Abstract
Background and Aims:
High flow nasal cannula (HFNC) has numerous advantages against conventional oxygen therapy delivery systems. However, there is limited evidence supporting the use of HFNC in endoscopic ultrasound (EUS) under procedural sedation. The aims of this study is to evaluate the efficacy of two different oxygen delivery devices, that is,HFNCand conventional nasal cannula on the oxygenation status of patients during procedural sedation for EUS.
Material and Methods:
Sixty adult patients undergoing EUS for various ailments were randomized to two groups group HFNC (n=30) and group nasal cannula [NC (n = 30)]. HFNC (AIRVO2, Fisher and Paykel Healthcare, New Zealand) was used on patients in the group HFNC. Respiratory status of the patients was assessed using pulse oximetry, respiratory rate, procedural airway complications, and oxygen therapy adjustments. The endoscopist assessed the ease of performing EUS at the end of the procedure and patient satisfaction score (PSS) was assessed by using a Likert score in the post-anesthesia care unit.
Results:
SpO2 measurements in the HFNC group during the procedure were marginally better compared to the NC group but this failed to reach statistical significance. Also, no significant association was found between both groups while comparing desaturation events (P = 0.499), patient satisfaction score (PSS) and endendoscopist’s satisfaction score (ESS) (P = 0.795). Both the groups were comparable in terms of airway manipulation, use of airway adjuncts, need to increase oxygen flow rate, endoscope removal, apneic episodes, hypotension, and bradycardia. No major complications were observed in either group.
Conclusion:
HFNC use in patients undergoing EUS is not superior when compared to conventional nasal cannula oxygen therapy. HFNC failed to show any significant impact on decreasing the risk of desaturation events and airway manipulation during the procedure.
Keywords: Cannula, endoscopy, endosonography, oxygen inhalation therapy, patient satisfaction
Introduction
Endoscopic ultrasound (EUS) is a commonly performed procedure in endoscopy suites wherein sedation and analgesia are essential for smooth initiation, maintenance, and completion of the procedure.[1] To accomplish this, propofol/opioid-based sedation is the most commonly used. The common adverse effects identified with propofol/opioid-based sedation are hypotension, apnoea/respiratory depression leading to desaturation episodes, unplanned intubation, and procedure termination.[2] Therefore, along with carefully titrated administration of analgesia/sedation, adjuvant oxygen administration is an integral part in such procedures to prevent respiratory adverse effects. Oxygen therapy can be given by various methods such as oxygen mask, nasal cannula, or advanced airway devices. The nasal cannula (NC) is most commonly used tool to deliver oxygen therapy in such scenarios.
Recently, there has been a growing interest in alternative oxygen therapy delivery systems, one of them being high flow nasal cannula (HFNC). These systems utilize higher gas flow rates than the standard nasal cannula. The use of HFNC as a respiratory support modality is increasing in the infant, pediatric, and adult populations as an alternative to non-invasive positive pressure ventilation. Humidified and warmed HFNC oxygen therapy has numerous improved physiological effects such as pharyngeal dead space washout, nasopharyngeal splinting, positive expiratory pressure effect, increased alveolar recruitment, better humidification, constant fraction of inspired oxygen, more comfort, and better tolerance by the patient.[3-6] These advantages are associated with reduced hypoventilation, atelectasis, and improved oxygenation.[7] There is limited evidence of the use of HFNC during procedural sedation in endoscopic procedures, hence we felt the need for this study. With these viewpoints, the present study was conducted with the primary objective to comparatively evaluate the efficacy of oxygenation (viz. desaturation events and respiratory rate) of two different oxygen delivery devices, that is, high flow nasal oxygen cannula and conventional nasal cannula on the oxygenation status of patients during propofol–fentanyl-based intravenous sedation for EUS. Secondary objectives of the study were any respiratory complications, total propofol consumption (TPC), endoscopist satisfaction score (ESS), and patient satisfaction score (PSS). A pilot study was conducted as a preliminary to a larger trial for assessing the proposed benefit of the HFNC device.
Material and Methods
The study was conducted in the gastroenterology (GE) center of a tertiary care hospital. Institute ethical committee clearance certification was sought and obtained before the study began. Informed written consent was obtained from all the study participants before including them in the study after explaining them implications of the study. This study was conducted on patients planned to undergo EUS for various indications. Patients with pre-existing respiratory impairment or illness, history of obstructive sleep apnea, coronary artery disease, anticipated difficult airway, pregnancy, and allergy to any of the drugs used during sedation were excluded from the study.
