Abstract
The aim of the present study was to determine the efficacy of an antibiotic-lock technique in preventing endoluminal catheter-related infection with gram-positive bacteria in neutropenic patients with hematologic malignancies. Patients with nontunneled, multilumen central venous catheters were assigned in a randomized, double-blinded manner to receive either 10 U of heparin per ml (57 patients) or 10 U of heparin per ml and 25 μg of vancomycin per ml (60 patients), which were instilled in the catheter lumen and which were allowed to dwell in the catheter lumen for 1 h every 2 days. Insertion-site and hub swabs were taken twice weekly. The primary and secondary end points of the trial were significant colonization of the catheter hub and catheter-related bacteremia, respectively. Significant colonization of the catheter hub occurred in nine (15.8%) patients receiving heparin (seven patients were colonized with Staphylococcus epidermidis, one patient was colonized with Staphylococcus capitis, and one patient was colonized with Corynebacterium sp.), whereas the catheter hubs of none of the patients receiving heparin and vancomycin were colonized (P = 0.001). Catheter-related bacteremia developed in four (7%) patients receiving heparin (three patients had S. epidermidis bacteremia and one patient had S. capitis bacteremia), whereas none of the patients in the heparin and vancomycin group had catheter-related bacteremia (P = 0.05). The times to catheter hub colonization and to catheter-related bacteremia by the Kaplan-Meier method were longer in patients receiving heparin and vancomycin than in patients receiving heparin alone (P = 0.004 and P = 0.06, respectively). Our study shows that a solution containing heparin and vancomycin administered by using an antibiotic-lock technique effectively prevents catheter hub colonization with gram-positive bacteria and subsequent bacteremia during chemotherapy-induced neutropenia in patients with hematologic malignancy.
Central venous catheters (CVCs) have become essential tools for the appropriate management of patients with cancer. These devices provide reliable access for administration of chemotherapeutic agents, blood products, parenteral nutrition, and antibiotics, but they are also a major cause of infection (4). Catheter-related infection causes significant morbidity and mortality in cancer patients, particularly during episodes of chemotherapy-induced neutropenia (6, 7, 10, 23, 28). It is estimated that the rate of septicemia associated with noncuffed CVCs ranges from 4 to 14 percent (18). The major pathogens that cause these infections are the gram-positive cocci, mainly coagulase-negative staphylococci (CoNS) (5, 29). The skin insertion site and the catheter hub are the most important sources of catheter colonization and subsequent catheter-related bacteremia (11, 18). In neutropenic patients with hematologic malignancies, the catheter hub, which is frequently manipulated by the sanitary personnel, is a major portal of entry for organisms (30). From the contaminated hub, the organisms migrate along the internal surface of the catheter, where they create a bloodstream infection.
The antibiotic-lock technique (ALT) consists of filling and closing of the catheter lumen with an antibiotic solution that acts locally and that allows the side effects and toxicity associated with systemic administration of antibiotics to be avoided (2). This method, which allows delivery of a high concentration of antibiotic inside the catheter, has been used to eradicate catheter-related bacteremia, mainly in patients receiving parenteral nutrition at home, although no comparative randomized trials have been performed to date (1, 15). We undertook a randomized, double-blind trial to determine the efficacy of an ALT in preventing endoluminal catheter-related infection with gram-positive bacteria during chemotherapy-induced neutropenia in patients with hematologic malignancies. The primary and secondary end points of the trial were significant colonization of the catheter hub and catheter-related bacteremia, respectively.
MATERIALS AND METHODS
Setting and study population.
The study was conducted at the hematology ward of a university hospital for adults in Barcelona, Spain. From April 1994 through March 1996, hospitalized patients with a nontunneled, multilumen, polyurethane CVC (Arrow International, Inc., Reading, Pa.) in place and who were to receive chemotherapy designed to produce severe neutropenia (<500 neutrophils per μl) were eligible for participation in the trial. Patients were excluded if they had clinical or microbiologic evidence of infection or had a known allergy to vancomycin. Patients already receiving antibiotics or parenteral nutrition were also excluded. Participants gave informed consent, and the study was approved by the Ethics Committee of our institution.
