Abstract
Aims
We aimed to study the progression of cardiac dysfunction in patients with lamin A/C mutations and explore markers of adverse cardiac outcome.
Methods and results
We followed consecutive lamin A/C genotype-positive patients divided into tertiles according to age. Patients underwent repeated clinical examinations, electrocardiograms (ECGs), and echocardiograms. We followed left ventricular (LV) and right ventricular (RV) size and function, and the severity atrioventricular-valve regurgitations. Outcome was death, LVAD implant, or cardiac transplantation. We included 101 patients [age 44 (29–54) years, 39% probands, 50% female]. We analysed 576 echocardiograms and 258 ECGs during a follow-up of 4.9 (interquartile range 2.5–8.2) years. The PR-interval increased at young age from 204 ± 73 to 212 ± 69 ms (P < 0.001), LV ejection fraction (LVEF) declined from middle age from 50 ± 12% to 47 ± 13% (P < 0.001), while LV volumes remained unchanged. RV function and tricuspid regurgitation worsened from middle age with accelerating rates. Progression of RV dysfunction [odds ratio (OR) 1.3, 95% confidence interval (CI) (1.03–1.65), P = 0.03] and tricuspid regurgitation [OR 4.9, 95% CI (1.64–14.9), P = 0.004] were associated with outcome when adjusted for age, sex, comorbidities, LVEF, and New York Heart Association functional class.
Conclusion
In patients with lamin A/C genotype, electrical disease started at young age. From middle age, LV function deteriorated progressively, while LV size remained unchanged. Worsening of RV function and tricuspid regurgitation accelerated in older age and were associated with outcome. Our systematic map on cardiac deterioration may help optimal monitoring and prognostication in lamin A/C disease.
Keywords: heart failure, echocardiography, genetic disease
Introduction
Mutations in the lamin A/C gene account for 5–8% of cases of familial dilated cardiomyopathy (DCM).1–3 Lamin A/C cardiomyopathy has autosomal dominant inheritance and complete age-related disease-penetrance.3,4 The disease is highly malignant with high risk of sudden cardiac death and end-stage heart failure.3,5
Lamin A/C cardiomyopathy commonly presents with atrial fibrillation (AF) and progresses with atrioventricular (AV) block and ventricular arrhythmias (VA). Heart failure occurs later, and often progresses to end-stage heart failure.3 Systematic reports on the cardiac disease progression in lamin A/C cardiomyopathy are lacking, although there have been suspected less left ventricular (LV) dilatation than in DCM of other aetiologies.6 In addition to medical treatment, cardiac resynchronization therapy, and circulatory assist devices are established in heart failure therapy.7 The success of device therapy is partly dependent on cardiac structure and function, including LV size, right ventricular (RV) function, and AV valve competence.8–12 The timing of intervention and follow-up of these patients may benefit from knowledge about the clinical course and cardiac structural and functional progression of lamin A/C cardiomyopathy.
We aimed to describe the individual cardiac electrical, structural, and functional progression in lamin A/C genotype-positive patients. We wanted to evaluate if disease progression were prognostic markers of heart failure outcome.
Methods
The authors do not have the authority to share the data used in the analyses described in this article. The approval of the Regional Committee for Medical Research Ethics limits sharing of data with researchers outside of Norway for purposes of reproducing the results or replicating the procedures. The data can only be made available to any additional researchers if a formal request is filled with the Regional Committee of Research Ethics and explicit consent is given from every study subject.
Study population
We conducted a single-centre, longitudinal cohort study. The patients were recruited from the Unit for Genetic Cardiac Diseases, Department of Cardiology, Oslo University Hospital Rikshospitalet, Oslo, Norway. We included consecutive lamin A/C genotype-positive patients with at least one clinical visit including an echocardiogram and followed them at our institution. Follow-up were performed according to clinical protocol. We defined probands as the first member of a family who sought medical attention due to cardiac or neuromuscular disease caused by a lamin A/C mutation. Family members were identified by cascade genetic screening. Inclusion was the first clinical visit at our centre. We stratified the patients by age into tertiles. The age tertiles were defined as younger, middle, and older.
We recorded demographical data, clinical characteristics including New York Heart Association (NYHA) functional class I–IV and comorbidities at each visit. Comorbidities were defined as having at least one of the following: hypertension, coronary artery disease, chronic obstructive pulmonary disease, peripheral arterial disease, cerebrovascular disease, and diabetes mellitus.13
Outcome
Outcome was defined as cardiac death, heart transplantation, or LV assist device (LVAD) placement. Last follow-up was the last visit before September 2019.
The study complied with the Declaration of Helsinki. The research protocol was approved by the regional committee for medical research ethics (REK no. 17.01.2008/S-0746a). All patients gave written informed consent.
