.
GGCT is overexpressed in PTC. (a) Scatter dot plots with connecting lines show the mRNA expression of GGCT in paired PTC and normal thyroid tissues (n = 27). (b) Western blot (WB) experiments show the protein expression of GGCT in 6 paired normal and tumor samples. (c) The quantifications of WB signal in each patient were normalized to β-actin and presented as histograms. (d) Representative images of immunohistochemistry (IHC) of GGCT in the normal thyroid tissues (right) and PTC tissues (left) (magnification, ×100, scar bar = 1000 μm). Solid black boxes indicate higher magnifications (×400) of the areas. (e) IHC scores of GGCT were calculated in PTC group (n = 178) and normal thyroid group (n = 82). GGCT protein levels in PTC and normal thyroid cell lines were assessed by WB (f) and quantified (g). (h) The level of secreted GGCT in patient’s serum before and 24 hours after surgical intervention was determined by ELISA assays (n = 26). The line connecting the 2 scatters denotes the change in GGCT secretion of the same patient before and after surgery. ***: P < 0.001.
High Level of GGCT Correlates With Unfavorable Clinicopathological Characteristics and Worse Outcomes
We focused on whether GGCT overexpression was responsible for worse PTC prognosis. Based on the IHC staining score, the patients were classified into low-GGCT (n = 67) and high-GGCT expression groups (n = 111). The Fishers exact or Chi-square test showed that GGCT expression was strongly associated with tumor histological type (P < 0.001), extrathyroidal extension (P = 0.035), primary tumor classification (P = 0.004), and TNM stage (P = 0.002) (Table 1). In addition, the Kaplan–Meier survival curve revealed a shorter DFS time in the high-GGCT expression group than in the low-GGCT expression group (Supplementary Fig. S2 (26)). These results infer that GGCT is closely correlated with tumor malignant properties and decreases DFS in PTC patients.
Table 1.
Relationship between GGCT expression and clinicopathological characteristics in 178 PTC tissues
| Clinicopathologic parameters | n | GGCT expression | P | |
|---|---|---|---|---|
| low | high | |||
| All cases | 178 (100%) | 67 (37.6%) | 111 (62.4%) | |
| Age, y | 0.848 | |||
| ≤55 | 60 (33.7%) | 22 (36.7%) | 38 (63.3%) | |
| >55 | 118 (66.3%) | 45 (38.1%) | 73 (61.9%) | |
| Gender | 0.159 | |||
| male | 69 (38.8%) | 20 (29.0%) | 49 (71.0%) | |
| female | 119 (61.2%) | 47 (39.5%) | 72 (60.5%) | |
| Histological type | <0.001 | |||
| classical | 106 (59.6%) | 39 (36.8%) | 67 (63.2%) | |
| follicular | 28 (15.7%) | 19 (67.9%) | 9 (32.1%) | |
| tall cell | 44 (24.7%) | 9 (20.5%) | 35 (79.5%) | |
| Multifocality | 0.218 | |||
| unifocal | 129 (72.5%) | 45 (34.9%) | 84 (65.1%) | |
| multifocal | 49 (27.5%) | 22 (44.9%) | 27 (55.1%) | |
| Extrathyroidal extension | 0.035 | |||
| no | 133 (74.8%) | 56 (42.1%) | 77 (57.9%) | |
| yes | 45 (25.2%) | 11 (24.4%) | 34 (75.6%) | |
| T classification | 0.004 | |||
| T1-T2 | 117 (65.8%) | 53 (45.3%) | 64 (54.7%) | |
| T3-T4 | 61 (34.2%) | 14 (23.0%) | 47 (77.0%) | |
| Lymph node metastasis | 0.395 | |||
| no | 87 (48.9%) | 30 (34.5%) | 57 (65.5%) | |
