Abstract
Simple Summary
In recent years, cryopreservation of fish sperm has been a rapidly evolving technique that contributes both to the improvement of genetic reproduction programs and the proper management of broodstock as well as to ensuring the viability of endangered species. However, this technique can cause significant damage to sperm, making the use of cryoprotectants and antioxidants in cryopreservation solutions imperative. The hormone melatonin has demonstrated positive effects on the cryopreservation of sperm in both farm animals and humans. Therefore, the plethora of research that has been conducted on animals and humans could be expanded to fish cryopreservation, making melatonin potentially a very promising alternative cryoprotectant.
Abstract
Cryopreservation is a technique that offers various advantages, especially in fish, among others, that makes the reproduction of species easier through a constant supply of sperm, synchronization of the gamete availability of both sexes, storage of semen for genetic improvement programs, reduction in the cost by eliminating the need to maintain male broodstock, and conserving the gametes of endangered species. However, freezing and warming procedures for cryopreservation lead to a reduction in the quality and viability of cryopreserved sperm because of oxidative stress. For this reason, the enrichment of extender media with antioxidants is a common method of cryopreservation of the semen of several fish species. Recently, many studies have been published for the protective role of antioxidants and especially of melatonin on male fertility preservation both in farm animals and humans, demonstrating the beneficial effects of melatonin as a sperm cryoprotectant. On the other hand, very few studies were conducted using melatonin as an antioxidant in different male fish species for semen cryopreservation. We conclude that the use of moderate concentrations of melatonin are beneficial to semen preservation, and the mechanisms through which melatonin acts positively on spermatozoa need to be further investigated to establish improvement protocols for cryopreservation in fish species.
Keywords: cryopreservation, fish, farm animals, human, semen, melatonin, antioxidant, oxidative stress
1. Introduction
The use of the technology of sperm cryopreservation offers many benefits extensively described in previous reviews for fish [1,2,3], farm animals [4,5,6], and humans [7,8]. In addition, extensive reviews have been published that include detailed protocols for fish species [9,10,11,12].
The development of fish sperm cryopreservation protocols for marine species is not as extensive for freshwater species, and the plethora of research work concerns the latter [2]. The main goal of most work on new species is to maintain stocks to ensure production, to optimize genetic improvement programs, and to properly manage offspring [2,13]; therefore, the development of cryopreservation protocols would help to achieve the above objectives [2]. In addition, cryopreservation of gametes can be used to protect endangered species [14].
The main goal of this brief review was to summarize the findings in the literature that refers to the supplementation of melatonin in cryopreservation media of semen, both in humans and farm animals, and to provide this knowledge in the cryopreservation of fish milt.
2. Cryodamage of Spermatozoa
The cryopreservation of sperm provokes a decrease in its quality and viability, mainly due to the increase in the production of reactive oxygen species (ROS) and the alteration of oxidative metabolism during the process of freezing and warming [15].
Although the sperm of fish, like all biological systems, are provided with protective antioxidants agents [16,17], in the cryopreservation technique, the antioxidant defense of the sperm is almost insufficient due to the reduced amount of these factors after the dilution of sperm [18,19]. As a consequence, during cryopreservation, an imbalance is observed between ROS production and the inherent antioxidant system [20,21], known as oxidative stress. Scientific studies in fish have shown that ROS production during cryopreservation contributes to the occurrence of lesions in sperm [22,23], resulting in lipid peroxidation (LPO) [24,25], DNA fragmentation [18,26], mitochondrial damage and dysfunction [23,27,28,29], protein oxidation [30], and loss or inactivation of enzymes associated with sperm motility [24,31,32]. Due to the aforementioned problems, it has become common practice to enrich the cryopreservation diluents of the sperm of many fish species with enzymatic and non-enzymatic antioxidants [18,33,34,35]; nevertheless, their use is often controversial.
3. Antioxidant Supplementation of Semen Extenders: The Case of Melatonin
Considering that the increased production of ROS during the cryopreservation process is partly responsible for the poor quality of sperm after thawing, various antioxidants have been proposed and tested for the cryopreservation of sperm of various terrestrial animals and fish [36]. Recent published studies focus on the protective role of various antioxidants, especially melatonin, in maintaining male fertility in both productive animals [37,38] and fish species [39], thus demonstrating the increased interest in this hormone.
Melatonin, the principal hormone secreted by the pineal gland, has been suggested as a free radical scavenger and antioxidant [40]. Mainly due to the fact of its amphiphilic nature that allows it to pass through all morphophysiological barriers of the cell; it is one of the most effective antioxidants protecting cells from oxidative stress caused by reactive species [41]. In addition, its lipophilic nature allows it to easily cross cell membranes and act directly in various organs including those of the reproductive system [42,43]. Of particular interest is the fact that melatonin’s metabolites, which are formed when the hormone functions as a scavenger, are likewise equally as good or better than the parent molecule in neutralizing toxic oxygen-based and nitrogen-based reactants [44].
The cytoprotective action of melatonin and its metabolites is due to the fact of its direct and indirect antioxidant properties [45]. The direct properties include the scavenging of both ROS and RNS (reactive nitrogen species) [46], while the indirect effects cover the stimulation of antioxidative enzymes and inhibition of pro-oxidative enzymes [47], probably through epigenetic mechanisms [48]. This molecule with its strong detoxifying effect at the mitochondrial level, could be an appropriate candidate for improving the quality of animal sperm during cryopreservation [49,50]. It has been observed that this substance could protect sperm from oxidative damage [51], maintain its viability [42,52], and reduce morphological abnormalities [53,54] and DNA fragmentation [55]. Improvement in sperm quality due to the high levels of endogenous melatonin has been found in humans [56], while in vitro treatment with this hormone can improve human sperm motility [56] and several quality parameters of ram [57] and pig [58] sperm. Finally, melatonin has been used as an additive antioxidant in the cryopreservation of sperm [59,60] helping to increase its quality after thawing [61].
4. Melatonin Supplementation in Farm Animals and Human Freezing Medium
The majority of recent studies evaluating the effect of melatonin on the cryopreservation of sperm focus on cattle compared to those on sheep, pigs, or goats [38].