Catheter insertion and care.
Catheters were inserted into the subclavian vein by physicians who wore masks, caps, sterile gloves, and surgical gowns and who used large sterile drapes. Study catheters were not exchanged over guidewires. At the time of catheter insertion, the skin insertion site was disinfected with 4% chlorhexidine gluconate (Hibiscrub; ICI Farma, Pontevedra, Spain), which was applied by scrubbing for at least 30 s. The insertion sites were covered with sterile gauze.
Catheter care included changing of the dressing, stock-cocks, and tubes every 48 h by registered nurses, who followed maximal barrier precautions. The insertion site was softly scrubbed with sterile gauze saturated with normal saline and 4% chlorhexidine gluconate for at least 30 s, rinsed with normal saline, and dried. Catheter lumens were assessed for occlusion and were flushed with 10 ml of 0.9% sodium chloride. In case of occlusion the lumen was flushed with either heparin or urokinase by a soft instillation-aspiration technique to reestablish patency. The insertion site and catheter hubs were protected with 1% chlorhexidine pomade (Hibitane crema; ICI Farma), dressed, and taped securely.
Study design.
Patients were randomized, on the day after completion of the corresponding chemotherapy course, to receive in a double-blind fashion either a solution containing heparin (Rovi, SA, Madrid, Spain) at 10 U/ml or heparin at 10 U/ml and vancomycin (Lilly, Madrid, Spain) at 25 μg/ml. For study solution allocation a computer-generated list of random numbers, which was available only to the pharmacist, was used. The stability of the heparin and vancomycin solution has been demonstrated previously (8). The hospital pharmacy prepared the solutions on a horizontal-airflow workbench using aseptic technique by a previously described procedure (25). The two solutions were indistinguishable to medical personnel and were dispensed in 20-ml vials that were numerically coded and that were kept refrigerated. The code list was kept in the hospital pharmacy and was opened only after the study was completed. The neutrophil count was determined daily, and when it was below 500 per μl the administration of the allocated solution was initiated. A 2.5-ml injection of the solution was administered through the stock-cocks in each catheter lumen and was allowed to dwell for approximately 1 h every 2 days. In all cases, the solution was then aspirated with a syringe and was discarded.
Swabs of the skin insertion site and the inner surfaces of the catheter hubs were obtained before randomization and twice weekly thereafter for culture. Swabs of the insertion site and catheter hub as well as blood specimens from a peripheral vein were also obtained for culture before the beginning of intravenous empirical antibiotic therapy for fever and neutropenia. Ceftazidime plus amikacin was the empirical antibiotic regimen most commonly used to treat febrile episodes that occurred during the study period. Vancomycin was added to the regimens of patients in whom infection with gram-positive bacteria was initially suspected and also those who had not improved after initial therapy for 48 h or who worsened before that time. In case of catheter removal, two 5-cm segments, a proximal subcutaneous segment and the tip, were sampled by a sterile technique for culture. Patients were monitored until any of the following criteria was present: recovery from neutropenia, administration of systemic vancomycin, colonization of the catheter hub, bacteremia caused by gram-positive bacteria, administration of parenteral nutrition, catheter removal, or death. The patients’ physicians and nurses, the clinical investigators, and the research microbiologists who processed all cultures were blinded to each study group.
Microbiologic studies.
