Significance
Strategies to reduce consumption of antimicrobial drugs are needed to contain the growing burden of antimicrobial resistance. Respiratory syncytial virus (RSV) is a prominent cause of upper and lower respiratory tract infections, as a single agent and in conjunction with bacterial pathogens, and may thus contribute to the burden of both inappropriately treated viral infections and appropriately treated polymicrobial infections involving bacteria. In a double-blind, randomized, placebo-controlled trial, administering an RSV vaccine to pregnant mothers reduced antimicrobial prescribing among their infants by 12.9% over the first 3 mo of life. Our findings implicate RSV as an important contributor to antimicrobial exposure among infants and demonstrate that this exposure is preventable by use of effective maternal vaccines against RSV.
Keywords: respiratory syncytial virus, antimicrobial prescribing, vaccination, antimicrobial resistance, randomized controlled trial
Abstract
Reductions in antimicrobial consumption are needed to mitigate the burden of antimicrobial resistance. Vaccines may have an important role to play in reducing antimicrobial consumption by preventing infections for which treatment is often prescribed, whether appropriately or inappropriately. However, limited understanding of the volume of antimicrobial treatment attributable to specific pathogens—and to viruses, in particular—presently hinders efforts to prioritize vaccines with the greatest potential to reduce antimicrobial consumption. In a double-blind trial undertaken across 11 countries, infants born to mothers who were randomized to receive an experimental vaccine against respiratory syncytial virus (RSV) experienced 12.9% (95% CI: 1.3 to 23.1%) lower incidence of antimicrobial prescribing over the first 3 mo of life than infants whose mothers were randomized to receive placebo. Vaccine efficacy against antimicrobial prescriptions associated with acute lower respiratory tract infections (LRTIs) was 16.9% (95% CI: 1.4 to 29.4%). Over the first 3 mo of life, maternal vaccination prevented 3.6 antimicrobial prescription courses for every 100 infants born in high-income countries and 5.1 courses per 100 infants in low- and middle-income countries, representing 20.2 and 10.9% of all antimicrobial prescribing in these settings, respectively. While LRTI episodes accounted for 69 to 73% of all antimicrobial prescribing prevented by maternal vaccination, striking vaccine efficacy (71.3% [95% CI: 28.1 to 88.6%]) was also observed against acute otitis media–associated antimicrobial prescription among infants in high-income countries. Our findings implicate RSV as a cause of substantial volumes of antimicrobial prescribing among young infants and demonstrate the potential for prevention of such prescribing through use of maternal vaccines against RSV.
Antimicrobial resistance (AMR) poses a severe threat to human health and well-being. As human consumption of antimicrobial drugs is an important source of selective pressure contributing to the emergence and expansion of AMR (1), strategies to reduce antimicrobial use in situations where it is avoidable or unnecessary are a focus of AMR action plans (2, 3). Vaccines against influenza, pneumococcus, and rotavirus have been found to prevent substantial antimicrobial consumption and prescribing (4–7). While understanding the potential for new vaccines to mitigate antimicrobial use and AMR burden could inform priority setting in vaccine development, evaluation, and approval (8), challenges surround efforts to define the extent of antimicrobial consumption preventable by specific vaccines (9). Microbiological diagnosis is seldom undertaken for common, nonsevere infections that precipitate the greatest volumes of prescribing (10, 11) and is often impractical, as many of the pathogens causing acute respiratory, febrile, and diarrheal infections are also prevalent among individuals without symptoms (12). Due to this challenge, substantial antimicrobial consumption is likely associated with viral infections for which treatment is unnecessary or inappropriate (7). Additionally, polymicrobial interactions contribute to the pathogenesis of numerous bacterial infections, as best understood in the context of influenza virus interactions with Streptococcus pneumoniae and Staphylococcus aureus (13–17). Such interaction pathways may further implicate viruses in the etiology of conditions that are appropriate to treat with antibiotics. Vaccines against respiratory viruses may thus have importance for preventing infections that lead to both appropriate and inappropriate antimicrobial use.
Respiratory syncytial virus (RSV) is a leading cause of severe acute lower respiratory tract infections (LRTIs) among infants globally (18). While RSV is also recognized as a prominent contributor to common nonsevere infections, such as acute otitis media [AOM (19)], the spectrum of clinical illnesses associated with RSV and its resulting impact on antimicrobial consumption are incompletely understood (20). Previous ecological studies in the United Kingdom have reported increases in pediatric antimicrobial prescribing during periods with greater RSV circulation and have estimated that RSV may account for a greater than or equal to threefold higher fraction of pediatric antimicrobial prescribing than influenza (21, 22). Facilitative interactions between RSV and S. pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and S. aureus have also been reported in the upper respiratory tract among children with and those without acute respiratory symptoms (23–27), and seasonal increases in respiratory and invasive bacterial disease activity are associated with dynamics of RSV transmission (28–31). These considerations have led to enthusiasm about the prospects for reducing antimicrobial consumption through RSV vaccination (32, 33).
A recent randomized trial administering a candidate RSV fusion (F) protein nanoparticle vaccine to pregnant mothers provided an opportunity to probe the burden of illness associated with RSV among young infants (34). Although the trial’s prespecified primary success criterion was not met and the vaccine was not licensed, infants whose mothers were randomized to receive the RSV F vaccine experienced 41.4% (95% CI: 18.0 to 58.1%) efficacy against medically significant RSV-associated LRTI during the first 90 d of life in an intention-to-treat (ITT) analysis including clinical trial–specific and hospital record data. During the first 180 d of life, infants whose mothers were assigned the RSV F vaccine experienced 24.7% (95% CI: –1.3 to 44.0%) and 32.2% (95% CI: 15.3 to 45.7%) efficacy against medically significant RSV-associated LRTI and hospitalized LRTI due to any cause, respectively. To better understand the contribution of RSV to antimicrobial prescribing among young infants and to test whether such prescribing can be prevented by maternal RSV vaccination, we revisited data from this trial assessing vaccine efficacy (VE) against antimicrobial prescription end points.
Results
Enrollment in the trial has been described previously (34) and proceeded from 3 December 2015 to 2 May 2018 across 87 sites in Argentina, Australia, Chile, Bangladesh, Mexico, New Zealand, the Philippines, South Africa, Spain, the United Kingdom, and the United States. We analyzed VE in an ITT framework, including data from 3,005 maternal participants and 2,978 infants randomized to the RSV F vaccine and 1,573 maternal participants and 1,546 infants randomized to placebo who were present in the final database following all safety follow-up and data monitoring. In total, 46 (of 3,051 [1.5%]) randomized mothers and 36 (of 3,014 [1.2%]) live-born infants in the RSV F vaccine arm and 12 (of 1,585 [0.8%]) randomized mothers and 19 (of 1,565 [1.2%]) live-born infants in the placebo arm were excluded from the ITT efficacy cohort based on incomplete study documentation or because data were not available from any study interaction. Baseline characteristics of maternal participants and infants were well balanced between the RSV F vaccine and placebo arms (Table 1), as described previously (34).
Table 1.
