Abstract
Simple Summary
Linseed has been utilized in ruminant nutrition to improve the production and quality of milk. In regard to the increasing concerns of consumers for the healthy aspects of foods as well as for animal welfare, in this research, we showed that linseed supplementation increased fat content but did not improve the milk concentration of odd- and branched-chain fatty acids (OBCFA), which are known to produce healthy effects in humans. Importantly, linseed did not show negative effects on animals’ health, suggesting it can be safely used in goats’ diet.
Abstract
The purpose of this study was to investigate the effects of linseed supplementation on milk yield and quality, serum biochemistry and, in particular, to evaluate its possible effects on the production of odd- and branched-chain fatty acids (OBCFA) in the milk of Cilentana grazing goats. Twelve pregnant Cilentana dairy goats were divided into two groups (CTR, control, and LIN, linseed supplementation group). After kidding, the goats had free access to the pasture and both groups received a supplement of 400 g/head of concentrate, but the one administered to the LIN group was characterized by the addition of linseed (in a ratio of 20% as fed) to the ingredients. During the trial, milk samples were taken from April to August in order to evaluate milk production, composition, and fatty acid profile. In addition, blood samples were taken for evaluating the effects of linseed supplementation on goats’ health status. The health status of the goats was not influenced by the linseed supplementation, as confirmed by blood analyses. Concerning the effects on milk, the supplementation positively affected (p < 0.001) milk production and fat percentage and the fatty acid profile was markedly influenced by the lipid supplementation. In particular, milk from the LIN group was characterized by significantly lower concentrations of saturated fatty acids (FA; p < 0.001) and higher proportions of monounsaturated FA, polyunsaturated FA, and conjugated linoleic acids (CLAs) than milk from the CTR group (p < 0.001). In contrast, the OBCFA were negatively influenced by the linseed supplementation (p < 0.0001). Further studies are needed to test the effects of different fat sources and other nutrients on the diets.
Keywords: OBCFA, linseed, fat supplementation, milk, blood profile
1. Introduction
As reported by the FAO [1], the total world production of goat milk in 2017 reached 18.7 million tons and its consumption is considerable in some Mediterranean countries. In recent years, the interest in goat milk has increased because of its functional and dietary properties and high nutritional value [2,3,4]. In addition to the widely known compounds that can produce beneficial effects on consumer health, such as polyunsaturated fatty acids (PUFA), Omega−3, and conjugated linoleic acids (CLAs), milk from ruminants contain some minor but important components of fat, such as the odd- and branched-chain fatty acids (OBCFA). Intestinal absorption of microbially produced OBCFA and their absorption by the mammary gland led to the appearance of OBCFA in the milk fat of lactating dairy cows. Different groups of rumen microbial species have unique OBCFA profiles [5]. Cellulolytic bacteria mainly synthesize iso fatty acids (FA), while amylolytic bacteria produce high levels of anteiso and odd-chain linear FA and relatively low levels of iso FA [6]. Given that lipid supplementation can influence the various steps that determine the FA profile of milk, few data are available about how the OBCFA profile of milk may be influenced by the presence of additional lipids in the diet. Therefore, the milk OBCFA profile could potentially be used as a noninvasive method to predict some aspects of ruminal function.
Several studies aimed to discuss the linkage between animal diet and rumen microbiota, and the related effects on milk quality. The content of OBCFA in goat milk fat depends on the characteristics of the diet, such as the forage-concentrate ratio [7], the integration of vegetable oil [8], its interaction with forage level [9], and the percentage of physically effective neutral detergent fiber (NDF) [10]. In Italy, dairy goat farming systems are characterized by a high variability: from intensive indoor systems, mostly of specialized breeds, to semiextensive and extensive outdoor systems, with local breeds, depending on the economic importance of the supply chain and environmental and race specificities [11,12].
The integration of fat in the diet of lactating dairy cows is an approach used to improve the energy of the ration. In early-lactating cows, this feeding practice limits the amount of negative energy balance needed to support milk production [13]. It is well-documented that the inclusion of supplemental lipids in the diet can alter the rumen environment, thus influencing microbial fermentation and volatile fatty acid (VFA) production [14]. One of the most interesting lipid supplements is linseed (Linum usitatissimum L.) since it contains high levels of alfa-linoleic acid (ALA; 50% to 60% of total fatty acids, FA) and a lower concentration of linoleic acid (LA) and saturated fatty acids (SFA) compared to soybean, sunflowers, cottonseed, and corn [15]. Despite linseed supplementation being shown to affect milk production and composition, no studies have focused on its effect on the production of OBCFA. For this reason, the purpose of this study was to investigate a possible increasing effect of linseed on the production of odd- and branched-chain fatty acids in milk. Additionally, since energy changes and lipid supplementation may affect metabolism, mainly liver function, a serum biochemistry profile including liver and kidney markers was performed to assess possible adverse effects of linseed supplementation in Cilentana grazing goats.
2. Materials and Methods
2.1. Animals, Diets, and Management
Twelve pregnant dairy goats (breed: Cilentana; calving: 3rd; body weight: 46.3 ± 1.2 kg) reared in a farm located in Salerno province (Italy), were equally divided into Control group (CTR) and linseed supplementation group (LIN), homogeneous for calving and milk production at the previous lactation (1260 ± 126 g/head/day). The experimental procedures were carried out at the Department of Veterinary Medicine and Animal Production (University of Napoli Federico II) and were approved by the local Bioethics Committee (protocol number: PG/2019/0070006). Goats were fed oat hay ad libitum, and concentrate in a ratio of 100, 200, and 300 g/head/day at 45, 30, and 15 days before kidding, respectively, using ultrasonography data to assess the state of pregnancy. After kidding (first week of March), every day, from 8:00 AM to 15.00 PM, the goats had free access to the pasture composed of 60% of Leguminosae and 40% of Graminae. In addition, both groups received 400 g/head/day of concentrate and the concentrate administered to the LIN group was characterized by the inclusion of linseed (20% a. f. within the ingredients). In order to keep the two diets isoproteic, a different amount and different ingredients were used for the control group (Table 1). During the experimental period, the goats from each group grazed in the daytime and were kept together in a barn after sunset to respect the habits of the farm and to avoid affecting animals’ feeding behavior. Each barn was equipped with individual self-closing feeders.
Table 1.