| yes | 91 (51.1%) | 37 (40.7%) | 54 (59.3%) | |
| TNM stage | 0.002 | |||
| I + II | 128 (71.9%) | 57 (44.5%) | 71 (55.5%) | |
| III + IV | 50 (28.1%) | 10 (20.0%) | 40 (80.0%) | |
| Braf-V600E mutation | 0.975 | |||
| no | 72 (40.4%) | 27 (37.5%) | 45 (62.5%) | |
| yes | 106 (59.6%) | 40 (37.7%) | 66 (62.3%) |
Knockdown of GGCT Alleviates the Proliferation, Migration, and Invasion of PTC Cells In Vitro and In Vivo
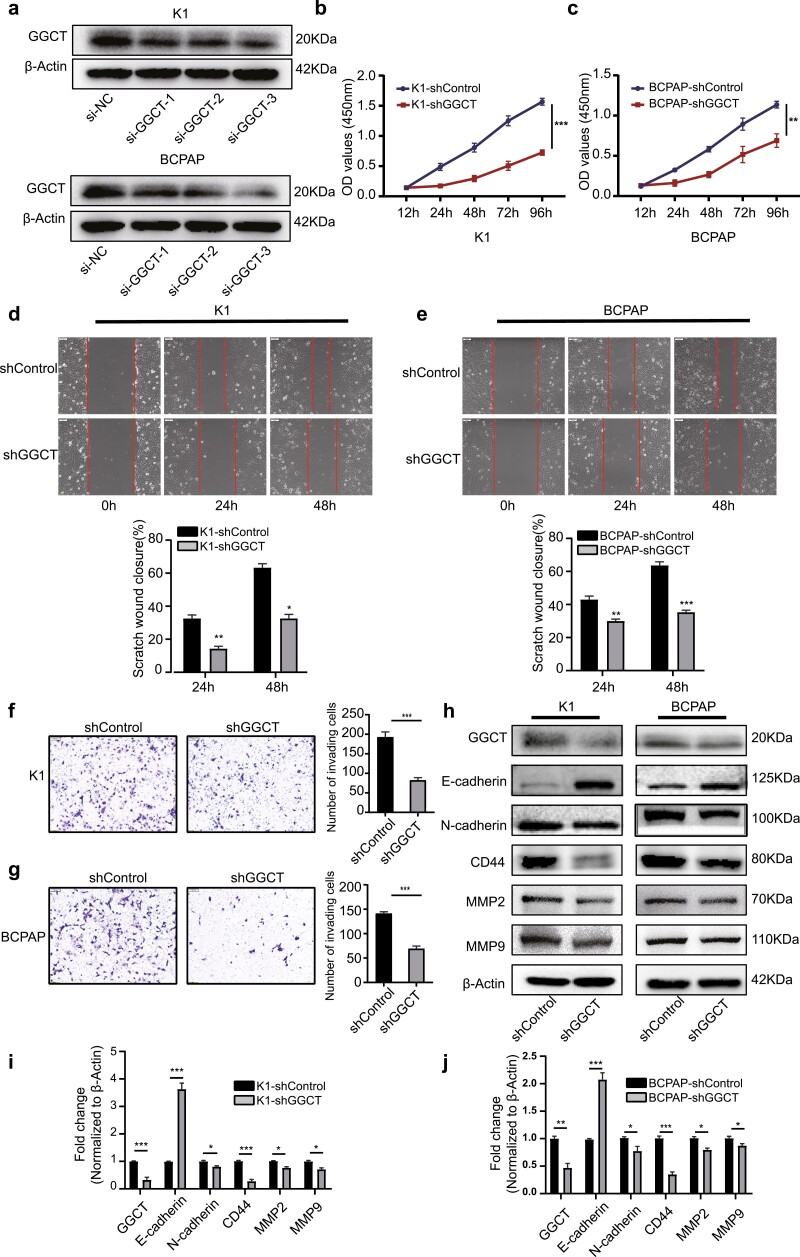
Given the findings that upregulation of GGCT was associated with poorly clinicopathological characteristics and worse outcomes in PTC patients, we postulated that GGCT might act as a tumor facilitator in PTC. To assess the effect of GGCT on the biological behaviors of PTC cells, we performed the RNA interference experiment with 3 distinct shRNA duplexes. Among them, shRNA-3 exhibited the best knockdown efficiency, as indicated by Western blot analysis, and thus was selected for the subsequent investigations (Fig. 2A). Firstly, we assessed the impact of GGCT on cell growth through the CCK8 assay. As shown in Fig. 2B and 2C, GGCT knockdown remarkably inhibits the proliferation of the K1 and BCPAP cell lines in a time-dependent manner. We next evaluated the effect of GGCT on cell migration and invasion via wound healing and Matrigel Transwell assays. The wound healing assay demonstrated that silencing of GGCT significantly retarded the closure of the wound gap after 24 hours and 48 hours (Fig. 2D and 2E). Also, shRNA-mediated abrogation of GGCT impeded the matrix penetration capability of K1 and BCPAP cell lines (Fig. 2F and 2G).
Figure 2.
Downregulation of GGCT decreases PTC cell proliferation and aggressiveness in vitro. (a) WB analysis validation for GGCT knockdown in K1 and BCPAP cells. (b-c) Cell proliferation determined by CCK-8 in K1 and BCPAP cells infected with sh-GGCT or sh-Control as indicated. (d-e) Wound healing assay comparing the migration ability between sh-GGCT and sh-Control PTC cells. (f-g) Transwell assay of K1 and BCPAP cells infected with sh-GGCT or sh-Control. (h) The abundance of EMT-related markers E-cadherin, N-cadherin, CD44, MMP2, and MMP9 was measured by WB in the K1 and BCPAP cells following GGCT knockdown. (i-j) Quantification of WB experiments was normalized to β-actin control. *: P < 0.05, **: P < 0.01, ***: P < 0.001.
Since epithelial-mesenchymal transition (EMT) has previously been proved to be critical for the acquisition of invasiveness in epithelial cancer (27), we questioned whether interference with GGCT expression inhibits cell proliferation, migration, and invasion by reversing the EMT phenotype. Indeed, after stable downregulation of GGCT in PTC cells, K1 and BCPAP cells underwent several morphologic alterations from mesenchymal spindle-like phenotype to epithelial polarized phenotype in contrast to the control cells (data not shown). Subsequently, the EMT-related proteins were examined by Western blot analysis, and the results showed a downregulation of mesenchymal markers (N-cadherin, CD44, MMP-2, MMP-9) and a concomitant upregulation of epithelial marker (E-cadherin) in sh-GGCT cells, heralding the EMT-promoting role of GGCT in PTC (Fig. 2H-2J).
