Abstract
Humans perceive objects and scenes consistently, even in situations where visual input is noisy and unstable. One of the mechanisms that underlies this perceptual stability is serial dependence, whereby the perception of objects or features at any given moment is pulled toward what was previously seen. Although recent findings from several studies have reported large individual differences in serial dependence, it is not clear how stable the serial dependence is within an individual. Here, we investigated the stability of serial dependence in orientation perception over two different days within the same observers. In addition, we also examined the visual field location specificity of perceptual serial dependence. On each trial, observers viewed a Gabor patch and then reported its apparent orientation by adjusting the orientation of a bar. For each observer, the Gabor was located in the foveal or peripheral (10° right or left eccentricity) visual field on both days or changed location from day to day. The results showed a very high degree of test-retest reliability in serial dependence measured across days within individual observers. Interestingly, this high within-subject consistency was only found when serial dependence was measured at the same visual field location. These results suggest that individual differences in serial dependence are stable across days, and that the spatiotemporal range in which the previous stimulus assimilates the perception of the current stimulus (the continuity field) may vary across different visual field locations in an observer-specific manner.
Keywords: sequential effect, individual difference, orientation, location specificity, visual history
Introduction
Visual input is noisy and discontinuous, and this poses a challenge for the visual system. Information at the retina can be noisy and unreliable because of external sources, such as snow and rain, object motion, occlusions from blinks or from other objects in the world. There are also internal sources of noise as well, including optical and neural noise. A challenge and computational goal for the visual system is that we want to perceive objects and scenes as being continuous and having stable identities that are consistent over time. How does the visual system achieve this representational stability?
One of the recently discovered mechanisms that supports perceptual stability is called serial dependence. Serial dependence is a phenomenon in which objects or features are perceived as being more similar to those that were seen in the last several seconds. This means that the perception of anything at any given moment is pulled toward what was previously seen, as long as those successive things are relatively similar (Alais, Leung, & Van der Burg, 2017; Cicchini, Anobile, & Burr, 2014; Collins, 2019; Collins, 2020; Corbett, Fischer, & Whitney, 2011; Fischer & Whitney, 2014; Kim, Burr, Cicchini, & Alais, 2020; Liberman, Manassi, & Whitney, 2014; Manassi, Liberman, Kosovicheva, Zhang, K., & Whitney, 2018; Taubert, Alais, & Burr, 2016; Suarez-Pinilla, Seth, & Roseboom, 2018). The spatiotemporal range in which the previous stimulus assimilates the perception of the current stimulus is called the continuity field (Fischer & Whitney, 2014). In the real world, serial dependence tends to be beneficial because the world is autocorrelated: things that were present a few moments ago tend to be present at this moment as well. Insofar as the visual system is able to match the serial dependence to the autocorrelations in the physical world, the visual system could stabilize perceptual representations by taking the prior knowledge about the temporal structure of the world into account. Serial dependence is beneficial for a host of other reasons, as well (Cicchini, Mikellidou, & Burr, 2018; Fischer & Whitney, 2014; Fornaciai & Park, 2018a; Manassi, Liberman, Chaney, & Whitney, 2017).
A defining characteristic of serial dependence is its location specificity: the perception of an object at one moment is pulled toward a previous object only when the sequential objects are nearby in space. Some researchers have found that serial dependence is largely retinotopic in its spatial specificity (Collins, 2019; Corbett et al., 2011). Other groups have found that there may be some additional component or some degree of spatiotopic/world-centered serial dependence (Fischer & Whitney, 2014; Mikellidou, Cicchini, & Burr, 2021). Even in those studies that found that serial dependence is predominantly retinotopic (Collins, 2019), the effect is not literally restricted to the retinal location of previous stimuli, unlike negative aftereffects (for reviews; see Kohn, 2007; Thompson & Burr, 2009; Webster, 2012). Instead, serial dependence spreads across some (but not all of) the visual field and is not tied to one highly specific location on the retina (Collins, 2019; Fischer & Whitney, 2014; Manassi et al., 2017; Manassi, Kristjánsson, & Whitney, 2019). Several groups have now reported that serial dependence is also gated by spatial attention (Fischer & Whitney, 2014; Fornaciai & Park, 2018a; Fornaciai & Park, 2018b; Fritsche & de Lange, 2019; Rafiei, Hansmann-Roth, Whitney, Kristjánsson, & Chetverikov, 2021). When attention is directed to a spatial location, there is strong serial dependence. Ignoring the same stimulus can result in no serial dependence and even some negative aftereffect (Fischer & Whitney, 2014; Rafiei et al., 2021). Collectively, it is clear that there are significant ways in which serial dependence is spatially tuned.
Another potentially defining characteristic of serial dependence is that there may be some degree of individual differences (Abrahamyan, Silva, Dakin, Carandini, & Gardner, 2016; Braun, Urai, & Donner, 2018; Kim & Alais, 2021; Manassi, Murai, & Whitney, 2018; Zhang & Alais, 2020). For example, Manassi and colleagues found that individual observers varied considerably in how much orientation serial dependence they perceived (Manassi, Murai, et al., 2018). Another group reported that the individual differences in serial dependence result from different weightings of cues available in the task (Zhang & Alais, 2020). What remains unclear from the previous studies is how stable—from day to day—the serial dependence is. One might expect that, if serial dependence is related to an individual observer's perceptions and decisions, it should be relatively stable from day to day. Another interesting possibility is that there may be interactions between the spatial tuning of serial dependence and the individual differences in the effect. There is accumulating evidence that spatial attention (Huang, Mo, & Li, 2012; O'Regan & Serrien, 2018) and position perception (Kosovicheva & Whitney, 2017; Wang, Murai, & Whitney, 2020) also show great individual differences that are stable over time—sometimes over months (Wexler, Duyck, & Mamassian, 2015; Wexler, 2018). If the serial dependence reflects internal representations about the spatiotemporal structure of the external world, the serial dependence should vary across individuals depending on the unique nature of representations within each individual.
