Abstract
Background
Preterm birth is the leading cause of death and disability in newborns worldwide. A wide variety of tocolytic agents have been utilized to delay birth for women in preterm labor. One of the earliest tocolytics utilized for this purpose was ethanol infusion, although this is not generally used in current practice due to safety concerns for both the mother and her baby.
Objectives
To determine the efficacy of ethanol in stopping preterm labor, preventing preterm birth, and the impact of ethanol on neonatal outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 May 2015) and reference lists of retrieved studies.
Selection criteria
We included randomized and quasi‐randomized studies. Cluster‐randomized trials and cross‐over design trials were not eligible for inclusion. We only included studies published in abstract form if there was enough information on methods and relevant outcomes. Trials were included if they compared ethanol infusion to stop preterm labor versus placebo/control or versus other tocolytic drugs.
Data collection and analysis
At least two review authors independently assessed studies for inclusion and risk of bias. At least two review authors independently extracted data. Data were checked for accuracy.
Main results
Twelve trials involving 1586 women met inclusion criteria for this review. One trial did not report on the outcomes of interest in this review.
Risk of bias of included studies: The included studies generally were of low quality based on inadequate reporting of methodology. Only three trials had low risk of bias for random sequence generation and one had low risk of bias for allocation concealment and participant blinding. Most studies were either high risk of bias or uncertain in these key areas.
Comparison 1: Ethanol versus placebo/control (two trials, 77 women)
Compared to controls receiving pain medications and dextrose solution, ethanol did not improve any of the primary outcomes: birth < 48 hours after trial entry (one trial, 35 women; risk ratio (RR) 0.93, 95% confidence interval (CI) 0.43 to 2.00), or neonatal mortality (one trial, 35 women; RR 1.06, 95% CI 0.31 to 3.58). Serious maternal adverse events and perinatal mortality were not reported by either of the two trials in this comparison. Maternal adverse events (overall) were not reported but one trial (42 women) reported that there were no maternal adverse events that required stopping or changing drug) in either group. One trial did report delay until delivery but this outcome was reported as a median with no mention of the standard deviation (median 19 days in ethanol group versus "less than 1" day in the glucose/water group). There were no differences in any secondary outcomes reported: preterm birth < 34 weeks or < 37 weeks; serious infant outcome; fetal alcohol syndrome/fetal alcohol spectrum disorder; or small‐for‐gestational age.
Comparison 2: Ethanol versus other tocolytic (betamimetics) (nine trials, 1438 women)
Compared to betamimetics (the only tocolytic used as a comparator in these studies), ethanol was associated with no clear difference in the rate of birth < 48 hours after trial entry (two trials, 130 women; average RR 1.12, 95% CI 0.53 to 2.37, Tau² = 0.19, I² = 59%), similar rates of perinatal mortality (six trials, 698 women; RR1.20, 95% CI 0.78 to 1.84), higher rates of neonatal mortality (eight trials, 1238 women; RR 1.43, 95% CI 1.02 to 2.02), higher rates of preterm birth < 34 weeks (two trials, 599 women; RR 1.56, 95% CI 1.11 to 2.19), higher rates of neonatal respiratory distress syndrome (three trials, 823 women; RR 1.76, 95% CI 1.33 to 2.33), and higher rates of low birthweight babies < 2500 g (five trials, 834 women; RR 1.30, 95% CI 1.09 to 1.54). These outcomes are likely all related to the lower incidence of preterm birth seen with other tocolytics, which for all these comparisons were betamimetics. Serious maternal adverse events were not reported in any of the nine trial reports. However, ethanol had a trend towards a lower rate of maternal adverse events requiring stopping or changing the drug (three trials, 214 women; RR 0.25, 95% CI 0.06 to 0.97). There were no differences in other secondary outcomes of preterm birth < 37 weeks, number of days delivery was delayed, or overall maternal adverse events.
Planned sensitivity analysis, excluding quasi‐randomized trials did not substantially change the results of the primary outcome analyses with the exception of neonatal mortality which no longer showed a clear difference between the ethanol and other tocolytic groups (3 trials, 330 women; RR 1.49, 95% CI 0.82 to 2.72).
Authors' conclusions
This review is based on evidence from twelve studies which were mostly low quality. There is no evidence that to suggest that ethanol is an effective tocolytic compared to placebo. There is some evidence that ethanol may be better tolerated than other tocolytics (in this case betamimetics), but this result is based on few studies and small sample size and therefore should be interpreted with caution. Ethanol appears to be inferior to betamimetics for preventing preterm birth in threatened preterm labor.
Ethanol is generally no longer used in current practice due to safety concerns for the mother and her baby. There is no need for new studies to evaluate the use of ethanol for preventing preterm birth in threatened preterm labour. However, it would be useful for long‐term follow‐up studies on the babies born to mothers from the existing studies in order to assess the risk of long‐term neurodevelopmental status.
Plain language summary
Ethanol (alcohol) for preventing preterm birth
Preterm birth is when a baby is born at less than 37 weeks' gestation. These babies are generally more ill and are less likely to survive than babies born at term. Preterm babies are also more likely to have some disability, and the earlier the baby is born the more likely they are to have problems. Even short‐term postponement of preterm birth can improve outcomes for babies, as this gives time for the mother to be given a steroid injection to help develop the baby's lungs prior to birth. Short‐term postponement of preterm birth may also give the chance to transfer the mother, if required, to somewhere where there is more expert care for the baby available.
Drugs used to try and stop labor are called tocolytics. These drugs are given to women experiencing preterm labor to try and stop or relax uterine contractions. One of the earliest drugs used to try and stop contractions was ethanol (also known as alcohol), although this is not generally used in current practice due to safety concerns for both the mother and her baby. In this review, we looked at the published studies to see if ethanol was effective in postponing labor and improving outcomes for babies, and also whether ethanol was better than other types of tocolytics used to postpone preterm labor and birth.
We searched for trials evidence on 31 May 2015 and found 12 trials total involving 1586 women, some comparing ethanol with a placebo and others comparing ethanol with other tocolytics (in this instance, all betamimetics). The trials included in this review were considered to be mostly low quality studies.
For our comparison of ethanol versus placebo control (two trials, 77 women). We found that ethanol was no better than placebo (sugar water) for any of the outcomes studied: birth <48 hours after trial entry (one trial, 35 women) or neonatal mortality (one trial, 35 women). Serious maternal adverse events and perinatal mortality was not reported. There was no differences between groups for other outcomes: preterm birth < 37 weeks or < 34 weeks, serious infant outcome, fetal alcohol syndrome/fetal alcohol spectrum disorder, or small‐for‐gestational age.
We also compared ethanol with other tocolytic drugs (nine trials involving 1438 women; all trials studied betamimetic drugs). We found that ethanol was worse than other betamimetic drugs at postponing birth until after 34 weeks' gestation and led to a higher rate of low birthweight babies, babies with breathing problems at birth and neonatal death (although there was no clear difference in neonatal deaths when we restricted our analyses to the better quality studies), However, we did find that, compared to betamimetics, ethanol was associated with a trend for fewer maternal side effects that required stopping or changing the drug, though this result is based on three small trials. There were no differences in other secondary outcomes of preterm birth < 37 weeks, number of days delivery was delayed, or overall maternal adverse events.
Overall, we found no evidence that ethanol was better than a placebo at postponing preterm labor and birth. Whilst there was some evidence to suggest that ethanol may be better tolerated than betamimetics, we found that ethanol was not as effective as betamimetics at postponing preterm labor and birth. None of the studies were long‐term ones and thus none of them reported on the risk of giving ethanol on the babies developing fetal alcohol syndrome, which can cause mental retardation.
There is no need for new studies to evaluate the use of ethanol for preventing preterm birth. However, it would be useful for long‐term follow‐up studies on the babies born to mothers from the existing studies in order to assess the risk of long‐term neurodevelopmental status.
Background
Description of the condition
Preterm birth is currently recognized as the leading cause of death in newborns worldwide (Howson 2012). According to the World Health Organization, preterm birth is defined as delivery prior to completion of 37 weeks' gestation (WHO 1977). Of the estimated 3.1 million neonatal deaths in 2010, 35% were directly caused by preterm birth complications (Howson 2012). The premature infants who survive are at risk for several perinatal morbidities, including respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage, sepsis, cerebral palsy, and other neurologic deficits (Gladstone 2011). Neonatal morbidity and mortality rates are inversely proportional to gestational age and birthweight. Thus, as gestational age increases, mortality rate decreases (Lawn 2010). Consequently, research efforts have focused on ways to prolong pregnancy when a woman presents in preterm labor. Research has shown that tocolytic drugs could improve perinatal outcomes by inhibiting uterine contractions during preterm labor (Conde‐Agudelo 2011). Though the ultimate goal would be to decrease the incidence of preterm birth, clinicians also use tocolysis as a short‐term treatment to delay birth long enough to administer corticosteroids to the mother for fetal lung maturation and to allow time to transfer the woman to a tertiary care facility (Roberts 2006). A wide variety of tocolytic agents have been utilized, including betamimetics, calcium channel blockers, oxytocin receptor antagonists, magnesium sulfate, and ethanol. Although ethanol is no longer used in clinical practice, this review aims to evaluate ethanol as a tocolytic in preterm labor.
Description of the intervention
Ethanol was previously used as a common tocolytic agent. Research has shown that it is an effective tocolytic when given in high concentrations (Schaefer 2007). However, ethanol has harmful effects on the development of the fetus at these high concentrations in maternal blood (ACOG 2011). Other research has shown that ethanol is an ineffective tocolytic in low concentrations. Both consumption and treatment with ethanol puts the fetus at risk for fetal alcohol syndrome, which is characterized by growth retardation, facial dysmorphia, central nervous system abnormalities, intellectual deficits, and various other birth defects. In addition, maternal adverse effects, including intoxication and withdrawal, were common. According to the American Congress of Obstetricians and Gynecologists (ACOG), no amount of ethanol consumption during pregnancy has been determined to be safe, and, therefore, women should avoid ethanol entirely during pregnancy (ACOG 2011). It is due to these recommendations that the practice of using ethanol as a tocolytic agent has been eliminated.