Specimens from the skin at the insertion site (an area of approximately 4 cm2) were obtained for culture with a moistened sterile swab (Biomedics, Madrid, Spain), as described previously (12). The catheter hub samples were taken by repeatedly rubbing the inner surface of the hub with a sterile cotton swab (Eurotubo; Industrias Aulabor, SA, Barcelona, Spain) before administration of the study solution. All samples were sent to the laboratory in transport medium and were plated onto blood agar plates. The outer surfaces of the catheter tips were cultured by the roll-plate technique and were rolled back and forth across the surfaces of blood agar plates at least four times, as described previously (14). The inner surface of the catheter tip was cultured by a modified quantitative method (11); 2 ml of Trypticase soy broth was passed across each lumen and was then diluted 10-fold, and each dilution was plated onto blood agar. Colony counting was carried out after incubation of the plates at 37°C in a 5% CO2 atmosphere for 48 h. Skin and catheter hub cultures were considered positive when ≥15 CFU was isolated. The criteria for positivity of the catheter tip culture were counts of ≥103 CFU by the quantitative method and counts of ≥15 CFU by the roll-plate method. Blood cultures were performed by routine methods (BACTEC NR 860 instrument; Johnston Laboratories, Inc., Towson, Md.). The bacteria were fully identified by conventional procedures and by an automated method (MicroScan; Baxter Healthcare, West Sacramento, Calif.). Antibiotic susceptibility studies were performed by the disk diffusion method and by the microdilution method.
Molecular typing.
Molecular typing was performed by pulsed-field gel electrophoresis (PFGE) after DNA restriction with SmaI and separation of fragments in a contour-clamped homogeneous electric field (CHEF-DRII) apparatus (Bio-Rad, Richmond, Calif.) with running conditions of 200 V and pulse times ranging from 1 to 30 s for 23 h at 14°C (3).
Definitions.
Significant colonization of the catheter hub was defined as the isolation of ≥15 CFU. Bacteremia attributed to luminal colonization was defined as the isolation of identical organisms from the catheter hub and from cultures of separate percutaneously drawn blood specimens; the organism’s identity was proven by molecular typing for patients with Staphylococcus epidermidis bacteremia. Catheter occlusion was defined as the inability to aspirate blood through the catheter and/or the inability to force infusate through the catheter with usual infusion pressure. Patients were considered to have a fever if the temperature was above 38°C.
Statistical analysis.
To assess the adequacy of randomization, groups were compared by the uncorrected chi-square test or, when appropriate, Fisher’s exact test for categorical variables and the Mann-Whitney test for continuous variables. The distributions of time until catheter hub colonization and time until catheter-related bacteremia were stimated by the Kaplan-Meier method and were compared across the two groups by the log rank test (Mantel-Cox test). Crude and adjusted hazard ratios could not be estimated because no events were observed in the heparin and vancomycin group. However, since randomization produced two groups of patients with comparable baseline characteristics, no indication of positive or negative confounding needed to be controlled for with multivariate Cox models. Statistical significance was established at an alpha value of 0.05. All P values are two-tailed.
The sample size of 116 patients was calculated to give an 80% power to detect a 20% difference in the rate of catheter hub colonization by the two treatments at a one-sided 0.05 level of significance. On the basis of our previous experience (11), it was estimated that about 25% of patients in the heparin group would develop catheter hub colonization. The effect of heparin and vancomycin treatment was thought to be clinically relevant if the catheter hub colonization rate was reduced to 5%. We performed an intention-to-treat analysis for all patients who received at least one dose of study solution.