Structural and functional progression by echocardiography
We analysed repeated echocardiograms and excluded echocardiograms on intravenous inotropes, on treatment with circulatory assist devices, and post-heart transplantation.
Echocardiograms were evaluated offline (Echo Pac® GE Healthcare version 2.02), by three expert echocardiographers (E.T.S., M.C., and M.R.), blinded to clinical data. The same echocardiographer analysed all repeated exams within one patient. We measured LV end-diastolic volume (LVEDV), end-systolic volume (LVESV), and LV ejection fraction (LVEF) by Simpson’s biplane method.14 LV global longitudinal strain (LVGLS) was assessed in a 16 segments model.15 Left atrial (LA) volume was calculated by the area length method indexed for body surface area as LA volume index (LAVI).14 RV size was measured as RV basal linear dimension (RVD).14 We assessed RV systolic function by tricuspid annular plane systolic excursion (TAPSE) and by 6 segments RV longitudinal strain (RVLS).14,16 Right atrial (RA) size was measured as RA area.14 We quantified mitral- and tricuspid valve regurgitation as mild, moderate, and severe.17
Electrical parameters and progression
We examined all available electrocardiograms (ECG) during follow-up and recorded heart rate, PR-interval, and occurrence of AV-block grade I–III. We excluded ECGs with ventricular pacing. Electrical progression was defined as an increase in PR-interval. Occurrence of AF was noted from ECG, exercise ECG, Holter monitorings, and implantable cardiac electronic devices. We noted time and indication of implanted cardiac electronic devices.
Statistics
Continuous variables were presented as mean ± (SD) or (SE), or as median with interquartile range (IQR) and were compared by one-way ANOVA or Kruskal–Wallis test as appropriate. Categorical data were presented as numbers (percentages) and compared by Fisher’s exact test.
Repeated echocardiographic and electrical parameters were analysed in a linear mixed model regression with random intercept. The effect of time on disease progression was compared across age tertiles by an interaction term with the middle tertile serving as the reference group. For evaluating cardiac chamber size progression, we excluded patients <age 18 to eliminate bias by physiologic growth. We compared the disease progression of probands vs. family members separately in each age tertile.
We used uni- and multivariate mixed model logistic regression with random intercept to test whether disease progression were markers for outcome. Univariate markers associated with outcome (P < 0.05) were included in a multivariate model adjusted for sex, and the evolution of age, comorbidities, LVEF, and NYHA class as time varying covariates.
We used a multivariable fractional polynomial linear regression model to fit an age curve for the expected PR-interval. Kaplan–Meier survival curves illustrated age at first episode of AF (STATA version 16.1, StataCorp LLC, TX, USA). Two-sided P-values <0.05 were considered significant.
Results
Clinical characteristic
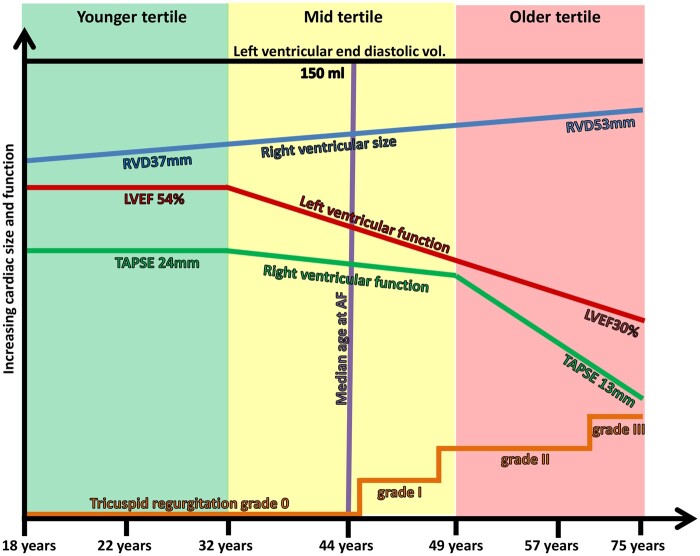
We included 101 lamin A/C genotype-positive patients [age 44 (IQR: 29–54) years, 39% probands, 50% female, 33 different families] with median follow-up 4.9 (IQR: 2.5–8.2) years (Table 1). Genetic testing revealed 15 different pathological lamin A/C mutations (Supplementary data online, Table S1). At inclusion, age tertiles were distributed as younger tertile <32 years, middle tertile 32–49 years, and older tertile >49 years (Figure 1). Patients in the older tertile were more often probands and had more severe cardiac disease as expected (Table 1).
Table 1.