The study was carried out as an open-label, prospective randomized comparative study over a period of 18 months from Jan 2018 to June 2019. Subjects were randomly divided into two groups, group HFNC (n = 30) and group NC (n = 30) based on concealed envelop allocation technique with explicit code randomization. Standard fasting guidelines were followed. All the patients were in left decubitus position and standard monitoring was placed. BIS disposable SomaSensor transducers were applied on the forehead (Invos 5100C, Covidien, USA) to monitor the depth of sedation. A crystalloid solution at 4 ml/kg/h was given during the procedure and continued in the recovery room.
HFNC was used for patients in the group HFNC and connected to the AIRVO2 system (Fisher & Paykel Healthcare Limited, Auckland, New Zealand). This system comprises of an air/oxygen blender, an active humidifier, a single heated circuit, and a nasal cannula. It delivers adequately heated and humidified oxygen up to 60 L/min of flow and set FiO2 (fraction of inspired oxygen). The FiO2 was set at 0.4 with temperature of 37°C and with a humidity of 100%. In group NC, a conventional NC (Adult nasal cannula, Flexicare Medical Limited, United Kingdom) was used at 6–7 l/min to deliver similar FiO2.[8]
The procedure was performed in the endoscopy suite by gastroenterologists of the institution who had adequate experience. After standard monitoring recommended by the American Society of Anesthesiologists (ASA) were attached, the patients were asked to position themselves into left decubitus position. All the patients received 1 μg/kg fentanyl and 1.5 mg/kg propofol bolus over 30 s, which was followed by an infusion of propofol (25–100mcg/kg/min) using a syringe pump to maintain a target BIS of 50–60 during the procedure. An adult endoscope was inserted with bite block in situ in all patients. In situations of sudden patient movement and difficulty in maneuvring the endoscope, a bolus of propofol 0.5mg/ kg was used.
Heart rate (HR), non-invasive mean arterial pressure (MAP), respiratory rate (RR), and oxygen saturation (SpO2) were noted every 5 min. Desaturation was defined as SpO2<92% for >10s, hypotension as MAP <60 mm Hg, and bradycardia as HR <50 beats per minute for the purpose of this study. Primary endpoint of the study was desaturation event. Secondary endpoints being the incidence of airway manipulation, use of airway adjuncts, need to increase oxygen flow rate, apnea episodes, hypotension, and bradycardia during the procedure.
The endoscopist assessed the ease of performing EUS at the end of the procedure as I-satisfactory, II-difficult, or III-impossible. The procedure was abandoned when respiratory depression (oxygen saturation level <92% for >10s) was encountered, the attending anesthesiologist would provide immediate airway support, and the procedure either resumed or abandoned at the discretion of the anesthesiologist. Hypotension and bradycardia were treated with fluid boluses and intravenous atropine 20 mcg/kg, respectively.
The patients were shifted to Post-Anesthesia Care Unit PACU after completion of the procedure and BIS >75. Patients were monitored in PACU for any complications (hypoxia, hemodynamic instability, shivering, nausea and vomiting, agitation). In case of any serious event, patients were transferred to High Dependency Unit (HDU) and treated by the attending physician. The Aldrete‘s scoring system was used for determining when patients could be safely discharged from the PACU.
PSS was graded on a five-point Likert scale (1 = poor, 2 = fair, 3 = good, 4 = very good, 5 = excellent). A score of ≥3 was considered satisfactory (favorable response) which was taken 6 h post-procedure.
Statistical analysis
Distribution of the continuous data was tested with the Kolmogorov–Smirnov one-sample test. Continuous variables with a normal distribution are expressed as mean % standard deviation (SD). Dichotomous data are expressed as numbers and percentages. For continuous variables, t-test has been used for comparing two groups. Chi-square/Fisher exact test is used for the categorical variables. Mixed factor repeated measures ANOVA with Tukey correction was used to find any significant impact of the use of HFNC (AIRVO) or NC on factors like respiratory rate and SpO2 at different time periods. This being a pilot study, with a 90% powered main trial and standardized difference of 0.2 (variable-SpO2 reading), a sample size of 28 was required per treatment arm (based on Non-central t-distribution).[9] We accepted a minimum number of subjects of 30 per group. Statistical analysis was performed using SPSS software (IBM SPSS Statistics 21, Chicago IL). Pvalue <0.01 was considered statistically significant.
