Abstract
The roles of arbuscular mycorrhizal fungi (AMF) and plant growth-promoting rhizobacteria (PGPR) in improving nutrition uptake and soil quality have been well documented. However, few studies have explored their effects on root morphology and soil properties. In this study, we inoculated Elymus nutans Griseb with AMF and/or PGPR in order to explore their effects on plant growth, soil physicochemical properties, and soil enzyme activities. The results showed that AMF and/or PGPR inoculation significantly enhanced aboveground and belowground vegetation biomass. Both single and dual inoculations were beneficial for plant root length, surface area, root branches, stem diameter, height, and the ratio of shoot to root, but decreased root volume and root average diameter. Soil total nitrogen, alkaline phosphatase, and urease activities showed significant growth, and soil electrical conductivity and pH significantly declined under the inoculation treatments. Specific root length showed a negative correlation with belowground biomass, but a positive correlation with root length and root branches. These results indicated that AMF and PGPR had synergetic effects on root morphology, soil nutrient availability, and plant growth.
Keywords: Arbuscular mycorrhizal fungi (AMF), Plant growth-promoting rhizobacteria (PGPR), Root morphology, Soil physicochemical properties, Plant-microbe interaction
Introduction
Almost 25% of the Earth’s biodiversity is composed of soil microorganisms that have interacted with animals, plants, and soil in ecosystems around the world for millions of years (Fierer, 2017; Wagg et al., 2019; Whitman, Coleman & Wiebe, 1998). Numerous beneficial microorganisms play critical roles in biogeochemical circulation that fulfill global carbon (C) and nutrient cycling that allow ecosystems to function and improve their productivity. However, the plant-microbe interaction has still been undervalued in studies on the direct plant-soil feedback effects and links between plant communities and soil microbes, such as in nutrient acquisition and hormone stimulation (Fierer, 2017; Morgan, Bending & White, 2005). Plant diversity has been shaped by microorganisms via their symbionts in terrestrial ecosystems (Wagg et al., 2014). Generally, rhizosphere microbes can assist the growth and development of plants by recycling nutrients, producing hormones, improving tolerance toward potentially hazardous compounds, keeping the soil healthy, and exercising other indispensable functions such as soil formation and decomposition of organic matter (Wallenstein, 2006). When plants lack essential mineral elements such as phosphorus (P) or nitrogen (N), this kind of symbiont can enhance and benefit plant growth. Soil microorganisms, including mutualists and pathogens, drive abiotic properties and regulate individual plant growth and species coexistence with successive mutual interactions (Li et al., 2020). This plant-associated microbiota involves various groups of organisms, including bacteria, archaea, and fungi, acting as a symbiont or pathogen (Berendsen, Pieterse & Bakker, 2012; Hussain & Khan, 2020; Vorholt, 2012).
Arbuscular mycorrhizal fungi (AMF), assigned to the Glomeromycotina phylum, are one of the most important components of the soil ecosystem. They maintain symbiotic relationships with over 70% of terrestrial plants and provide nutrients and water to plants in exchange for sugars through their arbuscules, which is where the exchange of necessary nutrients between host plants and fungi occurs (Schussler, Schwarzott & Walker, 2001; Wagg et al., 2019). AMF have the capacity to expand the exhaustion zone using an extensive hyphal network to acquire extra water and nutrients that can significantly improve a host plants’ fitness (Bonfante & Genre, 2010; Ezawa & Saito, 2018; Field & Pressel, 2018; van der Heijden et al., 2015). AMF also enhance the ability of host plants and adjacent plants that are connected with common mycorrhizal networks (CMN) to resist drought, heavy metals, and pathogens (Bonfante & Genre, 2010; Giovannetti et al., 2001; Pepe, Giovannetti & Sbrana, 2016). The area around the mycorrhizal hyphae, called the hyphosphere (Patel, Thakkar & Subramanian, 2015; Rasmann et al., 2017), contains helper bacteria that promote the plant-mycorrhizal fungus symbiotic associations, plant-growth-promoting rhizobacteria (PGPR) that work collaboratively with AMF to facilitate plant growth and productivity, and mycophagous bacteria that are dependent on fresh hyphaes (Yuan et al., 2021). PGPR include Azospirillum, Pseudomonas, Azotobacter, Klebsiella, Enterobacter, Alcaligens, Arthrobacter, Burkholderia, and Bacillus spp., which are important members of the plant-associated microbiome (Bashan et al., 2014; Muzammil et al., 2014). Bacillus spp. promotes plant growth by fixing N, solubilizing and mineralizing P and other nutrients, stimulating phytohormones, producing siderophores, inducing systemic resistance (ISR), and enhancing their tolerance to abiotic stresses (Bonfante & Genre, 2010; Saxena et al., 2020). Bacteria rely on lignin and cellulose-hydrolysed fungus to produce primary bacterial materials and provide nourishment to fungus for exchange (Clausen, 1996; Romaní et al., 2006). Fungal hyphae form hyphal networks to connect soil patches and build “fungal hyphae highways” for bacteria to transfer substrates (Warmink et al., 2011).
Numerous studies have shown that utilizing PGPR and AMF is a feasible ecological approach to enhance soil health and plant productivity (Aini, Yamika & Ulum, 2019; Vessey, 2003). AMF and PGPR symbionts can enhance resistance to salinity by shifting individual root morphology and root-to-shoot communication, keeping ion homeostasis, diminishing oxidative damage, and increasing photosynthetic capacity (Chandrasekaran et al., 2014; Egamberdieva et al., 2017; Pan et al., 2019), as well as significantly elevating aboveground biomass, stem branches, and plant height (Pan et al., 2020). Additionally, the combination of AMF and PGPR promoted the projected area, total volume, and total root length of trifoliate orange under limited P conditions (Wang et al., 2016), and aboveground organs under deficient organic N (Saia et al., 2015), N, P, potassium (K), and sulfur (S) concentrations in the rhizospheres of onion and maize (Mohamed et al., 2014).
However, there is still a lack of detailed insight and evidence to verify the key functions of AMF, PGPR, and their combined effects on the growth and development of dominant species of the grassland community. Elymus nutans Griseb is a dominant perennial species in the Qinghai-Tibet Plateau, China (Chu et al., 2016) that plays an important role in animal husbandry and the ecological conservation of this region. Therefore, this study was conducted to investigate the effects of AMF and PGPR on plant traits of Elymus nutans Griseb and the surrounding soil properties.
We hypothesized that: (1) AMF and PGPR could mutually symbiose and enhance the plant growth of Elymus nutans Griseb, and (2) the co-existence of AMF and PGPR could improve plant traits and soil quality better than their individual applications.
Materials and Methods
Plant materials
This pot experiment had a fully randomized one-factor experimental design with four treatments. The soil was excavated (0–15 cm depth) in March 2020 from the Qinghai-Tibet Plateau, China, (53°19′2.44″N and 13°51′48.03″E), which has a typical plateau continental climate and clay loam type soil. Precipitation is greatest between June and September (Wei et al., 2021). The sampled soil was air-dried for about two weeks and sieved through a 2 mm screen to ensure homogeneity. The sieved soil was sterilized at 121 °C for 2 h and put into a 2 L pot (17 cm height × 12 cm internal diameter × 14 cm external diameter) for the later experiment.