Baseline characteristics of 101 lamin A/C genotype patients
| Clinical data | Total, n = 101 | Younger tertile, n = 34 | Middle tertile, n = 34 | Older tertile, n = 33 | P |
|---|---|---|---|---|---|
| Follow-up time (years) | 4.4 (2.5–6.6) | 6.1 (3.4–9.6) | 2.6 (1.7–4.6) | 2.5 (2.5–3.0) | <0.01 |
| Clinical visits, n | 5 (3–9) | 6 (4–10) | 6 (4–9) | 4 (3–7) | 0.11 |
| Age at inclusion (years) | 44 (29–54) | 22 (15–29) | 44 (38–47) | 57 (55–60) | <0.001 |
| Female sex, n (%) | 50 (50) | 17 (50) | 19 (56) | 14 (42) | 0.57 |
| Proband, n (%) | 39 (39) | 3 (9) | 15 (44) | 21 (64) | <0.001 |
| Families | 33 | 18 | 20 | 15 | NA |
| NYHA I, n (%) | 70 (69) | 30 (88) | 27 (79) | 13 (41) | <0.001 |
| NYHA II, n (%) | 15 (15) | 2 (6) | 4 (12) | 9 (28) | 0.045 |
| NYHA III, n (%) | 12 (13) | 1 (3) | 3 (9) | 8 (25) | 0.03 |
| NYHA IV, n (%) | 3 (3) | 1 (3) | 0 (0) | 2 (6) | 0.31 |
| Electrophysiological | |||||
| AV-block I, n (%) | 14 (14) | 3 (10) | 7 (23) | 4 (14) | 0.39 |
| AV-block II, n (%) | 3 (3) | 0 (0) | 1 (3) | 2 (7) | 0.32 |
| AV-block III, n (%) | 27 (27) | 0 (0) | 9 (30) | 18 (62) | <0.001 |
| Atrial fibrillation, n (%) | 45 (45) | 4 (12) | 16 (47) | 25 (78) | <0.001 |
| Pacemaker, n (%) | 15 (15) | 0 (0) | 7 (20) | 8 (24) | <0.01 |
| ICD, n (%) | 2 (2) | 0 (0) | 0 (0) | 2 (6) | 0.10 |
| CRT-D, n (%) | 9 (9) | 0 (0) | 0 (0) | 9 (28) | <0.001 |
| Medication | |||||
| ACE inhibitor, n (%) | 19 (19) | 0 (0) | 5 (15) | 14 (43) | <0.001 |
| β-Blocker, n (%) | 23 (23) | 1 (3) | 8 (24) | 14 (44) | <0.001 |
| MCRA, n (%) | 6 (6) | 0 (0) | 1 (3) | 5 (16) | 0.01 |
| Comorbidities | |||||
| Hypertension, n (%) | 3 (3) | 0 (0) | 0 (0) | 3 (9) | 0.32 |
| Coronary artery disease, n (%) | 3 (3) | 0 (0) | 0 (0) | 3 (9) | 0.03 |
| Stroke, n (%) | 5 (5) | 1 (3) | 0 (0) | 4 (13) | 0.04 |
| COPD, n (%) | 1 (1) | 0 (0) | 1 (3) | 0 (0) | 0.65 |
| Diabetes, n (%) | 5 (5) | 1 (3) | 1 (3) | 3 (9) | 0.44 |
Values are mean ± standard deviation, median (IQR), or frequency (%). P-values by one-way ANOVA, Kruskal–Wallis test, or Fisher’s exact test as appropriate.
ACE inhibitor; angiotensin converting enzyme inhibitor, AV-block; atrioventricular block, BMI; body mass index, COPD; chronic obstructive pulmonary disease, CRT-D; cardiac resynchronization therapy defibrillator, DCM; dilated cardiomyopathy, MCRA; mineral corticoid receptor antagonist, NSVT; non-sustained ventricular tachycardia, NYHA; New York Heart Association functional class, VA; ventricular arrhythmia.
Figure 1.
Schematic figure showing structural and functional progression of lamin A/C cardiomyopathy with increasing age. Age tertiles are colour coded on the x-axis by younger (green), middle (yellow), and older (red) . LVEF; left ventricular ejection fraction, RVD; right ventricular basal linear dimension, TAPSE; tricuspid annular plane systolic excursion.
Structural and functional disease progression in lamin A/C
We obtained 576 echocardiographic exams during follow-up. LV systolic function deteriorated during follow-up without compensatory LV dilatation (Table 2 and Figure 1).
Table 2.