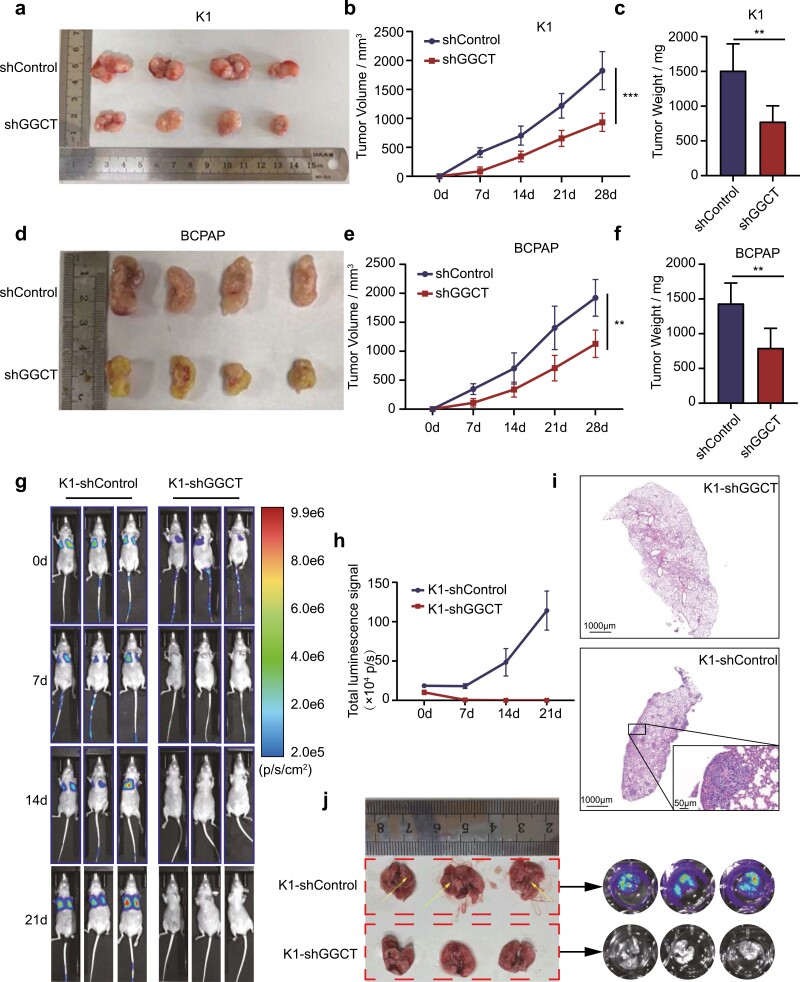
To determine whether GGCT is required for in vivo tumor growth and metastasis, the nude mouse subcutaneous xenotransplant tumor model and the tail-vein lung metastasis model was performed. As pointed out in Fig. 3A-3C, GGCT-knockdown K1 cells exhibited an evidently decreased tumor volume and tumor weight 3 weeks postmodeling compared with the control group. Similar results were yielded by the xenografts constructed using BCPAP cell lines which were stably infected with sh-GGCT or sh-NC lentivirus (Fig. 3D-F). Moreover, the efficiencies of GGCT knockdown in vivo and the EMT-associated markers were assessed by Western blot analyses, which proved the silencing of GGCT contributed to the reduction of tumor growth and reversion of EMT process (Supplementary Fig. S3 (26)). As for the experimental pulmonary metastasis assay, sh-GGCT-K1 or sh-NC-K1 cells expressing firefly luciferase were administered via tail vein injection. The successful aggregation of tumor cells into the lung in both sh-GGCT and sh-NC groups was confirmed at day 0 by bioluminescence imaging (Fig. 3G). With extended observation time, the bioluminescence signals in the sh-NC-K1 group rapidly increased and all mice in this group were forced to be sacrificed due to the tumor-associated cachexia up to 3 weeks post injection (Fig. 3G-3H). Surprisingly, none of the positive signals were captured in the sh-GGCT-K1 group throughout the observation period, demonstrating an entire abrogation of the formation of lung metastatic foci by GGCT repression (Fig. 3G-3H). These were also corroborated by an ex vivo imaging of the visceral organs in combination with H&E staining of lung tissues (Fig. 3I-3J). All above shed light on that abolishment of GGCT weakened the malignant potential of PTC cells in vitro and in vivo by reversing the EMT process.
Figure 3.
GGCT knockdown delays tumor growth and suppresses lung metastasis of PTC cells in vivo. (a and d) Representative images of K1 and BCPAP subcutaneous xenografts from nude mice in sh-GGCT and sh-Control groups. (b and e) Tumor volumes were measured every 7 days post implantation. (c and f) Comparison of dissected tumor weights in each group 28 days post tumor implantation. (g) Metastasis bioluminescence images of K1-sh-Control and K1-sh-GGCT every 7 days post tumor cell tail vein injection. (h) The line chart denotes luciferase bioluminescence emitted from the lungs in each group over time (0–21 days). (i) H&E staining for the lungs of K1-sh-Control and K1-sh-GGCT mice. The figure boxed in the lower right corner shows the metastatic foci with higher magnifications. (j) Gross anatomy (left) and ex vivo bioluminescence (right) images from K1-sh-Control and K1-sh-GGCT mice at day 21 after tumor cells challenge. **: P < 0.01, ***: P < 0.001.
MiR-205-5p Can Directly Bind to the 3′-UTR of GGCT and Regulate GGCT Expression
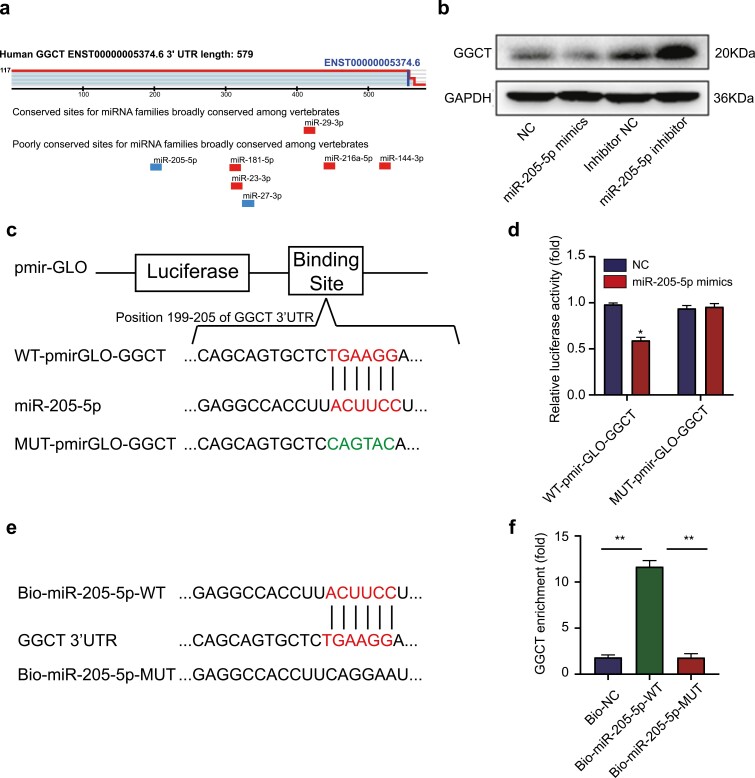
Evidence supports the crucial role that miRNAs play in various cancers through inhibition of their target genes (28). Interestingly, miR-205-5p, a potential tumor suppressor in multiple malignancies, was predicted to contain the binding site of the 3′-UTR of GGCT, as obtained by bioinformatics (Fig. 4A). We also noticed a significant downregulation of mir-205-5p expression in PTC tissues compared with normal thyroid tissues in the TCGA database (Supplementary Fig. S4 (26)). Further exploration showed that decreased expression of GGCT was observed in K1 cells transfected with mir-205-5p mimics, while increased expression in mir-205-5p inhibitor groups (Fig. 4B). To probe whether GGCT was the direct target of miR-205-5p, GGCT-3′-UTR with a wild-type (WT) or mutated (MUT) miR-205-5p binding motif was constructed and subcloned into the dual-luciferase vector (Fig. 4C). It was found that miR-205-5p reduced the luciferase activity of WT 3′-UTR -reporter, but not the MUT 3′-UTR-reporter (Fig. 4D). Moreover, biotin RNA-RNA pull-down assay revealed that GGCT mRNA were significantly enriched by using Bio-miR-205-5p-WT compared with Bio-miR-205-5p-MUT and Bio-NC (Fig. 4E-4F), indicating the interactions between miR-205-5p and GGCT.