RESULTS
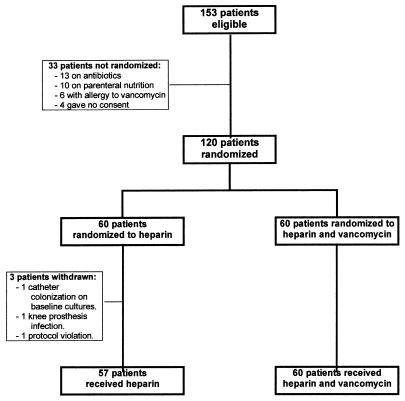
We recruited 120 consecutive patients; 60 were randomly assigned to receive heparin and 60 were randomly assigned to receive heparin and vancomycin (Fig. 1). Three patients assigned to receive heparin had to be withdrawn before receiving the drug because of CoNS colonization of the catheter skin insertion site and hub detected in baseline cultures (one patient), infection of knee prosthesis (one patient), and protocol violation (one patient). Overall, 57 patients received heparin and 60 patients received the solution of heparin and vancomycin. The baseline characteristics of the patients in each group were similar, and no significant differences were found with regard to age, sex, and underlying disease (Table 1). The type of catheter and the mean duration of catheterization prior to study inclusion were comparable for the two treatment groups. Catheters remained in place while the patients were in the study for similar mean durations for each treatment group; 11 days for patients who received heparin and 10 days for patients in the heparin and vancomycin group (P = 0.30) (Table 2). No catheter-related infection was observed in patients receiving heparin and vancomycin. Significant colonization of the catheter hub occurred in 9 (15.8%) of 57 patients treated with heparin but in none of 60 patients treated with heparin and vancomycin (P = 0.001). Catheter-related bacteremia occurred in 4 (7%) of 57 patients receiving heparin but in none of the 60 patients receiving heparin and vancomycin (P = 0.05). No colonization of the skin insertion site was detected during the study period. Bacteremias not attributable to catheter infection occurred at equal frequencies in both treatment groups; 13 (22.8%) patients in the heparin group and 15 (25%) patients in the heparin and vancomycin group (P = 0.35). In all these patients, cultures of catheter hub and skin insertion site swabs obtained at the time of bacteremia were negative. Six (10.5%) patients receiving heparin had catheter occlusion, whereas four (6.7%) of those receiving heparin and vancomycin had catheter occlusion (P = 0.52); in all these patients the catheter patency was reestablished by using heparin or urokinase flushings. None of the patients died during the study. One patient who had received heparin died of invasive pulmonary aspergillosis and one patient who had received heparin and vancomycin died of uncontrolled cancer 1 and 2 months after completion of the study, respectively.
FIG. 1.
Trial profile.
TABLE 1.
Baseline characteristics of patients and catheters by treatment group
| Characteristic | Treatment group
|
P valuea | |
|---|---|---|---|
| Heparin | Heparin and vancomycin | ||
| Sex (no. [%] of patients) | |||
| Male | 39 (68.4) | 31 (51.7) | 0.07 |
| Female | 18 (31.6) | 29 (48.3) | |
| Age | |||
| Mean (yr) | 44 | 42 | 0.5b |
| Median (yr) | 47 | 40 | |
| Tertiles (no. [%] of patients) | |||
| ≤30 | 14 (24.6) | 23 (38.3) | 0.2 |
| 31–52 | 24 (42.1) | 17 (28.3) | |
| ≥53 | 19 (33.3) | 20 (33.3) | |
| Underlying disease (no. [%] of patients)c | |||
| AML | 37 (64.9) | 33 (55.0) | 0.8 |
| CML | 6 (10.5) | 10 (16.7) | |
| ALL | 8 (14.0) | 9 (15.0) | |
| NHL | 4 (7.0) | 5 (8.3) | |
| HD | 2 (3.5) | 3 (5.0) | |
| Type of catheter (no. [%] of patients) | |||
| Double lumen | 35 (61.4) | 40 (66.7) | 0.6 |
| Triple lumen | 22 (38.6) | 20 (33.3) | |
| Duration of catheterization prior to randomization (days) | |||
| Mean | 24 | 25 | 0.9b |
| Median | 10 | 10 | |
P values are from the chi-square test or Fisher’s exact test unless otherwise indicated.
P values are from the Mann-Whitney test.
AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; ALL, acute lymphocytic leukemia; NHL, non-Hodgkin’s lymphoma; HD, Hodgkin’s disease.
TABLE 2.