Results
Sixty-seven patients were screened for enrolment in the pilot study; 3 patients did not consent. Of those, a total of 64 patients, aged more than 18 years undergoing EUS for various indications under propofol sedation were included in the study. Among 64 patients enrolled in the study, 32 were randomly assigned to the NC group and 32 were in the HFNC (AIRVO) group [Figure 1]. Among 60 patients who completed the study {mean age, 51 years [range, 19–83]; 51.70% Females 48.30% Men}, 30 received HFNC and 30 conventional oxygen therapy (NC). The majority of patients were male (~57%) in the NC group vs. HFNC group (40%). 60% of subjects in both groups had BMI in the range of 23–26. There were no comorbidities seen in 45% of the study subjects. Hypertension was seen in 25% of the study subjects, followed by both hypertension and type II DM (15%) [Table 1]. Distribution of diseases based on diagnosis is represented in Figure 2.
Figure 1.
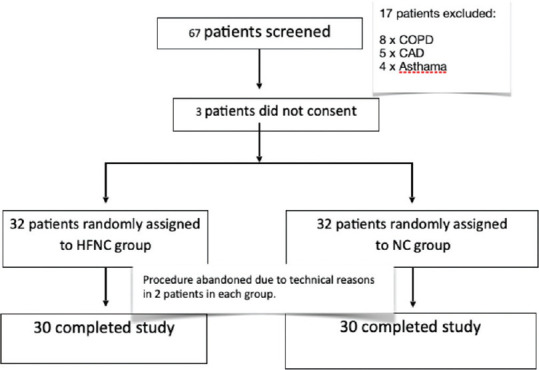
Consort
Table 1.
Patients demographic Characteristics, Hemodynamic Variables and Outcomes
| SN o | Parameter | HFNC n=30 | NC n=30 | T value/Chi square value | P value |
|---|---|---|---|---|---|
| 1 | Age (yrs) | 48.3 ±18.5 | 52.7 ±15.9 | -0.98 | 0.33 |
| 2 | Gender (male:female ratio) | 12:18(0.66) | 17:13 (1.31) | 1.67 | 0.2 |
| 3 | BMI | 23.83 ±2.8 | 24.9 ±2.4 | -1.6 | 0.12 |
| 4 | Hypertension n(%) | 10(33.3%) | 15 (50%) | 1.71 | 0.19 |
| 5 | Diabetes Mellitus n(%) | 6 (20%) | 6 (20%) | 0.0 | 1 |
| 6 | ASA Status (n) | ||||
| 1 | 9 | 10 | 0.74 | 0.69 | |
| 2 | 17 | 14 | |||
| 3 | 4 | 6 | |||
| 7 | TPC (mg/kg) | 2.56 ±0.27 | 2.66 ± 0.23 | -1.6 | 0.12 |
| 8 | Duration of Procedure (mins) | 38.1 ±6.4 | 41.4 ±10.3 | -1.5 | 0.14 |
| Baseline Recording | |||||
| 9 | HR (bpm) | 85 % 11 | 82 % 10 | 1.04 | 0.3 |
| 10 | MAP (mm Hg) | 90 ±8 | 86 ±9 | 1.68 | 0.09 |
| 11 | Sp02(%) | 99.3 ±0.5 | 99.4 ±0.9 | -0.66 | 0.51 |
| 12 | RR (/min) | 17.6 % 2.6 | 16.4 ±2.7 | 1.72 | 0.09 |
| Outcomes | |||||
| 13 | ESS n(%) | ||||
| 1 | 30 (100%) | 28 (93.3%) | 2.1 | 0.49 | |
| 2 | 0 | 2 (6.7%) | |||
| 14 | PSS n(%) | ||||
| 4 | 16(53.3%) | 18(60%) | 0.27 | 0.60 | |
| 5 | 14(46.7%) | 12(40%) | |||
| Desaturation during procedure | |||||
| 15 | Desaturation event (n) | 2 | 5 | 2.3 | 0.51 |
| 16 | Desaturation duration (sec) MEDIAN (IQR) | 15-20 | 15 (13.75-16.25) | - | - |
| 17 | Percent desaturation <90% MEDIAN (IQR) | 2-5 | 8.5 (6.5-10) | - | - |
Clii square/Fisher Exact test, BMI-Body mass index, ASA- American Association of Anaesthesia, TPC- Total- propofol consumption. HR- Heart rate. MAP- Mean arterial pressure, Sp02- pulse oximetry, RR- Respiratory ate, ESS- Endoscopist satisfaction score. PSS- Patient satisfaction score, IQR-Inter quartile range
Figure 2.