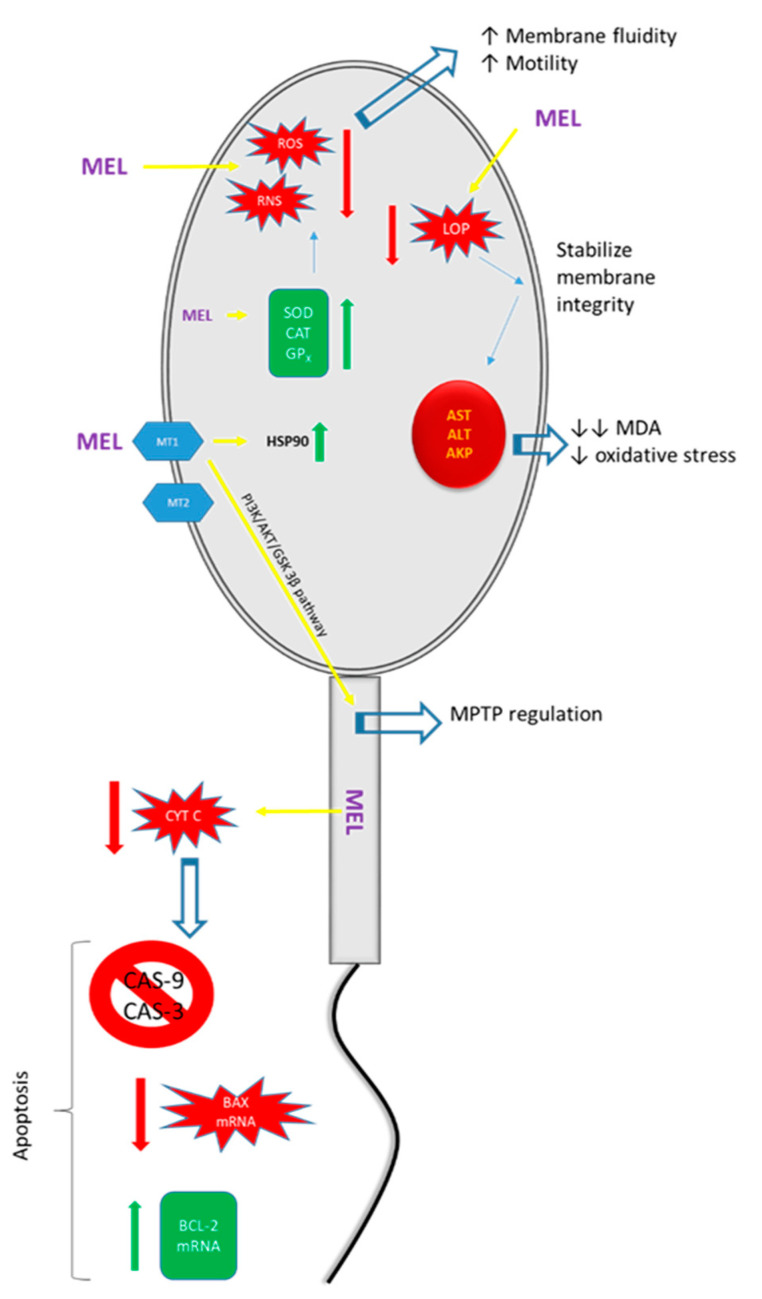
In summary, the effect of melatonin on sperm is shown in Table 1 and the pathway of action in Figure 1.
Table 1.
Effects of melatonin on sperm.
| Pathway of Action | Εffects on Spermatozoa | References |
|---|---|---|
| Reduction in excessive production of free radicals. | Positive effects on the function and morphometry parameters of sperm in humans and various farm animals. | [62,63,64] |
| Upregulation of the expression of heat shock protein (HSP) 90. | Resistance to stress factors in frozen–thawed sperm. | [62] |
| Upregulation of antioxidant enzymes, e.g., superoxide dismutase, glutathione peroxidase, and catalase. | Elimination of ROS levels causing preservation of membrane fluidity and motility. | [62,65,66] |
| Regulation of mitochondrial permeability transition pores (MPTPs) as a result of binding to the MT1 receptor and the activation of the PI3K/AKT/GSK 3β pathway. | Improvement in the quality and fertilizing capacity of frozen–thawed ram sperm. | [67] |
| Reduction of LPO production leads to (a) stabilization of membrane integrity and (b) prevention of leakage of intracellular enzymes, e.g., aspartate transaminase (AST), alanine transaminase (ALT), and phosphatase. | Decreased malondialdehyde (MDA) concentrations and oxidative stress. | [68,69] |
| Enhancement of the functions of antioxidant enzymes. | Protection against oxidative modifications of DNA. DNA becomes more resistant to fragmentation, reducing the rate of sperm degradation and enhancing its viability and functions. | [42,59,70,71] |
| Action as an anti-apoptotic molecule. |
|
[72] |
|
[73,74] |
Figure 1.
Illustration of the positive effects of melatonin (MEL) on spermatozoa. MT1: melatonin type 1 receptor; MT2: melatonin type 2 receptor; ROS: reactive oxygen species; RNS: reactive nitrogen species; LOP: lipid peroxidation; SOD: superoxide dismutase; CAT: catalase; GPX: glutathione peroxidase; HSP90: heat shock protein 90; AST: aspartate amino transferase; ALT: alanine aminotransferase; AKP: alkaline phosphatase; MDA: malondialdehyde; MPTP: mitochondrial permeability transition pore.
Several studies have shown that the cryoprotective effects of melatonin depend on its concentration [59,75]. In the international literature, many different concentrations have been defined as being optimal for the cryoprotection of sperm. Among others, some studies have shown that 1 and 2 mM are the optimal concentrations, while another study found that the best concentration was 0.25 mg/mL in various species [76]. In humans, a better range of sperm viability and motility were observed at a 3 mM melatonin concentration, and intracellular ROS levels were reduced [51]. The vitality of thawed human sperm was found to be improved after the supplementation of 0.1 mM melatonin, while it was adversely affected by other concentrations (i.e., 0.001 and 1 mM) [62]. Karimfar et al. [75] reported that the best protection for human sperm against cryopreservation damage was observed at a 0.01 mM melatonin concentration.
Studies have shown that parameters, such as membrane integrity, motility and velocity, capacitation, antioxidant protein quantity, and developmental competence of fresh and frozen sperm improved after administration of moderate melatonin concentrations [62,77,78,79]. The addition of melatonin to cryopreservation solutions of bovine sperm [71], sheep [80,81], goats [82,83], rams [70], buffalo [84], and pigs [85,86] increased the number of live sperm with normal quality after thawing, including the normal length and movement of the tail, and reduced morphological defects of the sperm. In addition, in farmed animals, the frozen sperm with membrane integrity showed greater motility [60,86].
