Abstract
The long-term in vivo cytogenetic effects of high-dose radiation exposure can be traced in accidentally irradiated persons, and particularly useful for developing strategies of monitoring and therapy of such patients, as well as for elucidating the fundamental aspects of hematopoiesis and radiobiology. Using 24-color fluorescent in situ hybridization (mFISH), we analysed the frequency and the spectrum of chromosomal aberrations (CA) in peripheral blood lymphocytes of the Chernobyl Nuclear Power Plant (NPP) accident victim 30, 31, 32 and 33 years after acute accidental exposure to high-dose gamma radiation of the whole body. Totally, 993 metaphase cells were analyzed (or 219, 272, 258, 244 cells each year), of which 297 were aberrant. Our study demonstrated a constant aberrant cell frequency at 28% in 2016–2018 years, while in 2019, a significant increase up to 35% occurred due to contribution of significantly elevated frequency of simple aberrations in the absence of evident recent genotoxic factors. Four clonal aberrations were detected, three of which persisted for more than one year at a frequency up to 2.5% of analyzed cells. The distribution of 731 breakpoints per individual chromosomes was nearly proportional to their physical length, excepting Chromosomes 13 and 20, which were significantly breakpoint-deficient compared to the genome median rate. Monitoring of the long-term effects on chromosomal instability caused by radiation exposure is important for understanding and predicting the long-term effects of ionizing radiation.
Keywords: chromosomal aberrations (CA), mFISH, exposure, Chernobyl Nuclear Power Plant (NPP) accident
INTRODUCTION
Cytogenetic analysis of chromosomal damage in peripheral blood lymphocytes is widely used for epidemiological molecular examination of irradiated people [1]. Radiation exposure causes both mutagenic and clastogenic effects, and can lead to DNA breakage cytogenetically manifesting as stable and unstable chromosomal aberrations (CA) [2]. Stable CAs present a sensitive, dose-dependent and long-lived biomarker of radiation exposure [3, 4]. Twenty-four-color fluorescent in situ hybridization (mFISH) or spectral karyotyping (SKY) can provide pan-chromosome detection of rearrangements and differentiation of complex and simple exchange events [5, 6, 7]. Accumulation of SKY and mFISH analyses of in vivo irradiated patients (accidentally, occupationally or therapeutically) may contribute to the establishment of common scoring and interpretation criteria of whole-genome aberration patterns, as well as the estimation of the in vivo effects on the dose reconstruction models, including immunogenicity, selective/mitotic advantage of some aberrations, elimination of stable translocations co-occurring with dicentric chromosomes in the same cells or clonal lymphocyte expansion under immune response [8, 9]. The mFISH analysis of peripheral blood lymphocytes revealed significant increase in frequencies of chromosomal abnormalities in the groups of experienced exposure to α- and γ- ionizing radiation [10, 11, 12, 13] and in patients after radiation therapy [14, 15]. Whole-genome profiles of chromosome aberrations in their peripheral blood lymphocytes were suitable for estimation of absorbed doses and quality of radiation even over 40 years after exposure [10, 11, 12].
One of the intriguing issues is how the human organism deals with the high percentage of rearranged cells persistent in hematopoietic tissue and circulation after irradiation. There is strong evidence of an elevated lifetime risk of hemato oncological diseases among those exposed to high-dose radiation, presumably conferred by induction of cancer-driving rearrangements [16]. Genetically altered cells can be destroyed by immune surveillance: a close correlation between cell killing and the induction of double-strand DNA breaks and CA has been reported [17, 18]. Studies on molecular karyotyping of lymphocytes in patients exposed to high-dose radiation are limited in the literature due to the relatively high cost and complexity of mFISH for routine monitoring and the rarity of accidental exposures. Evaluation of individual radiosensitivity of a person is among the most complex and vital tasks of radiomedicine. The use of mFISH can be particularly reasonable and productive in cases of high-dose irradiation with increased frequency and complexity of aberrations cryptic for routine chromosome painting. Long-term monitoring of chromosomal abnormalities in peripheral blood lymphocytes in response to uniform radiation exposure is of great interest, both from a practical and theoretical point of view.
The adherence of Chernobyl Nuclear Power Plant (NPP) accident victims to regular follow-up is low, especially decades after the accident; the age of these persons gets closer to their life expectancy. Therefore, even a single patient from this group monitored recurrently for several years provides a unique and valuable opportunity for radiobiological research. Our study enrolled one patient affected by external relatively uniform exposure to gamma-beta irradiation, application and incorporation of radionuclides, who suffered in 1986 acute radiation disease with the II-degree bone marrow syndrome. Currently, post-radiation injuries are compensated, the patient’s condition is satisfactory, with minor age-related complaints. In 2019 basal cell carcinoma was first diagnosed and excised. The aim of the study was to identify aberrant metaphases using the mFISH molecular karyotyping method and to describe the frequency and spectrum of CAs in peripheral blood lymphocytes of the patient 30, 31, 32 and 33 years after acute accidental exposure to high-dose gamma radiation of the whole body.
MATERIALS AND METHODS
Patient history
Patient Y, born in 1960, was the operator of the central hall of block 4 of the Chernobyl NPP. He worked at the NPP since 1983. During the accident (26 April 1986), he was in the utility room behind a thin partition 20 m from the reactor. The room was filled with steam, and he left the destroyed block in 20 minutes. According to the cytogenetic study, the average whole-body radiation dose was 3.2 Gy. After treatment in a specialized clinic, the patient was diagnosed with the following: acute radiation syndrome of III degree of severity from predominantly external relatively uniform gamma-beta-irradiation, application and incorporation of radionuclides (iodine-131 – 65 MBq), bone marrow syndrome of II degree, oropharyngeal syndrome II degree, local radiation lesions I, II and III degrees of severity of 8%, 20% and 5% of the surface of the skin, respectively (chest, forearms, abdomen, right thigh and both legs). In the long term after radiation damage the patient was subjected to multiple clinical examinations. As a consequence of local radiation injury, the distal phalanx of the first finger of the right foot was amputated in 1988. In the period of long-term effects, moderate transient leukopenia was noted, radiation cataract II degree of both eyes, hypertension, compensated subcortical encephalopathy and chronic gastritis in remission were diagnosed. In 2001, he was operated on for a leiomyoma of the lower section of esophagus (non-malignant tumor unrelated to radiation). Malignant oncological diseases were not detected until 2019, chemotherapy was not performed. In 2019, six tumors were found and excised on the skin of the back, cytologically classified as basal cell carcinoma. The total blood counts in 2019 were nearly normal: leukocytes—4.2 × 109/L, neutrophils—1.5 × 109/L, lymphocytes—2.1 × 109/L, monocytes—0.4 × 109/L, erythrocytes—4.86 × 1012/L, platelets—226 × 109/L. There was a slight increase in the level of gamma-glutamine transferase (65, reference 10–60 U/L), with normal values of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, as well as slightly increased levels of triglycerides (2.76, reference 0.4–1.7 mmol/L), cholesterol (6.47, reference 3.9–5.2 mmol/L) and glucose (6.17, reference 3.9–6.05 mmol/l). The C-reactive protein level was within the reference values. The patient does not smoke and denies other bad habits. Constantly taken medications include statins (Atorvastatinum) and antiplatelet agents (Cardiomagnyl). According to the patient, there were no X-ray, CT or MRI examinations, as well as acute viral infections at least 3 months before the cytogenetic tests in 2016–2019. The irradiation was a single one in 1986, after that he had no contact with sources of ionizing radiation.