Our goal in this article was to test for individual differences in serial dependence. In particular, we aimed to measure the test-retest reliability of serial dependence over time within individual observers. A second, complementary goal was to analyze the location specificity of serial dependence for individual observers. In essence, the goal here was to test whether observers have consistent serial dependence in their perception of a given feature and whether that consistency remains the same from day to day in a location-specific manner.
Methods
Participants
Seventy-six healthy volunteers participated in the experiments. All participants reported to have normal or corrected-to-normal vision. The experiments were conducted under the approval by the institutional review boards of the University of California at Berkeley. All participants provided written informed consent before the experiments.
Apparatus and stimuli
All the visual stimuli were presented on a CRT monitor (Sony Trinitron Multiscan G520; Resolution 1024 × 768 pixels; Refresh rate 60 Hz). Participants viewed the Gabor stimuli from a chin rest positioned 57.3 cm from the monitor in a dark booth. The Gabors (windowed sine wave gratings) had a peak contrast of 25% Michelson, a spatial frequency of 0.75 cycles per degree, and a 0.9° s.d. Gaussian contrast envelope. All the stimuli were created by MATLAB (MathWorks, R2017a) and the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997).
Procedure
In the present study, we tested serial dependence in orientation perception. The methods in this set of experiments were extensions of those previously reported (Fischer & Whitney, 2014). In each trial, a Gabor patch was presented briefly for 500 ms, followed by a noise patch for 1000 milliseconds. After a fixation point (250 ms), a manipulable test bar appeared, with which observers were able to continuously report the orientation of the just-seen Gabor patch. The Gabor patch could be presented at the fovea, or it could be presented in the right visual field or the left visual field (Figure 1).
Figure 1.
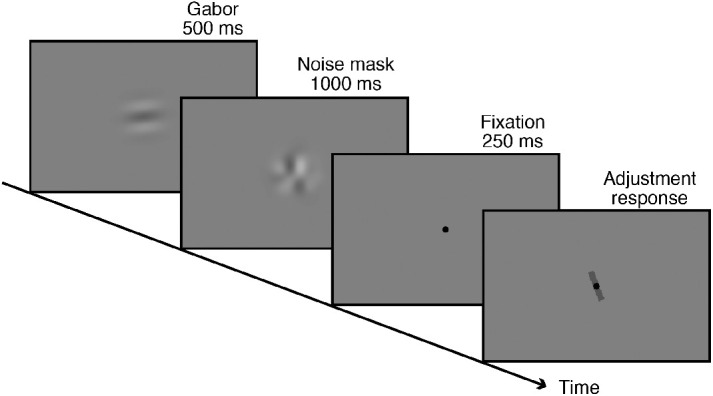
Schematic of experimental procedure. In each trial, a Gabor patch was presented for 500 ms, followed by a noise mask. After a fixation point of 250 ms, participants were asked to adjust the response bar to match its orientation to the Gabor they saw.
The experiment was conducted on two separate days to measure test-retest reliability. On the first day observers completed 500 trials. On a second day, separated in time by at least one day, but within eight days, each subject performed another 500 trials. The Gabor patches were presented either at the fovea or in the right peripheral or left peripheral visual field. The stimuli were always presented at the same location within the same day. The question here was whether the serial dependence remained consistent from one day to the next, and also whether the serial dependence at specific locations in the visual field transferred or were consistent across space. To test this, participants were divided into four groups, each participating in a different condition (Figure 2). The first and second groups participated in two foveal or two right peripheral conditions, respectively, to evaluate the test-retest reliability of serial dependence either at the fovea (N = 20) or in the periphery (N = 20). We further tested if the serial dependence is consistent within each individual across different locations. The third group participated in one foveal and one right peripheral condition (N=20), and the fourth group participated in one right peripheral and one left peripheral condition (N = 16). The order of experiments was counterbalanced across participants (Figure 2).
Figure 2.
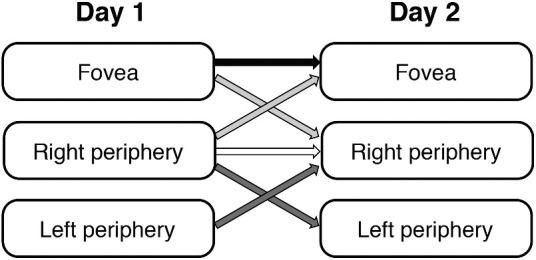
Condition assignments. Four groups of subjects participated in either (1) two foveal sessions, (2) two right peripheral sessions, (3) one foveal and one right peripheral session, or (4) one right peripheral and one left peripheral session.
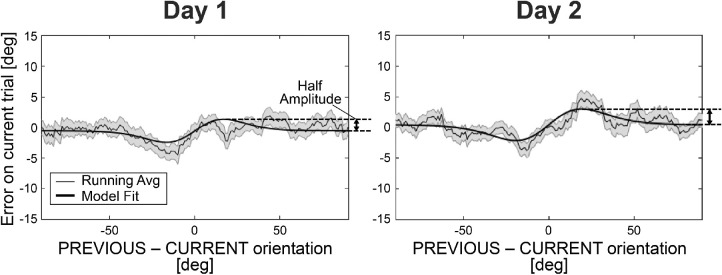
To measure the serial dependence, we used a familiar approach that many authors have used, originally introduced by Fisher and Whitney (2014). In Figure 3, the response error (y-axis; difference between response and stimulus orientation) was plotted against the difference in the orientation of the previous Gabor patch relative to the current Gabor patch (x-axis). Zero on the x axis means sequential Gabor patches that had identical orientations. Zero on the y-axis would be perfect reports of the orientation of the stimulus on each trial. In many previous studies (e.g., Fischer & Whitney, 2014; Fritsche, Mostert, & de Lange, 2017; Manassi et al., 2017; Collins, 2019; Fritsche & de Lange, 2019; Cicchini et al., 2021), the error, plotted as a function of the difference in sequential orientations, deviates from 0. We expected something similar here, with the response errors following a characteristic derivative of von Mises (S curve-like) shape, such that errors to the left of 0 are negative and to the right of 0 are positive. A positive slope in this derivative of von Mises function would indicate positive serial dependence, as has been reported in many papers (Cicchini, Mikellidou, & Burr, 2017; Cicchini et al., 2018). To quantify the degree of serial dependence, we measured the half amplitude of the best fitting derivative of von Mises function (peak-to-trough of the derivative of von Mises divided by two).