The scientific work on the role of melatonin in the mitigation of oxidative damage, mostly concerns humans and fewer farm animals. Significant progress has been made in understanding the action of melatonin against oxidative damage caused by cryopreservation [38]. In studies of farm animals, the available data focus on the positive effects of melatonin on sperm quality indicators but without clearly identifying the mechanisms by which it acts. It would therefore be crucial to further investigate these mechanisms in both fresh and frozen sperm, especially in sheep, goats, and pigs [38].
5. Studies Used Melatonin as an Antioxidant in Fish Sperm Cryopreservation
Very few studies have been conducted using melatonin as an antioxidant in different male fish species for semen cryopreservation compared to farm animal species.
In freshwater species, curimba (Prochilodus lineatus), melatonin was used as an additive in the sperm cryopreservation extender and no significant differences were found among different concentrations (i.e., 1, 2, and 3 mM), neither in motility parameters and spermatozoa morphological anomalies nor in fertilization capacity [87].
Recently, in the same fish, Prochilodus lineatus, Motta et al. [88] studied the antioxidant capacity of four different concentrations of melatonin (i.e., 2.00, 2.75, 3.50, and 4.25 mM) by determining the kinetic motility parameters and oxidative stress indices in sperm after thawing. The use of high concentrations of melatonin was shown to be harmful to the sperm of this species. In addition, 2.00 mM melatonin resulted in a higher curvilinear velocity (VCL) and linear velocity (VSL) and reduced catalysis (CAT). However, with the use of this dosage, no statistically significant differences were observed with the control in motility rate, membrane integrity, percentage of normal cells, lipid peroxidation, and fertilization and hatching rates.
Ferrão [89] examined the protective action of melatonin during sperm cryopreservation in F1 Senegalese sole using two different melatonin supplementations (i.e., 0.1 and 10 mM). In the post-thawed sperm, motility, viability, DNA fragmentation, lipid peroxidation, ROS, and apoptosis were examined. The 10 mM melatonin supplement exhibited significantly lower spermatozoa viability combined with higher percentages of late apoptotic cells dead by caspases. The post-thawed sperm motility decreased throughout post-activation time in the control and in the melatonin supplemented groups (i.e., 0.1 and 10 mM).
In Portugal, Félix et al. [90], for first time, evaluated the antioxidant capacity of three concentrations of melatonin (i.e., 0.001, 0.01, and 0.1 mM) during sperm cryopreservation of gilthead seabream (Sparus aurata). The motility of all parameters, which analyzed total motility (TM), progressive motility (PM), curvilinear velocity (VCL), straight line velocity (VSL), and linearity (LIN), were revealed to be influenced by melatonin. Regarding DNA fragmentation, no differences were observed between treatments in tail DNA (%), but olive tail movement was consistently higher at melatonin treatment. Moreover, they brought into account another parameter of the action of melatonin during cryopreservation. The effect of endogenously produced melatonin by night on the spermatozoa quality. The authors compared sperm samples that were collected at two different chronic points, mid-light and mid-dark. Interestingly, they concluded that the higher levels of endogenous melatonin production at night could have an important role in spermatozoa protection during cryopreservation [91].
Finally, Palhares et al. [92] verified the percentage of dead and live spermatozoa of frozen Brycon orbignyanus semen after freezing with extenders containing different concentrations of melatonin (i.e., 1 and 2 mM) and after three different dry shipper freezing times (i.e., 15 min, 12 h, and 24 h). The authors concluded that the addition of 2 mM of melatonin in freezing medium with the cryoprotectant methylglycol improved Brycon orbignyanus sperm quality regarding sperm kinetics, while the tested freezing times did not influence the quality of thawed semen. Additionally, the same group in Brazil [93], studied melatonin supplementation in different freezing curves determining the antioxidant enzyme activity and the peroxidation lipid and sperm characteristics of cryopreserved Brycon orbignyanus milt. Melatonin concentrations of 1 and 2 mM were examined, and significant differences in viability, morphology, motility, and fertilization rate in the solutions with melatonin were identified. Specifically, total motility, progressive motility, and motility time were significantly different. In terms of oxidative stress markers, the solutions with melatonin yielded the lowest values. The study concluded that 2 mM of melatonin is beneficial for Brycon orbignyanus semen based on the best sperm characteristics, peroxidation lipid, and antioxidant enzyme activity obtained during cryopreservation.
6. Conclusions: Lessons to Be Learned from Farm Animals and Human Studies
In the future, research should be carried out on the improvement of mechanisms that trigger the production of endogenous antioxidants, such as melatonin, to protect spermatozoa naturally from oxidative stress, as other research groups support. Definitely, the need for a description of appropriate and safe application methods in regard to male fertility cryopreservation in different fish species is required as soon as possible.
The findings of previous research papers on farm animals and humans are expected to be helpful for the improvement in cryopreservation protocols in fish species. The success of freezing protocols could be of help in strengthening the programs of intensive breeding and genetic improvement in aquaculture industry [94]. In particular, this will be achieved through transmission and application of acquired knowledge of farm animals and human semen cryopreservation to ensure the reproductive efficiency and productivity of fishes.
All of this knowledge could lead to the following conclusions:
Not all melatonin concentrations are optimal for the properties of sperm cryopreservation extenders;
Improvements in the use of melatonin as an antioxidant to moderate oxidative damage is more advanced in humans than in farm animals due to the spermatozoon’s smaller head which presents maximum cryostability [95];
Use of moderate concentrations of melatonin improves the quality of both fresh and frozen semen;
The positive effects of melatonin on spermatozoa have been proved in non-seasonal long-day and short-day breeders, which suggests that this action is not relevant by the hypothalamus–pituitary–testicular axis regulation;
Because of its low toxicity and commonly accepted antioxidant activity, melatonin could be a perfect candidate to enhance semen quality during cryopreservation;
The mechanisms through which melatonin acts positively on spermatozoa need further investigation.
The findings of the research on the use of melatonin as an antioxidant/cryoprotectant in the cryopreservation media support the idea that melatonin concentration may be dependent on the species and should be tested for different fish species.
In a nutshell, our efforts are to establish protocols with detailed descriptions that are generally accepted by the research community in the field of cryopreservation of marine species sperm. The role and contribution of melatonin in these protocols as an antioxidant/cryoprotectant remain to be explored with the important help of researchers in the topics of genetic, cryobiology, and physiology of reproduction.