How the intervention might work
The mechanism for the onset of parturition is complex. There are several known hormones and pathways that play a role in the initiation of labor. One of the most well‐understood pathways is the interaction between oxytocin and the uterus. The myometrium has four distinct physiologic phases during pregnancy. In the first phase, uterotropin hormones, such as estrogen, upregulate the production of contraction‐associated proteins (CAPs). These CAPs include the myometrial receptor for oxytocin, a hormone released from the posterior pituitary and considered the most potent agent in the stimulation of uterine contractions (Zeeman 1997). Through this mechanism, the receptors exponentially increase in number during pregnancy, peaking during early labor. The oxytocin/oxytocin‐receptor complex interacts with a G protein and results in an influx of calcium, stimulating uterine contractions. At high concentrations, ethanol has a tocolytic effect by acting as an inhibitor of oxytocin release (Schaefer 2007), thus reducing the strength of uterine contractions and decreasing the likelihood of preterm labor.
Why it is important to do this review
Early clinical findings supported ethanol as an effective tocolytic agent and it was a standard therapy for many years (Belinkoff 1950; Fuchs 1965; Fuchs 1967). However, ethanol is a teratogen and can affect the fetus at any gestational age (ACOG 2011). Therefore, it is important to assess the evidence surrounding the benefits and harms of the use of ethanol as a tocolytic for women in preterm labor systematically. Even though adverse‐effect profiles and potential adverse effects on the fetus have led to this therapy not being used clinically anymore, systematically reviewing the evidence to complete the Cochrane reviews of tocolytic therapies is important.
Other Cochrane reviews of tocolysis include reviews of magnesium sulfate (Crowther 2014), oxytocin receptor antagonists (Flenady 2014a), calcium channel blockers (Flenady 2014b), nitric oxide donors (Duckitt 2014), betamimetics (Neilson 2014), progestational agents (Su 2014), hydration (Stan 2013), relaxin (Bain 2013), cyclo‐oxygenase inhibitors (Reinebrant 2015), and combinations of drugs (Vogel 2014).
Objectives
To determine the efficacy of ethanol in stopping preterm labor, preventing preterm birth, and the impact of ethanol on neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized and quasi‐randomized studies. Cluster‐randomized trials and cross‐over trials were not eligible for inclusion. We only included studies published in abstract form if there is enough information on methods and relevant outcomes.
Types of participants
Pregnant women admitted to hospital for threatened preterm birth.
Types of interventions
We included any use of ethanol therapy for preterm labor.
This review included comparisons of:
ethanol versus placebo/usual care/no tocolytic;
ethanol versus a different tocolytic agent.
We planned to exclude trials that used ethanol in combination with another tocolytic agent, unless the use of ethanol was the only difference between the two groups (i.e. betamimetic versus betamimetic plus ethanol).
Types of outcome measures
Primary outcomes
The Cochrane Pregnancy and Childbirth Group's (PCG) Consensus statement, "Adopting a consistent approach to PCG systematic reviews on tocolysis for inhibiting preterm labour" was followed in development of these outcomes. We include the core outcomes in the consensus statement below. These core outcomes are denoted by an asterisk.
Maternal
Birth less than 48 hours after trial entry*.
Serious maternal adverse events (defined as death, cardiac arrest, respiratory arrest, admission to intensive care unit)*. None of the included trials reported on this outcome and thus no comparisons for it were performed.
Fetal/neonatal/infant
Perinatal mortality (fetal death and neonatal death up to 28 days).
Neonatal mortality.
Secondary outcomes
Maternal
Preterm birth less than 37 weeks.
Preterm birth less than 34 weeks*.
Number of days that delivery was delayed from start of therapy.
Maternal adverse events (overall)*.
Maternal adverse events (requiring stopping or changing drug).
Fetal/neonatal/infant
Serious infant outcome (defined as death or chronic lung disease (need for supplemental oxygen at 28 days of life or later), grade three or four intraventricular hemorrhage or periventricular leukomalacia, major neurosensory disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay/intellectual impairment (defined as developmental quotient (DQ) or intelligence quotient (IQ) less than two standard deviations below mean)))*.
Fetal alcohol syndrome/fetal alcohol spectrum disorder (defined by the trialist or involving brain damage, impaired growth, and head and face abnormalities).
Respiratory distress syndrome.
Birthweight.
Small‐for‐gestational age (birthweight below 10th percentile for gestational age or as defined by trial authors).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 May 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of identified trials.
We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreements through discussion. We created a study flow diagram to map out the number of records identified, included, and excluded.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted extract the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. We entered data into Review Manager (RevMan 2014), and checked them for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details. We were only successful at contacting one author to clarify an inclusion criteria for his trial (Caritis 1982).
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third review author.
(1) Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low risk of bias;
high risk of bias;
unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data)
For each included study and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study's prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study did not include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study, described any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there was risk of other bias.
(7) Overall risk of bias
We made explicit judgments about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented the results as summary risk ratio with 95% confidence interval (CI).
Continuous data
For continuous data, we used the mean difference with 95% CI where outcomes are measured in the same way between trials. We planned to use the standardized mean difference with 95% CI to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomized trial
Cluster‐randomized trials were not eligible for inclusion in this review.
Cross‐over trials
Cross‐over trials were not eligible for inclusion in this review.
Other unit of analysis issues
As women with multiple gestations are at high risk of preterm labor, it is reasonable to expect that trials will include women with multiple gestations. Had trial authors reported data for multiple gestations separately, we planned to perform a subgroup analysis of women with singleton gestations versus women with multiple gestations. We planned that if a trial excluded multiple gestations, we would include them in the singleton gestation subgroup analysis. For maternal outcomes, we would have counted the number of pregnant women in the trial for outcomes and utilized the number in the denominator. For neonatal/fetal outcomes, the number of individual fetuses/newborns would have been used as the population for outcomes and in the denominator. All of these would have been based on the level of detail reported in the individual trial.
If trials had included more than two treatment groups, we planned to select one pair of interventions for the analysis and exclude the others to avoid double counting as noted in theCochrane Handbook for Systematic Reviews of Interventions Section 16.5 (Higgins 2011).
We planned to follow the Pregnancy and Childbirth Group's consensus statement for tocolytic trials. However, as this review is mainly of historical context, we planned to separate the analyses into two comparisons: ethanol versus placebo/control and ethanol versus other tocolytic. As the consensus statement recommends comparisons only between classes of drugs, this is still consistent with this guidance as all of the comparisons that had relevant outcomes compared ethanol to betamimetic drugs. If in future updates, in the highly unlikely event of new trials being found for inclusion that compare ethanol versus a different class of tocolytic, we will separate out the comparisons by class. Because of this, we plan to use a subgroup analysis based on membrane status at admission within these two comparisons.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analyses.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we attempted to include all women randomized to each group in the analyses, and all women were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention). The denominator for each outcome in each trial is the number randomized minus any women whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I², and Chi²statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Had there been 10 or more studies in the meta‐analysis, we planned to investigate reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager (RevMan 2014). We used fixed‐effect meta‐analyses for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect (i.e. where trials were examining the same intervention, and we judged the trials' populations and methods sufficiently similar). If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analyses to produce an overall summary, if a mean treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the mean of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the mean treatment effect was not clinically meaningful, we would not have combined trials.
Where we used random‐effects analyses, we present the results as the mean treatment effect with 95% CI, and the estimates of Tau² and I² statistic.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
Women with multiple gestations versus women with singleton gestations (this subgroup analysis was not carried out due to insufficient data).
Women with intact membranes versus women with ruptured membranes. This subgroup analysis was included following current thoughts that preterm labor that accompanies premature preterm ruptured membranes may have a different pathophysiology than simple preterm labor. In addition, many older trials utilized nonspecific definitions of preterm labor for trial entry, such that the presence of regular contractions with minimal or no cervical dilation could qualify a woman for the diagnosis and trial entry. These women would be less likely to delivery preterm in general than women with ruptured membranes. Thus, this subgroup analysis was performed as the outcomes may be very different between them.
Subgroup analyses were restricted to primary outcomes only.
We assessed subgroup differences by interaction tests available within Review Manager (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² statistic.
Sensitivity analysis
We expected many of the trials to be older trials and thus may not robustly report facts related to the methodological quality of the trial. Consequently, we planned to perform a sensitivity analysis of trials found to be at overall low risk of bias versus all trials to explore if there is a risk of bias associated with the quality of some of the included trials. We planned to base this assessment of quality mainly on the risk of bias related to randomization, allocation concealment, and blinding parameters. However, since there were no trials assessed as a low risk of bias, this planned sensitivity analysis was not performed.
We also carried out sensitivity analysis where we included any quasi‐randomized trials, to explore the impact of their inclusion on the overall results.
Planned sensitivity analyses for the primary outcomes will be conducted in subsequent updates of this review (if necessary).
Results
Description of studies
Results of the search
See: Figure 1.
1.
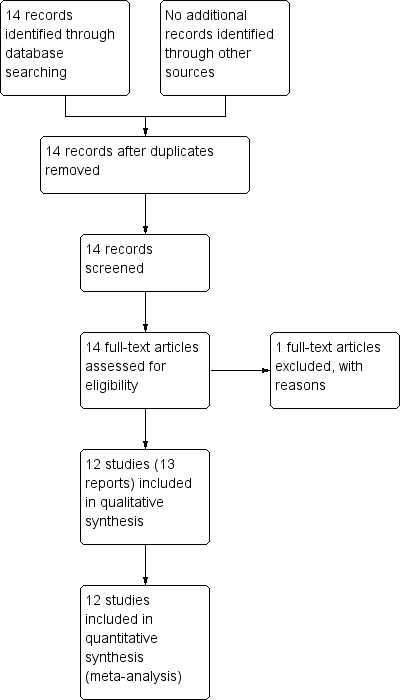
Study flow diagram.
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register identified 14 reports of 13 studies.
Included studies
Twelve trials involving 1586 women met the inclusion criteria for the review. One trial contributed no data to analyses (Steer 1977). Full details on the methods and populations of each trial are available in the Characteristics of included studies.