Baseline characteristics of maternal participants and their infants included within the ITT population by intended treatment
| Study population and attribute | Participants by intended treatment, no (%) | |
|---|---|---|
| RSV F vaccine | Placebo | |
| Mothers | ||
| n | 3,005 | 1,573 |
| Country | ||
| Argentina | 164 (5.5) | 83 (5.3) |
| Australia | 73 (2.4) | 40 (2.5) |
| Bangladesh | 63 (2.1) | 40 (2.5) |
| Chile | 19 (0.6) | 20 (1.3) |
| Spain | 26 (0.9) | 12 (0.8) |
| Mexico | 7 (0.2) | 4 (0.3) |
| New Zealand | 148 (4.9) | 88 (5.6) |
| Philippines | 174 (5.8) | 90 (5.7) |
| United Kingdom | 21 (0.7) | 11 (0.7) |
| United States | 708 (23.6) | 368 (23.4) |
| South Africa | 1,602 (53.3) | 817 (51.9) |
| Age, y | ||
| ≤24 | 1,225 (40.8) | 646 (41.1) |
| 25–29 | 932 (31.0) | 477 (30.3) |
| 30–34 | 597 (19.9) | 331 (21.0) |
| ≥35 | 251 (8.4) | 119 (7.6) |
| Gestational age at randomization, wk | ||
| ≤29 | 892 (29.7) | 471 (29.9) |
| 30–34 | 1,607 (53.5) | 832 (52.9) |
| ≥35 | 484 (16.1) | 263 (16.7) |
| Unknown | 22 (0.7) | 7 (0.4) |
| Risk behaviors at the time of randomization | ||
| Current smoking | 215 (7.2) | 108 (7.0) |
| Alcohol consumption | 10 (0.6) | 17 (0.6) |
| Recreational drug use | 7 (0.4) | 7 (0.2) |
| Infants | ||
| n | 2,978 | 1,546 |
| Country | ||
| Argentina | 162 (5.4) | 81 (5.2) |
| Australia | 73 (2.5) | 40 (2.6) |
| Bangladesh | 81 (2.7) | 37 (2.4) |
| Chile | 19 (0.6) | 20 (1.3) |
| Spain | 26 (0.9) | 12 (0.8) |
| Mexico | 7 (0.2) | 4 (0.3) |
| New Zealand | 146 (4.9) | 88 (5.7) |
| Philippines | 172 (5.8) | 89 (5.8) |
| United Kingdom | 21 (0.7) | 11 (0.7) |
| United States | 701 (23.5) | 366 (23.7) |
| South Africa | 1,570 (52.7) | 798 (51.6) |
| Birth characteristics | ||
| Sex, male | 1,538 (51.6) | 794 (51.4) |
| Gestational age <37 wk | 173 (5.8) | 92 (6.0) |
| Interval from randomization to delivery, wk | ||
| 0–3 | 385 (12.9) | 207 (13.4) |
| 4–7 | 1,278 (42.9) | 684 (44.2) |
| 8–11 | 1,154 (38.8) | 559 (36.2) |
| ≥12 | 161 (5.4) | 96 (6.2) |
Further descriptions of enrollment dates (SI Appendix, Table S1), eligibility criteria, and demographic attributes of the study population as well as comprehensive data on LRTI, safety surveillance, and immunogenicity end points are available in ref. 34.
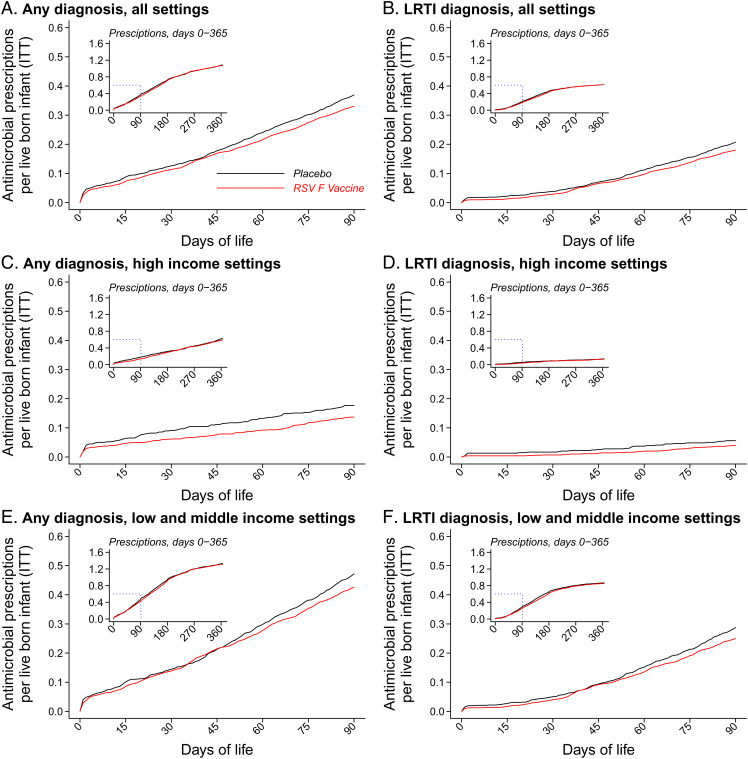
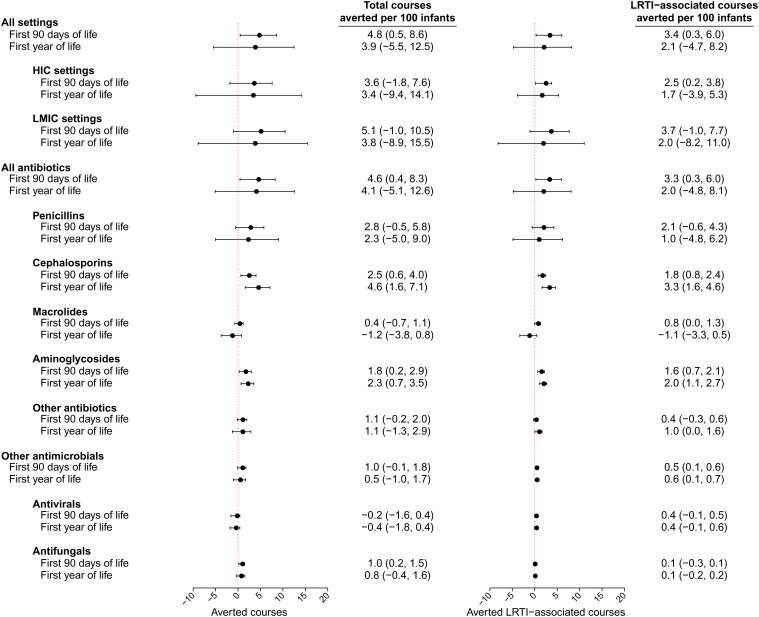
Over the first 90 d of life, incidence rates of antimicrobial prescribing among infants whose mothers were assigned the RSV F vaccine and placebo were 133.7 and 148.7 prescription courses per 100 person-years at risk, respectively (Fig. 1 and Table 2). The corresponding VE estimate against new antimicrobial prescription courses through 90 d of life was 12.9% (95% CI: 1.3 to 23.1%). Incidence rates of LRTI-associated antimicrobial prescription courses were 71.0 and 82.2 per 100 person-years during the first 90 d of life among infants whose mothers were assigned the RSV F vaccine and placebo, respectively, yielding 16.6% (95% CI: 1.4 to 29.4%) VE against this end point. Estimates of VE did not meet statistical significance thresholds in analyses covering the full (365-d) follow-up period for infants. Observed VE translated to prevention of 4.8 (95% CI: 0.5 to 8.6) new antimicrobial prescription courses per 100 infants during the first 90 d of life and 3.9 (95% CI: –5.5 to 12.5) courses per 100 infants during the first year of life (Fig. 2).