Author Contributions
Conceptualization, S.P. and A.I.A.; methodology, S.P.; software, A.I.A.; validation, A.E. and E.M.; formal analysis, S.P.; investigation, S.P., A.I.A., A.E., E.M.; resources, S.P., A.E., E.M.; data curation, S.P. and A.I.A.; writing—original draft preparation, S.P. and A.I.A.; writing—review and editing, S.P., A.E., E.M.; visualization, S.P. and A.I.A.; supervision, S.P.; project administration, A.E. and E.M.; funding acquisition, A.E. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cabrita E., Sarasquete C., Martínez-Páramo S., Robles V., Beirão J., Pérez-Cerezales S., Herráez M.P. Cryopreservation of Fish Sperm: Applications and Perspectives. J. Appl. Ichthyol. 2010;26:623–635. doi: 10.1111/j.1439-0426.2010.01556.x. [DOI] [Google Scholar]
- 2.Martinez-Paramo S., Horváth A., Labbe C., Zhang T., Robles V., Herraez P., Suquet M., Adams S., Viveiros A., Tiersch T.R., et al. Cryobanking of Aquatic Species. Aquaculture. 2017;472:156–177. doi: 10.1016/j.aquaculture.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnotti C., Cerqueira V., Lee-Estevez M., Farias J.G., Valdebenito I., Figueroa E. Cryopreservation and Vitrification of Fish Semen: A Review with Special Emphasis on Marine Species. Rev. Aquac. 2018;10:15–25. doi: 10.1111/raq.12145. [DOI] [Google Scholar]
- 4.Lv C., Wu G., Hong Q., Quan G. Spermatozoa Cryopreservation: State of Art and Future in Small Ruminants. Biopreserv. Biobank. 2019;17:171–182. doi: 10.1089/bio.2018.0113. [DOI] [PubMed] [Google Scholar]
- 5.Salinas M.B., Chuammitri P., Sringarm K., Boonyayatra S., Sathanawongs A. Current Perspectives on Ruminant Sperm Freezability: Harnessing Molecular Changes Related to Semen Quality through Omics Technologies. Veter. Integr. Sci. 2021;19:487–511. doi: 10.12982/VIS.2021.039. [DOI] [Google Scholar]
- 6.Yánez-Ortiz I., Catalán J., Rodríguez-Gil J.E., Miró J., Yeste M. Advances in Sperm Cryopreservation in Farm Animals: Cattle, Horse, Pig and Sheep. Anim. Reprod. Sci. 2021:106904. doi: 10.1016/j.anireprosci.2021.106904. in press . [DOI] [PubMed] [Google Scholar]
- 7.Di Santo M., Tarozzi N., Nadalini M., Borini A. Human Sperm Cryopreservation: Update on Techniques, Effect on DNA Integrity, and Implications for ART. Adv. Urol. 2012;2012:854837. doi: 10.1155/2012/854837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao Y., Sanger E., Saewu A., Leveille M.-C. Human Sperm Vitrification: The State of the Art. Reprod. Biol. Endocrinol. 2020;18:17. doi: 10.1186/s12958-020-00580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopeika E., Kopeika J., Zhang T. Cryopreservation of Fish Sperm. In: Day J.G., Stacey G.N., editors. Cryopreservation and Freeze-Drying Protocols. Volume 368. Humana Press; Totowa, NJ, USA: 2007. pp. 203–217. [DOI] [PubMed] [Google Scholar]
- 10.Cabrita E., Robles V., Herraez P. Methods in Reproductive Aquaculture: Marine and Freshwater Species. 1st ed. CRC Press; Boca Raton, FL, USA: 2008. [Google Scholar]
- 11.Tiersch T.R., Yang H., Jenkins J.A., Dong Q. Sperm Cryopreservation in Fish and Shellfish. Soc. Reprod. Fertil. Suppl. 2007;65:493–508. [PubMed] [Google Scholar]
- 12.Tiersch T.R. Introduction to the Second Edition. In: Tiersch T.R., Green C.C., editors. Cryopreservation in Aquatic Species. 2nd ed. World Aquaculture Society; Baton Rouge, LA, USA: 2011. pp. 1–17. [Google Scholar]
- 13.Exadactylos A., Arvanitoyannis I. Aquaculture Biotechnology for enhanced fish production for human consumption. In: Ray R.C., editor. Microbial biotechnology in Agriculture and Aquaculture. Volume II. Science Publishers Inc.; Enfield, NH, USA: 2006. [DOI] [Google Scholar]
- 14.Zhou G.-B., Zhu S.-E., Hou Y.-P., Jin F., Yang Q.-E., Yang Z.-Q., Quan G.-B., Tan H.-M. Vitrification of Mouse Embryos at Various Stages by Open-Pulled Straw (OPS) Method. Anim. Biotechnol. 2005;16:153–163. doi: 10.1080/10495390500263831. [DOI] [PubMed] [Google Scholar]
- 15.Marques L.S., Fossati A.A.N., Rodrigues R.B., da Rosa H.T., Izaguirry A.P., Ramalho J.B., Moreira J.C.F., Santos F.W., Zhang T., Streit D.P. Slow Freezing versus Vitrification for the Cryopreservation of Zebrafish (Danio rerio) Ovarian Tissue. Sci. Rep. 2019;9:15353. doi: 10.1038/s41598-019-51696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva S., Soares A., Batista A., Almeida F., Nunes J., Peixoto C., Guerra M. In Vitro and In Vivo Evaluation of Ram Sperm Frozen in Tris Egg-Yolk and Supplemented with Superoxide Dismutase and Reduced Glutathione: In Vitro and In Vivo Effect of the Addition of SOD and GSH. Reprod. Domest. Anim. 2011;46:874–881. doi: 10.1111/j.1439-0531.2011.01758.x. [DOI] [PubMed] [Google Scholar]
- 17.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrita E., Ma S., Diogo P., Martínez-Paramo S., Sarasquete C., Dinis M.T. The Influence of Certain Aminoacids and Vitamins on Post-Thaw Fish Sperm Motility, Viability and DNA Fragmentation. Anim. Reprod. Sci. 2011;125:189–195. doi: 10.1016/j.anireprosci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Paramo S., Diogo P., Dinis M.T., Herraez M.P., Sarasquete C., Cabrita E. Incorporation of Ascorbic Acid and α-Tocopherol to the Extender Media to Enhance Antioxidant System of Cryopreserved Sea Bass Sperm. Theriogenology. 2012;77:1129–1136. doi: 10.1016/j.theriogenology.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaliutina-Kolesova A., Xian M., Nian R. Antioxidant Defense System in Protein Fractions of Common Carp (Cyprinus carpio) Seminal Plasma. Czech J. Anim. Sci. 2019;64:265–271. doi: 10.17221/187/2018-CJAS. [DOI] [Google Scholar]