Chemical composition and fatty acid profile (mean ± SD) of feeds administered to goats.
| Chemical Composition g/kg DM |
CTR Concentrate | LIN Concentrate | Linseed | Oat Hay | Pasture |
|---|---|---|---|---|---|
| CP | 177.4 ± 4.75 | 178.6 ± 3.69 | 242.3 ± 5.83 | 88.4 ± 5.08 | 160 ± 20.04 |
| EE | 28.70 ± 2.66 | 67.40 ± 1.36 | 350.2 ± 7.45 | 19.4 ± 1.84 | 20.3 ± 1.40 |
| NDF | 262.5 ± 6.13 | 266.9 ± 8.51 | 259.3 ± 7.12 | 594.5 ± 12.35 | 465.9 ± 50.12 |
| ADF | 98.60 ± 6.90 | 101.4 ± 7.04 | 112.1 ± 3.41 | 312.7 ± 9.270 | 338.4 ± 15.25 |
| ADL | 26.70 ± 1.33 | 27.30 ± 1.51 | 54.10 ± 2.11 | 39.6 ± 2.98 | 50.0 ± 7.19 |
| PDIN g/kg DM | 116.0 ± 3.66 | 100.5 ± 2.90 | 168.3 ± 3.86 | 62.3 ± 4.52 | 112.2 ± 12.31 |
| PDIE g/kg DM | 100.8 ± 2.72 | 104.4 ± 3.11 | 133.6 ± 2.98 | 64.5 ± 3.47 | 76.4 ± 9.90 |
| Fatty acid profile % of total FA |
|||||
| SFA | 28.4 ± 1.13 | 26.6 ± 1.22 | 10.9 ± 0.31 | . | 17.7 ± 2.22 |
| MUFA | 15.8 ± 0.65 | 14.3 ± 0.25 | 22.2 ± 1.64 | . | 6.3 ± 0.74 |
| PUFA | 55.8 ± 2.61 | 59.12 ± 4.16 | 66.9 ± 2.51 | . | 76.0± 8.36 |
| C18:2 | 48.7 ± 2.34 | 37.9 ± 2.19 | 13.7 ± 0.78 | . | 26.4 ± 6.70 |
| C18:3 | 3.50 ± 0.12 | 18.3 ± 1.43 | 53.2 ± 4.82 | . | 41.9 ± 5.76 |
|
Concentrates ingredients
% as fed |
CTR | LIN | |||
| Soft wheat bran 26.6; Corn meal 15.0; Sunflower meal 14.5; Dried pulp beet 12.0; Fava bean 10.6; Corn gluten feed 7.0; Dried citrus pulp 6.5; Molasses 5.6; Vitamin-mineral premix 2.2 |
Soft wheat bran 30.0; Corn meal 23.0; Linseed 20.0; Dried citrus pulp 10.0; Dried pulp beet 8.0; Corn gluten feed 7.0; Vitamin–mineral premix 2.0. |
CTR: control group; LIN: linseed supplementation group; CP: crude protein; EE: ether extract; ADF: acid detergent fiber; ADL: acid detergent lignin; PDIN: protein digested in the small intestine when rumen-fermentable nitrogen is limiting; PDIE: protein digested in the small intestine when rumen-fermentable energy is limiting; MUFA: monounsaturated fatty acids; NDF: neutral detergent fiber; PUFA: polyunsaturated fatty acids; SFA: saturated fatty acids; UFL: unit feed for lactation.
Pasture samples were collected from four different areas (2.5 m2/area) and were cut at 3 cm from the ground. Then, the pasture samples were weighed and the four different samples from the different areas were dried using an air oven set at 65 °C, then were milled and stored until the analyses. According to Van Soest et al. [16] and AOAC [17], the chemical composition of feeds was analyzed, while the net energy was calculated as suggested by INRA [18]. Finally, according to Castro et al. [19], concentrates and pasture samples were analyzed using gas chromatography to determine the fatty acids profile (Table 1).
2.2. Milk Collection and Analysis
After kidding (first week of March), as in farm practice, milk was only suckled by kids, and by the end of April (final week), they completed weaning and goats were milked twice a day. Milk yield was recorded daily using a graduated cylinder after milking. Additionally, representative milk samples were collected monthly (for a total of 5 samples, from April to August) and stored at −20 °C until analysis. Then, the milk samples were analyzed for protein, fat, and lactose concentrations by the infrared method (Milkoscan 133B, Foss Matic, Hillerod, Denmark) standardized for goat milk. In addition, milk was extracted, methylated, and analyzed by liquid chromatography LCG as described by Tudisco et al. [20]. Briefly, milk fat was separated using a mixture of hexane and isopropane (3/2 v/v) [21]. The fatty acids methyl esters (FAMEs) were prepared by direct transesterification of the lipids with sulfuric acid and methanol (1:9, v/v) [22]. FA transmethylation and quantification were performed in the same way; additional standards for conjugated linoleic acid (CLA) isomers were purchased from Larodan (Larodan Fine Chemicals, AB, Limhamnsgardens Malmo, Sweden). The FAMEs were analyzed in a gas chromatograph (Agilent technologies, model 5890) fitted with an SP-2560 fused silica capillary column (100 m × 0.25 mm i.d. × 0.2 µm film thickness, Supelco, Inc., Bellefonte, PA, USA). The carrier gas, helium, was set at a constant pressure of 180 kPa, splitting flow of 50 mL/min, and injection volume of 1 µL. Column parameters: the initial temperature of the column was maintained at 170 °C for 15 min; then, with an increase of 5 °C/min, it was brought up to 240 °C. The total execution time was 64 min.
2.3. Blood Analysis
Five milliliters of blood were drawn at 7.00 am every 60 days (April, June, and August) from each goat via jugular venipuncture after a 12 h fast, and was collected into BD Vacutainer plastic tubes with clot activator and gel. Serum was obtained by centrifugation (1500× g for 15 min) and stored at −20 °C in 1.5 mL Eppendorf Safe-Lock Tubes until analysis. Serum samples were analyzed for the following: alanine amino transferase (ALT), aspartate amino transferase (AST), gamma glutamyl transferase (GGT), alkaline phosphatase (ALP), glucose (GLU), total protein (TP), creatinine (CREA), blood urea nitrogen (BUN), cholesterol (CHOL), and triglycerides (TRI). Analysis was performed using an automatic biochemical analyzer (AMS Auto lab, Diamond Diagnostics, West Point, UT, USA) using reagents from Spinreact (Girona, Spain).
2.4. Statistical Analysis
Feed data were analyzed using the one-way ANOVA with JMP software (version 11, PROC GLM, SAS 2000) according to the following model:
| yij = µ + Sj + εij | (1) |
where y = single datum, µ = general mean, S = sampling effect (j = 5; April, May, June, July, August), and ε = residual error.
Milk and blood data were analyzed using the two-way ANOVA with JMP software (version 11, PROC GLM, SAS 2000) according to the following model:
| yijk = µ + Di + Sj + (DS)ij + εijk | (2) |
where y = single datum, µ = general mean, D = effect of the dietary treatment (i = 2; CTR and LIN), S = sampling effect (j milk = 5; April, May, June, July, August; j blood = 3; April, June, August), DS = interaction between dietary treatment × sampling effect, and ε = residual error. The mean was statistically compared using Tukey’s test. Differences were considered statistically significant at p < 0.05.