Elymus nutans Griseb seeds were collected from Haibei Autonomous Prefecture, Qinghai Province, China (36°55′N, 100°57′E, 3,029 m a.s.l.). Before sowing, the seeds were surface-sterilized with 10% H2O2 and rinsed three times with sterile water (Schweiger, Baier & Mueller, 2014). The seeds were then sowed in a 3:2 mixture of clay:sand and laid in the climate chamber (20 °C, 12/12 h: light/dark, 60–70% r.h.). After two weeks, 50 Elymus nutans Griseb seedlings were transferred into experimental pots. They were stored in the dark at 4 °C for 3 d and then transferred to a greenhouse (Tomczak, Schweiger & Müller, 2016) where they were watered twice a week with a standard quantity of water (1,800 ml/week were added in each pot based on the experimental pot size).
AMF inoculum
The inoculum of mycorrhizal fungus Funneliformis mosseae (accession no. BGCYN05), obtained from the Beijing Academy of Agriculture and Forestry Sciences, was isolated from red clover and contained spores, mycelium, sand, and root fragments The inoculant used in this experiment was a rhizosphere soil mixture of spores, extrarhizoma hyphae, and root segments of infected plants, and contained 129 spores per gram. The mycorrhizal fungus spores were placed almost 2 cm below the soil surface before sowing the seeds.
PGPR inoculum
The Bacillus megaterium (accession no. ACCC10011) that were provided by the Institute of Agricultural Resources and Regional Planning (ACCC). Bacillus was grown in 10 g of beef extract peptone AGAR medium with peptone, 3 g of beef extract, 5.0 g of NaCl, and 1,000 mL of distilled water, then 20 g of agar was added. We sterilized the AGAR medium at 121 °C for 30 min, and Bacillus was grown overnight with constant shaking at 220 rpm (Constantino et al., 2008). We regulated the cell suspension to 109 CFU mL−1 and then used it as a standard inoculum. The seeds were immersed in a bacterial suspension before sowing, and the seedling were inoculated with 20 ml of same bacterial suspension after sowing.
Experimental design
Four treatments were used to explore the effects of fungus-bacteria symbiont on Elymus nutans Griseb, including one dual inoculation treatment (30 g-AMF inoculum and 25 ml-Bacillus suspension), two single inoculation treatments (25 ml-Bacillus suspension and 30 g AMF inoculum, respectively), and one controlled treatment (AMF and PGPR were both autoclaved). Each treatment included six replicates.
Determination of parameters
AMF colonization
Mycorrhizal infection was examined using Kormanik & McGraw’s (1982) method. The receiver plant roots were randomly collected per treatment, washed with distilled water, cut into 1 cm segments, immersed in 10% KOH, and heated in water under 90 °C for 60 min. The roots segments were watered to eliminate the alkali, and the remaining alkali was neutralized in 2% hydrochloric acid for 10 min. Then we added 0.01% acid fuchsin to stain the root segments, heated them in 90 °C for 30 mins after separating them from the acid solution, and then immersed them in the mixture solution of glycerol/lactic/water acid (1:1:1) for 24 h to destain them. After that, the treated samples were observed using a regular optical microscope in 40× to qualify the levels of mycorrhizal colonization (Dalpé & Séguin, 2013).
Analysis of parameters
Plant parts
The plant samples were separated into shoots and washed roots, dried at 60 °C for 72 h, and weighed to determine the biomass of dry shoots and roots (Bourles et al., 2020). To determine the total C and N content, leaf tissues were milled with a ball mill (Retsch MM400; Retsch, Haan, Germany), 0.15 g of the sieved plant sample was weighed, wrapped in the tin cup required by the instrument, and measured using an elemental analyzer (Vario MAX CNS; Elementar, Hanau, Germany). The plants were thoroughly washed with deionized water to remove all soil particles and dust, and were then divided into two parts: shoot and root parts. The shoot and root parts were then scanned using EPSON Perfection V700 PHOTO and WinRHIZO Pro software (Regent Instruments Inc., Quebec, Canada) to look at the shoot size, root/shoot ratio, root surface area, root length (mm), mean root diameter (mm), and root branches.
Soil parts
Soil total P content was measured using the sodium hydroxide melting-molybdenum antimony colorimetric method (Yang et al., 2018). Soil total C and N were milled using a ball mill (Retsch MM400; Retsch, Haan, Germany), and weighing 0.15 g of the sieved soil sample, wrapping it in the tin cup required by the instrument, and using the elemental analyzer (Vario MAX CNS, Elementar, Hanau, Germany). Soil ammonium N (NH4+-N) and nitrate N (NO3−-N) levels were measured after extraction using 50 mL of 2 mol/L KCl on a 10 g subsample and a potassium chloride leaching-flow-solution analyzer. Soil available P was measured using Xiao et al. (2018) and Yang et al. (2018). Soil urease enzyme (UE), and alkaline phosphatase activities (ALP) were examined using the Solarbio soil urease kit (Solarbio, BC0120, Beijing, China) and soil alkaline phosphatase kit (Solarbio, BC0280, Beijing, China), respectively.
Data analysis
Plant traits, soil properties, and soil enzyme activities were analyzed using a one-way analysis of variance (ANOVA) test in the “agricolae” package of R software. The correlation between specific root length and plant traits was analyzed using the “corrplot” package. The statistical analysis figures were produced with R software version 4.1.0 (Frew, Powell & Johnson, 2020). The differences were considered to be significant at a 0.05 level.
Results
Plant traits and nutrient uptake
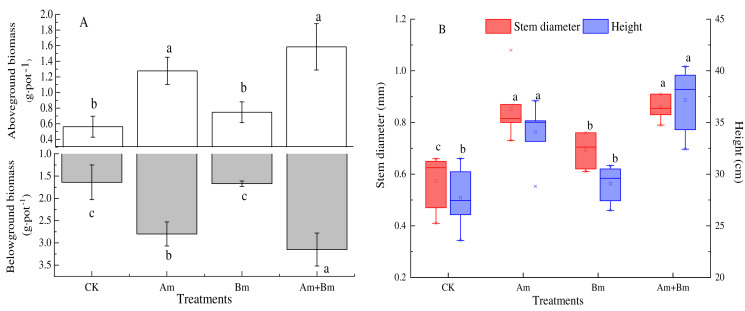
There were significant differences across all treatments (P < 0.05). The aboveground and belowground biomass from dual inoculation with AMF and Bacillus had maximum values of 3.15 g·pot−1 and 1.59 g·pot−1, respectively. Compared with the control group, mixed inoculations were twice as high for aboveground biomass and four times higher for belowground biomass (Fig. 1A).
Figure 1. Effect of different inoculations on the aboveground and belowground biomass (A) and stem diameter and height (B).
Different letters represent significant differences at P = 0.05 using one-way ANOVA. The error bars in the figure legends represent the standard deviation. CK, no inoculation; (Am), only AMF inoculation; Bm, only Bacillus inoculation; Am + Bm, both symbionts.
Stem diameter and plant height with inoculations were greater than those with no inoculation (CK) (P < 0.05) (Fig. 1B), and both stem diameter and height were significantly highest in the dual inoculation (Am + Bm) (P < 0.05) group, followed by only AMF inoculation (Am), and least in only Bacillus inoculation (Bm). The stem diameter and height values ranged from 0.57 mm to 0.86 mm and 27.72 to 37.19 cm, respectively. Single and dual inoculations (Table 1) increased the concentration of plant total C and N, but decreased plant total P compared with the CK group. There were no significant differences in plant total C and N (P > 0.05).