- 22.Balamurugan B., Ghosh S., Lone S., Prasad J., Das G., Katiyar R., Mustapha A.R., Kumar A., Verma M. Partial Deoxygenation of Extender Improves Sperm Quality, Reduces Lipid Peroxidation and Reactive Oxygen Species during Cryopreservation of Buffalo (Bubalus bubalis) Semen. Anim. Reprod. Sci. 2018;189:60–68. doi: 10.1016/j.anireprosci.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Figueroa E., Lee-Estevez M., Valdebenito I., Watanabe I.-S., Oliveira R.P.S., Romero J., Castillo R.L., Farías J.G. Effects of Cryopreservation on Mitochondrial Function and Sperm Quality in Fish. Aquaculture. 2019;511:634190. doi: 10.1016/j.aquaculture.2019.06.004. [DOI] [Google Scholar]
- 24.Klaiwattana P., Srisook K., Srisook E., Vuthiphandchai V., Neumvonk J. Effect of cryopreservation on lipid composition and antioxidant enzyme activity of seabass (Lates calcarifer) sperm. Iran. J. Fish. Sci. 2016;15:157–169. [Google Scholar]
- 25.Riesco M.F., Oliveira C., Soares F., Gavaia P.J., Dinis M.T., Cabrita E. Solea senegalensis Sperm Cryopreservation: New Insights on Sperm Quality. PLoS ONE. 2017;12:e0186542. doi: 10.1371/journal.pone.0186542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Öğretmen F., İnanan B.E., Kutluyer F., Kayim M. Effect of Semen Extender Supplementation with Cysteine on Postthaw Sperm Quality, DNA Damage, and Fertilizing Ability in the Common Carp (Cyprinus carpio) Theriogenology. 2015;83:1548–1552. doi: 10.1016/j.theriogenology.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.He S., Woods III L.C. Effects of Dimethyl Sulfoxide and Glycine on Cryopreservation Induced Damage of Plasma Membranes and Mitochondria to Striped Bass (Morone saxatilis) Sperm. Cryobiology. 2004;48:254–262. doi: 10.1016/j.cryobiol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Cabrita E., Robles V., Cuñado S., Wallace J.C., Sarasquete C., Herráez M.P. Evaluation of Gilthead Sea Bream, Sparus aurata, Sperm Quality after Cryopreservation in 5 mL Macrotubes. Cryobiology. 2005;50:273–284. doi: 10.1016/j.cryobiol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Figueroa E., Valdebenito I., Merino O., Ubilla A., Risopatrón J., Farias J.G. Cryopreservation of Atlantic Salmon Salmo salar Sperm: Effects on Sperm Physiology. J. Fish. Biol. 2016;89:1537–1550. doi: 10.1111/jfb.13052. [DOI] [PubMed] [Google Scholar]
- 30.Purdy P.H., Barbosa E.A., Praamsma C.J., Schisler G.J. Modification of Trout Sperm Membranes Associated with Activation and Cryopreservation. Implications for Fertilizing Potential. Cryobiology. 2016;73:73–79. doi: 10.1016/j.cryobiol.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Dietrich M.A., Arnold G.J., Fröhlich T., Otte K.A., Dietrich G.J., Ciereszko A. Proteomic Analysis of Extracellular Medium of Cryopreserved Carp (Cyprinus carpio L.) Semen. Comp. Biochem. Physiol. Part D Genom. Proteom. 2015;15:49–57. doi: 10.1016/j.cbd.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Nynca J., Arnold G.J., Fröhlich T., Ciereszko A. Cryopreservation-Induced Alterations in Protein Composition of Rainbow Trout Semen. Proteomics. 2015;15:2643–2654. doi: 10.1002/pmic.201400525. [DOI] [PubMed] [Google Scholar]
- 33.Lahnsteiner F., Mansour N., Kunz F.A. The Effect of Antioxidants on the Quality of Cryopreserved Semen in Two Salmonid Fish, the Brook Trout (Salvelinus fontinalis) and the Rainbow Trout (Oncorhynchus mykiss) Theriogenology. 2011;76:882–890. doi: 10.1016/j.theriogenology.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Figueroa E., Farias J.G., Lee-Estevez M., Valdebenito I., Risopatrón J., Magnotti C., Romero J., Watanabe I., Oliveira R.P.S. Sperm Cryopreservation with Supplementation of α-Tocopherol and Ascorbic Acid in Freezing Media Increase Sperm Function and Fertility Rate in Atlantic Salmon (Salmo salar) Aquaculture. 2018;493:1–8. doi: 10.1016/j.aquaculture.2018.04.046. [DOI] [Google Scholar]
- 35.Li P., Xi M., Du H., Qiao X., Liu Z., Wei Q. Antioxidant Supplementation, Effect on Post-Thaw Spermatozoan Function in Three Sturgeon Species. Reprod. Domest. Anim. 2018;53:287–295. doi: 10.1111/rda.13103. [DOI] [PubMed] [Google Scholar]
- 36.Sandoval-Vargas L., Silva Jiménez M., Risopatrón González J., Villalobos E.F., Cabrita E., Valdebenito Isler I. Oxidative Stress and Use of Antioxidants in Fish Semen Cryopreservation. Rev. Aquac. 2021;13:365–387. doi: 10.1111/raq.12479. [DOI] [Google Scholar]
- 37.Sun T.-C., Li H.-Y., Li X.-Y., Yu K., Deng S.-L., Tian L. Protective Effects of Melatonin on Male Fertility Preservation and Reproductive System. Cryobiology. 2020;95:1–8. doi: 10.1016/j.cryobiol.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Ofosu J., Qazi I.H., Fang Y., Zhou G. Use of Melatonin in Sperm Cryopreservation of Farm Animals: A Brief Review. Anim. Reprod. Sci. 2021;233:106850. doi: 10.1016/j.anireprosci.2021.106850. [DOI] [PubMed] [Google Scholar]