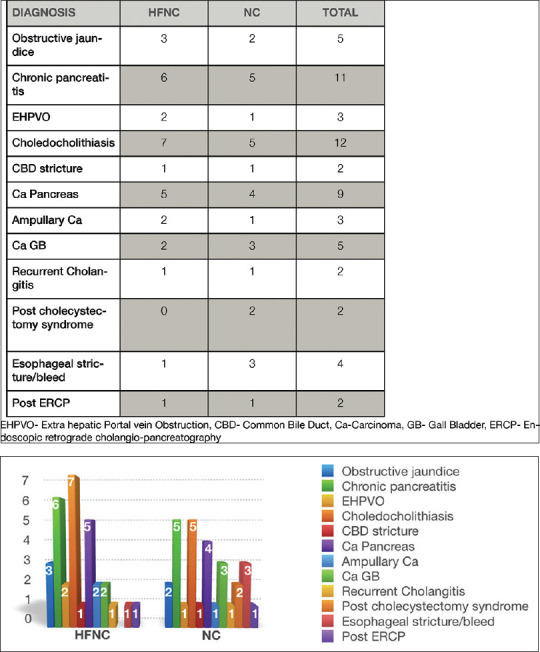
Diagnosis of Patients undergoing EUS
No significant association was found between both groups in desaturation events (DSE) (P = 0.51), TPC (P = 0.12), procedure duration (P = 0.14), PSSandESS (P =0.49 and 0.60, respectively) [Table 1].
The two groups were comparable for the respiratory rate which was higher in patients during the procedure compared to before and after procedure [Figure 3]. There was a greater decline in SPO2 during the procedure in the NC compared to the HFNC group but the difference was not statistically significant (P = 0.22) [Figure 4].
Figure 3.
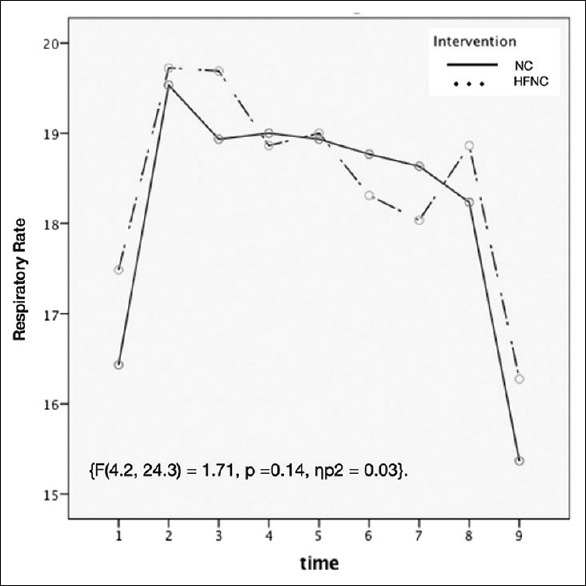
Line diagram of Respiratory Rate (RR) of two groups at different time periods. (1- baseline; After start of procedure: 2- 1min, 3–5 min, 4–10 min, 5–15 min, 6–20 mis, 7–30 min)
Figure 4.
