Abstract
Cardiac computed tomography (CT) was initially developed as a non-invasive diagnostic tool to detect and quantify coronary stenosis. Thanks to the rapid technological development, cardiac CT has become a comprehensive imaging modality which offers anatomical and functional information to guide patient management. This is the second of two complementary documents endorsed by the European Association of Cardiovascular Imaging aiming to give updated indications on the appropriate use of cardiac CT in different clinical scenarios. In this article, emerging CT technologies and biomarkers, such as CT-derived fractional flow reserve, perfusion imaging, and pericoronary adipose tissue attenuation, are described. In addition, the role of cardiac CT in the evaluation of atherosclerotic plaque, cardiomyopathies, structural heart disease, and congenital heart disease is revised.
Keywords: coronary computed tomography angiography (CCTA), cardiac computed tomography, myocardial ischaemia, fractional flow reserve, FFRCT, CT perfusion imaging, plaque imaging, structural heart disease, radiomics, artificial intelligence, cardiomyopathy
Graphical Abstract
Clinical applications of cardiac CT. For more details, please refer to Table 1 which summarizes the main applications of cardiac CT. CAD, coronary artery disease; CT, computed tomography; CTP, computed tomography perfusion; FFRCT, CT-derived fractional flow reserve; LAA, left atrial appendage; TAVI, transcatheter aortic valve implantation.
Introduction
This is the second of two complementary documents released by the European Association of Cardiovascular Imaging (EACVI) to provide recommendations on the evolving clinical applications of cardiac computed tomography (CT). The aim of this second document is to summarize the available evidence on coronary atherosclerotic plaque imaging, computational fluid dynamics, and perfusion imaging techniques as well as pericoronary adipose tissue (PCAT) attenuation, radiomics, and artificial intelligence. Finally, the key findings for a standardized coronary CT angiography (CCTA) report are described.
Methodology
The topic of this document was approved by the EACVI Scientific Document Committee. The writing committee comprised acknowledged experts in the field of cardiac CT. The writing committee discussed and approved the table of contents. This includes either well established applications of cardiac CT, or novel tools which have shown promising results for a potential implementation in the clinical arena. The evidence-based literature was searched in the electronic databases Medline/PubMed, Embase, and the Cochrane Library and afterwards reviewed by G.P. and A.R., with the restriction to English language. Both retrospective and prospective studies were considered eligible. Case reports, letters to the editor, and comments were excluded. The final decision on inclusion was reached by consensus between the two screening authors. Based on the collected data, the screening authors wrote the first draft of the article which was then circulated among all co-authors. Thereafter, each section was carefully reviewed by the entire writing committee until a consensus was reached for each potential application of cardiac CT. Thus, this consensus document reports the current and emerging clinical applications of cardiac CT agreed by the panel of experts and grounded on the best available evidence at present, as summarized in Table 1 and in Graphical Abstract.
Table 1.
EACVI key points on the clinical applications of cardiac CT
|
Plaque imaging and standardized CCTA report |
|
|
|
|
Pericoronary adipose tissue |
|
|
|
|
CT-derived fractional flow reserve (FFRCT) |
|
|
|
|
Myocardial perfusion imaging with CT |
|
|
|
|
Structural heart disease |
|
|
|
|
Cardiomyopathies |
|
|
|
|
Coronary anomalies and congenital heart disease |
|
|
|
| Coronary anatomy in aortic dissection, aortic aneurysms, and pulmonary embolism scans |
|
CAD, coronary artery disease; CAC-DRS, Coronary Artery Calcium—Data and Reporting System; CAC-RADS, Coronary Artery Disease—Reporting And Data System; CCTA, coronary computed tomography angiography; CMR, cardiac magnetic resonance; CRT, cardiac resynchronization therapy; CT, computed tomography; CTP, computed tomography perfusion; ECG, electrocardiogram; ECV, extracellular volume; FAI, fat attenuation index; FFRCT, computed tomography-derived fractional flow reserve; LAA, left atrial appendage; PCAT, pericoronary adipose tissue; TAVI, transcatheter aortic valve implantation; TOE, transoesophageal ecocardiography.
Plaque imaging with CT
Rationale
CCTA is the only non-invasive tool capable of robustly imaging the coronary atherosclerotic plaque that leads to myocardial infarction (MI) as well as stable angina pectoris. CCTA therefore can directly assess the disease process driving coronary artery disease (CAD): from plaque detection and characterization to identification of adverse plaque features, quantification of atherosclerotic plaque burden and assessment of stenosis severity as shown in Figure 1.
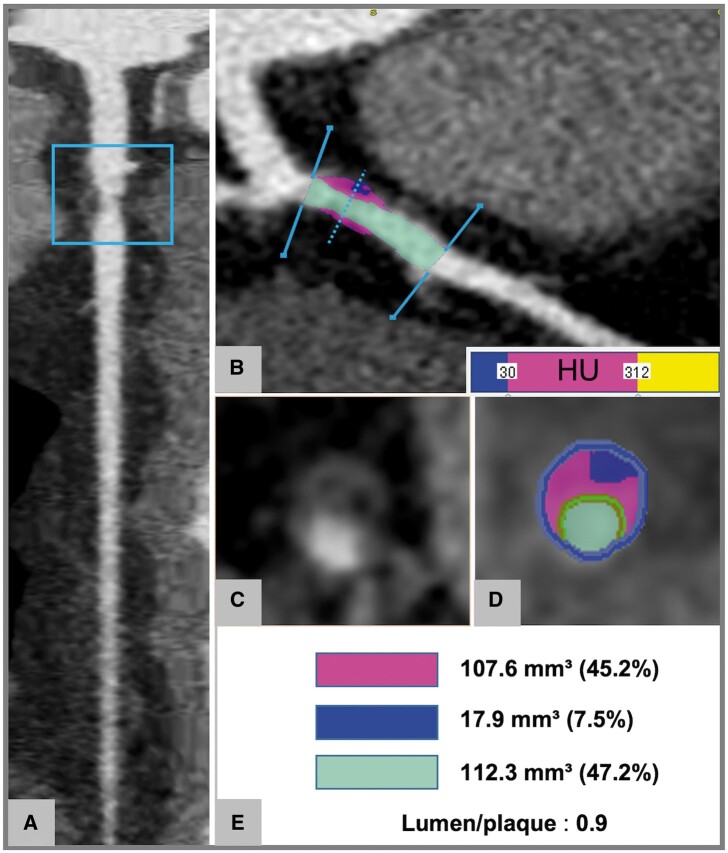
Figure 1.
Plaque characterization and quantitative assessment using semi-automated software. (A–E) Plaque of the proximal LAD. Straight (A) and curved (B) MPRs demonstrate a non-calcified plaque of the proximal LAD. The cross-section (C) shows a low-attenuation component of the plaque associated with positive remodelling and a rim of higher attenuation corresponding to the napkin-ring sign. The semi-automate software delineates inner (green) and outer (blue) vessel walls and automatically identifies components with attenuation <30 HU (blue), 31–312 HU (pink), and >312 HU (yellow). The lumen is highlighted in light blue (B and D). Quantitative data on area stenosis severity and volume of plaque subcomponents are provided in (E). HU, Hounsfield unit; LAD, left anterior descending artery; MPR, multiplanar reconstruction.
Non-obstructive plaque
An advantage of CCTA is its ability to image and quantify non-obstructive atherosclerotic plaque, a stage of the disease missed by conventional stress perfusion imaging techniques and stress echocardiography. Multiple studies have highlighted the prognostic importance of non-obstructive plaque detection. In the ICONIC (Incident Coronary Events Identified by Computed Tomography) study, 75% of culprit lesions identified by invasive coronary angiography (ICA) were non-obstructive on antecedent CCTA.1 In the SCOT-HEART (Scottish Computed Tomography of the Heart Trial) and PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trials, as many subsequent MIs were observed in patients with non-obstructive as obstructive CAD.2,3 Although more prospective data are needed, given its prognostic importance, non-obstructive plaque may be used to guide the prescription of statin and aspirin therapy.
Plaque burden
CT calcium scoring has provided a surrogate of coronary plaque burden for many years, however advances in CCTA software technology mean that quantification of both calcified and non-calcified coronary plaque is now possible. The approach can be either semi-quantitative, in terms of number of segments involved by atherosclerosis [segment involvement score (SIS), segment stenosis score (SSS), or CT adapted Leaman score],4,5 or quantitative through plaque burden quantification by semi-automated/automated software.6 Although such quantitative approach is attractive and has shown good correlation with the invasive evaluation by intravascular ultrasound,7 it is still time-consuming and requires validation, standardization, and automation before being implemented in routine clinical care.
Adverse plaque characteristics
CCTA can also characterize plaque type. Most simply, coronary plaques can be categorized into calcified, non-calcified, and partially calcified by visual assessment.8 A further step in plaque analysis consists in the visual identification of adverse plaque9,10 which include low-attenuation plaque (LAP), a marker of a large necrotic core, positive remodelling (PR), spotty calcification, and the napkin-ring sign11 as defined in Table 2. Adverse plaque features have been correlated with worse prognosis and can be used to identify patients at increased probability of developing a future acute event. As such, the PROMISE trial demonstrated that the presence of adverse plaque was associated with 70% increase of death, MI, and hospitalization for unstable angina after 2 years follow-up.12 In line with this, the SCOT-HEART trial showed that patients with adverse plaque characteristics had a three-fold increased rate of major cardiovascular events (MACE) at 5 years. However, this elevated risk was not independent of plaque burden as assessed by CT calcium score.13 A recent meta-analysis reported that the napkin-ring sign was associated with the highest risk of future MACE (HR: 5.06), followed by LAP (HR: 2.95) and PR (HR: 2.58).14 Spotty calcification showed the lowest correlation with MACE (HR: 2.25),14 likely due to its high prevalence and therefore low specificity.15 Table 3 summarizes the major studies reporting on the association between adverse plaque features and MACE.10,12,16–22 Furthermore, the EMERALD (Exploring the Mechanism of plaque Rupture in Acute coronary syndrome using coronary CT Angiography and computational fluid Dynamics) study demonstrated that integrating adverse haemodynamic plaque characteristics [i.e. haemodynamic forces acting on plaques like fractional flow reserve (FFR) derived from CCTA and its change across the lesion, wall shear stress, and axial plaque stress] to adverse plaque features improves the identification of culprit lesions for future acute coronary syndrome (ACS)23. Importantly, in all the studies the number of adverse plaques greatly exceeded the number of clinical events, highlighting that only a small minority of these adverse plaques causes a MI.12,13,24,25 However, at the patient level, subjects with a tendency to develop such lesions consistently appear to be at high risk. The next level is to consider not only the presence of high-risk plaques but their burden. In a sub-study of the SCOT-HEART trial, Williams et al.21 demonstrated that patients with total LAP burden >4% had five times higher risk of developing fatal or nonfatal MI and that this risk prediction was independent of calcium score as well as cardiovascular risk factors and the presence of luminal stenoses. Similarly, the ICONIC study showed that necrotic and fibro-fatty plaque volumes were higher in the ACS patients as compared to controls.11 Further work is required to confirm the prognostic utility of the LAP burden in different patient populations and to streamline methods for quantification.
Table 2.
Definitions of adverse plaque characteristics
| HRP feature | Definition |
|---|---|
| Low attenuation plaque | Presence of a central area within the plaque characterized by low CT attenuation <30 HU.10 |
| Positive remodelling or remodelling index (RI) | Presence of an outer vessel diameter which is >10% of the diameter of the reference normal segment within the same vessel (RI >1.1).10 |
| Spotty calcification | Small focal calcifications <3 mm diameter in any direction.10 |
| Napkin-ring sign | Central area of low CT attenuation that is apparently in contact with the lumen and is surrounded by a rim of higher attenuation.11 |
CT, computed tomography; HU, Hounsfield unit; RI, remodelling index.
Table 3.
Selection of studies investigating the prognostic value of qualitative adverse plaque characteristic detected by CCTA
| Trial | Motoyama et al.10 | Motoyama et al.18 | Nadjiri et al.19 | Feuchtner et al.16 | Ferencik et al.12 | Finck et al.17 | Williams et al.21 | Senoner et al.20 | Yamamoto et al.22 |
|---|---|---|---|---|---|---|---|---|---|
| Study design | Retrospective, observational study | Retrospective, observational study | Retrospective, observational study | Prospective, observational study | RCT | Prospective, observational study | RCT | Prospective, observational study | Prospective, observational study |
| Sample size | 1059 | 3158 | 1168 | 1469 | 4415 | 1615 | 1769 | 1430 | 2083 |
| Population | Suspected or known CAD | Suspected or known CAD | Suspected CAD | Suspected CAD, low to intermediate pre-test probability | Suspected CAD | Suspected CAD | Suspected CAD | Suspected CAD, low to intermediate pre-test probability | Suspected CAD |
| Follow-up (years) | 2.3 | 3.9 | 5.7 | 7.8 | 2.1 | 10.5 | 4.7 | 10.5 | 2 |
| Primary endpoint | ACS | ACS | Cardiac death, MI, unstable angina, and coronary revascularization | STEMI, NSTEMI, unstable angina | Death from any cause, MI, and hospitalization for unstable angina | Cardiac death or nonfatal MI | Fatal or nonfatal MI | Fatal and nonfatal MACE | Cardiac death, non-fatal acute coronary syndrome, and coronary revascularization >3 months after indexed CCTA |
| Rate of primary endpoint (%) | 1.4 | 2.8 | 3.9 | 2.8 | 3.0 | 3.1 | 2.3 | 3.9 | 3.5 |
| Plaque feature | LAP, PR | LAP, PR | PR, NRS, SC | LAP, NRS, SC | LAP, PR, NRS | LAP, NRS, SC | LAP | LAP, PR, NRS, SC | LAP, PR, NRS, SC |
| Adjusted hazard ratio (95% CI) | LAP and/or PR: 22.79 (6.91–75.17) |
|
|
|
|
|
LAP: 1.60 (1.10–2.34) |
|
≥2 adverse characteristics: 1.95 (1.13–3.34) |
ACS, acute coronary syndrome; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CI, confidence interval; LAP, low-attenuation plaque; MACE, major adverse cardiovascular events; MI, myocardial infarction; NRS, napkin-ring sign; NSTEMI, non-ST elevation myocardial infarction; PR, positive remodelling; RCT, randomized clinical trial; SC, spotty calcification; STEMI, ST elevation myocardial infarction.