Figure 4.
MiR-205-5p binds to 3′-UTR of GGCT and regulates the expression of GGCT. (a) Position of the miR-205-5p target site in 3′-UTR of human GGCT mRNA predicted by TargetScan (http://www.targetscan.org/). (b) Western blot analysis of the effect of miR-205-5p mimics or inhibitors on GGCT protein levels in K1 cells. (c) Schematic representation of the binding sites of miR-205-5p and the expression vectors construction of the wild type and mutant GGCT- 3′-UTR pmir-GLO plasmids used in luciferase reporter assays. (d) Luciferase reporter vectors WT-pmirGLO-GGCT (intact) or MUT-pmirGLO-GGCT (mutant) were transfected in K1 cells. The luciferase activity was measured and the values were normalized to Renilla luciferase activity. (e) Schematic of biotin-labeled RNA-RNA pull-down assays. (f) Expression of GGCT mRNA in biotin-labeled miRNA/mRNA complex was demonstrated by qRT-PCR. *: P < 0.05, **: P < 0.01.
MiR-205-5p Reverses the Pro-Malignant Phenotypes Induced by GGCT Dysregulation
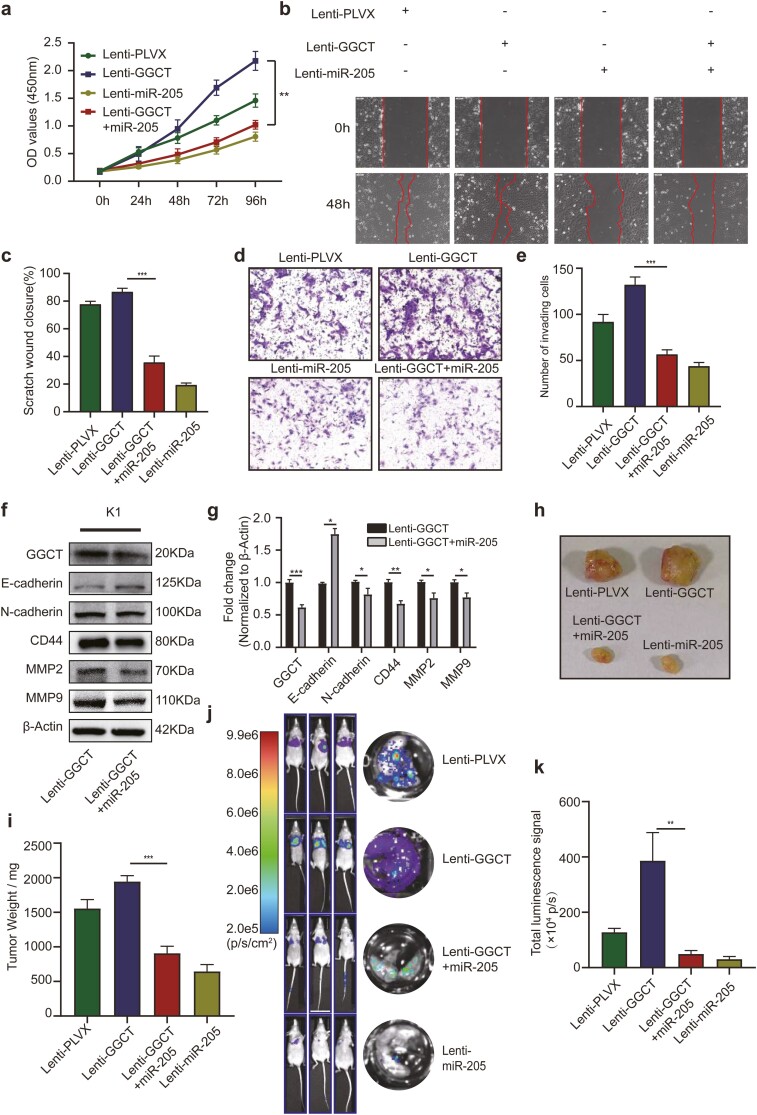
To corroborate that mir-205-5p reverses the GGCT-induced promotion of cell proliferation and invasion, pre-miR-205 was introduced into K1-GGCT cells by lentiviral overexpression. Overexpressing of miR-205 resulted in an attenuated proliferation ability of K1-GGCT cells (Fig. 5A). Also, upregulation of miR-205 in K1-GGCT cells leaded to the inhibition of migratory (Fig. 5B-5C) and invasive (Fig. 5D-5E) capacities, accompanying with increased expression of the epithelial markers and decreased mesenchymal markers (Fig. 5F). To further confirm if miR-205-5p eventually restores GGCT-mediated tumorigenicity and metastasis in vivo, K1-GGCT cells with or without miR-205 overexpression were administrated into immune-deficient nude mice. Introduction of miR-205 not only repressed tumor growth (Fig. 5G-5H) but diminished the metastatic lesions in the metastatic model (Fig. 5I-5J). Altogether, these findings suggested that miR-205-5p may have a role in suppression of the enhanced tumorigenicity and metastasis induced by GGCT, while the exact mechanism still requires more investigation.
Figure 5.