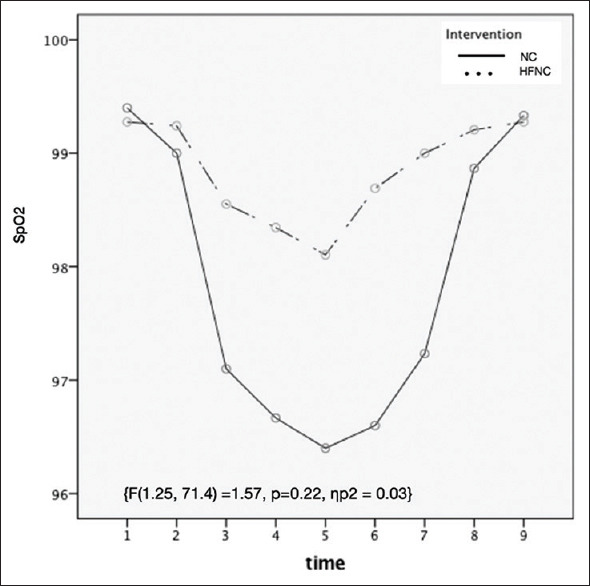
Line diagram of pulse oximetry (SpO2) of two groups at different time periods. (1- baseline; After start of procedure: 2- 1min, 3–5 min, 4–10 min, 5–15 min, 6–20 min, 7–30 min)
Mean Bi Spectral Index (BIS) at baseline in HFNC and NC group was 93.6 ± 2.6 and 93.9 ± 2.4, respectively. There was no significant difference in BIS values between the groups at various time intervals during the procedure. The complications during the procedure has been summarized in Table 2. Desaturation events, airway manipulation, use of airway adjuncts, need to increase oxygen flow rate, endoscope removal, apnea episodes, hypotension, and bradycardia were not significantly different in NC and HFNC groups (P>0.01). Though desaturation events and duration of desaturation are fewer and lesser in HFNC group as compared to the NC group but the difference could not reach statistical significance.
Table 2.
Complications during the procedure
| COMPLICATIONS | HFNC (N=30) | NC (N=30) | FISHER EXACT VALUE | P VALUE |
|---|---|---|---|---|
| Desaturation events (No of patients) | 2(2) | 8(5) | 2.37 | 0.499 |
| Airway manipulation | 0 | 5 | 1.45 | 0.210 |
| Use Of Airway Adjuncts | 0 | 1 | 1.40 | 1.000 |
| Need to increase oxygen flow rate | 2 | 5 | 1.49 | 0.420 |
| Endoscope removal | 0 | 2 | 2.84 | 0.490 |
| Apnoeic episodes | 2 | 5 | 1.49 | 0.420 |
| Hypotension /Bradycardia | 0 | 2 | 2.84 | 0.490 |
Discussion
EUS procedures are largely being done under intravenous sedation (IVS) world over and this has many advantages in terms of decreased morbidity, better procedural success and better patient and endoscopist satisfaction. However, it is well known that propofol /opioid-based intravenous sedation can cause inadequate gas exchange owing to hypoventilation and V-Q mismatch.[10-12] Propofol-based anesthesia largely entails propofol infusions governed by the anesthesiologist closely monitoring clinical signs to prevent adverse effects.[13,14] Inhibition of oropharyngeal reflex and decreased muscle tone causing upper airway obstruction (UAO) could be detrimental to the patient safety during deep IVS. Several maneuvres including airway adjuncts, jaw lift, neck tilt can be applied to maintain the upper airway patency. However, in the endoscopic procedures like EUS, due to sharing of airways and patient positioning, use of airway adjuncts, airway isolation as well as airway maneuvres in case of UAO is difficult. Supplemental use of O2 as a routine administration in all patients undergoing EUS is not universal.[15,16] Conventional oxygen therapy through nasal prongs has been used for decades for endoscopic procedures. Newer methods like HFNC are promising in reducing the procedure related morbidity. Both methods have been shown to deliver humidified oxygen and both are capable of generating some amount of unquantified CPAP or positive pressure in the oropharyngeal cavity leading to improved airway patency and higher inspired oxygen.[17,18,19,20] Although in patients with open mouth, the positive pressure generated at this flow rates could be insufficient to relieve UAO. In our study, we chose a FiO2 of 40% in order to minimise hypoxaemia during EUS. After the procedure a FiO2 of 35% was used in PACU. This study showed that both HFNC and NC delivered oxygen therapy achieves effective oxygenation of patients undergoing EUS under IVS.
In this study, we investigated the respiratory effect of HFNC and NC in patients undergoing EUS under sedation. We observed an increase in RR in both groups but the fall in SpO2 was more in the NC group. It is well known that propofol administration changes the respiratory dynamics causing an increase in RR and decrease in tidal volume. Although oxygen saturation depends upon various other factors and may or may not improve, this increase in RR maintains the minute ventilation and negates the effect of hypoventilation to maintain the oxygenation.[21]
Most of the published data on HFNC is in pediatric patients while evidence in adults undergoing intravenous sedation is poor.[22,23] HFNC has been proven to be better in oxygenation when compared to Venturi mask or conventional oxygen therapy.[24,25]However, in our study the de-saturation rate declined and resulted in marginally better oxygenation for a similar set of FiO2 with HFNC therapy when compared with NC group although the difference was not statistically significant.