Progression of CAD and effect of medications
Several studies demonstrated the ability of CCTA in monitoring the effects of anti-atherosclerotic medications on plaque volume and composition.26–28 As such, Table 4 summarizes the results of selected studies which investigated the impact of statins on coronary plaque burden by using serial CCTA scans.6,29–35 In particular, the PARADIGM (Progression of Atherosclerotic Plaque Determined by Computed Tomographic Angiography Imaging) study is a multicentre prospective registry, which included patients who underwent repeat CCTA with a median follow-up time of 3.8 years.36 Lee et al.6 demonstrated that in patients on statin treatment non-calcified plaques showed slower progression over time and increased conversion to calcified plaque compared with statin naïve subjects. Of note, patients with diabetes experienced more plaque progression, in terms of disease burden and adverse plaque features, than those without diabetes.37 Overall, total plaque burden (defined as plaque volume/vessel volume) was the strongest predictor of progression from non-obstructive to obstructive lesions after adjustment for drug use including statin.38
Table 4.
Selection of studies investigating the effect of statins using serial CCTA
| Trial | Inoue et al.30 | Zeb et al.35 | Lo et al.33 | Ausher et al.29 | Li et al.32 | Lee et al.6 | Lee et al.31 | van Rosendael et al.34 |
|---|---|---|---|---|---|---|---|---|
| Study design | Prospective, non- randomized study | Retrospective, observational study | Randomized, double-blind, placebo-controlled study | Prospective, open label, randomized clinical trial | Prospective, observational study | Multinational, observational registry | Multinational, observational registry | Multinational, observational registry |
| Sample size | 32 | 100 | 40 | 96 | 206 | 1255 | 654 | 857 |
| Population | Suspected CAD | Suspected CAD | Patients with HIV with no prior history of CAD | AMI | Suspected CAD | Suspected or known CAD | Suspected or known CAD | Suspected or known CAD |
| Therapy | Fluvastatin vs. control | Statin vs. no statin | Atorvastatin vs. placebo | Intensive vs. standard statin therapy | Intensive vs. moderate vs. no statin | Statin vs. no statin | Statin vs. no statin | Statin vs. no statin |
| Follow-up time | 12 months | 406 days | 12 months | 360 days | 18 months | 3.8 years | 3.9 years | 3.2 years |
| Quantitative plaque parameter | Total PV, LAP | Total PV, NCP, LAP | Total PV, NCP | Total PV, dense calcium volume | Total PV, LAP | Annualized changes in total PAV, calcified PAV | Annualized changes in normalized total PV and calcified PV | LAP, fibro-fatty plaque volume |
| Treatment effect | Statin therapy was associated with decrease in plaque and necrotic core volume | Statin therapy was associated with lower progression of LAPs and NCPs | Statin therapy reduced NCP volume and adverse plaque characteristics | Intensive statin therapy increased dense calcium volume | Statin therapy induced regression of LAP | Statin therapy was associated with slower progression of disease and increased plaque calcifications | Statin therapy was associated with disease progression limited to calcified PV | Statin therapy was associated with larger decrease of LAP and fibro-fatty plaque volume |
AMI, acute myocardial infarction; CAD, coronary artery disease; HIV, human immunodeficiency virus; LAP, low-attenuation plaque; NCP, non-calcified plaque; PAV, percentage atheroma volume; PV, plaque volume.
Standardized CCTA report
Standardization of CCTA reporting is recommended to assure an effective communication of the results to the referring physician. A complete overview of the different sections of a standardized cardiac CT report is presented in Supplementary data online, Figure S1. A section providing the evidence which supports the use of coronary calcium in clinical care is presented in the part 1 of this consensus document.
Key points
Reporting total Agatston calcium score and the corresponding risk category is appropriate (when available) as a surrogate of total plaque burden. The coronary calcium risk categories are as follows: no coronary calcium, minimal: 1–9 calcium score, mild : 10–99 calcium score, moderate: 100–299 calcium score, and severe: ≥300 calcium score.39
Reporting the severity of luminal stenoses, location, and extent of the coronary plaque is appropriate.
The following grading of luminal stenosis severity (percentage stenosis) is appropriate: no stenosis, minimal: <25%; mild: 25–49%; moderate: 50–69%; severe: 70–99%; occlusion.40
For patients presenting with coronary atherosclerotic plaque, description of plaque composition (i.e. calcified, non-calcified, and partially calcified) and reporting of adverse plaque characteristics are appropriate.
For patients presenting with coronary atherosclerotic plaque, reporting the overall plaque burden is appropriate. This can be provided by visual assessment as mild, moderate, or severe plaque burden (sub-optimal method) or by semi-quantitative approaches such as the SIS.
The use of the Coronary Artery Calcium—Data and Reporting System (CAC-DRS)41 and Coronary Artery Disease—Reporting And Data System (CAD-RADS)42 systems for standardized coronary calcium and CCTA report has been suggested by the Society of Cardiac Computed Tomography (SCCT) since they provide information on disease severity considering the presence, burden, and location of both non-obstructive and obstructive plaque.
Pericoronary adipose tissue
Rationale
Inflammation is a key factor in the development and progression of atherosclerosis.43 In the presence of vascular inflammation, the release of pro-inflammatory cytokines triggers lipolysis in the PCAT and blocks the differentiation of adipocytes thereby increasing the water/lipid ratio44 and PCAT attenuation. A recent study reported that PCAT values increased across three distinct stages of CAD (no disease vs. stable CAD vs. MI) independently of age, gender, risk factors, and coronary plaque burden.45
The fat attenuation index (FAI) captures changes in PCAT attenuation around standardized segments of the coronary arteries on CT images. FAI is measured by using dedicated software which considers local anatomy, biological factors, scan settings, and reconstruction parameters.46,47 Of note, few studies demonstrated that FAI is a dynamic value which is affected by anti-inflammatory and disease-modifying therapies.48–50
Prognostic value
In a post hoc analysis of outcome data from the CRISP-CT (Cardiovascular risk prediction using CT) study, an adjusted FAI value of >−70.1 Hounsfield unit was derived to identify patients at higher risk of all-cause mortality, cardiac mortality, and ACS.46
Despite these promising results, evaluation of PCAT attenuation/FAI is still considered a research tool. Further work is required to assess where measurements are best made and whether it provides clinical value above and beyond direct CT assessments of coronary plaque.
Key points
Limited data prevent recommendation on the role of PCAT attenuation/FAI.
Functional assessment of coronary stenoses with CT
Rationale
Documentation of myocardial ischaemia and the haemodynamic impact of luminal stenoses might help patient selection for elective invasive procedures, particularly in patients with recalcitrant symptoms and borderline obstructive stenoses.
CT-derived FFR
Technique
At the present time, invasive FFR is widely used during ICA to evaluate the haemodynamic significance of a coronary stenosis.51,52 Nevertheless, FFR can be estimated non-invasively by applying computational fluid dynamics to anatomic image data extracted from CCTA, without the need for additional imaging, modification of acquisition protocols, or administration of additional medications53 as detailed in Figure 2. Currently, the only technology for CT-derived FFR (FFRCT) approved for clinical use requires off-site analysis in a central core laboratory (Heartflow, Redwood City, CA, USA). Meanwhile, multiple applications have been developed allowing on-site analysis on a regular workstation with shorter computational time, although none of these are commercially available.54,55
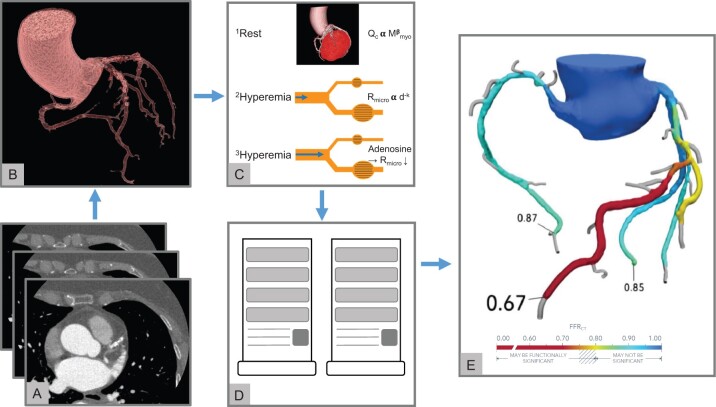
Figure 2.
FFRCT. (A–E) Calculation of FFRCT by using computational fluid dynamics principles. (A and B) Standard rest CCTA images (A) are used to create a 3-dimensional anatomic model of the coronary arteries (B). (C) A physiological model of the coronary microcirculation is derived according to the following principles: 1resting coronary flow is proportional to myocardial mass; 2microvascular resistance is inversely proportional to vessel size; and 3microvascular resistance is reduced to simulate maximal hyperaemia. (D and E) An off-site supercomputer is used to compute coronary blood flow by applying the physical laws of fluid dynamics (D) and calculated FFRCT throughout the entire coronary tree (E). FFRCT, CT-derived fractional flow reserve.
Diagnostic accuracy
A recent meta-analysis reported a pooled sensitivity of 85% and a pooled specificity of 75% of FFRCT at vessel level (n = 1247) in the detection of haemodinamically significant lesions as defined by invasive FFR.56 Table 5 summarizes the results of the major studies investigating the feasibility and diagnostic accuracy of FFRCT against invasive FFR in patients with chronic stable angina.57–64 The NXT (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps) trial showed improved specificity of FFRCT for the detection of haemodynamically significant stenoses at the patient level compared with 50% stenosis on CCTA (79% vs. 34%, respectively).60 Moreover, the PACIFIC (Comparison of Cardiac Imaging Techniques for Diagnosing Coronary Artery Disease) trial compared FFRCT vs. single-photon emission computed tomography and positron emission tomography demonstrating the highest diagnostic accuracy of FFRCT for the detection of vessel specific ischaemia, provided CCTA images were evaluable by FFRCT.57 Indeed, inadequate CCTA image quality is still a considerable limitation of FFRCT with a rejection rate of FFRCT in contemporary cohorts, ranging from 2.9% to 8.4% due to motion and slab artefacts.65 Diagnostic accuracy of FFRCT is not as good in patients with extensive coronary calcium,66 multi-vessel disease post ST myocardial infarction,67 and FFRCT values ranging between 0.7 and 0.8-the borderline lesions where it most likely to be used.68
Table 5.
Major studies evaluating the diagnostic performance of FFRCT at vessel level with FFR ≤ 0.80 as reference standard
| Trial | DISCOVER- FLOW58 | DeFACTO59,64 | NXT60 | PACIFIC57 | Coenen et al.62,63 | Ko et al.54 | Li et al.55 |
|---|---|---|---|---|---|---|---|
| Study design | Prospective, multicentre. international study | Prospective, multicentre. international study | Prospective, multicentre. international study | Prospective, single-centre study | Prospective/retrospective, multicentre. international study | Prospective, single-centre study | Retrospective, two-centre study |
| Company | HeartFlow | HeartFlow | HeartFlow | HeartFlow | Siemens | Canon | Pulse Medical |
| Name of CT-derived FFR | FFRCT | FFRCT | FFRCT | FFRCT | CT-FFR | CT-FFR | CT-QFR |
| Technique | CFD | CFD | CFD | CFD | Machine learning approach | CFD | CFD |
| Patients, n | 103 | 252 | 254 | 157 | 351 | 30 | 134 |
| All lesions | |||||||
| Vessels, n | 159 | 407 | 484 | 505 | 525 | 58 | 156 |
| AUC (95% CI) | 0.90a | N/A | 0.93 (0.91–0.95) | 0.94 (0.92–0.96) | 0.84 (0.80-0.87) | 0.88 (0.76–1.0) | 0.92a |
| Sensitivity (95% CI) | 87 (76–95) | 80 (73–86) | 84 (75–89) | 90 (84–95) | 81 (75–86) | 77 (51–92) | 87a |
| Specificity (95% CI) | 82 (73–89) | 61 (54–67) | 86 (82–89) | 86 (82–89) | 76 (71–81) | 86 (71–95) | 86a |
| PPV (95% CI) | 73 (61–83) | N/A | 61 (53–69) | 65 (57–73) | 70 (64–76) | 73 (48–89) | 82a |
| NPV (95% CI) | 92 (84–96) | N/A | 95 (93–97) | 96 (92–98) | 85 (81–90) | 89 (73–95) | 90a |
| Intermediate lesions | |||||||
| Vessels, n | 47 | 150 | N/A | N/A | 144b | N/A | N/A |
| Definition of intermediate lesions | 50–69% DS | 30–70% DS | N/A | N/A | 25–69% DS | N/A | N/A |
| AUC (95% CI) | N/A | 0.79 (0.72-0.87) | N/A | N/A | N/A | N/A | N/A |
| Sensitivity (95% CI) | 66a | 74 (57–88) | N/A | N/A | 87 (76–94) | N/A | N/A |
| Specificity (95% CI) | 88a | 67 (58–75) | N/A | N/A | 59 (47–70) | N/A | N/A |
| PPV (95% CI) | 66a | 41 (29–54) | N/A | N/A | 62 (51–72) | N/A | N/A |
| NPV (95% CI) | 88a | 90 (81–95) | N/A | N/A | 85 (73–93) | N/A | N/A |
95% confidence intervals not reported in the original study.
CFD technique.
AUC, area under curve; CFD, computational fluid dynamics; CI, confidence interval; CT, computed tomography; DeFACTO, Determination of Fractional Flow Reserve by Anatomic Computed Tomography Angiography; DISCOVER-FLOW, Diagnosis of Ischaemia-Causing Stenoses Obtained Via Non-invasive Fractional Flow Reserve; DS, diameter stenosis; FFR, fractional flow reserve; FFRCT, CT-derived fractional flow reserve; N/A, not available; NPV, negative predictive value; NXT, Analysis of Coronary Blood Flow Using CT Angiography: Next Steps; PACIFIC, Prospective Comparison of Cardiac PET/CT, SPECT/CT Perfusion Imaging and CT Coronary Angiography With Invasive Coronary Angiography; PPV, positive predictive value; QFR, quantitative flow ratio.