Lymphocyte culture and metaphase chromosome preparations
The leukocyte fraction of the blood was added to RPMI1640 medium (Glutamax, Gibco) supplemented with 2% phytohaemagglutinin (PHA) and antibiotics, cultured for 48 h with the addition of colchicine (0.05 mg/ml) two hours before the fixation start. Cells were treated with a hypotonic solution (0.56% KCl) for 10 min at 37°C and fixed in three changes of ice-cold acetic acid: methanol (1:3) mixture. An aliquot of fixed cells was dropped on the glass sides and air-dried.
Multicolor fluorescence in situ hybridization
Molecular karyotyping was performed using the mFISH 24-colour chromosome painting kit (‘24XCyte,’ MetaSystems, Germany) according to the manufacturer’s instructions. The slides were denatured in NaOH solution and hybridized with the ‘24XCyte probe’ for 24–48 h, followed by the washing in 72°C 0.4-fold SSC and room temperature 2-fold SSC-Tween 20. Slides were counterstained with DAPI/antifade (MetaSystems, Germany). Signal detection and subsequent metaphase analysis was performed using the ‘ISIS software’ (MetaSystems) based on unique chromosome-specific processed colors generated. Chromosome aberrations were described according to the International System of Human Cytogenetic Nomenclature [19], identical structural changes in at least two metaphases were considered as clonal abnormalities. Chromosomal bands were defined using inverted DAPI-staining of mFISH-painted chromosomes by the ‘ISIS software’ (Metasystems, GmbH, Germany).
Comet assay
Comet assay was carried out under alkaline and neutral conditions. Whole blood was mixed with 0.8% low-melting agarose, dropped on slides pre-coated with 1% normal-melting agarose and slides were sealed with coverslips. Then slides were immersed for 1 h in ice-cold lysis solution. For the alkaline assay, electrophoresis was performed for 20 min at electric field strength of 1 V/cm and a current of ~300 mA; for the neutral version of assay, slides underwent electrophoresis in for 10 minutes at 1 V/cm and current of 12 mA. Then slides were washed in PBS, immersed in 70% ethanol for 15 min and air-dried. Slides were stained with SYBR Green I, DNA-comets were visualized on an epifluorescent microscope (LOMO, Russia). The mean and median percentage of DNA in the tail (% DNA in tail) of 300 individual DNA-comets was determined using the CASP v.1.2.2 analysis software [20]. DNA comets with a small or existent head and a wide diffuse tail (‘hedgehogs’) were excluded from the total analysis and counted separately as percentage on 500 randomly selected nucleoids.
Statistical analysis
We performed Fisher’s exact test (Fisher’s φ-criterion) to compare aberrant cells and CAs in different years of the study. To compare chromosome mean frequencies of breakpoints per 1 Mb, t-test was used, with Bonferroni correction for multiple comparisons. All data were analyzed using Statistica software, version 7 (StatSoft, USA). For all analyses, P < 0.05 was considered statistically significant.
RESULTS
Chromosomal aberrations
Acute accidental whole-body exposure of the patient to gamma-irradiation of 3.2 Gy occurred in 1986. We examined chromosome rearrangements in peripheral blood lymphocytes 30, 31, 32 and 33 years after the accident using mFISH. A total of 993 mFISH-painted metaphase cells were analyzed, or 219, 272, 258 and 244 cells each year, respectively (Table 1). The frequency of aberrant cells was between 27–29% in 2016–2018, and significantly increased to 35% in 2019 (P < 0.05). Aberrant metaphase cells were divided into two groups according to the structure of CA, simple or complex (Figs 1–2). To simple CAs included chromatide and chromosome breaks, deletions and reciprocal translocations with one break at each chromosome, for example, chtb(6)(p21), chrb(14)(q23), del(10)(p11), t(11;17)(p15;q21). Aberrant metaphases carrying CA that involved two or more chromosomes or formed through at least three chromosomal breaks, were classified as complex ones, for example, exchanges between several chromosomes t(2;7;20)(p21q32;p12;q13.3), insertions ins(13;14)(q13;q12q32). Also cells carrying more than one simple or complex CA per genome, were attributed to the complex group, for example, 46,XY,t(1;5)(p32;p15),t(9;11)(q22;p11.2), 46,XY,t(3;11)(p22;p12),t(4;5;8;10)(q35;p13;p12;q24),dic(5;17)(p13;p13q21), 46,XY,inv(1)(q12p26),t(14;15)(p11.2;q11.2). The frequency of metaphases with simple CAs ranged from 19% to 27% in different years (Table 1). In 2018, the simple aberrant cell frequency was significantly lower than in 2017 and 2019, but not in 2016. Similarly to the total aberrant cell counts, the proportion of simply rearranged metaphases in 2019 was significantly increased compared to previous years (2016–2018). Frequencies of cells with complex aberrations were stable during four years of examinations, with statistically insignificant decline in 2017, it were 7.76, 5.15, 8.14 and 7.79 % in 2016, 2017, 2018 and 2019, accordingly.
Table 1.
Frequencies of aberrant metaphases with simple and complex aberrations
| Year | Metaphases | AM | % | AM with simple aberrations |
% | AM with complex aberrations | % |
|---|---|---|---|---|---|---|---|
| 2016 | 219 | 61 | 27.85 ± 3,03 | 44 | 20.09 ± 2.71 | 17 | 7.76 ± 1.81 |
| 2017 | 272 | 79 | 29.04 ± 2,75 | 65 | 23.90 ± 2.59 | 14 | 5.15 ± 1.34 |
| 2018 | 258 | 71 | 27.52 ± 2,78 | 50 | 19.38 ± 2.46** | 21 | 8.14 ± 1.7 |
| 2019 | 244 | 86 | 35.25 ± 3,06* | 67 | 27.46 ± 2.86* | 19 | 7.79 ± 1.72 |
AM - aberrant metaphases
*- statistically significant difference with 2016–2018, P < 0.05
**- statistically significant difference with 2017 and 2019, P < 0.05
Fig. 1.