Several authors have described the positive effects of the usage of HFNC oxygenation therapy against the conventional NC therapy.[26] Whereas in our study though, adverse events (episodes, hypotension, and bradycardia) and interventions (airway manipulation, use of airway adjuncts, need to increase oxygen flow rate, endoscope removal) required in NC group was higher compared to HFNC group but the difference was not significant. This benefit may be related to good compliance, better oxygenation, PEEP and in turn provided better respiratory support. In a prospective, observational study of 20 adults comparing HFNC with conventional oxygen therapy in patients with acute respiratory failure the use of the HFNC significantly reduced respiratory rate and improved partial pressure of oxygen in arterial blood (PaO2) values.[27] However, we did not measure the PaO2 values but found only marginal improvement in SpO2 values during the procedure. This study shows a marginal beneficial effect of HFNC in a mixed group of patients undergoing EUS which was not significant.
Deep sedation in endoscopic procedures has proven to be safe. Although number of side effects including desaturation, hypotension, bradycardia, and apnea have been reported in the literature but only a small percentage of patients experience these. This subset of patients invariably are sicker, had higher ASA class (III/IV), higher BMI and had lower BIS values.[28,29] The morbidity in this group of patients is reported to be 0.19% with no mortality. Also, the pooled OR reported for developing hypoxia or hypotension is 1.07 for EUS.[10] These have been implicated in frequent interruptions and lower satisfaction scores for endoscopist and patients.[2]Providing appropriate sedation is an art that encompasses the quality of examination, patient and physician satisfaction with the sedation.[30]They are better managed in the presence of an anesthesiologist when compared to nurse or endoscopist controlled sedation.[2,29] HFNC comprises of Air/O2 blender, an active humidifier and heated circuit which is capable of delivering warm and humidified O2 at high flows hence better tolerated unlike NC which just delivers dry oxygen. Rabbat et al. reported preference of HFNC therapy as the first alternative to standard oxygen therapy as it was found to have better comfort and better tolerance.[31,32] The results of our study however revealed similar patient satisfaction score in NC and HFNC groups. Since the level of comfort was identical in both groups as judged by PSS, one can possibly assume the two methods were tolerated well. Inadequate depth of anesthesia can cause coughing and retching during endoscope manipulation, whereas deep sedation can lead to airway obstruction, secretions aspiration, and desaturation events, both of which could lower ESS. Interventions like airway manipulation, use of airway adjuncts, increased oxygen flow rate, and endoscope removal though found lower in HFNC were statistically insignificant in between groups. The satisfaction of endoscopist was not different in NC group as compared to HFNC group.
The dosage of propofol was recorded, calculated, and compared between the groups. The method of administration of propofol was bolus dose, while induction followed by infusion and bolus for rescue therapy in case the anesthesia plane was inadequate. The anesthesiologist in-charge of the case closely monitored the BIS levels and insertion of endoscope was attempted once BIS was <60 coinciding with the peak effect of propofol. No significant difference was noted in total dose/kg and duration of procedure between the groups. The results of propofol-based sedation monitored with BIS in both HFNC and NC groups revealed that there was neither any significant association in BIS values nor in adverse events between both the groups. Hence, we conclude that propofol consumption in NC and HFNC group had no association with any of the outcomes.
There were several limitations in this study. Complete blinding was not possible. Furthermore, the patient population had normal BMI without any obvious difficult airway component which could have highlighted the benefit of HFNC in this population. We did not carry out a cost-effect analysis as hypoxia related adverse events are difficult to analyze in relation to the cost factor. Apneic episodes recordings are again subjective and unreliable. Further studies are needed to see the beneficial effect of HFNC in high-risk airway patient sub-group such as with Obesity, OSA and limited pulmonary reserves. Also, correlating the severity of hypoxia with blood gas analysis could be more valuable and may be considered in further studies.
