Abstract
Dermal substitutes provide a template for dermal regeneration and reconstruction. They constitutes an ideal clinical treatment for deep skin defects. However, rapid vascularization remains as a major hurdle to the development and application of dermal substitutes. Several bioactive factors play an important regulatory role in the process of angiogenesis and an understanding of the mechanism of achieving their effective delivery and sustained function is vital. Nanomaterials have great potential for tissue engineering. Effective delivery of bioactive factors (including growth factors, peptides and nucleic acids) by nanomaterials is of increasing research interest. This paper discusses the process of dermal substitute angiogenesis and the roles of related bioactive factors in this process. The application of nanomaterials for the delivery of bioactive factors to enhance angiogenesis and accelerate wound healing is also reviewed. We focus on new systems and approaches for delivering bioactive factors for enhancing angiogenesis in dermal substitutes.
Keywords: Nanomaterials, Bioactive factors, Dermal substitute, Angiogenesis, Wound healing, Regeneration, Tissue engineering, Vascularization
Highlights.
The role of bioactive factors in the process of dermal substitute angiogenesis is discussed.
Recent progress in the use of nanomaterials for delivery of bioactive factors to enhance angiogenesis and accelerate wound healing are systematically reviewed.
New systems and approaches for delivering nucleic acids for enhancing angiogenesis in dermal substitutes are described and summarized.
Background
Skin tissue defects triggered by various acute and chronic factors, including mechanical injury, burn, chronic ulcer and tumour resection, are often seen in clinical practice and represent a major public health, economic and social problem [1]. A dermal substitute is a dermal regeneration template that plays a crucial role in dermal reconstruction/regeneration. It constitutes an ideal treatment for deep skin defects. However, insufficient vascularization in dermal substitutes remains a challenge [2]. This deficiency is primarily manifested in the slow growth of new blood vessels during the early stages of implantation of the dermal substitute, which prolongs the latency to vascularization. Accordingly, accelerating the vascularization process is an urgent issue that needs to be addressed to enhance repair efficiency when using dermal substitutes [3].
To ensure rapid vascularization of dermal substitutes, various methods have been established to promote vascularization, including optimization of the scaffold structure [4], pre-seeding of vascular endothelial cells (ECs) or stem cells [5] and introduction of vasoactive factors [6]. The structure (i.e. pore size, porosity and connectivity) of dermal scaffold has a fundamental regulatory effect on the tissue cell growth induced by the scaffold. However, simply changing the structural parameters of the scaffold has a limited effect on vascularization. Pre-seeding cells or stem cells closely related to angiogenesis into the scaffold is important. Ultimately, the active components secreted by the cells indirectly affect the vascularization process of the scaffold [5]. The vascularization process taking place within the dermal substitute body from angiogenesis to maturation is closely regulated by various bioactive factors [7]. Enhancing the vascularization of dermal substitutes by delivering bioactive factors is an emerging strategy to promote wound healing. However, the use of bioactive factors in wound tissues is hampered by their rapid degradation [8,9]. To overcome this problem, another effective strategy for the delivery of bioactive factors uses nanomaterials to transfer the gene of a pro-angiogenic factor into wound cells, such that cells transfected with the target gene can continuously and stably express the factor within a certain period of time, thereby promoting angiogenesis. Genes encoding pro-angiogenic factors have achieved better wound closure than direct application of bioactive factors [10].
Nano-sized carriers are required for delivery of biologics that act intracellularly, such as peptides or nucleic acids. Small size would favour improved drug penetration into wound beds and increased intracellular uptake [11]. On the other hand, microparticle-mediated delivery would offer a more sustained therapeutic effect if only extracellular delivery is required because the lower surface-to-volume ratio would slow the release kinetics [12]. In this review, we summarise recent progress in the use of nanomaterials to deliver bioactive factors (including growth factors, peptides and nucleic acids) to improve dermis substitute angiogenesis (DSA) and accelerate wound healing. In particular, we focus on new systems and approaches for delivering bioactive factors to enhance angiogenesis in dermal substitutes. Additionally, important strategies are proposed to achieve effective delivery and sustained function of bioactive factors in the proteolytic environment of wounds.
Review
Dermal substitute angiogenesis in wound healing
The vascularization process of a dermal substitute implanted into a wound resembles the vascularization process of wound healing. It is highly complex, involving the interaction of various cells, including fibroblasts, ECs and pericytes, along with various bioactive factors including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) and angiopoietins, extracellular matrix and proteolytic enzymes (Fiigure 1). Vascularization is a two-stage process, i.e. angiogenesis followed by vascular maturation. In the angiogenesis stage, ECs proliferate to form primary vascular structures by budding, under the regulation of various factors. Wound healing can be roughly divided into three phases: inflammatory, proliferative and remodelling. In the first stage of wound healing, hypoxia causes rapid infiltration of inflammatory cells, including neutrophils, mast cells, lymphocytes and macrophages, and the formation of granulation tissue. Also, VEGF, primarily expressed during granulation tissue formation (from day 4 to 7) [13], is released by macrophages, ECs [13], platelets [14], activated epidermal cells and fibroblasts following tumour necrosis factor alpha (TNF-α) induction [15]. VEGF specifically binds to the VEGFR-2 receptor on the surface of ECs and promotes the migration, proliferation and primary lumen formation of ECs. In addition to vascular endothelial cells, other cells, including keratinocytes and macrophages, express VEGFRs and directly respond to VEGF [16]. However, the structure and function of the new blood vessel are immature, reflected in a narrow lumen, high permeability of the vessel wall and easy leakage [17,18]. Further vascular maturation improves the function of new blood vessels.
Figure 1.
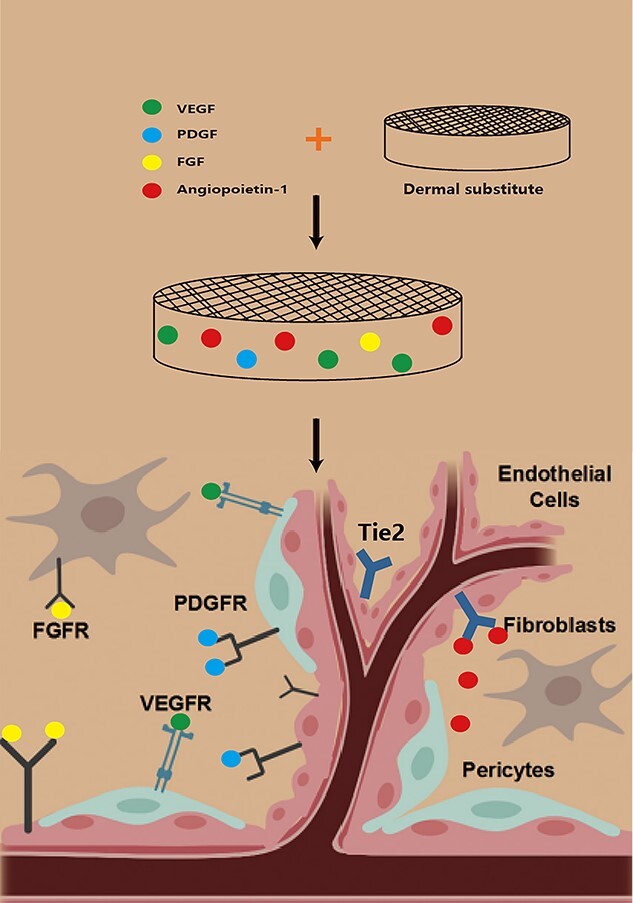
Summary diagram of the cellular and molecular components of dermal substitute angiogenesis. VEGF vascular endothelial growth factor receptor, PDGF platelet-derived growth factor, FGF fibroblast growth factor receptor, VEGFR vascular endothelial growth factor, PDGFR platelet-derived growth factor, FGFR fibroblast growth factor receptor
Additionally, endothelial progenitor cells (EPCs) continuously express chemokine receptor 4, a receptor of stromal cell-derived factor 1 (SDF-1). SDF-1 promotes the regeneration of injured blood vessels by mediating the migration and aggregation of EPCs [19]. FGF-2 is a multifunctional polypeptide released by fibroblasts, keratinocytes, ECs, smooth muscle cells and chondrocytes during the healing process [20]. Moreover, it promotes the growth, migration and differentiation of various cell types, including dermal fibroblasts, keratinocytes, ECs and melanocytes [21]. FGF-2 stimulates the expression of VEGF, granulation tissue formation and angiogenesis in wounds [22]. PDGF, EGF and hypoxia-inducible factor 1 (HIF-1) activation up-regulate the expression of VEGF in wounds [23,24]. HIF-1 is the core regulator of angiogenesis under hypoxia and directly participates in the entire angiogenesis process by influencing the expression of other growth factors [25,26]. Under hypoxia, the HIF-1α subunit migrates to the nucleus and binds to the HIF-1β subunit. The transcription initiation complex thus formed binds to the hypoxia response element (HBE) containing 5′-CGTG-3′ in the 5′ flanking sequence of the target gene, and triggers the transcription of related target genes, thereby balancing the oxygen demand of the body [27]. Related target genes are involved in angiogenesis and remodelling, as well as energy metabolism, cell migration and apoptosis [23].
Notably, pericytes play a vital role in vascular maturation. These heterogeneous cell groups, located in the vessel wall, are generally implicated in vascular rheology and maintenance of homeostasis, including blood flow regulation, angiogenesis, vascular system stability and vascular permeability. Generally, pericytes stabilize the newly formed vascular structure via direct contact with ECs or paracrine during a relatively late stage of vascularization, thus promoting the maturation of new blood vessels [28]. Pericytes are closely associated with ECs, which play crucial roles in the maintenance of blood vessels and angiogenesis. Physical interactions, through adhesion plaques, ‘peg-and-socket’ junctions and gap junctions are key for maintaining pericyte–endothelium attachment [29]. Activated ECs secrete PDGF-B via paracrine to recruit pericytes and bind to PDGF receptor β (PDGFRβ) expressed on pericytes [30,31]. Furthermore, the secretion of angiopoietin 1 (Ang-1) by pericytes mediates pericyte–endothelial attachment via the Tie2 receptor, which is expressed by ECs [32]. Disruption of the pericyte–EC interaction triggers a loss of pericyte coverage in the vessels and a leaky vasculature, resulting in haemorrhage and oedema [33].