Prognostic utility
Several follow-up studies have demonstrated both the prognostic value and clinical utility of FFRCT.69–73 The 5 years follow-up of the NXT trial showed that a FFRCT value of 0.8 or less was independently associated with MACE.69 In particular, each 0.05 unit decrease of FFRCT correlated with greater incidence of the primary endpoint.69 The PLATFORM (Prospective Longitudinal Trial of FFRCT: Outcome and Resource Impact) study compared two consecutive cohorts of patients with recent onset of chest pain evaluated with CCTA/FFRCT vs. usual care. The cohort that underwent CCTA and FFRCT had a lower number of ICA performed, a lower rate of non-obstructive CAD for those who were invasively investigated, and an overall lower cost compared with usual care.70 Furthermore, the ADVANCE (Assessing Diagnostic Value of Noninvasive FFRCT in Coronary Care) registry investigated the real-world utility of FFRCT showing that the prospective implementation of FFRCT after positive CCTA affected management recommendations in ∼ 67% of the patients. Only 2.6% of cases required further non-invasive diagnostic testing after FFRCT.73 The randomized FORECAST (Fractional Flow Reserve Derived From Computed Tomography Coronary Angiography in the Assessment and Management of Stable Chest Pain) trial demonstrated that a strategy guided by CCTA and selective FFRCT in patients with chronic coronary syndrome was comparable with standard clinical care in terms of cost and clinical outcomes, whilst reducing the number of ICA.74
Revascularization decision-making
The role of FFRCT has been also investigated in patients with complex CAD. In this context, the anatomical Syntax Score (SS) is commonly used to grade the severity and complexity of CAD on ICA and to help the heart team in decision-making between percutaneous coronary intervention and coronary artery by-pass graft.75,76 The SYNTAX (Synergy Between PCI With TAXus and Cardiac Surgery) II trial demonstrated the feasibility of deriving functional SS from CCTA and FFRCT with comparable results against ICA with pressure-wire assessment.75 Furthermore, the SYNTAX III trial reported that decision-making based on non-invasive functional SS was in high agreement with the decisions derived from the invasive approach.77 Adding the functional information provided by FFRCT to the anatomic data of CCTA changed the heart team recommendation in 7% of the cases and modified selection of vessel for revascularization in 12%.78 Future developments in novel post-processing software, which allows for the non-invasive recalculation of FFRCT after the virtual stent implantation, are waited.79
Key points
FFRCT could be considered to assess functional significance of moderately obstructive lesions if CCTA image quality is adequate and the results are likely to change patient management.
Myocardial perfusion imaging with CT
Technique
Myocardial perfusion imaging by computed tomography (CTP) allows for the evaluation of the distribution of myocardial blood flow (MBF) into the myocardium during rest and hyperaemia, thus providing information on the presence of myocardial ischaemia. Static perfusion protocols require the acquisition of a single set of images during the maximum myocardial enhancement, with either a single-energy or dual-energy approach.80 Dynamic perfusion protocols consist of multiple acquisitions over time of the same cardiac volume in order to evaluate the wash-in and wash-out of contrast material from the myocardium. This allows the construction of time-attenuation curves and, through the application of specific mathematical models, the quantification of MBF in each voxel of the myocardium.81 Examples of CTP acquisition protocols are shown in Figure 3. While for static CTP the effective dose is comparable with a regular CCTA with an average value of 5.93 mSv, the average radiation exposure of dynamic CTP is higher with an average value of 9.23 mSv.82 Nevertheless, new developments in CT hardware and software and tailored acquisition protocols appear promising in terms of dose reduction.83 At the present time there is no standardization regarding acquisition protocols and imaging analysis for CTP imaging.
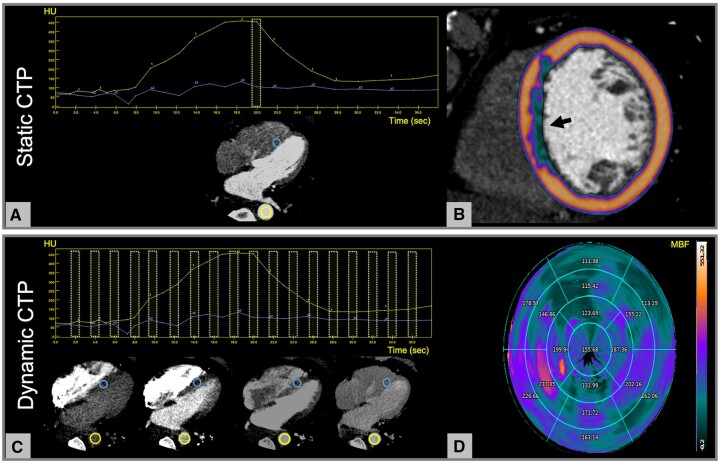
Figure 3.
CTP imaging during pharmacological stress. (A and B) Static CTP consists of a single acquisition of the entire heart at the time of maximum myocardial enhancement as shown by the yellow dotted box in (A). The image assessment is qualitative and relies on the visual comparison between the hypo-perfused area and a normal (remote) region of the myocardium. In this example, a colour-coded map representing the myocardial distribution of HU has been overlayed to the myocardium showing a subendocardial perfusion defect of the septum (arrow, B). (C and D) Dynamic CTP protocols are based on the acquisition of serial CT datasets of the whole myocardium at different time points, which allows the construction of time attenuation curves of the descending aorta (yellow circle) and the myocardium (blue circle, C). MBF is calculated in absolute terms for each single voxel of the myocardium by applying dedicated mathematical models to the time-attenuation curves. A representative polar map of the MBF (mL/100g/min) distribution based on 17-segment AHA model is shown in (D) demostrating reduced perfusion in the anterior and antero-lateral walls of the left ventricle. The numbers indicate the average perfusion value for each myocardial segment. AHA, American Heart Association; CT, computed tomography; CTP, CT perfusion; HU, Hounsfield unit; MBF, myocardial blood flow.
Diagnostic accuracy
A selection of studies reporting on feasibility and diagnostic performance of static and dynamic CTP are summarized in Tables 6 and 7, respectively.83–96 In a recent meta-analysis including 697 patients and 2118 corresponding vessels CTP presented a sensitivity of 81% and a specificity of 86% in the detection of functionally significant coronary stenoses as defined by FFR ≤0.80.97 In particular, CTP improved the diagnostic accuracy of CCTA alone by decreasing the number of false positive findings either in patients with suspected CAD97 or in patients with previous coronary stent implantation.98 Dynamic CTP has shown to have higher sensitivity but lower specificity than static CTP (85% vs. 72% and 81% vs. 90%, respectively).97
Table 6.
Selection of the available studies evaluating the diagnostic performance of single-energy static CTP
| Trial | Bettencourt et al. 84 | Rochitte et al. 85 | Cury et al. 86 | George et al.95 | Magalhaes et al.96 | Pontone et al. 83 |
|---|---|---|---|---|---|---|
| Study design | Prospective, single-centre study | Prospective, multicentre, international study | Randomized, multicentre, multivendor study | Prospective, multicentre, international study | Prospective, multicentre, international study | Prospective, single-centre study |
| Sample size | 101 | 381 | 110 | 381 | 381 | 147 |
| Clinical setting | Stable CAD | Stable CAD | Stable CAD | Stable CAD | Stable CAD | Stable CAD |
| CT scanner | 64-slice CT | 320-slice CT | Multivendor | 320-slice CT | 320-slice CT | 256-slice CT |
| Reference standard | FFR ≤ 0.80 or LM disease or vessel occlusion on ICA | QCA ≥ 50% and SPECT | SPECT | QCA | QCA ≥ 50% and SPECT | QCA > 80% and/or invasive FFR ≤0.80 |
| Level of analysis | Vessel (n = 303) | Vessel (n = 1143) | Patient (n = 110) | Vessel (n = 1143) | Vessel (n = N/A) | Vessel (n = 432) |
| Sensitivity (95% CI) | 55 (46–61) | 61 (55–67)a | 90 (71–100) | 78 (73–82) | 58 (51–64)a | 92 (87–97)a |
| Specificity (95% CI) | 95 (93–97) | 83 (80–86)a | 84 (77–91) | 62 (58–67) | 86 (83–88)a | 95 (92–97)a |
| PPV (95% CI) | 78 (66–88) | 52 (45–58)a | 36 (17–55) | 58 (53–63) | 55 (48–61)a | 87 (81–92)a |
| NPV (95% CI) | 87 (84–89) | 88 (85–90)a | 99 (97–100) | 81 (76–85) | 87 (84–90)a | 97 (95–99)a |
CCTA + CTP.
CAD, coronary artery disease; CI, confidence interval; CT, computed tomography; FFR, fractional flow reserve; ICA, invasive coronary angiography; LM, left main; N/A, not available; NPV, negative predictive value; PPV, positive predictive value; QCA, quantitative coronary angiography; SPECT, single-photon emission computed tomography.
Table 7.
Selection of the available studies evaluating the diagnostic performance of dynamic CTP
| Trial | Huber et al.87 | Rossi et al.88 | Coenen et al.89 | Pontone et al.90 | Alessio et al.91 | de Kneght et al.92 | Nous et al.93 | Kitagawa et al.94 |
|---|---|---|---|---|---|---|---|---|
| Study design | Prospective, single-centre study | Prospective, two-single-centre study | Prospective, two-single-centre study | Prospective, single-centre study | Prospective, single-centre study | Prospective, single-centre study | Prospective, multicentre, international study | Prospective, multicentre study |
| Sample size | 32 | 80 | 74 | 85 | 34 | 93 | 123 | 157 |
| Clinical setting | Suspected and stable CAD | Suspected and stable CAD | Suspected and stable CAD | Suspected and stable CAD | Suspected and stable CAD | Suspected and stable CAD | Suspected and stable CAD | Suspected or known CAD |
| CT scanner | 256-slice CT | 128-slice dual source CT (second generation) | 128-slice dual source CT (second generation) | 256-slice CT | 256-slice CT | 128-slice dual source CT (second generation) | 128-slice dual source CT (third generation) | 128-slice dual source CT (second and third generation) |
| Reference standard | ICA >75% and/or invasive FFR ≤0.75 | QCA >90% and/or invasive FFR ≤0.75 | Invasive FFR ≤0.80 | QCA ≥80% and/or invasive FFR ≤0.80 | PET | QCA ≥80% and/or invasive FFR ≤0.80 | QCA >90% and/or invasive FFR ≤0.80 | ICA >90% and/or FFR <0.80 |
| Level of analysis | Patient | Vessel (n = 210) | Vessel (n = 142) | Vessel (n = 255) | N/A | Vessel (n = 218) | Vessel (n = 289) | Vessel (n=442) |
| Cut-off value of MBF |
|
|
|
|
N/A |
|
|
|
| Sensitivity (95% CI) | 75 (56–89) | 88 (74–95) | 73 (61–86) | 73 (63–83)b | 75a | 84 (77–89) | 84 (75–92) | 73 (64–81)b |
| Specificity (95% CI) | 100 (94–100) | 90 (82–95) | 68 (56–80) | 86 (81–91)b | 71a | 88 (82–92) | 89 (85–93) | 72 (67–77)b |
| PPV (95% CI) | 100a | 77 (61–87) | 67 (55–80) | 72 (62–82)b | NA | 67 (54–79) | 73 (63–83) | 47 (42–53)b |
| NPV (95% CI) | 90a | 95 (90–98) | 74 (63–85) | 87 (81–92)b | NA | 95 (90–97) | 94 (91–97) | 89 (85–92)b |
a 95% CI not reported in the original study; b CCTA + CTP
CAD, coronary artery disease; CI, confidence interval; CT, computed tomography; FFR, fractional flow reserve; ICA, invasive coronary angiography; MBF, myocardial blood flow; N/A, not available; NPV, negative predictive value; PET, positron emission tomography; PPV, positive predictive value; QCA, quantitative coronary angiography.
With regard to dynamic CTP, a wide range of CT-derived MBF cut-off values has been reported by different groups, preventing the identification of a standardized threshold. The implementation of indexed MBF values, in which the ischaemic myocardium was normalized to either the remote myocardium or the 75th percentile of MBF, improved the diagnostic performance of CTP in selected cohorts.89,99,100
CTP vs. FFRCT
Several studies have reported a comparable diagnostic performance of dynamic CTP and FFRCT as well as other stress imaging modalities in the detection of functionally significant coronary lesions.89,90,101 Although FFRCT may have practical advantages in terms of acquisition protocol and dose saving, CTP imaging may be useful when the value of FFRCT is in the ‘grey area’ (i.e. 0.74–0.85)89 or when FFRCT cannot be calculated due to technical reasons (i.e. sub-optimal image quality, diffuse coronary calcifications).
Prognostic value
At the present time, data on the prognostic value of CTP is still scarce.102–106
Clinical utility
The multicenter prospective randomized CRESCENT (Comprehensive Cardiac CT Versus Exercise Testing in Suspected Coronary Artery Disease) II trial demonstrated that a comprehensive cardiac CT protocol including dynamic CTP is an efficient alternative to standard functional testing in patients with stable angina. At 6 months follow-up the rate of ICA with a European Society of Cardiology (ESC) class I indication for revascularization was higher in the cardiac CT group without increasing overall catheterization rate and with a lower rate of additional non-invasive testing.107 The results of the multicentre, randomized controlled CTP-PRO (impact of stress Cardiac computed Tomography myocardial Perfusion on downstream resources and PROgnosis in patients with suspected or known CAD: A multicentre international study) study will provide further insights into the cost-effectiveness of a CCTA+CTP strategy vs. usual care in intermediate–high risk patients with suspected or known CAD.108
Key points
CTP could represent an alternative for other stress tests to evaluate myocardial ischaemia although its exact clinical role remains to be determined.
Structural heart disease
Rationale
Cardiac CT has become crucial in the diagnosis of structural heart disease as well as in the selection of patients for interventional procedures, pre-procedural planning, device sizing, and post-procedural follow-up as shown in Figures 4 and 5. Specific protocols for the preparation, acquisition, and interpretation of cardiac CT in patients with structural heart diseases are reported in detail elsewhere.109–112
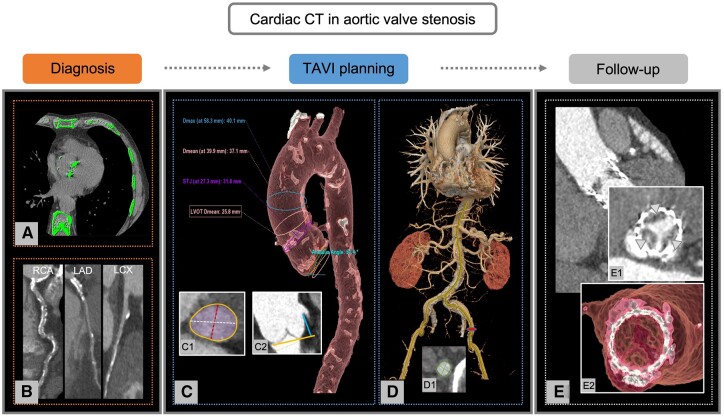
Figure 4.
Role of cardiac CT in patients with aortic valve stenosis. (A–E) Overview of the applications of cardiac CT in patients with aortic valve stenosis. (A and B) Diagnostic work-up of aortic valve stenosis. The severity of aortic valve stenosis can be estimated by the calcium load of aortic valve calculated according to the Agatston method on non-enhanced cardiac CT (A). In addition, CCTA can be used for CAD evaluation. In this example, curves MPRs show diffuse calcifications of RCA, LAD, and LCX (B). (C and D) Pre-procedural planning, device selection, and access sites prior to TAVI. Cardiac CT provides information on aortic root and thoracic aorta measurements (C), aortic annulus sizing with determination of maximum (white), and minimum (red) diameters, area (purple), and perimeter (orange, C1), as well as distance of the coronary ostia from the aortic valve plane (C2). In addition, CT allows the assessment of abdominal aorta and peripheral vascular access. In this case, a transfemoral vascular approach has been evaluated (D) with sizing of maximum and minimum diameters of the left femoral artery (D1). (E) Follow-up after TAVI. Cross-section (E1) and volume rendering (E2) of the prosthetic valve show an example of a patient with HALT (arrowheads). CAD, coronary artery disease; CCTA, computed tomography coronary angiography; CT, computed tomography; HALT, hypo-attenuated leaflet thickening; MPR, multiplanar reconstruction; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; TAVI, transcatheter aortic valve implantation.