Fig. 1.
Incidence of new antimicrobial prescription courses among infants within the ITT population by setting and occurrence of a linked LRTI diagnosis. We present cumulative incidence curves for the denominator of all live-born infants within the ITT population and stratify data for all countries (A and B), HICs (C and D), and LMICs (E and F). Red and black lines indicate observations among infants whose mothers were randomized to the RSV F vaccine and placebo, respectively; Insets plot data throughout the first year of life. We further stratify by drug class in SI Appendix, Fig. S1 and present all-antimicrobial and drug-stratified observations among maternal participants in SI Appendix, Figs. S2 and S3.
Table 2.
VE against antimicrobial prescriptions among infants within the ITT population
| Setting and end point | Through 90 d from birth | Through end of follow-up | ||||
|---|---|---|---|---|---|---|
| RSV F vaccine, no. of events per 100 person-y (no. of events) | Placebo, no. of events per 100 person-y (no. of events) | VE (95% CI), % | RSV F vaccine, no. of events per 100 person-y (no. of events) | Placebo, no. of events per 100 person-y (no. of events) | VE (95% CI), % | |
| All countries, person-y | 730 | 379 | 2,908 | 1,504 | ||
| All antimicrobial prescriptions | 133.7 (976) | 148.7 (563) | 12.9 (1.3–23.1) | 111.2 (3,234) | 112.8 (1,696) | 3.4 (–4.8–11.1) |
| All antimicrobial prescriptions for LRTI* | 71.0 (518) | 82.2 (311) | 16.6 (1.4–29.4) | 61.8 (1,797) | 62.4 (939) | 3.3 (–7.6–13.1) |
| HICs | 242 | 132 | 953 | 516 | ||
| All antimicrobial prescriptions | 55.8 (135) | 72.2 (95) | 20.2 (–10.1–42.2) | 62.8 (599) | 66.1 (341) | 5.2 (–14.2–21.3) |
| All antimicrobial prescriptions for LRTI* | 10.3 (25) | 20.5 (27) | 49.4 (3.5–73.5) | 10.4 (99) | 12.6 (65) | 13.2 (–30.6–42.4) |
| LMICs | 488 | 247 | 1,955 | 988 | ||
| All antimicrobial prescriptions | 172.3 (841) | 189.5 (468) | 10.9 (–2.1–22.2) | 134.8 (2,635) | 137.1 (1,355) | 2.8 (–6.5–11.3) |
| All antimicrobial prescriptions for LRTI* | 101.0 (493) | 115.0 (284) | 12.8 (–3.6–26.7) | 86.9 (1,698) | 88.5 (874) | 2.2 (–9.2–12.5) |
VE is estimated as one minus the hazard ratio fitted via Cox proportional hazards models, allowing for recurrent events and using gamma frailty terms to account for interindividual heterogeneity as well as differing baseline hazards within country-level strata. Further information on drugs prescribed is presented in SI Appendix, Table S2, and estimates of VE against prescribing associated with differing respiratory infection end points are presented in SI Appendix, Table S3. Data on antimicrobial consumption among mothers and VE estimates among mothers are presented in SI Appendix, Tables S4 and S5.
*LRTI was defined by the presence of at least one of the following signs or symptoms: cough, nasal flaring, chest indrawing, subcostal retractions, stridor, rales, rhonchi, wheezing, crackles or crepitations, apnea, hypoxemia (defined as peripheral O2 saturation of <95% at sea level or <92% at altitudes of >1,800 m), or tachypnea (defined as ≥70 breaths per minute at ages 0 to 59 d and ≥60 breaths per minute thereafter).
Fig. 2.
Total antimicrobial prescribing averted by the RSV F vaccine among infants within the ITT population. We plot the number of antimicrobial prescription courses averted over each period as points with lines signifying 95% CIs, derived from primary effect estimates presented in Tables 2 and 3. Estimates are stratified for all prescribing and LRTI-associated prescribing by HIC or LMIC setting and by drug class.
Among infants in high-income countries (HICs; Argentina, Australia, Chile, New Zealand, Spain, the United Kingdom, the United States), VEs were 20.2% (95% CI: –10.1 to 42.2%) and 49.4% (95% CI: 3.5 to 73.5%) against all antimicrobial prescription courses and LRTI-associated antimicrobial prescription courses, respectively, through 90 d of life (Table 2). In low- and middle-income countries (LMICs; Bangladesh, Mexico, the Philippines, South Africa), VEs were 10.9% (95% CI: –2.1 to 22.2%) and 12.8% (95% CI: –3.6 to 26.7%) against any and LRTI-associated antimicrobial prescription courses, respectively, over the same period. Among infants whose mothers were assigned placebo, incidence rates of any and LRTI-associated antimicrobial prescribing were roughly 2.6- and 5.6-fold higher in LMIC settings than HIC settings, respectively, over the first 90 d of life. Accounting for these differences in incidence of antimicrobial prescribing across settings, maternal vaccination prevented an estimated 3.6 (95% CI: –1.8 to 7.6) antimicrobial courses per 100 infants over the first 90 d of life in HICs and 5.1 (95% CI: –1.0 to 10.5) courses per 100 infants over the first 90 d of life in LMICs (Fig. 2). Considering only LRTI-associated prescribing, maternal vaccination was estimated to avert 2.5 (95% CI: 0.2 to 3.8) and 3.7 (95% CI: –1.0 to 7.7) prescription courses per 100 infants in HICs and LMICs, respectively, over the first 90 d of life. Thus, LRTI episodes accounted for 69 to 73% of all antimicrobial prescribing prevented over the first 3 mo of life by maternal RSV vaccination in both HIC and LMIC settings.
Estimates of VE against any acute respiratory infection–associated antimicrobial prescribing during the first 90 d of life were 28.0% (95% CI: –11.4 to 53.5%) and 8.0% (95% CI: –7.6 to 21.3%) in HIC and LMIC settings, respectively (SI Appendix, Table S1). For AOM-associated antimicrobial prescribing, VE over the first 90 d of life was 71.3% (95% CI: 28.1 to 88.6%) in HIC settings, where 9.9 AOM-associated prescriptions occurred per 100 person-years in the placebo arm. In contrast, protection was not evident in LMIC settings where incidence of this end point was markedly lower, with only 2.0 AOM-associated prescriptions per 100 person-years in the placebo arm.
Differences in VE estimates across HIC and LMIC settings were driven in large part by observations in the United States and South Africa, which accounted for 1,067 (23.6%) and 2,368 (52.3%) of 4,524 infants in the ITT efficacy cohort, respectively. Within the placebo arms, incidence rates of antimicrobial prescribing during the first 90 d of life were 55.8 and 175.8 new prescription courses per 100 person-years in the United States and South Africa, respectively (SI Appendix, Table S2). Marked differences were apparent in the clinical conditions precipitating antimicrobial prescriptions in the two settings, with LRTI accounting for 19.0 prescriptions per 100 person-years (34.1% of all prescribing) among US infants and 110.7 prescriptions per 100 person-years (63.0% of all prescribing) among South African infants within the placebo arms of both countries (SI Appendix, Table S3). In contrast, AOM accounted for 10.0 and 2.6 prescriptions per 100 person-years among US and South African infants in the placebo arms, respectively (17.9 and 1.5% of all prescribing in the two settings, respectively). During the first 90 d of life, near-equal volumes of LRTI-associated prescribing were prevented in the two settings (12.0 [95% CI: 3.5 to 15.9] and 13.1 [95% CI: –7.8 to 30.3] LRTI-associated courses prevented per 100 person-years in the United States and South Africa, respectively) (SI Appendix, Table S4). The incidence of AOM-associated antimicrobial prescribing prevented by maternal vaccination over the same period was estimated to be 6.6 (95% CI: 0.3 to 8.8) courses per 100 person-years among US infants vs. –0.6 (95% CI: –7.5 to 1.6) courses per 100 person-years among South African infants.