Conclusion
This study showed marginal improvement in respiratory functions in terms of oxygenation and decreased need for airway rescue maneuvres with HFNC as compared to NC in EUS procedures under intravenous sedation. However, no difference of statistical significance could be proven over conventional nasal oxygen therapy. HFNC use among the patients undergoing EUS was not found to be superior to conventional nasal cannula oxygen therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Yusoff IF, Raymond G, Sahai AV. Endoscopist administered propofol for upper-GI EUS is safe and effective:A prospective study in 500 patients. Gastrointest Endosc. 2004;60:356–60. doi: 10.1016/s0016-5107(04)01711-0. [DOI] [PubMed] [Google Scholar]
- 2.Berzin TM, Sanaka S, Barnett SR, Sundar E, Sepe PS, Jakubowski M, et al. A prospective assessment of sedation-related adverse events and patient and endoscopist satisfaction in ERCP with anesthesiologist-administered sedation. Gastrointest Endosc. 2011;73:710–7. doi: 10.1016/j.gie.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Ward JJ. High-flow oxygen administration by nasal cannula for adult and perinatal patients. Resp Care. 2013;58:98–122. doi: 10.4187/respcare.01941. [DOI] [PubMed] [Google Scholar]
- 4.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Brit J Anaesth. 2009;103:886–90. doi: 10.1093/bja/aep280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucangelo U, Vassallo FG, Marras E, Ferluga M, Beziza E, Comuzzi L, et al. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit Care Res Pract. 2012;2012:506382. doi: 10.1155/2012/506382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura M. High-flow nasal cannula oxygen therapy in adults. J Intensive Care. 2015;3:15. doi: 10.1186/s40560-015-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Brit J Anaesth. 2011;107:998–1004. doi: 10.1093/bja/aer265. [DOI] [PubMed] [Google Scholar]
- 8.Tokarczyk AJ, Katz J, Vender JS. Oxygen delivery systems, inhalation and respiratory therapy. In: Hagberg CA, editor. Hagberg and Benumof's Airway Management. 4th ed. Philadelphia: Elsevier; 2018. pp. 287–308. [Google Scholar]
- 9.Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25:1057–73. doi: 10.1177/0962280215588241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qadeer MA, Vargo JJ, Khandwala F, Lopez R, Zuccaro G. Propofol versus traditional sedative agents for gastrointestinal endoscopy:A meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1049–56. doi: 10.1016/s1542-3565(05)00742-1. [DOI] [PubMed] [Google Scholar]
- 11.Paspatis GA, Manolaraki MM, Vardas E, Theodoropoulou A, Chlouverakis G. Deep sedation for endoscopic retrograde cholangiopancreatography:Intravenous propofol alone versus intravenous propofol with oral midazolam premedication. Endoscopy. 2008;40:308–13. doi: 10.1055/s-2007-995346. [DOI] [PubMed] [Google Scholar]
- 12.Lee CK, Lee SH, Chung IK, Lee TH, Park SH, Kim EO, et al. Balanced propofol sedation for therapeutic GI endoscopic procedures:A prospective, randomized study. Gastrointest Endosc. 2011;73:206–14. doi: 10.1016/j.gie.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Fanti L, Agostoni M, Gemma M, Radaelli F, Conigliaro R, Beretta L, et al. Sedation and monitoring for gastrointestinal endoscopy:A nationwide web survey in Italy. Dig Liver Dis. 2011;43:726–30. doi: 10.1016/j.dld.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Paspatis GA, Manolaraki MM, Tribonias G, Theodoropoulou A, Vardas E, Konstantinidis K, et al. Endoscopic sedation in Greece:Results from a nationwide survey for the Hellenic Foundation of gastroenterology and nutrition. Dig Liver Dis. 2009;41:807–11. doi: 10.1016/j.dld.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Horiuchi A, Nakayama Y, Hidaka N, Ichise Y, Kajiyama M, Tanaka N. Low-dose propofol sedation for diagnostic esophagogastroduodenoscopy:Results in 10,662 adults. Am J Gastroenterol. 2009;104:1650–5. doi: 10.1038/ajg.2009.250. [DOI] [PubMed] [Google Scholar]
- 16.Levitzky BE, Lopez R, Dumot JA, Vargo JJ. Moderate sedation for elective upper endoscopy with balanced propofol versus fentanyl and midazolam alone:A randomized clinical trial. Endoscopy. 2012;44:13–20. doi: 10.1055/s-0031-1291421. [DOI] [PubMed] [Google Scholar]
