Abstract
Background and Aims:
There is a lack of basic science data on the effect of dexmedetomidine on the hypoxic chemosensory reflex with both depression and stimulation suggested. The primary aim of this study was to assess if dexmedetomidine inhibited the cellular response to hypoxia in rat carotid body glomus cells, the cells of the organs mediating acute hypoxic ventilatory response (AHVR). Additionally, we used a small sample of mice to assess if there was any large influence of subsedative doses of dexmedetomidine on AHVR.
Material and Methods:
In the primary study, glomus cells isolated from neonatal rats were used to study the effect of 0.1 nM (n = 9) and 1 nM (n = 13) dexmedetomidine on hypoxia-elicited intracellular calcium [Ca2%]i influx using ratiometric fluorimetry. Secondarily, whole animal unrestrained plethysmography was used to study AHVR in a total of 8 age-matched C57BL6 mice, divided on successive days into two groups of four mice randomly assigned to receive sub-sedative doses of 5, 50, or 500 μg.kg-1 dexmedetomidine versus control in a crossover study design (total n = 12 exposures to drug with n = 12 controls).
Results:
There was no effect of dexmedetomidine on the hypoxia-elicited increase in [Ca2%]i in glomus cells (a mean ± SEM increase of 95 ± 32 nM from baseline with control hypoxia, 124 ± 41 nM with 0.1 nM dexmedetomidine; P = 0.514). In intact mice, dexmedetomidine had no effect on baseline ventilation during air-breathing (4.01 ± 0.3 ml.g-1.min-1 in control and 2.99 ± 0.5 ml.g-1.min-1 with 500 μg.kg-1 dexmedetomidine, the highest dose; P = 0.081) or on AHVR (136 ± 19% increase from baseline in control, 152 ± 46% with 500 μg.kg-1 dexmedetomidine, the highest dose; P = 0.536).
Conclusion:
Dexmedetomidine had no effect on the cellular responses to hypoxia. We conclude that it unlikely acts via inhibition of oxygen sensing at the glomus cell. The respiratory chemoreflex effects of this drug remain an open question. In our small sample of intact mice, hypoxic chemoreflex responses and basal breathing were preserved.
Keywords: Carotid body, chemoreflexes, hypoxia, sedation, volatile
Introduction
Dexmedetomidine is a non-selective alpha2-adrenoceptor (α2) agonist,[1] and a full agonist for the α2b receptor subtype,[2] and has been in clinical use worldwide since ~2010. It is an analgesic hypnotic and shows volatile and intravenous anesthetic-sparing effects.
Many sedative (midazolam[3,4]), analgesic (opiate[5]) or anesthetic (volatile and intravenous) drugs[6,7,8] have the common effect of depressing the protective chemoreflex increase in ventilation in the face of hypoxemia (or hypercapnia; perhaps this latter being somewhat better preserved than the former[9]). In this context, there has been considerable debate over the effects of dexmedetomidine on respiratory control with some reports finding respiratory chemoreflex depression in common with other anesthetics,[10,11] while others suggesting no depressive effect, and even chemostimulation of hypoxic responses.[12,13]
In intact rabbits and rats respectively, Nishida, et al.[10] and Fernandes, et al.[11] reported that dexmedetomidine was depressive to chemoreflex control of breathing. One limitation of the Nishida et al.[10] study was that the animals continued to inhale up to 0.3% sevoflurane whilst dexmedetomidine was infused, which at these concentrations itself can modestly reduce the ventilatory response for hypoxia[14] (but not for CO2[9]).
In humans observations to date on respiratory effects of dexmedetomidine appear to be largely clinical observations confined to its use for sedation[15] or to facilitate ventilation in critical care,[16] and there appear to be no rigorous studies of its effects on the physiology of chemoreflex control. One early report suggested that dexmedetomidine depressed ventilatory response to CO2, but hypoxic responses were not examined.[17] In an infusion and bolus regimen sufficient to cause significant sedation in human volunteers, a preliminary report in abstract form suggests that dexmedetomidine impaired both hypoxic and hypercapnic ventilatory responses, equivalent to the effects of propofol.[18]
In contrast to these findings of chemoreflex depression, Nguyen, et al.[12] reported in dogs that high dose (up to 100 μg.kg-1) dexmedetomidine actually stimulated ventilation and chemoreflex responses. More recently, Nakatani, et al.[13] used a reductive approach to the problem, by studying more directly the influence of the drug on carotid sinus nerve activity from excised carotid bodies (rabbits) and reported an increase in response to hypoxia when perfused with high dose (1 nM at cell level) dexmedetomidine.