Reductions in prescribing among infants differed across drug classes (Table 3 and SI Appendix, Fig. S1). Overall, infants whose mothers were assigned the RSV F vaccine and placebo experienced 127.4 and 141.1 new antibiotic prescription courses per 100 person-years over the first 90 d of life, respectively, of which most were LRTI associated (70.4 and 81.4 prescription courses per 100 person-years in the two arms, respectively). These observations corresponded to 13.1% (95% CI: 1.2 to 23.5%) VE against all antibiotic prescription courses and 16.4% (95% CI: 1.3 to 29.3%) VE against LRTI-associated antibiotic prescription courses through 90 d of life. Drugs for which the greatest reductions in prescribing were observed included cephalosporins (with 28.0% [95% CI: 7.1 to 44.2%] VE against any prescribing and 46.2% [95% CI: 19.8 to 63.4%] VE against LRTI-associated prescribing over the first 90 d of life) and aminoglycosides (with 46.1% [95% CI: 19.9 to 64.0%] VE against any prescribing and 49.0% [95% CI: 24.0 to 65.8%] VE against LRTI-associated prescribing over the first 90 d of life). For both drug classes, reduced incidence of new prescription courses among infants whose mothers were assigned the RSV F vaccine remained evident through the end of follow-up. For penicillins, which accounted for 64.2% of all prescribing observed in the trial, VE was 11.2% (95% CI: –2.2 to 22.7%) over the first 90 d of life. Of the 4.8 (95% CI: 0.5 to 8.6) new antimicrobial prescription courses prevented per 100 infants over the first 90 d of life, the averted burdens of prescribing for penicillins, cephalosporins, and aminoglycosides totaled 2.8 (95% CI: –0.5 to 5.8), 2.5 (95% CI: 0.6 to 4.0), and 1.8 (95% CI: 0.2 to 2.9) courses, respectively (Fig. 2).
Table 3.
VE against antimicrobial prescriptions by drug class among infants within the ITT population
| End point and drug | Through 90d from birth | Through end of follow-up | ||||
|---|---|---|---|---|---|---|
| RSV F vaccine, no. of events per 100 person-y (no. of events) | Placebo, no. of events per 100 person-y (no. of events) | VE (95% CI), % | RSV F vaccine, no. of events per 100 person-y (no. of events) | Placebo, no. of events per 100 person-y (no. of events) | VE (95% CI), % | |
| All antimicrobial prescriptions, person-y | 730 | 379 | 2,908 | 1,504 | ||
| Any antibiotic | 127.4 (930) | 141.1 (534) | 13.1 (1.2–23.5) | 108.1 (3,145) | 109.8 (1,651) | 3.7 (–4.6–11.4) |
| Penicillins | 92.2 (673) | 101.7 (385) | 11.2 (–2.2–22.7) | 79.7 (2,317) | 80.9 (1,216) | 2.9 (–6.2–11.2) |
| Cephalosporins | 29.6 (216) | 36.2 (137) | 28.0 (7.1–44.2) | 17.2 (499) | 20.1 (302) | 22.9 (7.9–35.5) |
| Macrolides | 7.7 (56) | 9.5 (36) | 17.8 (–30.1–48.1) | 9.4 (274) | 8.1 (122) | −15.1 (–46.4–9.5) |
| Aminoglycosides | 21.5 (157) | 28.0 (106) | 25.3 (3.5–42.1) | 6.0 (175) | 8.1 (122) | 27.9 (8.3–43.3) |
| Other antibiotics | 10.3 (75) | 15.1 (57) | 29.2 (–4.4–51.9) | 8.0 (232) | 9.0 (136) | 11.8 (–14.0–31.8) |
| Antivirals, antifungals, antiprotozoans, and other antimicrobials | 7.3 (53) | 11.9 (45) | 35.3 (–2.5–59.2) | 3.7 (107) | 4.3 (65) | 12.7 (–23.9–38.5) |
| Antivirals | 3.2 (23) | 3.4 (13) | −20.7 (–184.2–48.7) | 1.7 (49) | 1.5 (22) | −25.8 (–121.0–28.3) |
| Antifungals | 4.1 (30) | 8.5 (32) | 48.0 (8.9–70.3) | 2.0 (58) | 2.9 (43) | 27.8 (–13.9–54.3) |
| All antimicrobial prescriptions for LRTI* | ||||||
| Any antibiotic | 70.4 (514) | 81.4 (308) | 16.4 (1.3–29.3) | 61.6 (1,791) | 62.2 (935) | 3.2 (–7.7–13.0) |
| Penicillins | 56.4 (412) | 63.4 (240) | 13.0 (–3.6–26.9) | 49.5 (1,439) | 49.6 (746) | 2.0 (–9.8–12.6) |
| Cephalosporins | 9.6 (70) | 15.3 (58) | 46.2 (19.8–63.4) | 5.9 (173) | 8.7 (131) | 38.1 (18.9–52.8) |
| Macrolides | 4.4 (32) | 7.7 (29) | 42.6 (–1.7–67.6) | 6.0 (175) | 4.8 (72) | −23.8 (–69.7–9.6) |
| Aminoglycosides | 7.4 (54) | 13.5 (51) | 46.1 (19.9–64.0) | 2.1 (62) | 4.1 (62) | 49.1 (26.8–64.6) |
| Other antibiotics | 1.4 (10) | 3.2 (12) | 48.0 (–32.7–79.6) | 1.3 (37) | 2.3 (35) | 44.6 (0.9–69.0) |
| Antivirals, antifungals, antiprotozoans, and other antimicrobials | 0.5 (4) | 2.6 (10) | 75.2 (10.4–93.2) | 0.3 (8) | 0.9 (13) | 64.9 (8.1–86.6) |
| Antivirals | 0.4 (3) | 2.1 (8) | 75.9 (–10.1–94.7) | 0.2 (6) | 0.7 (10) | 64.9 (–11.1–88.9) |
| Antifungals | 0.1 (1) | 0.5 (2) | 73.1 (–196.8–97.6) | 0.1 (2) | 0.2 (3) | 65.0 (–109.6–94.2) |
VE is estimated as one minus the hazard ratio fitted via Cox proportional hazards models, allowing for recurrent events and using gamma frailty terms to account for interindividual heterogeneity as well as differing baseline hazards within country-level strata. Further information on drugs prescribed is presented in SI Appendix, Table S2, and estimates of VE against prescribing associated with differing respiratory infection end points are presented in SI Appendix, Table S3. Data on antimicrobial consumption among mothers and VE estimates among mothers are presented in SI Appendix, Tables S4 and S5.