Promotion of DSA in wound healing via delivery of bioactive factors by nanomaterials
Bioactive factors promote vascularization. Besides effectively stimulating the aggregation and proliferation of ECs and EPCs, various bioactive factors promote the formation and development of new blood vessels. However, the effect of direct application of these expensive bioactive factors is often unsatisfactory, primarily due to their instability, easy inactivation and short half-life, making them difficult to be used for promoting vascularization. To ensure effective delivery and continuous functioning of angiogenic factors, common strategies involve controlled release of single or multiple active factors via a delivery system, including hydrogels, scaffold-like films, sponges, nanofibres (NFs), nanoparticles (NPs) and nanomicrospheres (NMs) [34,35] (Table 1).
Table 1.
Common bioactive factors delivery system used in wound healing research
| Bioactive factors | Delivery mode | Wound model | Results | References |
|---|---|---|---|---|
| VEGF bFGF |
PLGA NPs Liposomes |
Excisional wound in db/db mice. Deep second degree burns in rats. |
Promoted faster wound healing, re-epithelialization, granulation tissue formation, and neovascularization. Liposomes can effectively protect the biological activity of bFGF, significantly promote wound vascularization and shorten healing time. |
[37] [62] [43] |
| PCL microspheres | In vivo matrigel plug angiogenesis assay in 8-week-old Sprague–Dawley rats. | Significantly increase the expression of angiogenic genes (bFGF and VEGFA). | [42] | |
| PDGF-BB Angiogenin GM-CSF SDF-1α Ginsenoside Rg1 LL37 |
PLLA NFs scaffold containing PDGF-BB Microspheres Heparinized collagen-chitosan porous scaffold Heparinized gelatin-chitosan scaffold ROS-responsive NPs SDF-1α Collagen/chitosan-gelatin microspheres PLGA NPs |
Mid-sagittal incisions on Sprague Dawley rats skin flap model. Subcutaneous implantation in New Zealand rabbit. Subcutaneous implantation in Sprague–Dawley rats. Full-thickness dorsal wound in mice. Full-thickness dorsal wound in Sprague–Dawley rats. Full-thickness excisional skin wounds (8 mm) in 6–7 week old mice. |
Successfully realized the sustained release of PDGF-BB, enhance cell migration and angiogenesis in vivo. Enhanced the angiogenesis. Successfully realized the controlled release of GM-CSF, promote vascularization. SDF-1α was effectively released and targeted to the wound; promoted the chemotaxis of BM-MSCs to the wound, induced wound angiogenesis and accelerated wound healing. Promote the proliferation of EC and specifically enhance the expression of VEGF; promoted HUVEC migration and tube formation. Induced faster wound closure, increased collagen production, significantly up-regulated the expression of IL-6 and VEGF, increased angiogenesis, and regulated inflammatory wound response. |
[47] [52] [54] [56] [6] [62] |
| AP-57 | AP-57-NP in heat-sensitive hydrogel (AP-57-NPs-H) | Full-thickness excisional skin wounds (2 × 2 cm) in Sprague Dawley rats. | Promoted granulation tissue formation, increased collagen deposition and promoted angiogenesis. | [63] |
| VEGF-PDGF-BB VEGF-bFGF bFGF-VEGF-EGF-PDGF |
PLGA NP(PBGF-BB)-CS/PEO NFs (VEGF) Poly(ether)urethane-polydimethylsiloxane/fibrin-based scaffold containing PLGA NPs loaded with VEGF and bFGF Hyaluronic NFs loaded with bFGF and VEGF-loaded gelatine NPs Collagen NFs loaded with EGF and PDGF-loaded gelatine NPs |
Full-thickness excisional wound (5 mm diameter) in Sprague Dawley rats. Full-thickness dorsal wound (8 mm diameter) in 12-week-old db/db mice. Excisional wound (15 mm diameter) in male Sprague Dawley rats STZ induced. |
Significantly accelerate the wound healing process by promoting angiogenesis, increasing re-epithelization and controlling granulation tissue formation. Induced complete re-epithelialization, enhanced granulation tissue formation/maturation, promoted vascularization, collagen deposition, and accelerated wound closure. Improve the proliferation of HUVEC and the formation of vascular-like tubular structures in vitro, promote the re-epithelization, dermal reconstruction, and the formation of mature vessels. |
[49] [44] [38] |
VEGF vascular endothelial growth factor receptor, PDGF platelet-derived growth factor, FGF fibroblast growth factor receptor, PLGA poly (lactic-co-glycolic acid), GM-CSF granulocyte-macrophage colony-stimulating factor, PDGF-BB platelet-derived growth factor-BB, bFGF basicfibroblast growth factor, ROS reactive oxygen species, SDF stromal cell derived factor, NPs nanoparticles, EGF epidermal growth factor, HUVEC human umbilical vein vascular endothelial cell
Nanomaterial delivery systems loaded with growth factors
Using growth factors to induce both angiogenesis and enhance wound closure has been intensively studied. Endogenous growth factors play a critical role in orchestrating the wound healing process and are essential for DSA and effective wound healing (Figure 2) [20].
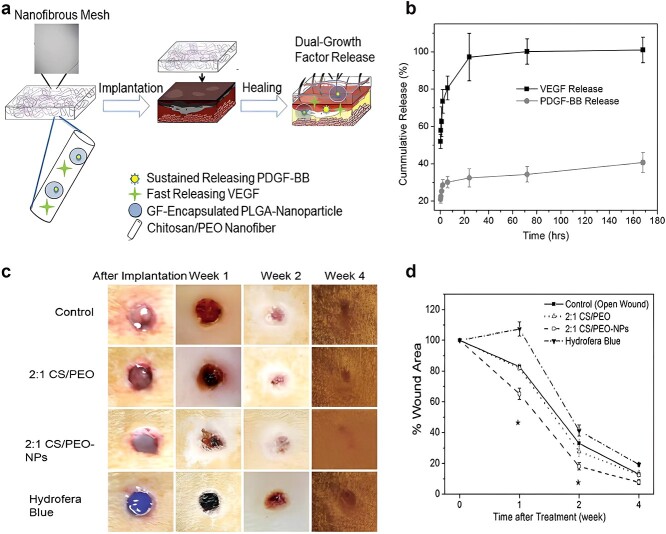
Figure 2.
Dual growth factor releasing multi-functional nanofibers for wound healing. (a) Schematic illustration of the nanoparticle embedded electrospun nanofibers loaded with two growth factors for the wound healing. (b) Growth factor release kinetics from nanofibers and nanoparticles within fibers as determined by ELISA. (c) Representative macroscopic appearance of wound closure at 0, 1, 2, and 4 weeks after treatment of skin wound after 0, 1, 2, and 4 weeks. (d) Quantitative measurement of wound size reduction. (*p<0.05, **p<0.01). PDGF-BB platelet-derived growth factor-BB, VEGF vascular endothelial growth factor, GF growth factor, CS chitosan, PEO poly (ethylene oxide), PLGA poly-lactic-co-glycolic acid [49]. (Copyright 2013 Acta Biomaterialia Inc.)
VEGF, a glycosylated secretory polypeptide factor, is a powerful angiogenic factor that can stimulate the mitosis of human umbilical vein endothelial cells (HUVECs) and angiogenesis, as well as improve the permeability of monolayer endothelium. Based on the different shearing methods of mRNA, VEGF produces at least five different protein forms. Among them, VEGF121, VEGF145 and VEGF165 are soluble proteins, which directly act on vascular ECs and promote their proliferation. Golub et al. [36] embedded VEGF in poly (lactic-co-glycolic acid) (PLGA) nanospheres to construct a controlled release system that could release VEGF within 2–4 days. In vivo studies showed that this system was beneficial to induce vascular ingrowth. Chereddy et al. [37] reported positive effects of VEGF-loaded PLGA NPs on wound healing and angiogenesis. The release of VEGF from PLGA-VEGF NPs followed a biphasic pattern (sustained release after initial sudden release). Most studies have focused on the vascularization of a single growth factor, while the vascularization process in vivo is often complex, involving multiple bioactive factors. Therefore, it is essential to investigate the synergy and antagonism between different pro-angiogenic factors, as well as the mechanism of sequential regulation of the vascularization process, to resolve the early vascularization of dermal scaffolds. The collagen (Col)–hyaluronic acid (HA)–gelatine (Col–HA–GN) nanofibrous membrane developed by Lai et al. displayed similar mechanical properties to human skin, releasing various angiogenic growth factors (VEGF, PDGF, basic fibroblast growth factor [bFGF] and EGF) in a programmable manner for up to 1 month. The delivery of EGF and bFGF in the early stage was expected to accelerate epithelialization and vasculature sprouting, while the release of PDGF and VEGF in the late stage was intended to induce maturation of blood vessels. The results showed that the Col–HA membrane with four types of growth factor NF constructs (Col–HAw/4GF) improved HUVEC proliferation and the formation of vascular-like tubular structures in vitro and also promoted re-epithelization, dermal reconstruction and the formation of mature vessels in streptozotocin (STZ)-induced diabetic rats [38].
FGFs are a type of multifunctional cell growth factor, being both mitogens of fibroblasts and chemokines of ECs, which effectively promote angiogenesis [20]. Among them, bFGF (or FGF-2) is an important member of the family. bFGF exists in the basement membrane and subendothelial extracellular matrix. It stimulates the proliferation of fibroblasts and capillary ECs, thereby promoting angiogenesis and wound healing [22,39]. Mesoporous silica NPs (MSNs) can be used for controlled drug release by fine-tuning the surface area-to-volume ratio, pore size and surface properties. Zhang et al. delivered bFGF from MSNs with a formulation that allowed release over 20 days. MSNs represent a new system for the delivery of growth factors, assist blood vessel regeneration and potentiate angiogenesis [40]. Li et al. fabricated biodegradable GN microspheres containing bFGF, and incorporated them into a porous Col/cellulose nanocrystals scaffold as a platform for long-term release (up to 14 days) and consequent angiogenic promotion. In a subcutaneous implantation model, bFGF-releasing GN microspheres significantly increased the number of new blood vessels compared to FGF-2 from the scaffold alone or control group [41]. Arunkumar et al. [42] developed bFGF-loaded poly (ε- caprolactone) (PCL) microspheres for sustained release of bFGF and evaluated their angiogenic potential. In vitro bFGF release studies showed controlled release for up to 30 days. HUVECs treated with bFGF–PCL-microspheres significantly upregulated the expression of angiogenic genes (bFGF and VEGFA). Xu et al. developed liposomes with a hydrogel core of silk fibroin (SF-LIP) by gelation of liquid SF inside vesicles [43]. SF-LIP encapsulated bFGF with high efficiency and improved the stability of bFGF in wound fluid 3-fold relative to free bFGF. Furthermore, bFGF-incorporated SF-LIP not only accelerated wound closure in mice with deep second-degree scald but also induced angiogenesis by improving the stability of bFGF. Losi et al. [44] integrated NPs loaded with VEGF and bFGF into fibrin-based polymer scaffolds. The scaffold comprising two growth factors induced complete re-epithelialization, enhanced granulation tissue formation/maturation, promoted vascularization and Col deposition and accelerated wound closure.