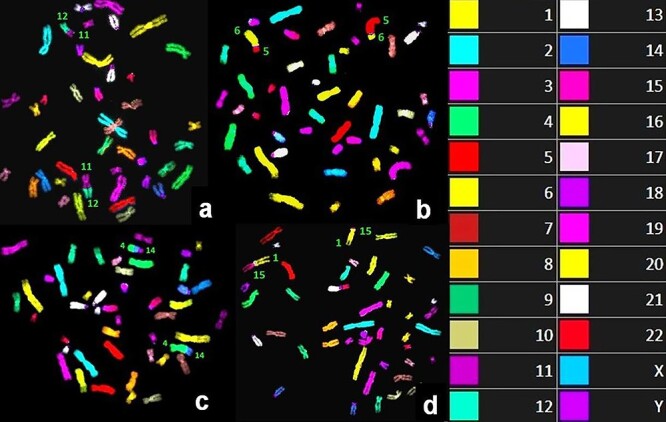
Example mFISH-painted metaphases with simple CA; chromosomes participating in rearrangements are indicated with respective chromosome numbers. Notice balanced chromosomal translocations between chromosomes 11 and 12 (a); 5 and 6 (b); 4 and 14 (c); 1 and 15 (d). The right box indicates the color code of mFISH-painted chromosomes.
Fig. 2.
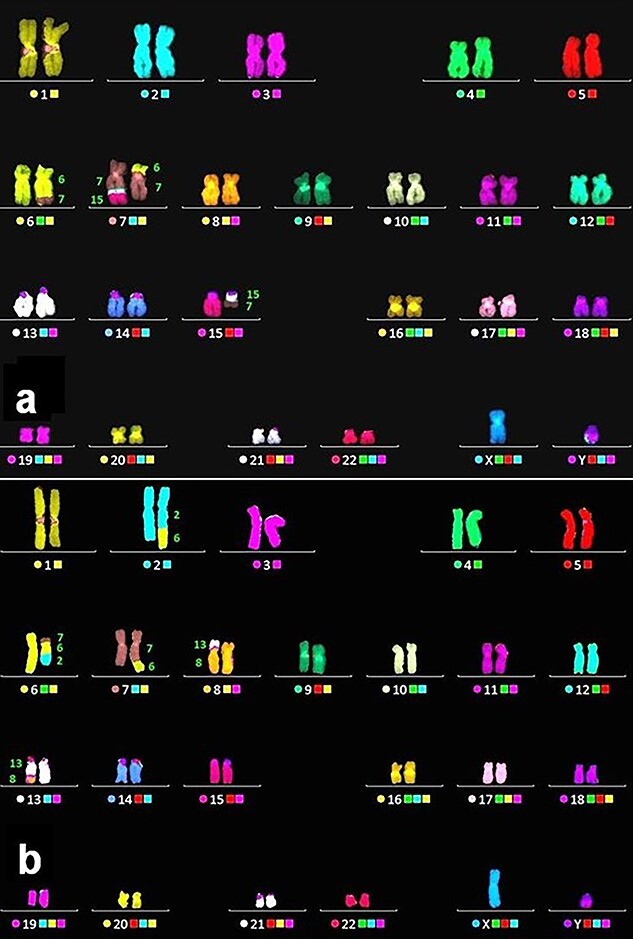
Representative mFISH karyograms with complex CA; components of marker chromosomes are indicated with respective numbers. Two chromosomal translocations with homologues of chromosome 7 (a): between chromosomes 6–7 and 7–15; two chromosomal translocations (b): between three chromosomes (2–6 - 7) and two chromosomes (8–13).
When the total number of CA detected in all metaphases was analysed, the same significant increase in 2019 was revealed compared to 2016–2018 (Table 2). These CA included chromatid and chromosome fragments, large deletions, simple and complex translocations, insertions, inversions and dicentric chromosomes. Translocations were the main contributors to the overall level of CA, constituting approximately 86–91% of all detected CA in different years. With a few unitary exceptions, all identified translocations were reciprocal. The frequencies of other type aberrations accounted for: 1–7% deletions and chromosome breaks, 1–5% chromatid breaks, 1–2% dicentrics, 1–3% insertions, 0–2% inversions of all CA. Chromatid type exchanges, such as inter(intra)chromatid interchromosome exchanges (e.g. tetraradial), were not detected. The total frequencies of all translocations identified in 2016, 2017, 2018 and 2019 were 31.96 ± 3.20%, 31.25 ± 2.81%, 31.78 ± 2.90% and 36.89 ± 3.09%, respectively, with a statistically significant increase in 2019 compared to other years. Fluctuations in the frequencies of other rearrangement types (chromatid and chromosomal fragments, deletions, insertions, inversions and dicentrics) were statistically insignificant.
Table 2.
Frequencies of different type of chromosome aberrations
| Year | All cells | All CAs |
Cht | Chr and Del | Tr | Ins | Inv | Dic | |
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 219 | n % |
79 36.07 ± 3.24 |
4 1.83 ± 0.9 |
1 0.46 ± 0.46 |
70 31.96 ± 3.15 |
2 0.91 ± 0.64 |
1 0.46 ± 0.46 |
1 0.46 ± 0.46 |
| 2017 | 272 | n % |
93 34.19 ± 2.88 |
1 0.37 ± 0.37 |
4 1.47 ± 0.73 |
85 31.25 ± 2.81 |
1 0.37 ± 0.37 |
0 0 |
2 0.74 ± 0.52 |
| 2018 | 258 | n % |
95 36.82 ± 3.00 |
1 0.39 ± 0.39 |
7 2.71 ± 1.01 |
82 31.78 ± 2.90 |
3 1.16 ± 0.67 |
1 0.39 ± 0.39 |
1 0.39 ± 0.39 |
| 2019 | 244 | n % |
101 41.39 ± 3.15* |
1 0.41 ± 0.41 |
5 2.05 ± 0.91 |
90 36.89 ± 3.09* |
1 0,41 ± 0.41 |
2 0.82 ± 0.58 |
2 0.82 ± 0.58 |
All cells—all cells analyzed, All CAs—all chromosomal aberrations, Cht—chromatid breaks, Chr and Del—chromosome breaks and deletions, Tr—translocations, Ins—insertion, Inv—inversion, Dic—dicentric chromosome. n—number of cells, %—frequency of CAs. *statistically significant differences with 2016–2018, Fisher’s φ-criterion, P < 0.05.
Aberrant clones
According to ISCN criterion [19], four aberrant cell clones were identified, each carrying one balanced translocation. In one of them, an additional pericentric inversion of Chromosome 3 was observed. The largest clone with t(4;8) (Fig. 3a) persisted throughout the four years of investigations with the frequency of up to 2.3%. The clones with inv(3) and t(14;15) (Fig. 3b) and with t(2;2) (Fig. 3c) were detectable only in two different years; clone with t(1;15)—only in 2019 (Fig. 3d, Table 3).