- 39.Félix F., Oliveira C.C.V., Cabrita E. Antioxidants in Fish Sperm and the Potential Role of Melatonin. Antioxidants. 2020;10:36. doi: 10.3390/antiox10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng X., Li Y., Li S., Zhou Y., Gan R.-Y., Xu D.-P., Li H.-B. Dietary Sources and Bioactivities of Melatonin. Nutrients. 2017;9:367. doi: 10.3390/nu9040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardeland R., Cardinali D.P., Srinivasan V., Spence D.W., Brown G.M., Pandi-Perumal S.R. Melatonin—A Pleiotropic, Orchestrating Regulator Molecule. Prog. Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Reiter R.J., Tan D.-X., Manchester L.C., Paredes S.D., Mayo J.C., Sainz R.M. Melatonin and Reproduction Revisited. Biol. Reprod. 2009;81:445–456. doi: 10.1095/biolreprod.108.075655. [DOI] [PubMed] [Google Scholar]
- 43.Maitra S.K., Hasan K.N. The Role of Melatonin as a Hormone and an Antioxidant in the Control of Fish Reproduction. Front. Endocrinol. 2016;7:38. doi: 10.3389/fendo.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galano A., Tan D.X., Reiter R.J. On the Free Radical Scavenging Activities of Melatonin’s Metabolites, AFMK and AMK. J. Pineal Res. 2013;54:245–257. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 45.Reiter R.J., Rosales-Corral S., Tan D.X., Jou M.J., Galano A., Xu B. Melatonin as a Mitochondria-Targeted Antioxidant: One of Evolution’s Best Ideas. Cell. Mol. Life Sci. 2017;74:3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan D.-X., Chen L.D., Poeggeler B., Manchester L.C., Reiter R. Melatonin: A Potent Endogenous Hydroxyl Radical Scavenger. Endocr. J. 1993;1:57–60. [Google Scholar]
- 47.Reiter R.J., Tan D., Sainz R.M., Mayo J.C., Lopez-Burillo S. Melatonin: Reducing the Toxicity and Increasing the Efficacy of Drugs. J. Pharm. Pharmacol. 2010;54:1299–1321. doi: 10.1211/002235702760345374. [DOI] [PubMed] [Google Scholar]
- 48.Korkmaz A., Rosales-Corral S., Reiter R.J. Gene Regulation by Melatonin Linked to Epigenetic Phenomena. Gene. 2012;503:1–11. doi: 10.1016/j.gene.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 49.Da Silva C.M.B., Macías-García B., Miró-Morán A., González-Fernández L., Morillo-Rodriguez A., Ortega-Ferrusola C., Gallardo-Bolaños J.M., Stilwell G., Tapia J.A., Peña F.J. Melatonin Reduces Lipid Peroxidation and Apoptotic-like Changes in Stallion Spermatozoa: Melatonin Reduces Lipid Peroxidation. J. Pineal Res. 2011;51:172–179. doi: 10.1111/j.1600-079X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen X.-J., Zhang Y., Jia G.-X., Meng Q.-G., Bunch T.-D., Liu G.-S., Zhu S.-E., Xhou G.-B. Effect of Melatonin Supplementation on Cryopreserved Sperm Quality in Mouse. Cryo Lett. 2016;37:115–122. [PubMed] [Google Scholar]
- 51.Najafi A., Adutwum E., Yari A., Salehi E., Mikaeili S., Dashtestani F., Abolhassani F., Rashki L., Shiasi S., Asadi E. Melatonin Affects Membrane Integrity, Intracellular Reactive Oxygen Species, Caspase3 Activity and AKT Phosphorylation in Frozen Thawed Human Sperm. Cell Tissue Res. 2018;372:149–159. doi: 10.1007/s00441-017-2743-4. [DOI] [PubMed] [Google Scholar]
- 52.Reiter R., Rosales-Corral S., Manchester L., Tan D.-X. Peripheral Reproductive Organ Health and Melatonin: Ready for Prime Time. Int. J. Mol. Sci. 2013;14:7231–7272. doi: 10.3390/ijms14047231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aripin S.A., Jintasataporn O., Yoonpundh R. Effects of Melatonin in Clarias macrocephalus Male Broodstock First Puberty; Proceedings of the International Conference of Aquaculture Indonesia (ICAI); Bandung, Indonesia. 1 January 2014. [Google Scholar]
- 54.Aripin S.-A., Jintasatap O., Yoonpundh R. Effects of Exogenous Melatonin and Zinc Amino Acid on Male Clarias macrocephalus Broodstock. Asian J. Sci. Res. 2018;11:515–521. doi: 10.3923/ajsr.2018.515.521. [DOI] [Google Scholar]
- 55.Sarabia L., Maurer I., Bustos-Obregón E. Melatonin Prevents Damage Elicited by the Organophosphorous Pesticide Diazinon on Mouse Sperm DNA. Ecotoxicol. Environ. Saf. 2009;72:663–668. doi: 10.1016/j.ecoenv.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 56.Ortiz A., Espino J., Bejarano I., Lozano G.M., Monllor F., García J.F., Pariente J.A., Rodríguez A.B. High Endogenous Melatonin Concentrations Enhance Sperm Quality and Short-Term in Vitro Exposure to Melatonin Improves Aspects of Sperm Motility: Melatonin Improves Sperm Quality. J. Pineal Res. 2010;50:132–139. doi: 10.1111/j.1600-079X.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 57.Casao A., Vega S., Palacín I., Pérez-Pe R., Laviña A., Quintín F., Sevilla E., Abecia J., Cebrián-Pérez J., Forcada F., et al. Effects of Melatonin Implants During Non-Breeding Season on Sperm Motility and Reproductive Parameters in Rasa aragonesa Rams: Melatonin on Ram Sperm Motility and Reproductive Parameters. Reprod. Domest. Anim. 2008;45:425–432. doi: 10.1111/j.1439-0531.2008.01215.x. [DOI] [PubMed] [Google Scholar]
- 58.Jang H., Kim Y., Kim B., Park I., Cheong H., Kim J., Park C., Kong H., Lee H., Yang B. Ameliorative Effects of Melatonin against Hydrogen Peroxide-Induced Oxidative Stress on Boar Sperm Characteristics and Subsequent In Vitro Embryo Development: Effect of Melatonin against Oxidative Stress in Pig Germ Cell. Reprod. Domest. Anim. 2010;45:943–950. doi: 10.1111/j.1439-0531.2009.01466.x. [DOI] [PubMed] [Google Scholar]