Figure 3.
A representative participant's results. In each plot, the response error in the current trial (ordinate) was plotted against the difference between the previous and current Gabor orientations (abscissa). Zero on the abscissa indicates no response error. A derivative of von Mises function was fitted to the data, and half of the peak-to-trough amplitude was used to quantify the magnitude of serial dependence. Shaded regions represent SEM.
Results
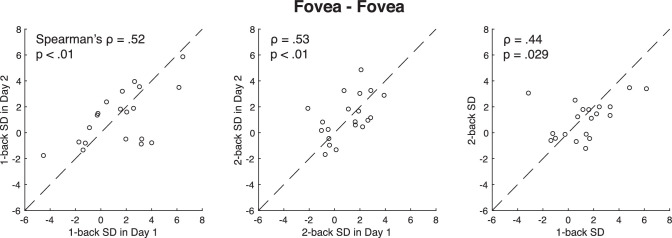
We measured the orientation serial dependence amplitude on day one and separately on day two for each subject (see Figure 3 for a representative observer). In one of the conditions, we measured serial dependence at the fovea across the two days. The results showed that the serial dependence on day one was significantly correlated with the serial dependence on day two (Spearman's ρ = 0.52, p < 0.01, BF10 = 18.15; Figure 4), indicating a high degree of test-retest reliability. Serial dependence measured on sequential days was therefore highly consistent.
Figure 4.
The test-retest reliability of the serial dependence at the fovea. Each dot represents a single participant's data. Left panel: the one-back serial dependence on day one is correlated with the one-back serial dependence on day two (Spearman's ρ = 0.52), indicating a high degree of test-retest reliability. Middle panel: the test-retest reliability of two-back serial dependence (ρ = 0.53). Two-back serial dependence is the serial dependence of the current error on the stimulus presented two trials ago. Right panel: the consistency of one-back and two-back serial dependence (ρ = 0.44). Collectively, the results indicate that there is strong test-retest reliability of serial dependence at the fovea.
It is worth noting that the average serial dependence effect collapsed across observers was positive and significant, consistent with previous reports (Alais et al., 2017; Cicchini et al., 2018; Collins, 2019; Corbett et al., 2011; Fischer & Whitney, 2014; Kim et al., 2020; Liberman et al., 2014; Manassi, Liberman, et al., 2018; Suarez-Pinilla et al., 2018). However, a minority of observers perceived negative serial dependence. This echoes previous reports (Abrahamyan et al., 2016; Braun et al., 2018; Manassi, Murai, et al., 2018; Zhang & Alais, 2020) and is an unsurprising consequence of the fact that the Gabor stimulus was presented at the fovea and had no superimposed noise; both of these factors can reduce the serial dependence effect (Cicchini et al., 2018; Kim & Alais, 2021). Despite a noise-free Gabor patch at the fovea, there was still a significant overall positive serial dependence and significant individual differences that were consistent from day to day (Figure 4).
We also measured serial dependence on the two-back stimulus. The two back serial dependence is serial dependence of the current report toward the stimulus presented two trials ago, which is about 10 seconds in the past. Previous research consistently reports finding two-back serial dependence (Fischer & Whitney, 2014; Manassi, Liberman, et al., 2018). We found significant test-retest reliability for two-back serial dependence (ρ = 0.53, p < 0.01, BF10 = 4.95; Figure 4). Interestingly, the correlation between the one-back serial dependence and the two-back serial dependence was significant (ρ = 0.44, p < 0.05, BF10 = 2.25).
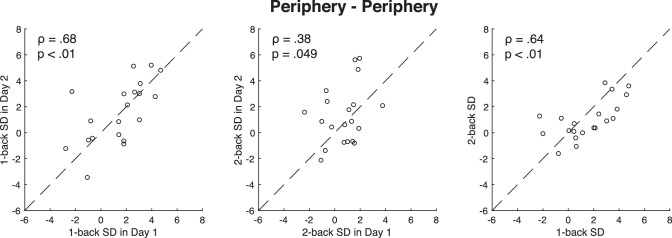
In another condition, we measured serial dependence in the right periphery on day one and then measured serial dependence again in the right periphery on day two. We found a strong test-retest reliability of one-back serial dependence across the two days (ρ = 0.68; p < 0.001; Figure 5, BF10 = 26.84). The test-retest reliability of two-back serial dependence between the two days was trending significant (ρ = 0.38, p = 0.049, BF10 = 1.43). The data in Figures 4 and 5 suggest that serial dependence is highly reliable and consistent within each individual, if measured within the same general region of the visual field—either the fovea or the periphery. Similar to the serial dependence at the fovea, the correlation between one-back serial dependence and two-back serial dependence was significant (ρ = 0.64, p < 0.01, BF10 = 13.76).
Figure 5.
The test-retest reliability of the serial dependence in the peripheral visual field. Each dot represents a single participant's data. The results indicate that, when measured at the same location in the periphery, serial dependence is consistent over time within single observers.