3. Results
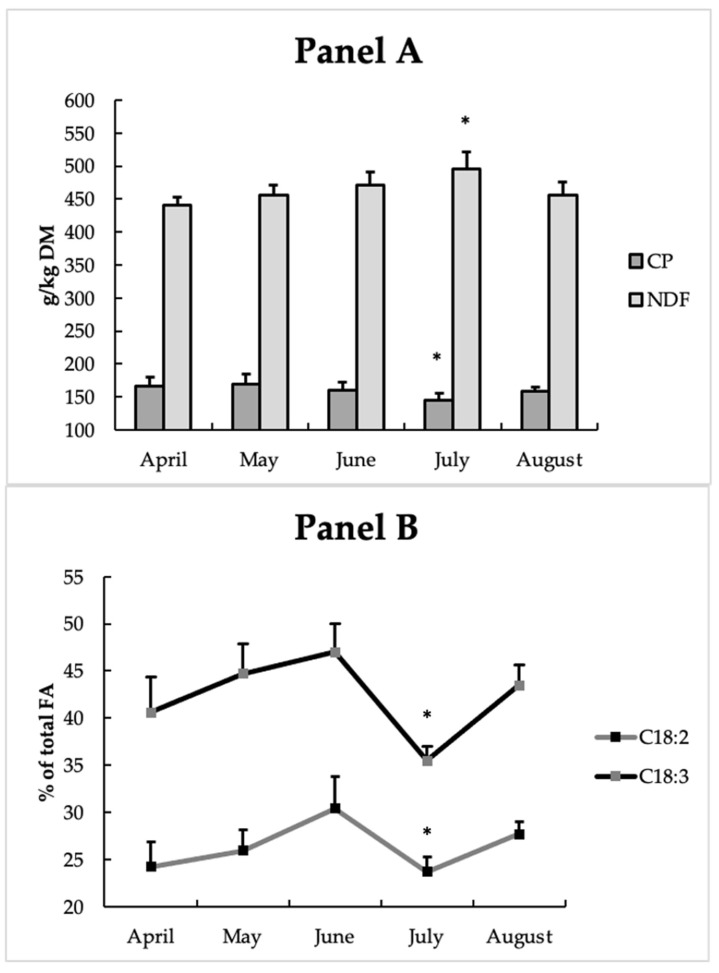
As Figure 1 shows, the pasture’s chemical composition was affected by the month of sampling; in particular, NDF was significantly (p < 0.05) higher in July, and an opposite trend was observed for CP (p < 0.05). In addition, C18:2 and C18:3 were significantly (p < 0.05) lower in July compared to the other months of sampling.
Figure 1.
Pasture crude protein (CP) and neutral detergent fibre (NDF; Panel A) and fatty acid profile (Panel B) during the experimental period (mean ± SD). FA: fatty acids; DM: dry matter. Pasture composition: Leguminosae (in a ratio of 60% Trifolium alexandrinum, Vicia spp.) and Graminae (in a ratio of 40% Bromus catharticus, Festuca arundinacea, Lolium perenne). * p < 0.05.
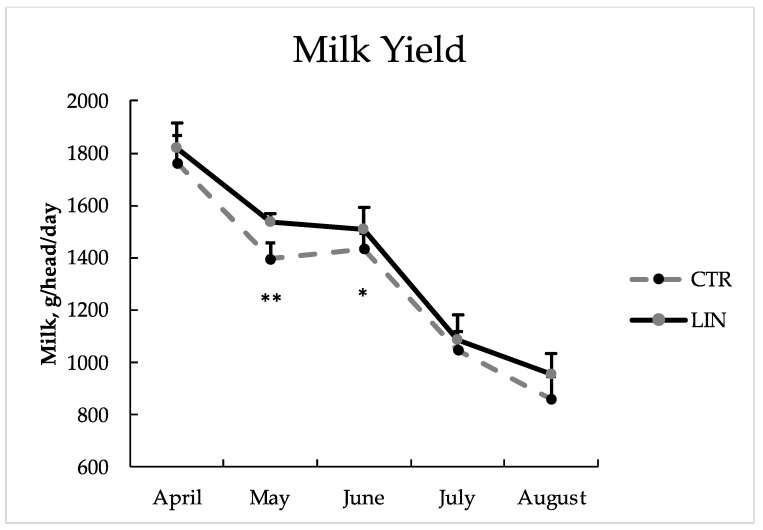
The LIN group showed a significantly (p < 0.001) higher milk yield (Table 2), expressed as g/head/day, compared to the CTR group (1358 vs. 1291 for LIN and CTR, respectively) and, as Figure 2 shows, such a difference was particularly noticeable in May and June. Additionally, the control group produced significantly less milk fat compared to the treated group (p < 0.001; 3.05% vs. 4.10%). No differences between groups were observed for protein and lactose content. The significant (p < 0.001) differences registered for lactose content during the trial (time effect) reflect a physiological trend not ascribable to the different dietary treatment between groups.
Table 2.
Goats’ milk production and composition (mean value, g/head/day) of control (CTR) and linseed supplementation (LIN) groups.
| Milk Production | Fat | Protein | Lactose | |||||
|---|---|---|---|---|---|---|---|---|
| CTR | LIN | CTR | LIN | CTR | LIN | CTR | LIN | |
| Mean value | 1291.38 | 1358.72 | 40.77 | 57.13 | 42.28 | 46.75 | 52.96 | 56.24 |
| April | 1763.33 | 1818.33 | 56.42 | 63.28 | 58.90 | 59.46 | 75.47 | 84.01 |
| May | 1395.20 | 1536.39 | 51.34 | 72.52 | 45.76 | 52.85 | 61.39 | 65.60 |
| June | 1434.17 | 1506.67 | 45.75 | 70.51 | 44.89 | 50.32 | 56.22 | 60.27 |
| July | 1046.67 | 1085.21 | 30.25 | 37.77 | 33.28 | 35.70 | 40.30 | 41.13 |
| August | 858.33 | 953.33 | 23.43 | 41.37 | 28.67 | 34.32 | 33.73 | 35.27 |
| RMSE | 86.004 | 8.14 | 3.74 | 2.81 | ||||
| p-value | ||||||||
| Group | *** | *** | NS | NS | ||||
| Time | *** | *** | NS | *** | ||||
| GxT | NS | NS | NS | * | ||||
*** p < 0.001; * p < 0.05. NS: not significant; RMSE: root mean square error.
Figure 2.
Goats’ milk production (mean ± SD) during the experimental period of control (CTR) and linseed supplementation (LIN) groups. ** p < 0.01; * p < 0.05.