Outcomes for study patients by treatment group
| Outcome | Treatment group
|
P valuea | |
|---|---|---|---|
| Heparin | Heparin and vancomycin | ||
| Duration of catheterization while in the study (days) | |||
| Mean | 11 | 10 | 0.3b |
| Median | 11 | 9 | |
| Significant colonization of catheter hub (no. [%] of patients) | |||
| No | 48 (84.2) | 60 (100.0) | 0.001 |
| Yes | 9 (15.8) | 0 | |
| Catheter-related bacteremia (no. [%] of patients) | |||
| No | 53 (93.0) | 60 (100.0) | 0.05 |
| Yes | 4 (7.0) | 0 | |
| Other reasons for ending the study (no. [%] of patients) | |||
| Neutropenia recovery | 19 (35.2) | 22 (36.7) | 0.92 |
| Systemic vancomycin | 33 (61.1) | 35 (58.3) | |
| Parenteral nutrition | 2 (3.7) | 3 (5.0) | |
P values are from the chi-square test or Fisher’s exact test unless otherwise indicated.
P values are from the Mann-Whitney test.
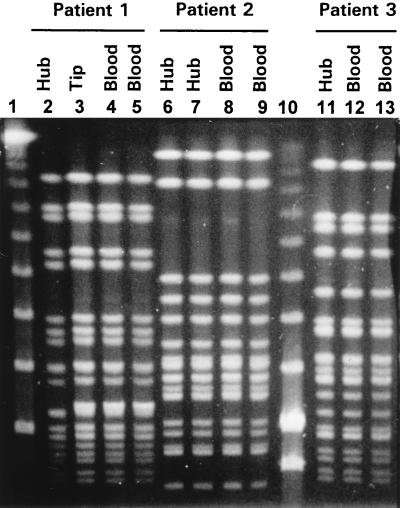
The organisms that caused catheter hub colonization were S. epidermidis (n = 7 patients), Staphylococcus capitis (n = 1), and Corynebacterium sp. (n = 1), and those that caused catheter-related bacteremia were S. epidermidis (n = 3) and S. capitis (n = 1). In each of the three patients with catheter-related S. epidermidis bacteremia, molecular typing by PFGE showed that the blood and hub isolates were identical (Fig. 2). In one of these patients the catheter had to be removed because of breakthrough bacteremia, and the catheter tip grew the same strain. No vancomycin-resistant organism was isolated from any source during the study period.
FIG. 2.
PFGE of genomic DNAs of S. epidermidis strains isolated from catheter segments and corresponding blood cultures. Lanes 2 to 9 and 11 to 13 show that the S. epidermidis strains isolated from each patient had identical DNA patterns. Lanes 1 and 10, bacteriophage lambda ladder PFGE marker (New England, BioLabs, Beverly, Mass.).
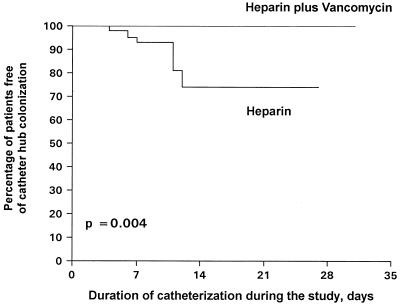
Figure 3 shows Kaplan-Meier curves for catheter hub colonization by treatment group. In the heparin and vancomycin group, all patients remained free of catheter hub colonization at the end of follow-up. In contrast, in the heparin group, the proportion of patients free of colonization of the catheter hub decreased to 74.6% at the end of the study. The survival distributions compared by the log rank test were significantly different (P = 0.004).
FIG. 3.
Rates of survival free of catheter hub colonization by treatment group.
Figure 4 shows Kaplan-Meier curves for catheter-related bacteremia by treatment group. In the heparin and vancomycin group, all patients remained free of bacteremia at the end of the study. Conversely, in the heparin group the proportion of patients free of catheter-related bacteremia decreased to 88% at the end of the study (P = 0.06).
FIG. 4.
Rates of survival free of catheter-related bacteremia by treatment group.
DISCUSSION
Our prospective, randomized, double-blind trial shows that an ALT with vancomycin can decrease the frequency of endoluminal catheter-related gram-positive bacterial infection during episodes of chemotherapy-induced neutropenia in patients with hematologic malignancies.