Cardiac structural and functional progression in 101 patients with lamin A/C mutation by 576 echocardiographic assessments and 258 ECG
| Variable | Observed values at inclusion, n = 101 | Observed values at last follow-up, n = 94 | Progression rate/1 year (SE) | P for progression |
|---|---|---|---|---|
| LVEF (%) | 50 ± 12 | 47 ± 13 | −0.5 (0.1) | <0.001 |
| Younger tertile | 54 ± 10 | 53 ± 12 | −0.2 (0.1) | 0.13 |
| P for interaction | <0.01 | |||
| Middle tertile | 51 ± 11 | 49 ± 10 | −0.8 (0.2) | <0.001 |
| P for interaction | 0.60 | |||
| Older tertile | 43 ± 13 | 38 ± 12 | −1.0 (0.3) | <0.001 |
| LVGLS (%) | −15.5 ± 4.7 | −14.5 ± 4.5 | 0.2 (0.1) | <0.01 |
| Younger tertile | −17.4 ± 4.2 | −16.9 ± 4.1 | 0.02 (0.1) | 0.70 |
| P for interaction | <0.01 | |||
| Middle tertile | −16.5 ± 4.3 | −14.2 ± 3.3 | 0.3 (0.1) | <0.001 |
| P for interaction | 0.94 | |||
| Older tertile | −13.0 ± 4.4 | −11.8 ± 4.4 | 0.3 (0.2) | 0.051 |
| LVEDV (mL) | 136 ± 45 | 138 ± 43 | 0.2 (0.3) | 0.60 |
| Younger tertile | 120 ± 23 | 131 ± 35 | 0.2 (0.5) | 0.68 |
| P for interaction | 0.72 | |||
| Middle tertile | 149 ± 51 | 138 ± 41 | −0.1 (0.5) | 0.90 |
| P for interaction | 0.01 | |||
| Older tertile | 145 ± 47 | 146 ± 54 | −2.5 (0.7) | <0.001 |
| LAVI (mL/m2) | 45 ± 22 | 50 ± 25 | 1.4 (0.2) | <0.001 |
| Younger tertile | 30 ± 5 | 36 ± 10 | 1.0 (0.2) | <0.001 |
| P for interaction | 0.08 | |||
| Middle tertile | 42 ± 15 | 51 ± 21 | 1.7 (0.3) | <0.001 |
| P for interaction | 0.17 | |||
| Older | 61 ± 28 | 69 ± 29 | 2.4 (0.5) | <0.001 |
| MR, Grades 0–3 | 0.7 ± 0.8 | 1.0 ± 0.8 | 0.04 (0.01) | <0.001 |
| Younger tertile | 0.3 ± 0.6 | 0.5 ± 0.6 | 0.02 (0.01) | <0.01 |
| P for interaction | <0.01 | |||
| Middle tertile | 0.6 ± 0.7 | 1.0 ± 0.8 | 0.07 (0.01) | <0.001 |
| P for interaction | 0. 51 | |||
| Older tertile | 1.1 ± 0.7 | 1.4 ± 0.80 | 0.05 (0.02) | 0.03 |
| RVD (mm) | 41 ± 7 | 43 ± 9 | 0.2 (0.06) | <0.001 |
| Younger tertile | 37 ± 6 | 39 ± 5 | 0.1 (0.1) | 0.18 |
| P for interaction | 0.21 | |||
| Middle tertile | 42 ± 6 | 44 ± 6 | 0.3 (0.09) | 0.001 |
| P for interaction | 0.48 | |||
| Older tertile | 45 ± 8 | 48 ± 11 | 0.2 (0.1) | 0.15 |
| TAPSE (mm) | 23 ± 6 | 21 ± 6 | −0.3 (0.06) | <0.001 |
| Younger tertile | 24 ± 5 | 23 ± 5 | −0.03 (0.8) | 0.70 |
| P for interaction | <0.01 | |||
| Middle tertile | 24 ± 7 | 23 ± 6 | −0.4 (0.1) | <0.001 |
| P for interaction | <0.01 | |||
| Older tertile | 21 ± 5 | 16 ± 5 | −0.9 (0.1) | <0.001 |
| RVLS (%) | −19.7 ± 5.8 | −17.8 ± 5.9 | 0.3 (0.1) | <0.001 |
| Younger tertile | −21.5 ± 5 | −21.4 ± 4.1 | 0.1 (0.1) | 0.40 |
| P for interaction | 0.03 | |||
| Middle tertile | −20.8 ± 5.0 | −17.6 ± 4.7 | 0.4 (0.1) | <0.01 |
| P for interaction | 0.27 | |||
| Older tertile | −17.1 ± 6.3 | −13.5 ± 5.9 | 0.6 (0.1) | <0.001 |
| RA area (cm2) | 21 ± 8 | 24 ± 9 | 0.5 (0.06) | <0.001 |
| Younger tertile | 16 ± 5 | 18 ± 7 | 0.1 (0.09) | 0.13 |
| P for interaction | <0.001 | |||
| Middle tertile | 20 ± 4 | 24 ± 6 | 0.6 (0.1) | <0.001 |
| P for interaction | <0.001 | |||
| Older tertile | 26 ± 9 | 31 ± 10 | 1.3 (0.2) | <0.001 |
| TR, Grades 0–3 | 1.0 ± 0.8 | 1.4 ± 0.9 | 0.06 (0.01) | <0.001 |
| Younger tertile | 0.7 ± 0.7 | 0.9 ± 0.6 | 0.03 (0.01) | 0.001 |
| P for interaction | 0.047 | |||
| Middle tertile | 1.1 ± 0.5 | 1.5 ± 0.8 | 0.06 (0.01) | <0.001 |
| P for interaction | 0.01 | |||
| Older tertile | 1.4 ± 0.9 | 2.0 ± 0.8 | 0.1 (0.02) | <0.001 |
| Electrical parameters | ||||
| PR-interval (ms) | 204 ± 73 | 211 ± 69 | 4 (0.5) | <0.001 |
| Younger tertile | 177 ± 54 | 185 ± 58 | 3 (0.5) | <0.001 |
| P for interaction | <0.001 | |||
| Middle tertile | 224 ± 83 | 248 ± 74 | 11 (1.7) | <0.001 |
| P for interaction | 0.42 | |||
| Older tertile | a | a | 9 (2.3) | <0.001 |
Observed data expressed as mean ± SD. Progression rate expressed as mean (SE). P values for yearly progression rate and for time-interaction in linear mixed models statistics.