Table 1. Effect of different inoculations on plant traits and soil properties.
| Treatments | Plant traits | Soil properties | |||||
|---|---|---|---|---|---|---|---|
| Total carbon (%) | Total nitrogen (%) | Total phosphorus (g·kg−1) | Total carbon (%) | Total nitrogen (%) | Total phosphorus (g·kg−1) | Available phosphorus (g·kg−1) | |
| CK | 42.64 ± 0.33a | 2.10 ± 0.16a | 0.06 ± 0.02b | 3.43 ± 0.12a | 0.21 ± 0.02ab | 0.43 ± 0.14a | 2.26 ± 0.18a |
| Am | 42.96 ± 0.41a | 1.98 ± 0.17a | 0.02 ± 0.002a | 3.45 ± 0.06a | 0.22 ± 0.02a | 0.46 ± 0.06a | 2.21 ± 0.13a |
| Bm | 42.65 ± 0.38a | 1.97 ± 0.17a | 0.04 ± 0.01b | 3.43 ± 0.06a | 0.19 ± 0.01b | 0.48 ± 0.04a | 2.12 ± 0.10a |
| Am + Bm | 42.64 ± 0.64a | 1.89 ± 0.22a | 0.03 ± 0.003a | 3.46 ± 0.04a | 0.21 ± 0.01ab | 0.47 ± 0.01a | 2.85 ± 0.46a |
Note:
Different letters represent significant differences at P = 0.05 in using one-way ANOVA. Data (average ± SE, n = 6) in the same column with different letters indicates significant differences according to LSD test (P < 0.05). CK, no inoculation; (Am), only AM fungi inoculation; Bm, only bacillus inoculation; Am + Bm, both symbionts.
Dual inoculation with AMF and PGPR did not significantly affect mycorrhizal colonization (Table 2). There were statistically significant differences across the four treatments in root length, root surface area, root branches, and root average diameter (P < 0.05). Inoculations with AMF and/or PGPR enhanced root length, root surface area, shoot to root ratio, and root branches (P < 0.05). However, root volume and root average diameter showed the greatest discrepancy in the Am and Bm groups, respectively. In the presence of AMF, the root length, root surface area, and root branches were much greater than in the other groups, and Bm had a positive effect on root surface area and root to shoot ratio (P < 0.05).
Table 2. Effect of different inoculations on the root length and root surf area (a) and root/shoot radio and root branches (b) and root volume and root average diameter.
| Treatments | Root colonization | Root length cm | Root surf area cm2 | Root volume cm3 | Root branches | Root ADV mm | Root to shoot ratio | Specific root length |
|---|---|---|---|---|---|---|---|---|
| CK | 0 ± 0b | 318.56 ± 68.5c | 786.34 ± 82.28b | 161.18 ± 14.03a | 1,313.11 ± 334.57b | 8.41 ± 1.13a | 0.35 ± 0.07b | 5.94 ± 1.88a |
| Am | 59.77 ± 10.44a | 610.87 ± 77.2a | 883.65 ± 64.79a | 105.27 ± 14.55c | 4,160.78 ± 1594.83a | 4.75 ± 0.55c | 0.45 ± 0.04a | 4.82 ± 0.58a |
| Bm | 0 ± 0b | 446.82 ± 100b | 880.37 ± 78.69a | 146.39 ± 34.53b | 2,265.39 ± 879.79 b | 6.64 ± 1.43b | 0.44 ± 0.06ab | 6.01 ± 1.14a |
| Am + Bm | 58.07 ± 9.37a | 712.70 ± 113a | 859.8 ± 51.54ab | 86.32 ± 17.16c | 3,629.00 ± 667.67a | 4.00 ± 0.67c | 0.51 ± 0.12a | 4.67 ± 1.31a |
Note:
Different letters represent significant differences at P = 0.05 in using one-way ANOVA. CK, no inoculation; Am, only AM fungi inoculation; Bm, only bacillus inoculation; AM + BM, both symbionts.
Soil properties
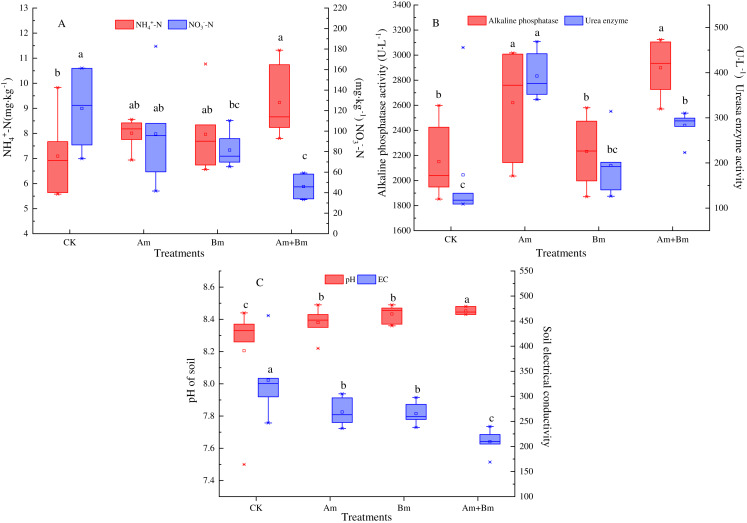
Single AMF inoculation significantly improved the content of soil total N (Table 1), which was decreased in the Bm group (P < 0.05). Am and Bm treatments showed less soil available P compared with the control group. Inoculations significantly enhanced the content of soil NH4+-N but decreased soil NO3−-N (Fig. 2A).
Figure 2. Effect of different inoculations on the soil ammonium nitrogen and nitrate nitrogen content (A), alkaline phosphatase and urea enzyme (B), and pH and electrical conductivity (C).
Different letters represent significant differences at P = 0.05 using one-way ANOVA. The error bars in the figure legends represent the standard deviation. CK, no inoculation; (Am), only AMF inoculation; Bm, only Bacillus inoculation; Am + Bm, both symbionts.
The ALP and UE activity results showed that single or multiple inoculations enhanced ALP activities, and ALP was greater in the presence of AMF than in CK and Bm treatments, which increased to 2,620.76 U·L−1 and 2,899.35 U·L−1, respectively. In the meantime, AMF enhanced UE activities more than other treatments by about 218.07 U·L−1 (P < 0.05) (Fig. 2B). The dual inoculation group (Am + Bm) had the lowest pH value but the highest electrical conductivity, while the control group had the opposite results (Fig. 2C).
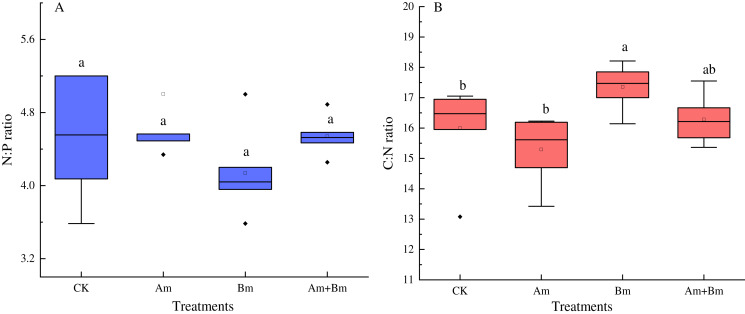
The N:P ratio was not significantly influenced by any inoculations (Fig. 3A), but there were significant differences in C: N ratio across all treatments (P < 0.05) (Fig. 3B), and PGPR inoculation promoted the C:N ratio, but decreased N:P ratio.
Figure 3. Effect of different inoculations on the N:P ratio (A) and C:N ratio (B).