Figure 5.
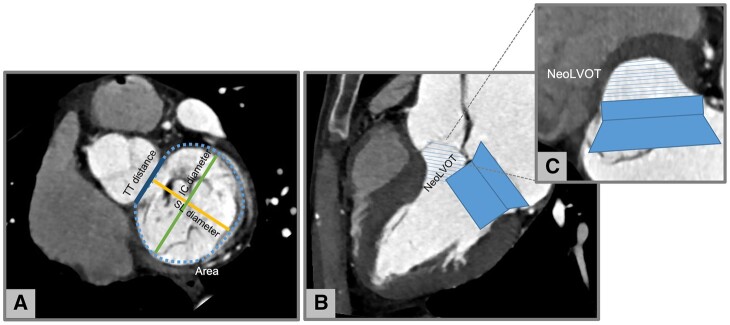
Neo LVOT assessment in native mitral regurgitation with CT. (A) Measurement of mitral annulus size: area, SL, IC, and TT diameters. (B) Virtual valve implantation and (C) identification of the neo LVOT. Images by courtesy of Antonio Esposito and Anna Palmisano, San Raffaele IRCCS, Milan (Italy). CT, computed tomography; IC, inter-commissural; LVOT, left ventricular outflow tract; SL, septal–lateral; TT, trigone to trigone.
Aortic valve stenosis
Diagnosis of aortic valve stenosis
CT calcium score of the aortic valve can be quantified on non-enhanced, electrocardiogram (ECG)-triggered cardiac CT by using the Agatston method113 allowing for flow-independent assessments of aortic stenosis severity when echocardiography measurements are discordant as recommended in the recent ESC heart valve guidelines.114,115 Sex-specific thresholds for differentiating moderate from severe aortic stenosis have been proposed (≥2065 Agatston for men and ≥1274 for women) and validated in a large international study, demonstrating excellent agreement with concordant echocardiography and providing powerful prognostic information.116
Diagnostic work-up before transcatheter aortic valve implantation
Cardiac CT is the preferred imaging modality to select patients suitable for transcatheter aortic valve implantation (TAVI) allowing for more precise measurements of the aortic annulus than 2D echocardiography, thereby optimizing the sizing and selection of the aortic valve prostheses.117 As a consequence, a CT-guided approach resulted in better patient outcomes and less paravalvular regurgitation rate than echocardiography.118,119 In addition, CT provides ancillary information on the distance of the coronary ostia from the aortic valve plane, the number and length of aortic cusps, aortic root and ascending aorta dimensions, optimal fluoroscopic projection109,110 as well as the grade and distribution of annular and sub-annular calcifications.120,121 Finally, CT offers information regarding the optimal vascular access route providing detail on vessel size, tortuosity, course, calcifications, and stenosis which are the main determinants of vascular complications during the procedure.109,110 The information provided by cardiac CT in the context of TAVI is summarized in Figure 4.
The assessment of CAD and comorbidities is another fundamental step in the diagnostic work-up of patients undergoing TAVI.122,123 A recent meta-analysis including 1275 patients reported a sensitivity of 95% and a specificity of 65% for CCTA in the detection of obstructive CAD in TAVI candidates.124 As such, the 2021 ESC guidelines for the management of valvular heart disease suggested that CCTA could be used to rule-out CAD in patients at low risk of atherosclerosis thanks to its high negative predictive value.125Table 8 reports a selection of the major studies investigating the diagnostic performance of CCTA for pre-TAVI coronary assessment.126–133
Table 8.
Major studies evaluating the diagnostic performance of CCTA at patient level for the detection of obstructive CAD (coronary stenosis ≥ 50%) in TAVI candidates
| Trial | Pontone et al.131 | Andreini et al.126 | Hamdan et al.127 | Harris et al. 128 | Opolski et al.130 | Matsumoto et al.129 | Rossi et al.132 | Strong et al.133 |
|---|---|---|---|---|---|---|---|---|
| Sample size | 60 | 325 | 115 | 100 | 475 | 60 | 140 | 200 |
| Prevalence of obstructive CAD (%) | 43 | N/A | 43 | 73 | 57 | 40 | 41 | 34 |
| Sensitivity (95% CI) | 88 (76–100) | 89a | 96a | 98 (92–100) | 98 (95–99) | 91a | 91 (81–96) | 100 (94–100) |
| Specificity (95% CI) | 88 (77–99) | 90a | 73a | 55 (35–74) | 37 (30–44) | 58a | 54 (44–65) | 42 (33–50) |
| PPV (95% CI) | 85 (72–99) | 80a | 72a | 85 (76–92) | 67 (62–72) | 59a | 58 (48–68) | 47 (44–51) |
| NPV (95% CI) | 91 (81–100) | 95a | 96a | 93 (69–99) | 94 (86–98) | 91a | 90 (78–95) | 100 (92–100) |
| Coronary stents included | + | + | + | + | + | - | - | - |
| CABG included | + | + | + | + | + | - | - | - |
95% confidence intervals not reported in the original study.
CABG, coronary artery by-pass graft; CAD, coronary artery disease; CI, confidence intervals; N/A, not available; NPV, negative predictive value; PPV, positive predictive value.
Post-procedural follow-up
Although cardiac CT is not routinely performed after TAVI, its use is advocated when there is concern regarding valve thrombosis, infective endocarditis, or structural degeneration. Regarding endocarditis, CT is particularly helpful in the detection of aortic root abscesses and pseudoaneurysms. In patients with valve thrombosis, cardiac CT allows for the detection of hypoattenuating leaflet thickening and restricted leaflet motion, which are often signs of leaflet thrombus.134
Mitral valve disease
Diagnosis of mitral valve disease
CT has shown promising results in identifying the aetiology,135,136 in detecting mitral valve prolapse,137 and in quantifying the regurgitation volume by a combined approach with echocardiography.138
Diagnostic work-up before percutaneous mitral valve interventions
Thanks to the recent advancements in percutaneous mitral valve interventions, CT is playing an increasing role in the pre-procedural work-up of patients with mitral valve disease, helping determine device suitability111,139 as demonstrated in Figure 5. Specifically, cardiac CT has been used to evaluate the mitral annulus size and geometry, the relationship of the mitral valve with the circumflex coronary artery and the distribution of mitral annular calcifications.111 Pre-procedural CT based simulation has shown to be helpful in identifying patients at higher risk for post-implant left ventricular outflow tract obstruction.140,141 Ancillary information that can be further extracted from CT images are the optimal fluoroscopic view for the percutaneous procedure142 and the appropriate access point for the transapical puncture.143
Atrial fibrillation
In the context of atrial fibrillation, cardiac CT has been widely used in the pre-procedural planning of catheter ablation, providing detailed anatomic delineation of left atrium and pulmonary veins.144 In addition, delayed imaging (60–90 s) with CT has been proposed as an alternative method to rule out left atrial appendage (LAA) thrombus thanks to its high sensitivity of 100% and specificity of 99%.145 Of note, anatomic features of the left atrium as detected on cardiac CT have been correlated with patient outcomes following cryoablation.146
LAA closure
Nowadays cardiac CT is considered a valid alternative to the reference standard transoesophageal echocardiography (TOE) for pre-procedural planning prior to LAA closure.112 Thanks to its high isotropic spatial resolution and 3D datasets, cardiac CT is ideal for the evaluation of LAA morphology and dimensions, as well as its relationship with surrounded structures, thereby guiding appropriate device selection.112 In addition, CT appears a promising alternative to TOE for exclusion of LAA thrombus before the procedure145 and for device surveillance after LAA closure.147
Key points
CT calcium score of the aortic valve is appropriate to assess disease severity in patients with discordant echocardiographic measurements.
Cardiac CT is appropriate prior to TAVI to assess the size of aortic annulus and its distance from the coronary ostia, the anatomy and dimensions of the aortic root, and the distribution of annular and sub-annular calcifications.
Cardiac CT is appropriate prior to TAVI to select the optimum vascular access route.
In the absence of obstructive CAD on CCTA, catheterization could be avoided.
Post-TAVI cardiac CT is appropriate when there is concern of valve thrombosis, infective endocarditis, or structural valve degeneration.
Cardiac CT is appropriate for pre-procedural planning of transcatheter mitral valve intervention.
Cardiac CT is appropriate for pre-procedural planning of atrial fibrillation and ventricular tachycardia ablation.
Cardiac CT with delayed acquisition (60–90 s) is appropriate as an alternative to TOE to rule-out left atrium and LAA thrombosis.
Cardiac CT is appropriate for pre-procedural planning of LAA closure.
Cardiomyopathies
Rationale
Cardiac CT can be helpful in the setting of cardiomyopathies as shown in Figure 6.
Figure 6.
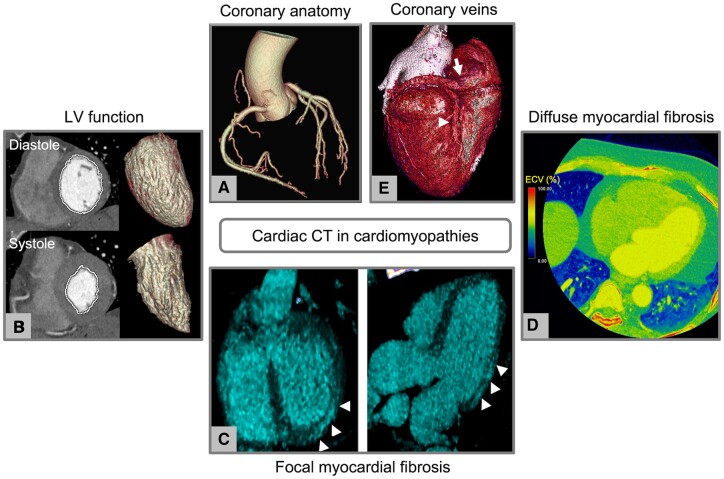
Cardiac CT in cardiomyopathies. (A–E) Overview of the potential information derived from cardiac CT in patients with cardiomyopathies. (A) Evaluation of coronary anatomy; (B) functional assessment with calculation of ventricular volumes and ejection fraction; (C) identification of focal myocardial fibrosis with late iodine enhancement technique (arrowheads); (D) assessment of diffuse fibrosis using extracellular volume measurement; and (E) assessment of the coronary venous system prior to cardiac resynchronization therapy (arrow, coronary sinus; arrowhead, posterior interventricular vein). CT, computed tomography.
Functional evaluation
Cardiac CT can reliably quantify cardiac volumes and ejection fraction of left and right ventricle, with excellent accuracy compared with the reference standard cardiac magnetic resonance (CMR).148–151 A retrospectively ECG-gated acquisition throughout the cardiac cycle is required, hence leading to an average radiation dose of ∼10 mSv in men and 11 mSv in women.150 ECG-tube current modulation is often implemented to minimize radiation exposure.
CAD assessment
Current ESC guidelines on acute and chronic heart failure recommend CCTA in patients with low-intermediate pre-test probability of CAD or in those with an equivocal non-invasive stress test in order to exclude the diagnosis of CAD (Class IIa).152
Focal and diffuse fibrosis
While CMR is the imaging modality of choice for cardiomyopathy phenotyping and risk assessment,153 cardiac CT may alternatively be used in patients with CMR contraindications. Late iodine CT enhancement imaging has shown good agreement with CMR in the localization and pattern recognition of myocardial fibrosis in patients with heart failure.154–157 Most recently, the feasibility of extracellular volume (ECV) calculation, which is a surrogate marker of diffuse myocardial fibrosis, has been also demonstrated using different CT technologies and CT protocols.158–160 Several studies demonstrated a good correlation between CT-derived ECV and CMR-derived ECV in heart failure patient cohorts.161–163 Furthermore, when compared with healthy subjects, CT-derived ECV was significantly higher in patients with hypertrophic cardiomyopathy, dilated cardiomyopathy, sarcoidosis, and amyloidosis.159 Currently, no studies on the prognostic role of CT-derived ECV are available in patients with cardiomyopathies.
Treatment planning before cardiac resynchronization therapy
Cardiac CT can visualize accurately the coronary venous system which can be helpful for left ventricle lead implantation before cardiac resynchronization therapy (CRT), particularly in cases where the coronary sinus has previously proved difficult to intubate.164 Little data are available on the role of cardiac CT for the assessment of scar location165 and of phrenic nerve anatomy in relation to target veins166 before CRT.
Key points
CCTA is appropriate in patients with reduced ejection fraction and low–intermediate pre-test probability of CAD.
In selected cases when echocardiography and/or CMR are unavailable or not interpretable, cardiac CT could be helpful for the evaluation of cardiac volumes, ejection fraction, and regional wall motion abnormalities.
In selected cases when CMR is unavailable or not interpretable, late iodine enhancement CT imaging could be helpful to identify the aetiology of cardiomyopathy.
Cardiac CT is appropriate in the evaluation of coronary vein anatomy before left ventricle lead placement.
Limited data prevent recommendation on the role of CT-derived ECV for the detection of diffuse fibrosis.
Limited data prevent recommendation on the role of late iodine CT enhancement imaging for myocardial scar detection and localization before CRT.
Coronary anomalies
CCTA provides detailed information on coronary artery architecture and relationship to the surrounding cardiac structures, with superior ability to ICA.167 In particular, CCTA is highly accurate in the detection of anomalous coronary arteries and in the classification of high-risk anatomic features (i.e. slit-like ostium, inter-arterial course, intramural course and acute take-off angle) which have been associated with an increased risk of myocardial ischaemia, ventricular arrhythmias and sudden cardiac death.168
Congenital heart disease
Cardiac CT is increasingly used for the initial diagnosis of congenital heart diseases (CHD) in neonates and children as demonstrated in Figure 7.169 Cardiac CT offers precise anatomical and functional information of cardiac structures, great vessels, and associated abnormalities of lung parenchyma.169–171 Moreover, rapid acquisition allows for minimal motion artefacts, reducing the need for sedation whilst preserving image quality. In addition, thanks to evolving technical developments and implementation of tailored protocols, radiation dose can be substantially reduced.172 Beyond diagnosis, cardiac CT is becoming a common tool to plan interventional and surgical procedures and to evaluate postoperative anatomy and complications as shown in Figure 8.171 Due to the complexity and heterogeneity of CHD, specific training and expertise are essentials in this setting.172
Figure 7.