Induction of miR-205-5p attenuates GGCT-mediated PTC cell proliferation, migration, invasion, EMT process, tumorigenic ability, and distant metastatic potential to the lung. (a) Cell viability was examined by CCK8 after overexpression of miR-205-5p in K1-Control or K1-GGCT cell lines. Cell migration (b-c) and invasion (d-e) potentials were determined by wound healing and transwell assays. (f) Expression of epithelial marker (E-cadherin) and mesenchymal markers (N-cadherin, CD44, MMP2 and MMP9) was analyzed by Western blotting after miR-205-5p overexpression in K1-GGCT cells. (g) Quantification of WB experiments was normalized to β-Actin control. (h) Tumorigenic ability was assessed after miR-205-5p overexpression in K1-GGCT cells. (i) Dissected tumor weights in each group were depicted as dot plots. (j) Tumor lung metastasis was assayed after miR-205-5p overexpression in K1-GGCT cells. (k) The histogram denotes luciferase bioluminescence emitted from the lungs in each group 21 days post tumor injection. **: P < 0.01, ***: P < 0.001.
GGCT Interacts With CD44 and Inhibits the Degradation of CD44
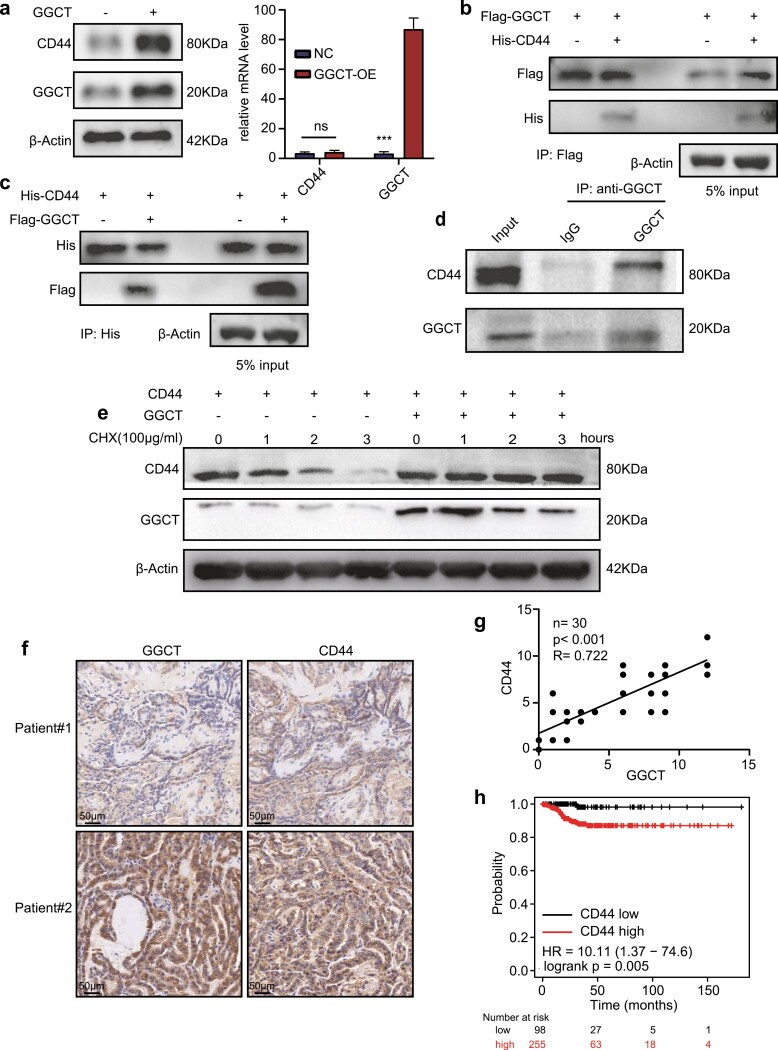
Considerable evidence indicates that the adhesion molecule CD44 plays a vital role in mediating chemoresistance, cell proliferation, and EMT (25). As the preceding data indicated that CD44 expression was positively influenced by GGCT, we were intrigued by which mechanism this phenomenon occurred. As depicted in Fig. 6A, overexpression of GGCT significantly upregulated the CD44 protein, but had no effect on the mRNA level of CD44. Subsequently, co-immunoprecipitation assays were conducted in HEK293T cell extracts. We co-expressed CD44-his with vector or GGCT-flag in HEK293T cells. We saw a co-precipitated CD44-his when GGCT-flag was pulled down (Fig. 6B). Also, the interaction was further substantiated by reciprocal co-immunoprecipitations (Fig. 6C). Consistently, by immunoprecipitating GGCT with anti-GGCT, CD44 protein was successfully precipitated, confirming the existence of an endogenous GGCT-CD44 complex in K1 cells (Fig. 6D). Subsequently, a cycloheximide (CHX) chase experiment was conducted to explore the function of GGCT on CD44 stability by blocking de novo protein biosynthesis. An obvious degradation of CD44 protein was observed in HEK293T cells transfected with vector plasmid, but not in cells transfected with GGCT-encoding plasmid (Fig. 6E). Furthermore, to evaluate the relationship of GGCT and CD44 expression, we carried out IHC analyses of human PTC simples, and observed that CD44 was positively correlated with GGCT protein levels (Fig. 6F-6G). Finally, an analysis of public databases demonstrated that high expression of CD44 was correlated with worse DFS of patients with PTC (Fig. 6H). Collectively, these data signified that GGCT positively regulates CD44 expression by inhibiting its protein degradation.
Figure 6.
GGCT interacts with and stabilizes CD44. (a) K1 cells were transfected with GGCT or control vectors. The western blot showed the protein level of GGCT and CD44 (left) and the RT-qPCR was conducted to measure the mRNA level of GGCT and CD44 (right). (b and c) Flag-GGCT or vector plasmids were co-expressed with His-CD44 or vector plasmids in HEK-293T cells. Cell lysates were immunoprecipitated with anti-Flag or anti-His antibodies, followed by immunoblotting. (d) The endogenous interaction of GGCT and CD44 in K1 cells was detected by Co-IP and western blot assays. (e) CD44 was co-expressed with vector or GGCT in K1 cells. After 24 hours, cells were challenged with CHX (100 μg/ml) for 0 to 3 hours, followed by western blot assays. (f) Representative images of GGCT and CD44 IHC staining in tumor sections from the same patient. (g) The figure shows the correlation between GGCT and CD44 IHC scores, with the Pearson correlation coefficients (R), p value as well as simple numbers (n) in the upper corner. (h) The disease-free survival (DFS) of CD44 expression in PTC. The analysis was conducted using the K-M Plotter online tool (http://kmplot.com/analysis/). ***: P < 0.001.