Different letters represent significant differences at P = 0.05 using one-way ANOVA. The error bars in the figure legends represent the standard deviation. CK, no inoculation; (Am), only AMF inoculation; Bm, only Bacillus inoculation; Am + Bm, both symbionts.
Correlations among traits and related variables
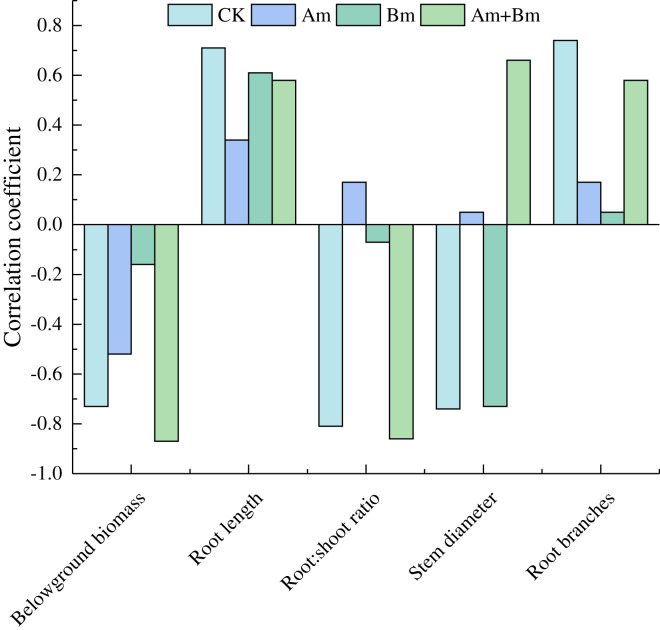
Pearson analysis between specific root length and plant traits showed that specific root length was positively correlated with root length and root branches, but was negatively correlated with belowground biomass (Fig. 4).
Figure 4. Pearson correlations in specific root length and root variables across different inoculations.
CK, no inoculation; (Am), only AMF inoculation; Bm, only Bacillus inoculation; Am + Bm, both symbionts.
Discussion
Plant trait responses to inoculations
Plant-soil microorganism interactions play an essential role in nutrient acquisition and ecosystem functions. However, few studies have focused on soil microorganism responses, especially fungi-bacteria co-occurrence, to plant root growth. In this study, we quantified how plant-associated microbial symbionts affected plant growth and traits, as well as changes in soil physicochemical properties. The aboveground and belowground biomass of the experiment species were significantly promoted in the presence of AMF and Bacillus compared to a single symbiont (Fig. 1). The AMF-PGPR symbiont increased P and N acquisition (Aliasgharzad, Neyshabouri & Salimi, 2006), indicating the positive and synergistic effects of combining AMF and PGPR in the host plant (Bourles et al., 2020; Shockley, McGraw & Garrett, 2004; Xie et al., 2020). PGPR + AMF also influenced AMF colonization, which was suggested by earlier studies that found that multiple inoculations increase AMF colonization (Aini, Yamika & Ulum, 2019; Constantino et al., 2008; Juge et al., 2012) as well as root length and resource acquisition. In general, higher root hydraulic conductivity and proliferation rate were demonstrated by a higher specific root length (Rewald Email et al., 2013). We observed that specific root length decreased with microbial inoculations (but was increased by Bm). This may be due to that AMF mycelium replace the absorption function of the root system, and that the specific compounds secreted by PGPR and AMF provide more nutrition and eliminate toxic ions in the rhizosphere, which facilitates the host to develop a thicker root system and larger absorption area (Brundrett, 2002; Solomon & Rajeshkanna, 2013). Therefore, it is not necessary for Elymus nutans Griseb inoculated with AMF and PGPR to develop a larger specific root length as a substitute for its diameter in order to obtain more resources (Hodge, 2004).
Our results found that height, root, biomass, and root surface area increased following the mixed inoculation, which confirmed the results found by Toro, Azcon & Barea (1997), Ma, Rajkumar & Freitas (2009) and Xie et al. (2020). Additionally, our findings also showed that the presence of AMF and PGPR promoted biomass and N and P accumulation in plant tissues. This is possibly due to AMF and PGPR effectively influencing the calculation of N compounds (like amino acids and soluble proteins) in the host plant (Xie et al., 2020), and their coexistence mediates the production of phytohormones or enzymatic activities to further root evolution and growth (Abdel-Rahman & El-Naggar, 2014), as well as enhance the foundation and development of rhizobial or mycorrhizal symbioses (Patten & Glick, 2002). In our findings, the root:shoot ratio was significantly magnified by mixed or single inoculations, while root biomass, length, branches, and root surface area in dual inoculations were significantly higher than in single inoculations (Table 1), demonstrating that mycorrhizal plants have more advanced root systems, as well as more potential for nutrient acquisition, and the co-occurrence of PGPR and mycorrhizae have benefits for plant growth and improve each other’s development. This shows that PGPR facilitate mycorrhizae hyphal growth when colonizing the host root (Artursson, Finlay & Jansson, 2006; Bianciotto et al., 2001; Hildebrandt et al., 2006; Jeffries et al., 2003; Pivato et al., 2009), increasing the amount and/or length of lateral roots (Chamam et al., 2013; Combes-Meynet et al., 2011) by mediating the hormone pathways and balances (Dodd et al., 2010; Moubayidin, Di Mambro & Sabatini, 2009; Peret et al., 2012; Stepanova & Alonso, 2009) and modifying root morphology (Aloni et al., 2006). It has been well documented that greater special root length is positively correlated with higher resource absorbing efficiency in root systems assimilating nutrients (particularly N and P) from the soil (Cantarel et al., 2015; Comas, Bouma & Eissenstat, 2002; Larson & Funk, 2016; Legay et al., 2014), which can also diminish nitrous oxide release and N extracted from the soil (Abalos et al., 2014; de Vries & Bardgett, 2016; Moreau et al., 2015).
Soil property responses to inoculations
There is evidence of a tradeoff between different N forms (NO3−-N and NH4+-N) uptake among coexisting grass species that have no relationship with root morphology (Maire et al., 2009). Roots and their related mycorrhizal fungi regulate the long-term soil C pool by impacting organic substance decadence (Clemmensen et al., 2013; Phillips, Brzostek & Midgley, 2013) and promoting soil aggregation (Rillig et al., 2015). Additionally, the mixed inoculation of AMF and Bacillus significantly increased soil NH4+-N compared to the single inoculations of AMF or Bacillus in our results (Fig. 2B), which may be linked to PGPR’s potential in enhancing the NO3−-N assimilation rate (Sondergaard, Schulz & Palmgren, 2004) as well as the nitrification limitations of the AMF and PGPR combination (Arif et al., 2016). Consequently, the consumption and absorption of NH+4-N also was lower and better than NO−3-N (Hawkins, Johansen & George, 2000). Soil ALP and UE for both inoculations performed significantly better than the single symbiont in our study, and these results were consistent with recent research (El-Sawah et al., 2021; Zai et al., 2014). AMF may contribute to facilitating soil ALP activity, and its propagules have capacities to synthesize and release soil enzymes (Wang et al., 2006). Additionally, PGPR contribute to P mobilization (Krey et al., 2011), indicating that microorganisms increase the activity of phosphatase, as well as catalyze the hydrolysis of organic P into inorganic P that can be absorbed by plants. At the same time, they can secrete metabolites into the soil matrix during growth and reproduction, promote soil humification, accelerate the degradation of organic matter, and increase the content of organic matter (Mazzoncini et al., 2010). The increase of soil UE also promotes the N concentration in rhizosphere soil. Consequently, the promotion of soil enzyme activities could significantly facilitate the decomposition of organic matter and the remobilization of nutrients in rhizosphere soil (Zai et al., 2014). Inoculations significantly increased soil pH but decreased soil electrical conductivity compared with the control group, and there is similar evidence that Glomalin released by AMF can promote soil physicochemical properties (Mazzoncini et al., 2010).