PDGF-BB locally delivered by peptide NFs reduced the infarct size after ischaemia/reperfusion [45]. This was partly because PDGF promoted Col synthesis, recruited pericytes through neovascularization and regulated the maturation of infarcted vasculature [46]. However, a major obstacle to the successful clinical application of PDGF is the delivery system. Slow-release is key to the in vivo application of PDGF to promote tissue repair. Jin et al. [47] designed a poly-L-lactide NF scaffold containing PDGF-BB microspheres and achieved sustained release of PDGF-BB. By controlling the molecular weight of the microspheres over the range of 6. 5–64 kDa, release rates of PDGF were regulated over periods of weeks to months in vitro. In vivo experiments confirmed that this controlled release scaffold enhanced cell migration and angiogenesis, which might be related to the induction of chemokine-related genes. Simultaneous application of multiple growth factors may provide the extra benefit needed for use in the clinical environment [48]. Xie et al. [49] successfully developed a biomimetic NF scaffold loaded with NPs, which released VEGF and PDGF-BB via a relay mode. Chitosan and polyethylene oxide were electrospun into NF meshes as mimics of the extracellular matrix. VEGF was loaded within the NFs to promote short-term angiogenesis. Furthermore, PDGF-BB-encapsulated PLGA NPs were embedded inside the NFs for sustained release of PDGF-BB to accelerate tissue regeneration and remodelling. A preliminary study using a full-thickness rat skin wound model demonstrated that a bionic NF scaffold significantly accelerated the wound healing process by promoting angiogenesis, increasing re-epithelization and controlling granulation tissue formation. In another study, an electrospun NF scaffold of Col and HA was created that released FGF-2, EGF, VEGF and PDGF-BB with varying release kinetics. The delivery of EGF and bFGF during the early stage was expected to accelerate epithelialization and vasculature sprouting, while the release of PDGF and VEGF in the late stage was intended to induce blood vessel maturation. This construct accelerated wound closure and the maturation of blood vessels in the wound beds of rats with STZ-induced diabetes [38].
Activation of EGF up-regulates VEGF expression in the wound. Chu et al. [50] used a modified double-emulsion method with PLGA as the carrier to prepare recombinant human epidermal growth factor (rhEGF) NPs. The rhEGF NPs were ~193. 5 nm in diameter, and the particle size distribution was uniform and dispersible. The encapsulation efficiency was 85.6% and rhEGF release lasted 24 h. The results showed that controlled release of rhEGF encapsulated in the NPs enhanced the stimulatory effects of rhEGF on cell proliferation, accelerating angiogenesis and shortening the wound healing time.
The delivery of exogenous growth factors has been shown to promote wound closure and angiogenesis in animal studies. However, mixed results from clinical trials and the prohibitive cost of the therapies have slowed clinical adoption of these treatments. Nevertheless, research continues to develop more effective growth factor therapies for wound healing. Future studies including growth factor-based gene therapy and biomaterial-based drug delivery systems might revolutionize treatment of chronic wounds.
Nanomaterial delivery systems loaded with other peptides and proteins
Angiogenin is implicated in virtually the entire process from angiogenesis to maturation and regulates angiogenesis [51]. Angiopoietin was grafted onto a Col–chitosan porous scaffold to construct a biologically active scaffold with controlled release of angiogenin. The scaffold rapidly induced blood vessel growth [52]. However, insufficient stability of the ‘scaffold-loaded protein’ controlled release system, expensive bioactive factors and a short half-life in vivo limit its further application. Wang et al. [53] believed that introducing the plasmid DNA of angiogenin into the scaffold, to obtain a new gene-active dermal scaffold, might be a feasible approach for effective delivery of angiogenin and continuous promotion of vascularization. Despite angiogenin exerting a powerful angiogenesis-promoting effect, research on its application in the field of tissue engineering (TE) is still in the exploratory stage.
Sun et al. [54] grafted granulocyte-macrophage colony-stimulating factor (GM-CSF) onto heparinized GN–chitosan scaffold by chemical crosslinking and achieved controlled release of GM-CSF. In vivo implantation experiments confirmed that this controlled release scaffold promoted macrophage aggregation and vascularization. Kohara et al. [55] embedded the neuropeptide substance P into a biodegradable hydrogel comprising silver ions and achieved controlled release of substance P. In vivo experiments showed that this controlled release system was more beneficial for promoting blood vessels than subcutaneous injection of substance P. Tang et al. [56] synthesised reactive oxygen species (ROS)-responsive NPs containing SDF-1α. These NPs were intravenously infused into mice with full-thickness skin defects. The experimental results revealed that SDF-1α was effectively released and targeted the wound, hence promoting chemotaxis of bone marrow mesenchymal stem cells to the wound and its surroundings, inducing wound angiogenesis and accelerating wound healing.
Traditional Chinese medicine ingredients have also been used to promote angiogenesis. For example, puerarin activates VEGF, HIF-1 and nitric oxide synthase (NOS) for pro-vascularization effects [57]. Ginsenoside Rgl enhances VEGF expression in human ECs by activating the glucocorticoid receptor-related phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway and B-catenin-T cell factor-related signal pathway [58]. Panax notoginseng saponins promote the proliferation of ECs and specifically enhance the expression of VEGF mRNA by activating the PI3K/Akt pathway. Application of P. notoginseng extract to zebrafish enhanced vascularization [59]. Our research group evaluated the angiogenesis-promoting effect of P. notoginseng saponin Rg1 in vitro. Here, ginsenoside Rg1 was incorporated into a Col/chitosan-GN microsphere (CC-GMS) scaffold. The Rg1 released from the CC-GMS scaffold did not lose its activity, had a significant effect on HUVEC proliferation and promoted HUVEC migration and tube formation [6].
LL37 is an endogenous human host defence peptide that regulates wound healing and angiogenesis and prevents infection [60]. The antimicrobial and angiogenic activity of LL37 renders it a potential drug candidate for wound healing therapy [61]. Although topical application of free LL37 did not improve wound healing in mice, encapsulating LL37 in PLGA NPs enabled sustained release of LL37 [62]. PLGA–LL37 NPs induced faster wound closure, increased Col production, significantly up-regulated the expression of interleukin (IL)-6 and VEGF, increased angiogenesis and regulated the inflammatory response. These effects were attributed to the combined action of lactic acid release from NPs after degradation and LL37 release from NPs. AP-57 is another antibacterial peptide. AP-57 NPs were encapsulated in a heat-sensitive hydrogel (AP-57-NPs-H) to promote skin wound repair. The AP-57-NPs-H displayed prolonged release of AP-57, low cytotoxicity and high antioxidant activity in vitro. In vivo wound healing testing demonstrated that AP-57-NPs-H promoted skin wound healing via granulation tissue formation, thereby increasing Col deposition and promoting angiogenesis in wound tissue [63].
Nanomaterial delivery systems loaded with nucleic acids
Currently, DSA gene therapy mostly uses bioactive factors as therapeutic genes. Two main methods are used to deliver target genes to target cells, i.e. a viral vector delivery system (VVDM) and a non-viral vector delivery system (nVVDM) (Table 2). Viral vectors, which include adenovirus, lentivirus and retrovirus vectors, transfect cells in large quantities, leading to high transfection rates and life-long expression of the transgenes or regulation of the host genome. However, insertion mutations and other adverse effects have been reported during post-transfection periods after viral gene delivery [64], resulting in uncertainty and controversy regarding the application of viral vectors for tissue repair and regeneration. The nVVDMs include plasmid injection, gene gun, electroporation, cationic polymers, liposome and liposome complexes. Non-viral vectors have several advantages over viral delivery, including lower immunogenicity and toxicity, better cell specificity, better modifiability and higher productivity [65]. They are a better alternative to deliver genes for the repair and regeneration of damaged tissues. However, the transfection efficiency of a non-viral vector is often lower, thereby limiting its application compared to a viral vector [66] and the efficiency varies depending on the type of vector and target cell (Table 3). Furthermore, as an emerging drug delivery system, DNA nanomaterials have great potential to achieve drug delivery [67,68]. The delivery system of DNA nanomaterials has unique characteristics such as uniform size, clear shape, accurate spatial positioning, surface functionalization of specific sites, excellent biocompatibility and so on. Therefore, it has incomparable advantages in biomedical applications [69,70].
Table 2.
Different types of gene delivery and their characteristics
| Type | Method | Principle | Feature | References | |
|---|---|---|---|---|---|
| Non-viral vector delivery | Chemical transfection |
Calcium phosphate | Complex of calcium phosphate and DNA is endocytosed by cell via absorbtion to cell membrane. | Easy to operate but with low repeatability. Not fit for every types of cell. |
[71] |
| Cationic polymer-liposome | Complex of liposome with positive electricity and negative phosphate group of DNA is endocytosed by cell. | Wild applicability, high transfection efficiency and repeatability. Need serum. Transfection effects vary with cell type. |
[95,96] | ||
| Physical transfection |
Electroporation | High-pulse voltage destroys cell membrane potential, DNA enters cells by pores in membrane. | Wild applicability but with high cell fatality rate. Considerable quantity of DNA and cells. |
[88] | |
| DNA injection | Using microscope to inject DNA into cell nucleus directly. | Limited quantity of transfected cells. Often applied to gene engineering modification or embryonic cells of transgenic animals. |
[86] | ||
| Gene gun | Using gold or tungsten as the probe and helium pressure as the power to carry DNA plasmid into cells. | Can carry multiple genes at the same time. Short gene expression time. Surrounding cells damage. |
[87–89] | ||
| Viral vector delivery | Biological transfection |
Virus induction | Combining exogenous genes to chromosome via infecting host cells. | Can be applied to cells that are difficult to transfect, primary cells, cells in vivo. | [64] |
| Retrovirus | Small target gene. Cells should be in mitotic phase. Security should be considered. |
[72] | |||
| Adenovirus | Can be applied to cells that are difficult to transfect. Security should be considered. |
[73,74] | |||
Table 3.