Hypoxia causes membrane depolarization through closure of leak potassium (TASK; TWIK-related acid-sensitive potassium) channels and results in an increased intracellular calcium ([Ca2%]i) and subsequent neurotransmitter release to activate the carotid sinus nerve and initiate autocrine/paracrine signaling pathways.[19,20] Thus the findings of Nakatani, et al.[13] and Nguyen, et al.[12] might imply that the glomus cells (the hypoxia sensing element of the carotid body) may be stimulated by dexmedetomidine. Yet, little is currently known about how dexmedetomidine might act on the glomus cell. The answer to this question is of key mechanistic importance, as the excitability of these cells (and the function of their background TASK-like channels) has been shown to be depressed by a variety of inhalational anesthetics[21,22,23] and similarly depressed by intravenous agents[24,25,26]: consistent with the known effects of all these drugs at whole body level. Verifying Nakatini et al.’s[13] results of stimulation of the response would make dexmedetomidine quite a unique drug in this context.
There is plausible reason to suppose that dexmedetomidine could have a direct glomus cell effect: α2-adrenoceptors have been demonstrated by radio-binding assays on rabbit glomus cells.[27]
We wished to determine whether dexmedetomidine augments the hypoxia-induced Ca2% influx into carotid body glomus cells (as predicted by the results of Nguyen, et al.[12] and Nakatani, et al.[13]). A contrary result of depression would be more consistent with the findings of Nishida, et al.[10] and Fernandes, et al.[11] Thus our null hypothesis (statistically) was that dexmedetomidine would have no effect on hypoxia-induced cellular Ca2% influx in isolated glomus cells.
In an additional set of experiments, we also tested the effects of dexmedetomidine on the chemoreflex responses of a small sample of intact mice (unrestrained plethysmography), simply to assess if our results in isolated glomus cells (i.e., null effect or depression or stimulation of response) were consistent across the hierarchies of cellular organization from cell to organism, and across species.
Material and Methods
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act, 1986. Our reporting conforms to ARRIVE guidelines. The work had the approval of the local animal ethics committee and was performed under a project license to KJB (all authors also possess personal animal licenses for rodent work).
Primary study: isolated glomus cell responses to hypoxia
Neonatal Sprague-Dawley rats (P11-14) were used for the isolated glomus cell study, supplied by Harlan (Blackthorn, Oxfordshire, UK).
The methods used have been extensively previously described.[23,28] Briefly, carotid bifurcations were dissected in situ from rat pups anesthetized with 2-4% isoflurane and placed in ice-cold Dulbecco’s phosphate-buffered saline. Microdissection of the carotid body was carried out ex vivo, and after enzymatic treatment, the tissue was triturated to isolate individual cells in suspension. Primary cell cultures were plated onto coverslips and incubated for 2 h prior to loading with the cell permeant acetoxymethyl ester variant of indo-1 dye for exactly 60 min. After microscopic identification of a glomus cell, [Ca2%]i was estimated in these cells using ratiometric fluorimetry.
Each cell was sequentially superfused with 37°C Tyrode’s solution equilibrated with 5% CO2 in balanced air (euoxia) for 5–10 min and 5% CO2 with 1% O2 in balanced N2 (mild hypoxia) or 5% CO2 in balanced N2 (severe hypoxia) for periods of 1–3 min each, returning to euoxia each time for between 5 and 10 min. This exposure sequence protocol was repeated in both control and drug so that each cell acted as its own control. Dexmedetomidine (Tocris Bioscience, Bristol, UK) was added to the superfusate in the concentrations 0.1 nM and 1 nM (the same concentration range as used by Nakatini et al.[13]), randomly assigned by coin flip.
Each cell under investigation was exposed to hypoxia only once (with euoxic periods either side of the hypoxic stimulus) and on any given day, only one severity of hypoxic stimulus was used on a batch of cells.
These levels of gas input yield average O2 tensions of 10 mmHg and 3 mm Hg in the cell perfusion dish for mild and severe hypoxia, respectively, as measured using O2 optodes (Presens, Regensburg, Bavaria, Germany).[29] These stimuli elicit robust activation of glomus cells and probably mimic tissue conditions during whole-body exposures to hypoxia of ~ 50 mmHg end-tidal.[30]
The end-point was the change in intracellular calcium concentration ([Ca2%]i, nM) in response to hypoxia, calculated as the average value over the period of hypoxia, subtracted from the mean of [Ca2%]i values in the 30 sec euoxic period before the switch into hypoxia, and compared for each cell in the presence and absence of dexmedetomidine. Values for individuals cells were averaged to yield mean ± SD for statistical analysis (see below). Values in results and figures are presented as mean ± SEM, for clarity (SDs can be readily derived from each SEM using the square root of the n quoted).
Secondary study: Breathing responses of intact mice
After the primary study, we wished to assess if our results were also observed in intact animals. Our purpose here was not to provide a complete analysis of modest effects of dexmedetomidine on breathing responses using a large sample size, but rather to assess if dexmedetomidine had as large an effect on hypoxic chemoreflex response as did other anesthetics. We used a small sample of 8 C57BL6 inbred mice (7-8 weeks old; Harlan). All animals compared were kept in identical housing conditions.