Data not shown due to <6 observations.
LAVI; left atrial volume index, LVEDV; left ventricular end-diastolic volume, LVEF; left ventricular ejection fraction, LVGLS; global longitudinal strain, MR; mitral regurgitation, RA area; right atrial area, RVD; right ventricular basal linear dimension, RVLS; right ventricular longitudinal strain, TR; tricuspid regurgitation, TAPSE; tricuspid annular plane systolic excursion.
RV systolic function deteriorated along with a progressive RV dilatation (Table 2 and Figure 1). LA and RA size increased along with increased mitral- and tricuspid regurgitation (Table 2).
Comparison of LV and RV structural and functional progression in different age-tertiles
LV systolic function remained unchanged in the younger tertile, but deteriorated in the middle and older age tertiles with an average LVEF reduction of 1% per year (Table 2 and Figure 1). LV volume did not change (Table 2 and Figure 1).
LA size increased with similar rates in all tertiles and pathological LAVI values were reached in the younger tertile (Table 2). Mitral regurgitation progressed in all age groups with faster progression in the middle and older tertiles (Table 2).
RV and RA size and RV function remained unchanged in the younger tertile, and worsened from the middle tertile. The deterioriation of TAPSE and RA dilatation accelerated with increasing age (Table 2 and Figure 1). Tricuspid regurgitation progressed in all age groups with accelerated rates with increasing age (Table 2).
We observed no clear differences in cardiac disease progression in probands compared with family members (data not shown).
Electrical disease
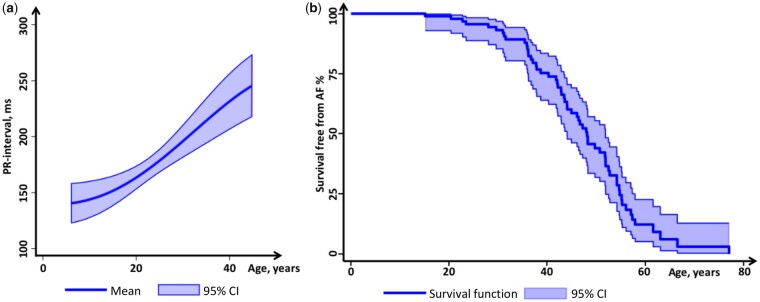
We analysed in all 258 ECGs (Table 2). The PR-interval increased during follow-up in all age groups with faster progression rates in the middle and older tertile (Table 2 and Figure 2a). The frequency of AF increased from 44% at inclusion to 62% during follow-up and occurred at median age 44 (range 15–77) years (Figures 1 and 2b).
Figure 2.
PR interval and survival from atrial fibrillation, according to age in 101 lamin A/C genotype-positive patients. (a) Mathematical-derived curve showing the expected PR-interval (y-axis) according to age (x-axis). (b) Kaplan–Meier survival curve displaying survival free from AF according to age.
Frequency of implantable cardiac electronic devices increased during follow-up from n = 26 (26%) (Table 1) to n = 52 (56%) (pacemakers: n = 2, ICDs: n = 20, and CRT-D: n = 30).
Association between structural progression and outcome
Eighteen patients had adverse cardiac outcome during follow-up [younger tertile n = 3 (10%), middle tertile n = 3 (10%), and older tertile n = 12 (44%), P = 0.001].
Worsening of LV and RV function, increasing ventricular and atrial sizes, worsening of AV-valvular regurgitations, NYHA functional class, increasing age, and proband status were all associated with increased odds for outcome in univariate analysis (Table 3). RV and LV dilatation, RV dysfunction by RVLS, and increase in AV-regurgitations remained significant markers for outcome when adjusted for sex, evolution of age, LVEF, comorbidities, and NYHA class (Table 3).