Fig. 3.
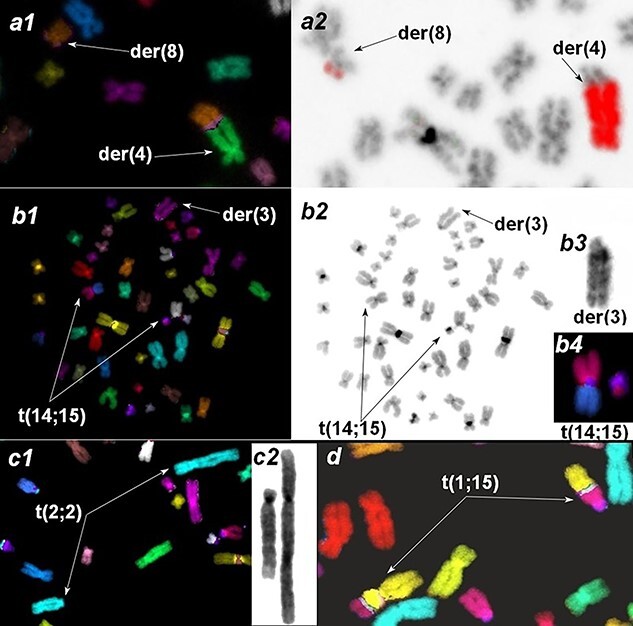
Clonal aberrations in peripheral blood lymphocytes. Derivative chromosomes are indicated with arrows. a. translocation between chromosomes 4 and 8: a1—the fragment of mFISH-painted metaphase, a2—verification of the translocation using whole-chromosome 4 painting probe. b. Clone with two CAs, translocation of Chromosomes 14 and 15 and inversion of Chromosome 3. b1: mFISH painted metaphase. b2. Inverted DAPI image of a metaphase. b3. Inverted DAPI staining of der3. b4. Translocation of Chromosomes 14 and 15 painted with mFISH probe. c. Translocation between two Chromosome 2 homologs. c1. mFISH metaphase fragment. c2. inverted DAPI image of Chromosome 2 derivates. d. Fragment of mFISH-painted metaphase with clonal translocation of Chromosomes 1 and 15.
Table 3.
Karyotype and frequency of aberrant clones in different years
| Karyotype of the aberrant clones | Number of clonal cells (M ± m, %) | |||
|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | |
| 46,XY,t(4;8)(q35;q12) | 2.24 ± 1.01 | 0.37 ± 0.37 | 1.53 ± 0.77 | 1.22 ± 0.71 |
| 46,XY,inv(3)(p26q12),t(14;15)(p11.2;q11.2) | 1.36 ± 0.64 | 0 | 0.77 ± 0.55 | 0 |
| 46,XY,t(2;2)(p13;q37) | 0.91 ± 0.64 | 0.37 ± 0.37 | 0 | 0 |
| 46,XY,t(1;15)(q21;q22) | 0 | 0 | 0 | 0.82 ± 0.58 |
Chromosome breakage rates and breakpoint distribution
MFISH analysis allowed us to identify a total of 731 breakpoints of CA with inverted DAPI-banding resolution, affecting each of the chromosomes. To assess breakpoint distribution between individual chromosomes, the mean frequencies of breakpoints per 1 Mb per 100 cells were calculated (Fig. 4). These were found to meet the criteria of normal distribution, ranging from 0.0066 (Chromosome 13) to 0.01725 (Chromosome 17), with the genome mean of 0.0125 ± 0.0005. The chromosome 13 and 20 values were beyond two standard deviations from the genome mean and fall outside 95% of the basic data, therefore, these events could be considered belonging to another general population. When genome mean frequencies (G, Fig. 4) were compared with specific values for chromosomes 13 and 20 using the t-test, significant difference was found (P < 0.0001), even after adjusting for multiple comparisons.
Fig. 4.
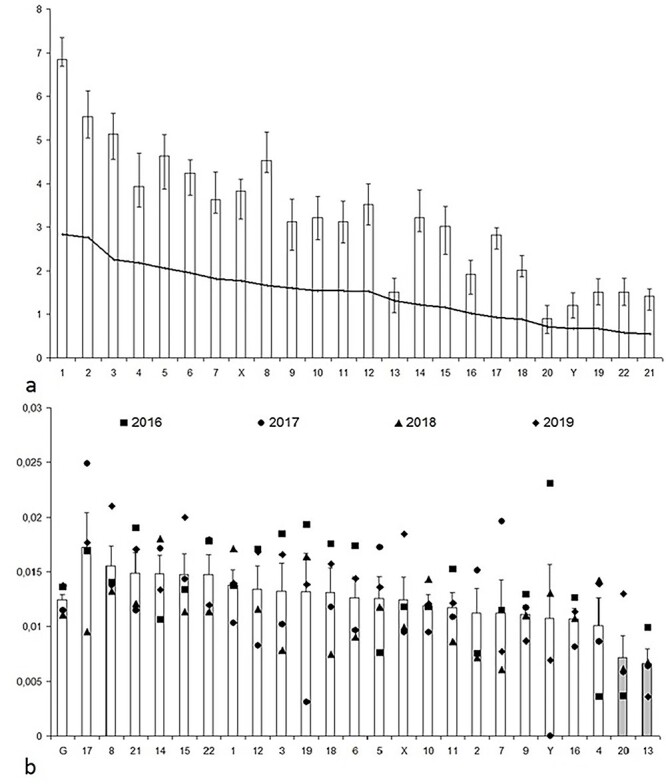
Distribution of the mean frequencies of aberration breakpoints on individual chromosomes per 1 Mb per 100 cells. Columns and vertical bars—means and standard errors for every one chromosome. a—mean breakpoint frequencies per chromosome (2016–2019) in descending order of the chromosome physical length. The line—approximated trend line. b—mean breakpoint frequencies per 1 Mb (2016–2019) for the entire genome (G) and individual chromosomes (X, Y, 1–22) in ascending order of the breakpoint number per chromosome. The differences are statistically significant for chromosome 13 and 20 (grey histogram columns).
To clarify the localization of breakpoints on chromosomes, we mapped pooled breakpoints to the ideogram (Fig. 5) and revealed their uneven distribution on some of the chromosomes. The regions that accumulated breakpoints (11 and more breakpoints) were regarded as ‘hot spots,’ including 1p32–p36.1 (18 times), 3p21–22 (17 times), 5q31–q35 (17 times), 6p21–p22 (11 times), 8q11.2–q13 (15 times), 10q24–q26 (16 times), 12p13 (12 times), 14p10–q13 (14 times), 14q24 (11times).