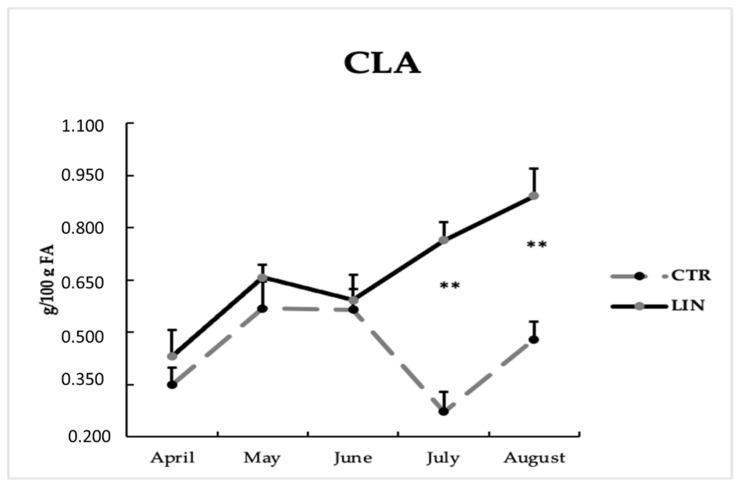
In Table 3, the milk fatty acid profile is reported. With the exception of the n6-n3 ratio and C18:2 (t10, c12) n6 content, all classes of fatty acids were significantly (p < 0.001) different between groups. The LIN group revealed a higher concentration of all classes of fatty acids, except for SFA, which was significantly (p < 0.001) higher in the CTR group (73.19 vs. 69. 47 g/100 g of total FA for CTR and LIN groups, respectively). CLAs were significantly (p < 0.001) higher in the LIN group compared to the CTR group (0.735 vs. 0.429 g/100 g of total FA), as Figure 3 shows.
Table 3.
Goats’ milk fatty acid profile (mean value, g/100 g of total fatty acids) of control (CTR) and linseed supplementation (LIN) groups.
| CTR | LIN | RMSE | p-Value | |||
|---|---|---|---|---|---|---|
| Group (G) | Time (T) | GxT | ||||
| SFA | 73.19 | 69.47 | 3.452 | *** | *** | NS |
| MUFA | 23.43 | 26.10 | 2.994 | *** | *** | NS |
| PUFA | 3.32 | 4.20 | 0.454 | *** | *** | *** |
| PUFA n3 | 1.16 | 1.38 | 0.177 | *** | *** | *** |
| PUFA n6 | 1.81 | 2.20 | 0.263 | *** | *** | *** |
| n6/n3 | 1.54 | 1.63 | 0.239 | NS | *** | *** |
| C18:2 (c9, t11) | 0.338 | 0.610 | 0.1648 | *** | ** | ** |
| C18:2 (t10, c12) n6 | 0.091 | 0.108 | 0.1184 | NS | NS | NS |
| CLAs | 0.429 | 0.735 | 0.243 | *** | ** | ** |
SFA: saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; CLAs: conjugated linoleic acids. *** p < 0.001; ** p < 0.01; NS: not significant; RMSE: root mean square error.
Figure 3.
Goats’ CLAs during the experimental period (mean ± SD) of control (CTR) and linseed supplementation (LIN) groups. FA: fatty acids; CLA: conjugated linoleic acids. ** p < 0.01.
Odd- and branched-chain fatty acid content is reported in Table 4. The total OBCFA was significantly affected by dietary treatment (p < 0.001; 3.21 vs. 2.84 g/100 g of total FA for CTR and LIN group, respectively) and an opposite trend was seen for iso C13:0 (0.028 vs. 0.034 for CTR and LIN group, respectively, p < 0.001).
Table 4.
Goats’ odd- and branched-chain fatty acid content (mean value, g/100 g of total fatty acids) of control (CTR) and linseed supplementation (LIN) groups.
| CTR | LIN | RMSE | p-Value | |||
|---|---|---|---|---|---|---|
| Group (G) | Time (T) | GxT | ||||
| iso C13:0 | 0.028 | 0.034 | 0.007 | *** | NS | NS |
| iso C14:0 | 0.088 | 0.094 | 0.013 | NS | NS | NS |
| iso C15:0 | 0.176 | 0.193 | 0.038 | NS | NS | NS |
| iso C16:0 | 0.024 | 0.029 | 0.037 | NS | * | NS |
| iso C17:0 | 0.027 | 0.034 | 0.049 | NS | ** | NS |
| anteiso C:13 | 0.0032 | 0.0040 | 0.004 | NS | NS | NS |
| anteiso C:15 | 0.363 | 0.383 | 0.006 | NS | NS | NS |
| anteiso C:17 | 0.391 | 0.404 | 0.083 | NS | NS | NS |
| C15:0 | 0.823 | 0.609 | 0.053 | *** | *** | *** |
| C17:0 + cis9 C17:1 | 0.924 | 0.676 | 0.091 | *** | *** | *** |
| Total OBCFA | 3.21 | 2.84 | 0.203 | *** | *** | *** |
OBCFA: odd- and branched-chain fatty acids. *** p < 0.001; ** p < 0.01; * p < 0.05; NS: not significant; RMSE: root mean square error.
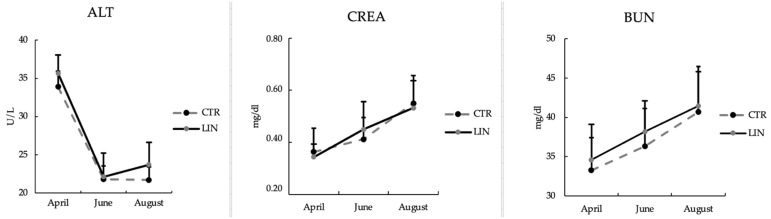
The blood parameters are depicted in Table 5 and Figure 4. The fat supplementation did not determine significant changes in blood parameters’ levels, with the exception of triglycerides, which were significantly (p < 0.01) higher in the treated group compared to the control group (24.34 vs. 18.49 mg/dl). Despite no differences being observed between groups for the other parameters, liver and renal function seemed to be significantly (p < 0.05) affected by time; in fact, ALT significantly (p < 0.0001) decreased during the trial, whereas CREA and BUN significantly (p < 0.001) increased during the experimental period (Figure 4).
Table 5.
Goats’ serum biochemistry (mean value) of control (CTR) and linseed supplementation (LIN) groups.
| AST | ALT | GGT | ALP | GLU | TP | CREA | BUN | CHOL | TRY | |
|---|---|---|---|---|---|---|---|---|---|---|
| U/L | U/L | U/L | U/L | mg/dL | g/dL | mg/dL | mg/dL | mg/dL | mg/dL | |
| CTR | 64.74 | 20.94 | 34.55 | 208.17 | 51.57 | 6.97 | 0.500 | 36.74 | 61.14 | 18.49 |
| LIN | 68.28 | 22.07 | 36.44 | 215.63 | 54.12 | 6.95 | 0.422 | 38.07 | 61.52 | 24.34 |
| RMSE | 7.333 | 2.473 | 5.121 | 39.542 | 5.934 | 0.564 | 0.142 | 5.237 | 8.261 | 4.852 |
| Group (G) | NS | NS | NS | NS | NS | NS | NS | NS | NS | ** |
| Time (T) | NS | *** | NS | NS | NS | NS | ** | ** | NS | NS |
| GxT | NS | NS | NS | NS | NS | NS | * | NS | NS | * |
AST: aspartate amino transferase; ALT: alanine amino transferase; GGT: gamma glutamyl transferase; ALP: alkaline phosphatase; GLU: glucose; TP: total protein; CREA: creatinine; BUN: blood urea nitrogen; CHOL: cholesterol; TRY: triglycerides. *** p < 0.001; ** p < 0.01; * p < 0.05; NS: not significant; RMSE: root mean square error.