- 59.Succu S., Berlinguer F., Pasciu V., Satta V., Leoni G.G., Naitana S. Melatonin Protects Ram Spermatozoa from Cryopreservation Injuries in a Dose-Dependent Manner. J. Pineal Res. 2011;50:310–318. doi: 10.1111/j.1600-079X.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 60.Ashrafi I., Kohram H., Ardabili F.F. Antioxidative Effects of Melatonin on Kinetics, Microscopic and Oxidative Parameters of Cryopreserved Bull Spermatozoa. Anim. Reprod. Sci. 2013;139:25–30. doi: 10.1016/j.anireprosci.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Domínguez-Rebolledo Á.E., Fernández-Santos M.R., Bisbal A., Ros-Santaella J.L., Ramón M., Carmona M., Martínez-Pastor F., Garde J.J. Improving the Effect of Incubation and Oxidative Stress on Thawed Spermatozoa from Red Deer by Using Different Antioxidant Treatments. Reprod. Fertil. Dev. 2010;22:856–870. doi: 10.1071/RD09197. [DOI] [PubMed] [Google Scholar]
- 62.Deng S.-L., Sun T.-C., Yu K., Wang Z.-P., Zhang B.-L., Zhang Y., Wang X.-X., Lian Z.-X., Liu Y.-X. Melatonin Reduces Oxidative Damage and Upregulates Heat Shock Protein 90 Expression in Cryopreserved Human Semen. Free Radic. Biol. Med. 2017;113:347–354. doi: 10.1016/j.freeradbiomed.2017.10.342. [DOI] [PubMed] [Google Scholar]
- 63.Zhu Z., Li R., Lv Y., Zeng W. Melatonin Protects Rabbit Spermatozoa from Cryo-Damage via Decreasing Oxidative Stress. Cryobiology. 2019;88:1–8. doi: 10.1016/j.cryobiol.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Mehaisen G.M.K., Partyka A., Ligocka Z., Niżański W. Cryoprotective Effect of Melatonin Supplementation on Post-Thawed Rooster Sperm Quality. Anim. Reprod. Sci. 2020;212:106238. doi: 10.1016/j.anireprosci.2019.106238. [DOI] [PubMed] [Google Scholar]
- 65.García J.J., Reiter R.J., Guerrero J.M., Escames G., Yu B.P., Oh C.S., Muñoz-Hoyos A. Melatonin Prevents Changes in Microsomal Membrane Fluidity during Induced Lipid Peroxidation. FEBS Lett. 1997;408:297–300. doi: 10.1016/S0014-5793(97)00447-X. [DOI] [PubMed] [Google Scholar]
- 66.Reiter R.J., Tan D., Osuna C., Gitto E. Actions of Melatonin in the Reduction of Oxidative Stress: A Review. J. Biomed. Sci. 2000;7:444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 67.Fang Y., Zhao C., Xiang H., Jia G., Zhong R. Melatonin Improves Cryopreservation of Ram Sperm by Inhibiting Mitochondrial Permeability Transition Pore Opening. Reprod. Domest. Anim. 2020;55:1240–1249. doi: 10.1111/rda.13771. [DOI] [PubMed] [Google Scholar]
- 68.Perumal P., Chang S., Baruah K.K., Srivastava N. Administration of Slow Release Exogenous Melatonin Modulates Oxidative Stress Profiles and In Vitro Fertilizing Ability of the Cryopreserved Mithun (Bos frontalis) Spermatozoa. Theriogenology. 2018;120:79–90. doi: 10.1016/j.theriogenology.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 69.ChaithraShree A.R., Ingole S.D., Dighe V.D., Nagvekar A.S., Bharucha S.V., Dagli N.R., Kekan P.M., Kharde S.D. Effect of Melatonin on Bovine Sperm Characteristics and Ultrastructure Changes Following Cryopreservation. Vet. Med. Sci. 2020;6:177–186. doi: 10.1002/vms3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pool K.R., Rickard J.P., Tumeth E., de Graaf S.P. Treatment of Rams with Melatonin Implants in the Non-Breeding Season Improves Post-Thaw Sperm Progressive Motility and DNA Integrity. Anim. Reprod. Sci. 2020;221:106579. doi: 10.1016/j.anireprosci.2020.106579. [DOI] [PubMed] [Google Scholar]
- 71.El-Raey M., Badr M.R., Rawash Z.M., Darwish G.M. Evidences for the Role of Melatonin as a Protective Additive During Buffalo Semen Freezing. Am. J. Anim Vet. Sci. 2014;9:252–262. doi: 10.3844/ajavsp.2014.252.262. [DOI] [Google Scholar]
- 72.El-Raey M., Badr M.R., Assi M.M., Rawash Z.M. Effect of melatonin on buffalo bull sperm freezability, ultrastructure changes and fertilizing potentials. Assiut Vet. Med. J. 2015;61:201–208. [Google Scholar]
- 73.Feng T.-Y., Li Q., Ren F., Xi H.-M., Lv D.-L., Li Y., Hu J.-H. Melatonin Protects Goat Spermatogonial Stem Cells against Oxidative Damage during Cryopreservation by Improving Antioxidant Capacity and Inhibiting Mitochondrial Apoptosis Pathway. Oxid. Med. Cell. Longev. 2020;2020:5954635. doi: 10.1155/2020/5954635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaudhary S.C., Aeksiri N., Wanangkarn A., Liao Y.-J., Inyawilert W. Effects of Melatonin on Cryopreserved Semen Parameters and Apoptosis of Thai Swamp Buffalo Bull (Bubalus bubalis) in Different Thawing Conditions. Adv. Anim. Vet. Sci. 2020;9:238–245. doi: 10.17582/journal.aavs/2021/9.2.238.245. [DOI] [Google Scholar]
- 75.Karimfar M., Niazvand F., Haghani K., Ghafourian S., Shirazi R., Bakhtiyari S. The Protective Effects of Melatonin against Cryopreservation-Induced Oxidative Stress in Human Sperm. Int. J. Immunopathol Pharm. 2015;28:69–76. doi: 10.1177/0394632015572080. [DOI] [PubMed] [Google Scholar]