Discussion
In the present study, we identified a novel miR-205-5p/GGCT/CD44 signaling pathway, which plays a crucial functional role in PTC pathogenesis. A high level of GGCT was tightly correlated with that of more aggressive clinical characteristics and worse outcomes. GGCT depletion by RNA interference–mediated proliferation, migration, invasion, and EMT suppression of PTC cells in vitro, and reduced tumor growth and metastasis to lung in murine xenograft and tail vein metastatic models, suggesting that GGCT might function as a tumor facilitator. The mechanism of action linking GGCT and miR-205-5p lies in the directly binding to the 3′-UTR of GGCT, which subsequently leads to transcriptional repression of GGCT expression and counteracts the pro-oncogenic effect of this protein. In addition, we found that CD44, a non-kinase transmembrane glycoprotein, which is considered to play a key role in tumorigenesis and metastasis, could directly bind to GGCT protein and lead to inhibition of its degradation.
GGCT, (γ-glutamylcyclotransferase, 188 amino acids, 21 kDa), an enzyme participating in glutathione metabolism, has already been detected to be upregulated in various types of malignancies, including colorectal cancer (29), prostate cancer (30), ovarian cancer (12), and breast cancer (31). However, few studies on GGCT have been reported in thyroid cancer research. Intriguingly, a previous study based on bioinformatics showed a 7-gene panel including GGCT exhibited significantly superior accuracy in predicting relapse-free survival and the status of immunocyte infiltration in PTC (32). Nevertheless, it remained unknown if GGCT upregulation is simply a concomitant product of tumor progression or is selectively upregulated during cancer initiation and development, and thereby serves as an oncogenic accelerator. Our data show that the upregulation of GGCT is not only connected with unfavorable prognosis and clinicopathological characteristics, but significantly affects the biological behavior of PTC, suggesting a role of GGCT in PTC tumorigenesis and metastasis. Interestingly, a significant reduction in serum GGCT levels was observed in postoperative samples compared with preoperative samples, heralding a possible tendency for GGCT to function as a putative indicator of diagnosis for PTC. However, whether the serological alteration is PTC-specific or induced by surgical stress, and whether it is transient or permanent, remains unknown. Inclusion of more patients (including patients with benign thyroid nodules or nonthyroid malignancies) and longer period of observations with larger number of serum collection time points could be beneficial to address this. In addition, as we have shown in loss-of-function assays, GGCT knockdown reduced the proliferation, migration, and invasion capacities and blocked EMT process of PTC cells in vitro. Further in vivo experiments strengthened evidence that GGCT deficiency drastically inhibited tumor growth and experimental metastases. These findings highlight an indispensable role of GGCT in PTC tumorigenesis and metastasis.
Despite these promising findings, the possible mechanism as well as the upstream and downstream signaling of GGCT remain ill-defined. As previously noted in a series of reports, besides the classic oncogenes like BRAF or RET, dysregulation of miRs is implicated in the tumorigenicity and progression of PTC (21). Among them, miR-205-5p has been proposed to exert tumor suppressor functions via repression of the downstream target gene expression, such as YAP1 (33), VEGFA (22), and CCNB2 (34). In this study, we revealed GGCT is a novel target gene of miR-205-5p through the dual-luciferase reporter and RNA-RNA pull-down assays. Downregulation of miR-205-5p could partly explain the increased expression of GGCT in PTC tissues. More importantly, treatment with miR-205-5p by lentiviral strategy led to decreased expression of GGCT and reverted the malignant phenotypes mediated by GGCT. Notably, despite the expression of GGCT decreased after miR-205-5p introduction, the magnitude of that was much less pronounced than the direct interference by sh-lentivirus, inferring the existence of additional regulatory mechanisms such as competitive endogenous RNA (ceRNA) molecules between miR-205-5p and GGCT. This may also explain why overexpression of miR-205-5p alone does not effectively prevent the formation of lung metastases in experimental metastatic models as direct GGCT knockdown does. More studies are urgently needed to elucidate the regulating networks between miR-205-5p and GGCT. Furthermore, since a single miR member may bind to multiple target genes simultaneously and a single mRNA can be regulated by multiple miRs, whether the introduction of miR-205-5p will impede the expression of other tumor suppressor genes and whether more specific GGCT-binding miRs exist require further research.
To further understanding the downstream mechanisms underlying GGCT-mediated pro-proliferative and pro-metastatic phenotypes, we focused then on an EMT-specific marker—CD44—whose expression was altered concomitant with GGCT. CD44 is a cell adhesion glycoprotein that plays a role in cancer progression and metastasis and has been widely utilized as a cancer stem cell (CSC) marker in thyroid cancer (35) and various other types of malignancies (36-38). In this study, we employed exogenous and endogenous co-IP assays and found that GGCT could bind to CD44. Further CHX chase assay demonstrated that GGCT plays a role in stabilizing CD44 to prevent its degradation. Clinically, the protein levels of GGCT were positively related to CD44 expression, demonstrating a vital GGCT-CD44 signaling axis in tumorigenesis and metastasis in PTC. Yet, exactly how GGCT binds to CD44 and affects its protein stability remains unclear. We can currently only speculate that it may be attributed to the catalytic glutaminyl cyclization function of GGCT (9). GGCT was functionally similar to glutaminyl cyclase (QC, glutaminyl-peptide cyclotransferase [QPCT]) and its iso-enzyme iso-QC (also known as QPCTL), which belong to an enzyme family that catalyze the formation of pyroglutamate (pE-) at protein’s N-terminus by converting glutamate/glutamine into pE-peptides (39). The pE-residue confers stability against N-terminal degradation by proteases (40, 41). Interestingly, a previous study by Astrid et al found that the N-terminus of CCL2, a major chemokine involved in recruiting myeloid derived mesenchymal cells (MDSCs) to the tumor microenvironment, can be modified to a pE-residue by both QC and iso-QC in thyroid cancer (42). The pE-modification confers the resistance to proteolytic degradation of CCL2, subsequently affects tumor tumorigenesis by modulating cancer cells per se and their microenvironment niche (42). By analyzing the protein sequence of CD44, a conserved glutamine at the N-terminus of CD44 (amino acid position: 21) just following the signal peptide was found, as analogous to other pE-modified molecules, suggesting the possibility of the pE-modification of CD44. Further research with the aid of pE-modified-specific antibodies or mass spectrometry should be conducted to confirm our conjecture.