Despite important advances in preventive measures (12, 13, 20–22, 26), catheter-related infection remains a significant clinical problem in many institutions. Two randomized studies with children have analyzed a preventive method of endoluminal catheter-related infection based on catheter flushing, but they had conflicting results. Schwartz et al. (25) used a solution of heparin and vancomycin to flush tunneled CVCs and compared its efficacy with that of heparin alone in 45 children with oncologic or hematologic disorders. Daily flushing with heparin and vancomycin significantly decreased the frequency of catheter-related bacteremia attributed to luminal colonization with gram-positive organisms that were susceptible to vancomycin. On the other hand, a recent randomized, open trial (24) that included 55 children with cancer and 8 children receiving total parenteral nutrition for bowel disorders found that this flush solution had no effect on the risk of catheter-related bacteremia caused by gram-positive bacteria. It should be noted that in both studies neither surveillance cultures of the catheter hub nor molecular typing of the organisms was performed. In addition, a flush solution containing minocycline and EDTA has successfully been used to prevent recurrent catheter infections in three patients with serious diseases (19).
Our study involved 117 adult patients with hematologic malignancies and was designed to prevent colonization of the catheter hub as the entry point for microorganisms that lead to endoluminal catheter-related bacteremia during neutropenia. We used an ALT which allows delivery of a high concentration of the antibiotic inside the catheter lumen. By this straightforward technique, colonization of the catheter hub and catheter-related bacteremia of endoluminal origin were successfully prevented. Previous data have shown that there is a link between the number of organisms colonizing a catheter and the risk of infection (27). As recently pointed out by Raad (17), most catheters are colonized with organisms embedded in a biofilm, but infection appears to depend on whether the organisms on the catheter surface, particularly those in a planktonic free-floating phase, exceed a certain quantitative threshold. Since biofilm bacteria are relatively resistant to antibiotics, it can be speculated that our preventive ALT eradicated the organisms soon after they were introduced into the hub from the hands of sanitary personnel in some cases, while in others the ALT probably interfered with the multiplication and release of free-floating organisms.
Recently, the emergence and spread of vancomycin resistance among gram-positive organisms have become matters of great concern (9, 16). A limitation of our study could be that the prophylactic use of vancomycin could lead to the selection of such resistant organisms. However, we believe that this possibility is unlikely. By using the ALT, the drug is applied locally and does not come into contact with patients’ blood. It is administered at a high concentration rather than at the subinhibitory concentrations that seem most prone to lead to the emergence of resistance. Moreover, it is important that our study involved patients with hematologic malignancies and that the ALT with vancomycin was not used for the whole duration of catheterization. It was applied only during episodes of chemotherapy-induced neutropenia, when the risk of serious complications following catheter-related bacteremia is particularly high (7, 23). In the critically ill population described here, the increase in the numbers of infections caused by gram-positive organisms observed in many institutions, which has been related in part to a significant increase in catheter-related infections (5), has led to the widespread empirical use of systemic vancomycin.
In summary, our study demonstrates that a solution containing heparin and vancomycin administered by using an ALT effectively prevents colonization of the catheter hub and endoluminal catheter-related bacteremia during episodes of chemotherapy-induced neutropenia in patients with hematologic malignancies. Furthermore, this prophylactic strategy can be used as a basis for conducting future randomized trials with other classes of antibiotics.
ACKNOWLEDGMENTS
We are grateful to J. L. Pontón for preparing the study solutions and M. A. Domínguez for performing the molecular typing of organisms. We thank the nursing staff of the Clinical Hematology Service for valuable cooperation.
This study was supported in part by grant 93/1081 from Fondo de Investigación Sanitaria, Madrid, Spain.