Fig. 5.
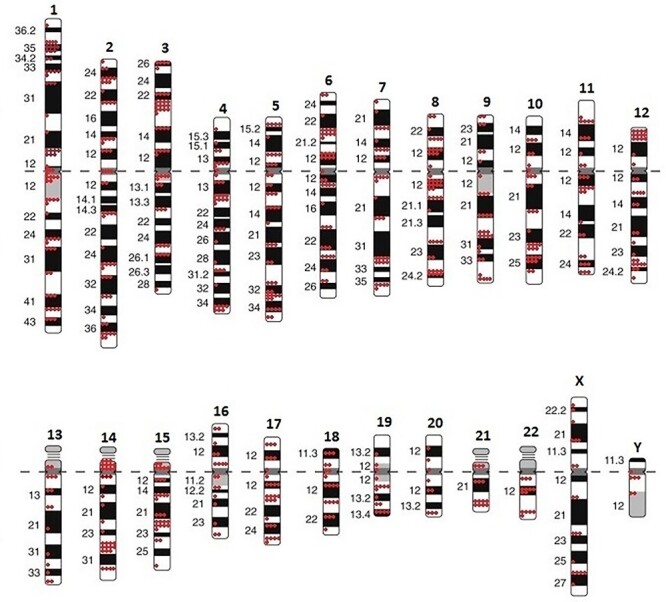
The ideogram of human’s karyotype with 731 breakpoints, identified in lymphocytes peripheral blood of exposed patient in 2016–2019 years.
Comet assay test
To estimate the recent genotoxic exposure, we performed neutral and alkaline comet assay analysis of DNA double- and single-strand breaks in 2019. There was 7.2% (median 2.3%) value of DNA in the tail (‘% DNA in the tail’) in the blood cells of the patient in alkaline comet assay. It was significantly higher than in interlaboratory control for healthy patients (2.6 ± 0.5%, 1.6–4.1%). The patient’s blood cells exhibited a widely scattered pattern of comets, with the presence of cells with high DNA damage, comet images from the slide of the blood cells of a patient are shown in Fig. 6. Comets with small or no visible head and containing more 70% DNA in the tail were judged the ‘hedgehog.’ The count of ‘hedgehog’ comets was higher in the irradiated patient compared to the interlaboratory control with healthy men (10.1% vs 0.5 ± 0.3%). In the neutral version of assay, the degree of DNA damage in blood cells of the patient was also higher than the average value for healthy subjects: 14.8% DNA in tail (median 14.0%) vs 7.2 ± 2.2%.
Fig. 6.
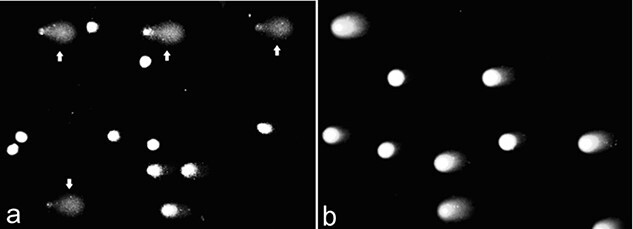
Alkaline (a) and neutral (b) comet assay images of patient’s blood cells (‘hedgehog’ comets indicated by arrows).
DISCUSSION
The background (spontaneous) level of CA in the human population is conditioned by the permanent influence of external and internal mutagenic factors of physical (various types of radiation, mainly natural background radiation, extreme temperature), chemical (chemical mutagens, environmental pollutants, certain drugs) and biological (viruses) nature. The spontaneous level of CA in human peripheral blood lymphocytes stained by Azur-Eosin is estimated to be 1–3%, in rare cases reaching 6% [21]. According to Hande et al. and Wahab et al. [10, 13], the frequency of translocations detectable by the mFISH analysis of metaphases from healthy donors is about 1%.
The increased aberrant metaphases frequency, rearrangements at tumor-associated chromosomal loci and clonal expansion of malignantly transformed aberrant cells can be involved in oncological processes and, possibly, be their predictors and biomarkers [22]. Indeed, a high background frequency of translocations in peripheral blood lymphocytes has been detected in a patient with head and neck cancer (8.6%), mFISH analysis was performed before the start of radiation therapy, chemotherapy drugs were not used, among other possible genotoxic effects only smoking is reported [14]. In our study, a statistically significant increase in the total amount of aberrant cells in a patient’s lymphocytes was observed in the same year, 2019, when basal cell skin carcinoma was first detected, it remains unclear however whether these events were related.
After irradiation, the amount of aberrant metaphases in human peripheral blood lymphocytes increases in a dose-dependent manner, with an extensive discussion about the stability of radiation-induced transmissible CA [23, 24]. Currently, there is just a fragmentary information available about the chromosomal repertoire, structure and dynamics of radiation-induced aberrations persisting in vivo at the resolution of molecular karyotyping. Hande et al. [10] have performed mFISH analyses in the group of Mayak Production Association workers who experienced prolonged occupational exposure to α- and γ- ionizing radiation, and the highest average level of translocations of about 9% was in the group of Highly exposed Pu Workers. In another study, the frequency of aberrant metaphases long after exposure was even lower. The mFISH analysis of peripheral blood lymphocytes revealed significant differences in frequencies of chromosomal abnormalities between the group of New Zealand nuclear test veterans and age matched controls (3.9%), with the maximum individual values of CA frequencies rarely exceeding 10% [13]. Importantly, this study used 72-hour lymphocyte culture, which increases the probability of accumulation of aberrant cells in vitro and may affect the frequency of CA. Whole-genome cytogenetic effects of therapeutic radiation have been reported in two patients receiving Cobalt 60 radiotherapy (25 fractions of 2 Gy) as a treatment for head and neck cancer [14]. Immediately after the end of radiation therapy, 37% and 18% of CA were observed in these patients and their number decreased after 4–6 months to 28.5 and 16%, respectively. No further cytogenetic studies were conducted and it is not known how long this level of CA was maintained in patients. Another mFISH analysis has been performed on blood lymphocytes of a patient receiving the ablative radioactive 131 iodine therapy of papillary thyroid carcinoma [15]. Twenty-one years after two courses of local-body irradiation of 48 mCi and 392 mCi (according to the authors’ calculations, the second dose corresponded to 1.3–2.9 Gy), the mFISH method revealed 10.83% of cells with CA, with the chromosomal translocation frequency of 8.83% (65 out of 600 analyzed metaphase cells, were found to be aberrant). In our study, a cytogenetically assessed dose of 3.2 Gy, received approximately 30 years ago, in the absence of evident repeated genotoxic effects resulted in extremely high frequency of aberrant cells (28%) in 2016–2018 yy with an increase up to 35% in 2019, which is consistent with whole-body exposure. Out of 993 metaphase cells analyzed, 297 cells were found to be aberrant. Some authors reported the persistence of simple CA (mainly translocations) over many years [25, 26], and the others consider their slow decrease [27, 28]. Our data are consistent with [10] and indicate that complex, but not simple, CA retained at the constant frequency within three decades after occupational exposure despite the patient’s healthy status. The number of simple CAs has changed in the last year of the study (2019) and it is not yet clear whether this is due to temporary fluctuations, e.g. physiological lymphocytosis or it is pathological lymphocytosis of the aberrant cells.