Table 3.
Odds for adverse cardiac outcome (cardiac transplantation or LVAD, or death from heart disease) in 101 lamin A/C genotype patients in univariate and multivariate mixed model logistic regression
| Univariate |
Multivariate Adjusted for age, sex, LVEF, NYHA functional class, and comorbidities |
|||||
|---|---|---|---|---|---|---|
| OR | CI | P | OR | CI | P | |
| Age per year | 1.3 | 1.21–1.42 | <0.001 | NA | NA | NA |
| Female | 0.4 | 0.06–2.80 | 0.36 | NA | NA | NA |
| Proband | 160 | 2.2–11 925 | 0.02 | 3.5 | 0.6–21 | 0.17 |
| NYHA I–IV | 30 | 11–84 | <0.001 | NA | NA | NA |
| LVEF per 5% | 2.5 | 1.9–3.3 | <0.001 | NA | NA | NA |
| LVGLS per % | 1.5 | 1.3–1.7 | <0.001 | NA | NA | NA |
| LVEDV per 10 mL | 1.5 | 1.2–1.9 | 0.001 | 1.2 | 1.006–1.4 | 0.04 |
| LAVI per 5 cm3/m2 | 1.6 | 1.3–2.0 | <0.001 | 1.0 | 0.8–1.1 | 0.65 |
| MR per 0–3 | 20 | 6.1–69 | <0.001 | 2.4 | 1.004–6.0 | 0.049 |
| RVLS per % | 1.7 | 4.7–42 | <0.001 | 1.3 | 1.03–1.65 | 0.03 |
| RVD per 3 mm | 3.0 | 1.4–2.1 | <0.001 | 1.5 | 1.1–2.1 | 0.01 |
| RA area per 5 cm2 | 4.6 | 2.5–8.8 | <0.001 | 1.3 | 0.8–2.0 | 0.28 |
| TR per 0–3 | 158 | 11.3–2182 | <0.001 | 5.0 | 1.64–14.8 | <0.01 |
| TAPSE per 3 mm | 3.1 | 2.1–4.6 | <0.001 | 1.4 | 0.96–2.0 | 0.08 |
P values by mixed models logistic regression analyses. Comorbidities included hypertension, coronary artery disease, peripheral arterial disease, cerebrovascular disease, and diabetes mellitus.
Parameters LAVI, LVEDV LVEF, RA area, RVD, and TAPSE are all continuous scale parameters.
LAVI; left atrial volume index, LVEDV; left ventricular end-diastolic volume, LVEF; left ventricular ejection fraction, LVGLS; left ventricular global longitudinal strain, MR; mitral regurgitation, NYHA; New York Heart Association functional class, OR; odds ratio, RA; right atrium, RVD; right ventricular basal linear dimension, RVLS; right ventricular longitudinal strain, TAPSE; tricuspid annular plane systolic excursion, TR; tricuspid regurgitation.
Discussion
We describe the progressive and dynamic cardiac deterioration in patients with lamin A/C genotypes. At young age electrical progression was most predominant, followed by decline in LV systolic function from middle age. LV systolic function deteriorated without compensatory LV dilatation, indicating an important structural difference in patients with lamin A/C compared with DCM of other aetiologies. RV dysfunction progressed at accelerating rates during the middle and older tertiles and was together with RV dilatation, and AV-valvular regurgitations associated with adverse cardiac outcome independent of the traditional selection criteria for heart transplantation or LVAD implantation. We provide hereby a comprehensive map of the expected cardiac deterioration in a cohort of patients with lamin A/C genotypes. Our findings implicate a closer monitoring of RV parameters in advanced disease as markers of adverse outcome and may be valuable in planning of LVAD and heart transplantation in lamin A/C patients.
Structural and functional disease progression in lamin A/C
LV function declined in patients in the middle and older tertiles with no obvious compensatory LV dilatation. These findings suggest different LV progression in lamin A/C cardiomyopathy compared with typical DCM and support the suggestion of lamin A/C cardiomyopathy as a hypokinetic non-DCM.6,18 In heart failure, compensatory LV dilatation restore stroke volume.19 A decline in LV systolic function without compensatory LV dilatation as in lamin A/C, may lead to a steeper decline in stroke volumes and earlier activation of neuro-hormonal systems known to stimulate heart failure progression20 and may explain the rapid clinical deterioration seen in clinical practice.
LA size reached pathological values already in the younger tertile. Whether the high prevalence of AF explains the increase in atrial size or vice versa the atrial cardiomyopathy induce AF is unknown.