Whole body plethysmography was performed by placing mice in plexigas chambers (Buxco Electronics, Wilmington, North Carolina, USA), which were continually flushed with gas mixtures at a flow rate of 2 l.min-1. Pressure changes were measured with a differential pressure transducer, the signal amplified, filtered and converted from analogue-to-digital through a preamplifier. Signal processing was performed by Finepointe software (Buxco Electronics, Wilmington, North Carolina, USA). Oxygen and carbon dioxide concentration within the chamber were measured by continuous sampling from a side-port (Datex, Helsinki, Finland).
Animals were acclimatized to the chambers for a period of 30 minutes prior to experimentation. All experiments were conducted at room temperature, between 0800 and 1400, on animals housed in a dual light cycle aligned with ambient. Hypoxic stimulus was performed with two 5-min step changes from air into 10% O2, 3% CO2, 87% N2 (“isocapnic hypoxia”; see Supplementary material (1.4MB, tif) ) with periods of euoxia in between.
The first 30 sec of data after the switch was excluded to allow for the gas concentration in the chamber to equilibrate and the ‘peak’ minute ventilation calculated as the mean ventilation achieved within the subsequent 90 sec after the switch into hypoxia (i.e., average of data 30-120 sec after entry into hypoxia). The AHVR was the difference between this peak value and the mean resting minute ventilation over the 90-s period of euoxic breathing before the gas switch. The increase in minute ventilation was indexed to (i.e., divided by) the resting minute ventilation to give AHVR as a percentage increase from baseline, to account for variations in absolute levels of resting breathing between mice.
Sustained hypoxia can result in a secondary decline in ventilation towards baseline, after the initial peak. This “hypoxic ventilatory decline” (HVD) has been extensively studied and if it arose in mouse ventilation we planned to estimate its magnitude as previously described in human studies.[4] First, the difference between the ‘peak’ ventilation (described above) and the ventilation in the last 90 sec of hypoxic exposure was calculated. Second, this value was expressed as a proportion (%) of the AHVR such that a high % indicates significant HVD.
A total of 8 age-and weight-matched male mice were used and on successive days were randomized in two groups of 4 to receive either dexmedetomidine subcutaneously (5 μg.kg-1, 50 μg.kg-1 or 500 μg.kg-1) or vehicle (equivalent volume NaCl 0.9%) in age-matched control animals. Thus in this crossover design, each mouse was studied three times, being randomly subjected to either drug or control on any given day. We wished to select a range of doses, where the highest was sub-sedative, but which had proven analgesic or behavioral properties. Savola and Virtanen have confirmed that a dose of ~500 μg.kg-1 reduces spontaneous motor activity and is analgesic in mice, but that there is no overt sedation.[31] Sallinen et al.[32] confirmed a reduction in motor activity including fine grooming movements, but no sedation, at dexmedetodine doses of 30 μg.kg-1. Doses of up to ~500 μg.kg-1 have been used as antinociceptive, but not sedative, in many other experiments in mice.[33]
The reason for our approach was that most if not all drugs known to induce sedation also depresses the hypoxic chemoreflex by a non-specific effect of sedation. It is by studying concentrations just below those needed to induce sedation that any specific effects of drug can be discerned with confidence.
Before commencing the respiratory studies, we first estimated the inspired CO2 that would maintain a near-isocapnic state during hypoxic exposure. A separate cohort of four wildtype mice were exposed to 5 min of air or 10% O2, 3% CO2 with 87% N2. At the end of this exposure, the animals were killed and immediately exsanguinated into a heparinized tube. Blood gas analysis (GEM 3000, Instrumentation Laboratory, Warrington, UK) was carried out on the samples immediately (the time between killing and analysis was ~5 sec). There was no significant difference in the exsanguinated blood gas analysis of pH comparing the isocapnic hypoxic stimulus (pCO2 5.00 ± 0.09 kPa; pH 7.38 ± 0.01) and air (CO2 5.73 ± 0.19 kPa; pH 7.35 ± 0.02), confirming that 3% inspired CO2 approximated isocapnia (P = 0.916 and P = 0.139 for pCO2 and pH. respectively). Furthermore, a separate cohort of 8 mice were studied for their breathing responses to hypoxia (10% and 12% O2) against four different background inspired CO2 concentrations: no added CO2 (poikilocapnia), 1%, 3% and 4% CO2. In human studies,[34,35] isocapnia appears characterized by a concentration of inspired CO2 which (a) elicits a measurable AHVR, such that (b) there is a clear graded response to hypoxia, with AHVR in 10% O2 greater than that in 12% O2, and (c) does not change the baseline ventilation post-hypoxia compared with pre-hypoxia baseline. Data in Supplementary Material (1.4MB, tif) shows that only 3% CO2 fulfilled all these criteria in mice.