Two trials compared ethanol with a control/placebo of glucose or dextrose in water (Watring 1976; Zlatnik 1972). The Steer 1977 trial compared ethanol with magnesium sulfate, and also had a small third arm of placebo using 5% dextrose (n = 9). To avoid double counting the ethanol group in two different comparisons, and given the small size of the groups and clear difference in the interventions, we opted to overcome the unit of analysis error issue by selecting one of the pair‐wise comparisons (ethanol versus magnesium sulfate) and not including the dextrose group comparison. However, as this trial's reported "success" outcome was defined as stopping contractions and at least a day going by before they started again, and did not report any of our predefined outcomes, it did not contribute to any analyses. The remaining nine studies compared ethanol with a betamimetic ‐ salbutamol (Boyd 1978; Reynolds 1978; Sims 1978; Spearing 1979), terbutaline (Caritis 1982), nylidrin (Castren 1975), fenoterol (Forster 1987 (Part II); Forster 1987 (Part III)), or ritodrine (Lauersen 1977). The Spearing 1979 trial had a third group that received ethanol plus indomethacin. This group was excluded from the analyses.
The trials did not use a single standard definition of preterm labor or successful therapy.
One trial (Forster 1987 (Part II)) reported mean delay of delivery but did not report standard deviations. As it was one of the larger trials (210 women), we believed it important to include for this outcome. After consultation with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and a statistician, we imputed a range of standard deviations from 1.0 up to the largest reported standard deviation of a trial reporting that outcome (Sims 1978) of 24.4. No values within this range significantly altered the results seen. Thus, values similar to the majority of standard deviations in that comparison (˜3.3) were utilized in the final analysis. This limits conclusions from this comparison somewhat (Analysis 2.6).
2.6. Analysis.
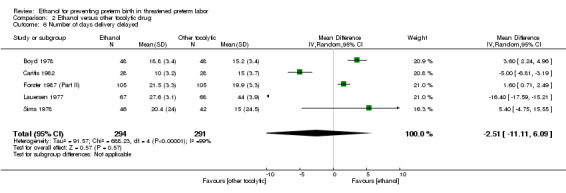
Comparison 2 Ethanol versus other tocolytic drug, Outcome 6 Number of days delivery delayed.
Four trials excluded women with ruptured membranes (Forster 1987 (Part II); Steer 1977; Watring 1976; Zlatnik 1972). Of the other trials, two specified outcomes for those with intact versus ruptured membranes (Caritis 1982; Sims 1978), allowing for the subgroup analyses.
Three trials excluded multiple gestations (Sims 1978; Steer 1977; Watring 1976), and two reported outcomes separately for singleton and multiple gestations (Lauersen 1977; Spearing 1979). However, since there were so few women in the trials with multiple gestations and the outcome reported was birth < 72 hours, which was not a prespecified outcome, we did not perform that planned subgroup analysis.
Six trials (Castren 1975; Forster 1987 (Part II); Forster 1987 (Part III); Reynolds 1978; Spearing 1979; Steer 1977) had randomization methods that qualified as quasi‐randomized. In the sensitivity analysis, these trials were excluded.
Excluded studies
One trial was excluded as it did not report trial data regarding ethanol as a tocolytic (Waltman 1969). For more information please see Characteristics of excluded studies.
Risk of bias in included studies
In general, the quality of trial reporting was low. This is likely due to the years of publication of many of the studies being well prior to the CONSORT (Consolidated Standards of Reporting Trials) criteria (Schulz 2010), or any attempts at standard reporting of trials. Thus, the assessment of risk of bias in several areas is unclear simply because they are not stated (see Figure 2; Figure 3).
2.
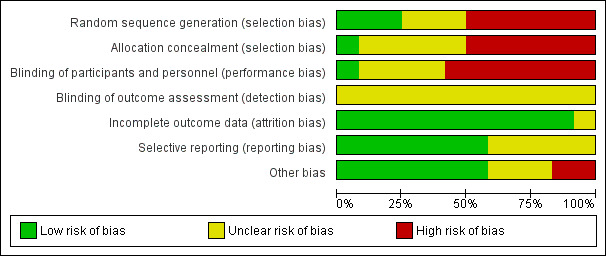
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
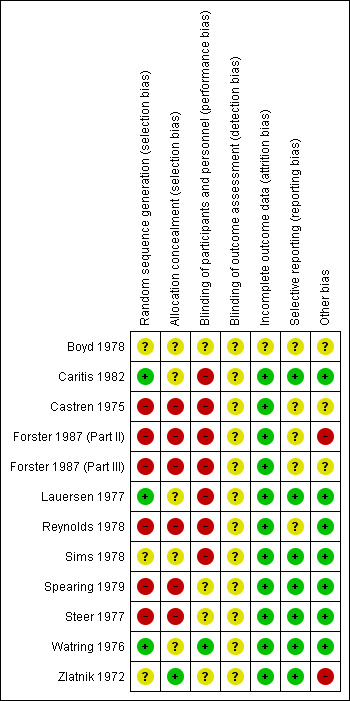
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Random sequence generation
Only three trials had a low risk of bias for random sequence generation. (Caritis 1982; Lauersen 1977; Watring 1976). The Zlatnik 1972 trial stated that assignments were "random" in envelopes without any details about how randomization occurred. Six of the trials (Castren 1975; Forster 1987 (Part II); Forster 1987 (Part III); Reynolds 1978; Spearing 1979; Steer 1977) contained high risk of bias for random sequence generation. This is due to generally stating that randomization/allocation was based on alternating allocation or based on last names.
Allocation concealment
While all three of the above trials (Caritis 1982; Lauersen 1977; Watring 1976) used envelopes for allocation, none stated if the envelopes were sequentially numbered or opaque and thus all were unclear in the risk of allocation bias. A fourth had low risk of bias for allocation concealment however, only stated that assignments were "random" in the envelopes (Zlatnik 1972). Six of the trials (Castren 1975; Forster 1987 (Part II); Forster 1987 (Part III); Reynolds 1978; Spearing 1979; Steer 1977) contained high risk of bias for allocation concealment. Often this was due to "alternating" allocation or randomization based on surnames or record numbers.
Blinding
Only the Watring 1976 trial stated that it was "single blind" in the title. However, it was not stated in the trial report which group was blinded. The control group in this study were given morphine or secobarbital so may have appeared similarly "intoxicated" as the ethanol group. Seven trials stated that they were unable to blind providers or women (Caritis 1982; Castren 1975; Forster 1987 (Part II); Forster 1987 (Part III); Lauersen 1977; Reynolds 1978; Sims 1978). In the remaining trials, blinding was not stated for these groups, however it is likely that blinding in a study of ethanol such as this would be difficult. No trial separately reported if the outcomes assessors were blinded, thus all were rated as unclear.
Attrition, reporting, and other sources of bias
Generally, the trials had low risk of bias for attrition and reporting and did not have other sources of bias. The Forster 1987 (Part II) trial initially had a placebo group but after noting "no change" in the first 10 participants, these women were then split into groups one and two for the remainder of the study. This may have led to other bias. The Zlatnik 1972 trial also was rated as a potentially high risk of other bias due to only stopping the trial "when the plot of data indicated one treatment superior to the other" and that while they stated the demographic characteristics were the same for the two groups, they did not show these data.
Thus, none of the trials would easily be categorized as low risk of bias. The Caritis 1982; Lauersen 1977; Watring 1976; Zlatnik 1972 trials appear to be at a lower risk of bias than the rest of the trials of lower quality. As these trials reported different outcomes in different comparisons and they are not clearly low risk of bias, no subgroup analyses based on trial quality was performed.
Effects of interventions
Ethanol versus placebo/control ‐ comparison 1 (two trials, 77 women)
Primary outcomes
The two trials included in this comparison (Watring 1976; Zlatnik 1972), did not report on any of the same outcomes. Both trials excluded women with ruptured membranes so the only subgroup of relevance is that of women with intact membranes for these analyses. There were no significant differences in any of the primary outcomes with data: birth < 48 hours after trial entry (one trial, 35 women; risk ratio (RR) 0.93, 95% confidence interval (CI) 0.43 to 2.00 (Analysis 1.1)); neonatal mortality (one trial, 35 women; RR 1.06, 95% CI 0.31 to 3.58 (Analysis 1.3)). Both of these results were from the Watring 1976 trial. Perinatal mortality was not reported in either trial. Serious maternal adverse events were also not reported.
1.1. Analysis.
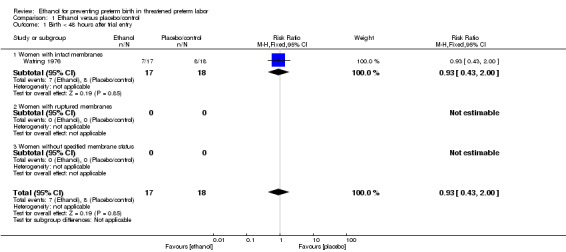
Comparison 1 Ethanol versus placebo/control, Outcome 1 Birth < 48 hours after trial entry.
1.3. Analysis.
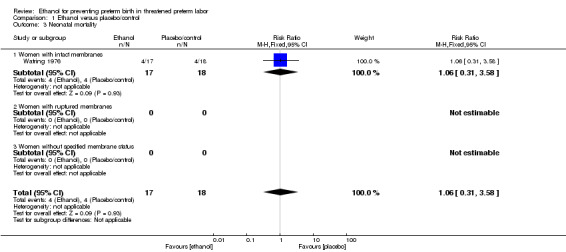
Comparison 1 Ethanol versus placebo/control, Outcome 3 Neonatal mortality.
Secondary outcomes
There were no differences in this comparison for any of the secondary outcomes that were reported, although all comparisons were based on only one of the trials: preterm birth < 37 weeks (RR 1.29, 95% CI 0.72 to 2.31 (Analysis 1.4)); preterm birth < 34 weeks (RR 1.69, 95% CI 0.69 to 4.16 (Analysis 1.5)); number of days delivery was delayed (mean difference (MD) ‐3.43 days, 95% CI ‐22.74 to 15.88 (Analysis 1.6)); respiratory distress syndrome (RR 0.85, 95% CI 0.27 to 2.64 (Analysis 1.9)), or birthweight < 2500 g (RR 1.41, 95% CI 0.81 to 2.46 (Analysis 1.10)), all from the Watring 1976 trial (one trial, 35 women).