REFERENCES
- 1.Benoit J L, Carandang G, Sitrin M, Arnow P M. Intraluminal antibiotic treatment of central venous catheter infections in patients receiving parenteral nutrition at home. Clin Infect Dis. 1995;21:1286–1288. doi: 10.1093/clinids/21.5.1286. [DOI] [PubMed] [Google Scholar]
- 2.Capdevila J A, Gavalda J, Pahissa A. Antibiotic-lock technique: usefulness and controversies. Antimicrob Infect Dis News. 1996;15:9–13. [Google Scholar]
- 3.Domínguez M A, Liñares J, Pulido A, Pérez J L, De Lencastre H. Molecular tracking of coagulase-negative staphylococcal isolates from catheter-related infections. Microb Drug Resist. 1996;2:423–429. doi: 10.1089/mdr.1996.2.423. [DOI] [PubMed] [Google Scholar]
- 4.Eastridge B J, Lefor A T. Complications of indwelling venous access devices in cancer patients. J Clin Oncol. 1995;13:233–238. doi: 10.1200/JCO.1995.13.1.233. [DOI] [PubMed] [Google Scholar]
- 5.González-Barca E, Fernández-Sevilla A, Carratalà J, Grañena A, Gudiol F. Prospective study of 288 episodes of bacteremia in neutropenic cancer patients in a single institution. Eur J Clin Microbiol Infect Dis. 1996;15:291–296. doi: 10.1007/BF01695660. [DOI] [PubMed] [Google Scholar]
- 6.Groeger J S, Lucas A B, Thaler H T, Friedlander-Klar H, Brown A E, Kiehn T E, Armstrong D. Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Ann Intern Med. 1993;119:1168–1174. doi: 10.7326/0003-4819-119-12-199312150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Guiot H F L, Visser L G, Barge R M Y, Bosboom R, van de Klunder J A M. Fatal meningitis due to catheter-related Staphylococcus epidermidis bacteraemia in a granulocytopenic patient without predisposing trauma. Eur J Clin Microbiol Infect Dis. 1994;13:772–775. doi: 10.1007/BF02276065. [DOI] [PubMed] [Google Scholar]
- 8.Henrickson K J, Powell K R, Schwartz C L. A dilute solution of vancomycin and heparin retains antibacterial and anticoagulant activities. J Infect Dis. 1988;157:600–601. doi: 10.1093/infdis/157.3.600. [DOI] [PubMed] [Google Scholar]
- 9.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogenously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 10.Howell P B, Walters P E, Donowitz G R, Farr B M. Risk factors for infection of adult patients with cancer who have tunnelled central venous catheters. Cancer. 1995;75:1367–1375. doi: 10.1002/1097-0142(19950315)75:6<1367::aid-cncr2820750620>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Liñares J, Sitges-Serra A, Garau J, Pérez J L, Martin R. Pathogenesis of catheter sepsis: a prospective study with quantitative and semiquantitative cultures of catheter hub and segments. J Clin Microbiol. 1985;21:357–360. doi: 10.1128/jcm.21.3.357-360.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maki D G, Ringer M, Alvarado C J. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet. 1991;338:339–343. doi: 10.1016/0140-6736(91)90479-9. [DOI] [PubMed] [Google Scholar]
- 13.Maki D G, Stolz S M, Wheeler S, Mermel L A. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127:257–266. doi: 10.7326/0003-4819-127-4-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Maki D G, Weise C E, Sarafin H W. A semiquantitative culture method for identifying intravenous catheter related infection. N Engl J Med. 1977;296:1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- 15.Messing B, Man R, Colimon R, Thuillier F, Beliah M. Antibiotic-lock technique is an effective treatment of bacterial catheter-related sepsis during parenteral nutrition. Clin Nutr. 1990;9:220–225. doi: 10.1016/0261-5614(90)90023-l. [DOI] [PubMed] [Google Scholar]
- 16.Murray B E. Vancomycin-resistant enterococci. Am J Med. 1997;102:284–293. doi: 10.1016/S0002-9343(99)80270-8. [DOI] [PubMed] [Google Scholar]