Statistical analysis
Factorial analysis of variance was used to assess the influence of different doses of dexmedetomidine on the endpoints for both isolated carotid body and whole animal studies (IBM-SPSS version 20.0, IBM-SPSS Science Inc., Chicago, Illinois, USA). For the former, the end-point was [Ca2%]i and there were the following factors: hypoxia (two levels, one for each stimulus level, mild or severe) and dexmedetomidine (two levels, one for each dose). For the latter, the end-point was minute ventilation and there was one factor, dexmedetomidine (three levels, one for each dose). Where significance was suggested (P < 0.05 was taken as level of statistical significance) post-hoc Bonferroni tests were applied (where this P value was adjusted by dividing by the number of multiple comparisons).
Results
Primary study: Isolated glomus cell responses to hypoxia
Figure 1 shows a typical glomus cell [Ca2%]i recording of hypoxia, with and without dexmedetomidine. The drug did not appear greatly to influence the magnitude of [Ca2%]i response in either direction (stimulation or depression). With milder levels of hypoxia [Figure 1a], we noticed a more ‘spiky’ [Ca2%]i response, whereas severe hypoxia generally yielded a more stable [Ca2%]i response pattern [Figure 1b], consistent with previous reports.[36,37] Our method of calculating glomus cells hypoxic response by average [Ca2%]i values over the hypoxic period thus took into account this range of cell response types.
Figure 1.
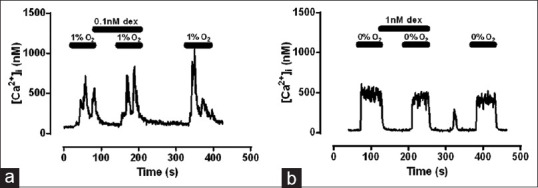
Example traces of intracellular [Ca2%]i glomus cell responses to ~60 sec exposures to hypoxia; (a) mild hypoxia; (b) severe hypoxia. There is no apparent influence of dexmedetomidine in either hypoxic exposure, and this is confirmed by quantitative analysis (see text)
Quantitative analysis confirmed the appearances. Hypoxia increased [Ca2%]i as expected in the absence of dexmedetomidine; severe hypoxia elicited a greater increase in [Ca2%]i than mild hypoxia (189 ± 41 nM, n = 21, versus 95 ± 41 nM, n = 9; P = 0.009). However, the magnitude of increase in [Ca2%]i evoked by mild and severe hypoxia was not influenced by dexmedetomidine (ANOVA, P = 0.512) and there was no interaction between level of hypoxia and drug dose (P = 0.544).
Secondary study: Breathing responses of intact mice
Figure 2 shows a typical plethysmographic output of mice breathing quietly in euoxia, and the effect of hypoxia, with and without dexmedetomidine. Minute ventilation rose abruptly, as expected, with hypoxia and even during this short exposure, some ‘hypoxic ventilatory decline’ (HVD) was evident with sustained hypoxia (a phenomenon recognized in rodents[38,39] and modelled elsewhere[40]). This HVD gave the appearance of a depressive influence of dexmedetomidine on ventilation (i.e., the bottom row in Figure 2 gives appearance of lower ventilation values with drug than control; analyzed further below). There was appropriate return of ventilation to pre-hypoxic levels on return to euoxia. Also evident is the variability in control (no dexmedetomidine) responses between the groups of mice for each experiment. Overall, any effect of dexmedetomidine seemed at best modest. Only at the highest dose was there a suggestion of a reduced response during hypoxia, but this must be offset against what appeared to be a small reduction in baseline ventilation.
Figure 2.
Influence of dexmedetomidine on hypoxic responses in mice. Each row shows the results for one drug concentration: top to both, from low dose (5 μg.kg-1), middle dose (50 μg.kg-1) and high dose (500 μg.kg-1) dexmedetomidine. The first column is the control data for that dose (dark lines), the middle column the with-drug data (red lines). In these first two columns, each trace shows the data for a single mouse (individual data points omitted for clarity). The last column panels show the averages for the drug dose control values (black lines) and with-drug (red lines; points are 30-sec averages, mean ± SEM)
The behavior of the mice at all doses of dexmedetomidine was superficially normal (we did not test subtle motor movements or pain responses). Immediately after experimentation, they fed normally. It was therefore concluded that even at the highest doses, dexmedetomidine was sub-sedative, as intended.
Figure 3 shows the quantitative analysis and averaged results for all mice across the three protocols for both pre-hypoxic resting ventilation and AHVR. There was no significant interaction between day and AVHR (P = 0.855). Analysis of variance confirmed that dexmedetomidine had no significant effect on resting, air-breathing minute ventilation at any dose compared with control, (P = 0.291). Although in Figure 3 (bottom panel) it might appear that low dose dexmedetomidine depresses AHVR while higher doses augment it, there were no statistically significant dose dependent effects on normalized (P = 0.559) or absolute hypoxic ventilatory response (P = 0.997). While there was indeed an increase in mean magnitude of HVD with increasing dose of dexmedetomidine [Table 1], this did not reach statistical significance (P = 0.127), and the effect on the mean value may be due to one animal with an especially pronounced reduction in AHVR in the 500 μg.kg-1 group [see Figure 2].