Figure 4.
Indicators of liver and kidney status (mean ± SD) of goats during the experimental period of control (CTR) and linseed supplementation (LIN) groups. ALT: alanine amino transferase; CREA: creatinine; BUN: blood urea nitrogen.
4. Discussion
As already evidenced in previous studies, linseed supplementation significantly improved milk yield in goats [23,24], as well as in dairy cows [25,26] and in dairy sheep [27]. On the contrary, other authors registered a similar milk yield with and without linseed supplementation in the diets [28,29,30]. In the present trial, a significantly higher fat production was registered in the experimental group, similar to Renna et al. [31], and in contrast with Loor et al. [32] and Almeida et al. [33], who found no differences in milk fat content with linseed supplementation in the diets. As reported by Abuelfatah et al. [34], the inclusion of linseed in the diet of goats appears to be able to increase the molar proportion of acetate in the rumen, which may be responsible for the fat increase in milk. Indeed, these differences could be attributable to the shape of the administered linseed. As reported by Chilliard et al. [35], the treatment to which linseed is often subjected (i.e., micronization, extrusion, or heat treatments) can affect milk production and milk fat concentration, sometimes even leading to a decrease. In fact, these treatments affect the oil release rate compared to the whole seeds, thus leading to an increase in the production of trans FA in the rumen and, therefore, a decrease in the fat content of the milk [36].
Milk protein and lactose were unaffected by linseed supplementation, as previously reported by Chilliard et al. [35]. As reported by Sarrazin et al. [37], the levels of fat inclusion in the diet can affect milk production; moderate inclusion levels increase milk production by improving feed efficiency while higher inclusion levels negatively affect feed intake, consequently depressing ruminal function and decreasing milk yield [38]. As reported by Chilliard et al. [39], when lipids are ingested or directly infused into the rumen, the decrease in feed intake is even greater. These negative effects are proportional to the polyunsaturated fatty acid content of the lipids. These effects on feed intake have long since been attributed to the negative effects of lipid infusion on rumen digestion [40] or to palatability problems associated with high-fat diets.
The milk fatty acid profile was markedly influenced by lipid supplementation. In particular, milk from the LIN group was characterized by lower concentrations of SFA and higher proportions of MUFA, PUFA, and CLAs than milk from the CTR group, as previously observed by Nudda et al. [23] and Tudisco et al. [24].
The milk content of CLAs increased in the experimental group. As reported by Dilzer and Park [41], in the last twenty years, CLAs have acquired the interest of the scientific community for their anticarcinogenic effects, but they are also known to reduce the risk of cardiovascular diseases, reduce body fat, and modulate immune and inflammatory responses. CLAs are intermediates of the biohydrogenation in the rumen mammary [42] of C18:2 cis-9 cis-12 and C18: 3 linolenic acids, which is present in high concentrations in linseed. In addition, CLAs were also a product of a Δ9-desaturase, the stearoyl-CoA desaturase (SCD) in the mammary gland [43]. Other studies reported an increase in CLAs in milk in different ruminants’ species, such as Bernard et al. [28] in goats, Bu et al. [44] and Ferlay et al. [45] in cows, and Gomez-Cortes et al. [46] and Kholif et al. [47] in ewes. Chillard et al. [29] reported that CLAs’ concentration in goat milk after the administration of linseed and sunflower oil achieved a peak after two weeks of supplementation and persisted for at least ten weeks before decreasing. Contrastively, in dairy cows, Roy et al. [48] reported that the supplementation with linseed oil led to an increase in CLAs in milk that persisted throughout lactation. In our trial, we recorded a similar trend for the first three months of observation (April, May, and June) and a significant increase in CLAs in the treated group (Figure 3) during the deterioration of the pasture (July), particularly regarding the two precursors of CLAs (C18:2 and C18:3). Thus, we may speculate that, in grazing goats, linseed supplementation produces a beneficial effect when the pasture has a low PUFA concentration.
This study examined the relationship between milk OBCFA and dietary composition to improve our knowledge of the interactions occurring with lipid supplementation. The dietary treatment affected only a few OBC fatty acids and, except for C13:0 iso, linseed reduced the OBCFA concentration in milk. These results could be attributable to the negative effects of the fat supplement on the rumen bacteria. The inhibitory effects of long-chain fatty acids on rumen bacteria are well-documented and are greater by increasing the unsaturation of long-chain fatty acids. Importantly, there are several differences between bacteria, for example, the growth of cellulolytic strains is lower than that of amylolytic ones [49].
Hence, when the fat source contains high amounts of C18: 2 n-6 and C18: 3 n-3 (e.g., sunflower oil and linseeds, respectively), effects on a bacterial population can be expected. As reported by Maia et al. [50] and Cívico et al. [51], linseed oil produces an inhibitory effect on some rumen bacterial populations and, particularly, cellulolytic bacteria are negatively affected by PUFA. Indeed, the main bacteria susceptible to unsaturated FA are the cellulolytic ones, particularly to alfa-linolenic acid [52], which is the main fatty acid supplied by linseed oil. Additionally, cellulolytic bacteria participate in the predominant rumen biohydrogenation pathway; hence, it might be expected that linseed oil could positively affect the rumen biohydrogenation intermediate levels in milk fat while lowering its iso FA content according to the analysis of milk OBCFA. Dairy cows that were fed supplemental fat rich in C18:3 n-3 [53,54] had lower proportions of milk OBCFA. In agreement with this hypothesis, and according to our results, Bernard et al. [28] found a decrease in the majority of iso FA content in milk fat relating to an increase in the proportions of total trans C18: 1 and total conjugate C18: 2 by adding linseed oil to the diet of dairy goats. The nutritional treatment significantly reduced the milk concentrations of C15: 0 and C17: 0 and this could be considered a nonpositive result. In fact, these fatty acids are considered biomarkers of rumen microbial fermentation and microbial de novo lipogenesis and the mammary gland plays a pivotal role in their synthesis [55]. Recently, the importance of C15: 0 and C17: 0, in terms of human health, has been highlighted. These fatty acids are able to reduce the risk of cardiovascular diseases [56] and the incidence of diabetes type 2 [57]; in addition, C15:0 reduces the inflammatory state [58]. Humans are not able to synthetize these fatty acids, but they can intake it with dairy products.