Clonal aberrations can be frequently observed in irradiated patients and healthy controls [15, 24, 29, 30], as well as in aged persons [31] and hypothetically reflect a stem cell pool depletion or an outgrowth of a particular lymphocyte population due to a premalignant process in a hematopoietic stem/progenitor cell or an immunological stimulation of a lymphocyte subpopulation. The time of persistence and dynamics of circulating clones are yet to be determined. In our study, four low-frequency clones were detected, three of which persisted for more than one year. The largest clone was detected each of the four years at a frequency never exceeding 2.5% of analysed cells. Given short cultivation time (48 h) and mitogen-induced synchronization of cell cycle, the identified clonal cells are likely descendants of irradiated stem or progenitor cells and long-lived T-lymphocytes at their first in vitro mitosis. All revealed clonal aberrations have not been associated with any oncohematological diseases, while the balanced translocation t(1;15)(q21;q22) detected in 2 cells in 2019 involves the 1q arm, which is rearranged in 1/3 of multiple myeloma patients and can influence prognosis [32]. Alterations at the band 1q21 and CKS1B hyperexpression were associated with poor prognosis in plasma-cell dyscrasias [33]. Unlike this clone, malignant clones are typically characterized by a high frequency and imbalanced karyotype, such as the acute malignant myeloma clone recently reported by us in another Chernobyl victim [34], however, the patient of the present study does not manifest any signs of hematooncological diseases. Since clone t(1;15) was only detected in the 2019 year, the dynamics of its frequency remained unknown.
When analyzing non-clonal aberrations, we observed an increase in the frequency of damage in the bands: 12p13, 5q33, changes in which are associated with diseases such as acute and chronic myeloid leukemia, chronic lymphocytic leukemia, myelodysplastic syndrome, multiple myeloma and lymphoma [35]. Reciprocal translocations may not always be balanced at the molecular level: rejoining of double-strand breaks is error-prone, and deletions ranging up to 8 kb have been observed at the splice sites as a result of rejoining. The position where the rearrangements occur towards essential genes may affect the fate of the cell [27]. Obviously, stochastic fluctuation and methodological bias of DAPI-banding can largely underlie these results. But the ‘hot’ chromosomal loci is predictably located near the T-cell receptor locus (14q11.2) in our study, which confirms the numerous studies in which the TCR mutant frequency was significantly higher than in control non-irradiated individuals, including of Chernobyl clean-up workers [36, 37].
Based on the stochastic nature of radiation-induced DNA damage, partial genome painting biodosimetry uses the widely accepted assumption of random break distribution along the genome. The mean of frequencies of breakpoints per 1 Mb for each chromosome were nearly proportional to chromosome physical length, with the exception of chromosomes 13 and 20, which were significantly more breakpoint-deficient compared to the genome median rate. Some inconsistency has been found with other studies in relative frequencies of chromosome-specific aberrations [24, 38, 39]. One of the reasons for this inconsistency is the use of different cell types: while the most of studies were performed on ex-vivo irradiated peripheral blood lymphocytes or other cultured cells analyzed immediately after rearrangement induction, we used cells most likely descendant from in vivo irradiated hematopoietic stem cells or progenitor cells decades after exposure. It was also hypothesized that less compact active chromatin has higher probability of being affected by radiation-induced damage due to the secondary effect of lowering the physical density of DNA per unit volume [7]. This can be partly supported by our results. The most gene-poor chromosomes 13 and 4 exhibited lower breakpoint frequency; contrary, gene-rich chromosomes 17 and 22 were among the most frequently affected ones. However, the most active chromosome 19 and relatively gene-rich chromosome 20 had intermediate and significantly decreased rearrangement rates, respectively. These trends may become more pronounced or disappear as the number of analyzed cells increases. Early hematopoiesis, lineage commitment and immune cell maturation are accompanied by widespread 3D nucleome reorganization [8, 40, 41], therefore, the exact impact of spatial chromosome interactions should be clarified in future radiation studies.
Since CA can be detected only in dividing mitogen-stimulated T-lymphocytes, we performed a comet assay, which characterizes the whole population of nuclear blood cells. Comet assay detects the bulk of single- and double-strand DNA breaks formed by genotoxic agents as well as during replication, repair and other cellular processes, while singular chromosome and chromatid breaks revealed by mFISH analysis are far beyond the resolution of comet assay.
Alkaline and neutral comet assays revealed elevated DNA damage and atypical comets in blood cells of the patient compared to control donors, which is consistent with early data reporting persistence of DNA damage over a long period after irradiation in workers exposed to γ-radiation. Pelevina et al. [42] using neutral comet assay showed that the level of double-strand DNA breaks in lymphocytes of the Chernobyl cleanup workers was higher as compared with the control population cohort. Plapert et al. [43] found that DNA damage persisted after a longer period in blood cells of a person who worked as a liquidator in the Chernobyl area immediately after the reactor accident. A high level of DNA damage was recorded in leukocytes of a worker incidentally exposed to γ-radiation [44], which was still elevated compared to control values. At the same time, the rate of the cells with micronucleus and all types of chromatid aberrations did not differ from healthy individuals, and only the amount of chromosome type aberrations was significantly elevated [44]. The present study has confirmed this fact, the balanced translocations had accounted for 86–89% of all CA. And the mean frequency of acentric fragments was comparable to population mean value, and there were no chromatid exchange aberrations, which are usually associated with chemical mutagenesis (xenobiotics, environmental and industrial pollution) revealed, which raises the questions about the source of observed DNA damage. Apoptosis is the main radiation-induced cell death mechanism in cells from myeloid and lymphoid lineages, which occurs within a few hours following exposure and does not require entering into mitosis [45]. Moreover the high level of atypical (apoptotic) comets observed in our analysis can be attributed to early apoptotic DNA fragmentation or genetic instability, since it is impossible to distinguish between genotoxic DNA damage and early stages of apoptosis using comet assay [46]. The patient in our study may have sufficient normal hematopoietic cells to compensate and maintain normal hematopoiesis. Genetically altered cells and minor cell clones may be eradicated by apoptosis immune surveillance or acquire additional genetic hits driving clonal expansion and clinically evident malignancy.