Conclusion
We evaluated the effects of AMF and PGPR on root morphology, plant growth, and soil properties. A dual inoculation of AMF and PGPR was the most effective for improving plant growth regulation, nutrient acquisition, and soil properties, and should be used as bio-fertilizer to promote local forage production and soil quality in the Qinghai-Tibet Plateau, and provide practical guidance for agricultural management.
Supplemental Information
Acknowledgments
We appreciate the reviewers for their practical comments that improved the quality of this article. We would also like to thank Xiaoting Wei, Bing Han, and Fengyan Jiang for their help in sampling.
Funding Statement
This study was supported by the National Natural Science Foundation of China (Grant Number 31971746), the Ministry of Science and Technology of China (Grant No. 2016YFC0501902), the Major science and technology projects in Qinghai Province (2018-NK-A2) and the Platform of Adaptive Management on Alpine Grassland-livestock System (2020-ZJ-T07). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Lu Yu conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Hui Zhang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Wantong Zhang performed the experiments, prepared figures and/or tables, and approved the final draft.
Kesi Liu analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Miao Liu conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Xinqing Shao conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.
References
- Abalos et al. (2014).Abalos D, De Deyn GB, Kuyper TW, van Groenigen JW. Plant species identity surpasses species richness as a key driver of N2O emissions from grassland. Global Change Biology. 2014;20:265–275. doi: 10.1111/gcb.12350. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman & El-Naggar (2014).Abdel-Rahman SSA, El-Naggar AI. Promotion of rooting and growth of some types of bougainvilleas cutting by plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) in combination with Indole-3-Butyric acid (IBA) International Journal of Science and Research. 2014;3:97–108. [Google Scholar]
- Aini, Yamika & Ulum (2019).Aini N, Yamika WSD, Ulum B. Effect of nutrient concentration, PGPR and AMF on plant growth, yield, and nutrient uptake of hydroponic lettuce. International Journal of Agriculture and Biology. 2019;21:175–183. doi: 10.17957/IJAB/15.0879. [DOI] [Google Scholar]
- Aliasgharzad, Neyshabouri & Salimi (2006).Aliasgharzad N, Neyshabouri MR, Salimi G. Effects of arbuscular mycorrhizal fungi and Bradyrhizobium japonicum on drought stress of soybean. Biologia. 2006;61:S324–S328. doi: 10.2478/s11756-006-0182-x. [DOI] [Google Scholar]
- Aloni et al. (2006).Aloni R, Aloni E, Langhans M, Ullrich CI. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Annals of Botany. 2006;97:883–893. doi: 10.1093/aob/mcl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif et al. (2016).Arif MS, Riaz M, Shahzad SM, Yasmeen T, Akhtar MJ, Riaz MA, Jassey VEJ, Bragazza L, Buttler A. Associative interplay of plant growth promoting rhizobacteria (Pseudomonas aeruginosa QS40) with nitrogen fertilizers improves sunflower (Helianthus annuus L.) productivity and fertility of aridisol. Applied Soil Ecology. 2016;108:238–247. doi: 10.1016/j.apsoil.2016.08.016. [DOI] [Google Scholar]
- Artursson, Finlay & Jansson (2006).Artursson V, Finlay RD, Jansson JK. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environmental Microbiology. 2006;8(1):1–10. doi: 10.1111/j.1462-2920.2005.00942.x. [DOI] [PubMed] [Google Scholar]
- Bashan et al. (2014).Bashan Y, De-Bashan LE, Prabhu SR, Hernandez J. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013) Plant and Soil. 2014;378(1–2):1–33. doi: 10.1007/s11104-013-1956-x. [DOI] [Google Scholar]
- Berendsen, Pieterse & Bakker (2012).Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trends in Plant Science. 2012;17(8):478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Bianciotto et al. (2001).Bianciotto V, Andreotti S, Balestrini R, Bonfante P, Perotto S. Extracellular polysaccharides are involved in the attachment of Azospirillum brasilense and Rhizobium leguminosarum to arbuscular mycorrhizal structures. European Journal of Histochemistry. 2001;45(1):39–49. doi: 10.4081/1612. [DOI] [PubMed] [Google Scholar]
- Bonfante & Genre (2010).Bonfante P, Genre A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nature Communication. 2010;1(1):64. doi: 10.1038/ncomms1046. [DOI] [PubMed] [Google Scholar]
- Bourles et al. (2020).Bourles A, Guentas L, Charvis C, Gensous S, Majorel C, Crossay T, Cavaloc Y, Burtet-Sarramegna V, Jourand P, Amir H. Co-inoculation with a bacterium and arbuscular mycorrhizal fungi improves root colonization, plant mineral nutrition, and plant growth of a Cyperaceae plant in an ultramafic soil. Mycorrhiza. 2020;30(1):121–131. doi: 10.1007/s00572-019-00929-8. [DOI] [PubMed] [Google Scholar]
- Brundrett (2002).Brundrett MC. Coevolution of roots and mycorrhizas of land plants. New Phytologist. 2002;154(2):275–304. doi: 10.1046/j.1469-8137.2002.00397.x. [DOI] [PubMed] [Google Scholar]
- Cantarel et al. (2015).Cantarel AAM, Pommier T, Desclos-Theveniau M, Diquelou S, Dumont M, Grassein F, Kastl E, Grigulis K, Laine P, Lavorel S. Using plant traits to explain plant-microbe relationships involved in nitrogen acquisition. Ecology. 2015;96(3):788–799. doi: 10.1890/13-2107.1. [DOI] [PubMed] [Google Scholar]
- Chamam et al. (2013).Chamam A, Sanguin H, Bellvert F, Meiffren G, Comte G, Wisniewski-Dyé F, Bertrand C, Prigent-Combaret C. Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum-Oryza sativa association. Phytochemistry. 2013;87(12):65–77. doi: 10.1016/j.phytochem.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran et al. (2014).Chandrasekaran M, Boughattas S, Hu S, Oh S, Sa T. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza. 2014;24(8):611–625. doi: 10.1007/s00572-014-0582-7. [DOI] [PubMed] [Google Scholar]
- Chu et al. (2016).Chu XT, Fu JJ, Sun YF, Xu YM, Miao YJ, Xu YF, Hu TM. Effect of arbuscular mycorrhizal fungi inoculation on cold stress-induced oxidative damage in leaves of Elymus nutans Griseb. South African Journal of Botany. 2016;104:21–29. doi: 10.1016/j.sajb.2015.10.001. [DOI] [Google Scholar]
- Clausen (1996).Clausen CA. Bacterial associations with decaying wood: a review. International Biodeterioration and Biodegradation. 1996;37(1–2):101–107. doi: 10.1016/0964-8305(95)00109-3. [DOI] [Google Scholar]