Mitral regurgitation worsened in all ages. The progressive regurgitation despite unchanged LV volumes may seem contradictory, but may be explained by the combination of progressive LV dysfunction, dyssynchrony, and mitral annular dilatation even without LV dilation.21
We found an accelerating deterioration of RV function and increase in tricuspid regurgitation in the middle and older tertiles. RV disease progression may be explained by progressive increase in RV afterload due to elevated LV filling pressures, and at later stages, chronic RV volume overload due to severe tricuspid regurgitation.22 Chronic AF leading to atrial and annular dilatation23 may worsen tricuspid regurgitation and transvalvular pacing leads can further increase the unfavourable situation.24
Together, the progressively failing LV without compensatory LV dilatation, accompanied by RV disease and worsening of tricuspid regurgitation, all contribute to low systemic stroke volumes. These factors may contribute to the high incidence of end-stage heart failure in patients with lamin A/C genotypes.3
Electrical progression in lamin A/C disease
The PR-interval increased across all age tertiles with increased progression rate from the middle tertile resulting in the frequent need of cardiac pacing.3 Studies have shown early replacement fibrosis in the AV junction in lamin A/C cardiomyopathy1 supported by findings of septal late gadolinium enhancement and septal dysfunction.25 Conduction delays leading to LV dyssynchrony and an inadequate chronotropic response may further reduce cardiac output.
Clinical implications
During the younger tertile, we observed an increasing risk of AF accompanied by increasing LA volumes, while other structural features were stable. Management of young lamin A/C patients should therefore focus on Holter monitoring to detect asymptomatic AF which may warrant anticoagulation therapy.26 Furthermore, it is important to detect AV-block and VA leading to therapeutic measures including device therapy.
From around 40 years of age, we observed increasing cardiac LV and RV structural and functional deterioration, accompanied by increased severity of AV valve regurgitations. RV deterioration was a strong marker for adverse cardiac outcome independent of other typical markers indicating need of LVAD or heart transplant. In addition to management of electrical disease, management of adult lamin A/C patients should focus on assessment of both LV and RV size and function.
LV dilatation was uncommon in lamin A/C cardiomyopathy and the clinician should be aware that an end-stage phenotype can occur without LV dilatation. Nevertheless, occurrence of LV dilatation should be regarded as sign of poor prognosis. The lack of severe LV dilatation in lamin A/C cardiomyopathy may have additional clinical relevance. Smaller hearts may respond better to cardiac resynchronization therapy.12 Furthermore, smaller LV size and progressive RV dysfunction and tricuspid regurgitation may imply non-optimal effect of LVAD therapy.8,9,11,27 Future studies should evaluate the effect and optimal timing of LVAD treatment in lamin A/C patients.
Limitations
This was a single-centre longitudinal cohort study conducted in a tertiary reference centre with inherent limitations. Subtle structural progression may overlap with echocardiographic measurement variability and may be difficult to identify clinically. The number of outcomes was limited and potential outcome markers from his study should be validated in a separate cohort.
We did not include CMR examinations in this study due to the high frequency of cardiac devices at older ages and thereby missing data.
Conclusion
We provided a comprehensive map of cardiac disease progression in patients with lamin A/C genotypes. Cardiac disease progressed slowly at younger age, mainly with electrical disease including AV block and further AF. With increasing age, LV function gradually deteriorated with a subsequent rapid RV deterioration to end-stage heart failure. We observed limited compensatory LV dilatation, suggesting disease mechanisms different from other DCM. These findings may have impact on prognostication, clinical management, follow-up, and timing of interventions in patients with lamin A/C disease.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Funding
This work was funded by the Norwegian Research Council [203489/030].
Conflict of interest: none declared.