- 17.Raad I. Intravascular-catheter-related infections. Lancet. 1998;351:893–898. doi: 10.1016/S0140-6736(97)10006-X. [DOI] [PubMed] [Google Scholar]
- 18.Raad I, Bodey G P. Infectious complications of indwelling vascular catheters. Clin Infect Dis. 1992;15:197–210. doi: 10.1093/clinids/15.2.197. [DOI] [PubMed] [Google Scholar]
- 19.Raad I, Buzaid A, Rhyne J, Hachem R, Darouiche R, Safar H, Albitar M, Sheretz R J. Minocycline and ethylenediaminetetraacetate for the prevention of recurrent vascular catheter infections. Clin Infect Dis. 1997;25:149–151. doi: 10.1086/514518. [DOI] [PubMed] [Google Scholar]
- 20.Raad I, Darouiche R, Dupuis J, Abi-Said D, Gabrielli A, Hachem R, Wall M, Harris R, Jones J, Buzaid A, Robertson C, Shenaq S, Curling P, Burke T, Ericson C the Texas Medical Center Catheter Study Group. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. Ann Intern Med. 1997;127:267–274. doi: 10.7326/0003-4819-127-4-199708150-00002. [DOI] [PubMed] [Google Scholar]
- 21.Raad I, Darouiche R, Hachem R, Mansouri M, Bodey G P. The broad-spectrum activity and efficacy of catheters coated with mynocicline and rifampin. J Infect Dis. 1996;173:418–424. doi: 10.1093/infdis/173.2.418. [DOI] [PubMed] [Google Scholar]
- 22.Raad I, Hohn D C, Gilbreath B J, Suleiman N, Hill L A, Bruso P A, Marts K, Mansfield P F. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15:231–238. [PubMed] [Google Scholar]
- 23.Raad I, Narro J, Khan A, Tarrand J, Vartivarian S, Bodey G P. Serious complications of vascular catheter-related Staphylococcus aureus bacteremia in cancer patients. Eur J Clin Microbiol Infect Dis. 1992;11:675–682. doi: 10.1007/BF01989970. [DOI] [PubMed] [Google Scholar]
- 24.Rackoff W R, Weiman M, Jakobowski D, Hirschl R, Stallings V, Bilodeau J, Danz P, Bell L, Lange B. A randomized, controlled trial of the efficacy of a heparin and vancomycin solution in preventing central venous catheter infections in children. J Pediatr. 1995;127:147–151. doi: 10.1016/s0022-3476(95)70276-8. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz C, Henrickson K J, Roghmann K, Powell K. Prevention of bacteremia attributed to luminal colonization of tunneled central venous catheters with vancomycin-susceptible organisms. J Clin Oncol. 1990;8:1591–1597. doi: 10.1200/JCO.1990.8.9.1591. [DOI] [PubMed] [Google Scholar]
- 26.Segura M, Alvarez-Lerma F, Tellado J M, Jiménez-Ferreres J, Oms L, Rello J, Baró T, Sánchez R, Morera A, Mariscal D, Marrugat J, Sitges-Serra A. A clinical trial on the prevention of catheter-related sepsis using a new hub model. Ann Surg. 1996;223:363–369. doi: 10.1097/00000658-199604000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheretz R J, Raad I I, Balani A, Koo L, Rand K. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol. 1990;28:76–82. doi: 10.1128/jcm.28.1.76-82.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tacconelli E, Tumbarello M, Pittiruti M, Leone F, Lucia M B, Cauda R, Ortona L. Central venous catheter-related sepsis in a cohort of 366 hospitalised patients. Eur J Clin Microbiol Infect Dis. 1997;16:203–209. doi: 10.1007/BF01709582. [DOI] [PubMed] [Google Scholar]
- 29.Tenney J H, Moody M R, Newman K A, Schimpff S C, Wade J C, Costerton J W, Reed W P. Adherent microorganisms on lumenal surfaces of long-term intravenous catheters. Importance of Staphylococcus epidermidis in patients with cancer. Arch Intern Med. 1986;146:1949–1954. [PubMed] [Google Scholar]
- 30.Weightman N C, Simpson E M, Speller D C E, Mott M G, Oakhill A. Bacteraemia related to indwelling central venous catheters: prevention, diagnosis and treatment. Eur J Clin Microbiol Infect Dis. 1988;7:125–129. doi: 10.1007/BF01963064. [DOI] [PubMed] [Google Scholar]