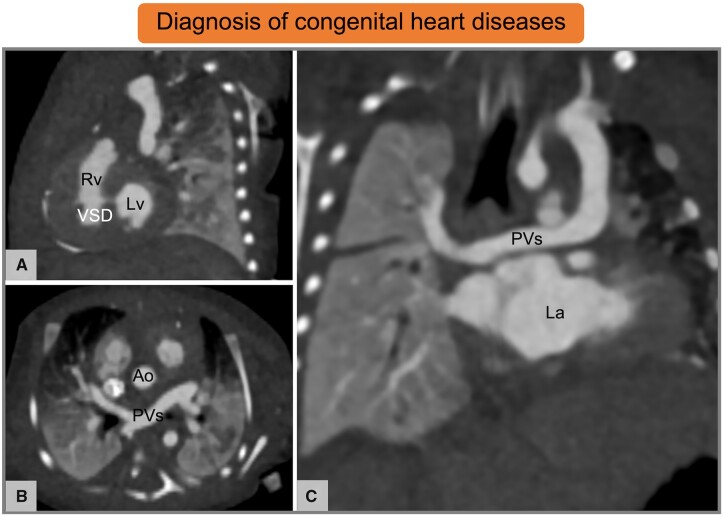
Cardiac CT in neonate with suspected congenital heart disease. (A–C) Ten-day-old female patient with partial anomalous pulmonary venous return and a small muscular VSD. Images by courtesy of Francesco Bianco, Ospedale Riuniti Ancona, Ancona (Italy). Ao, aorta; CT, computed tomography; La, left atrium; Lv, left ventricle; PVs, pulmonary veins; Rv, right ventricle; VSD, ventricular septal defect.
Figure 8.
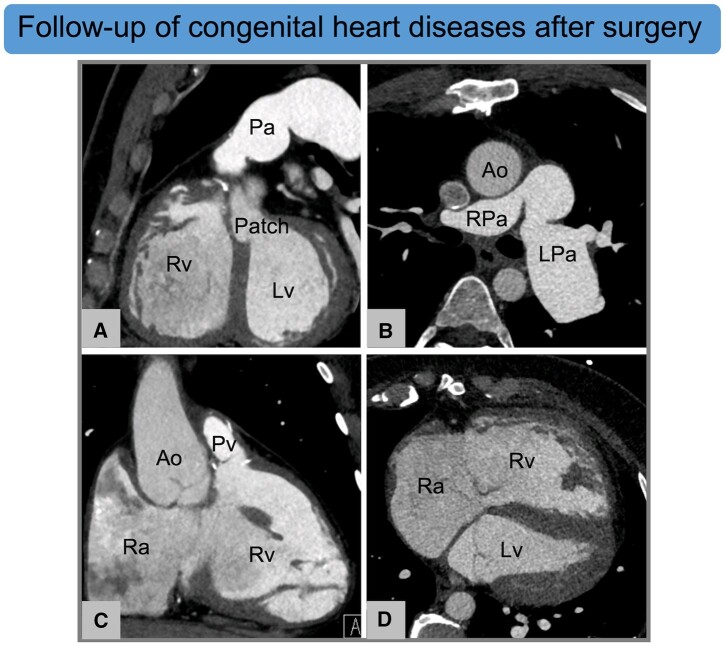
Cardiac CT for the postoperative follow-up of congenital heart disease. (A–D) Thirty-one-year-old male with repaired Tetralogy of Fallot, postsurgical LPa aneurism and Rv dilatation. Ventricular septal defect has been repaired by using a patch (A). Images by courtesy of Francesco Bianco, Ospedale Riuniti Ancona, Ancona (Italy). Ao, aorta; CT, computed tomography; LPa, left pulmonary artery; Lv, left ventricle; Pa, pulmonary artery; Pv, pulmonary vein; Ra, right atrium; Rpa, right pulmonary artery; Rv, right ventricle.
Key points
Cardiac CT is appropriate in patients with suspected or known coronary artery anomalies.
Cardiac CT could be appropriate in neonates and children with suspected or known CHD in the presence of complex anatomy, extra-cardiac findings, CMR incompatible device, or poor CMR image quality.
Cardiac CT is appropriate in neonates, children, and adult patients for coronary artery imaging.
Cardiac CT is appropriate in neonates, children, and adult patients to plan interventional and surgical procedures.
Evaluation of coronary anatomy in aortic dissection, aortic aneurysms, and pulmonary embolism scans
Since scans performed for aortic dissection and pulmonary embolism have been reported to be positive only in a small proportion of patients, evaluation of coronary arteries could be beneficial to explain the presenting symptoms.173 In addition, if aortic dissection is the final diagnosis, knowledge of coronary atherosclerotic burden is essential to decide for concomitant coronary artery by-pass surgery. Similarly, thoracic aortic aneurisms are often associated with CAD174 and, therefore, coronary evaluation is critical also in this category of patients.
Performing a single-phase, ECG-triggered CT angiography can improve image quality, thus allowing for the evaluation of coronary arteries as well as facilitating the detection of pulmonary emboli in small branches and a more accurate estimation of ascending aorta diameters. Nevertheless, an obstacle to the implementation of this approach is the requirement of a specific training for the interpreting physician.
Key points
ECG-triggered CT angiography is appropriate in patients presenting with symptoms of aortic dissection and pulmonary embolism to allow for the evaluation of coronary arteries (depending on the CT technology available and the training of the interpreting physician).
ECG-triggered CT angiography is appropriate at the first diagnosis and follow-up after repair of thoracic aortic aneurysm to allow for the evaluation of coronary arteries (depending on the CT technology available and the training of the interpreting physician).
Radiomics and artificial intelligence
Rationale
The amount of imaging data has increased dramatically in recent years and so therefore has the quantitative information that can be extracted from them. The traditional image analysis approach based on visual assessment and interpretation of radiological images guided by previous training and experience may not capture all the information contained within these scans. In this context, radiomics and artificial intelligence (AI) techniques have been used at different levels, from image post-processing to diagnosis and prognosis. Although a detailed overview of the basic principles of radiomics and AI techniques is beyond the aim of this article, a brief explanation of these novel methods is provided in Table 9.175–177
Table 9.
Radiomics and data analytic methods applied to cardiac imaging
| Technique | Definition175–177 |
|---|---|
| Radiomics | Process by which quantitative features describing the texture and spatial complexity of the tissue are extracted from CT images. |
| Machine learning | Sub-division of artificial intelligence, which uses computer algorithms to learn from data analysis, without being programmed for that specific task. Differently from conventional multivariable analysis based on probability theory, it derives pure statistical models to detect non-linear relationships between a large number of potential predictor variables. |
| Deep learning | Sub-division of machine learning, which uses deep neural networks to solve difficult problems. |
CT, computed tomography.
Image post-processing
Deep learning (DL) methods have been investigated to improve the fully automated segmentation of different cardiac structures. Validated applications include quantification of coronary calcium178 and epicardial adipose tissue,179 where DL techniques demonstrate good agreement with corresponding manual assessments. Similarly, on-site computation of FFRCT based on machine learning (ML) showed equal performance to FFRCT derived from the conventional computed flow dynamics approach in the detection of haemodynamically significant stenoses while reducing processing time.62
Diagnosis
Radiomics and ML algorithms have been used to optimize the extraction of information from cardiac CT images to facilitate CAD diagnosis. Examples of this application include automated stenosis grading,180 plaque phenotyping,181,182 CAD-RADS classification,183 detection of lesion-specific ischaemia,184 and identification of the napkin-ring sign.181
Prognosis
The accuracy of ML algorithms to predict adverse outcome has been evaluated in several studies.185,186 In a recent study including 66 636 asymptomatic patients , Nakanishi et al.185 demonstrated that a ML model including clinical variables, coronary calcium score, and CT variables extracted from non-contrast acquisition was superior to Atherosclerotic Cardiovascular Disease score and coronary calcium score alone in the prediction of cardiovascular and CAD death.
Key points
Several AI algorithms, mainly involved in automated image segmentation, have been already implemented in the clinical routine.
Summary
In recent years, developments in CT technology have increased dramatically the amount of information that can be derived from cardiac CT. CCTA offers unrivalled information about coronary atherosclerotic plaque, with its ability to identify, characterize, and quantify coronary atherosclerosis allowing for accurate patient risk stratification and monitoring of treatment effect. Emerging technologies, such as CTP imaging and computational flow dynamics, have overcome some of the limitations of CCTA, thus improving the identification of patients who might benefit from coronary revascularization. In near future, the next generation of randomized clinical trials will likely incorporate cardiac CT features extracted using radiomics or artificial intelligence-based models. These are likely to expand yet further the clinical applications of cardiac CT described in this consensus document.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Conflict of interest: G.P. receives Speaker honorarium and/or research grant from GE Heatchare, BRACCO, Bhoeringer. M.R.D. has received speaker fees from Pfizer and Novartis. He has received consultancy fees from Novartis, Jupiter Bioventures and Silence therapeutics. K.N. has received unresticted institutional research support from Siemens Healthineers, Bayer, and HeartFlow Inc. He has received consultancy fees from Siemens Medical Solutions USA and honoraria for lectures by Bayer. He owns stock options at Lumen Therapeutics. P.M.-H. is shareholder of Neumann Medical Ltd. B.C. is supported by research grant from Abbott. He has received honoraria for lectures by Philips. He is part of the data safety monitoring board/advosry board of Daichi Sankyo and Lilly. The remaining authors have nothing to disclose.
Funding
M.R.D. is supported by the British Heart Foundation (FS/14/78/31020) and is the recipient of the Sir Jules Thorn Award for Biomedical Research 2015 (15/JTA). K.N. is supported by research grants from the NIH/NHLBI (NIH R01-HL141712; NIH R01- HL146754).
Supplementary Material
Contributor Information
Gianluca Pontone, Centro Cardiologico Monzino IRCCS, Via C. Parea 4, 20138 Milan, Italy.
Alexia Rossi, Department of Nuclear Medicine, University Hospital, Zurich, Switzerland; Center for Molecular Cardiology, University of Zurich, Zurich, Switzerland.
Marco Guglielmo, Centro Cardiologico Monzino IRCCS, Via C. Parea 4, 20138 Milan, Italy.
Marc R Dweck, Centre for Cardiovascular Sciences, University of Edinburgh, Edinburgh, UK.
Oliver Gaemperli, Heart Clinic, Hirslanden Hospital, Zurich, Switzerland.
Koen Nieman, Department of Radiology and Division of Cardiovascular Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Francesca Pugliese, Department of Cardiology, Barts Heart Centre, Barts Health NHS Trust, London, UK; Centre for Cardiovascular Medicine and Devices, William Harvey Research Institute, Queen Mary University of London, London, UK.
Pal Maurovich-Horvat, MTA-SE Cardiovascular Imaging Research Group, Medical Imaging Centre, Semmelweis University, Budapest, Hungary.
Alessia Gimelli, Fondazione CNR/Regione Toscana “Gabriele Monasterio”, Pisa, Italy.
Bernard Cosyns, Department of Cardiology, CHVZ (Centrum voor Hart en Vaatziekten), ICMI (In Vivo Cellular and Molecular Imaging) Laboratory, Universitair ziekenhuis Brussel, Brussel, Belgium.
Stephan Achenbach, Department of Cardiology, Friedrich-Alexander-University of Erlangen, Erlangen, Germany.