- 17.Locke RG, Wolfson MR, Shaffer TH, Rubenstein SD, Greenspan JS. Inadvertent administration of positive end-distending pressure during nasal cannula flow. Pediatrics. 1993;91:135–8. [PubMed] [Google Scholar]
- 18.Courtney SE, Pyon KH, Saslow JG, Arnold GK, Pandit PB, Habib RH. Lung recruitment and breathing pattern during variable versus continuous flow nasal continuous positive airway pressure in premature infants:An evaluation of three devices. Pediatrics. 2001;107:304–8. doi: 10.1542/peds.107.2.304. [DOI] [PubMed] [Google Scholar]
- 19.Dutta S. High-flow nasal cannula versus nasal continuous positive airway pressure in the management of apnea of prematurity. Pediatrics. 2002;109:718–9. doi: 10.1542/peds.109.4.718. [DOI] [PubMed] [Google Scholar]
- 20.Woodhead DD, Lambert DK, Clark JM, Christensen RD. Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation:A prospective, randomized, masked, crossover trial. J Perinatol. 2006;26:481–5. doi: 10.1038/sj.jp.7211543. [DOI] [PubMed] [Google Scholar]
- 21.Hagiwara A, Matsuura N, Ichinohe T. Comparison of changes in respiratory dynamics immediately after the start of propofol sedation with or without midazolam. J Oral Maxillofac Surg. 2018;76:52–9. doi: 10.1016/j.joms.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 22.Sago T, Harano N, Chogyoji Y, Nunomaki M, Shiiba S, Watanabe S. A nasal high flow system prevents hypoxia in dental patients under intravenous sedation. J Oral Maxillofac Surg. 2015;73:1058–64. doi: 10.1016/j.joms.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Teng WN, Ting CK, Wang YT, Hou MC, Chang WK, Tsou MY, et al. High-flow nasal cannula and mandibular advancement bite block decrease hypoxic events during sedative Esophagogastroduodenoscopy:A randomized clinical trial. Biomed Res Int. 2019;2019:4206795. doi: 10.1155/2019/4206795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maggiore SM, Idone FA, Vaschetto R, Festa R, Cataldo A, Antonicelli F, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190:282–8. doi: 10.1164/rccm.201402-0364OC. [DOI] [PubMed] [Google Scholar]
- 25.Hernández G, Vaquero C, González P, Subira C, Frutos-Vivar F, Rialp G, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients:A randomized clinical trial. JAMA. 2016;315:1354–61. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 26.Schumann R, Natov NS, Rocuts-Martinez KA, Finkelman MD, Phan TV, Hegde SR, et al. High-flow nasal oxygen availability for sedation decreases the use of general anesthesia during endoscopic retrograde cholangiopancreatography and endoscopic ultrasound. World J Gastroenterol. 2016;22:10398–405. doi: 10.3748/wjg.v22.i47.10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sztrymf B, Messika J, Mayot T, Lenglet H, Dreyfuss D, Ricard JD. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure:A prospective observational study. J Crit Care. 2012;27:324–9. doi: 10.1016/j.jcrc.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 28.Agostoni M, Fanti L, Gemma M, Pasculli N, Beretta L, Testoni PA. Adverse events during monitored anesthesia care 107 for GI endoscopy:An 8-year experience. Gastrointest Endosc. 2011;74:266–75. doi: 10.1016/j.gie.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Guidance for the use of propofol sedation for adult patients undergoing Endoscopic Retrograde Cholangiopancreatography (ERCP) and other complex upper GI endoscopic procedures. Joint Royal College of Anaesthetists (RCoA) and British Society of Gastroenterology (BSG) Working Party report, April 2011. https://www.bsg.org.uk/Guidance-for-the-use-of-propofol-sedation-for-adult-patients-undergoing-Endoscopic-Retrograde-Cholangiopancreatography-ERCP-and-other-complex-upper-.html.
- 30.Bell GD. Preparation, premedication, and surveillance. Endoscopy. 2004;36:23–31. doi: 10.1055/s-2004-814117. [DOI] [PubMed] [Google Scholar]
- 31.Rabbat A, Blanc K, Lefebvre A, Lorut C. Nasal high flow oxygen therapy after extubation:The road is open but don't drive too fast! J Thorac Dis. 2016;8:e1620–4. doi: 10.21037/jtd.2016.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell DM, Shah PS, Shah V, Kelly EN. Nasal continuous positive airway pressure from high flow cannula versus infant flow for preterm infants. J Perinatol. 2006;26:546–9. doi: 10.1038/sj.jp.7211561. [DOI] [PubMed] [Google Scholar]