*LRTI was defined by the presence of at least one of the following signs or symptoms: cough, nasal flaring, chest indrawing, subcostal retractions, stridor, rales, rhonchi, wheezing, crackles or crepitations, apnea, hypoxemia (defined as peripheral O2 saturation of <95% at sea level or <92% at altitudes of >1,800 m), or tachypnea (defined as ≥70 breaths per minute at ages 0 to 59 d and ≥60 breaths per minute thereafter).
Of all trial participants, South African infants experienced the greatest reductions in prescribing of cephalosporins and aminoglycosides (SI Appendix, Tables S5 and S6). As compared with 10.0 and 13.4 prescriptions of cephalosporins and aminoglycosides, respectively, per 100 person-years among US infants aged 0 to 90 d whose mothers were assigned placebo, rates of prescribing of these drugs among South African infants in the placebo arm were 32.3 and 30.9 courses per 100 person-years, respectively (SI Appendix, Tables S5 and S6). Among US infants, VE against prescribing of penicillins was 42.9% (95% CI: 5.4 to 65.5%) over the first 90 d of life, similar to the estimate in other high-income settings (VE = 38.2% [95% CI: –18.0 to 67.6%]). Whereas significant protection against prescribing of penicillins was not observed in South Africa, it should be noted that penicillins accounted for a higher proportion of all antimicrobial prescribing in that setting (81.9 vs. 62.0% in the placebo arms of South Africa and the United States, respectively), with 4.2-fold higher rates of new prescriptions among South African infants (144.0 vs. 34.6 prescriptions per 100 children annually in the placebo arms). The absolute incidence of penicillin prescribing prevented by maternal vaccination was similar over the first 90 d of life between the two settings, with 14.8 (95% CI: 1.9 to 22.7) and 12.1 (95% CI: –11.5 to 32.0) prescriptions prevented per 100 infants annually in the US and South African trial cohorts, respectively.
Across all settings, we further estimated 75.2% (95% CI: 10.4 to 93.2%) and 64.9% (95% CI: 8.1 to 86.6%) VE against LRTI-associated prescription of antivirals, antifungals, antiprotozoans, and other antimicrobials during the first 90 d and through end of follow-up, respectively, among infants (Table 3). Infants whose mothers were randomized to the RSV F vaccine experienced 48.0% (95% CI: 8.9 to 59.2%) VE against all antifungal prescribing over the first 90 d of life, amounting to 1.0 (95% CI: 0.2 to 1.5) course averted per 100 infants (Fig. 2). We did not identify evidence of differences in rates of new antimicrobial prescription courses for maternal participants assigned the RSV F vaccine or placebo overall or for specific drugs (SI Appendix, Figs. S2 and S3 and Tables S7 and S8).
Discussion
In this randomized, double-blinded, multisite trial, infants of mothers assigned the RSV F vaccine received fewer antimicrobial prescription courses over the first 90 d of life than infants of mothers assigned placebo. While estimated efficacy of the RSV F vaccine against RSV-associated, medically significant LRTI in this trial did not meet the prespecified criterion for success, our findings implicate RSV as a major contributor to antimicrobial prescribing among young infants and demonstrate that this prescribing is preventable by maternal vaccination. This evidence of protection against all-cause antimicrobial prescribing end points is particularly noteworthy given that the bulk of prescribing is associated with nonsevere conditions against which vaccination may be expected to offer lower degrees of protection (35); indeed, expanded ITT analyses including trial-specific and hospital record data revealed incrementally higher VE point estimates of 53.8, 58.6, and 73.7% for end points of medically significant RSV-associated LRTI, RSV-associated LRTI with hospitalization, and RSV-associated LRTI with severe hypoxemia, respectively (34). Future RSV vaccine candidates achieving higher efficacy may achieve greater reductions than were evident in this trial, underscoring the importance of RSV vaccine development as a strategy to reduce antimicrobial consumption and AMR.
In this trial, the RSV F vaccine yielded the most noteworthy effects on prescribing of cephalosporins and aminoglycosides. These findings were most clearly apparent among South African infants in site-specific analyses, although it should be noted that stratification by both drug and setting resulted in underpowered analyses for most trial sites. While estimated VE against prescribing of penicillins did not exclude the possibility of no effect, it is important to note that penicillins were the most commonly prescribed antibiotic class in this study. Point estimates indicating prevention of 2.8 penicillin prescriptions per 100 infants over the first 90 d of life accounted for over half the estimated vaccine-attributable reduction in all antimicrobial prescribing in the trial. Among US infants who experienced 42.9% (95% CI: 5.4 to 65.5%) VE against prescribing of penicillins during the first 90 d of life, penicillins accounted for nearly all prescribing prevented by maternal vaccination. Our findings likely reflect international guidelines for the management of severe pneumonia with ampicillin/amoxicillin (or penicillin) and gentamicin as the first-line treatment and ceftriaxone as a second-line therapy (36). The extended duration over which we identified protection against LRTI-associated prescribing of certain drugs may have been anticipated from adverse event monitoring in the trial, which revealed 24.6, 32.4, and 15.0% lower incidence of all-cause pneumonia, ear infection, and rhinitis, respectively, through the first year of life among infants whose mothers were assigned vaccination (34). Whereas most prescribing of aminoglycosides occurred during the first 90 d of life, the same was not true for prescribing of other drugs against which we identified protection through the end of follow-up.
In an earlier publication (34), expanded ITT analyses of the primary trial end points identified greater VE against all RSV-associated LRTI end points in LMICs as compared with HICs. In comparing findings for these microbiologically specific end points with findings from the present analysis, it is important to consider that VE against antimicrobial use end points is influenced not only by protection against RSV infection and progression to disease, but also by antimicrobial prescribing practices and burden of RSV-associated disease, each of which may differ across settings. In the placebo arm of this trial, incidence rates of antimicrobial prescribing due to any cause and due to LRTI were 2.6- and 5.6-fold higher in LMICs than HICs, respectively, during the first 90 d of life (189.5 vs. 72.2 prescriptions per 100 child-years and 115.0 vs. 20.5 prescriptions per 100 child-years, respectively), indicating differences in the burden associated with respiratory pathogens beyond RSV. Whereas our 20.2% VE point estimate against antimicrobial prescriptions among HIC infants through 90 d of life translated to prevention of 3.6 prescriptions per 100 infants, a 10.9% VE point estimate in LMIC settings translated to prevention of 5.1 prescriptions per 100 infants. Among infants in the United States and South Africa specifically, maternal vaccination prevented 3.0 and 3.2 LRTI-associated prescriptions, respectively, per 100 infants over the first 90 d of life, despite substantial differences in VE point estimates in the two settings (63.4% in the United States vs. 11.9% in South Africa). Pathways by which RSV infection contributes to common, nonsevere illnesses that account for the majority of antimicrobial prescribing merit further investigation. While some antimicrobial treatment may occur for LRTI episodes directly attributable to RSV, a substantial proportion may also occur for bacterial infections precipitated by prior and possibly, mild or asymptomatic RSV infection in the respiratory tract (37–39). It remains to be determined to what extent the observed efficacy of the RSV F vaccine against RSV-associated LRTI in this trial resulted from prevention of acquisition of RSV or protection against progression to medically significant illness and how these modes of protection may impact risk of diseases that result in antimicrobial prescriptions. Regardless of the specific mechanisms involved, however, our findings signify a similar burden of vaccine-preventable antimicrobial prescribing in the United States and other HICs as compared with South Africa and other LMICs in the context of substantially differing incidence of antimicrobial prescribing due to other causes.