Monitoring of the long-term effects on chromosomal instability caused by radiation exposure is important for understanding and predicting the long-term effects of ionizing radiation. Future research implementing high-throughput sequencing techniques (next-generation, single-molecule and long-read sequencing) could provide more detailed characterization of aberrations and breakpoint mapping with single-nucleotide resolution and allow to depict the complex nature of radiation-induced DNA damage and malignant diseases.
Contributor Information
Victoriya Nikitina, State Research Center Burnasyan Federal Medical Biophysical Center of Federal Medical Biology Agency of Russia, 123128 Zhivopisnaya str., 46, Moscow, Russia.
Vladimir Nugis, State Research Center Burnasyan Federal Medical Biophysical Center of Federal Medical Biology Agency of Russia, 123128 Zhivopisnaya str., 46, Moscow, Russia.
Tatiyana Astrelina, State Research Center Burnasyan Federal Medical Biophysical Center of Federal Medical Biology Agency of Russia, 123128 Zhivopisnaya str., 46, Moscow, Russia.
Diana Zheglo, Federal State Budgetary Scientific Institution “Research Centre for Medical Genetics”, 115522, Moskvorechye str., 1, Moscow, Russia.
Irina Kobzeva, State Research Center Burnasyan Federal Medical Biophysical Center of Federal Medical Biology Agency of Russia, 123128 Zhivopisnaya str., 46, Moscow, Russia.
Mariya Kozlova, State Research Center Burnasyan Federal Medical Biophysical Center of Federal Medical Biology Agency of Russia, 123128 Zhivopisnaya str., 46, Moscow, Russia.
Irina Galstyan, State Research Center Burnasyan Federal Medical Biophysical Center of Federal Medical Biology Agency of Russia, 123128 Zhivopisnaya str., 46, Moscow, Russia.
Elena Lomonosova, State Research Center Burnasyan Federal Medical Biophysical Center of Federal Medical Biology Agency of Russia, 123128 Zhivopisnaya str., 46, Moscow, Russia.
Aliy Zhanataev, Research Zakusov Institute of Pharmacology, 125315 Baltyiskaya str., 8, Moscow, Russia.
Tatiyana Karaseva, State Research Center Burnasyan Federal Medical Biophysical Center of Federal Medical Biology Agency of Russia, 123128 Zhivopisnaya str., 46, Moscow, Russia.
Alexander S Samoylov, State Research Center Burnasyan Federal Medical Biophysical Center of Federal Medical Biology Agency of Russia, 123128 Zhivopisnaya str., 46, Moscow, Russia.
CONFLICT OF INTEREST
The authors declare they have no conflict of interest.
FUNDING
This work was supported by the Federal target program ‘Provision of nuclear and radiation safety for 2016–2025 for the period up to 2030’ A.I. Burnazyan Medical Biophysical Center FMBA of Russia.
References
- 1. International Atomic Energy Agency safety standards . Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies (EPR-Biodosimetry--2011(S)). International Atomic Energy Agency Vienna International Centre PO Box 100, A-1400 Vienna, Austria, 2014. http://www-ns.iaea.org/standards/ (2014). [Google Scholar]
- 2. Cornforth M-N, Loucas B-D. A cytogenetic profile of radiation damage. Radiat Res 2019;191:1–19. [DOI] [PubMed] [Google Scholar]
- 3. Braselmann H, Kulka U, Baumgartner A et al. SKY and FISH analysis of radiation-induced chromosome aberrations: a comparison of whole and partial genome analysis. Mutat Res 2005;578:124–33. [DOI] [PubMed] [Google Scholar]
- 4. Lucas J-N, Awa A, Straume T et al. Rapid translocation frequency analysis in humans decades after exposure to ionizing radiation. Int J Radiat Biol 1992;62:53–63. [DOI] [PubMed] [Google Scholar]
- 5. Balajee AS, Hadjidekova V. Retrospective cytogenetic analysis of unstable and stable chromosome aberrations in the victims of radiation accident in Bulgaria. Mutat Res 2021;861–2:503295. [DOI] [PubMed] [Google Scholar]
- 6. Greulich K-M, Kreja L, Heinze B et al. Rapid detection of radiation-induced chromosomal aberrations in lymphocytes and hematopoietic progenitor cells by mFISH. Mutat Res 2000;452:73–81. [DOI] [PubMed] [Google Scholar]
- 7. Loucas B-D, Shuryak I, Cornforth M-N. Three-color chromosome painting as seen through the eyes of mFISH: another look at radiation-induced exchanges and their conversion to whole-genomeequivalency. Front Oncol 2016;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kloetgen A, Thandapani P, Tsirigos A et al. 3D chromosomal landscapes in hematopoiesis and immunity. Trends Immunol 2019;40:809–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tucker J, Senft J. Analysis of naturally occurring and radiation-induced breakpoint locations in human chromosomes 1, 2 and 4. Radiat Res 1994;140:31–6. [PubMed] [Google Scholar]
- 10. Hande M-P, Azizova T-V, Burak L-E et al. Complex chromosome aberrations persist in individuals many years after occupational exposure to densely ionizing radiation: an mFISH study. Genes Chromosomes Cancer 2005;44:1–9. [DOI] [PubMed] [Google Scholar]
- 11. Osovets S-V, Sotnik N-V, Meineke V et al. Threshold limits for biological indication of prolonged radiation exposure using mFISH. Health Phys 2014;106:677–81. [DOI] [PubMed] [Google Scholar]
- 12. Sotnik N-V, Osovets S-V, Scherthan H et al. mFISH analysis of chromosome aberrations in workers occupationally exposed to mixed radiation. Radiat Environ Biophys 2014;53:347–54. [DOI] [PubMed] [Google Scholar]
- 13. Wahab M-A, Nickless E-M, Najar-M'kacher R et al. Elevated chromosome translocation frequencies in New Zealand nuclear test veterans. Cytogenet Genome Res 2008;121:79–87. [DOI] [PubMed] [Google Scholar]
- 14. Pouzoulet F, Roch-Lefèvre S, Giraudet A-L et al. Monitoring translocations by M-FISH and three-color FISH painting techniques: a study of two radiotherapy patients. J Radiat Res 2007;48:425–34. [DOI] [PubMed] [Google Scholar]
- 15. Livingston G-K, Escalona M, Foster A et al. Persistent in vivo cytogenetic effects of radioiodine therapy: a 21-year follow-up study using multicolor FISH. J Radiat Res 2018;59:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol 2002;30:513–28. [DOI] [PubMed] [Google Scholar]
- 17. Cornforth M-N, Bedford J-S. A quantitative comparison of potentially lethal damage repair and the rejoining of interphase chromosome breaks in low passage normal human fibroblasts. Radiat Res 1987;111:385–405. [PubMed] [Google Scholar]
- 18. Cornforth M-N, Durante M. Radiation quality and intra-chromosomal aberrations: size matters. Mutat Res Genet Toxicol Environ Mutagen 2018;836:28–35. [DOI] [PubMed] [Google Scholar]
- 19. Shaffer L-G, McGowan-Jordan J, Schmid M. ISCN 2013: An International System for Human Cytogenetic Nomenclature. Karger: International Standing Committee on Human Cytogenetic, 2013, 140. [Google Scholar]
- 20. CASPLab (1.0.1) Krzysztof Końca . https://casplab.com (November 23, 2013).