Supplementary Material
References
- 1. Arbustini E, Pilotto A, Repetto A, Grasso M, Negri A, Diegoli M. et al. Autosomal dominant dilated cardiomyopathy with atrioventricular block: a lamin A/C defect-related disease. J Am Coll Cardiol 2002;39:981–90. [DOI] [PubMed] [Google Scholar]
- 2. Jacoby D, McKenna WJ.. Genetics of inherited cardiomyopathy. Eur Heart J 2012;33:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TP. et al. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur Heart J 2018;39:853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pasotti M, Klersy C, Pilotto A, Marziliano N, Rapezzi C, Serio A. et al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol 2008;52:1250–60. [DOI] [PubMed] [Google Scholar]
- 5. van Berlo JH, de Voogt WG, van der Kooi AJ, van Tintelen JP, Bonne G, Yaou RB. et al. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: do lamin A/C mutations portend a high risk of sudden death? J Mol Med 2005;83:79–83. [DOI] [PubMed] [Google Scholar]
- 6. Taylor MR, Fain PR, Sinagra G, Robinson ML, Robertson AD, Carniel E. et al. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J Am Coll Cardiol 2003;41:771–80. [DOI] [PubMed] [Google Scholar]
- 7. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V. et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 8. Dang NC, Topkara VK, Mercando M, Kay J, Kruger KH, Aboodi MS. et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant 2006;25:1–6. [DOI] [PubMed] [Google Scholar]
- 9. Potapov EV, Stepanenko A, Dandel M, Kukucka M, Lehmkuhl HB, Weng Y. et al. Tricuspid incompetence and geometry of the right ventricle as predictors of right ventricular function after implantation of a left ventricular assist device. J Heart Lung Transplant 2008;27:1275–81. [DOI] [PubMed] [Google Scholar]
- 10. Puwanant S, Hamilton KK, Klodell CT, Hill JA, Schofield RS, Cleeton TS. et al. Tricuspid annular motion as a predictor of severe right ventricular failure after left ventricular assist device implantation. J Heart Lung Transplant 2008;27:1102–7. [DOI] [PubMed] [Google Scholar]
- 11. Kawabori M, Kurihara C, Conyer R, Sugiura T, Critsinelis AC, Lee VV. et al. A left ventricular end-diastolic dimension less than 6.0 cm is associated with mortality after implantation of an axial-flow pump. J Thorac Cardiovasc Surg 2019;157:2302–10. [DOI] [PubMed] [Google Scholar]
- 12. Rickard J, Brennan DM, Martin DO, Hsich E, Tang WH, Lindsay BD. et al. The impact of left ventricular size on response to cardiac resynchronization therapy. Am Heart J 2011;162:646–53. [DOI] [PubMed] [Google Scholar]
- 13. Metra M, Zaca V, Parati G, Agostoni P, Bonadies M, Ciccone M. et al. ; Heart Failure Study Group of the Italian Society of Cardiology. Cardiovascular and noncardiovascular comorbidities in patients with chronic heart failure. J Cardiovasc Med (Hagerstown) 2011;12:76–84. [DOI] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 15. Edvardsen T, Haugaa KH.. Imaging assessment of ventricular mechanics. Heart 2011;97:1349–56. [DOI] [PubMed] [Google Scholar]
- 16. Haugaa KH, Basso C, Badano LP, Bucciarelli-Ducci C, Cardim N, Gaemperli O. et al. ; EACVI Scientific Documents Committee, EACVI Board members and external reviewers. Comprehensive multi-modality imaging approach in arrhythmogenic cardiomyopathy – an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017;18:237–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA. et al. ; Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611–44. [DOI] [PubMed] [Google Scholar]
- 18. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M. et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2016;37:1850–8. [DOI] [PubMed] [Google Scholar]
- 19. Ertl G, Gaudron P, Neubauer S, Bauer B, Horn M, Hu K. et al. Cardiac dysfunction and development of heart failure. Eur Heart J 1993;14 Suppl A:33–7. [DOI] [PubMed] [Google Scholar]
- 20. Packer M. Evolution of the neurohormonal hypothesis to explain the progression of chronic heart failure. Eur Heart J 1995;16 Suppl F:4–6. [DOI] [PubMed] [Google Scholar]
- 21. Donal E, De Place C, Kervio G, Bauer F, Gervais R, Leclercq C. et al. Mitral regurgitation in dilated cardiomyopathy: value of both regional left ventricular contractility and dyssynchrony. Eur J Echocardiogr 2009;10:133–8. [DOI] [PubMed] [Google Scholar]
- 22. Rana BS, Robinson S, Francis R, Toshner M, Swaans MJ, Agarwal S. et al. Tricuspid regurgitation and the right ventricle in risk stratification and timing of intervention. Echo Res Pract 2019;6:r25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Najib MQ, Vinales KL, Vittala SS, Challa S, Lee HR, Chaliki HP.. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography 2012;29:140–6. [DOI] [PubMed] [Google Scholar]
- 24. Al-Bawardy R, Krishnaswamy A, Bhargava M, Dunn J, Wazni O, Tuzcu EM. et al. Tricuspid regurgitation in patients with pacemakers and implantable cardiac defibrillators: a comprehensive review. Clin Cardiol 2013;36:249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hasselberg NE, Edvardsen T, Petri H, Berge KE, Leren TP, Bundgaard H, Haugaa KH.. Risk prediction of ventricular arrhythmias and myocardial function in Lamin A/C mutation positive subjects. Europace 2014;16:563–71. [DOI] [PubMed] [Google Scholar]
- 26. van Rijsingen IA, Bakker A, Azim D, Hermans-van Ast JF, van der Kooi AJ, van Tintelen JP, van den Berg MP. et al. Lamin A/C mutation is independently associated with an increased risk of arterial and venous thromboembolic complications. Int J Cardiol 2013;168:472–7. [DOI] [PubMed] [Google Scholar]
- 27. Klotz S, Naka Y, Oz MC, Burkhoff D.. Biventricular assist device-induced right ventricular reverse structural and functional remodeling. J Heart Lung Transplant 2005;24:1195–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.