References
- 1. Chang HJ, Lin FY, Lee SE, Andreini D, Bax J, Cademartiri F et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol 2018;71:2511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SCOT-HEART Investigators; Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924–33. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135:2320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–70. [DOI] [PubMed] [Google Scholar]
- 5. Mushtaq S, De Araujo Goncalves P, Garcia-Garcia HM, Pontone G, Bartorelli AL, Bertella E et al. Long-term prognostic effect of coronary atherosclerotic burden: validation of the computed tomography-Leaman score. Circ Cardiovasc Imaging 2015;8:e002332. [DOI] [PubMed] [Google Scholar]
- 6. Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging 2018;11:1475–84. [DOI] [PubMed] [Google Scholar]
- 7. Papadopoulou SL, Neefjes LA, Schaap M, Li HL, Capuano E, van der Giessen AG et al. Detection and quantification of coronary atherosclerotic plaque by 64-slice multidetector CT: a systematic head-to-head comparison with intravascular ultrasound. Atherosclerosis 2011;219:163–70. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol 2006;47:1655–62. [DOI] [PubMed] [Google Scholar]
- 9. Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319–26. [DOI] [PubMed] [Google Scholar]
- 10. Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49–57. [DOI] [PubMed] [Google Scholar]
- 11. Maurovich-Horvat P, Hoffmann U, Vorpahl M, Nakano M, Virmani R, Alkadhi H. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc Imaging 2010;3:440–4. [DOI] [PubMed] [Google Scholar]
- 12. Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol 2018;3:144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol 2019;73:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nerlekar N, Ha FJ, Cheshire C, Rashid H, Cameron JD, Wong DT et al. Computed tomographic coronary angiography-derived plaque characteristics predict major adverse cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Imaging 2018;11:e006973. [DOI] [PubMed] [Google Scholar]
- 15. Williams MC, Earls JP, Hecht H. Quantitative assessment of atherosclerotic plaque, recent progress and current limitations. J Cardiovasc Comput Tomogr 2021. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 16. Feuchtner G, Kerber J, Burghard P, Dichtl W, Friedrich G, Bonaros N et al. The high-risk criteria low-attenuation plaque <60 HU and the napkin-ring sign are the most powerful predictors of MACE: a long-term follow-up study. Eur Heart J Cardiovasc Imaging 2017;18:772–9. [DOI] [PubMed] [Google Scholar]
- 17. Finck T, Stojanovic A, Will A, Hendrich E, Martinoff S, Hausleiter J et al. Long-term prognostic value of morphological plaque features on coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2020;21:237–48. [DOI] [PubMed] [Google Scholar]
- 18. Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 2015;66:337–46. [DOI] [PubMed] [Google Scholar]
- 19. Nadjiri J, Hausleiter J, Jahnichen C, Will A, Hendrich E, Martinoff S et al. Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. J Cardiovasc Comput Tomogr 2016;10:97–104. [DOI] [PubMed] [Google Scholar]
- 20. Senoner T, Plank F, Barbieri F, Beyer C, Birkl K, Widmann G et al. Added value of high-risk plaque criteria by coronary CTA for prediction of long-term outcomes. Atherosclerosis 2020;300:26–33. [DOI] [PubMed] [Google Scholar]
- 21. Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish Computed Tomography of the HEART). Circulation 2020;141:1452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamamoto H, Kihara Y, Fujimoto S, Daida H, Kobuke K, Iwanaga Y et al. Predictive value of the coronary artery calcium score and advanced plaque characteristics: post hoc analysis of the PREDICT registry. J Cardiovasc Comput Tomogr 2021;15:148–53. [DOI] [PubMed] [Google Scholar]
- 23. Lee JM, Choi G, Koo BK, Hwang D, Park J, Zhang J et al. Identification of high-risk plaques destined to cause acute coronary syndrome using coronary computed tomographic angiography and computational fluid dynamics. JACC Cardiovasc Imaging 2019;12:1032–43. [DOI] [PubMed] [Google Scholar]
- 24. Dweck MR, Maurovich-Horvat P, Leiner T, Cosyns B, Fayad ZA, Gijsen FJH et al. Contemporary rationale for non-invasive imaging of adverse coronary plaque features to identify the vulnerable patient: a Position Paper from the European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2020;21:1177–83. [DOI] [PubMed] [Google Scholar]
- 25. Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andelius L, Mortensen MB, Norgaard BL, Abdulla J. Impact of statin therapy on coronary plaque burden and composition assessed by coronary computed tomographic angiography: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2018;19:850–8. [DOI] [PubMed] [Google Scholar]
- 27. Lee J, Nakanishi R, Li D, Shaikh K, Shekar C, Osawa K et al. Randomized trial of rivaroxaban versus warfarin in the evaluation of progression of coronary atherosclerosis. Am Heart J 2018;206:127–30. [DOI] [PubMed] [Google Scholar]
- 28. Win TT, Nakanishi R, Osawa K, Li D, Susaria SS, Jayawardena E et al. Apixaban versus warfarin in evaluation of progression of atherosclerotic and calcified plaques (prospective randomized trial). Am Heart J 2019;212:129–33. [DOI] [PubMed] [Google Scholar]
- 29. Auscher S, Heinsen L, Nieman K, Vinther KH, Logstrup B, Moller JE et al. Effects of intensive lipid-lowering therapy on coronary plaques composition in patients with acute myocardial infarction: assessment with serial coronary CT angiography. Atherosclerosis 2015;241:579–87. [DOI] [PubMed] [Google Scholar]
- 30. Inoue K, Motoyama S, Sarai M, Sato T, Harigaya H, Hara T et al. Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging 2010;3:691–8. [DOI] [PubMed] [Google Scholar]
- 31. Lee SE, Sung JM, Andreini D, Budoff MJ, Cademartiri F, Chinnaiyan K et al. Differential association between the progression of coronary artery calcium score and coronary plaque volume progression according to statins: the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging (PARADIGM) study. Eur Heart J Cardiovasc Imaging 2019;20:1307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Z, Hou Z, Yin W, Liu K, Gao Y, Xu H et al. Effects of statin therapy on progression of mild noncalcified coronary plaque assessed by serial coronary computed tomography angiography: a multicenter prospective study. Am Heart J 2016;180:29–38. [DOI] [PubMed] [Google Scholar]
- 33. Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015;2:e52-63–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Rosendael AR, van den Hoogen IJ, Gianni U, Ma X, Tantawy SW, Bax AM et al. Association of statin treatment with progression of coronary atherosclerotic plaque composition. JAMA Cardiol 2021;6:1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeb I, Li D, Nasir K, Malpeso J, Batool A, Flores F et al. Effect of statin treatment on coronary plaque progression—a serial coronary CT angiography study. Atherosclerosis 2013;231:198–204. [DOI] [PubMed] [Google Scholar]
- 36. Lee SE, Chang HJ, Rizvi A, Hadamitzky M, Kim YJ, Conte E et al. Rationale and design of the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography IMaging (PARADIGM) registry: a comprehensive exploration of plaque progression and its impact on clinical outcomes from a multicenter serial coronary computed tomographic angiography study. Am Heart J 2016;182:72–9. [DOI] [PubMed] [Google Scholar]
- 37. Kim U, Leipsic JA, Sellers SL, Shao M, Blanke P, Hadamitzky M et al. Natural history of diabetic coronary atherosclerosis by quantitative measurement of serial coronary computed tomographic angiography: results of the PARADIGM study. JACC Cardiovasc Imaging 2018;11:1461–71. [DOI] [PubMed] [Google Scholar]
- 38. Lee SE, Sung JM, Andreini D, Al-Mallah MH, Budoff MJ, Cademartiri F et al. Differences in progression to obstructive lesions per high-risk plaque features and plaque volumes with CCTA. JACC Cardiovasc Imaging 2020;13:1409–17. [DOI] [PubMed] [Google Scholar]
- 39. Shaw LJ, Blankstein R, Bax JJ, Ferencik M, Bittencourt MS, Min JK et al. Society of cardiovascular computed tomography / North American society of cardiovascular imaging—expert consensus document on coronary CT imaging of atherosclerotic plaque. J Cardiovasc Comput Tomogr 2020;15:93–109. [DOI] [PubMed] [Google Scholar]
- 40. Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342–58. [DOI] [PubMed] [Google Scholar]
- 41. Hecht HS, Blaha MJ, Kazerooni EA, Cury RC, Budoff M, Leipsic J et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr 2018;12:185–91. [DOI] [PubMed] [Google Scholar]
- 42. Cury RC, Abbara S, Achenbach S, Agatston A, Berman DS, Budoff MJ et al. CAD-RADS(TM) coronary artery disease—reporting and data system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2016;10:269–81. [DOI] [PubMed] [Google Scholar]
- 43. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 44. Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017;9: eaal2658. [DOI] [PubMed] [Google Scholar]
- 45. Lin A, Nerlekar N, Yuvaraj J, Fernandes K, Jiang C, Nicholls SJ et al. Pericoronary adipose tissue computed tomography attenuation distinguishes different stages of coronary artery disease: a cross-sectional study. Eur Heart J Cardiovasc Imaging 2021;22:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392:929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oikonomou EK, Antonopoulos AS, Schottlander D, Marwan M, Mathers C, Tomlins P et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: integration in clinical care as a prognostic medical device. Cardiovasc Res 2021;117:2677–90. [DOI] [PubMed] [Google Scholar]
- 48. Oikonomou EK, Desai MY, Marwan M, Kotanidis CP, Antonopoulos AS, Schottlander D et al. Perivascular fat attenuation index stratifies cardiac risk associated with high-risk plaques in the CRISP-CT study. J Am Coll Cardiol 2020;76:755–7. [DOI] [PubMed] [Google Scholar]
- 49. Elnabawi YA, Oikonomou EK, Dey AK, Mancio J, Rodante JA, Aksentijevich M et al. Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation index. JAMA Cardiol 2019;4:885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dai X, Yu L, Lu Z, Shen C, Tao X, Zhang J. Serial change of perivascular fat attenuation index after statin treatment: insights from a coronary CT angiography follow-up study. Int J Cardiol 2020;319:144–9. [DOI] [PubMed] [Google Scholar]
- 51. De Bruyne B, Baudhuin T, Melin JA, Pijls NH, Sys SU, Bol A et al. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation 1994;89:1013–22. [DOI] [PubMed] [Google Scholar]
- 52. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U et al. ; ESC Scientific Document Group. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165.30165437 [Google Scholar]
- 53. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61:2233–41. [DOI] [PubMed] [Google Scholar]
- 54. Ko BS, Cameron JD, Munnur RK, Wong DTL, Fujisawa Y, Sakaguchi T et al. Noninvasive CT-derived FFR based on structural and fluid analysis: a comparison with invasive FFR for detection of functionally significant stenosis. JACC Cardiovasc Imaging 2017;10:663–73. [DOI] [PubMed] [Google Scholar]
- 55. Li Z, Zhang J, Xu L, Yang W, Li G, Ding D et al. Diagnostic accuracy of a fast computational approach to derive fractional flow reserve from coronary CT angiography. JACC Cardiovasc Imaging 2020;13:172–5. [DOI] [PubMed] [Google Scholar]
- 56. Pontone G, Guaricci AI, Palmer SC, Andreini D, Verdecchia M, Fusini L et al. Diagnostic performance of non-invasive imaging for stable coronary artery disease: a meta-analysis. Int J Cardiol 2020;300:276–81. [DOI] [PubMed] [Google Scholar]
- 57. Driessen RS, Danad I, Stuijfzand WJ, Raijmakers PG, Schumacher SP, van Diemen PA et al. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol 2019;73:161–73. [DOI] [PubMed] [Google Scholar]
- 58. Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 2011;58:1989–97. [DOI] [PubMed] [Google Scholar]
- 59. Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Norgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol 2014;63:1145–55. [DOI] [PubMed] [Google Scholar]
- 61. Sand NPR, Veien KT, Nielsen SS, Norgaard BL, Larsen P, Johansen A et al. Prospective comparison of FFR derived from coronary CT angiography with SPECT perfusion imaging in stable coronary artery disease: the ReASSESS study. JACC Cardiovasc Imaging 2018;11:1640–50. [DOI] [PubMed] [Google Scholar]
- 62. Coenen A, Kim YH, Kruk M, Tesche C, De Geer J, Kurata A et al. Diagnostic accuracy of a machine-learning approach to coronary computed tomographic angiography-based fractional flow reserve: result from the MACHINE consortium. Circ Cardiovasc Imaging 2018;11:e007217. [DOI] [PubMed] [Google Scholar]
- 63. Coenen A, Lubbers MM, Kurata A, Kono A, Dedic A, Chelu RG et al. Fractional flow reserve computed from noninvasive CT angiography data: diagnostic performance of an on-site clinician-operated computational fluid dynamics algorithm. Radiology 2015;274:674–83. [DOI] [PubMed] [Google Scholar]
- 64. Nakazato R, Park HB, Berman DS, Gransar H, Koo BK, Erglis A et al. Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity: results from the DeFACTO study. Circ Cardiovasc Imaging 2013;6:881–9. [DOI] [PubMed] [Google Scholar]
- 65. Pontone G, Weir-McCall JR, Baggiano A, Del Torto A, Fusini L, Guglielmo M et al. Determinants of rejection rate for coronary CT angiography fractional flow reserve analysis. Radiology 2019;292:597–605. [DOI] [PubMed] [Google Scholar]
- 66. Norgaard BL, Gaur S, Leipsic J, Ito H, Miyoshi T, Park SJ et al. Influence of coronary calcification on the diagnostic performance of CT angiography derived FFR in coronary artery disease: a substudy of the NXT trial. JACC Cardiovasc Imaging 2015;8:1045–55. [DOI] [PubMed] [Google Scholar]
- 67. Gaur S, Taylor CA, Jensen JM, Botker HE, Christiansen EH, Kaltoft AK et al. FFR derived from coronary CT angiography in nonculprit lesions of patients with recent STEMI. JACC Cardiovasc Imaging 2017;10:424–33. [DOI] [PubMed] [Google Scholar]
- 68. Cook CM, Petraco R, Shun-Shin MJ, Ahmad Y, Nijjer S, Al-Lamee R et al. Diagnostic accuracy of computed tomography-derived fractional flow reserve: a systematic review. JAMA Cardiol 2017;2:803–10. [DOI] [PubMed] [Google Scholar]
- 69. Ihdayhid AR, Norgaard BL, Gaur S, Leipsic J, Nerlekar N, Osawa K et al. Prognostic value and risk continuum of noninvasive fractional flow reserve derived from coronary CT angiography. Radiology 2019;292:343–51. [DOI] [PubMed] [Google Scholar]
- 70. Douglas PS, De Bruyne B, Pontone G, Patel MR, Norgaard BL, Byrne RA et al. ; PLATFORM Investigators. 1-year outcomes of FFRCT-guided care in patients with suspected coronary disease: the PLATFORM study. J Am Coll Cardiol 2016;68:435–45. [DOI] [PubMed] [Google Scholar]
- 71. Norgaard BL, Terkelsen CJ, Mathiassen ON, Grove EL, Botker HE, Parner E et al. Coronary CT angiographic and flow reserve-guided management of patients with stable ischemic heart disease. J Am Coll Cardiol 2018;72:2123–34. [DOI] [PubMed] [Google Scholar]
- 72. Patel MR, Norgaard BL, Fairbairn TA, Nieman K, Akasaka T, Berman DS et al. 1-year impact on medical practice and clinical outcomes of FFRCT: the ADVANCE registry. JACC Cardiovasc Imaging 2020;13(1 Pt 1):97–105. [DOI] [PubMed] [Google Scholar]