This trial also revealed substantial VE against AOM-related antimicrobial prescribing among infants in HIC settings but no protection against this end point among LMIC infants. Incidence of AOM-associated antimicrobial prescribing was 5.0-fold higher within the placebo cohort of infants in HIC settings than LMIC settings (9.9 vs. 2.0 prescriptions per 100 child-years). Underdiagnosis of AOM in LMICs is widely recognized (40) and may contribute to these differences. Previous studies have implicated RSV as the most prominent viral etiology of AOM (19), particularly during the first year of life (41), substantiating the biological basis for our findings in HIC settings.
It is unsurprising that this trial did not reveal effects of the RSV F vaccine against antimicrobial prescribing among maternal participants. First exposure to RSV typically occurs early in life, and young adults are frequently reexposed and thus, relatively resistant to clinically important disease involving this pathogen (20, 42). Relative to observations among infants, acute respiratory infections and LRTIs were less prominent causes of antimicrobial prescribing among maternal participants; prophylaxis for group B Streptococcus or labor-related surgeries accounted for a high proportion of all antimicrobial prescribing within this group.
The 12.9% VE against all-cause antimicrobial prescribing estimated in this trial should be considered in the context of other interventions aiming to reduce antimicrobial exposure. When successful, intensive antimicrobial stewardship campaigns targeted to health care providers have reduced all-cause outpatient antibiotic prescribing by orders of only 3 to 6% (43, 44) and have proven difficult to implement in a cost-effective manner (45). While some RSV-attributable antimicrobial prescribing observed in this trial is unlikely to provide clinical benefit (46), facilitative interactions between bacteria and RSV in the upper airway (23–27) contribute to bacterial disease pathogenesis, ultimately necessitating antimicrobial use, which would not be preventable through stewardship. Moreover, prompt antibiotic treatment—before microbial etiology can be established—is recommended for children with severe LRTI owing to the risk for rapid clinical deterioration in cases with bacterial etiology (36). Thus, benefits of RSV vaccination likely encompass reductions in both appropriate and inappropriate antimicrobial prescribing. Prior studies have estimated that pneumococcal conjugate vaccines and rotavirus vaccines prevent 20 and 11% of antibiotic-treated episodes of acute respiratory infection and diarrhea, respectively, among young children (5); during the influenza season, all-cause antimicrobial prescribing was reduced by 42% in communities randomized to receive influenza vaccination in a cluster-randomized trial (47). These findings underscore the relative importance of vaccination as a strategy to control antimicrobial use, in addition to reducing disease burden, and suggest that maternal RSV vaccination could have a quantitatively similar impact to other vaccine programs.
Our study has limitations. Results of this post hoc secondary analysis should be viewed as hypothesis generating, as the trial was not powered for determination of effects against antimicrobial prescribing, and our analyses were not adjusted for multiplicity. As enrollment was timed relative to the season of peak RSV transmission in each setting, estimates do not necessarily reflect the overall burden of disease or antimicrobial prescribing associated with RSV, particularly at older ages when infants in our study were unlikely to be exposed to RSV. The trial excluded maternal participants with high-risk pregnancies, whose infants may comprise an important risk group for RSV infection. Larger studies are needed to estimate smaller vaccine effect sizes with appropriate precision throughout the first year of life, as protection from maternally derived immunity wanes and RSV may cause less prescribing during later infancy. Immunogenicity analyses within the trial cohort estimated 31- to 49-d half-lives for palivizumab-competitive antibody, anti–F protein immunoglobulin G (IgG), and RSV A/B microneutralization titers (34); in a smaller phase II immunogenicity study, antibody levels among infants whose mothers were assigned the RSV F vaccine and placebo converged around 180 d after birth (48).
Future studies are warranted to better define the contribution of RSV to antimicrobial prescribing among infants, including the extent to which RSV acts as a primary or coinfecting pathogen in LRTI and other syndromes for which antimicrobial drugs are often prescribed. Averting antimicrobial prescribing is of particular importance for assessments of the public health impact and value proposition of RSV vaccines, which may ultimately prevent fewer severe disease cases and deaths than other vaccines, such as those targeting S. pneumoniae, rotavirus, and influenza, due to differences in the global burden of these pathogens (49, 50). Our findings that RSV contributes to antimicrobial prescribing among young infants and that this prescribing is preventable by maternal vaccination suggest that assessments of efficacy and impact on antimicrobial prescribing should be included in the evaluation of interventions against RSV and other viral respiratory pathogens.
Materials and Methods
Procedures.
Trial design and primary safety and efficacy end points have been reported previously (34). Briefly, healthy women ages 18 to 40 y with low-risk singleton pregnancies were recruited and were randomized to receive RSV F vaccination or placebo at between 28 and 36 wk of gestation before the start of the typical RSV season in their location (SI Appendix, Table S9). Participants and trial staff were blinded to vaccine or placebo assignment throughout the trial; investigators worked with unblinded datasets for this secondary analysis. Detailed eligibility criteria are included in the original study protocol (34).
Maternal participants were randomized to receive a 0.5-mL intramuscular injection, which was either a 120-µg dose of RSV F protein, adsorbed to a 0.4-mg dose of aluminum as the phosphate salt, or a placebo formulation buffer without aluminum. Block randomization was conducted by each participating site using an interactive web-based randomization system; blocks were stratified by trial site and maternal age (18 to 28 or 29 to 40 y). The trial followed a group-sequential design, assigning treatment at a 1:1 (vaccine/placebo) ratio during the first (2015 to 2016) RSV season and a 2:1 ratio thereafter based on a benign safety profile in the first season.
The original trial design targeted a sample size of up to 8,618 third-trimester pregnant subjects to meet power requirements for the primary end point of RSV-associated medically significant LRTI in the first 90 d of life. Enrollment was terminated after a third RSV season, when an informational analysis was consistent with the presence of efficacy and the number of maternal participants in the vaccine arm exceeded 3,000 (a minimum target for detection of safety signals involving severe adverse events).
Trial Objectives, End Points, and Monitoring.
This post hoc secondary analysis was undertaken to determine VE against new antimicrobial prescription courses among infants during the first 90 d of life and through end of follow-up (scheduled around 365 d of life). We also assessed VE against new antimicrobial prescription courses among maternal participants through the end of follow-up (scheduled around 180 d postdelivery). We considered new antimicrobial prescription courses to have occurred any day when individuals received a prescription for one or more antimicrobial drugs (as multiple drugs could be prescribed as part of the same treatment course). Monitoring of all prescriptions for maternal participants and infants occurred as a routine component of adverse event surveillance in the trial. Investigators were responsible for recording medical indications for each new prescription ordered in consultation with children’s primary clinical care providers and parents or guardians in instances where prescriptions were not ordered by study personnel.