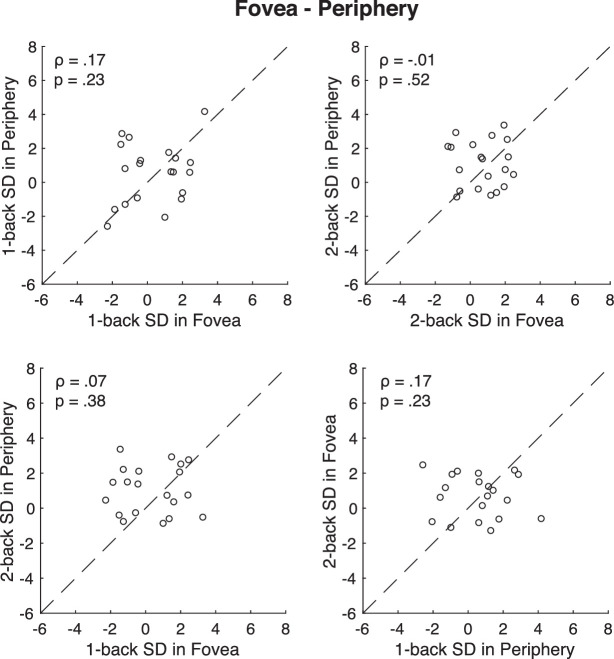
Next, we analyzed whether individual differences in serial dependence at the fovea were correlated with those in the right periphery (Figure 6). The correlation between foveal and peripheral one-back serial dependence was positive but not significant (ρ = 0.17, p = 0.23, BF10 = 0.67). The correlation between foveal and peripheral two-back serial dependence was also not significant (ρ = −0.01, p = 0.52, BF10 = 0.26). Given the high reliability and consistency of serial dependence measures as described above, this result suggests that the serial dependence may be location-specific. That is, serial dependence measured across days within a given location in the visual field was consistent. However, those who show stronger serial dependence at the fovea did not necessarily show stronger serial dependence in the periphery. The correlation between one-back serial dependence and two-back serial dependence was not significant (one-back serial dependence in fovea vs. two-back serial dependence in periphery: ρ = 0.07, p = 0.38, BF10 = 0.25; one-back serial dependence in periphery vs. two-back serial dependence in fovea: ρ = 0.17, p = 0.228, BF10 = 0.31).
Figure 6.
The correlation between foveal and peripheral serial dependence. Each dot represents a single participant's data. The correlations are low, indicating that there is little consistency in the measured serial dependence when it is measured at different locations in the visual field.
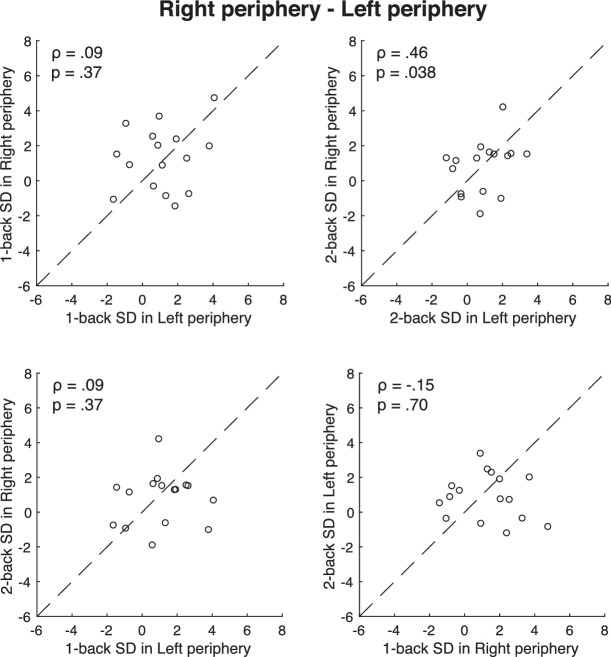
A remaining question from the previous analysis is whether serial dependence in orientation perception is location-specific or eccentricity-specific. In the previous analysis, we measured serial dependence at the fovea and serial dependence in the right periphery. To control for the possibility of eccentricity-specific effects, we also measured serial dependence in the left periphery, as well as the right periphery. In this condition, serial dependence was measured in the right periphery on one day and the left periphery on the other day (see Methods). We found that the individual differences in the amplitude of serial dependence in the left and right visual fields were distinct (Figure 7). The correlation between right and left one-back serial dependence was not significant (ρ = 0.09, p = 0.37, BF10 = 0.44). The correlation between right and left two-back serial dependence was marginally significant (ρ = 0.46, p = 0.038, BF10 = 0.83). Serial dependence was consistent within but not across the vertical meridian and is therefore location-specific, not just eccentricity-specific. This held for both the one-back and two-back serial dependence. The correlation between one-back serial dependence and two-back serial dependence was not significant (one-back serial dependence in left periphery vs. two-back serial dependence in right periphery: ρ = 0.09, p = 0.37, BF10 = 0.31; one-back serial dependence in right periphery vs. two-back serial dependence in left periphery: ρ = −0.15, p = 0.70, BF10 = 0.32).
Figure 7.
The correlation between serial dependence measured across the right and the left visual fields. Each dot represents a single participant's data. The correlations are modest or negative, indicating that there is relatively little consistency in the measured serial dependence when it is measured in different visual fields.
Figures 4 through 7 present an array of correlations quantifying the consistency of serial dependence. Because there are so many correlations, the overarching picture may not be intuitive or obvious. To better visualize the pattern of the results across all conditions, Figure 8 shows the correlations as an organized heatmap. The left column includes all conditions in which the serial dependence was measured at the same retinal location. The right column includes all conditions in which serial dependence was measured at different retinal locations. The test-retest correlations seem to be consistently higher for the same location group (left column) than for the different location group (right column), suggesting that the serial dependence is location-specific. To test this more rigorously, we conducted a mini meta-analysis (Goh, Hall, & Rosenthal, 2016).
Figure 8.