Figure 3.

Quantitative analysis of the plethysmography data. (a) pre-hypoxic resting, air-breathing minute ventilation (values from first column, Fig. 2; absolute level in l.min-1 ± SEM). (b) AHVR (shown as % increase from resting ventilation ± SEM)
Table 1.
Values of HVD (Mean±SEM) expressed as % of AHVR. See text for further detail. Effect of drug concentration was NS (P=0.127, ANOVA)
| Condition | HVD (%) | SEM | n |
|---|---|---|---|
| No drug (control) | 22.4 | 6.5 | 12 |
| Dexmed 5 µg.kg-1 | 13.2 | 6.6 | 4 |
| Dexmed 50 µg.kg-1 | 33.7 | 16.9 | 4 |
| Dexmed 500 µg.kg-1 | 50.7 | 25.4 | 4 |
Discussion
The main result of this study is that dexmedetomidine has no effect (i.e., neither stimulation nor depression) on the glomus cell response to mild or severe hypoxia. If dexmedetomidine does have any influence on chemoreflex control (whether inhibitory[10,11] or stimulatory[12,13]) then our results confirm that this effect is not via a direct action on the glomus cell itself. If drug effects do exist, then alternative mechanisms for these could be at the level of the glomus cell-afferent nerve synapse (e.g. an effect of drug on neurotransmitter secretion), or due to central drug effects. Thus, our result could be consistent with those of Nakatini et al.,[13] who reported response stimulation by dexmedetomidine in an isolated carotid body preparation, an experimental setup which retains synaptic transmission.
The result of our additional set of observations in intact mice is consistent with our main results in the carotid body. Thus, while we did not find that dexmedetomidine is a depressive drug to the ventilatory chemoreflex (as was suggested by Nishida[10] and Fernandes[11]), we also did not find that it actually stimulates chemoreflex function (as was suggested by Nguyen[12] and Nakatini[13]). However, this ‘neutral’ effect, if reflected in future human studies, nevertheless implies a potentially beneficial therapeutic profile for clinical practice, since during sedation or anesthesia it is highly desirable to maintain chemoreflex function as a protective response.
That said, we cannot exclude the possibility of a central effect of dexmedetomidine, even in our intact animal study. We noted that HVD persists with increasing doses of dexmedetomidine [Table 1]. Whereas in humans, HVD may originate in the peripheral chemoreceptor[41] in animals there is compelling evidence that it may be of central origin.[42] Normally HVD is studied over hypoxic exposures of 20-30 min, whereas our methods used hypoxic exposure time of only 5 min: if dexmedetomidine has a pronounced effect in increasing HVD, our methodology would not have detected this. Therefore, future human studies should explore the effect of drug on both acute and sustained hypoxia.
Even in theory it is not fully established how α2-adrenoceptors (the main putative target for dexmedetomidine) could influence the hypoxic chemoreflex loop. The same study that identified α2-adrenoceptors existing on glomus cells in rabbits,[27] showed that guanabenz (like dexmedetomidine, an α2-adrenoceptor agonist), reduced carotid body hypoxic chemosensory discharge. However, this study was conducted in an intact preparation without control of local blood flow or blood pressure, and it is possible that changes in these might have influenced the result (noting that Nakatani et al.[13] reported an opposite, stimulatory effect for dexmedetomidine).
There were several limitations of our study. Our sample of intact mice in our secondary study was small. We designed our power analysis to detect only a large change in effect size (25%; similar to effect size of subanesthetic volatile agents[6,7]). Based on our recorded variance in minute ventilation (1 ml g-1 min-1; see Supplementary Material (1.4MB, tif) ), this showed that 4 animals in each group would be sufficient (α = 5%, β = 80%) to detect a 2 ml g-1 min-1 change in AHVR.[43] Thus this subset of experiments cannot be regarded as definitive (i.e., could still be a type 2 statistical error), but rather as excluding only large effects comparable to other agents at subanesthetic doses. Taken together with the work of other authors, the physiological results to date demonstrate considerable heterogeneity. At various levels of cell organization from single glomus cell to isolated carotid body to whole organisms, and across species (rabbits, rats, mice and preliminary human data), some groups report depression,[10,11,16,18] whereas others report stimulation,[12,13] and now where we report here a neutral effect. This degree of heterogeneity itself suggests that dexmedetomidine is unlikely to demonstrate the inhibition of hypoxic chemoreflex response that other anesthetic agents do.