1.4. Analysis.
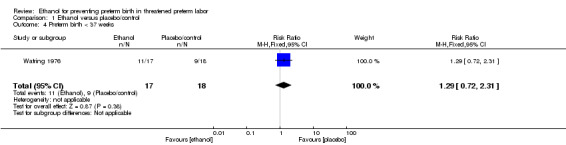
Comparison 1 Ethanol versus placebo/control, Outcome 4 Preterm birth < 37 weeks.
1.5. Analysis.
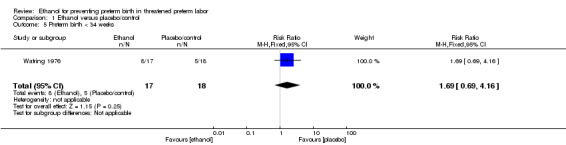
Comparison 1 Ethanol versus placebo/control, Outcome 5 Preterm birth < 34 weeks.
1.6. Analysis.
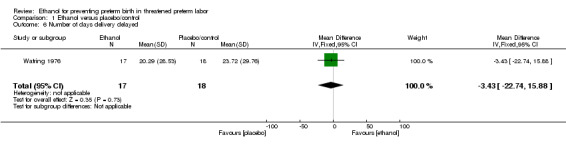
Comparison 1 Ethanol versus placebo/control, Outcome 6 Number of days delivery delayed.
1.9. Analysis.
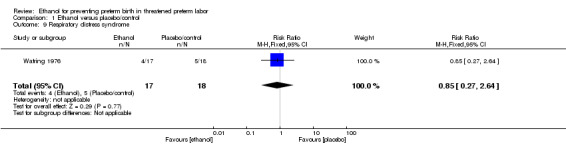
Comparison 1 Ethanol versus placebo/control, Outcome 9 Respiratory distress syndrome.
1.10. Analysis.
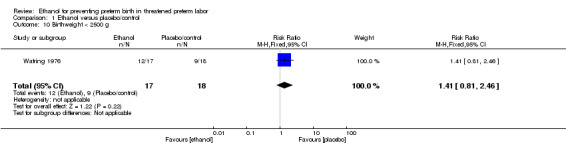
Comparison 1 Ethanol versus placebo/control, Outcome 10 Birthweight < 2500 g.
The risk of maternal adverse events requiring stopping or changing the drug was not estimable due to there being no events in either arm for the one trial (42 women) (Analysis 1.8) that reported this (Zlatnik 1972). Additionally, the Zlatnik 1972 trial did report delay until delivery but reported it as a median with no mention of the standard deviation (median 19 days in ethanol group versus "less than 1" day in the glucose/water group). No data were presented for the other prespecified secondary outcomes: maternal adverse events overall; serious infant outcome; fetal alcohol syndrome/fetal alcohol spectrum disorder; small‐for‐gestational age.
1.8. Analysis.
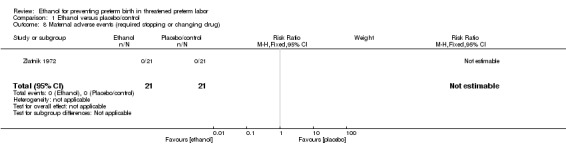
Comparison 1 Ethanol versus placebo/control, Outcome 8 Maternal adverse events (required stopping or changing drug).
Ethanol versus other tocolytic (betamimetics) ‐ comparison 2 (nine trials, 1438 women)
The Steer 1977 trial defined success as contractions stopping and not restarting for 24 hours. As it did not have any reported outcomes prespecified in this review, it did not contribute to the analyses. Thus, all comparisons below compare ethanol to a betamimetic drug.
Primary outcomes
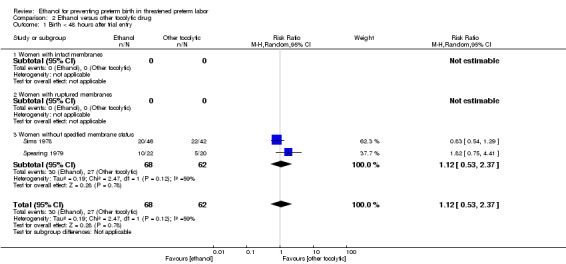
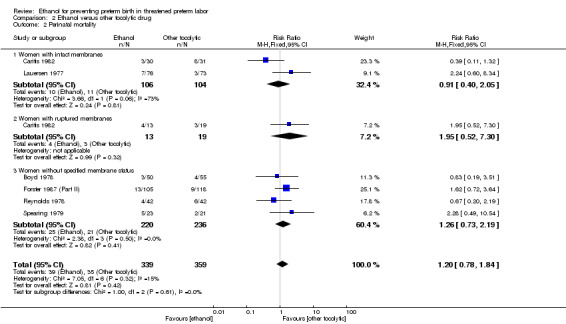
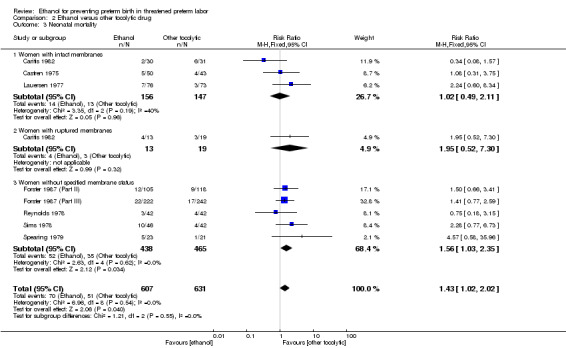
Compared to other tocolytic drugs (all involved a comparison of ethanol to betamimetics), ethanol treatment was associated with no clear difference in births < 48 hours after trial entry (three trials, 192 women; average RR 1.12, 95% CI 0.53 to 2.37, Tau² = 0.19; I² = 59% Analysis 2.1,) or perinatal mortality (six trials, 698 women; RR 1.20, 95% CI 0.78 to 1.84, Analysis 2.2), but higher rates of neonatal mortality (eight trials, 1238 women; RR 1.43, 95% CI 1.02 to 2.02, Analysis 2.3)). Serious maternal adverse events were not reported
2.1. Analysis.
Comparison 2 Ethanol versus other tocolytic drug, Outcome 1 Birth < 48 hours after trial entry.
2.2. Analysis.
Comparison 2 Ethanol versus other tocolytic drug, Outcome 2 Perinatal mortality.
2.3. Analysis.
Comparison 2 Ethanol versus other tocolytic drug, Outcome 3 Neonatal mortality.
Subgroup analysis
For the subgroup analysis comparing women with intact membranes versus those with ruptured membranes, only one trial presented some data stratified by ruptured or intact membranes (Caritis 1982). Two other trials excluded women with ruptured membranes (Castren 1975; Lauersen 1977) and thus are included in the subgroup of studies limited to women with intact membranes. The other trials either did not specify membrane status in their inclusion/exclusion criteria or in their reporting. These are included in the "women without specified membrane status" subgroup. We carried out subgroup analysis by membrane status, comparing women with intact membranes, those with ruptured membranes and those without specified membrane status. We were able to carry out these subgroup analyses for perinatal mortality and neonatal mortality but these analyses did not change the results ‐ there were no significant subgroup interactions: perinatal mortality (test for subgroup differences: Chi² = 1.00, df = 2 (P = 0.61), I² = 0%); neonatal mortality (test for subgroup differences: Chi² = 1.21, df = 2 (P = 0.55), I² = 0%).
Sensitivity analysis
Excluding quasi‐randomized trials did not significantly change the results of the primary outcome analyses with the exception of making the results for neonatal mortality no longer statistically significant (3 trials, 330 women; RR 1.49, 95% CI 0.82 to 2.72).
Secondary outcomes
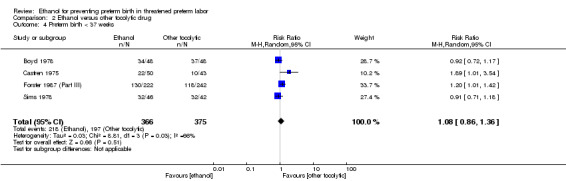
There was no clear difference in the rate of preterm birth < 37 weeks between the group of women who were given ethanol, compared to those women who received betamimetics (four trials, 741 women; average RR 1.08, 95% CI 0.86 to 1.36, random‐effects analysis, Tau² = 0.03; I² = 66% (Analysis 2.4)). In contrast, ethanol was associated with higher rates of preterm birth < 34 weeks (two trials, 599 women; RR 1.56, 95% CI 1.11 to 2.19 (Analysis 2.5)). There was no clear difference between the ethanol group and the betamimetic control in terms of the number of days delivery was delayed (five trials, 585 women; MD ‐2.51, 95% CI ‐11.11 to 6.09, random‐effects analysis, Tau² = 91.57; Heterogeneity: Tau² = 91.57; I² = 99% (Analysis 2.6)), or overall maternal adverse events (four trials, 350 women, average RR 0.99, 95% CI 0.46 to 2.11, random‐effects analysis, Tau² = 0.50; I² = 88% (Analysis 2.7)). Compared to betamimetics, however, ethanol was associated with a trend towards a lower rate of maternal adverse events requiring stopping or changing the drug (three trials, 214 women; RR 0.25, 95% CI 0.06 to 0.97, Analysis 2.8).
2.4. Analysis.
Comparison 2 Ethanol versus other tocolytic drug, Outcome 4 Preterm birth < 37 weeks.
2.5. Analysis.
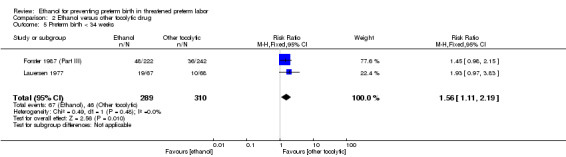
Comparison 2 Ethanol versus other tocolytic drug, Outcome 5 Preterm birth < 34 weeks.