During the experimental period, the pasture underwent a progressive modification due to the phenological stage of the plants, which led to changes in its chemical characteristics. As lactation progressed, there was a deterioration of the nutritional quality of the pasture, as can be seen in Figure 1, showing an increase in fiber and a reduction in the content of proteins and fatty acids, the concentration of which decreased in mature grass. Along with the lactation progress, there was an increase in neutral detergent fiber (NDF) in the pasture, thus suggesting a positive association between OBCFA and dietary fiber, as previously observed by Bas et al. [59] in dairy goats. These researchers highlighted dietary NDF as the most important factor of variation in the lipid composition of bacteria.
Concerning the effects of the linseed supplementation on goats‘ health during the experimental period, some serum biomarkers of renal and hepatic function changed. In particular, ALT decreased, while creatinine and bilirubin increased over time. We can observe that the temporal decrease in ALT shown in both groups followed the same trend of the lactation curve, as previously reported by Nudda et al. [23] in goats. The temporal increases in creatinine and bilirubin during the study were also observed in both groups. Generally, an increase in creatinine is associated with a decrease in renal function but, in this case, the registered values were not outside the physiological range reported for this species [60]. This consideration could indicate that no kidney lesions occurred, and since the creatinine concentration in the blood is generated by muscle metabolism, its increase during lactation could be due to a normal muscle turnover.
The differences found between groups for triglycerides are difficult to explain and, although the LIN group had a higher triglycerides value than the CTR group, both results reflect a physiological rather than a pathological condition, and are in line with previous studies conducted under the same experimental conditions [61].
5. Conclusions
Linseed supplementation was able to improve milk yield and fat content but not the milk concentration of the odd- and branched-chain fatty acids. Thus, the trial hypothesis has been only partially confirmed. Importantly, serum biochemistry showed no negative effects of linseed supplementation at the tested intake. Since the fatty acid composition in milk, as well as in other dairy products, has nutritional benefits in terms of human health, further studies using different experimental conditions might reveal a possible milk OBCFA improvement by linseed supplementation in goats’ diet.
Author Contributions
Conceptualization, N.M. and F.I.; methodology, M.W, R.T., and P.I.; software, B.D.; formal analysis, N.M., R.T., P.I., R.A., and P.T.; resources, F.I.; data curation, N.M. and B.D.; writing—original draft preparation, N.M., P.L., and F.I.; writing—review and editing, R.T., P.L., and G.E.; supervision, F.I. and M.W.; project administration, F.I. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the local Bioethics Committee (protocol number: PG/2019/0070006).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Food and Agriculture Organization of the United Nations (FAO) Food and Agriculture Organization of the United Nations Statistical Databases. 2019. [(accessed on 16 February 2019)].
- 2.de Asís Ruiz Morales F., Castel Genís J.M., Mena Guerrero Y. Current status, challenges and the way forward for dairy goat production in Europe. Asian-Australas. J. Anim. Sci. 2019;32:1256–1265. doi: 10.5713/ajas.19.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller B.A., Lu C.D. Current status of global dairy goat production: An overview. Asian-Australas. J. Anim. Sci. 2019;32:1219–1232. doi: 10.5713/ajas.19.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sepe L., Argüello A. Recent advances in dairy goat products. Asian-Australas. J. Anim. Sci. 2019;32:1306–1320. doi: 10.5713/ajas.19.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlaeminck B., Fievez V., Tamminga S., Dewhurst R.J., van Vuuren A., De Brabander D., Demeyer D. Milk odd- and branched-chain fatty acids in relation to the rumen fermentation pattern. J. Dairy Sci. 2006;89:3954–3964. doi: 10.3168/jds.S0022-0302(06)72437-7. [DOI] [PubMed] [Google Scholar]
- 6.Fievez V., Colman E., Castro-Montoya J.M., Stefanov I., Vlaeminck B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function—An update. Anim. Feed Sci. Technol. 2012;172:51–65. doi: 10.1016/j.anifeedsci.2011.12.008. [DOI] [Google Scholar]
- 7.Serment A., Schmidely P., Giger-Reverdin S., Chapoutot P., Sauvant D. Effects of the percentage of concentrate on rumen fermentation, nutrient digestibility, plasma metabolites, and milk composition in mid-lactation goats. J. Dairy Sci. 2011;94:3960–3972. doi: 10.3168/jds.2010-4041. [DOI] [PubMed] [Google Scholar]
- 8.Bernard L., Shingfield K.J., Rouel J., Ferlay A., Chilliard Y. Effect of plant oils in the diet on performance and milk fatty acid composition in goats fed diets based on grass hay or maize silage. Br. J. Nutr. 2009;101:213–224. doi: 10.1017/S0007114508006533. [DOI] [PubMed] [Google Scholar]
- 9.Mele M., Serra A., Buccioni A., Conte G., Pollicardo A., Secchiari P. Effect of soybean oil supplementation on milk fatty acid composition from Saanen goats fed diets with different forage: Concentrate ratios. Ital. J. Anim. Sci. 2008;7:297–311. doi: 10.4081/ijas.2008.297. [DOI] [Google Scholar]
- 10.Li F., Li Z., Li S., Ferguson J.D., Cao Y., Yao J., Sun F., Wang X., Yang T. Effect of dietary physically effective fiber on ruminal fermentation and the fatty acid profile of milk in dairy goats. J. Dairy Sci. 2014;97:2281–2290. doi: 10.3168/jds.2013-6895. [DOI] [PubMed] [Google Scholar]
- 11.Todaro M., Scatassa M.L., Giaccone P. Multivariate factor analysis of Girgentana goat milk composition. Ital. J. Anim. Sci. 2005;4:403–410. doi: 10.4081/ijas.2005.403. [DOI] [Google Scholar]
- 12.Infascelli L., Tudisco R., Iommelli P., Capitanio F. Milk quality and animal welfare as a possible marketing lever for the economic development of rural areas in southern Italy. Animals. 2021;11:1059. doi: 10.3390/ani11041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmquist D.L., Jenkins T.C. Fat in lactation rations: Review. J. Dairy Sci. 1980;63:1–14. doi: 10.3168/jds.S0022-0302(80)82881-5. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson J.D., Sklan D., Chalupa W.V., Kronfeld D.S. Effects of hard fats on in vitro and in vivo rumen fermentation, milk production, and reproduction in dairy cows. J. Dairy Sci. 1990;73:2864–2879. doi: 10.3168/jds.S0022-0302(90)78974-6. [DOI] [PubMed] [Google Scholar]