- 73. Fairbairn TA, Nieman K, Akasaka T, Norgaard BL, Berman DS, Raff G et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: lessons from the ADVANCE registry. Eur Heart J 2018;39:3701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Curzen N, Nicholas Z, Stuart B, Wilding S, Hill K, Shambrook J et al. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: the FORECAST randomized trial. Eur Heart J 2021; 42: 3844–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Collet C, Miyazaki Y, Ryan N, Asano T, Tenekecioglu E, Sonck J et al. Fractional flow reserve derived from computed tomographic angiography in patients with multivessel CAD. J Am Coll Cardiol 2018;71:2756–69. [DOI] [PubMed] [Google Scholar]
- 76. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219–27. [PubMed] [Google Scholar]
- 77. Collet C, Onuma Y, Andreini D, Sonck J, Pompilio G, Mushtaq S et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J 2018;39:3689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Andreini D, Modolo R, Katagiri Y, Mushtaq S, Sonck J, Collet C et al. ; SYNTAX III REVOLUTION Investigators. Impact of fractional flow reserve derived from coronary computed tomography angiography on heart team treatment decision-making in patients with multivessel coronary artery disease: insights from the SYNTAX III REVOLUTION trial. Circ Cardiovasc Interv 2019;12:e007607. [DOI] [PubMed] [Google Scholar]
- 79. Modi BN, Sankaran S, Kim HJ, Ellis H, Rogers C, Taylor CA et al. Predicting the physiological effect of revascularization in serially diseased coronary arteries. Circ Cardiovasc Interv 2019;12:e007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Patel AR, Bamberg F, Branch K, Carrascosa P, Chen M, Cury RC et al. Society of cardiovascular computed tomography expert consensus document on myocardial computed tomography perfusion imaging. J Cardiovasc Comput Tomogr 2020;14:87–100. [DOI] [PubMed] [Google Scholar]
- 81. Rossi A, Merkus D, Klotz E, Mollet N, de Feyter PJ, Krestin GP. Stress myocardial perfusion: imaging with multidetector CT. Radiology 2014;270:25–46. [DOI] [PubMed] [Google Scholar]
- 82. Danad I, Szymonifka J, Schulman-Marcus J, Min JK. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur Heart J Cardiovasc Imaging 2016;17:836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pontone G, Baggiano A, Andreini D, Guaricci AI, Guglielmo M, Muscogiuri G et al. Stress computed tomography perfusion versus fractional flow reserve CT derived in suspected coronary artery disease: the PERFECTION study. JACC Cardiovasc Imaging 2019;12(8 Pt 1):1487–97. [DOI] [PubMed] [Google Scholar]
- 84. Bettencourt N, Chiribiri A, Schuster A, Ferreira N, Sampaio F, Pires-Morais G et al. Direct comparison of cardiac magnetic resonance and multidetector computed tomography stress-rest perfusion imaging for detection of coronary artery disease. J Am Coll Cardiol 2013;61:1099–107. [DOI] [PubMed] [Google Scholar]
- 85. Rochitte CE, George RT, Chen MY, Arbab-Zadeh A, Dewey M, Miller JM et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J 2014;35:1120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cury RC, Kitt TM, Feaheny K, Blankstein R, Ghoshhajra BB, Budoff MJ. et al. A randomized, multicenter, multivendor study of myocardial perfusion imaging with regadenoson CT perfusion vs single photon emission CT. J Cardiovasc Comput Tomogr 2015;9:103–12 e1-2. [DOI] [PubMed] [Google Scholar]
- 87. Huber AM, Leber V, Gramer BM, Muenzel D, Leber A, Rieber J et al. Myocardium: dynamic versus single-shot CT perfusion imaging. Radiology 2013;269:378–86. [DOI] [PubMed] [Google Scholar]
- 88. Rossi A, Dharampal A, Wragg A, Davies LC, van Geuns RJ, Anagnostopoulos C et al. Diagnostic performance of hyperaemic myocardial blood flow index obtained by dynamic computed tomography: does it predict functionally significant coronary lesions? Eur Heart J Cardiovasc Imaging 2014;15:85–94. [DOI] [PubMed] [Google Scholar]
- 89. Coenen A, Rossi A, Lubbers MM, Kurata A, Kono AK, Chelu RG et al. Integrating CT myocardial perfusion and CT-FFR in the work-up of coronary artery disease. JACC Cardiovasc Imaging 2017;10:760–70. [DOI] [PubMed] [Google Scholar]
- 90. Pontone G, Baggiano A, Andreini D, Guaricci AI, Guglielmo M, Muscogiuri G et al. Dynamic stress computed tomography perfusion with a whole-heart coverage scanner in addition to coronary computed tomography angiography and fractional flow reserve computed tomography derived. JACC Cardiovasc Imaging 2019;12:2460–71. [DOI] [PubMed] [Google Scholar]
- 91. Alessio AM, Bindschadler M, Busey JM, Shuman WP, Caldwell JH, Branch KR. Accuracy of myocardial blood flow estimation from dynamic contrast-enhanced cardiac CT compared with PET. Circ Cardiovasc Imaging 2019;12:e008323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. de Knegt MC, Rossi A, Petersen SE, Wragg A, Khurram R, Westwood M et al. Stress myocardial perfusion with qualitative magnetic resonance and quantitative dynamic computed tomography: comparison of diagnostic performance and incremental value over coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging 2020. https://doi.org/10.1093/ehjci/jeaa270. [DOI] [PubMed] [Google Scholar]
- 93. Nous FMA, Geisler T, Kruk MBP, Alkadhi H, Kitagawa K, Vliegenthart R et al. Dynamic myocardial perfusion CT for the detection of hemodynamically significant coronary artery disease. JACC Cardiovasc Imaging 2022; 15: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kitagawa K, Nakamura S, Ota H, Ogawa R, Shizuka T, Kubo T et al. Diagnostic performance of dynamic myocardial perfusion imaging using dual-source computed tomography. J Am Coll Cardiol 2021;78:1937–49. [DOI] [PubMed] [Google Scholar]
- 95. George RT, Mehra VC, Chen MY, Kitagawa K, Arbab-Zadeh A, Miller JM et al. Myocardial CT perfusion imaging and SPECT for the diagnosis of coronary artery disease: a head-to-head comparison from the CORE320 multicenter diagnostic performance study. Radiology 2014;272:407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Magalhaes TA, Kishi S, George RT, Arbab-Zadeh A, Vavere AL, Cox C et al. Combined coronary angiography and myocardial perfusion by computed tomography in the identification of flow-limiting stenosis - The CORE320 study: an integrated analysis of CT coronary angiography and myocardial perfusion. J Cardiovasc Comput Tomogr 2015;9:438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Celeng C, Leiner T, Maurovich-Horvat P, Merkely B, de Jong P, Dankbaar JW et al. Anatomical and functional computed tomography for diagnosing hemodynamically significant coronary artery disease: a meta-analysis. JACC Cardiovasc Imaging 2019;12:1316–25. [DOI] [PubMed] [Google Scholar]
- 98. Andreini D, Mushtaq S, Pontone G, Conte E, Collet C, Sonck J et al. CT perfusion versus coronary CT angiography in patients with suspected in-stent restenosis or CAD progression. JACC Cardiovasc Imaging 2020;13:732–42. [DOI] [PubMed] [Google Scholar]
- 99. Kono AK, Coenen A, Lubbers M, Kurata A, Rossi A, Dharampal A et al. Relative myocardial blood flow by dynamic computed tomographic perfusion imaging predicts hemodynamic significance of coronary stenosis better than absolute blood flow. Invest Radiol 2014;49:801–7. [DOI] [PubMed] [Google Scholar]
- 100. Rossi A, Wragg A, Klotz E, Pirro F, Moon JC, Nieman K et al. Dynamic computed tomography myocardial perfusion imaging: comparison of clinical analysis methods for the detection of vessel-specific Ischemia. Circ Cardiovasc Imaging 2017;10: e005505. [DOI] [PubMed] [Google Scholar]
- 101. Takx RA, Blomberg BA, El Aidi H, Habets J, de Jong PA, Nagel E et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2015;8: e002666. [DOI] [PubMed] [Google Scholar]
- 102. Nakamura S, Kitagawa K, Goto Y, Omori T, Kurita T, Yamada A et al. Incremental prognostic value of myocardial blood flow quantified with stress dynamic computed tomography perfusion imaging. JACC Cardiovasc Imaging 2019;12:1379–87. [DOI] [PubMed] [Google Scholar]
- 103. van Assen M, De Cecco CN, Eid M, von Knebel Doeberitz P, Scarabello M, Lavra F et al. Prognostic value of CT myocardial perfusion imaging and CT-derived fractional flow reserve for major adverse cardiac events in patients with coronary artery disease. J Cardiovasc Comput Tomogr 2019;13:26–33. [DOI] [PubMed] [Google Scholar]
- 104. Nakamura S, Kitagawa K, Goto Y, Takafuji M, Nakamori S, Kurita T et al. Prognostic value of stress dynamic computed tomography perfusion with computed tomography delayed enhancement. JACC Cardiovasc Imaging 2020;13:1721–34. [DOI] [PubMed] [Google Scholar]
- 105. Chen MY, Rochitte CE, Arbab-Zadeh A, Dewey M, George RT, Miller JM et al. Prognostic value of combined CT angiography and myocardial perfusion imaging versus invasive coronary angiography and nuclear stress perfusion imaging in the prediction of major adverse cardiovascular events: the CORE320 multicenter study. Radiology 2017;284:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dewey M, Rochitte CE, Ostovaneh MR, Chen MY, George RT, Niinuma H et al. Prognostic value of noninvasive combined anatomic/functional assessment by cardiac CT in patients with suspected coronary artery disease—comparison with invasive coronary angiography and nuclear myocardial perfusion imaging for the five-year-follow up of the CORE320 multicenter study. J Cardiovasc Comput Tomogr 2021;15:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lubbers M, Coenen A, Kofflard M, Bruning T, Kietselaer B, Galema T et al. Comprehensive cardiac CT with myocardial perfusion imaging versus functional testing in suspected coronary artery disease: the multicenter, randomized CRESCENT-II trial. JACC Cardiovasc Imaging 2018;11:1625–36. [DOI] [PubMed] [Google Scholar]
- 108. Pontone G, De Cecco C, Baggiano A, Guaricci AI, Guglielmo M, Leiner T et al. Design of CTP-PRO study (impact of stress cardiac computed tomography myocardial perfusion on downstream resources and PROgnosis in patients with suspected or known coronary artery disease: a multicenter international study). Int J Cardiol 2019;292:253–7. [DOI] [PubMed] [Google Scholar]
- 109. Francone M, Budde RPJ, Bremerich J, Dacher JN, Loewe C, Wolf F et al. CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting—a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol 2020;30:2627–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2019;13:1–20. [DOI] [PubMed] [Google Scholar]
- 111. Weir-McCall JR, Blanke P, Naoum C, Delgado V, Bax JJ, Leipsic J. Mitral valve imaging with CT: relationship with transcatheter mitral valve interventions. Radiology 2018;288:638–55. [DOI] [PubMed] [Google Scholar]
- 112. Korsholm K, Berti S, Iriart X, Saw J, Wang DD, Cochet H et al. Expert recommendations on cardiac computed tomography for planning transcatheter left atrial appendage occlusion. JACC Cardiovasc Interv 2020;13:277–92. [DOI] [PubMed] [Google Scholar]
- 113. Pawade T, Sheth T, Guzzetti E, Dweck MR, Clavel MA. Why and how to measure aortic valve calcification in patients with aortic stenosis. JACC Cardiovasc Imaging 2019;12:1835–48. [DOI] [PubMed] [Google Scholar]
- 114. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ et al. ; ESC Scientific Document Group. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 115. Baumgartner HC, Hung JC-C, Bermejo J, Chambers JB, Edvardsen T, Goldstein S et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2017;18:254–75. [DOI] [PubMed] [Google Scholar]
- 116. Pawade T, Clavel MA, Tribouilloy C, Dreyfus J, Mathieu T, Tastet L et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging 2018;11:e007146. [DOI] [PubMed] [Google Scholar]
- 117. Jabbour A, Ismail TF, Moat N, Gulati A, Roussin I, Alpendurada F et al. Multimodality imaging in transcatheter aortic valve implantation and post-procedural aortic regurgitation: comparison among cardiovascular magnetic resonance, cardiac computed tomography, and echocardiography. J Am Coll Cardiol 2011;58:2165–73. [DOI] [PubMed] [Google Scholar]
- 118. Jilaihawi H, Kashif M, Fontana G, Furugen A, Shiota T, Friede G et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol 2012;59:1275–86. [DOI] [PubMed] [Google Scholar]
- 119. Binder RK, Webb JG, Willson AB, Urena M, Hansson NC, Norgaard BL et al. The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: a prospective, multicenter, controlled trial. J Am Coll Cardiol 2013;62:431–8. [DOI] [PubMed] [Google Scholar]
- 120. Hansson NC, Leipsic J, Pugliese F, Andersen HR, Rossi A, Simonato M et al. Aortic valve and left ventricular outflow tract calcium volume and distribution in transcatheter aortic valve replacement: influence on the risk of significant paravalvular regurgitation. J Cardiovasc Comput Tomogr 2018;12:290–7. [DOI] [PubMed] [Google Scholar]
- 121. Barbanti M, Yang TH, Rodes Cabau J, Tamburino C, Wood DA, Jilaihawi H et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013;128:244–53. [DOI] [PubMed] [Google Scholar]
- 122. Hinton J, Gough S, Ahmed H, Gabara L, Rawlins J, Calver A et al. Frequency and impact of incidental findings on computed tomography during work-up for transcatheter aortic valve implantation: single centre experience and review of the literature. Br J Radiol 2019;92:20190344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Faroux L, Guimaraes L, Wintzer-Wehekind J, Junquera L, Ferreira-Neto AN, Del Val D et al. Coronary artery disease and transcatheter aortic valve replacement: JACC state-of-the-art review. J Am Coll Cardiol 2019;74:362–72. [DOI] [PubMed] [Google Scholar]
- 124. van den Boogert TPW, Vendrik J, Claessen B, Baan J, Beijk MA, Limpens J et al. CTCA for detection of significant coronary artery disease in routine TAVI work-up : a systematic review and meta-analysis. Neth Heart J 2018;26:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J et al. ; ESC/EACTS Scientific Document Group; ESC Scientific Document Group.. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2021. Online ahead of print. [Google Scholar]
- 126. Andreini D, Pontone G, Mushtaq S, Bartorelli AL, Ballerini G, Bertella E et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in 325 consecutive patients referred for transcatheter aortic valve replacement. Am Heart J 2014;168:332–9. [DOI] [PubMed] [Google Scholar]
- 127. Hamdan A, Wellnhofer E, Konen E, Kelle S, Goitein O, Andrada B et al. Coronary CT angiography for the detection of coronary artery stenosis in patients referred for transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr 2015;9:31–41. [DOI] [PubMed] [Google Scholar]
- 128. Harris BS, De Cecco CN, Schoepf UJ, Steinberg DH, Bayer RR, Krazinski AW et al. Dual-source CT imaging to plan transcatheter aortic valve replacement: accuracy for diagnosis of obstructive coronary artery disease. Radiology 2015;275:80–8. [DOI] [PubMed] [Google Scholar]
- 129. Matsumoto S, Yamada Y, Hashimoto M, Okamura T, Yamada M, Yashima F et al. CT imaging before transcatheter aortic valve implantation (TAVI) using variable helical pitch scanning and its diagnostic performance for coronary artery disease. Eur Radiol 2017;27:1963–70. [DOI] [PubMed] [Google Scholar]
- 130. Opolski MP, Kim WK, Liebetrau C, Walther C, Blumenstein J, Gaede L et al. Diagnostic accuracy of computed tomography angiography for the detection of coronary artery disease in patients referred for transcatheter aortic valve implantation. Clin Res Cardiol 2015;104:471–80. [DOI] [PubMed] [Google Scholar]