Common nucleic acids used in wound healing studies by VVDM and nVVDM
| Nucleic acids | Delivery mode | Wound model | Results | References |
|---|---|---|---|---|
| VEGF | Recombinant adenovirus vector | Excisional wounds of streptozotocin-induced diabetic mice. | Significantly accelerate wound closure; promote angiogenesis. | [73] |
| rAAV vectors | Full-thickness excisional skin wound in diabetic mice. | Accelerated stimulation of angiogenesis, reepithelization, synthesis and maturation of extracellular matrix. | [74,75] | |
| pXC1 containing adenovirus type 5 sequences and a Rous sarcoma virus promoter |
Full-thickness excisional wounds (1. 4 cm in diameter) in 8-week-old db/db mice. | Accelerate wound closure by stimulating angiogenesis, epithelialization and collagen deposition. | [76] | |
| Adenovirus vector Electroporation Cationic polymers, (N,N,N-TMC) Copolymer-protected gene vectors |
Full-thickness excisional skin wounds (3–5 mm diameter) in 10-week-old db/db mice. Rat skin flap model. Full-thickness excisional skin wounds (3 cm diameter) in a porcine model. Full-thickness excisional skin wounds (12 mm diameter) in 6–8-week-old db/db mice. |
Enhanced angiogenesis and lymphangiogenesis; significantly accelerated wound healing. Significantly increased the expression of VEGF. Significant increase in the number of newly-formed and mature vessels and the fastest dermal regeneration; increased the tensile strength of the repaired tissue. The transient release of VEGF up to 3 weeks; promoted the formation of blood vessels. |
[77] [95] [98] [99] |
|
| FGF4-VEGF PDGF-BB HGF Ang1 |
AAV vector Adenovirus vector Adenovirus vector rAAV vectors |
Full-thickness excisional skin wounds (4 mm diameter) in 14-week-old db/db mice. Full-thickness excisional skin wounds (8 mm diameter) in db/db mice. Skin flap transplantation model in Sprague–Dawley rats. Incisional skin wound in 14-week-old db/db mice. |
Significantly increased granulation tissue formation, vascularity and dermal matrix deposition. Enhance wound healing, improve neovascularization and recruit EPCs to the epithelial wound. Improve the survival rate of flap; both the number of CD31-positive vessels and the expression of VEGF were significantly increased. Improved the healing process, stimulate reepithelization and collagen maturation, increase breaking strength and significantly augment the number of new vessels. |
[77] [79] [80][81] |
| eNOS HIF-1α TGF-β1 IGF-I—KGF |
Adenoviral vector in a fibrin scaffold Electroporation Pegylated poly-l-lysine (PLL-g-PEG) polymer Electroporation Liposome gene transfer |
Full-thickness excisional skin wounds (6 mm diameter) on the ear of New Zealand white rabbits. Full-thickness excisional skin wounds (5 mm diameter) in db/db mice. Full-thickness excisional skin wounds (10 mm diameter) in Sprague–Dawley rats Full-thickness excisional skin wounds (7 × 7 mm) in db/db mice Full-thickness scald burn on back of Sprague–Dawley rats |
Enhanced eNOS expression, inflammatory response, and increased the rate of re-epithelialization. Up-regulated the expressions of VEGF, PGF, PDGF and angiopoietin and promoted the epithelization of the wound surface and angiogenesis. Transiently induced gene expression of angiogenesis-related genes Acta2, Pecam1 and VEGF; enhance the number endothelial cells and smooth muscle cells Promoted epithelial regeneration, granulation tissue formation, angiogenesis. Increase the concentration of VEGF, thereby increasing the formation of new blood vessels; improved epidermal regeneration |
[85] [93] [101] [94] [105] |
VEGF vascular endothelial growth factor receptor, rAAV recombinant adeno-associated virus, HIF-1 hypoxia-inducible factor-1, HGF hepatocyte growth factor, nVVDM non-viral vector delivery system, TMC trimethyl chitosan chloride, FGF fibroblast growth factor, Ang1 angiopoietin-1, eNOS endothelial nitric oxide synthase, TGF transforming growth factor, IGF insulin-like growth factor, KGF keratinocyte growth factor
Viral gene delivery
Viral gene transfer has been used to enhance the expression of bioactive factors during wound healing, specifically the expression of bioactive factors involved in angiogenesis. Deodato et al. [73] found that recombinant adeno-associated virus (rAAV) vectors effectively transferred VEGF165 to rat skin and gene expression from these vectors was sustained and persistent over time. Histological examination of treated wounds revealed accelerated remodelling of epidermis and dermis and the formation of a thick granular layer comprising numerous newly formed capillaries and vessels of larger size, which shortened the healing time. Galeano et al. [74] also discovered that the r AAV vector was highly effective for gene transfer to skin tissue. The rAAV-VEGF165 gene improved wound healing in diabetic mice through the stimulation of angiogenesis and re-epithelization, synthesis and maturation of the extracellular matrix. Moreover, the rAAV encoding human VEGF165 increased the breaking strength of the wound and enhanced the wound content of VEGF. Brem et al. [75] constructed an adenoviral vector by cloning human VEGF165 into the multiple cloning sites of an adenovirus shuttle vector (pXC1) with adenovirus type 5 sequences (bp 22–5, 790) and a Rous sarcoma virus promoter. The ADV/VEGF accelerated wound closure by stimulating angiogenesis, epithelialization and Col deposition. Saaristo et al. [76] overexpressed vascular VEGF-C via an adenoviral vector to improve the healing of full-thickness punch biopsy wounds in genetically diabetic (db/db) mice. They found that VEGF-C enhanced angiogenesis and lymphangiogenesis in the wound and significantly accelerated wound healing compared to control wounds. Numerous studies have combined gene therapy with several bioactive factors to act at different levels throughout the complex vascularization process. Jazwa et al. [77] observed the fastest healing in mice injected with bicistronic AAV-FGF4-IRES-VEGF-A vector (day 17). This was accompanied by significantly increased granulation tissue formation, vascularity and dermal matrix deposition. Mechanistically, FGF4 stimulated matrix metalloproteinase-9 (MMP-9) and VEGF receptor-1 expression in dermal fibroblasts of mice, thereby enhancing their migration when delivered in combination with VEGF-A. Liu et al. [78] showed that the combined use of VEGF165 and bFGF cDNA improved flap vitality and neovascularization.
PDGF-B is another common bioactive factor that promotes angiogenesis. Keswani et al. [79] confirmed that adenoviral-mediated overexpression of PDGF-B enhanced wound healing, improved neovascularization and recruited EPCs to the epithelial wound in diabetic mouse models. EPC recruitment was positively correlated with neovascularization and wound healing, and PDGF-B-mediated augmentation of EPC recruitment to the wound bed might be a fundamental mechanism underlying these findings. Hepatocyte growth factor (HGF) is a potent angiogenic factor that stimulates the production of blood vessels in ischaemic tissue. Studies have used adenovirus expressing HGF for gene therapy to enhance flap survival. In the HGF virus group, the number of CD31-positive vessels and VEGF expression were significantly upregulated [80].
Ang-1 applied to wounds via gene therapy improves wound healing and promotes skin tissue regeneration and angiogenesis. Bitto et al. [81] discovered that rAAV-Ang-1 significantly improved the healing process, stimulating re-epithelization and Col maturation, increasing breaking strength and significantly increasing the number of new vessels. However, Ang-1 gene transfer did not modify the downregulated expression of VEGF mRNA and protein in diabetic mice. In contrast, Ang-1 upregulated the expression of endothelial nitric oxide synthase (eNOS) and augmented wound nitrate content as well as VEGFR-2 immunostaining and protein expression. Ang-1 gene transfer did not alter vascular permeability. This strongly indicates that Ang-1 gene transfer enhanced delayed wound repair in diabetes by inducing angiogenesis in a VEGF-independent manner.
Several lines of evidence indicate that nitric oxide plays a role in normal wound repair [82,83]. Wound closure was delayed by 31% in iNOS knockout mice compared to wild-type animals. One study discovered that the application of an adenovirus vector with human iNOS cDNA (AdeNOS) could completely reverse the delayed wound healing seen in iNOS-deficient mice, promoting wound angiogenesis [84]. Breen et al. [85] evaluated whether a fibrin scaffold enhanced the delivery of adenovirus encoding eNOS, which is one of the enzymes responsible for NO production; they observed greater NO production and enhanced healing. It was concluded that fibrin delivery of AdeNOS enhanced eNOS expression, the inflammatory response and the rate of re-epithelialization.
Non-viral gene delivery
Non-viral gene transfer is an area of major interest due to its biological safety and unlimited gene-size transferability [65]. Several commonly used non-viral vectors are summarised below.
Naked DNA injection
Gene therapy using plasmid DNA encoding VEGF is a promising strategy to promote vascularization. O’Toole et al. [86] evaluated three plasmids encoding different VEGF isoforms (VEGF A165, VEGF B167 and VEGF B186). The survival of flaps treated with VEGF A165 or B167 cDNA was significantly greater than that of controls. However, no difference in the number or size of blood vessels was observed through angiography and histology, indicating that the protective effect of microvascular repair and prevention of microvascular regression by VEGF were sufficient to enhance flap survival. The efficacy of plasmid injection was not high due to the fragility of the naked plasmid structure in the extracellular environment and to charge rejection [87,88].
Gene gun
The gene gun uses 1–5 μm of gold or tungsten particles as the probe and helium pressure as the power source to transport various DNA plasmids via ballistic micro-emission to skin cells [87]. The gene gun can simultaneously transport multiple genes or even tissue. However, the gene expression time is short (high expression within 1–3 days after injection) and genes penetrating epidermal cells account for ~10% of the total; they also damage surrounding cells [89]. Dileo et al. [90] devised a specially enhanced gene gun that could efficiently express genes in the epidermis, dermis and muscle, with expression peaking at 24 h, spanning at least 1 week and causing little damage to surrounding tissues. In short, the application of a gene gun is frequently limited due to low transfection rate and low activity of manipulated cells, causing poor angiogenesis, tissue repair and regeneration.