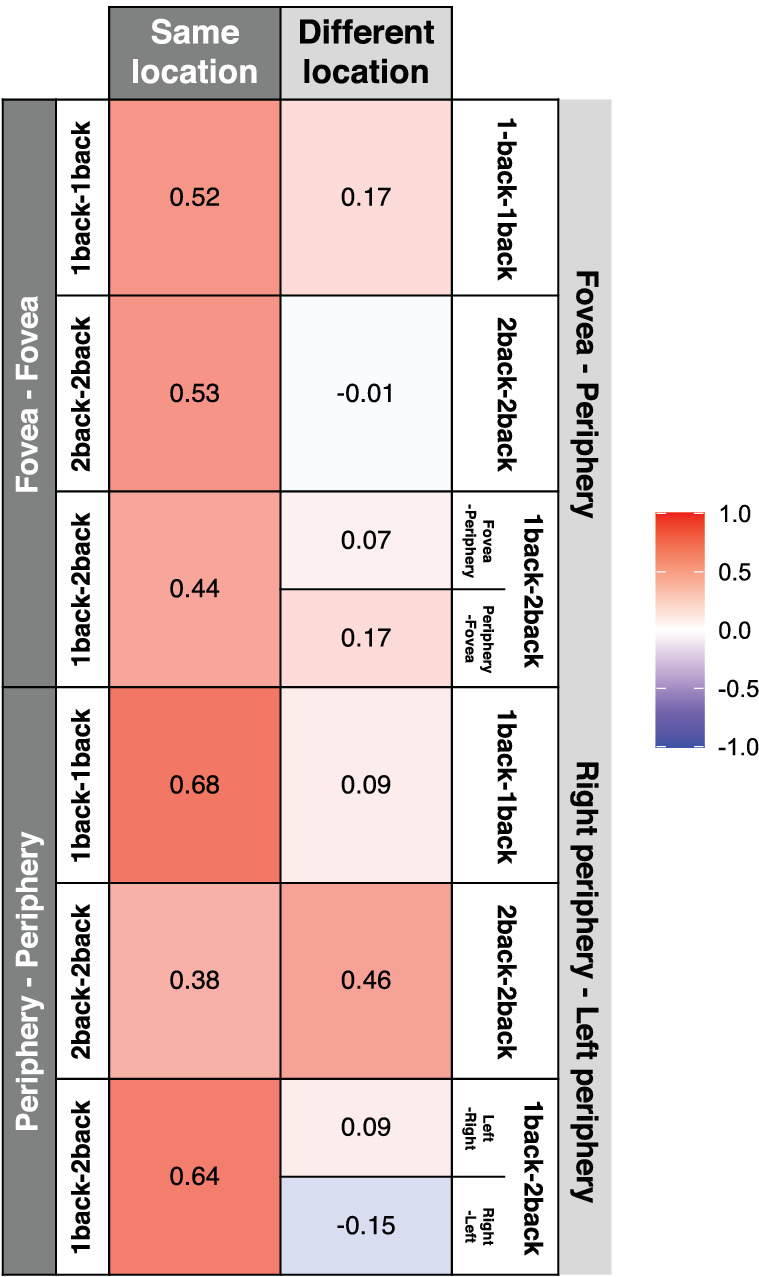
Meta-analysis of the test-retest reliability of serial dependence. Each cell in the matrix shows a correlation of the serial dependence measured between two days. The left column is the set of conditions in which the serial dependence was measured at the same retinal location. The right column is the collection of conditions in which serial dependence was measured in different retinal locations. The weighted sample combined Z test (Goh, et al., 2016) for the “same location” conditions was Zsame = 3.40 (p < 0.001). The weighted sample combined Z test for the “different location” conditions was Zdifferent = 0.77 (n.s.). The difference between these was significant (Zsame − Zdifferent = 2.63, p < 0.01), indicating that there is significantly more individual observer consistency in serial dependence measured at specific retinal locations. This location specificity occurs even when eccentricity is controlled, for peripheral (left versus right) visual field locations (Zsame − Zdifferent = 1.84, p = 0.066, two-tailed).
First, a separate meta-analysis was performed within each location condition (same and different locations, left and right columns in Figure 8). We used fixed effects in which the mean effect size (i.e., mean correlation) was weighted by sample size (Goh et al., 2016). We did this because the number of subjects in the Right-Left condition and the other three conditions was different (Fovea-Fovea, Periphery-Periphery, and Fovea-Periphery: N = 20; Right periphery –Left periphery: N = 16). All correlations were Fisher's z transformed for analyses and converted back to Spearman correlations for presentation. When the stimuli were presented in the same location, the serial dependence between the two days showed a significant positive correlation (M r = 0.54, p < 0.001). On the other hand, when the stimuli were presented in different locations, no significant correlation was found (M r = 0.11, n.s.). The difference between these was significant (using the combined test from Goh, et al., 2016, Zsame − Zdifferent = 2.63, p < 0.01).
We also used permutation methods to test whether the reliability of serial dependence was significantly different between the same and different location conditions. To calculate significance, we generated a null distribution of location-specific differences in test-retest correlations (Zsame − Zdifferent), using a permutation analysis. We randomly shuffled each test-retest correlation relative to the category labels (Location: Same and Different; Visual field: Fovea-Fovea, Periphery-Periphery, Fovea-Periphery, and Right periphery–Left periphery; N-back correlation: one-back-one-back, two-back-two-back, and one-back-two-back) and recalculated the Zsame − Zdifferent for each iteration of the shuffled data. We iterated this procedure 10,000 times to generate a null distribution of Zsame − Zdifferent. The significance level of the original Zsame − Zdifferent was calculated using this null distribution in a two-tailed manner. This permutation test confirmed that the location-specific difference in test-retest reliability was significant (p < 0.001). This result again confirms that the serial dependence was location specific.