- Clemmensen et al. (2013).Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, Stenlid J, Finlay RD, Wardle DA, Lindahl BD. Roots and associated fungi drive Long-Term carbon sequestration in boreal forest. Science. 2013;339(6127):1615–1618. doi: 10.1126/science.1231923. [DOI] [PubMed] [Google Scholar]
- Comas, Bouma & Eissenstat (2002).Comas LH, Bouma TJ, Eissenstat DM. Lingking root traits to potential growth rate in six temperate tree species. Oecologia. 2002;132(1):34–43. doi: 10.1007/s00442-002-0922-8. [DOI] [PubMed] [Google Scholar]
- Combes-Meynet et al. (2011).Combes-Meynet E, Pothier JF, Moenne-Loccoz Y, Prigent-Combaret C. The Pseudomonas secondary metabolite 2,4-diacetylphloroglucinol is a signal inducing rhizoplane expression of Azospirillum genes involved in plant-growth promotion. Molecular Plant-Microbe Interaction. 2011;24(2):271–284. doi: 10.1094/MPMI-07-10-0148. [DOI] [PubMed] [Google Scholar]
- Constantino et al. (2008).Constantino M, Gomez-Alvarez R, Alvarez-Solis JD, Geissen V, Huerta E, Barba E. Effect of inoculation with rhizobacteria and arbuscular mycorrhizal fungi on growth and yield of capsicum Chinense Jacquin. Journal of Agriculture and Rural Development in the Tropics and Subtropics. 2008;109:169–180. doi: 10.1017/S0021859615000714. [DOI] [Google Scholar]
- Dalpé & Séguin (2013).Dalpé Y, Séguin SM. Microwave-assisted technology for the clearing and staining of arbuscular mycorrhizal fungi in roots. Mycorrhiza. 2013;23(4):333–340. doi: 10.1007/s00572-012-0472-9. [DOI] [PubMed] [Google Scholar]
- de Vries & Bardgett (2016).de Vries FT, Bardgett RD. Plant community controls on short-term ecosystem nitrogen retention. New Phytologist. 2016;210(3):861–874. doi: 10.1111/nph.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd et al. (2010).Dodd IC, Zinovkina NY, Safronova VI, Belimov AA. Rhizobacterial mediation of plant hormone status. Annuals of Applied Biology. 2010;157(3):361–379. doi: 10.1111/j.1744-7348.2010.00439.x. [DOI] [Google Scholar]
- Egamberdieva et al. (2017).Egamberdieva D, Wirth S, Jabborova D, Rasanen LA, Liao H. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. Journal of Plant Interactions. 2017;12(1):100–107. doi: 10.1080/17429145.2017.1294212. [DOI] [Google Scholar]
- El-Sawah et al. (2021).El-Sawah AM, El-Keblawy A, Ali DFI, Ibrahim HM, El-Sheikh MA, Sharma A, Hamoud YA, Shaghaleh H, Brestic M, Skalicky M. Arbuscular mycorrhizal fungi and plant Growth-Promoting rhizobacteria enhance soil key enzymes, plant growth, seed yield, and qualitative attributes of guar. Agriculture. 2021;11(3):194. doi: 10.3390/agriculture11030194. [DOI] [Google Scholar]
- Ezawa & Saito (2018).Ezawa T, Saito K. How do arbuscular mycorrhizal fungi handlephosphate? New insight into fine-tuning of phosphate metabolism. New Phytologist. 2018;220(4):1116–1121. doi: 10.1111/nph.15187. [DOI] [PubMed] [Google Scholar]
- Field & Pressel (2018).Field KJ, Pressel S. Unity in diversity: structural and functional insights into the ancient partnerships between plants and fungi. New Phytologist. 2018;220(4):996–1011. doi: 10.1111/nph.15158. [DOI] [PubMed] [Google Scholar]
- Fierer (2017).Fierer N. Embracing the unknown: disentangling the complexities of the soil microbiome. Nature Reviews Microbiology. 2017;15(10):579–590. doi: 10.1038/nrmicro.2017.87. [DOI] [PubMed] [Google Scholar]
- Frew, Powell & Johnson (2020).Frew A, Powell JR, Johnson SN. Aboveground resource allocation in response to root herbivory as affected by the arbuscular mycorrhizal symbiosis. Plant and Soil. 2020;447(11):463–473. doi: 10.1007/s11104-019-04399-x. [DOI] [Google Scholar]
- Giovannetti et al. (2001).Giovannetti M, Fortuna P, Citernesi AS, Morini S, Nuti MP. The occurrence of anastomosis formation and nuclear exchange in intact arbuscular mycorrhizal networks. New Phytologist. 2001;151(3):717–724. doi: 10.1046/j.0028-646x.2001.00216.x. [DOI] [PubMed] [Google Scholar]
- Hawkins, Johansen & George (2000).Hawkins HJ, Johansen A, George E. Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant and Soil. 2000;226(2):275–285. doi: 10.1023/A:1026500810385. [DOI] [Google Scholar]
- Hildebrandt et al. (2006).Hildebrandt U, Ouziad F, Marner FJ, Bothe H. The bacterium Paenibacillus validus stimulates growth of the arbuscular mycorrhizal fungus Glomus intraradices up to the formation of fertile spores. FEMS Microbiology Letters. 2006;254(2):258–267. doi: 10.1111/j.1574-6968.2005.00027.x. [DOI] [PubMed] [Google Scholar]
- Hodge (2004).Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist. 2004;162(1):9–24. doi: 10.1111/j.1469-8137.2004.01015.x. [DOI] [Google Scholar]
- Hussain & Khan (2020).Hussain T, Khan AA. Bacillus subtilis HussainT-AMU and its Antifungal activity against Potato Black scurf caused by Rhizoctonia solani on seed tubers. Biocatalysis and Agricultural Biotechnology. 2020;23(3):101443. doi: 10.1016/j.bcab.2019.101443. [DOI] [Google Scholar]
- Jeffries et al. (2003).Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biology and Fertility of Soils. 2003;37(1):1–16. doi: 10.1007/s00374-002-0546-5. [DOI] [Google Scholar]
- Juge et al. (2012).Juge C, Prevost D, Bertrand A, Bipfubusa M, Chalifour FP. Growth and biochemical responses of soybean to double and triple microbial associations with Bradyrhizobium, Azospirillum and arbuscular mycorrhizae. Applied Soil Ecology. 2012;61:147–157. doi: 10.1016/j.apsoil.2012.05.006. [DOI] [Google Scholar]
- Kormanik & McGraw (1982).Kormanik PP, McGraw AC. Quantification of vesicular-arbuscular mycorrhizae in plant roots. In: Schenck NC, editor. Methods and principles of mycorrhizal research. St. Paul: American Phytopathological Society; 1982. pp. 37–45. [Google Scholar]
- Krey et al. (2011).Krey T, Caus M, Baum C, Ruppel S, Eichler-Löbermann B. Interactive effects of plant growth-promoting rhizobacteria and organic fertilization on P nutrition of Zea mays L. and Brassica napus L. Journal of Plant Nutrition and Soil Science. 2011;174(4):602–613. doi: 10.1002/jpln.200900349. [DOI] [Google Scholar]
- Larson & Funk (2016).Larson JE, Funk JL. Seedling root responses to soil moisture and the identification of a belowground trait spectrum across three growth forms. New Phytologist. 2016;210(3):827–838. doi: 10.1111/nph.13829. [DOI] [PubMed] [Google Scholar]
- Legay et al. (2014).Legay N, Baxendale C, Grigulis K, Krainer U, Kastl E, Schloter M, Bardgett RD, Arnoldi C, Bahn M, Dumont M. Contribution of above- and below-ground plant traits to the structure and function of grassland soil microbial communities. Annuals of Botany. 2014;114(5):1011–1021. doi: 10.1093/aob/mcu169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2020).Li JH, Xie S, Wilson GWT, Cobb AB, Tang S, Guo L, Wang K, Deng B. Plant-microbial interactions facilitate grassland species coexistence at the community level. Oikos. 2020;129(4):533–543. doi: 10.1111/oik.06609. [DOI] [Google Scholar]