Unusually, we conducted some (cell) studies in rats and other (plethysmography) studies in mice. We did not use carotid bodies from mice in our primary study, because glomus cells isolation is technically extremely challenging. Although single cell data from mice has been reported, this has been after the culmination of several years of work for a single paper.[44] Conversely, had our rat glomus cells in the primary experiments yielded a positive result (i.e., inhibition or stimulation), then we would have logically studied rats in the plethysmogram. Having established no drug effect at glomus cell level, we took the opportunity to assess if a different species produced a different result at whole-organism level. We note that previous workers have also used a similar mix of species to study dexmedetomidine on different aspects of function.[31]
In the plethysmography experiments, we were careful to employ real time gas analysis to ensure that hypoxic input was stable over time and equivalent across the protocols. Although we do not report on hypercapnic responses, we did employ the technique of adding 3% CO2 to the inspirate to ensure constancy of CO2 (isocapnia)[45,46] during the hypoxic exposures (as otherwise there would have been hypocapnia, which would have acted as a brake to ventilatory response, and therefore a confounder).
In relation to humans and patients, the study of dexmedetomidine on ventilatory chemoreflexes in humans remains sparse and we can find no full report of investigation on its effects on AHVR, and this would seem a gap that seems important to fill. The full report of Danielson, et al.[18] is therefore to be welcomed.
In summary, we can confidently exclude that dexmedetomidine does not have any direct action on glomus cell response to hypoxia. If it has any chemoreflex influence at all (depressive or stimulatory) it is possible that this is exerted at the level of the glomus cell-afferent nerve synapse, or more centrally in the nervous system. However, taken together with the heterogenous results of others, our results in mice suggest that subsedative doses are not depressive to the same extent as are other anesthetic agents.
Financial support and sponsorship
This work was funded by grants from the Medical Research Council, British Journal of Anaesthesia, National Institute of Academic Anaesthesia, Difficult Airway Society (UK) and a personal Higher Education Funding Council (England) New Blood Clinical Senior Lectureship award to JJP.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Defining an optimal isocapnic stimulus (see text for further detail). This array represents the change in minute ventilation to a variety of inspired gas mixtures (period of hypoxia black bar). Increasing background CO2 concentration top to bottom. This highlights a number of important observations: (1) The response to hypoxia in poikilopcapnia is small or absent; (2) the magnitude of response with 1% CO2 across the range of O2 concentrations is relatively small and produces little by way of a graded response between 10% and 12% O2. This suggests these two CO2 stimuli unlikely maintain isocapnia (i.e, a hypoxia-evoked hypocapnia likely blunting any ventilatory response of magnitude). Then: (3) with 4% CO2, post-hypoxic ventilation does not return to pre-hypoxic baseline values on return to euoxia and HVD appears absent, which suggests this stimulus is too high (ie, an ongoing acid/CO2 chemostimulus) and represents hypercapnia rather than isocapnia. It is only with 3% CO2 stimulus that a characteristic response to hypoxia is seen (with HVD), reminiscent of the human response to isocapnic hypoxia when using dynamic end-tidal forcing. All data points are mean ± (n = 8 per stimulus).
References
- 1.Paris A, Tonner PH. Dexmedetomidine in anaesthesia. Curr Opinion Anaesthesiol. 2005;18:412–8. doi: 10.1097/01.aco.0000174958.05383.d5. [DOI] [PubMed] [Google Scholar]
- 2.Peltonen JM, Pihlavisto M, Scheinin M. Subtype-specific stimulation of [35S] GTPgammaS binding by recombinant alpha2-adrenoceptors. Eur J Pharmacol. 1998;355:275–9. doi: 10.1016/s0014-2999(98)00518-4. [DOI] [PubMed] [Google Scholar]
- 3.Dahan A, Ward DS. Effect of i.v. midazolam on the ventilatory response to sustained hypoxia in man. Br J Anaesth. 1991;66:454–7. doi: 10.1093/bja/66.4.454. [DOI] [PubMed] [Google Scholar]
- 4.Nagyova B, Dorrington KL, Robbins PA. Effects of midazolam and flumazenil on ventilation during sustained hypoxia in humans. Respir Physiol. 1993;94:51–9. doi: 10.1016/0034-5687(93)90056-g. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright CR, Henson LC, Ward DS. Effects of alfentanil on the ventilatory response to sustained hypoxia. Anesthesiology. 1998;89:612–9. doi: 10.1097/00000542-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Knill RL, Gelb AW. Ventilatory responses to hypoxia and hypercapnia during halothane sedation and anesthesia in man. Anesthesiology. 1978;49:244–51. doi: 10.1097/00000542-197810000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Pandit JJ. The variable effect of low-dose volatile anaesthetics on the acute ventilatory response to hypoxia in humans:A quantitative review. Anaesthesia. 2002;57:632–43. doi: 10.1046/j.1365-2044.2002.02604.x. [DOI] [PubMed] [Google Scholar]