Discussion
Our goal was to measure the reliability of serial dependence over time within individual observers and to measure whether the serial dependence is spatially specific. First, we found significant individual differences in serial dependence that are stable across days. Observers who perceive strong orientation serial dependence on one day also display strong serial dependence on a following day, when measured at a specific spatial location. Second, we also found that serial dependence was strongly location-specific. This echoes previous research, which has indicated that serial dependence is either strongly retinotopic or both retinotopic and spatiotopic (Fischer & Whitney, 2014; Collins, 2019; Mikellidou et al., 2021). Our results go beyond previous demonstrations of spatial tuning, though. That serial dependence is retinotopically tuned does not require that there are variations in the magnitude of serial dependence across the visual field within single observers. Indeed, serial dependence could be uniform and consistent across subjects even though it is retinotopically tuned. Our results, however, show that serial dependence is lumpy throughout the visual field: different observers have different degrees of serial dependence at different positions in their visual field. The variation in serial dependence strength is not simply explained by eccentricity (Figure 7).
Spatial tuning
A large number of previous reports have found that serial dependence is spatially specific for various stimulus features (Collins, 2019; Corbett et al., 2011; Fischer & Whitney, 2014; Manassi et al., 2019; Mikellidou et al., 2021). Corbett et al. (2011) demonstrated that the serial dependence in numerosity perception is more affected if the previous stimulus was presented in the same visual field as the current stimulus. Fornaciai and Park (2018b) also reported that the serial dependence in numerosity perception is spatially narrowly tuned. Manassi, Liberman, et al. (2018) demonstrated that the serial dependence in object categorization also occurs within a certain spatial window. As for the serial dependence in orientation perception, several studies have reported spatial tuning, but whether it is strongly retinotopic or both retinotopic and spatiotopic is still under debate (Collins, 2019; Fischer & Whitney, 2014; Mikellidou et al., 2021). As such, the majority of studies have found that serial dependence is strongly spatially tuned.
Our study extends this spatial aspect of serial dependence, showing that the spatial specificity is not just in terms of eccentricity, but is also location specific. Previous studies have investigated how the distance between the previous and current stimuli affects serial dependence. In these studies, the spatial window within which perception is smoothed over time (Continuity Fields; Fischer & Whitney, 2014) is typically shown by averaging out across all possible stimulus locations. The present study, in contrast, suggests that such spatial operators (Continuity Fields) could be different across different visual field locations. This could explain why some observers have strong serial dependence at some retinal locations but not other locations. This is not due to a foveal versus periphery difference; instead, it reveals that serial dependence in orientation perception is tied very closely to visual processing, per se. Even though orientation serial dependence is a manifestation of a kind of memory, this memory is intimately linked to particular regions of particular observer visual fields.
Stability of individual differences in serial dependence
A more important goal of this study was to test for the stability of serial dependence over time, within specific observers. We found that individual observers were highly consistent in the amount of serial dependence that they displayed from day to day. This could be an adaptive process for a particular observer's visual system, establishing a degree of stability in perceptual interpretations tuned to the autocorrelations in the world that they experience.
The possible benefits of serial dependencies are manifold (Cicchini et al., 2017; Cicchini et al., 2018; Fischer & Whitney, 2014; Kiyonaga, Scimeca, Bliss, & Whitney, 2017; Manassi, Liberman, et al., 2018). In general, insofar as the world is autocorrelated, serial dependence can introduce autocorrelations in representations that match the temporal statistics of the world. For example, it can improve the efficiency of representation, the speed with which information is represented, reaction times, and the ability to discriminate stimuli that are repeated (Cicchini et al., 2014; Liberman et al., 2018; Manassi, Liberman, et al., 2018). Serial dependence also gives the visual system flexibility of internal templates that observers use to selectively weigh and filter incoming information, and leads to more adaptable and sensitive perception and perceptual decisions (Murai & Whitney, 2021). Furthermore, serial dependence can also change the appearance of the world, making the world seem continuous (Fischer & Whitney, 2014, Manassi & Whitney, 2022).
At this point, readers might expect that if the temporal structure of the world is relatively stable, especially for particular types of visual information (e.g., autocorrelations in the encountered orientation statistics), then serial dependencies that the brain generates should be very consistent across observers. That is, insofar as observers match the temporal statistics of the world, and the world is constant across observers, observers should agree with each other. However, this overlooks the important point that observers are, in fact, very different from one another, with genetic and physiological differences, as well as different experiences, different biases, and different degrees of internal noise.
For example, individual observers might have very different ways in which they interact with, navigate, or gaze at the world. They may therefore have different temporal statistical distributions of stimuli that they encounter. There are other reasons to expect individual differences, as well. For example, there are substantial individual differences in sensitivity to different kinds of visual information, ranging from localization and position (Kosovicheva & Whitney, 2017; Wang et al., 2020) to pattern, shape, object, and face recognition (Duchaine & Nakayama, 2006; Russell, Duchaine, & Nakayama, 2009; Wilmer, 2017). And, these can vary throughout the visual field (Wang et al., 2020): different parts of the visual field can have higher or lower sensitivity (and different degrees of internal noise). Given these observer and location-specific individual differences, serial dependence would be expected to vary across individuals. For example, variations in sensitivity around the visual field within single observers (Wang et al., 2020) could result in systematic and stable variations in serial dependence.
To some extent, this appears to be true. Cicchini et al. (2017) found that serial dependence in orientation perception was stronger at oblique (non-cardinal) orientations, where sensitivity is lower. Using an individual differences approach, one might expect that sensitivity, or perhaps sensory reliability or uncertainty, is correlated with serial dependence amplitude. Figure 9 supports this relationship between serial dependence amplitude and individual observer uncertainty. Data sets for this analysis were drawn from only the left and right periphery conditions, where the eccentricity of the stimuli was equated. To quantify uncertainty, we measured the standard deviation of the residual adjustment errors after the serial dependence effect was regressed out. To further ensure that the residual adjustment error was unbiased, we used independent sets of data for the X and Y axes in Figure 9 (collected on independent days). Interestingly, there was a relationship between serial dependence and uncertainty, but it was only significant when measured at the same visual field location (ρ = 0.482, p < 0.001; this still holds when controlling for random effects of n-back and subject). Moreover, the relationship was significantly stronger in the same location than when uncertainty and serial dependence were measured at different locations (permutation test, p = 0.045). These results address several important questions. First, they confirm the presence of individual differences in both serial dependence and uncertainty, and they confirm that the measured serial dependence was location specific. Second, because eccentricity was controlled and equated in the experiment (only peripheral left versus right visual field locations were compared), the individual differences in serial dependence were not likely due to cortical magnification, resolution, or other eccentricity-dependent effects. Third, the location specificity of both serial dependence and uncertainty suggest that observer eye movements did not play a role or impact the results, because they would have tended to eliminate any location specificity. Finally, the location specificity of the relationship between serial dependence and uncertainty hints at a possible origin of some of the individual differences and suggests that variations in sensitivity around the visual field within single observers (e.g., Wang et al., 2020) might result in systematic and stable variations in serial dependence.