- Ma, Rajkumar & Freitas (2009).Ma Y, Rajkumar M, Freitas H. Inoculation of plant growth promoting bacterium Achromobacter xylosoxidans strain Ax10 for the improvement of copper phytoextraction by Brassica juncea. Journal of Environmental Management. 2009;90:831–837. doi: 10.1016/j.jenvman.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Maire et al. (2009).Maire V, Gross N, Da Silveira Pontes L, Picon-Cochard C, Soussana JO. Trade-off between root nitrogen acquisition and shoot nitrogen utilization across 13 co-occurring pasture grass species. Functional Ecology. 2009;23:668–679. doi: 10.1111/j.1365-2435.2009.01557.x. [DOI] [Google Scholar]
- Mazzoncini et al. (2010).Mazzoncini M, Canali S, Giovannetti M, Castagnoli M, Tittarelli F, Antichi D, Nannelli R, Cristani C, Bàrberi P. Comparison of organic and conventional stockless arable systems: a multidisciplinary approach to soil quality evaluation. Applied Soil Ecology. 2010;44:124–132. doi: 10.1016/j.apsoil.2009.11.001. [DOI] [Google Scholar]
- Mohamed et al. (2014).Mohamed AA, Eweda WEE, Heggo AM, Hassan EA. Effect of dual inoculation with arbuscular mycorrhizal fungi and sulphur-oxidising bacteria on onion (Allium cepa L.) and maize (Zea mays L.) grown in sandy soil under greenhouse conditions. Annals of Agricultural Sciences. 2014;59:109–118. doi: 10.1016/j.aoas.2014.06.015. [DOI] [Google Scholar]
- Moreau et al. (2015).Moreau D, Pivato B, Bru D, Busset H, Deau F, Celine F, Annick Matejicek FSLP, Mougel AC. Plant traits related to nitrogen uptake inuence plant-microbe competition. Ecology. 2015;8:2300–2310. doi: 10.1890/14-1761.1. [DOI] [PubMed] [Google Scholar]
- Morgan, Bending & White (2005).Morgan JAW, Bending GD, White PJ. Biological costs and benefits to plant-microbe interactions in the rhizosphere. Journal of Experimental Botany. 2005;56:1729–1739. doi: 10.1093/jxb/eri205. [DOI] [PubMed] [Google Scholar]
- Moubayidin, Di Mambro & Sabatini (2009).Moubayidin L, Di Mambro R, Sabatini S. Cytokinin-auxin crosstalk. Trends in Plant Science. 2009;14:557–562. doi: 10.1016/j.tplants.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Muzammil et al. (2014).Muzammil S, Graillon C, Saria R, Mathieu F, Lebrihi A, Compant S. The Saharan isolate Saccharothrix algeriensis NRRL B-24137 induces systemic resistance in Arabidopsis thalianaseedlings against Botrytis cinerea. Plant and Soil. 2014;374:423–434. doi: 10.1007/s11104-013-1864-0. [DOI] [Google Scholar]
- Pan et al. (2020).Pan J, Huang C, Peng F, Zhang W, Luo J, Ma S, Xue X. Effect of arbuscular mycorrhizal fungi (AMF) and plant growth-promoting bacteria (PGPR) inoculations on Elaeagnus angustifolia L. in saline soil. Applied Sciences. 2020;10:945. doi: 10.3390/app10030945. [DOI] [Google Scholar]
- Pan et al. (2019).Pan J, Peng F, Xue X, You Q, Zhang W, Wang T, Huang C. The growth promotion of two Salt-Tolerant plant groups with PGPR inoculation: a meta-analysis. Sustainability-Basel. 2019;11(2):378. doi: 10.3390/su11020378. [DOI] [Google Scholar]
- Patel, Thakkar & Subramanian (2015).Patel RR, Thakkar VR, Subramanian BR. A Pseudomonas guariconensis strain capable of promoting growth and controlling collar rot disease in Arachis hypogaea L. Plant and Soil. 2015;390:369–381. doi: 10.1007/s11104-015-2436-2. [DOI] [Google Scholar]
- Patten & Glick (2002).Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Applied and Environmental Microbiology. 2002;68:3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe, Giovannetti & Sbrana (2016).Pepe A, Giovannetti M, Sbrana C. Different levels of hyphal self-incompatibility modulate interconnectedness of mycorrhizal networks in three arbuscular mycorrhizal fungi within the Glomeraceae. Mycorrhiza. 2016;26:325–332. doi: 10.1007/s00572-015-0671-2. [DOI] [PubMed] [Google Scholar]
- Peret et al. (2012).Peret B, Li G, Zhao J, Band LR, Voss U, Postaire O, Doan-Trung L, Da Ines O, Casimiro I, Lucas M. Auxin regulates aquaporin function to facilitate lateral root emergence. Nature Cell Biology. 2012;14:991–998. doi: 10.1038/ncb2573. [DOI] [PubMed] [Google Scholar]
- Phillips, Brzostek & Midgley (2013).Phillips RP, Brzostek E, Midgley MG. The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytologist. 2013;199:41–51. doi: 10.1111/nph.12221. [DOI] [PubMed] [Google Scholar]
- Pivato et al. (2009).Pivato B, Offre P, Marchelli S, Barbonaglia B, Mougel C, Lemanceau P, Berta G. Bacterial effects on arbuscular mycorrhizal fungi and mycorrhiza development as influenced by the bacteria, fungi, and host plant. Mycorrhiza. 2009;19(2):81–90. doi: 10.1007/s00572-008-0205-2. [DOI] [PubMed] [Google Scholar]
- Rasmann et al. (2017).Rasmann S, Bennett A, Biere A, Karley A, Guerrieri E. Root symbionts: powerful drivers of plant above- and belowground indirect defenses. Insect Science. 2017;24(6):947–960. doi: 10.1111/1744-7917.12464. [DOI] [PubMed] [Google Scholar]
- Rewald Email et al. (2013).Rewald Email B, Shelef O, Ephrath JE, Rachmilevitch S. Ecophysiology and Responses of Plants Under Salt Stress. New York: Springer; 2013. Adaptive plasticity of Salt-Stressed root systems. In ecophysiology and responses of plants under salt stress; pp. 169–201. [Google Scholar]
- Rillig et al. (2015).Rillig MC, Aguilar-Trigueros CA, Bergmann J, Verbruggen E, Veresoglou SD, Lehmann A. Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytologist. 2015;205(4):1385–1388. doi: 10.1111/nph.13045. [DOI] [PubMed] [Google Scholar]
- Romaní et al. (2006).Romaní AM, Fischer H, Mille-Lindblom C, Tranvik LJ. Interactions of bacteria and fungi on decomposing litter: differential extracellular enzyme activities. Ecology. 2006;87(10):2559–2569. doi: 10.1890/0012-9658(2006)87[2559:IOBAFO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Saia et al. (2015).Saia S, Rappa V, Ruisi P, Abenavoli MR, Sunseri F, Giambalvo D, Frenda AS, Martinelli F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front in Plant Science. 2015;6(116):51. doi: 10.3389/fpls.2015.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena et al. (2020).Saxena AK, Kumar M, Chakdar H, Anuroopa N, Bagyaraj DJ. Bacillus species in soil as a natural resource for plant health and nutrition. Journal of Applied Microbiology. 2020;128:1583–1594. doi: 10.1111/jam.14506. [DOI] [PubMed] [Google Scholar]