Electroporation
Electroporation is a physical process that temporarily creates micropores in the cell membrane under the action of a pulsed electric field, resulting in a significant increase in the exchange of molecules inside and outside the cell. It is conducive to the absorption of various drugs, genetic materials, proteins and other macromolecules. Glasspool-Malone et al. [91] found that the transfection efficiency of naked DNA transferred by electric pulses was 115-fold higher. The optimal electroporation protocol had no adverse effect on wound healing and might promote wound healing in gene therapy [88]. Liu et al. [92] transferred a Gwiz-CA5 plasmid into the full-thickness defect wound surface of diabetic mice via electroporation to stimulate the production of HIF-la and up-regulate the expression of VEGF, placental growth factor (PGF), PDGF and angiopoietin, thus promoting epithelization of the wound surface and angiogenesis. Ferraro et al. [93] used direct injection and electroporation methods to transfect the VEGF plasmid into the defect wounds of rats. The expression of VEGF in the electroporation gene transfection model was significantly upregulated, which promoted angiogenesis and wound healing. Impaired angiogenesis is a major clinical issue that affects wound healing in diabetic patients. Improving angiogenesis is a reasonable approach to increase healing of diabetic wounds. Studies have successfully used syringe electrodes for electroporation. For example, electrotherapy for delivery of the TGF-β1 gene promoted epithelial regeneration, granulation tissue formation and angiogenesis and enhanced diabetic wound healing in db/db mice [94].
Cationic polymers and liposome transfer
The key requirements of non-viral gene therapy for DSA include efficient biocompatibility and high transfection efficiency [95,96]. Derivatives of natural cationic polymers, including N,N,N-trimethyl chitosan chloride (TMC), are frequently used in TE research. Plasmid DNA encoding VEGF-165/TMC complexes were loaded into bilayered porous Col–chitosan/silicone membrane dermal equivalents (BDEs) and used for the treatment of full-thickness burn wounds. The DNA released from the BDEs remained in its supercoiled structure and transfected HEK293 cells in vitro with less efficiency. Studies demonstrated a significant increase in the number of newly formed and mature vessels and dermal regeneration was fastest in the TMC/pDNA-VEGF group. Also, the tensile strength of the repaired tissue increased with prolongation of the post-grafting period, resulting in ~70% normal skin at 105 days [97]. Reckhenrich et al. [98] activated an FDA-approved Col scaffold for dermal regeneration by incorporating copolymer-protected gene vectors to induce temporary release of VEGF. In vitro studies noted transient release of VEGF for up to 3 weeks. The gene-activated skin scaffolds applied to a full-thickness nude mouse skin defect model promoted the formation of blood vessels in the skin construct. McKnight et al. [99] examined the revascularization potential of plasmid DNA encoding VEGF within a polycation complex (jet PEI) mixed with the human fibrin sealant CROSSEAL. The study used a rat fasciocutaneous flap model and statistically significant enhancement of the survival of fasciocutaneous flaps compared to the control group was demonstrated. The enhanced survival was linked to an improvement in flap revascularization. Therefore, this study demonstrated the potential of gene therapy to enhance revascularization of the fasciocutaneous flap.
In another study, 100- and 60-μm porous and nonporous HA-MMP hydrogels with encapsulated reporter (pGFPluc) or proangiogenic (pVEGF) plasmids were used to investigate scaffold-mediated gene delivery for local gene therapy in a mouse model of diabetic wound healing. The study showed that the delivery of pDNA/PEI polyplexes positively promoted granulation tissue formation, even when the DNA did not encode an angiogenic protein. However, pVEGF delivery did not further enhance the angiogenic response, although the presence of transfected cells demonstrated a potential use of polyplex-loaded porous hydrogels for local gene delivery in the treatment of diabetic wounds [100]. HIF-1a is the main oxygen-dependent regulatory subunit of heterodimer transcription factor HIF-1, which regulates angiogenesis in other physiological pathways. Thiersch et al. [101] described a system of transient gene expression based on pegylated poly-l-lysine (PLL-g-PEG) polymer-mediated plasmid DNA delivery in vitro. The PLL-g-PEG polymer prevented cytotoxicity of the highly cationic polymer, indicating that it could be used for effective transport and delivery of therapeutic DNA [102–104]. Moreover, PLL-g-PEG polymer-mediated HIF-1α plasmid DNA delivery transiently induced the expression of the angiogenesis-related genes Acta2 and Pecam1, as well as the HIF-1α target gene VEGF, in vivo. Furthermore, HIF-1α gene delivery increased the number of ECs and smooth muscle cells (precursors of mature blood vessels) during wound healing.
Jeschke and Klein [105] confirmed that gene transfer of two genes might be more effective than single-gene therapy. They delivered insulin-like growth factor I (IGF-I) and keratinocyte growth factor (KGF) together via liposome gene transfer. In contrast to the control and single-gene groups, IGF-I/KGF cDNA gene transfer increased the concentration of VEGF, thereby increasing the formation of new blood vessels. Moreover, GF-I/KGF cDNA gene transfer exhibited the most rapid linear wound re-epithelization, indicating enhanced epidermal regeneration. Therefore, gene therapy with multiple genes is feasible and the transfer of multiple genes can enhance and accelerate physiological and biological effects. Elsewhere, PEI was mixed with pDNA in solution, emulsified with PELA and PEG, electrospun into fibres and lyophilized. Multiple releases of polyplexes of VEGF and bFGF plasmids from electrospun fibrous scaffolds led to the regeneration of mature blood vessels in an animal model of subcutaneous wounds [106]. In vitro investigations of HUVECs revealed that sustained release of pDNA from fibrous mats promoted cell attachment, viability and transfection, as well as protein expression and extracellular secretion of Col IV and laminin. The density of mature vessels was significantly enhanced by combined treatment with pbFGF and pVEGF compared to those incorporating pDNA alone.
Advanced non-viral gene delivery systems mediated by DSA include gene-activated scaffold platforms [107,108], genetically engineered stem cell therapies [109–112] and microRNA and siRNA delivery [113,114]. For future clinical applications, transfection efficiency, cytotoxicity, economy and ease of use should be considered. Based on these considerations, non-viral gene delivery systems for vascularization face the following challenges. Firstly, regarding the design of new non-viral vectors, an optimal balance between transfection rate and cytotoxicity has yet to be achieved. When applying non-viral gene delivery systems for DSA, achieving sufficient therapeutic effects while minimizing side effects remains a major challenge. Second, better understanding of the physiological and pathological effects of different genes involved in DSA is needed, along with the selection of appropriate genes and combinations thereof. A few genes have demonstrated excellent TRR-promoting effects, including FGF2, PDGF and VEGF, for angiogenesis.
Tetrahedral framework nucleic acids
Tetrahedral framework nucleic acids (tFNAs) are self-assembled nucleic acids that can be easily synthesised and used; they have favourable safety profiles due to the biological nature of nucleic acids (Figure 3) [115,116]. Self-assembled tFNAs comprise four single-stranded DNAs based on complementary base pairings [117,118]. tFNAs can be taken up in abundance, unassisted, through caveolin-mediated endocytosis [119,120], promoting cell proliferation and migration and reducing inflammatory reactions [121,122]. In addition, tFNAs may influence various signalling pathways, such as the Wnt and Nrf2 pathways [121,123]. Zhu et al. [124] showed that tFNAs can increase secretion of VEGF and bFGF in HSF cells and reduce the production of TNF-α and IL-1β in HaCaT cells by activating the AKT-signalling pathway. Zhao et al. [125] modified tFNAs using two different angiogenic DNA aptamers to derive the aptamer-tFNA nanostructures tFNA-Apt02 and tFNA-AptVEGF. Their study confirmed that tFNA-Apt02 and tFNA-AptVEGF had greater ability to accelerate EC proliferation and migration, tubule formation, spheroid sprouting and angiogenesis in vivo. There are a few reports indicating that nucleic acid nanophase materials can directly affect the skin wound healing process without delivery assistance.
Figure 3.
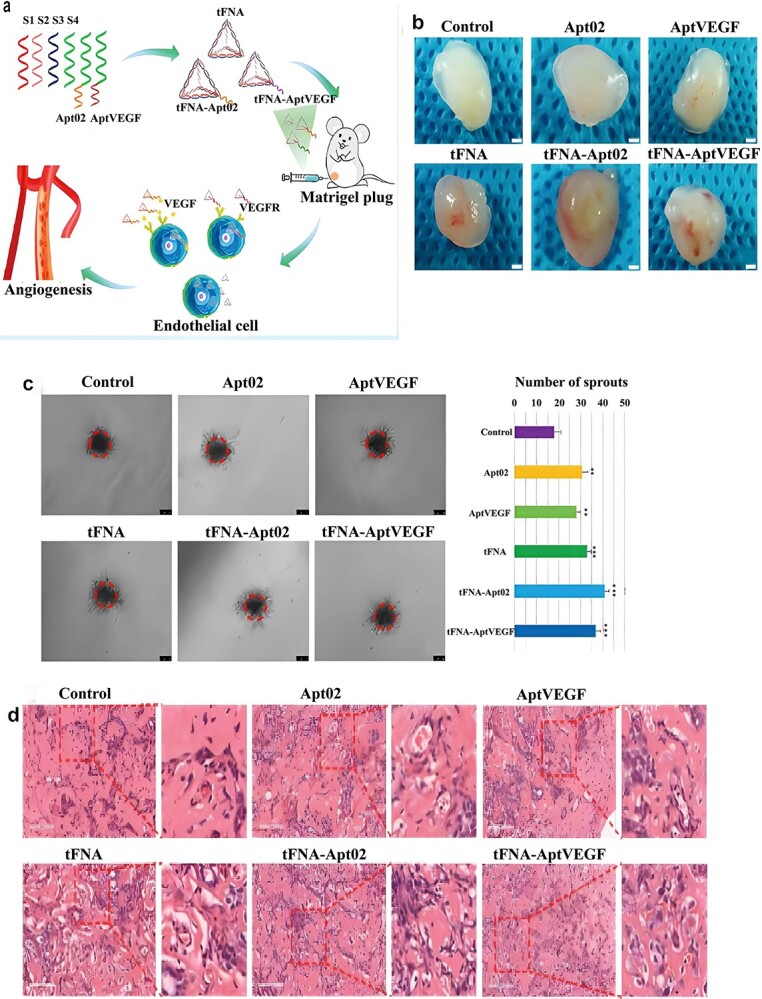
Angiogenic Aptamer-modified tetrahedral framework nucleic acid promotes angiogenesis in vitro and in vivo. (a) Schematic diagram of tFNA, tFNA-Apt02, and tFNA-AptVEGF promoting angiogenesis in vivo. (b) Sprouting assay after treatment with nanoparticles. And semiquantification analysis of the number of sprouts. (c) Photographs of a Matrigel plug assay after treatment with nanoparticles (ss-DNA, Apt02, AptVEGF, tFNA, tFNA-Apt02, and tFNA-AptVEGF). Scale bars are 500 μm. (d) H&E staining of the gel plug assay and plenty of capillaries can be seen. Scale bars are 100 μm. And quantification analysis of the number of blood vessels with red blood cells. (*p<0.05, **p<0.01). tFNA tetrahedral framework nucleic acid, tFNA-Apt02 tetrahedral framework nucleic acid-aptamer 02 nanostructures, tFNA-AptVEGF tetrahedral framework nucleic acid-aptamer, VEGF nanostructures, VEGF vascular endothelial growth factor, VEGFR vascular endothelial growth factor receptor [125]. (Copyright 2021 American Chemical Society)
In short, to ensure effective delivery and a sustained effect of pro-angiogenesis factors, advanced drug delivery systems, including various nanocarriers, are necessary to avoid degradation of bioactive factors by proteases for sustained release during vascularization, unless high and repeated doses are administered. Extensive preclinical data indicates that nanomedicines can be used to vascularize dermal substitutes and promote wound healing (Table 4). Various genes, scaffolds and nanospheres have been used for this purpose. However, at present, nanomedicines for accelerating the vascularization of dermal substitutes are still at the animal experiment stage, thus limiting clinical application. There are two main reasons for this. Firstly, there is no validated pre-clinical animal model of chronic wound healing that correlates well with human wound healing in the clinic. While many approaches have been used to promote vascularization and accelerate wound healing in animal models, there is no consensus regarding the model with the closest correspondence to compromised wound healing in humans [126,127]. Second, effective dermal substitute vascularization is a temporal process that can be compromised by disease or infection in a number of ways, including increased proteolysis [128], alterations in the inflammatory response [129] and ROS signalling [130]. It is difficult to envisage that treatment with any single agent could address more than a few of these factors, pointing to the need for combination therapies tailored to the patient’s particular wound and diagnostic assays to determine when a particular compound should be used. The biology of wound healing and any compromised mechanisms must be considered when designing and developing treatments and delivery platforms for dermal substitute vascularization.