Conclusions
In summary, we reveal a novel signaling axis, miR-205-5p/GGCT/CD44, that is involved in the carcinogenesis and progression of PTC. This is the first attempt to uncover the biological function of GGCT and the mechanisms behind this protein in PTC. Usage of miR-205-mimics or GGCT inhibitors as potential therapeutics for PTC may hold great promise for clinical applications.
Glossary
Abbreviations
- CCK8
cell counting kit-8
- CHX
cycloheximide
- Co-IP
co-immunoprecipitation
- DFS
disease-free survival
- ELISA
enzyme-linked immunosorbent assay
- EMT
epithelial-mesenchymal transition
- FBS
fetal bovine serum
- fLuc
firefly-luciferase
- GGCT
γ-glutamylcyclotransferase
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- iso-QC
iso-glutaminyl cyclase
- miR
microRNA
- MUT
mutated
- pE-
pyroglutamate
- PTC
papillary thyroid cancer
- QC
glutaminyl cyclase
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- ROS
reactive oxygen species
- RPMI
Roswell Park Memorial Institute medium
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- shRNA
short hairpin RNA
- TBST
Tris-buffered saline with Tween-20
- UTR
untranslated region
- VEGFA
vascular endothelial growth factor A
- WT
wild-type
Financial Support
Y.D. is supported by National Natural Science Foundation of China (No.81802676). X.R.L. is supported by Wuhan Youth Cadre Project (2017zqnlxr01 and 2017zqnlxr02), Clinical Research Physician Program of Tongji Medical College, Huazhong University of Science and Technology (HUST) (5001540018) and Natural Science Foundation of Hubei Province of China (H2015038). X.H.L. is supported by Educational Commission of Hubei (WJ2019M124), Hubei Province Health and Family Planning Scientific Research Project (WJ2021Q051), Frontier project of applied basic research in Wuhan (2020020601012250).
Author Contributions
Y.D. and X.H.L. contributed to the conception of the study. H.L. and H.Z. performed the experiment. J.W. and G.W. contributed significantly to data analysis. S.L., T.X., M.D., X.Y., Y.W., and X.C. contributed to data collection and assistance in the performing of the experiment. H.L., H.Z., and X.H.L. wrote the manuscript. Y.D., X.R.L., and X.H.L. contributed to the supervision of the research process and provided research funding support.
Disclosures
The authors declare that there is no conflict of interest.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Laetitia G, Sven S, Fabrice J. Combinatorial therapies in thyroid cancer: an overview of preclinical and clinical progresses. Cells. 2020;9(4):830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid. 2016;26(11):1541-1552. [DOI] [PubMed] [Google Scholar]
- 3. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783-2795. [DOI] [PubMed] [Google Scholar]
- 4. Capdevila J, Galofré JC, Grande E, et al. Consensus on the management of advanced radioactive iodine-refractory differentiated thyroid cancer on behalf of the Spanish Society of Endocrinology Thyroid Cancer Working Group (GTSEEN) and Spanish Rare Cancer Working Group (GETHI). Clin Transl Oncol. 2017;19(3):279-287. [DOI] [PubMed] [Google Scholar]
- 5. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelly LM, Barila G, Liu P, et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci USA. 2014;111(11):4233-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. 2016;23(3):R143-R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martínez-Aguilar J, Clifton-Bligh R, Molloy MP. Proteomics of thyroid tumours provides new insights into their molecular composition and changes associated with malignancy. Sci Rep. 2016;6:23660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oakley AJ, Yamada T, Liu D, Coggan M, Clark AG, Board PG. The identification and structural characterization of C7orf24 as gamma-glutamyl cyclotransferase. An essential enzyme in the gamma-glutamyl cycle. J Biol Chem. 2008;283(32):22031-22042. [DOI] [PubMed] [Google Scholar]
- 10. Kageyama S, Iwaki H, Inoue H, et al. A novel tumor-related protein, C7orf24, identified by proteome differential display of bladder urothelial carcinoma. Proteomics Clin Appl. 2007;1(2):192-199. [DOI] [PubMed] [Google Scholar]
- 11. Taniguchi K, Matsumura K, Ii H, et al. Depletion of gamma-glutamylcyclotransferase in cancer cells induces autophagy followed by cellular senescence. Am J Cancer Res. 2018;8(4):650-661. [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Wu T, Wang Y, et al. γ-Glutamyl cyclotransferase contributes to tumor progression in high grade serous ovarian cancer by regulating epithelial-mesenchymal transition via activating PI3K/AKT/mTOR pathway. Gynecol Oncol. 2018;149(1):163-172. [DOI] [PubMed] [Google Scholar]
- 13. Jiang Z, Zhang C, Gan L, et al. iTRAQ-based quantitative proteomics approach identifies novel diagnostic biomarkers that were essential for glutamine metabolism and redox homeostasis for gastric cancer. Proteomics Clin Appl. 2019;13(4):e1800038. [DOI] [PubMed] [Google Scholar]
- 14. Kageyama S, Ii H, Taniguchi K, et al. Mechanisms of tumor growth inhibition by depletion of γ-glutamylcyclotransferase (GGCT): a novel molecular target for anticancer therapy. Int J Mol Sci. 2018;19(7):2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Taniguchi K, Kageyama S, Moyama C, et al. γ-Glutamylcyclotransferase, a novel regulator of HIF-1α expression, triggers aerobic glycolysis. Cancer Gene Ther. 2022;29:37-48. doi: 10.1038/s41417-020-00287-0 [DOI] [PubMed] [Google Scholar]