Figure 9.
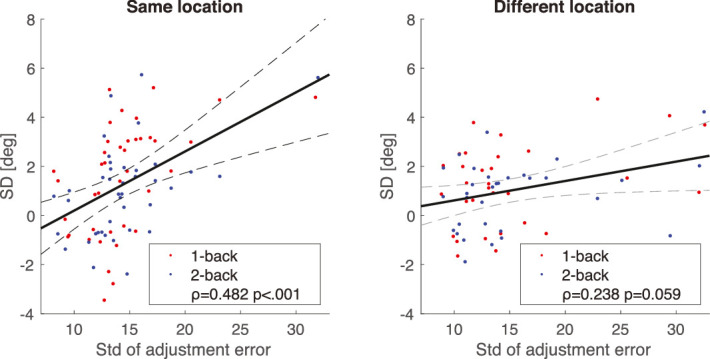
Serial dependence in orientation perception is correlated with uncertainty in a location-dependent manner. Uncertainty (X axis) was operationalized as the standard deviation of the residual adjustment error after regressing out serial dependence (essentially, this is the scatter of the data points around the observer-specific derivative-of-von-Mises fits like those in Figure 3). The serial dependence (Y axis) and uncertainty (X axis) were measured on separate days to avoid confounding information in the two axes. Red data points indicate one-back conditions, and blue data represent two-back conditions. Serial dependence was correlated with uncertainty for Gabor patches at the same location (ρ = 0.482, p < 0.001). Moreover, the relationship between serial dependence and uncertainty was significantly stronger at the same versus different locations (p = 0.045, permutation test). These results indicate that there are individual differences in both uncertainty and serial dependence, and that they are both location-specific. This hints at a possible source of serial dependence in the observer-specific variation in sensitivity around the visual field at isoeccentric locations.
The individual differences found here raise more general questions about the relative roles of genetics and experience in serial dependence. In particular, how perceptual learning, experience, cultural differences, and development may impact the formation and stability of serial dependence remains an interesting future avenue of investigation. Although these questions are beyond the scope of this paper, the present findings do provide an intriguing glimpse into the possibility that serial dependence could be strongly tuned to individual, cultural, and circumstantial differences. In any case, our results suggest that individual observers do, in fact, have very different degrees of serial dependence, and that it is consistent and stable from day to day. This supports the idea that serial dependence may be an individual observer-specific mechanism that serves an individual observer-specific function in improving and stabilizing perception.
Acknowledgments
Supported in part by the Japan Society for the Promotion of Science (15K16008) to A.K. and the National Institutes of Health (R01CA236793) to D.W. We also thank Robin Stewart for supporting data collection.
Commercial relationships: none.
Corresponding author: Aki Kondo.
Email: kondo.aki@aoni.waseda.jp.
Address: Waseda Institute for Advanced Study, 1st floor, Nishi-Waseda Bldg. 1-21-1 Nishi Waseda, Shinjuku-ku, Tokyo 169-8050, Japan.
References
- Alais, D., Leung, J., & Van der Burg, E. (2017). Linear summation of repulsive and attractive serial dependencies: Orientation and motion dependencies sum in motion perception. Journal of Neuroscience, 37(16), 4381–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamyan, A., Silva, L. L., Dakin, S. C., Carandini, M., & Gardner, J. L. (2016). Adaptable history biases in human perceptual decisions. Proceedings of the National Academy of Sciences of the United States of America, 113(25), E3548–E3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard, D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10(4), 433–436. [PubMed] [Google Scholar]
- Braun, A., Urai, A. E., & Donner, T. H. (2018). Adaptive History Biases Result from Confidence-Weighted Accumulation of Past Choices. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 38(10), 2418–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchini, G. M., Anobile, G., & Burr, D. C. (2014). Compressive mapping of number to space reflects dynamic encoding mechanisms, not static logarithmic transform. Proceedings of the National Academy of Sciences of the United States of America, 111(21), 7867–7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchini, G. M., Benedetto, A., & Burr, D. C. (2021). Perceptual history propagates down to early levels of sensory analysis. Current Biology, 31: 1245–1250.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchini, G. M., Mikellidou, K., & Burr, D. (2017). Serial dependencies act directly on perception. Journal of Vision, 17, 1–9. [DOI] [PubMed] [Google Scholar]
- Cicchini, G. M., Mikellidou, K., & Burr, D. C. (2018). The functional role of serial dependence. Proceedings. Biological Sciences/The Royal Society, 285(1890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, T. (2019). The perceptual continuity field is retinotopic. Scientific Reports, 9(1), 18841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, T. (2020) Serial dependence alters perceived object appearance. Journal of Vision, 20(13):9, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett, J. E., Fischer, J., & Whitney, D. (2011). Facilitating stable representations: serial dependence in vision. PloS One, 6(1), e16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine, B., & Nakayama, K. (2006). The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia, 44(4), 576–585. [DOI] [PubMed] [Google Scholar]
- Fischer, J., & Whitney, D. (2014). Serial dependence in visual perception. Nature Neuroscience, 17(5), 738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaciai, M., & Park, J. (2018a). Attractive Serial Dependence in the Absence of an Explicit Task. Psychological Science, 29(3), 437–446. [DOI] [PubMed] [Google Scholar]
- Fornaciai, M., & Park, J. (2018b). Serial dependence in numerosity perception. Journal of Vision, 18, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche, M., & de Lange, F. P. (2019). The role of feature-based attention in visual serial dependence. Journal of Vision, 19, 1–13. [DOI] [PubMed] [Google Scholar]