Table 4.
Comparison of different delivery systems for nanomaterials
| Delivery systems | Bioactive factors | Advantages | Drawbacks | References |
|---|---|---|---|---|
| Degradable polymers (Nanoparticle, nanofibers, etc.) Cationic polymers Liposomes |
VEGF, bFGF, PDGF, LL37 NO Small interfering RNA, LL37, VEGF plasmid, PDGF plasmid bFGF, VEGF plasmid, PDGF plasmid, Ang-1 plasmid, KGF |
Versatile degradation kinetics. Controlled drug release properties. Inherent wound healing property by supplying lactate byproducts. Intrinsic bio-adhesiveness to the mucosal surface of wound. Increased residence time due to similar lipoid composition to the skin. Superior drug penetration into skin. Strong hydrophobic interaction with drugs. Easy surface modification of liposomal membrane. |
Collateral tissue damage caused by acidic byproducts. Dose limit due to their inherent cytotoxicity. Usage of organic solvent or oil for particle formation. Drug leakage. Coalescence issue |
[20,37,38,45] [62,98,99,131] [41,105] |
| DNA nanomaterials | Tetrahedral framework nucleic acids | Good mechanical properties. Structural stability against nuclease degradation. Having the ability to access cells. |
Mostly confined to in vitro studies. | [115,116,124,125] |
VEGF vascular endothelial growth factor receptor, bFGF basicfibroblast growth factor, PDGF platelet-derived growth factor, Ang1 angiopoietin-1, KGF keratinocyte growth factor
Conclusions
Among the various innovative strategies for the delivery of bioactive factors for vascularization of dermal substitutes, nano-scaffold-mediated bioactive factors are particularly well-suited for accelerating vascularization and improving wound healing, because the scaffold can act as a barrier against infections and maintain a moist environment, in addition to serving as a ‘bioactive factor reservoir’ providing specific biochemical agents depending on the wound stage. Furthermore, cell-laden scaffolds can exploit the power of cell therapy and TE and are particularly advantageous for the regeneration of large-area skin defects. Regarding cell-based dermal substitutes, vascularization is preferred over delivering soluble bioactive factors because the cells can respond to the local environment and release multiple factors. Future studies should focus on ways to enhance the stability, efficacy and specificity of delivered bioactive factors. Microbial infection is a major challenge for wound management. Bacterial metabolites, such as enzymes, degrade bioactive factors and form biofilms that interfere with the diffusion and receptor binding of bioactive factors. Use of an antibacterial membrane or bioactive factor delivery device at the wound site would be beneficial. Molecular engineering technology could improve the affinity of bioactive factors for matrices and molecules, which would increase the specificity of bioactive factors. Research progress in these and related areas is expected over the coming decades.
Contributor Information
Tingting Weng, Department of Burns & Wound Care Centre, the Second Affiliated Hospital of Zhejiang University School of Medicine Hangzhou 310002, China; Key Laboratory of The Diagnosis and Treatment of Severe Trauma and Burn of Zhejiang Province, Hangzhou 310002,China.
Jialiang Wang, Department of Burns & Wound Care Centre, the Second Affiliated Hospital of Zhejiang University School of Medicine Hangzhou 310002, China; Key Laboratory of The Diagnosis and Treatment of Severe Trauma and Burn of Zhejiang Province, Hangzhou 310002,China.
Min Yang, Department of Burns & Wound Care Centre, the Second Affiliated Hospital of Zhejiang University School of Medicine Hangzhou 310002, China; Key Laboratory of The Diagnosis and Treatment of Severe Trauma and Burn of Zhejiang Province, Hangzhou 310002,China.
Wei Zhang, Department of Burns & Wound Care Centre, the Second Affiliated Hospital of Zhejiang University School of Medicine Hangzhou 310002, China; Key Laboratory of The Diagnosis and Treatment of Severe Trauma and Burn of Zhejiang Province, Hangzhou 310002,China.
Pan Wu, Department of Burns & Wound Care Centre, the Second Affiliated Hospital of Zhejiang University School of Medicine Hangzhou 310002, China; Key Laboratory of The Diagnosis and Treatment of Severe Trauma and Burn of Zhejiang Province, Hangzhou 310002,China.
Chuangang You, Department of Burns & Wound Care Centre, the Second Affiliated Hospital of Zhejiang University School of Medicine Hangzhou 310002, China; Key Laboratory of The Diagnosis and Treatment of Severe Trauma and Burn of Zhejiang Province, Hangzhou 310002,China.
Chunmao Han, Department of Burns & Wound Care Centre, the Second Affiliated Hospital of Zhejiang University School of Medicine Hangzhou 310002, China; Key Laboratory of The Diagnosis and Treatment of Severe Trauma and Burn of Zhejiang Province, Hangzhou 310002,China.
Xingang Wang, Department of Burns & Wound Care Centre, the Second Affiliated Hospital of Zhejiang University School of Medicine Hangzhou 310002, China; Key Laboratory of The Diagnosis and Treatment of Severe Trauma and Burn of Zhejiang Province, Hangzhou 310002,China.
Abbreviations
Ang-1: Angiopoietin 1; bFGF: basic fibroblast growth factor; CC-GMS: Collagen/chitosan-gelatin microsphere; CC-GMS: Collagen/ chitosan-gelatin microsphere; Col: Collagen; DSA: Dermis substitute angiogenesis; ECs: Endothelial cells; EGF: Epidermal growth factor; eNOS: endothelial nitric oxide synthase; EPCs: Endothelial progenitor cells; FGF: Fibroblast growth factor; GM-CSF: Granulocyte-macrophage colony-stimulating factor; GN: gelatin; HA: hyaluronic acid; HGF: Hepatocyte growth factor; HIF-1: Hypoxia-inducible factor-1; HUVEC: Human umbilical vein vascular endothelial cell; IGF-I: Insulin-like growth factor I; IL-6: interleukin 6; KGF: keratinocyte growth factor; MMP-9: Metalloproteinase-9; MSNs: Mesoporous silica nanoparticles; NFs: Nanofibers; NOS: Nitric oxide synthase; NPs: Nanoparticles; nVVDM: Non-viral vector delivery system; PDGF: Platelet-derived growth factor; PLL-g-PEG: Pegylated poly-l-lysine; rhEGF: recombinant human epidermal growth factor; PGF: Placental growth factor; PLA: Poly (lactic acid); PLGA: Poly (lactic-co-glycolic acid); PCL: Poly (ε- caprolactone); PLL-g-PEG: Pegylated poly-l-lysine; PI3K/Akt pathway: Phosphatidylinositol 3-kinase/protein kinase B pathway; rAAV: Recombinant adeno-associated virus; rhEGF: recombinant human epidermal growth factor; ROS: reactive oxygen species; SDF-1; Stromal cell derived factor-1; SF-LIP: silk fibroin liposome; STZ: streptozotocin ;TE: Tissue engineering; tFNAs: Tetrahedral framework nucleic acids; TMC: Trimethyl chitosan chloride; TNF: tumour necrosis factor; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; VVDM: Viral vector delivery system; IRES: Internal ribosomal entry side; FDA: Food and drug Administration; iNOS: Inducible nitric oxide synthase; PEI: polyethyleneimine; TRR: Tissue repair and regeneration; PEG: polyethyleneglycol; PELA: Poly (ethylene glycol)-poly(L-lactide).
Authors’ contributions
TW and XW designed the major structure of this review. TW and XW conducted the search on related literature. TW, JW, MY, WZ, PW, CY, CH and XW were involved in the literature review. All authors were involving in drafting the article or revising it critically for important intellectual content and all authors approved the final version to be published. All authors read and approved the final manuscript.
Funding
This work was financially supported by the National key research and development project (2016YFC1100800, 2016YFC1100803), the National Natural Science Foundation of China (81772069, 81401591, 81801911) and the Zhejiang Provincial Basic Public Welfare Research Program (LGF19H150008).
Conflict of interest
The authors declared that they have no conflicts of interest to this work.
Data Availability
All data or related information supporting the conclusions of the review is included in the article.