- 16. He Z, Wang S, Shao Y, et al. Ras downstream effector GGCT alleviates oncogenic stress. iScience. 2019;19:256-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vosgha H, Ariana A, Smith RA, Lam AK. miR-205 targets angiogenesis and EMT concurrently in anaplastic thyroid carcinoma. Endocr Relat Cancer. 2018;25(3):323-337. [DOI] [PubMed] [Google Scholar]
- 18. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522-531. [DOI] [PubMed] [Google Scholar]
- 19. Hu X, Wang Y, Liang H, et al. miR-23a/b promote tumor growth and suppress apoptosis by targeting PDCD4 in gastric cancer. Cell Death & Dis. 2017;8(10):e3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao J, Li L, Yang T. MiR-216a-3p suppresses the proliferation and invasion of cervical cancer through downregulation of ACTL6A-mediated YAP signaling. J Cell Physiol. 2020;235(12): 9718-9728. [DOI] [PubMed] [Google Scholar]
- 21. Mastronikolis N, Tsiambas E, Roukas D, et al. Micro-RNAs signatures in papillary thyroid carcinoma. J BUON. 2020;25(5):2144-2146. [PubMed] [Google Scholar]
- 22. Salajegheh A, Vosgha H, Md Rahman A, Amin M, Smith RA, Lam AK. Modulatory role of miR-205 in angiogenesis and progression of thyroid cancer. J Mol Endocrinol. 2015;55(3):183-196. [DOI] [PubMed] [Google Scholar]
- 23. Yang J, Huang Y, Dong B, Dai Y. Long noncoding RNA DLEU2 drives the malignant behaviors of thyroid cancer through mediating the miR-205-5p/TNFAIP8 axis. Endocr Connect. 2021;10(4):471-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou J, Cao L, Chen Z. Differentiation of benign thyroid nodules from malignant thyroid nodules through miR-205-5p and thyroid-stimulating hormone receptor mRNA. Hormones. 2021;20(3):571-580. [DOI] [PubMed] [Google Scholar]
- 25. Chen C, Zhao S, Karnad A, Freeman JW. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol. 2018;11(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li H. Supplemental materials for “MiR-205-5p/GGCT attenuates growth and metastasis of papillary thyroid cancer by regulating CD44”. Figshare. Posted February 16, 2022. 10.6084/m9.figshare.17261258.v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212-226. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi RU, Prieto-Vila M, Kohama I, Ochiya T. Development of miRNA-based therapeutic approaches for cancer patients. Cancer Sci. 2019;110(4):1140-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang Q, Zhou Y, Li Y, Liao Z. GGCT promotes colorectal cancer migration and invasion via epithelial-mesenchymal transition. Oncol Lett. 2020;20(2):1063-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takagi H, Ii H, Kageyama S, et al. Blockade of γ-glutamylcyclotransferase enhances docetaxel growth inhibition of prostate cancer cells. Anticancer Res. 2019;39(9):4811-4816. [DOI] [PubMed] [Google Scholar]
- 31. Matsumura K, Nakata S, Taniguchi K, et al. Depletion of γ-glutamylcyclotransferase inhibits breast cancer cell growth via cellular senescence induction mediated by CDK inhibitor upregulation. BMC Cancer. 2016;16(1):748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang L, Wang Y, Li X, et al. Identification of a recurrence signature and validation of cell infiltration level of thyroid cancer microenvironment. Front Endocrinol. 2020;11:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li D, Wang Q, Li N, Zhang S. miR-205 targets YAP1 and inhibits proliferation and invasion in thyroid cancer cells. Mol Med Rep. 2018;18(2):1674-1681. [DOI] [PubMed] [Google Scholar]
- 34. Wang X, Zhang H, Jiao K, et al. Effect of miR-205 on proliferation and migration of thyroid cancer cells by targeting CCNB2 and the mechanism. Oncol Lett. 2020;19(3):2568-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Falco V, Tamburrino A, Ventre S, et al. CD44 proteolysis increases CREB phosphorylation and sustains proliferation of thyroid cancer cells. Cancer Res. 2012;72(6):1449-1458. [DOI] [PubMed] [Google Scholar]
- 36. Gomez KE, Wu F, Keysar SB, et al. Cancer cell CD44 mediates macrophage/monocyte-driven regulation of head and neck cancer stem cells. Cancer Res. 2020;80(19):4185-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martincuks A, Li PC, Zhao Q, et al. CD44 in ovarian cancer progression and therapy resistance-a critical role for STAT3. Front Oncol. 2020;10:589601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ding R, Li G, Yao Y, et al. Transgelin-2 interacts with CD44 to regulate Notch1 signaling pathway and participates in colorectal cancer proliferation and migration. J Physiol Biochem. 2021. doi: 10.1007/s13105-021-00843-8 [DOI] [PubMed] [Google Scholar]
- 39. Stephan A, Wermann M, von Bohlen A, et al. Mammalian glutaminyl cyclases and their isoenzymes have identical enzymatic characteristics. FEBS J. 2009;276(22):6522-6536. [DOI] [PubMed] [Google Scholar]
- 40. Logtenberg MEW, Jansen JHM, Raaben M, et al. Glutaminyl cyclase is an enzymatic modifier of the CD47- SIRPα axis and a target for cancer immunotherapy. Nat Med. 2019;25(4):612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu Z, Weng L, Zhang T, et al. Identification of glutaminyl cyclase isoenzyme isoQC as a regulator of SIRPα-CD47 axis. Cell Res. 2019;29(6):502-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kehlen A, Haegele M, Menge K, et al. Role of glutaminyl cyclases in thyroid carcinomas. Endocr Relat Cancer. 2013;20(1): 79-90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.