2.7. Analysis.
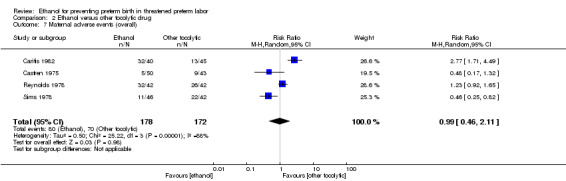
Comparison 2 Ethanol versus other tocolytic drug, Outcome 7 Maternal adverse events (overall).
2.8. Analysis.
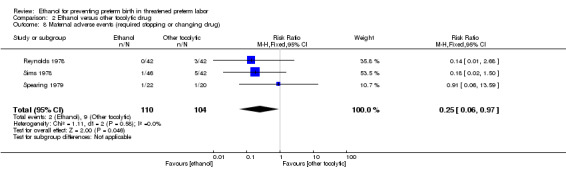
Comparison 2 Ethanol versus other tocolytic drug, Outcome 8 Maternal adverse events (required stopping or changing drug).
Ethanol use was associated with higher rates of neonatal respiratory distress syndrome (three trials, 823 women; RR 1.76, 95% CI 1.33 to 2.33 (Analysis 2.9)), and higher rates for babies with a birthweight of less than < 2500 g (five trials, 834 women; RR 1.30, 95% CI 1.09 to 1.54 (Analysis 2.10)).
2.9. Analysis.
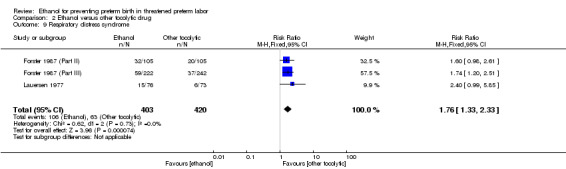
Comparison 2 Ethanol versus other tocolytic drug, Outcome 9 Respiratory distress syndrome.
2.10. Analysis.
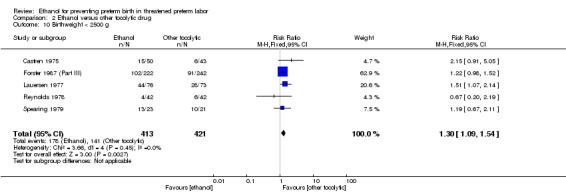
Comparison 2 Ethanol versus other tocolytic drug, Outcome 10 Birthweight < 2500 g.
These outcomes are likely all related to the lower incidence of preterm birth seen with other tocolytics, which for all these comparisons were betamimetics. The results for neonatal mortality must be viewed with additional caution as some trials did not report both perinatal mortality and neonatal mortality. Due to high measures of heterogeneity, random‐effects models were used for the outcomes of preterm birth < 37 weeks, number of days delivery delayed, and overall maternal adverse events (Analysis 2.4; Analysis 2.6; Analysis 2.7).
Discussion
Summary of main results
While early clinical findings supported the use of ethanol as a tocolytic (Fuchs 1965), concerns about its effectiveness and for fetal safety lead to it falling out of favor (ACOG 2011). Consequently, the trials available for this review are all fairly dated with the most recent one being published in 1987. There was much inconsistency in the data reported in these studies. In addition, all of these trials were completed before the routine use of antenatal corticosteroids, which may be responsible for some of the higher rates of neonatal mortality and respiratory distress syndrome seen in these trials.
Twelve studies involving 1586 women were available for review. In two of these studies with 77 women, ethanol was compared with placebo/control. The majority, nine studies with 1438 women, compared ethanol with another tocolytic (in this instance, all betamimetic agents). Spearing 1979 had a third group that received ethanol plus indomethacin and they excluded this arm. Steer 1977 compared ethanol and magnesium but contributed no data to the review.
Compared to placebo, ethanol was no different for birth < 48 hours after trial entry, perinatal mortality, neonatal mortality, or any of the secondary outcomes of preterm birth < 37 or < 34 weeks' gestation, adverse events, or neonatal outcomes.
Compared to betamimetic drugs, ethanol had a higher risk of neonatal mortality (although this difference was not apparent when our analysis excluded quasi‐RCTs). Compared to betamimetics, ethanol was not different in regards to birth < 48 hours or perinatal mortality, preterm birth < 37 weeks, or number of days delivery was delayed. However, ethanol was associated with a higher rate of preterm birth < 34 weeks and respiratory distress syndrome and low birthweight babies. Compared to betamimetics though, ethanol was associated with a trend towards a lower rate of maternal side effects requiring stopping the drug.
From the available data, there is no evidence of ethanol being superior to placebo and it appears to be inferior to betamimetics in many aspects. Subgroup analysis (for our comparison of ethanol versus betamimetic) did not substantially change these findings, although the number of trials for subgroup analyses was limited. The only advantage shown for ethanol was a trend suggesting that it may be better tolerated than betamimetic therapy, although this is based on few trials with small samples sizes so this result should be interpreted with caution.
Overall completeness and applicability of evidence
There were no data available to investigate any possible association between ethanol as a tocolytic and fetal alcohol syndrome. Alcohol use in pregnancy is the leading cause of preventable mental retardation (ACOG 2011). None of the studies identified using ethanol as a tocolytic contained long‐term neurodevelopmental follow‐up of the offspring. This does open a potential avenue for future research. Long‐term follow‐up of the participants in these studies could provide information about any possible cognitive effect that ethanol may have had on the exposed fetuses during labor.
The evidence obtained is relatively old and is complete as ethanol use is essentially obsolete currently due to fetal alcohol syndrome concerns. Thus, it is unlikely any new randomized controlled trials on the topic area will be forthcoming. As such, the evidence's applicability is limited because the therapy is no longer in clinical use.
Quality of the evidence
The quality of the evidence mostly has a high risk of bias, with very few studies that are of at least moderate quality. No truly low 'Risk of bias' studies were identified. Much of the assessments were based on uncertainty because trials in that era did not clearly state all methods for the study as they were before CONSORT.
Potential biases in the review process
As this is a historical therapy no longer used, it is possible that reports were graded more harshly. The two Forster studies Forster 1987 (Part II); Forster 1987 (Part III) were in German and it is possible that the translations missed some information. These reasons are both felt to be unlikely.
Agreements and disagreements with other studies or reviews
We found no difference in outcomes of ethanol compared to placebo, but when compared mainly to betamimetics, that betamimetics were superior in delaying delivery and reducing preterm birth. This corroborates the Cochrane review of betamimetics that found they were superior to placebo/control at delaying delivery (Neilson 2014). This review found that, compared to betamimetics though, ethanol was associated with a trend towards a lower rate of maternal side effects requiring stopping the drug. This is consistent with observations in other studies which report that, compared to placebo, betamimetics are associated with higher levels of side effects resulting in the need for a change in medication (Haas 2012).
Authors' conclusions
Implications for practice.
Historically, ethanol was used as a common tocolytic agent but is no longer used in practice due to safety concerns for both the mother and her baby. There is no evidence that ethanol is superior to placebo or to betamimetic drugs.
Implications for research.
While there is no current need for research regarding ethanol as a tocolytic, long‐term follow‐up studies on offspring from older trials to assess the risk of long‐term neurodevelopmental status would be useful.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Ethanol versus placebo/control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth < 48 hours after trial entry | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.43, 2.00] |
| 1.1 Women with intact membranes | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.43, 2.00] |
| 1.2 Women with ruptured membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Women without specified membrane status | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Perinatal mortality | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Women with intact membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Women with ruptured membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Women without specified membrane status | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Neonatal mortality | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.31, 3.58] |
| 3.1 Women with intact membranes | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.31, 3.58] |
| 3.2 Women with ruptured membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Women without specified membrane status | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Preterm birth < 37 weeks | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.72, 2.31] |
| 5 Preterm birth < 34 weeks | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.69, 4.16] |
| 6 Number of days delivery delayed | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐3.43 [‐22.74, 15.88] |
| 7 Maternal adverse events (overall) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Maternal adverse events (required stopping or changing drug) | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Respiratory distress syndrome | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.27, 2.64] |
| 10 Birthweight < 2500 g | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.81, 2.46] |
Comparison 2. Ethanol versus other tocolytic drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth < 48 hours after trial entry | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.53, 2.37] |
| 1.1 Women with intact membranes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Women with ruptured membranes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Women without specified membrane status | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.53, 2.37] |
| 2 Perinatal mortality | 6 | 698 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.78, 1.84] |
| 2.1 Women with intact membranes | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.40, 2.05] |
| 2.2 Women with ruptured membranes | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.52, 7.30] |
| 2.3 Women without specified membrane status | 4 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.73, 2.19] |
| 3 Neonatal mortality | 8 | 1238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.02, 2.02] |
| 3.1 Women with intact membranes | 3 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.49, 2.11] |
| 3.2 Women with ruptured membranes | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.52, 7.30] |
| 3.3 Women without specified membrane status | 5 | 903 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.03, 2.35] |
| 4 Preterm birth < 37 weeks | 4 | 741 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.86, 1.36] |
| 5 Preterm birth < 34 weeks | 2 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.11, 2.19] |
| 6 Number of days delivery delayed | 5 | 585 | Mean Difference (IV, Random, 95% CI) | ‐2.51 [‐11.11, 6.09] |
| 7 Maternal adverse events (overall) | 4 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.46, 2.11] |
| 8 Maternal adverse events (required stopping or changing drug) | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 0.97] |
| 9 Respiratory distress syndrome | 3 | 823 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.33, 2.33] |
| 10 Birthweight < 2500 g | 5 | 834 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.09, 1.54] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boyd 1978.