References
- 1. Jarbrink K, Ni G, Sonnergren H, Schmidtchen A, Pang C, Bajpai R, et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frueh FS, Menger MD, Lindenblatt N, Giovanoli P, Laschke MW. Current and emerging vascularization strategies in skin tissue engineering. Crit Rev Biotechnol. 2017;37:613–25. [DOI] [PubMed] [Google Scholar]
- 3. Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–11. [DOI] [PubMed] [Google Scholar]
- 4. Wang X, Wu P, Hu X, You C, Guo R, Shi H, et al. Polyurethane membrane/knitted mesh-reinforced collagen-chitosan bilayer dermal substitute for the repair of full-thickness skin defects via a two-step procedure. J Mech Behav Biomed Mater. 2016;56:120–33. [DOI] [PubMed] [Google Scholar]
- 5. Hendrickx B, Vranckx JJ, Luttun A. Cell-based vascularization strategies for skin tissue engineering. Tissue Eng Part B Rev. 2011;17:13–24. [DOI] [PubMed] [Google Scholar]
- 6. Zheng YR, Feng ZZ, You CG, Jin YY, Hu XL, Wang XG, et al. In vitro evaluation of Panax notoginseng Rg1 released from collagen/chitosan-gelatin microsphere scaffolds for angiogenesis. Biomed Eng Online. 2013;12:134–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phelps EA, Garcia AJ. Update on therapeutic vascularization strategies. Regen Med. 2009;4:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care (New Rochelle). 2013;2:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, et al. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132:991–6. [DOI] [PubMed] [Google Scholar]
- 10. Oryan A, Alemzadeh E, Zarei M. Basic concepts, current evidence, and future potential for gene therapy in managing cutaneous wounds. Biotechnol Lett. 2019;41:889–98. [DOI] [PubMed] [Google Scholar]
- 11. Kim HS, Sun X, Lee JH, Kim HW, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209–39. [DOI] [PubMed] [Google Scholar]
- 12. Chen W, Palazzo A, Hennink WE, Kok RJ. Effect of particle size on drug loading and release kinetics of Gefitinib-loaded PLGA microspheres. Mol Pharm. 2017;14:459–67. [DOI] [PubMed] [Google Scholar]
- 13. Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–52. [PMC free article] [PubMed] [Google Scholar]
- 14. Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A. 1997;94:663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jinnin M, Ihn H, Mimura Y, Asano Y, Yamane K, Tamaki K. Regulation of fibrogenic/fibrolytic genes by platelet-derived growth factor C, a novel growth factor, in human dermal fibroblasts. J Cell Physiol. 2005;202:510–7. [DOI] [PubMed] [Google Scholar]
- 16. Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle). 2014;3:647–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anisimov A, Tvorogov D, Alitalo A, Leppanen VM, An Y, Han EC, et al. Vascular endothelial growth factor-angiopoietin chimera with improved properties for therapeutic angiogenesis. Circulation. 2013;127:424–34. [DOI] [PubMed] [Google Scholar]
- 18. DiPietro LA. Angiogenesis and wound repair: when enough is enough. J Leukoc Biol. 2016;100:979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. [DOI] [PubMed] [Google Scholar]
- 20. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. [DOI] [PubMed] [Google Scholar]
- 21. Xiong S, Zhang X, Lu P, Wu Y, Wang Q, Sun H, et al. A Gelatin-sulfonated silk composite scaffold based on 3D printing technology enhances skin regeneration by stimulating epidermal growth and dermal neovascularization. Sci Rep. 2017;7:4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu J, Ye J, Zhu J, Xiao Z, He C, Shi H, et al. Heparin-based Coacervate of FGF2 improves dermal regeneration by asserting a synergistic role with cell proliferation and endogenous facilitated VEGF for cutaneous wound healing. Biomacromolecules. 2016;17:2168–77. [DOI] [PubMed] [Google Scholar]
- 23. Ruthenborg RJ, Ban JJ, Wazir A, Takeda N, Kim JW. Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1. Mol Cells. 2014;37:637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Desmet CM, Preat V, Gallez B. Nanomedicines and gene therapy for the delivery of growth factors to improve perfusion and oxygenation in wound healing. Adv Drug Deliv Rev. 2018;129:262–84. [DOI] [PubMed] [Google Scholar]
- 25. Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. [DOI] [PubMed] [Google Scholar]
- 26. Mazure NM, Brahimi-Horn MC, Pouyssegur J. Protein kinases and the hypoxia-inducible factor-1, two switches in angiogenesis. Curr Pharm Des. 2003;9:531–41. [DOI] [PubMed] [Google Scholar]
- 27. Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–20. [DOI] [PubMed] [Google Scholar]
- 28. Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–8. [DOI] [PubMed] [Google Scholar]
- 29. Diaz-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–69. [DOI] [PubMed] [Google Scholar]
- 30. Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, et al. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–57. [DOI] [PubMed] [Google Scholar]
- 32. Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S, et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat Commun. 2017;8:16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gianni-Barrera R, Butschkau A, Uccelli A, Certelli A, Valente P, Bartolomeo M, et al. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis. 2018;21:883–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gainza G, Villullas S, Pedraz JL, Hernandez RM, Igartua M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomedicine. 2015;11:1551–73. [DOI] [PubMed] [Google Scholar]
- 35. Berthet M, Gauthier Y, Lacroix C, Verrier B, Monge C. Nanoparticle-based dressing: the future of wound treatment? Trends Biotechnol. 2017;35:770–84. [DOI] [PubMed] [Google Scholar]
- 36. Golub JS, Kim YT, Duvall CL, Bellamkonda RV, Gupta D, Lin AS, et al. Sustained VEGF delivery via PLGA nanoparticles promotes vascular growth. Am J Physiol-Heart C. 2010;298:H1959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chereddy KK, Lopes A, Koussoroplis S, Payen V, Moia C, Zhu H, et al. Combined effects of PLGA and vascular endothelial growth factor promote the healing of non-diabetic and diabetic wounds. Nanomedicine. 2015;11:1975–84. [DOI] [PubMed] [Google Scholar]
- 38. Lai HJ, Kuan CH, Wu HC, Tsai JC, Chen TM, Hsieh DJ, et al. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014;10:4156–66. [DOI] [PubMed] [Google Scholar]
- 39. Claffey KP, Abrams K, Shih SC, Brown LF, Mullen A, Keough M. Fibroblast growth factor 2 activation of stromal cell vascular endothelial growth factor expression and angiogenesis. Lab Invest. 2001;81:61–75. [DOI] [PubMed] [Google Scholar]
- 40. Zhang J, Postovit LM, Wang D, Gardiner RB, Harris R, Abdul M, et al. In situ loading of basic fibroblast growth factor within porous silica nanoparticles for a prolonged release. Nanoscale Res Lett. 2009;4:1297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li W, Lan Y, Guo R, Zhang Y, Xue W, Zhang Y. In vitro and in vivo evaluation of a novel collagen/cellulose nanocrystals scaffold for achieving the sustained release of basic fibroblast growth factor. J Biomater Appl. 2015;29:882–93. [DOI] [PubMed] [Google Scholar]
- 42. Arunkumar P, Dougherty JA, Weist J, Kumar N, Angelos MG, Powell HM, et al. Sustained release of basic fibroblast growth factor (bFGF) encapsulated Polycaprolactone (PCL) microspheres promote angiogenesis in vivo. Nanomaterials (Basel). 2019;9:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu HL, Chen PP, ZhuGe DL, Zhu QY, Jin BH, Shen BX, et al. Liposomes with silk fibroin hydrogel Core to stabilize bFGF and promote the wound healing of mice with deep second-degree scald. Adv Healthc Mater. 2017;6:1–13. [DOI] [PubMed] [Google Scholar]
- 44. Losi P, Briganti E, Errico C, Lisella A, Sanguinetti E, Chiellini F, et al. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013;9:7814–21. [DOI] [PubMed] [Google Scholar]
- 45. Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, et al. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315–23. [DOI] [PubMed] [Google Scholar]
- 47. Jin Q, Wei G, Lin Z, Sugai JV, Lynch SE, Ma PX, et al. Nanofibrous scaffolds incorporating PDGF-BB microspheres induce chemokine expression and tissue neogenesis in vivo. PLoS One. 2008;3:e1729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xie ZW, Paras CB, Weng H, Punnakitikashem P, Su LC, Vu K, et al. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater. 2013;9:9351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chu Y, Yu D, Wang P, Xu J, Li D, Ding M. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010;18:499–505. [DOI] [PubMed] [Google Scholar]
- 51. Gao XW, Xu ZP. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin. 2008;40:619–24. [DOI] [PubMed] [Google Scholar]
- 52. Shi HF, Han CM, Mao ZW, Ma L, Gao CY. Enhanced angiogenesis in porous collagen-chitosan scaffolds loaded with Angiogenin. Tissue Eng Pt A. 2008;14:1775–85. [DOI] [PubMed] [Google Scholar]
- 53. Pan SC, Chiu HY. Response to Wang et al. Comments on "Angiogenin, an angiogenic factor with potential for tissue engineering applications". Wound Repair Regen 2014; 22: 289–9. [DOI] [PubMed] [Google Scholar]
- 54. Sun HF, Wang XG, Hu XL, Yu WJ, You CG, Hu H, et al. Promotion of angiogenesis by sustained release of rhGM-CSF from heparinized collagen/chitosan scaffolds. J Biomed Mater Res B. 2012;100b:788–98. [DOI] [PubMed] [Google Scholar]
- 55. Kohara H, Tajima S, Yamamoto M, Tabata Y. Angiogenesis induced by controlled release of neuropeptide substance P. Biomaterials. 2010;31:8617–25. [DOI] [PubMed] [Google Scholar]
- 56. Tang T, Jiang H, Yu Y, He F, Ji SZ, Liu YY, et al. A new method of wound treatment: targeted therapy of skin wounds with reactive oxygen species-responsive nanoparticles containing SDF-1 alpha. Int J Nanomedicine. 2015;10:6571–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang S, Chen S, Shen Y, Yang D, Liu X, Sun-Chi AC, et al. Puerarin induces angiogenesis in myocardium of rat with myocardial infarction. Biol Pharm Bull. 2006;29:945–50. [DOI] [PubMed] [Google Scholar]