- 15.Davidson L.A., Wang N., Shah M.S., Lupton J.R., Ivanov I., Chapkin R.S. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis. 2009;30:2077–2084. doi: 10.1093/carcin/bgp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and non starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74:3583–3598. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 17.AOAC . Official Methods of Analysis. 19th ed. Association of Official Analytical Chemists; Arlington, VA, USA: 2012. [Google Scholar]
- 18.INRA . In: Alimentation des Ruminants. Jarrige R., editor. INRA Editions; Versailles, France: 1978. [Google Scholar]
- 19.Castro T., Manso T., Jimeno V., Del Alamo M., Mantecón A.R. Effects of dietary sources of vegetable fats on performance of dairy ewes and conjugated linoleic acid (CLA) in milk. Small Rumin. Res. 2009;84:47–53. doi: 10.1016/j.smallrumres.2009.05.005. [DOI] [Google Scholar]
- 20.Tudisco R., Morittu V.M., Addi L., Moniello G., Grossi M., Musco N., Grazioli R., Mastellone V., Pero M.E., Lombardi P., et al. Influence of pasture on stearoyl-coa desaturase and mirna 103 expression in goat milk: Preliminary results. Animals. 2019;9:606. doi: 10.3390/ani9090606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara A., Radin N.S. Lipid extraction of tissues with a low-toxicity solvent. Annal. Biochem. 1978;90:420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 22.Christie W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. In: Christie W.W., editor. Advances in Lipid Methodology-two. Oily Press; Dundee, UK: 1993. pp. 69–111. [Google Scholar]
- 23.Nudda A., Battacone G., Atzori A.S., Dimauro C., Rassu S.P.G., Nicolussi P., Bonelli P., Pulina G. Effect of extruded linseed supplementation on blood metabolic profile and milk performance of Saanen goats. Animal. 2013;7:1464–1471. doi: 10.1017/S1751731113000931. [DOI] [PubMed] [Google Scholar]
- 24.Tudisco R., Chiofalo B., Lo Presti V., Morittu V.M., Moniello G., Grossi M., Musco N., Grazioli R., Mastellone V., Lombardi P., et al. Influence of feeding linseed on SCD activity in grazing goat mammary glands. Animals. 2019;9:786. doi: 10.3390/ani9100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuentes M.C., Calsamiglia S., Sánchez C., González A., Newbold J.R., Santos J.E.P., Rodríguez-Alcalá L.M., Fontecha J. Effect of extruded linseed on productive and reproductive performance of lactating dairy cows. Livest. Sci. 2008;113:144–154. doi: 10.1016/j.livsci.2007.03.005. [DOI] [Google Scholar]
- 26.Ariza J.M., Meignan T., Madouasse A., Beaudeau F., Bareille N. Effects on milk quantity and composition associated with extruded linseed supplementation to dairy cow diets. Sci. Rep. 2019;9:17563. doi: 10.1038/s41598-019-54193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mughetti L., Sinesio F., Acuti G., Antonini C., Moneta E., Peparaio M., Trabalza-Marinucci M. Integration of extruded linseed into dairy sheep diets: Effects on milk composition and quality and sensorial properties of Pecorino cheese. Anim. Feed Sci. Technol. 2012;178:27–39. doi: 10.1016/j.anifeedsci.2012.09.005. [DOI] [Google Scholar]
- 28.Bernard L., Bonnet M., Leroux C., Shingfield K.J., Chilliard Y. Effect of sunflower-seed oil and linseed oil on tissue lipid metabolism, gene expression, and milk fatty acid secretion in alpine goats fed maizesilage-based diets. J. Dairy Sci. 2009;92:6083–6094. doi: 10.3168/jds.2009-2048. [DOI] [PubMed] [Google Scholar]
- 29.Chilliard Y., Ferlay A., Rouel J., Lamberet G. A review of nutritional and physiological factors affecting goat milk lipid synthesis and lipolysis. J. Dairy Sci. 2003;86:1751–1770. doi: 10.3168/jds.S0022-0302(03)73761-8. [DOI] [PubMed] [Google Scholar]
- 30.Gómez-Cortés P., Cívico A., De la Fuente M., Núñez Sánchez N., Peña Blanco F., Martínez Marín A. Effects of dietary concentrate composition and linseed oil supplementation on the milk fatty acid profile of goats. Animal. 2018;12:2310–2317. doi: 10.1017/S1751731118000381. [DOI] [PubMed] [Google Scholar]
- 31.Renna M., Lussiana C., D’Agostino M., Mimosi A., Fortina R. Extruded linseed supplementation in dairy goat diet: Effects on productive performance and fatty acid profile of bulk milk, fresh and ripened cheese. J. Anim. Vet. Adv. 2013;12:1550–1564. [Google Scholar]
- 32.Loor J.J., Ferlay A., Ollier A., Doreau M., Chilliard Y. Relationship among trans and conjugated fatty acids and bovine milk fat yield due to dietary concentrate and linseed oil. J. Dairy Sci. 2005;88:726–740. doi: 10.3168/jds.S0022-0302(05)72736-3. [DOI] [PubMed] [Google Scholar]
- 33.Almeida O.C., Ferraz M.V.C., Susin I., Gentil R.S., Polizel D.M., Ferreira E.M., Barroso J.P.R., Pires A.V. Plasma and milk fatty acid profiles in goats fed diets supplemented with oils from soybean, linseed or fish. Small Rumin. Res. 2019;170:125–130. doi: 10.1016/j.smallrumres.2018.11.002. [DOI] [Google Scholar]
- 34.Abuelfatah K., Zuki A.B., Goh Y., Sazili A.Q., Abubakr A. Effects of feeding whole linseed on ruminal fatty acid composition and microbial population in goats. Anim. Nutr. 2016;2:323–328. doi: 10.1016/j.aninu.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chilliard Y., Martin C., Rouel J., Doreau M. Milk fatty acids in dairy cows fed whole crude linseed, extruded linseed or linseed oil, and their relationship with methane output. J. Dairy Sci. 2009;92:5199–5211. doi: 10.3168/jds.2009-2375. [DOI] [PubMed] [Google Scholar]
- 36.Dhaka V., Gulia N., Ahlawat K.S., Khatkar B.S. Trans fats-sources, health risks and alternative approach—A review. J. Food Sci. Technol. 2011;48:534–541. doi: 10.1007/s13197-010-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarrazin P., Mustafa A.F., Chouinard P.Y., Raghavan G., Sotocinal S. Performance of dairy cows fed roasted sunflower seed. J. Sci. Food Agric. 2004;84:1179–1185. doi: 10.1002/jsfa.1802. [DOI] [Google Scholar]
- 38.Gonthier C., Mustafa A.F., Ouellet D.R., Chouinard P.Y., Berthiaume R., Petit H.V. Feeding micronized and extruded flaxseed to dairy cows: Effects on blood parameters and milk fatty acid composition. J. Dairy Sci. 2005;88:748–756. doi: 10.3168/jds.S0022-0302(05)72738-7. [DOI] [PubMed] [Google Scholar]