- 8.Blouin RT, Seifert HA, Babenco HD, Conard PF, Gross JB. Propofol depresses the hypoxic ventilatory response during conscious sedation and isohypercapnia. Anesthesiology. 1993;79:1177–82. doi: 10.1097/00000542-199312000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Pandit JJ. Effect of low dose inhaled anaesthetic agents on the ventilatory response to carbon dioxide in humans:A quantitative review. Anaesthesia. 2005;60:461–9. doi: 10.1111/j.1365-2044.2004.04088.x. [DOI] [PubMed] [Google Scholar]
- 10.Nishida T, Nishimura M, Kagawa K, Hayashi Y, Mashimo T. The effects of dexmedetomidine on the ventilatory response to hypercapnia in rabbits. Int Care Med. 2002;28:969–75. doi: 10.1007/s00134-002-1338-y. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes FC, Ferreira HC, Cagido VR, Carvalho GM, Pereira LS, Faffe DS, et al. Effects of dexmedetomidine on respiratory mechanics and control of breathing in normal rats. Resp Physiol Neurobiol. 2006;154:342–50. doi: 10.1016/j.resp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen D, Abdul-Rasool I, Ward D, Hsieh J, Kobayashi D, Hadlock S, et al. Ventilatory effects of dexmedetomidine, atipamezole, and isoflurane in dogs. Anesthesiology. 1992;76:573–9. doi: 10.1097/00000542-199204000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Nakatani H, Kim C, Sakamoto A. Low-dose dexmedetomidine facilitates the carotid body response to low oxygen tension in vitro via alpha2-adrenergic receptor activation in rabbits. Eur J Anaesthesiol. 2012;29:570–6. doi: 10.1097/EJA.0b013e328356fba5. [DOI] [PubMed] [Google Scholar]
- 14.Nishizawa T, Suzuki H, Sagara S, Kanai T, Yahagi N. Dexmedetomidine versus midazolam for gastrointestinal endoscopy:A meta-analysis. Dig Endosc. 2015;27:8–15. doi: 10.1111/den.12399. [DOI] [PubMed] [Google Scholar]
- 15.Huang Z, Chen YS, Yang ZL, Liu JY. Dexmedetomidine versus midazolam for the sedation of patients with non-invasive ventilation failure. Intern Med. 2012;51:2299–305. doi: 10.2169/internalmedicine.51.7810. [DOI] [PubMed] [Google Scholar]
- 16.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans: I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–33. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Sarton E, Dahan A, Teppema L, van den Elsen M, Olofsen E, Berkenbosch A, et al. Acute pain and central nervous system arousal do not restore impaired hypoxic ventilatory response during sevoflurane sedation. Anesthesiology. 1996;85:295–303. doi: 10.1097/00000542-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Danielson A, Ebberyd A, Cedborg A, Mkrtchian S, Christensson E, Ullman J, et al. Sedation with dexmedetomidine or propofol impairs control of breathing in healthy male volunteers. A randomized cross-over study, Abstract: Anesthesiology 2014 Conference. New Orleans. 2014 [Google Scholar]
- 19.Kumar P, Prabhakar NR. Peripheral chemoreceptors:Function and plasticity of the carotid body. Comp Physiol. 2011;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurse CA. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J Physiol. 2014;592:3419–26. doi: 10.1113/jphysiol.2013.269829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandit JJ, Buckler KJ. Differential effects of halothane and sevoflurane on hypoxia-induced intracellular calcium transients of neonatal rat carotid body type I cells. Br J Anaesth. 2009;103:701–10. doi: 10.1093/bja/aep223. [DOI] [PubMed] [Google Scholar]
- 22.Pandit JJ, Winter V, Bayliss R, Buckler KJ. Differential effects of halothane and isoflurane on carotid body glomus cell intracellular Ca2+and background K+channel responses to hypoxia. Adv Exp Med Biol. 2010;669:205–8. doi: 10.1007/978-1-4419-5692-7_41. [DOI] [PubMed] [Google Scholar]
- 23.Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–42. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponte J, Sadler CL. Effect of thiopentone, etomidate and propofol on carotid body chemoreceptor activity in the rabbit and the cat. Br J Anaesth. 1989;62:41–5. doi: 10.1093/bja/62.1.41. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson MM, Lindahl SG, Eriksson LI. Effect of propofol on carotid body chemosensitivity and cholinergic chemotransduction. Anesthesiology. 2005;102:110–6. doi: 10.1097/00000542-200501000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Akada S, Fagerlund MJ, Lindahl SG, Sakamoto A, Prabhakar NR, Eriksson LI. Pronounced depression by propofol on carotid body response to CO2 and K+-induced carotid body activation. Resp Physiol Neurobiol. 2008;160:284–8. doi: 10.1016/j.resp.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Kou YR, Ernsberger P, Cragg PA, Cherniack NS, Prabhakar NR. Role of α2-adrenergic receptors in the carotid body response to isocapnic hypoxia. Respir Physiol. 1991;83:353–64. doi: 10.1016/0034-5687(91)90054-m. [DOI] [PubMed] [Google Scholar]