- 131. Pontone G, Andreini D, Bartorelli AL, Annoni A, Mushtaq S, Bertella E et al. Feasibility and accuracy of a comprehensive multidetector computed tomography acquisition for patients referred for balloon-expandable transcatheter aortic valve implantation. Am Heart J 2011;161:1106–13. [DOI] [PubMed] [Google Scholar]
- 132. Rossi A, De Cecco CN, Kennon SRO, Zou L, Meinel FG, Toscano W et al. CT angiography to evaluate coronary artery disease and revascularization requirement before trans-catheter aortic valve replacement. J Cardiovasc Comput Tomogr 2017;11:338–46. [DOI] [PubMed] [Google Scholar]
- 133. Strong C, Ferreira A, Teles RC, Mendes G, Abecasis J, Cardoso G et al. Diagnostic accuracy of computed tomography angiography for the exclusion of coronary artery disease in candidates for transcatheter aortic valve implantation. Sci Rep 2019;9:19942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yanagisawa R, Hayashida K, Yamada Y, Tanaka M, Yashima F, Inohara T et al. Incidence, predictors, and mid-term outcomes of possible leaflet thrombosis after TAVR. JACC Cardiovasc Imaging 2016;10:1–11. [DOI] [PubMed] [Google Scholar]
- 135. Kim K, Kaji S, An Y, Yoshitani H, Takeuchi M, Levine RA et al. Mechanism of asymmetric leaflet tethering in ischemic mitral regurgitation: 3D analysis with multislice CT. JACC Cardiovasc Imaging 2012;5:230–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Killeen RP, Arnous S, Martos R, Abbara S, Quinn M, Dodd JD. Chronic mitral regurgitation detected on cardiac MDCT: differentiation between functional and valvular aetiologies. Eur Radiol 2010;20:1886–95. [DOI] [PubMed] [Google Scholar]
- 137. Feuchtner GM, Alkadhi H, Karlo C, Sarwar A, Meier A, Dichtl W et al. Cardiac CT angiography for the diagnosis of mitral valve prolapse: comparison with echocardiography1. Radiology 2010;254:374–83. [DOI] [PubMed] [Google Scholar]
- 138. van Rosendael PJ, van Wijngaarden SE, Kamperidis V, Kong WKF, Leung M, Ajmone Marsan N et al. Integrated imaging of echocardiography and computed tomography to grade mitral regurgitation severity in patients undergoing transcatheter aortic valve implantation. Eur Heart J 2017;38:2221–6. [DOI] [PubMed] [Google Scholar]
- 139. Blanke P, Naoum C, Webb J, Dvir D, Hahn RT, Grayburn P et al. Multimodality imaging in the context of transcatheter mitral valve replacement: establishing consensus among modalities and disciplines. JACC Cardiovasc Imaging 2015;8:1191–208. [DOI] [PubMed] [Google Scholar]
- 140. Yoon SH, Bleiziffer S, Latib A, Eschenbach L, Ancona M, Vincent F et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. JACC Cardiovasc Interv 2019;12:182–93. [DOI] [PubMed] [Google Scholar]
- 141. Blanke P, Naoum C, Dvir D, Bapat V, Ong K, Muller D et al. Predicting LVOT obstruction in transcatheter mitral valve implantation: concept of the Neo-LVOT. JACC Cardiovasc Imaging 2017;10:482–5. [DOI] [PubMed] [Google Scholar]
- 142. Blanke P, Dvir D, Naoum C, Cheung A, Ye J, Theriault-Lauzier P et al. Prediction of fluoroscopic angulation and coronary sinus location by CT in the context of transcatheter mitral valve implantation. J Cardiovasc Comput Tomogr 2015;9:183–92. [DOI] [PubMed] [Google Scholar]
- 143. Blanke P, Park JK, Grayburn P, Naoum C, Ong K, Kohli K et al. Left ventricular access point determination for a coaxial approach to the mitral annular landing zone in transcatheter mitral valve replacement. J Cardiovasc Comput Tomogr 2017;11:281–7. [DOI] [PubMed] [Google Scholar]
- 144. Jongbloed MR, Bax JJ, Lamb HJ, Dirksen MS, Zeppenfeld K, van der Wall EE et al. Multislice computed tomography versus intracardiac echocardiography to evaluate the pulmonary veins before radiofrequency catheter ablation of atrial fibrillation: a head-to-head comparison. J Am Coll Cardiol 2005;45:343–50. [DOI] [PubMed] [Google Scholar]
- 145. Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging 2013;6:185–94. [DOI] [PubMed] [Google Scholar]
- 146. Kocyigit D, Yalcin MU, Gurses KM, Selin A, Turk G, Canpolat U et al. Pulmonary vein orientation is independently associated with outcomes following cryoballoon-based atrial fibrillation ablation. J Cardiovasc Comput Tomogr 2018;12:281–5. [DOI] [PubMed] [Google Scholar]
- 147. Saw J, Fahmy P, DeJong P, Lempereur M, Spencer R, Tsang M et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur Heart J Cardiovasc Imaging 2015;16:1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D'Andrea A et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging 2015;16:280. [DOI] [PubMed] [Google Scholar]
- 149. Habib G, Bucciarelli-Ducci C, Caforio ALP, Cardim N, Charron P, Cosyns B et al. ; EACVI Scientific Documents Committee; Indian Academy of Echocardiography. Multimodality imaging in restrictive cardiomyopathies: an EACVI expert consensus document in collaboration with the "Working Group on myocardial and pericardial diseases" of the European Society of Cardiology Endorsed by The Indian Academy of Echocardiography. Eur Heart J Cardiovasc Imaging 2017;18:1090–121. [DOI] [PubMed] [Google Scholar]
- 150. Kaniewska M, Schuetz GM, Willun S, Schlattmann P, Dewey M. Noninvasive evaluation of global and regional left ventricular function using computed tomography and magnetic resonance imaging: a meta-analysis. Eur Radiol 2017;27:1640–59. [DOI] [PubMed] [Google Scholar]
- 151. Pickett CA, Cheezum MK, Kassop D, Villines TC, Hulten EA. Accuracy of cardiac CT, radionucleotide and invasive ventriculography, two- and three-dimensional echocardiography, and SPECT for left and right ventricular ejection fraction compared with cardiac MRI: a meta-analysis. Eur Heart J Cardiovasc Imaging 2015;16:848–52. [DOI] [PubMed] [Google Scholar]
- 152. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M et al. ; ESC Scientific Document Group. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 153. Pontone G, Guaricci AI, Andreini D, Solbiati A, Guglielmo M, Mushtaq S et al. Prognostic benefit of cardiac magnetic resonance over transthoracic echocardiography for the assessment of ischemic and nonischemic dilated cardiomyopathy patients referred for the evaluation of primary prevention implantable cardioverter-defibrillator therapy. Circ Cardiovasc Imaging 2016;9: e004956. [DOI] [PubMed] [Google Scholar]
- 154. Ohta Y, Kitao S, Yunaga H, Fujii S, Mukai N, Yamamoto K et al. Myocardial delayed enhancement CT for the evaluation of heart failure: comparison to MRI. Radiology 2018;288:682–91. [DOI] [PubMed] [Google Scholar]
- 155. Chang S, Han K, Youn JC, Im DJ, Kim JY, Suh YJ et al. Utility of dual-energy CT-based monochromatic imaging in the assessment of myocardial delayed enhancement in patients with cardiomyopathy. Radiology 2018;287:442–51. [DOI] [PubMed] [Google Scholar]
- 156. Im DJ, Youn JC, Lee HJ, Nam K, Suh YJ, Hong YJ et al. Role of cardiac computed tomography for etiology evaluation of newly diagnosed heart failure with reduced ejection fraction. J Clin Med 2020;9:2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lee HJ, Im DJ, Youn JC, Chang S, Suh YJ, Hong YJ et al. Assessment of myocardial delayed enhancement with cardiac computed tomography in cardiomyopathies: a prospective comparison with delayed enhancement cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 2017;33:577–84. [DOI] [PubMed] [Google Scholar]
- 158. Bandula S, White SK, Flett AS, Lawrence D, Pugliese F, Ashworth MT et al. Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced CT: validation against histologic findings. Radiology 2013;269:396–403. [DOI] [PubMed] [Google Scholar]
- 159. Lee HJ, Im DJ, Youn JC, Chang S, Suh YJ, Hong YJ et al. Myocardial extracellular volume fraction with dual-energy equilibrium contrast-enhanced cardiac CT in nonischemic cardiomyopathy: a prospective comparison with cardiac MR imaging. Radiology 2016;280:49–57. [DOI] [PubMed] [Google Scholar]
- 160. Treibel TA, Fontana M, Steeden JA, Nasis A, Yeung J, White SK et al. Automatic quantification of the myocardial extracellular volume by cardiac computed tomography: synthetic ECV by CCT. J Cardiovasc Comput Tomogr 2017;11:221–6. [DOI] [PubMed] [Google Scholar]
- 161. Ohta Y, Kishimoto J, Kitao S, Yunaga H, Mukai-Yatagai N, Fujii S et al. Investigation of myocardial extracellular volume fraction in heart failure patients using iodine map with rapid-kV switching dual-energy CT: segmental comparison with MRI T1 mapping. J Cardiovasc Comput Tomogr 2020;14:349–55. [DOI] [PubMed] [Google Scholar]
- 162. Wang R, Liu X, Schoepf UJ, van Assen M, Alimohamed I, Griffith LP et al. Extracellular volume quantitation using dual-energy CT in patients with heart failure: comparison with 3T cardiac MR. Int J Cardiol 2018;268:236–40. [DOI] [PubMed] [Google Scholar]
- 163. Nacif MS, Kawel N, Lee JJ, Chen X, Yao J, Zavodni A et al. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology 2012;264:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Donal E, Delgado V, Bucciarelli-Ducci C, Galli E, Haugaa KH, Charron P et al. ; 2016–18 EACVI Scientific Documents Committee. Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: an expert consensus document from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2019;20:1075–93. [DOI] [PubMed] [Google Scholar]
- 165. Behar JM, Rajani R, Pourmorteza A, Preston R, Razeghi O, Niederer S et al. Comprehensive use of cardiac computed tomography to guide left ventricular lead placement in cardiac resynchronization therapy. Heart Rhythm 2017;14:1364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang YJ, Liu L, Zhang MC, Sun H, Zeng H, Yang P. Imaging of pericardiophrenic bundles using multislice spiral computed tomography for phrenic nerve anatomy. J Cardiovasc Electrophysiol 2016;27:961–71. [DOI] [PubMed] [Google Scholar]
- 167. Ghadri JR, Kazakauskaite E, Braunschweig S, Burger IA, Frank M, Fiechter M et al. Congenital coronary anomalies detected by coronary computed tomography compared to invasive coronary angiography. BMC Cardiovasc Disord 2014;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Grani C, Buechel RR, Kaufmann PA, Kwong RY. Multimodality imaging in individuals with anomalous coronary arteries. JACC Cardiovasc Imaging 2017;10:471–81. [DOI] [PubMed] [Google Scholar]
- 169. Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP et al. ; ESC Scientific Document Group. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J 2021;42:563–645. [DOI] [PubMed] [Google Scholar]
- 170. Sachdeva R, Valente AM, Armstrong AK, Cook SC, Han BK, Lopez L et al. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT/SCMR/SOPE 2020 appropriate use criteria for multimodality imaging during the follow-up care of patients with congenital heart disease: a report of the American College of Cardiology Solution Set Oversight Committee and appropriate use criteria task force, American Heart Association, American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Pediatric Echocardiography. J Am Coll Cardiol 2020;75:657–703. [DOI] [PubMed] [Google Scholar]
- 171. Han BK, Rigsby CK, Hlavacek A, Leipsic J, Nicol ED, Siegel MJ et al. ; North American Society of Cardiac Imaging. Society of Cardiovascular Computed T, Society of Pediatric R, North American Society of Cardiac I. Computed tomography imaging in patients with congenital heart disease part I: rationale and utility. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT): endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J Cardiovasc Comput Tomogr 2015;9:475–92. [DOI] [PubMed] [Google Scholar]
- 172. Han BK, Rigsby CK, Leipsic J, Bardo D, Abbara S, Ghoshhajra B et al. ; North American Society of Cardiac Imaging. Society of Cardiovascular Computed T, Society of Pediatric R, North American Society of Cardiac I. Computed tomography imaging in patients with congenital heart disease, part 2: technical recommendations. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT): endorsed by the Society of Pediatric Radiology (SPR) and the North American Society of Cardiac Imaging (NASCI). J Cardiovasc Comput Tomogr 2015;9:493–513. [DOI] [PubMed] [Google Scholar]
- 173. Burris AC, Bourg JA, Raff GL, Chinnaivan KM, ,et al. Triple rule out versus coronary CT angiography in patients with acute chest pain: results from the ACIC consortium. JACC Cardiovasc Imaging 2015;8:817–25. [DOI] [PubMed] [Google Scholar]
- 174. Ito S, Akutsu K, Tamori Y, Sakamoto S, Yoshimuta T, Hashimoto H et al. Differences in atherosclerotic profiles between patients with thoracic and abdominal aortic aneurysms. Am J Cardiol 2008;101:696–9. [DOI] [PubMed] [Google Scholar]
- 175. Kolossvary M, De Cecco CN, Feuchtner G, Maurovich-Horvat P. Advanced atherosclerosis imaging by CT: radiomics, machine learning and deep learning. J Cardiovasc Comput Tomogr 2019;13:274–80. [DOI] [PubMed] [Google Scholar]
- 176. Kolossváry M, Kellermayer M, Merkely B, Maurovich-Horvat P. Cardiac computed tomography radiomics: a comprehensive review on radiomic techniques. J Thorac Imaging 2018;33:26–34. [DOI] [PubMed] [Google Scholar]
- 177. Al'Aref SJ, Anchouche K, Singh G, Slomka PJ, Kolli KK, Kumar A et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur Heart J 2019;40:1975–86. [DOI] [PubMed] [Google Scholar]
- 178. Wolterink JM, Leiner T, Takx RA, Viergever MA, Isgum I. Automatic coronary calcium scoring in non-contrast-enhanced ECG-triggered cardiac CT with ambiguity detection. IEEE Trans Med Imaging 2015;34:1867–78. [DOI] [PubMed] [Google Scholar]
- 179. Commandeur F, Goeller M, Betancur J, Cadet S, Doris M, Chen X et al. Deep learning for quantification of epicardial and thoracic adipose tissue from non-contrast CT. IEEE Trans Med Imaging 2018;37:1835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kang D, Dey D, Slomka PJ, Arsanjani R, Nakazato R, Ko H et al. Structured learning algorithm for detection of nonobstructive and obstructive coronary plaque lesions from computed tomography angiography. J Med Imaging 2015;2:014003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kolossvary M, Karady J, Szilveszter B, Kitslaar P, Hoffmann U, Merkely B et al. Radiomic features are superior to conventional quantitative computed tomographic metrics to identify coronary plaques with napkin-ring sign. Circ Cardiovasc Imaging 2017;10: e006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Al’Aref SJ, Singh G, Choi JW, Xu Z, Maliakal G, van Rosendael AR et al. A boosted ensemble algorithm for determination of plaque stability in high-risk patients on coronary CTA. JACC Cardiovasc Imaging 2020;13:2162–73. [DOI] [PubMed] [Google Scholar]
- 183. Muscogiuri G, Chiesa M, Trotta M, Gatti M, Palmisano V, Dell'Aversana S et al. Performance of a deep learning algorithm for the evaluation of CAD-RADS classification with CCTA. Atherosclerosis 2020;294:25–32. [DOI] [PubMed] [Google Scholar]
- 184. Dey D, Gaur S, Ovrehus KA, Slomka PJ, Betancur J, Goeller M et al. Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: a multicentre study. Eur Radiol 2018;28:2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Nakanishi R, Slomka PJ, Rios R, Betancur J, Blaha MJ, Nasir K et al. Machine learning adds to clinical and CAC assessments in predicting 10-year CHD and CVD deaths. JACC Cardiovasc Imaging 2021;14:615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Motwani M, Dey D, Berman DS, Germano G, Achenbach S, Al-Mallah MH et al. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J 2017;38:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.