- 39.Chilliard Y., Doreau M., Gagliostro G., Elmeddah Y. Addition de lipides protégés (encapsulés ou savons de calcium) à la ration de vaches laitières. Effets sur les performances et la composition du lait (Protected (encapsulated or calcium salts) lipids in dairy cow diets. Effects on production and milk composition) INRA Prod. Anim. 1993;6:139–150. (In French) [Google Scholar]
- 40.Michalet-Doreau B., Martin C., Doreau M. Optimisation de la digestion des parois végétales dans le rumen: Quantification des ineractions digestives (Optimisation of fibre ruminal digestion: Interactions of fibre digestion with other components) Renc. Rech. Rum. 1997;4:103–112. (In French) [Google Scholar]
- 41.Dilzer A., Park Y. Implication of conjugated linoleic acid (CLA) in human health, critical reviews. Food Sci. Nutr. 2012;52:488–513. doi: 10.1080/10408398.2010.501409. [DOI] [PubMed] [Google Scholar]
- 42.Griinari J.M., Corl B.A., Lacy S.H., Chouinard P.Y., Nurmela K.V., Bauman D.E. Conjugated linoleic acid is synthesized endogenously in lactating dairy cows by Delta (9)-desaturase. J. Nutr. 2000;130:2285–2291. doi: 10.1093/jn/130.9.2285. [DOI] [PubMed] [Google Scholar]
- 43.Tudisco R., Calabrò S., Cutrignelli M.I., Moniello G., Grossi M., Gonzalez O.J., Piccolo V., Infascelli F. Influence of organic systems on Stearoyl-CoA desaturase in goat milk. Small Rumin. Res. 2012;106:37–42. doi: 10.1016/j.smallrumres.2012.04.031. [DOI] [Google Scholar]
- 44.Bu D.P., Wang J.Q., Dhiman T.R., Liu J. Effectiveness of oils rich in linoleic and linolenic acids to enhance conjugated linoleic acid in milk from dairy cows. J. Dairy Sci. 2007;90:998–1007. doi: 10.3168/jds.S0022-0302(07)71585-0. [DOI] [PubMed] [Google Scholar]
- 45.Ferlay A., Doreau M., Martin C., Chilliard Y. Effects of incremental amounts of extruded linseed on the milk fatty acid composition of dairy cows receiving hay or corn silage. J. Dairy Sci. 2013;96:6577–6595. doi: 10.3168/jds.2013-6562. [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Cortes P., Bach A., Luna P., Juarez M., de la Fuente M.A. Effects of extruded linseed supplementation on n-3 fatty acids and conjugated linoleic acid in milk and cheese from ewes. J. Dairy Sci. 2009;92:4122–4134. doi: 10.3168/jds.2008-1909. [DOI] [PubMed] [Google Scholar]
- 47.Kholif S.M., Morsy T.A., Abedo A.A., El-Bordeny N., Abdo M.M. Milk production and composition, milk fatty acid profile, nutrients digestibility and blood composition of dairy buffaloes fed crushed flaxseed in early lactation. Egypt. J. Nutr. Feeds. 2011;14:385–394. [Google Scholar]
- 48.Roy A., Ferlay A., Shingfield K.J., Chilliard Y. Examination of the persistency of milk fatty acid composition responses to plant oils in cows given different basal diets, with particular emphasis on trans-C18:1 fatty acids and isomers of conjugated linoleic acid. Anim. Sci. 2006;82:479–492. doi: 10.1079/ASC200658. [DOI] [Google Scholar]
- 49.Vlaeminck B., Fievez V., Cabrita A.R.J., Fonseca A.J.M., Dewhurst R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006;131:389–417. doi: 10.1016/j.anifeedsci.2006.06.017. [DOI] [Google Scholar]
- 50.Maia M.R.G., Chaudhary L.C., Figueres L., Wallace R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek. 2007;91:303–314. doi: 10.1007/s10482-006-9118-2. [DOI] [PubMed] [Google Scholar]
- 51.Cívico A., Núñez Sánchez N., Gómez-Cortés P., de la Fuente M.A., Peña Blanco F., Juárez M., Schiavone A., Martínez Marín A.L. Odd- and branched-chain fatty acids in goat milk as indicators of the diet composition. Ital. J. Anim. Sci. 2017;16:68–74. doi: 10.1080/1828051X.2016.1263547. [DOI] [Google Scholar]
- 52.Yang S.L., Bu D.P., Wang J.Q., Hu Z.Y., Li D., Wei H.Y., Zhou L.Y., Loor J.J. Soybean oil and linseed oil supplementation affect profiles of ruminal microorganisms in dairy cows. Animal. 2009;3:1562–1569. doi: 10.1017/S1751731109990462. [DOI] [PubMed] [Google Scholar]
- 53.Collomb M., Sieber R., Bütikofer U. CLA isomers in milk fat from cows fed diets with high levels of unsaturated fatty acids. Lipids. 2004;39:355–364. doi: 10.1007/s11745-004-1239-x. [DOI] [PubMed] [Google Scholar]
- 54.Loor J.J., Doreau M., Chardigny J.M., Ollier A., Sebedio J.L., Chilliard Y. Effects of ruminal or duodenal supply of fish oil on milk fat secretion and profiles of trans-fatty acids and conjugated linoleic acid isomers in dairy cows fed maize silage. Anim. Feed Sci. Technol. 2005;119:227–246. doi: 10.1016/j.anifeedsci.2004.12.016. [DOI] [Google Scholar]
- 55.Massart-Leën A.M., Peeters G., Vandeputte-Van Messom G., Roets E., Burvenich C. Effects of valerate and isobutyrate on fatty acid secretion by the isolated perfused mammary gland of the lactating goat. Reprod. Nutr. Dev. 1986;26:801–814. doi: 10.1051/rnd:19860505. [DOI] [PubMed] [Google Scholar]
- 56.Jenkins B., West J.A., Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (c15:0) and heptadecanoic Acid (c17:0) in health and disease. Molecules. 2015;20:2425–2444. doi: 10.3390/molecules20022425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hodge A.M., English D.R., O’Dea K., Sinclair A.J., Makrides M., Gibson R.A., Giles G.G. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: Interpreting the role of linoleic acid. Am. J. Clin. Nutr. 2007;86:189–197. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- 58.Venn-Watson S., Lumpkin R., Dennis E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020;10:8161. doi: 10.1038/s41598-020-64960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bas P., Archimède H., Rouzeau A., Sauvant D. Fatty acid composition of mixed-rumen bacteria: Effect of concentration and type of forage. J. Dairy Sci. 2003;86:2940–2948. doi: 10.3168/jds.S0022-0302(03)73891-0. [DOI] [PubMed] [Google Scholar]
- 60.Piccione G., Casella S., Lutri L., Vazzana I., Ferrantelli V., Caola G. Reference values for some haematological, haematochemical, and electrophoretic parameters in the Girgentana goat. Turk. J. Vet. Anim. Sci. 2010;34:197–204. [Google Scholar]
- 61.Tudisco R., Musco N., Pero M.E., Morittu V.M., Grossi M., Mastellone V., Cavaliere G., Wanapat M., Infascelli F., Lombardi P. Influence of dietary hydrogenated palm oil supplementation on serum biochemistry and progesterone levels in dairy goats. Anim. Nutr. 2019;5:286–289. doi: 10.1016/j.aninu.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.