| Methods | Design: this study randomized and analyzed 96 women in preterm labor. The methods of allocation/randomization are unknown. Sample size: 96 (not calculated before trial). Setting: the study was conducted at the Institute of Obstetrics & Gynaecology, Queen Charlotte's Hospital in London. |
|
| Participants | Patients in spontaneous preterm labor having 6 or more uterine contractions per hour between the 27th and 35th week of pregnancy were included in the study. Patients with cervical dilation of more than 4 cm or uterine bleeding were excluded. A total of 96 women were entered into the trial. | |
| Interventions | IV salbutamol: an initial dose of 5 ug/minute being increased to 50 ug/minute until contractions ceased or side effects precluded further increments. IV ethanol: an initial dose of 30 mL/hour for 2 hours followed by 3 mL/hour. Each regimen was continued for 12 hours and repeated up to 5 times if labor had not ceased. No oral therapy was used. |
|
| Outcomes | Delivery prevented before the 37th week of gestation. Delivery within 24 hours of admission to the study. |
|
| Notes | Only have the abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "allocated randomly", but it is unclear how. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Not stated. |
Caritis 1982.
| Methods | Design: this was a randomized controlled trial. Sample size: 92 (not prespecified). Setting: Magee‐Women's Hostpital in Pittsburgh. |
|
| Participants | Women were included in the study if the following conditions were met. (1) Regular painful uterine contractions were documented by a tocodynamometer and occurred at least every 5 to 7 minutes, lasted at least 30 seconds, and occurred for a minimum of 1 hour. (2) The gestational age was between 20 and 36 weeks in women with intact membranes and between 24 and 34 weeks in women with ruptured membranes. (3) There were no obstetric or medical contraindications to the inhibition of labor or to the use of the labor‐inhibiting drug. Women were excluded if they had received another labor‐inhibiting drug for the current episode of preterm labor or if cervical dilation was greater than 5 cm. Patients with rupture of membranes were analyzed as a subgroup. This study randomized 92 women, but only analyzed 88 women (4 women were withdrawn because they did not fulfil the inclusion criteria). |
|
| Interventions | IV ethanol: a loading dose of 7.5 mL/kg/hour of 10% ethanol in 5% dextrose was infused for 2 hours. This was followed by a maintenance infusion of ethanol at a rate of 1.5 mL/kg/hour for 10 hours. If labor recurred, a second or third course of ethanol was given. For repeated courses, the reloading dose was reduced by 10% for each hour less than 10 elapsing from the end of the previous maintenance infusion. IV terbutaline: terbutaline was diluted with physiologic saline solution to a concentration of 20 ug/mL. Physiologic saline solution (400 mL) was infused over 20 minutes before the administration of terbutaline. The rate of infusion of terbutaline was started at 5 ug/minute and was increased by 5 ug/minute every 20 minutes to a maximal dosage of 30 ug/minute or until uterine contractions were abolished or maternal side effects occurred. Once contractions were abolished, the infusion was maintained at that rate for 1 hour, then, the rate of infusion was reduced to the minimal dose (5‐10 ug/minute) required to inhibit labor and was maintained at that rate for an additional 8 hours. If labor recurred during the 8‐hour maintenance infusion, the rate was increased until labor again subsided, and the entire maintenance infusion was again initiated. All women with intact membranes in whom labor was inhibited received 5 mg of terbutaline orally 30 minutes before the end of the IV maintenance infusion. This dosage was repeated 4 times daily for 5 days. No oral medication was given to women with rupture of membranes. |
|
| Outcomes | Completely successful: pregnancy maintained beyond 36 weeks. Partially successful: prevented delivery or progression of cervical dilation during IV therapy, at least until a mature L/S ratio was obtained or until a full course of betamethasone could be given to the mother to enhance fetal lung maturation. Failed: side effects or drug intolerance led to discontinuation of an assigned drug treatment, and the patient continued to experience labor. |
|
| Notes | Bethamethasone (12 mg) was given IM 2 times, 24 hours apart to all women. A course was considered to be complete 36 hours after the first injection. If treatment failed, a second labor‐inhibiting drug, magnesium sulfate, was started. Women with rupture of membranes: Objective was to maintain pregnancy for 36 hours. Thus, no further labor‐inhibiting therapy was given after 36 hours, but induction of labor was not encouraged until labor recurred or signs of chorioamnionitis or fetal distress were detected. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Neither physician nor patient was aware of the treatment modality until informed consent had been signed. Then a sealed envelope containing 1 of 2 randomly assigned treatment protocols was opened and the treatment was identified. Thus, no one aware until after randomization. However, unclear if the envelope was opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Knew after enveloped was opened. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Low risk | 4 of the 92 women were excluded in the review because they did not meet the inclusion criteria. |
| Other bias | Low risk | None. |
Castren 1975.
| Methods | Design: the study was a "quasi" randomized controlled trial. Sample Size:194 (not prespecified). Setting: the trials were performed at the University Central Hospital of Helsinki (nylidrin versus ethanol) and the Midwifery Institute Hospital (isoxuprine versus placebo). |
|
| Participants | Partcipants in this study were pregnant women between 24 and 36 weeks' gestation in premature labor. Inclusion criteria were intact membranes and active uterine contractions. | |
| Interventions | Treatment with the randomized protocol was started on admission. Treatment was as follows. University Central Hospital of Helsinki: Nylidrin (50 mg IV over 6 hours, then approximately 65 mg IM over 57 hours, then 6 mg PO every 5 hours) versus ethanol (128 mL IV over 6 hours, then 40 mL of cognac every 6 hours, which was prescribed to continue at home upon discharge). Midwifery Institute Hospital: isoxuprine (25 mg IV over 1 hour, then 160 mg IM over 60 hours, then 10 mg PO every 4 hours continued after discharge) versus placebo (followed the protocol as described for nylidrin). |
|
| Outcomes | The reported outcome as "successful" treatment defined as the premature contractions were arrested and pregnancy prolonged such that the birthweight was >/= 2500 g, or that the pregnancy was prolonged by 7 days and the pregnancy continued until at least the 37th week of gestational age. Thus this trial contained the outcome of preterm birth < 37 weeks only. | |
| Notes | We analyzed the women from the University Central Hospital of Helsinki since these women were the group randomized to nylidrin or ethyl alcohol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternating based on admission. |
| Allocation concealment (selection bias) | High risk | Alternating allocation based on admission. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Based on protocol, it was not possible to blind participants or providers, especially with women who became intoxicated with ethanol. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Method of reporting makes it unclear if all outcomes are reported for the comparisons. No prespecified outcomes stated in Methods. |
| Other bias | Unclear risk | It was reported that the study obtained their pharmaceuticals directly from the pharmaceutical companies. It was unclear whether these were paid for or not. |
Forster 1987 (Part II).
| Methods | Design: this was a parallel randomized study. Sample size: 223 (not prespecified). Setting: Germany. |
|
| Participants | Women were included in this study if they were pregnant and experiencing premature labor. The exclusion criteria were not specified. | |
| Interventions | This was a parallel study with 3 groups. Group 1: fenoterol (1‐3 ug/minute). Group 2: ethanol 50 in a fructose solution (20 gtt/minute). Group 3: placebo (glucose 50), however the first 10 women showed no change with placebo and were therefore divided into Groups 1 and 2 for the rest of the study. |
|
| Outcomes | Measured outcomes included birthweight, length of gestation extended, mortality rate, morbidity rate in relation to RDS. | |
| Notes | Review was originally written in German. Information here is found on the Cochrane Pregnancy and Childbirth Group Translation Form and original report tables. 1 patient's pregnancy ended in abortion, but it appears that they were included in the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were split into groups by last name, not randomized. |
| Allocation concealment (selection bias) | High risk | Last name would be known on admission. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants and personnel during treatment if women were intoxicated with ethanol. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no attrition bias noted in the translation document. |
| Selective reporting (reporting bias) | Unclear risk | Not stated in translation form. |
| Other bias | High risk | There were originally 3 groups but the study designers divided the placebo group into the 2 study groups when there was no change from placebo in the study. |
Forster 1987 (Part III).
| Methods | Design: this was a parallel randomized study. Sample size: 464 (not prespecified). Setting: Germany. |
|
| Participants | Women were included in this study if they were pregnant and experiencing premature labor. The exclusion criteria was not specified. All women were approximately 32 weeks' gestation. | |
| Interventions | There were 4 treatment groups. Group 1: long‐term ethanol. Group 2: long‐term partusisten. Group 3: short‐term ethanol. Group 4: short‐term partusisten. Due to a limited translation form, we combined the data for the long term and short term for both treatments. |
|
| Outcomes | What determined a success was based on the length of the gestation period; the weight, Apgar score, RDS mortality, maturation level, neonatal morbidity; prolongation index and success score. | |
| Notes | Review was originally written in German. Information here is found on the Cochrane Pregnancy and Childbirth Group Translation Form. 2 women' participation ended in abortion, but it appears that they were included in the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were split into groups by last name, not randomized. |
| Allocation concealment (selection bias) | High risk | Last name would be known on admission. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants and personnel during treatment if women were intoxicated with ethanol. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated in translation form. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to be no attrition bias noted in the translation document. |
| Selective reporting (reporting bias) | Unclear risk | Not stated in translation form. |
| Other bias | Unclear risk | Not stated in translation form. |
Lauersen 1977.