- 21. Bochkov N-P, Chebotarev A-N. Human heredity and environmental mutagens. M: Medicine 1989;269. [Google Scholar]
- 22. Bonassi S, Norppa H, Ceppi M et al. Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: results from a pooled cohort study of 22 358 subjects in 11 countries. Carcinogenesis 2008;29:1178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Durante M, George K, Wu H et al. Karyotypes of human lymphocytes exposed to high-energy iron ions. Radiat Res 2002;158:581–90. [DOI] [PubMed] [Google Scholar]
- 24. Ohtaki K. G-banding analysis of radiation-induced chromosome damage in lymphocytes of Hiroshima A-bomb survivors. Jpn J Hum Genet 1992;37:245–62. [DOI] [PubMed] [Google Scholar]
- 25. Salassidis K, Georgiadou-Schumacher V, Braselmann H et al. Chromosome painting in highly irradiated Chernobyl victims: a follow-up study to evaluate the stability of symmetrical translocations and the influence of clonal aberrations for retrospective dose estimation. Int J Radiat Biol 1995;68:257–62. [DOI] [PubMed] [Google Scholar]
- 26. Lindholm C, Edwards A. Long-term persistence of translocations in stable lymphocytes from victims of a radiological accident. Int J Radiat Biol 2004;80:559–66. [DOI] [PubMed] [Google Scholar]
- 27. Bauchinger M, Schmid E, Braselmann H. Time-course of translocation and dicentric frequencies in a radiation accident case. Int J Radiat Biol 2001;77:553–7. [DOI] [PubMed] [Google Scholar]
- 28. Matsumoto K, Ramsey M, Nelson D et al. Persistence of radiation-induced translocations in human peripheral blood determined by chromosome painting. Radiat Res 1998;149:602–13. [PubMed] [Google Scholar]
- 29. George K, Durante M, Willingham V et al. Chromosome aberrations of clonal origin are present in astronauts' blood lymphocytes. Cytogenet Genome Res 2004;104:245–51. [DOI] [PubMed] [Google Scholar]
- 30. Kusunoki Y, Kodama Y, Hirai Y et al. Cytogenetic and immunologic identification of clonal expansion of stem cells into T and B lymphocytes in one atomic-bomb survivor. Blood 1995;86:2106–12. [PubMed] [Google Scholar]
- 31. Tang G, Medeiros L-J, Wang S-A. How I investigate clonal cytogenetic abnormalities of undetermined significance. Int J Lab Hematol 2018;40:385–91. [DOI] [PubMed] [Google Scholar]
- 32. Bessmeltsev S-S. Multiple myeloma (pathogenesis, clinical features, diagnosis, differential diagnosis). Part I Clinical Oncohematology 2013;6:237–57. [Google Scholar]
- 33. Hanamura I, Stewart J-P, Huang Y et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood 2006;108:1724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samoylov A-S, Bushmanov A-Y, Udalov Y-D et al. Acute myeloid leukemia, prostate and skin cancer in acute radiation syndrome survivor after the 1986 Chernobyl nuclear accident: case report. Radiat Prot Dosim 2018;182:85–9. [DOI] [PubMed] [Google Scholar]
- 35. Atlas of genetics and cytogenetics in oncology and haematology . http://atlasgeneticsoncology.org/Bands/12p13.html, 1. indexed on: sam. juin 5 15:14:00 CEST 2021. http://atlasgeneticsoncology.org/Bands/5q33.html, indexed on: sam. juin 5 15:07:51 CEST 2021. https://markerdb.ca/conditions/3994?page=3, last update: Aug. 2020.
- 36. Akleyev A-A, Blinova E-A, Dolgushin I-I. Immunological status of chronically exposed persons with increased level of TCR mutations. Radiat Environ Biophys 2019;58:81–8. [DOI] [PubMed] [Google Scholar]
- 37. Saenko A-S, Zamulaeva I-A, Smirnova S-G et al. Determination of somatic mutant frequencies at glycophorin a and t-cell receptor loci for biodosimetry of acute and prolonged irradiation. Appl Radiat Isot 2000;52:1145–8. [DOI] [PubMed] [Google Scholar]
- 38. Barquinero J-F, Knehr S, Braselmann H et al. DNA-proportional distribution of radiation-induced chromosome aberrations analysed by fluorescence in situ hybridization painting of all chromosomes of a human female karyotype. Int J Radiat Biol 1998;74:315–23. [DOI] [PubMed] [Google Scholar]
- 39. Foster H-A, Estrada-Girona G, Themis M et al. Relative proximity of chromosome territories influences chromosome exchange partners in radiation-induced chromosome rearrangements in primary human bronchial epithelial cells. Mutat Res 2013;756:66–77. [DOI] [PubMed] [Google Scholar]
- 40. Hu G, Cui K, Fang D et al. Transformation of accessible chromatin and 3D nucleome underlies lineage commitment of early T cells. Immunity 2018;48:227–242.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu H. Probabilities of radiation-induced inter- and intrachromosomal exchanges and their dependence on the DNA content of the chromosome. Radiat Res 2001;156:603–6. [DOI] [PubMed] [Google Scholar]
- 42. Pelevina I-I, Afanasieva G-G, Aleshchenko A-V et al. The molecular and cellular consequences of the Chernobyl accident. Biophysics 2011;56:577–603. [PubMed] [Google Scholar]
- 43. Plappert U, Raddatz K, Roth S et al. DNA-damage detection in man after radiation exposure – the comet assay – its possible application for human biomonitoring. Stem Cells Suppl 1995;1:215–22. [PubMed] [Google Scholar]
- 44. Garaj-Vrhovac V, Kopjar N, Razem D et al. Application of the alkaline comet assay in biodosimetry: assessment of in vivo DNA damage in human peripheral leukocytes after a gamma radiation incident. Radiat Prot Dosim 2002;98:407–16. [DOI] [PubMed] [Google Scholar]
- 45. Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol 2010;31:363–72. [DOI] [PubMed] [Google Scholar]
- 46. Wada S, Khoa TV, Kobayashi Y et al. Detection of radiation-induced apoptosis using the comet assay. J Vet Med Sci 2003;65:1161–6. [DOI] [PubMed] [Google Scholar]