We also evaluated VE against new antimicrobial prescription courses for LRTI, AOM, and all (upper or lower) acute respiratory tract infections based on medical indications recorded for each antimicrobial prescription. We considered LRTI to encompass illness episodes where the presence of one or more of the following signs or symptoms was noted: cough, nasal flaring, chest indrawing, subcostal retractions, stridor, rales, rhonchi, wheezing, crackles or crepitations, apnea, hypoxemia (defined as peripheral O2 saturation of <95% at sea level or <92% at altitudes of >1,800 m), or tachypnea (defined as ≥70 breaths per minute at ages 0 to 59 d and ≥60 breaths per minute thereafter). Passive surveillance for LRTI proceeded as follows; maternal participants or infants’ guardians were instructed to contact study staff, preferably <72 h from symptoms onset, if their infants experienced any LRTI symptoms (as listed above) or poor feeding/failure to feed, lethargy, or irritability. Maternal participants were likewise encouraged to contact study staff if they themselves experienced cough, nasal congestion, fever with one or more respiratory symptoms, runny nose, pharyngitis, or dyspnea. For each illness episode alerted to study staff, a visit was scheduled for medical assessment within 72 h. As an additional active surveillance measure, study staff members made telephone calls to maternal participants or infants’ guardians (at minimum) every 7 d during the first 180 d of life to determine whether infants had recently experienced any acute respiratory symptoms or adverse events. From approximately day 180 to 364 of life, adverse events, including respiratory infections and any attendant antibiotic use, were ascertained through passive reporting or at scheduled visits as detailed below.
Study staff recorded medication history for all other causes via interview of parents and review of medical records at scheduled follow-up visits (at 14, 35, 60, 90, 112, 180, 252, and 364 d after delivery for infants; for maternal participants, at 7, 14, and 28 d after randomization, at delivery, and 35 and 180 d after delivery) and at unscheduled follow-up visits in case of any medically attended adverse event. Infants’ primary pediatricians were also notified before or shortly after delivery that infants were participating in an investigational study and were encouraged (along with parents/guardians of infants) to report adverse events to study staff. We report total antimicrobial prescriptions for individual drugs by country, follow-up period, and randomization arm in SI Appendix, Tables S10 and S11 for infants and maternal participants, respectively.
Statistical Analysis.
The ITT efficacy analysis population for this study included maternal participants who underwent randomization and their live-born infants for whom complete informed consent documentation was available, regardless of treatment errors or other protocol deviations. Consistent with the primary trial analysis (34), the ITT efficacy analysis population was limited to infants and maternal participants who were randomized, received a study treatment, and completed one or more study visits from which efficacy could be calculated, meaning that source data on prescription medications were available to and were obtained by study staff. We truncated individuals’ observations at dates of trial exit if this preceded the end of scheduled follow-up.
We used Cox proportional hazards models to compute hazard ratios for infants and maternal participants assigned the RSV F vaccine vs. placebo for each outcome. Analyses accommodated recurrent events via the Andersen–Gill method (51); we used gamma frailty distributions to account for heterogeneity among individuals in the context of repeated measures (52). We allowed for country-specific baseline hazards (via regression strata) to address imbalance in the distribution of countries among maternal participants assigned the RSV F vaccine and placebo, which resulted from the midstudy transition from a 1:1 to 2:1 vaccine/placebo randomization scheme. We measured VE as . We computed 95% CIs without adjustment for multiplicity and considered estimates with 2.5-percentile lower-bound VE > 0% to provide statistically significant evidence of protection. However, as the primary trial design was not explicitly powered for these exploratory end points, we caution that estimates for which CIs include the null value of zero should not be considered to definitively rule out clinically meaningful vaccine effects. Relevant considerations for the interpretation of statistical testing and effect size measurement in the context of secondary data analyses have been described elsewhere (53, 54). We estimated vaccine-avertible incidence of antimicrobial prescribing by multiplying VE estimates against observed incidence within the placebo cohort. We conducted all analyses in R (version 4.0.5; R Foundation for Statistical Computing) and used the survival (55) package to fit proportional hazards models.
Ethics Declarations.
Secondary analyses of the deidentified trial data reported in this manuscript were considered nonhuman subjects research (exempt from review) by the Committee for Protection of Human Subjects at the University of California, Berkeley.
Supplementary Material
Acknowledgments
We thank Dr. Padmini Srikantiah for support. This study was funded by Bill & Melinda Gates Foundation Grant OPP1190803. The sponsor had no role in the preparation, review, or approval of the manuscript or the decision to submit the manuscript for publication.
Footnotes
Competing interest statement: J.A.L. discloses receipt of grants and honoraria from Pfizer unrelated to this research. L.F.F., I.C., and J.C. are employees of Novavax.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2112410119/-/DCSupplemental.
Data Availability
Analysis code has been deposited in GitHub (https://github.com/joelewnard/rsv_abx) (56). Previously published data were also used for this work (34). Data needed to replicate results of the analysis are presented in SI Appendix, Tables S10 and S11, with stratification by outcome, setting, treatment arm, and follow-up interval. Public sharing of original individual-level records from this trial was not permitted by regulatory and institutional review boards. Requests for data access should be made to L.F.F. (lfries@novavax.com); sharing of data will be contingent on both parties signing a disclosure agreement.
References
- 1.Olesen S. W., Lipsitch M., Grad Y. H., Response to comment on “The distribution of antibiotic use and its association with antibiotic resistance.” eLife 8, e47124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, US national action plan (2019). https://www.cdc.gov/drugresistance/us-activities/national-action-plan.html. Accessed 22 February 2022.
- 3.The World Bank, Drug-resistant infections: A threat to our economic future (2017). https://www.worldbank.org/en/topic/health/publication/drug-resistant-infections-a-threat-to-our-economic-future. Accessed 22 February 2022.