| Methods | Design: randomized controlled trial. Sample size: 135 women with threatened premature labor from 3 collaborating centers (not prespecified). Setting: 3 collaborating centers‐ New York, NY; Cleveland, OH, and East Meadows, NY‐ all in United States. |
|
| Participants | Patient included in this study fulfilled the following criteria: (1) regular uterine contractions of 30‐60 seconds' duration at least once every 10 minutes and clinically judged as premature labor; (2) pregnancy between 20 and 36 weeks; (3) estimated fetal weight below 2500 g; (4) uterine fundus above the umbilicus; (5) membranes intact and not bulging; (6) cervical effacement; and (7) cervical dilation not exceeding 4 cm. Exclusion criteria were as follows: (1) premature separation of the placenta, (2) pre‐eclampsia, chronic renal disease, chronic hypertension, cardiac disease, diabetes mellitus, and other severe maternal diseases, (3) presence of a dead or malformed fetus, (4) placenta previa if bleeding was severe enough to require intervention, and (5) any maternal or fetal complication that required immediate delivery. |
|
| Interventions | Patients were randomized to either the ritodrine group (68 women) or ethanol group (67 women). Ritodrine group: IV infusion of 50 ug/minute of ritodrine, which was increased by 50 ug every 10 minutes until adequate uterine relaxation occurred, with a maximum infusion rate of 350 ug/minute. The optimal dose for each patient was maintained for 12 hours. 30 minutes prior to termination of infusion, oral ritodrine was started and women were discharged on ritodrine orally in doses ranging from 20 to 60 mg daily maintained for 4 weeks or until 38 weeks' gestation, whichever came first. Ethanol group: a loading dose infusion of 7.5 mL/kg/hour ethanol was given for 2 hours, followed by maintenance dose of 1.5 mL/kg/hour for 10 hours. If premature labor recurred after the ethanol infusion, up to 2 additional courses following the same protocol were permitted. |
|
| Outcomes | Measured outcomes included (1) delivery postponed for more than 72 hours, (2) time gained, (3) gestational age at time of delivery, (4) perinatal mortality, and (5) infant morbidity in relation to RDS. | |
| Notes | 150 randomized initially, later 15 found to not meet inclusion criteria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly drawn sealed envelopes were used for randomization. |
| Allocation concealment (selection bias) | Unclear risk | Likely low risk as stated sealed envelopes. However, unclear if the envelope was opaque thus rated as Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants and personnel during treatment if women were intoxicated with ethanol. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Low risk | No reporting bias. |
| Other bias | Low risk | None identified. |
Reynolds 1978.
| Methods | Design: randomized controlled trial. Sample size: 84 women (not prespecified). Setting: not specified but author from Hammersmith Hospital, London. |
|
| Participants | In this study, 84 women in premature labor were selected for the trial. Inclusion criteria were gestational age between 20 and 37 weeks and contractions occurring at least every 10 minutes. Exclusions from participation included major maternal complications, the cervix dilated more than 5 cm, amnionitis, or significant hemorrhage. | |
| Interventions | Group 1: salbutamol infusion until titrated to 40 ug/minute, uterine contractions ceased, or patient became tachycardic. 200 mg sodium phenobarbitone also given to women in this group. Group 2: ethanol 10‐hour treatment according to the Fuchs 1967 protocol. |
|
| Outcomes | The criterion for success was an inhibition of labor for 24 hours. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were alternated to the 2 groups. |
| Allocation concealment (selection bias) | High risk | Alternate allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind participants and personnel during treatment if women were intoxicated with ethanol. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | No prespecified outcomes other than inhibition of labor for 24 hours. |
| Other bias | Low risk | None identified. |
Sims 1978.
| Methods | Design: randomized trial. Sample size: 100 women (not prespecified). Only 88 of the 100 were analyzed. 12 women were withdrawn (8 sets of twins, 2 < 27 weeks, 1 placenta previa, 1 lost records). Setting: Institute of Obstetrics & Gynaecology, Queen Charlotte's Hospital in London. |
|
| Participants | All women admitted in labor between 27 and 35 weeks' gestation were invited to join the study whether the membranes were intact or ruptured. The diagnosis of labor was made on a history of 6 or more contractions per hour. Patients were not considered in cases of multiple pregnancies, if postponement of delivery was contraindicated, or if cervix was more than 4 cm dilated. |
|
| Interventions | IV ethanol: a solution of 10% v/v ethanol in 5% dextrose in water was prepared. The solution was administered intravenously at the rate of 15 mL/kg of body weight over the first 2‐hour period. A maintenance infusion of 1.5 mL/kg of body weight per hour was administered for a further 10 hours. If contractions reappeared after discontinuation, the treatment was repeated. IV salbutamol: a solution of 4 mg salbutamol in 500 mL of 5% dextrose in water was prepared. The solution was administered intravenously at 5 ug of salbutamol per minute. The infusion rate was increased by 5 ug of salbutamol per minute every 10 minutes until either (a) contractions were abolished, (b) a maximum rate of 50 ug per minute was attained, or (c) until unacceptable side effects occurred. The infusion rate was maintained at the lowest rate to stop contractions for a total of 12 hours (24 hours for ruptured membranes). If contractions reappeared the treatment was repeated. Betamethasone therapy: each patient received either 4 mg of betamethasone IM or saline placebo IM according to a randomized scheme. These injections were repeated 8 hourly for 6 doses. |
|
| Outcomes | Delay delivery for at least 24 hours. Delay delivery for at least 48 hours. Delivery after 37 weeks. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States there was a "randomized list", but there is not enough detail. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Low risk | No reporting bias. |
| Other bias | Low risk | None. |
Spearing 1979.
| Methods | Design: randomized trial. Sample size: 66 women (not prespecified). Only 62 of the 66 were analyzed. 4 women were withdrawn due to drug administration protocol violations. Setting: University of Bristol, UK. |
|
| Participants | Patients were admitted to the trial if they were in labor (regular painful contractions occurring at least once every 10 minutes) between 26 and 35 completed weeks of gestation inclusive, and in whom arrest of labor was considered advisable. Those women with multiple pregnancy, threatened premature labor with show, and suspected rupture of membranes were included. Patients with antepartum hemorrhage, known fetal abnormality, and gross hydramnios were considered reasons for exclusion. |
|
| Interventions | Treatment was selected in sequence in the labor room. IV ethanol: ethanol was prepared as a 10% solution in 5% dextrose. A loading dose of 15 mL/kg of body weight was infused over 2 hours followed by a maintenance dose of 1.5 mL/kg of body weight. IV ethanol + indomethacin: ethanol was given as previously stated. 100 mg of indomethacin was given as a rectal suppository, and a similar dose was given after 12 hours. After 24 hours, this was continued orally with 25 mg every 6 hours for 48 hours after cessation of contractions. IV salbutamol: 5 mL of salbutamol to 500 mL of 5% dextrose was prepared, giving a concentration of 10 ug/mL. Infusion began at 20 drops/minute increased by increments of 10 drops/minute every 10 minutes until contractions ceased. The maximum infusion rate was 80 drops/minute or less if the maternal pulse rate exceeded 140 bpm or other maternal distress occurred (palpitations or hypotension). With cessation of contractions the infusion was maintained at the successful level for 6 hours and then gradually decreased. Treatment was continued with oral salbutamol 4 mg four times a day and continued for at least 48 hours. 2 infusions of any treatment were allowed. |
|
| Outcomes | Success: uterine activity ceased and did not return until 36 weeks or later, or returned only after 48 hours free of labor. | |
| Notes | In the cases of failure, treatment was changed. Only the data from the IV ethanol and IV salbutamol were included in our analysis because it was unclear whether the effects of the ethanol + indomethacin were due to the Indomethacin or the combination of the 2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Treatment was selected in sequence in the labor room, and the patient's name was added to a list which indicated the treatment to be given." |
| Allocation concealment (selection bias) | High risk | "Selected in sequence." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Low risk | No reporting bias. |
| Other bias | Low risk | None. |
Steer 1977.
| Methods | Design: randomized controlled trial. Sample size: 71 (not prespecified). Setting: Sloane Hospital for Women of the Columbia‐Presbyterian Medical Center in New York. |
|
| Participants | Patients in the study included those who met all of the following criteria: (1) there was a painful, identifiable contraction pattern with a frequency of 5 minutes or less, (2) the duration of the pregnancy was less than 37 weeks, (3) the estimated fetal weight was less than 2500 g, (4) the amnion was intact, and (5) cervical dilation was less than 5 cm. Patients with a fever due to amnionitis or those with bleeding greater than "show" were excluded from the study. |
|
| Interventions | IV ethanol: a solution of 9.5% v/v ethanol in 5% dextrose in water was administered intravenously at the rate of 15 mL/kg of body weight over the first 2 hours. A maintenance infusion rate of 1.5 mL/kg of body weight per hour was administered until signs of labor had subsided or labor had progressed to an irreversible stage. If contractions reappeared after discontinuation, the procedure was repeated. IV magnesium sulfate: a 2% maintenance solution was prepared by adding 200 mL of 10% magnesium sulfate to 800 mL of 5% dextrose in water. 4 g of magnesium sulfate was infused intravenously, slowly enough to avoid flushing and vomiting. A constant infusion of the 2% maintenance solution was then given at 100 mL/hour. This continued until labor had subsided or labor had progressed to an irreversible stage. The rate was reduced if magnesium toxicity was observed. If contractions reappeared, the procedure was repeated. IV dextrose in water: a solution of 5% dextrose in water was infused intravenously at 100 mL/hour. |
|
| Outcomes | Success: contractions stopped and a period of at least 24 hours went by before contractions occurred again. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patient received treatment according to the last digit of their hospital number (not truly random). A small group of women were chosen at random to receive the dextrose in water for a control group. |
| Allocation concealment (selection bias) | High risk | Last digit of medical record number. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Low risk | No reporting bias. |
| Other bias | Low risk | None. |
Watring 1976.
| Methods | Design: single‐blind randomized trial. Sample size: 35 women (not prespecified). Setting: Tripler Army Medical Center in San Francisco, CA in the United States. |
|
| Participants | Criteria for inclusion in this study were as follows. (1) a living, single gestation between 24 and 36 weeks. (2) a history of painful uterine contractions less than 10 minutes apart for 1 hour or more. (3) regular uterine contractions as determined by palpation and tokodynamometer. (4) greater than 50% effacement of the cervix with or without dilation. (5) absence of complications such as bleeding, premature rupture of membranes, and fever. (6) no history of incompetent cervical os. | |
| Interventions | Study group: received an IV infusion of 5% ethanol in 5% dextrose in water. A loading dose of 15 cc/lb/2 hour and a maintenance dose of 10% of the total loading dose per hour until the contractions had ceased for at least 6 hours. If contractions were still present after 4 hours or if they recurred within 24 hours, one‐half of the loading dose was given in 1 hour and the maintenance dose continued for a total of 12 hours. After contractions ceased, women were kept on bed rest for 24 hours. Then, women were on a 24 ambulation period. If no contractions occurred, patient was discharged. Control group: usually treated with either morphine sulfate or secobarbital and ambulated on the same schedule. |
|
| Outcomes | Success: patient remained without contractions for 72 hours following treatment. | |
| Notes | If contractions recurred for a third time, no attempt was made to stop labor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Groups of 10 cards, 5 treatment and 5 control, were placed in an envelope from which they were drawn by a disinterested, third party. When all cards had been drawn, another group of 10 cards was placed in the envelope and the process repeated. |
| Allocation concealment (selection bias) | Unclear risk | Drawn from envelope. However, unclear if the envelope was opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Single blind" but unclear which group was actually blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Low risk | No reporting bias. |
| Other bias | Low risk | None. |
Zlatnik 1972.