- 28.Buckler KJ, Vaughan-Jones RD. Effects of acidic stimuli on intracellular calcium in isolated type I cells of the neonatal rat carotid body. Pflügers Archiv. 1993;425:22–7. doi: 10.1007/BF00374499. [DOI] [PubMed] [Google Scholar]
- 29.Huskens N, O'Donohoe P, Wickens JR, McCullagh JSO, Buckler KJ, Pandit JJ. A method for continuous and stable perfusion of tissue and single cell preparations with accurate concentrations of volatile anaesthetics. J Neurosci Methods. 2016;258:87–93. doi: 10.1016/j.jneumeth.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Acker H, Lubbers DW, Purves MJ. Local oxygen tension field in the glomus caroticum of the cat and its change at changing arterial pO2. Pflügers Archiv. 1971;329:136–55. doi: 10.1007/BF00586988. [DOI] [PubMed] [Google Scholar]
- 31.Savola JM, Virtanen R. Central alpha 2-adrenoceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medetomidine. Eur J Pharmacol. 1991;195:193–9. doi: 10.1016/0014-2999(91)90535-x. [DOI] [PubMed] [Google Scholar]
- 32.Sallinen J, Link RE, Haapalinna A, Viitamaa T, Kulatunga M, Sjöholm B, et al. Genetic alteration of alpha 2C-adrenoceptor expression in mice:Influence on locomotor, hypothermic, and neurochemical effects of dexmedetomidine, a subtype-nonselective alpha 2-adrenoceptor agonist. Mol Pharmacol. 1997;51:36–46. doi: 10.1124/mol.51.1.36. [DOI] [PubMed] [Google Scholar]
- 33.Jang Y, Yeom MY, Kang ES, Kang JW, Song HK. The antinociceptive effect of dexmedetomidine modulates spleen cell immunity in mice. Int J Med Sci. 2014;11:226–33. doi: 10.7150/ijms.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang PJ, Bascom DA, Robbins PA. Extended models of the ventilatory response to sustained isocapnic hypoxia in humans. J Appl Physiol. 1997;82:667–77. doi: 10.1152/jappl.1997.82.2.667. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Robbins PA. Methodological and physiological variability within the ventilatory response to hypoxia in humans. J Appl Physiol. 2000;88:1924–32. doi: 10.1152/jappl.2000.88.5.1924. [DOI] [PubMed] [Google Scholar]
- 36.Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994;476:423–8. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dasso LLT, Buckler KJ, Vaughan-Jones RD. Interactions between hypoxia and hypercapnic acidosis on calcium signaling in carotid body type I cells. Am J Physiol. 2000;279:L36–42. doi: 10.1152/ajplung.2000.279.1.L36. [DOI] [PubMed] [Google Scholar]
- 38.Bissonnette JM, Knopp SJ. Developmental changes in the hypoxic ventilatory response in C57BL/6 mice. Respir Physiol. 2001;128:179–86. doi: 10.1016/s0034-5687(01)00271-7. [DOI] [PubMed] [Google Scholar]
- 39.Maxova H, Vizek M. Biphasic ventilatory response to hypoxia in unanesthetized rats. Physiol Res. 2001;50:91–6. [PubMed] [Google Scholar]
- 40.Bascom DA, Pandit JJ, Clement ID, Robbins PA. Effects of different levels of end-tidal PO2 on ventilation during isocapnia in humans. Respir Physiol. 1992;88:299–311. doi: 10.1016/0034-5687(92)90004-g. [DOI] [PubMed] [Google Scholar]
- 41.Robbins PA. Hypoxic ventilatory decline:Site of action. J Appl Physiol. 1995;79:373–4. doi: 10.1152/jappl.1995.79.2.373. [DOI] [PubMed] [Google Scholar]
- 42.Ward DS, Berkenbosch A, DeGoede J, Olievier CN. Dynamics of the ventilatory response to central hypoxia in cats. J Appl Physiol. 1990;68:1107–13. doi: 10.1152/jappl.1990.68.3.1107. [DOI] [PubMed] [Google Scholar]
- 43.Lehr R. Sixteen S-squared over D-squared:A relation for crude sample size estimates. Stat Med. 1992;11:1099–102. doi: 10.1002/sim.4780110811. [DOI] [PubMed] [Google Scholar]
- 44.Turner PJ, Buckler KJ. Oxygen and mitochondrial inhibitors modulate both monomeric and heteromeric TASK-1 and TASK-3 channels in mouse carotid body type-1 cells. J Physiol. 2013;591:5977–98. doi: 10.1113/jphysiol.2013.262022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slingo ME, Turner PJ, Christian HC, Buckler KJ, Robbins PA. The von Hippel-Lindau Chuvash mutation in mice causes carotid body hyperplasia and enhanced ventilatory sensitivity to hypoxia. J Appl Physiol. 2014;116:885–92. doi: 10.1152/japplphysiol.00530.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishiguro T, Iwase M, Kanamaru M, Izumizaki M, Ohshima Y, Homma I. Impaired ventilation and metabolism response to hypoxia in histamine H1 receptor-knockout mice. Respir Physiol Neurobiol. 2006;154:331–41. doi: 10.1016/j.resp.2006.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