- Fritsche, M., Mostert, P., & de Lange, F. P. (2017). Opposite Effects of Recent History on Perception and Decision, Current Biology, 27(4), 590–595. [DOI] [PubMed] [Google Scholar]
- Goh, J. X., Hall, J. A., & Rosenthal, R. (2016). Mini meta-analysis of your own studies: Some arguments on Why and a primer on How. Social and Personality Psychology Compass, 10, 535–549. [Google Scholar]
- Huang, L., Mo, L., & Li, Y. (2012). Measuring the interrelations among multiple paradigms of visual attention: an individual differences approach. Journal of Experimental Psychology. Human Perception and Performance, 38(2), 414–428. [DOI] [PubMed] [Google Scholar]
- Kim, S., Burr, D., Cicchini, G., & Alais, D. (2020). Serial dependence in perception requires conscious awareness. Current Biology, 30(6), R257–R258. [DOI] [PubMed] [Google Scholar]
- Kim, S., & Alais, D. (2021). Individual differences in serial dependence manifest when sensory uncertainty is high. Vision Research, 188, 274–282. [DOI] [PubMed] [Google Scholar]
- Kiyonaga, A., Scimeca, J. M., Bliss, D. P., & Whitney, D. (2017). Serial dependence across perception, attention, and memory. Trends in Cognitive Science, 21(7), 493–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, A. (2007). Visual Adaptation: Physiology, Mechanisms, and Functional Benefits. Journal of Neurophysiology, 97(5), 3155–3164. [DOI] [PubMed] [Google Scholar]
- Kosovicheva, A., & Whitney, D. (2017). Stable individual signatures in object localization. Current Biology: CB, 27(14), R700–R701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman, A., Fischer, J., & Whitney, D. (2014). Serial dependence in the perception of faces. Current Biology: CB, 24(21), 2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman, A., Manassi, M., & Whitney, D. (2018). Serial dependence promotes the stability of perceived emotional expression depending on face similarity. Attention, Perception & Psychophysics, 80(6), 1461–1473. [DOI] [PubMed] [Google Scholar]
- Manassi, M., Kristjánsson, Á., & Whitney, D. (2019). Serial dependence in a simulated clinical visual search task. Scientific Reports, 9(1), 19937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manassi, M., Liberman, A., Chaney, W., & Whitney, D. (2017). The perceived stability of scenes: serial dependence in ensemble representations. Scientific Reports, 7(1), 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manassi, M., Liberman, A., Kosovicheva, A., Zhang, K., & Whitney, D. (2018). Serial dependence in position occurs at the time of perception. Psychonomic Bulletin & Review, 25(6), 2245–2253. [DOI] [PubMed] [Google Scholar]
- Manassi, M., Murai, Y., & Whitney, D. (2018). Serial Dependence on a Large Scale. Journal of Vision, 18(10), 1153–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manassi, M. & Whitney, D. (2022). Illusion of stability through active serial dependence. Science Advances, 8(2), eabk2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikellidou, K., Cicchini, G. M., & Burr, D. C. (2021). Perceptual history acts in world-centred coordinates. i-Perception, 12(5), 20416695211029301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai, Y. & Whitney, D. (2021) Serial dependence revealed in history-dependent perceptual templates. Current Biology, 31, 3185–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan, L., & Serrien, D. J. (2018). Individual Differences and Hemispheric Asymmetries for Language and Spatial Attention. Frontiers in Human Neuroscience, 12, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli, D. G. (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision, 10(4), 437–442. [PubMed] [Google Scholar]
- Rafiei, M., Hansmann-Roth, S., Whitney, D., Kristjánsson, Á., & Chetverikov, A. (2021). Optimizing perception: Attended and ignored stimuli create opposing perceptual biases. Attention, Perception & Psychophysics, 83(3), 1230–1239. [DOI] [PubMed] [Google Scholar]
- Russell, R., Duchaine, B., & Nakayama, K. (2009). Super-recognizers: People with extraordinary face recognition ability. Psychonomic Bulletin & Review, 16(2), 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Pinilla, M., Seth, A. K., & Roseboom, W. (2018). Serial dependence in the perception of visual variance. Journal of Vision, 18(7):4, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert, J., Alais, D., & Burr, D. (2016). Different coding strategies for the perception of stable and changeable facial attributes. Scientific Reports, 6, 32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P., & Burr, D. (2009). Visual aftereffects. Current Biology: CB, 19(1), R11–R14. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Murai, Y., & Whitney, D. (2020). Idiosyncratic perception: a link between acuity, perceived position and apparent size. Proceedings. Biological Sciences /The Royal Society, 287(1930), 20200825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler, M. (2018). Multidimensional internal dynamics underlying the perception of motion. Journal of Vision, 18(5):7, 1–12. [DOI] [PubMed] [Google Scholar]
- Wexler, M., Duyck, M., & Mamassian, P. (2015). Persistent states in vision break universality and time invariance. Proceedings of the National Academy of Sciences, USA, 112(48), 14990–14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, M. A. (2012). Evolving concepts of sensory adaptation. F1000 Biology Reports, 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmer, J. B. (2017). Individual differences in face recognition: a decade of discovery. Current Directions in Psychological Science, 26, 225–230. [Google Scholar]
- Zhang, H., & Alais, D. (2020). Individual difference in serial dependence results from opposite influences of perceptual choices and motor responses. Journal of Vision, 20, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]