| Methods | Design: randomized controlled trial. Sample size: 42 women (not prespecified). Setting: New York Hospital‐Cornell Medical Center in the United States. |
|
| Participants | Patients admitted to the hospital with threatened premature labor were included in the study if stopping labor was deemed advisable. Patients were excluded from the study if delivery seemed imminent, the membranes were ruptured, or the patient was bleeding actively or had some other factor which contraindicated the continuation of pregnancy. |
|
| Interventions | IV ethanol: 9.5% ethanol solution. IV glucose: 5% glucose in water. Patients received 15 mL/kg of the assigned solution intravenously over a 2 hour period as a loading dose and then 1.5 mL/kg per hour as a maintenance dose for an additional 6 to 10 hours. If labor actively progressed so that delivery seemed imminent, the ethanol was discontinued. If labor stopped, but later recurred, the assigned solution was again administered as outlined above. |
|
| Outcomes | Success: delivery did not occur within 72 hours. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "there was random assignment of the two treatments to the numbered envelopes". |
| Allocation concealment (selection bias) | Low risk | Used serially numbered, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias. |
| Selective reporting (reporting bias) | Low risk | No reporting bias. |
| Other bias | High risk | Stopped the study "when the plot of data indicated one treatment superior to the other". Also states that "there were no significant differences between the two groups in regard to age, marital status, race, parity, weeks of gestation, or cervical dilation at the start of treatment, but the data is not shown". |
bpm: beats per minute IM: intramuscular IV: intravenous RDS: respiratory distress syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Waltman 1969 | This study is not a randomized controlled trial. |
Differences between protocol and review
None.
Contributions of authors
David Haas (DMH) and Amanda Morgan (AM) originally conceived the protocol.
DMH, AM, Samantha Deans, and Frank Schubert subsequently developed the final protocol, abstracted and entered data, wrote and approved the final version of this review.
DMH is the guarantor for this review.
Declarations of interest
David M Haas: none known
Amanda Morgan: none known
Samantha Deans: none known
Frank Schubert: none known
New
References
References to studies included in this review
Boyd 1978 {published data only}
- Boyd IE, Chamberlain GV, Lewis PJ, Sims CD. Comparative trial of salbutamol and ethanol in preterm labour [abstract]. British Journal of Clinical Pharmacology 1978;5:360P‐1P. [DOI] [PubMed] [Google Scholar]
Caritis 1982 {published data only}
- Caritis SN, Carson D, Greebon D, McCormick M, Edelstone DI, Mueller‐Heubach E. A comparison of terbutaline and ethanol in the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1982;142:183‐90. [DOI] [PubMed] [Google Scholar]
Castren 1975 {published data only}
- Castren O, Gummerus M, Saarikoski S. Treatment of imminent premature labour. Acta Obstetricia et Gynecologica Scandinavica 1975;54:95‐100. [DOI] [PubMed] [Google Scholar]
Forster 1987 (Part II) {published data only}
- Forster F, During R. Comparison of the effectivity of Partusisten(Regtrademark) and ethanol tocolysis Part II: Short term tocolysis with Partusisten (Regtrademark) or ethanol. Zentralblatt fur Gynakologie 1987;109:843‐9. [PubMed] [Google Scholar]
Forster 1987 (Part III) {published data only}
- Forster F, During R. Comparison of the effectivity of Partusisten (Regtrademark) and ethanol tocolysis Part III: Comparison of long term and short term tocolysis, using Partusisten (Regtrademark) or ethanol. Zentralblatt fur Gynakologie 1987;109:1177‐84. [PubMed] [Google Scholar]
Lauersen 1977 {published data only}
- Fuchs F. Prevention of prematurity. American Journal of Obstetrics and Gynecology 1976;126:809‐20. [DOI] [PubMed] [Google Scholar]
- Lauersen NH, Merkatz IR, Tejani N, Wilson KH, Roberson A, Mann LI, et al. Inhibition of premature labor: A multicenter comparison of ritodrine and ethanol. American Journal of Obstetrics and Gynecology 1977;127:837‐45. [DOI] [PubMed] [Google Scholar]
Reynolds 1978 {published data only}
- Reynolds JW. A comparison of salbutamol and ethanol in the treatment of premature labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1978;18:107‐9. [Google Scholar]
Sims 1978 {published data only}
- Sims CD, Chamberlain GVP, Boyd IE, Lewis PJ. A comparison of salbutamol and ethanol in the treatment of preterm labour. British Journal of Obstetrics and Gynaecology 1978;85:761‐6. [DOI] [PubMed] [Google Scholar]
Spearing 1979 {published data only}
- Spearing G. Alcohol, indomethacin and salbutamol. A comparative trial of their use in preterm labour. Obstetrics & Gynecology 1979;53:171‐4. [PubMed] [Google Scholar]
Steer 1977 {published data only}
- Steer CM, Petrie RH. A comparison of magnesium sulfate and alcohol for the prevention of premature labor. American Journal of Obstetrics and Gynecology 1977;129:1‐4. [DOI] [PubMed] [Google Scholar]
Watring 1976 {published data only}
- Watring WG, Benson WL, Wiebe RA, Vaughn DL. Intravenous alcohol: a single blind study in the prevention of premature delivery: a preliminary report. Journal of Reproductive Medicine 1976;16:35‐8. [PubMed] [Google Scholar]
Zlatnik 1972 {published data only}
- Zlatnik FJ, Fuchs F. A controlled study of ethanol in threatened premature labour. American Journal of Obstetrics and Gynecology 1972;112:610‐2. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Waltman 1969 {published data only}
- Waltman R, Nigrin G, Bonura F, Pipat C. Ethanol in prevention of hyperbilirubinaemia in the newborn. A controlled trial. Lancet 1969;2:1265‐7. [DOI] [PubMed] [Google Scholar]
Additional references
ACOG 2011
- ACOG. American College of Obstetricians and Gynecologists. Committee on Health Care for Underserved Women. Committee opinion no. 496: At‐risk drinking and alcohol dependence: obstetric and gynecologic implications. Obstetrics and Gynecology 2011;118(2 Pt 1):383‐8. [DOI] [PubMed] [Google Scholar]
Bain 2013
- Bain E, Heatley E, Hsu K, Crowther CA. Relaxin for preventing preterm birth. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010073.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Belinkoff 1950
- Belinkoff S, Hall OW Jr. Alcohol during labor. American Journal of Obstetrics and Gynecology 1950;59:429‐32. [Google Scholar]
Conde‐Agudelo 2011
- Conde‐Agudelo A, Romero R, Kusanovic JP. Nifedipine in the management of preterm labor: a systematic review and meta‐analysis. American Journal of Obstetrics and Gynecology 2011;204(2):134.e1‐134.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 2014
- Crowther CA, Brown J, McKinlay CJD, Middleton P. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD001060.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duckitt 2014
- Duckitt K, Thornton S, O'Donovan OP, Dowswell T. Nitric oxide donors for treating preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD002860.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flenady 2014a
- Flenady V, Reinebrant HE, Liley HG, Tambimuttu EG, Papatsonis DNM. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD004452.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flenady 2014b
- Flenady V, Wojcieszek AM, Papatsonis DNM, Stock OM, Murray L, Jardine LA, et al. Calcium channel blockers for inhibiting preterm labour and birth. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD002255.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuchs 1965
- Fuchs F. Treatment of threatened premature labour with alcohol. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1011‐3. [Google Scholar]
Fuchs 1967
- Fuchs F, Fuchs AF, Poblete VF, Risk A. Effect of alcohol on threatened premature labor. American Journal of Obstetrics and Gynecology 1967;99:627‐37. [DOI] [PubMed] [Google Scholar]
Gladstone 2011
- Gladstone M, Neilson JP, White S, Kafulafula G, Broek N. Post‐neonatal mortality, morbidity, and developmental outcome after ultrasound‐dated preterm birth in rural Malawi: a community‐based cohort study. PLoS Medicine 2011;8:e1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haas 2012
- Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta‐analysis. BMJ 2012;344:e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howson 2012
- Howson CP, Kinney MV, Lawn JE. March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012. [Google Scholar]
Lawn 2010
- Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C, for the GAPPS Review Group. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy and Childbirth 2010;10(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neilson 2014
- Neilson J, West HM, Dowswell T. Betamimetics for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD004352.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinebrant 2015
- Reinebrant HE, Pileggi‐Castro C, Romero CLT, dos Santos RAN, Kumar S, Souza JP, Flenady V. Cyclo‐oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD001992.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2006
- Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Schaefer 2007
- Schaefer C. Drugs During Pregnancy and Lactation. 2nd Edition. Elsevier BV, 2007. [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stan 2013
- Stan C, Boulvain M, Pfister R, Hirsbrunner‐Almagbaly P. Hydration for treatment of preterm labour. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD003096.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Su 2014
- Su LL, Samuel M, Chong YS. Progestational agents for treating threatened or established preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD006770.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vogel 2014
- Vogel JP, Nardin J, Dowswell T, West HM, Oladapo OT. Combination of tocolytic agents for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD006169.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1977
- Anon. WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstetricia et Gynecologica Scandinavica 1977;56(3):247‐53. [PubMed] [Google Scholar]
Zeeman 1997
- Zeeman GG, Khan‐Dawood FS, Dawood MY. Oxytocin and its receptor in pregnancy and parturition: current concepts and clinical implications. Obstetrics and Gynecology 1997;89(5 Pt 2):873‐83. [DOI] [PubMed] [Google Scholar]