- 76.Appiah M.O., He B., Lu W., Wang J. Antioxidative Effect of Melatonin on Cryopreserved Chicken Semen. Cryobiology. 2019;89:90–95. doi: 10.1016/j.cryobiol.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Tamura H., Jozaki M., Tanabe M., Shirafuta Y., Mihara Y., Shinagawa M., Tamura I., Maekawa R., Sato S., Taketani T., et al. Importance of Melatonin in Assisted Reproductive Technology and Ovarian Aging. Int. J. Mol. Sci. 2020;21:1135. doi: 10.3390/ijms21031135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pool K.R., Rickard J.P., Pini T., de Graaf S.P. Exogenous Melatonin Advances the Ram Breeding Season and Increases Testicular Function. Sci. Rep. 2020;10:9711. doi: 10.1038/s41598-020-66594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pool K.R., Rickard J.P., de Graaf S.P. Melatonin Improves the Motility and DNA Integrity of Frozen-Thawed Ram Spermatozoa Likely via Suppression of Mitochondrial Superoxide Production. Domest. Anim. Endocrinol. 2021;74:106516. doi: 10.1016/j.domaniend.2020.106516. [DOI] [PubMed] [Google Scholar]
- 80.Ashrafi I., Kohram H., Naijian H., Bahreini M., Poorhamdollah M. Protective effect of melatonin on sperm motility parameters on liquid storage of ram semen at 5 °C. Afr. J. Biotechnol. 2011;10:6670–6674. doi: 10.5897/AJB11.1020. [DOI] [Google Scholar]
- 81.Rateb S.A., Khalifa M.A., El-Hamid I.S.A., Shedeed H.A. Enhancing Liquid-Chilled Storage and Cryopreservation Capacities of Ram Spermatozoa by Supplementing the Diluent with Different Additives. Asian-Australasan J. Anim. Sci. 2020;33:1068–1076. doi: 10.5713/ajas.19.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medrano A., Contreras C.F.B., Herrera F.M., Alcantar-Rodriguez A.M. Melatonin as an Antioxidant Preserving Sperm from Domestic Animals. Asian Pac. J. Reprod. 2017;6:241. doi: 10.4103/2305-0500.217317. [DOI] [Google Scholar]
- 83.El-Battawy K. Preservation of goat semen at 5 °C with emphasis on its freezability and the impact of melatonin. Int. J. Vet. Sci. Res. 2019;5:35–38. doi: 10.17352/ijvsr.000039. [DOI] [Google Scholar]
- 84.Inyawilert W., Rungruangsak J., Liao Y., Tang P., Paungsukpaibool V. Melatonin Supplementation Improved Cryopreserved Thai Swamp Buffalo Semen. Reprod. Domest. Anim. 2021;56:83–88. doi: 10.1111/rda.13851. [DOI] [PubMed] [Google Scholar]
- 85.Maldjian A., Pizzi F., Gliozzi T., Cerolini S., Penny P., Noble R. Changes in Sperm Quality and Lipid Composition during Cryopreservation of Boar Semen. Theriogenology. 2005;63:411–421. doi: 10.1016/j.theriogenology.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 86.Martín-Hidalgo D., Barón F.J., Bragado M.J., Carmona P., Robina A., García-Marín L.J., Gil M.C. The Effect of Melatonin on the Quality of Extended Boar Semen after Long-Term Storage at 17 °C. Theriogenology. 2011;75:1550–1560. doi: 10.1016/j.theriogenology.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 87.De Lima Assis I., Palhares P.C., Machado G.J., da Silva Souza J.G., de Souza França T., de Oliveira Felizardo V., Murgas L.D.S. Effect of Melatonin on Cryopreserved Sperm of Prochilodus lineatus (Characiformes) Cryo Lett. 2019;40:152–158. [PubMed] [Google Scholar]
- 88.Motta N.C., Egger R.C., Monteiro K.S., de Oliveira A.V., Solis Murgas L.D. Effects of Melatonin Supplementation on the Quality of Cryopreserved Sperm in the Neotropical Fish Prochilodus lineatus. Theriogenology. 2022;179:14–21. doi: 10.1016/j.theriogenology.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 89.Ferrão M.L.P. Master Thesis. University of Algarve; Faro, Portugal: Nov, 2020. The Role of Melatonin in Sperm from Two Aquaculture Fish Species with Reproductive Problems: Solea senegalensis and Anguilla anguilla. [Google Scholar]
- 90.Félix F., Antunes R., Leoni L., Oliveira C.C.V., Cabrita E. Is there a melatonin protective effect during Gilthead seabream sperm cryopreservation?; Proceedings of the Aquaculture Europe 21; Funchal, Portugal. 4–7 October 2021; pp. 381–382. [Google Scholar]
- 91.Len J.S., Koh W.S.D., Tan S.-X. The Roles of Reactive Oxygen Species and Antioxidants in Cryopreservation. Biosci. Rep. 2019;39:BSR20191601. doi: 10.1042/BSR20191601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palhares P.C., de Lima Assis I., da Silva Souza J.G., de Souza França T., Egger R.C., de Jesus Paula D.A., Murgas L.D.S. Effect of Melatonin Supplementation to a Cytoprotective Medium on Post-Thawed Brycon orbignyanus Sperm Quality Preserved during Different Freezing Times. Cryobiology. 2020;96:159–165. doi: 10.1016/j.cryobiol.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 93.Palhares P.C., Assis I.D.L., Machado G.J., de Freitas R.M.P., de Freitas M.B.D., Paula D.A.J., Carneiro W.F., Motta N.C., Murgas L.D.S. Sperm Characteristics, Peroxidation Lipid and Antioxidant Enzyme Activity Changes in Milt of Brycon orbignyanus Cryopreserved with Melatonin in Different Freezing Curves. Theriogenology. 2021;176:18–25. doi: 10.1016/j.theriogenology.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 94.Martsikalis P.V., Gkafas G.A., Palaiokostas C., Exadactylos A. Genomics Era on Breeding Aquaculture Stocks. In: Lembo G., Mente E., editors. Organic Aquaculture. Springer; Berlin/Heidelberg, Germany: 2019. [DOI] [Google Scholar]
- 95.Gao D., Mazur P., Critser J.K. Fundamental Cryobiology of Mammalian Spermatozoa. In: Karow A.M., Critser J.K., editors. Reproductive Tissue Banking: Scientific Principles. Academic Press; Cambridge, MA, USA: 1997. pp. 263–328. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.