- Schussler, Schwarzott & Walker (2001).Schussler A, Schwarzott D, Walker C. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Research. 2001;105:1413–1421. doi: 10.1017/S0953756201005196. [DOI] [Google Scholar]
- Schweiger, Baier & Mueller (2014).Schweiger R, Baier MC, Mueller C. Arbuscular Mycorrhiza-Induced shifts in foliar metabolism and photosynthesis mirror the developmental stage of the symbiosis and are only partly driven by improved phosphate uptake. Molecular Plant Microbe Interaction. 2014;27:1403–1412. doi: 10.1094/MPMI-05-14-0126-R. [DOI] [PubMed] [Google Scholar]
- Shockley, McGraw & Garrett (2004).Shockley FW, McGraw RL, Garrett HE. Growth and nutrient concentration of two native forage legumes inoculated with Rhizobium and Mycorrhiza in Missouri, USA. Agroforestry Systems. 2004;60:137–142. doi: 10.1023/B:AGFO.0000013269.19284.53. [DOI] [Google Scholar]
- Solomon & Rajeshkanna (2013).Solomon JSK, Rajeshkanna KN. Microbiological Research in Agroecosystem Management. New Delhi: Springer India; 2013. Efficacy of AMF and PGPR inoculants on maize (Zea mays L.) plant growth and their rhizosphere soil properties; pp. 155–173. [DOI] [Google Scholar]
- Sondergaard, Schulz & Palmgren (2004).Sondergaard TE, Schulz A, Palmgren MG. Energization of transport processes in plants. Roles of the plasma membrane H+-ATPase. Plant Physiology. 2004;136:2475–2482. doi: 10.1104/pp.104.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova & Alonso (2009).Stepanova AN, Alonso JM. Ethylene signaling and response: where different regulatory modules meet. Current Opinion in Plant Biology. 2009;12(5):548–555. doi: 10.1016/j.pbi.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Tomczak, Schweiger & Müller (2016).Tomczak VV, Schweiger R, Müller C. Effects of arbuscular mycorrhiza on plant chemistry and the development and behavior of a generalist herbivore. Journal of Chemical Ecology. 2016;42(12):1247–1258. doi: 10.1007/s10886-016-0785-9. [DOI] [PubMed] [Google Scholar]
- Toro, Azcon & Barea (1997).Toro M, Azcon R, Barea JM. Improvement of arbuscular mycorrhiza development by inoculation of soil with phosphate-solubilizing rhizobacteria to improve rock phosphate bioavailability (32P) and nutrient cycling. Applied and Environmental Microbiology. 1997;63(11):4408–4412. doi: 10.1128/aem.63.11.4408-4412.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden et al. (2015).van der Heijden MGA, Martin FM, Selosse M, Sanders IR. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist. 2015;205(4):1406–1423. doi: 10.1111/nph.13288. [DOI] [PubMed] [Google Scholar]
- Vessey (2003).Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil. 2003;255(2):571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- Vorholt (2012).Vorholt JA. Microbial life in the phyllosphere. Nature Reviews Microbiology. 2012;10:828–840. doi: 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- Wagg et al. (2014).Wagg C, Bender SF, Widmer F, van der Heijden MGA. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:5266–5270. doi: 10.1073/pnas.1320054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagg et al. (2019).Wagg C, Schlaeppi K, Banerjee S, Kuramae EE, van der Heijden MGA. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nature Communication. 2019;10:4841. doi: 10.1038/s41467-019-12798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein (2006).Wallenstein MD. Modern soil microbiology. In: Elsas JDV, Trevors JT, Wellington EMH, editors. Modern Soil Microbiology. Boca Raton: CRC Press; 2006. p. 683. Soil Science Society of America Journal. [Google Scholar]
- Wang et al. (2006).Wang F, Lin X, Yin R, Wu L. Effects of arbuscular mycorrhizal inoculation on the growth of Elsholtzia splendens and Zea mays and the activities of phosphatase and urease in a multi-metal-contaminated soil under unsterilized conditions. Applied Soil Ecology. 2006;31:110–119. doi: 10.1016/j.apsoil.2005.03.002. [DOI] [Google Scholar]
- Wang et al. (2016).Wang P, Wu S, Wen M, Wang Y, Wu Q. Effects of combined inoculation with Rhizophagus intraradices and Paenibacillus mucilaginosus on plant growth, root morphology, and physiological status of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under different levels of phosphorus. Scientia Horticulturae. 2016;205:97–105. doi: 10.1016/j.scienta.2016.04.023. [DOI] [Google Scholar]
- Warmink et al. (2011).Warmink JA, Nazir R, Corten B, van Elsas JD. Hitchhikers on the fungal highway: the helper effect for bacterial migration via fungal hyphae. Soil Biology and Biochemistry. 2011;43(4):760–765. doi: 10.1016/j.soilbio.2010.12.009. [DOI] [Google Scholar]
- Wei et al. (2021).Wei XT, Shi YN, Qin FW, Zhou HK, Shao XQ. Effects of experimental warming, precipitation increase and their interaction on AM fungal community in an alpine grassland of the Qinghai-Tibetan Plateau. European Journal of Soil Biology. 2021;102:103272. doi: 10.1016/j.ejsobi.2020.103272. [DOI] [Google Scholar]
- Whitman, Coleman & Wiebe (1998).Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(12):6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao et al. (2018).Xiao L, Huang Y, Zeng Q, Zhao J, Zhou J. Soil enzyme activities and microbial biomass response to crop types on the terraces of the Loess Plateau, China. Journal of Soils and Sediments. 2018;18(5):1971–1980. doi: 10.1007/s11368-018-1969-4. [DOI] [Google Scholar]
- Xie et al. (2020).Xie M, Zou Y, Wu Q, Zhang Z, Kuča K. Single or dual inoculation of arbuscular mycorrhizal fungi and rhizobia regulates plant growth and nitrogen acquisition in white clover. Plant, Soil and Environment. 2020;66(6):287–294. doi: 10.17221/234/2020-PSE. [DOI] [Google Scholar]
- Yang et al. (2018).Yang C, Li J, Zhang F, Liu N, Zhang YJ. The optimal Redfield N: P ratio caused by fairy ring fungi stimulates plant productivity in the temperate steppe of China. Fungal Ecology. 2018;34:91–98. doi: 10.1016/j.funeco.2018.05.007. [DOI] [Google Scholar]
- Yuan et al. (2021).Yuan MM, Kakouridis A, Starr E, Nguyen N, Shi S, Pett-Ridge J, Nuccio E, Zhou J, Firestone M. Fungal-bacterial cooccurrence patterns differ between arbuscular mycorrhizal fungi and nonmycorrhizal fungi across soil niches. MBio. 2021;12(2):e03509. doi: 10.1128/mBio.03509-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zai et al. (2014).Zai X, Hao Z, Zhao H, Qin P. Rhizospheric niche of beach plum seedlings colonized by arbuscular mycorrhizal fungi (in Chinese) Scientia Silvae Sinicae. 2014;50:41–48. doi: 10.11707/j.1001-7488.20140107. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files.