- 4.Neuzil K. M., Mellen B. G., Wright P. F., Mitchel E. F. Jr., Griffin M. R., The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N. Engl. J. Med. 342, 225–231 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Lewnard J. A., Lo N. C., Arinaminpathy N., Frost I., Laxminarayan R., Childhood vaccines and antibiotic use in low- and middle-income countries. Nature 581, 94–99 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fireman B., et al. , Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr. Infect. Dis. J. 22, 10–16 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Smith E. R., et al. , Reducing antibiotic use in ambulatory care through influenza vaccination. Clin. Infect. Dis. 71, e726–e734 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vekemans J., et al. , Leveraging vaccines to reduce antibiotic use and prevent antimicrobial resistance: A WHO action framework. Clin. Infect. Dis. 73, e1011–e1017 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight G. M., et al. , Mathematical modelling for antibiotic resistance control policy: Do we know enough? BMC Infect. Dis. 19, 1011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming-Dutra K. E., et al. , Prevalence of inappropriate antibiotic prescriptions among us ambulatory care visits, 2010-2011. JAMA 315, 1864–1873 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Rogawski E. T., et al. , Use of antibiotics in children younger than two years in eight countries: A prospective cohort study. Bull World Health Organ 95, 49–61 (2017). Correction in: Bull World Health Organ 95, 164 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewnard J. A., Uses of pathogen detection data to estimate vaccine direct effects in case-control studies. J. R. Soc. Interface 17, 20200161 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madhi S. A., Klugman K. P.; Vaccine Trialist Group, A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 10, 811–813 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard L. M., et al. , Nasopharyngeal pneumococcal density during asymptomatic respiratory virus infection and risk for subsequent acute respiratory illness. Emerg. Infect. Dis. 25, 2040–2047 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter N. D., et al. ; Active Bacterial Core Surveillance Team, Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin. Infect. Dis. 50, 175–183 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Iverson A. R., et al. , Influenza virus primes mice for pneumonia from Staphylococcus aureus. J. Infect. Dis. 203, 880–888 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullers J. A., The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 12, 252–262 (2014). [DOI] [PubMed] [Google Scholar]
- 18.O’Brien K. L., et al. ; Pneumonia Etiology Research for Child Health (PERCH) Study Group, Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet 394, 757–779 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heikkinen T., Thint M., Chonmaitree T., Prevalence of various respiratory viruses in the middle ear during acute otitis media. N. Engl. J. Med. 340, 260–264 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Hall C. B., et al. , The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 360, 588–598 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick T., et al. , Community-based antibiotic prescribing attributable to respiratory syncytial virus and other common respiratory viruses in young children: A population-based time-series study of Scottish children. Clin. Infect. Dis. 72, 2144–2153 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Taylor S., et al. , Modelling estimates of the burden of respiratory syncytial virus infection in children in the UK. BMJ Open 6, e009337 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung J., Seo E., Yoo R. N., Sung H., Lee J., Clinical significance of viral-bacterial codetection among young children with respiratory tract infections: Findings of RSV, influenza, adenoviral infections. Medicine (Baltimore) 99, e18504 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morpeth S. C., et al. , Impact of viral upper respiratory tract infection on the concentration of nasopharyngeal pneumococcal carriage among Kenyan children. Sci. Rep. 8, 11030 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeMuri G. P., Gern J. E., Eickhoff J. C., Lynch S. V., Wald E. R., Dynamics of bacterial colonization with Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis during symptomatic and asymptomatic viral upper respiratory tract infection. Clin. Infect. Dis. 66, 1045–1053 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgene M. F., et al. , Staphylococcus aureus colonization and non-influenza respiratory viruses: Interactions and synergism mechanisms. Virulence 9, 1354–1363 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brealey J. C., et al. , Bacterial colonization dynamics associated with respiratory syncytial virus during early childhood. Pediatr. Pulmonol. 55, 1237–1245 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Jansen A. G. S. C., et al. , Invasive pneumococcal and meningococcal disease: Association with influenza virus and respiratory syncytial virus activity? Epidemiol. Infect. 136, 1448–1454 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberger D. M., Klugman K. P., Steiner C. A., Simonsen L., Viboud C., Association between respiratory syncytial virus activity and pneumococcal disease in infants: A time series analysis of US hospitalization data. PLoS Med. 12, e1001776 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberger D. M., et al. , Influence of pneumococcal vaccines and respiratory syncytial virus on alveolar pneumonia, Israel. Emerg. Infect. Dis. 19, 1084–1091 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberger D. M., et al. , Seasonal drivers of pneumococcal disease incidence: Impact of bacterial carriage and viral activity. Clin. Infect. Dis. 58, 188–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipsitch M., Siber G. R., How can vaccines contribute to solving the antimicrobial resistance problem? MBio 7, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clift C., Salisbury D. M., Enhancing the role of vaccines in combatting antimicrobial resistance. Vaccine 35, 6591–6593 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madhi S. A., et al. ; Prepare Study Group, Respiratory syncytial virus vaccination during pregnancy and effects in infants. N. Engl. J. Med. 383, 426–439 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halloran M. E., Struchiner C. J., Longini I. M. Jr., Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am. J. Epidemiol. 146, 789–803 (1997). [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization, Revised WHO classification and treatment of childhood pneumonia at health facilities: Evidence summaries (2014). https://apps.who.int/iris/bitstream/handle/10665/137319/9789241507813_eng.pdf;jsessionid=D10ED3233B1B87B7B791577A041151BC?sequence=1. Accessed 22 February 2022. [PubMed]
- 37.Beadling C., Slifka M. K., How do viral infections predispose patients to bacterial infections? Curr. Opin. Infect. Dis. 17, 185–191 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Fleming D. M., Pannell R. S., Elliot A. J., Cross K. W., Respiratory illness associated with influenza and respiratory syncytial virus infection. Arch. Dis. Child. 90, 741–746 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verhoeven D., Xu Q., Pichichero M. E., Differential impact of respiratory syncytial virus and parainfluenza virus on the frequency of acute otitis media is explained by lower adaptive and innate immune responses in otitis-prone children. Clin. Infect. Dis. 59, 376–383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li M. G., Hotez P. J., Vrabec J. T., Donovan D. T., Is chronic suppurative otitis media a neglected tropical disease? PLoS Negl. Trop. Dis. 9, e0003485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas E., et al. , Burden of respiratory syncytial virus infection during the first year of life. J. Infect. Dis. 223, 811–817 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Falsey A. R., Hennessey P. A., Formica M. A., Cox C., Walsh E. E., Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352, 1749–1759 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Hallsworth M., et al. , Provision of social norm feedback to high prescribers of antibiotics in general practice: A pragmatic national randomised controlled trial. Lancet 387, 1743–1752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finkelstein J. A., et al. , Impact of a 16-community trial to promote judicious antibiotic use in Massachusetts. Pediatrics 121, e15–e23 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Naylor N. R., et al. , Is antimicrobial stewardship cost-effective? A narrative review of the evidence. Clin. Microbiol. Infect. 23, 806–811 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Pinto L. A., et al. , Azithromycin therapy in hospitalized infants with acute bronchiolitis is not associated with better clinical outcomes: A randomized, double-blinded, and placebo-controlled clinical trial. J. Pediatr. 161, 1104–1108 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Loeb M., et al. , Effect of influenza vaccination of children on infection rates in Hutterite communities: A randomized trial. JAMA 303, 943–950 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Muňoz F. M., et al. , Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. J. Infect. Dis. 220, 1802–1815 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Walker C. L. F., et al. , Global burden of childhood pneumonia and diarrhoea. Lancet 381, 1405–1416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi T., et al. ; RSV Global Epidemiology Network, Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 390, 946–958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersen P. K., Gill R. D., Cox’s regression model for counting processes: A large sample study. Ann. Stat. 10, 1100–1120 (2007). [Google Scholar]
- 52.McGilchrist C. A., Aisbett C. W., Regression with frailty in survival analysis. Biometrics 47, 461–466 (1991). [PubMed] [Google Scholar]
- 53.Hernán M. A., Causal analyses of existing databases: No power calculations required. J. Clin. Epidemiol., 10.1016/j.jclinepi.2021.08.028 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wasserstein R. L., Lazar N. A., The ASA statement on p-values: Context, process, and purpose. Am. Stat. 70, 129–133 (2016). [Google Scholar]
- 55.Therneau T. M., survival: A Package for Survival Analysis in R. R Package version 3.2-11 (2021). https://cran.r-project.org/package=survival. Accessed 22 February 2022.
- 56.J. A. Lewnard, "rsv_abx" repository, trialAnalysis.R. GitHub. https://github.com/joelewnard/rsv_abx. Deposited 22 February 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Analysis code has been deposited in GitHub (https://github.com/joelewnard/rsv_abx) (56). Previously published data were also used for this work (34). Data needed to replicate results of the analysis are presented in SI Appendix, Tables S10 and S11, with stratification by outcome, setting, treatment arm, and follow-up interval. Public sharing of original individual-level records from this trial was not permitted by regulatory and institutional review boards. Requests for data access should be made to L.F.F. (lfries@novavax.com); sharing of data will be contingent on both parties signing a disclosure agreement.