- 58. Leung KW, Pon YL, Wong RNS, Wong AST. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281:36280–8. [DOI] [PubMed] [Google Scholar]
- 59. Hong SJ, Wan JB, Zhang Y, Hu G, Lin HC, Seto SW, et al. Angiogenic effect of Saponin extract from Panax notoginseng on HUVECs in vitro and zebrafish in vivo. Phytother Res. 2009;23:677–86. [DOI] [PubMed] [Google Scholar]
- 60. Xhindoli D, Pacor S, Benincasa M, Scocchi M, Gennaro R, Tossi A. The human cathelicidin LL-37 a pore-forming antibacterial peptide and host-cell modulator. BBA-Biomembranes. 2016;1858:546–66. [DOI] [PubMed] [Google Scholar]
- 61. Vandamme D, Landuyt B, Luyten W, Schoofs L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012;280:22–35. [DOI] [PubMed] [Google Scholar]
- 62. Chereddy KK, Her CH, Comune M, Moia C, Lopes A, Porporato PE, et al. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. J Control Release. 2014;194:138–47. [DOI] [PubMed] [Google Scholar]
- 63. Li XL, Fan RR, Tong AP, Yang MJ, Deng JJ, Zhou LX, et al. In situ gel-forming AP-57 peptide delivery system for cutaneous wound healing. Int J Pharm. 2015;495:560–71. [DOI] [PubMed] [Google Scholar]
- 64. Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–58. [DOI] [PubMed] [Google Scholar]
- 65. Park HJ, Yang F, Cho SW. Nonviral delivery of genetic medicine for therapeutic angiogenesis. Adv Drug Deliv Rev. 2012;64:40–52. [DOI] [PubMed] [Google Scholar]
- 66. Lu CH, Chang YH, Lin SY, Li KC, Hu YC. Recent progresses in gene delivery-based bone tissue engineering. Biotechnol Adv. 2013;31:1695–706. [DOI] [PubMed] [Google Scholar]
- 67. Okholm AH, Kjems J. DNA nanovehicles and the biological barriers. Adv Drug Deliv Rev. 2016;106:183–91. [DOI] [PubMed] [Google Scholar]
- 68. Jones MR, Seeman NC, Mirkin CA. Nanomaterials. Programmable materials and the nature of the DNA bond. Science 2015; 347: 1260901. [DOI] [PubMed] [Google Scholar]
- 69. Angell C, Xie S, Zhang L, Chen Y. DNA nanotechnology for precise control over drug delivery and gene therapy. Small. 2016;12:1117–32. [DOI] [PubMed] [Google Scholar]
- 70. Lee DS, Qian H, Tay CY, Leong DT. Cellular processing and destinies of artificial DNA nanostructures. Chem Soc Rev. 2016;45:4199–225. [DOI] [PubMed] [Google Scholar]
- 71. Kwon M, Firestein BL. DNA transfection: calcium phosphate method. Methods Mol Biol. 2013;1018:107–10. [DOI] [PubMed] [Google Scholar]
- 72. Wu X, Gong Y, Ding X, Cheng G, Yan W, She X, et al. Retrovirus-mediated transfection of the tissue-type plasminogen activator gene results in increased thrombolysis of blood clots. Biochem Genet. 2019;57:234–47. [DOI] [PubMed] [Google Scholar]
- 73. Deodato B, Arsic N, Zentilin L, Galeano M, Santoro D, Torre V, et al. Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene Ther. 2002;9:777–85. [DOI] [PubMed] [Google Scholar]
- 74. Galeano M, Deodato B, Altavilla D, Cucinotta D, Arsic N, Marini H, et al. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003;46:546–55. [DOI] [PubMed] [Google Scholar]
- 75. Brem H, Kodra A, Golinko MS, Entero H, Stojadinovic O, Wang VM, et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol. 2009;129:2275–87. [DOI] [PubMed] [Google Scholar]
- 76. Saaristo A, Tammela T, Farkkila A, Karkkainen M, Suominen E, Yla-Herttuala S, et al. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol. 2006;169:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jazwa A, Kucharzewska P, Leja J, Zagorska A, Sierpniowska A, Stepniewski J, et al. Combined vascular endothelial growth factor-a and fibroblast growth factor 4 gene transfer improves wound healing in diabetic mice. Genet Vaccines Ther. 2010;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu PY, Liu K, Wang XT, Badiavas E, Rieger-Christ KM, Tang JB, et al. Efficacy of combination gene therapy with multiple growth factor cDNAs to enhance skin flap survival in a rat model. DNA Cell Biol. 2005;24:751–7. [DOI] [PubMed] [Google Scholar]
- 79. Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D, et al. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen. 2004;12:497–504. [DOI] [PubMed] [Google Scholar]
- 80. Rah DK, Yun IS, Yun CO, Lee SB, Lee WJ. Gene therapy using hepatocyte growth factor expressing adenovirus improves skin flap survival in a rat model. J Korean Med Sci. 2014;29:S228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bitto A, Minutoli L, Galeano MR, Altavilla D, Polito F, Fiumara T, et al. Angiopoietin-1 gene transfer improves impaired wound healing in genetically diabetic mice without increasing VEGF expression. Clin Sci (Lond). 2008;114:707–18. [DOI] [PubMed] [Google Scholar]
- 82. Schaffer MR, Tantry U, Gross SS, Wasserburg HL, Barbul A. Nitric oxide regulates wound healing. J Surg Res. 1996;63:237–40. [DOI] [PubMed] [Google Scholar]
- 83. Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yamasaki K, Edington HD, McClosky C, Tzeng E, Lizonova A, Kovesdi I, et al. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest. 1998;101:967–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Breen AM, Dockery P, O'Brien T, Pandit AS. The use of therapeutic gene eNOS delivered via a fibrin scaffold enhances wound healing in a compromised wound model. Biomaterials. 2008;29:3143–51. [DOI] [PubMed] [Google Scholar]
- 86. O'Toole G, MacKenzie D, Lindeman R, Buckley MF, Marucci D, McCarthy N, et al. Vascular endothelial growth factor gene therapy in ischaemic rat skin flaps. Br J Plast Surg. 2002;55:55–8. [DOI] [PubMed] [Google Scholar]
- 87. Jeschke MG, Herndon DN, Baer W, Barrow RE, Jauch KW. Possibilities of non-viral gene transfer to improve cutaneous wound healing. Curr Gene Ther. 2001;1:267–78. [DOI] [PubMed] [Google Scholar]
- 88. Byrnes CK, Malone RW, Akhter N, Nass PH, Wetterwald A, Cecchini MG, et al. Electroporation enhances transfection efficiency in murine cutaneous wounds. Wound Repair Regen. 2004;12:397–403. [DOI] [PubMed] [Google Scholar]
- 89. Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2009;35:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dileo J, Miller TE, Jr, Chesnoy S, Huang L. Gene transfer to subdermal tissues via a new gene gun design. Hum Gene Ther. 2003;14:79–87. [DOI] [PubMed] [Google Scholar]
- 91. Glasspool-Malone J, Somiari S, Drabick JJ, Malone RW. Efficient nonviral cutaneous transfection. Mol Ther. 2000;2:140–6. [DOI] [PubMed] [Google Scholar]
- 92. Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, et al. Age-dependent impairment of HIF-1alpha expression in diabetic mice: correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008;217:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ferraro B, Cruz YL, Coppola D, Heller R. Intradermal delivery of plasmid VEGF(165) by electroporation promotes wound healing. Mol Ther. 2009;17:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lee PY, Chesnoy S, Huang L. Electroporatic delivery of TGF-beta1 gene works synergistically with electric therapy to enhance diabetic wound healing in db/db mice. J Invest Dermatol. 2004;123:791–8. [DOI] [PubMed] [Google Scholar]
- 95. Samal SK, Dash M, Van Vlierberghe S, Kaplan DL, Chiellini E, van Blitterswijk C, et al. Cationic polymers and their therapeutic potential. Chem Soc Rev. 2012;41:7147–94. [DOI] [PubMed] [Google Scholar]
- 96. Paliwal R, Palakurthi S. Zein in controlled drug delivery and tissue engineering. J Control Release. 2014;189:108–22. [DOI] [PubMed] [Google Scholar]
- 97. Guo R, Xu S, Ma L, Huang A, Gao C. The healing of full-thickness burns treated by using plasmid DNA encoding VEGF-165 activated collagen-chitosan dermal equivalents. Biomaterials. 2011;32:1019–31. [DOI] [PubMed] [Google Scholar]
- 98. Reckhenrich AK, Hopfner U, Krotz F, Zhang Z, Koch C, Kremer M, et al. Bioactivation of dermal scaffolds with a non-viral copolymer-protected gene vector. Biomaterials. 2011;32:1996–2003. [DOI] [PubMed] [Google Scholar]
- 99. McKnight CD, Winn SR, Gong X, Hansen JE, Wax MK. Revascularization of rat fasciocutaneous flap using CROSSEAL with VEGF protein or plasmid DNA expressing VEGF. Otolaryngol Head Neck Surg. 2008;139:245–9. [DOI] [PubMed] [Google Scholar]
- 100. Tokatlian T, Cam C, Segura T. Porous hyaluronic acid hydrogels for localized nonviral DNA delivery in a diabetic wound healing model. Adv Healthc Mater. 2015;4:1084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Thiersch M, Rimann M, Panagiotopoulou V, Ozturk E, Biedermann T, Textor M, et al. The angiogenic response to PLL-g-PEG-mediated HIF-1alpha plasmid DNA delivery in healthy and diabetic rats. Biomaterials. 2013;34:4173–82. [DOI] [PubMed] [Google Scholar]
- 102. Ramsay E, Hadgraft J, Birchall J, Gumbleton M. Examination of the biophysical interaction between plasmid DNA and the polycations, polylysine and polyornithine, as a basis for their differential gene transfection in-vitro. Int J Pharm. 2000;210:97–107. [DOI] [PubMed] [Google Scholar]
- 103. Zaitsev S, Cartier R, Vyborov O, Sukhorukov G, Paulke BR, Haberland A, et al. Polyelectrolyte nanoparticles mediate vascular gene delivery. Pharm Res. 2004;21:1656–61. [DOI] [PubMed] [Google Scholar]
- 104. Lee H, Jeong JH, Park TG. PEG grafted polylysine with fusogenic peptide for gene delivery: high transfection efficiency with low cytotoxicity. J Control Release. 2002;79:283–91. [DOI] [PubMed] [Google Scholar]
- 105. Jeschke MG, Klein D. Liposomal gene transfer of multiple genes is more effective than gene transfer of a single gene. Gene Ther. 2004;11:847–55. [DOI] [PubMed] [Google Scholar]
- 106. He S, Xia T, Wang H, Wei L, Luo X, Li X. Multiple release of polyplexes of plasmids VEGF and bFGF from electrospun fibrous scaffolds towards regeneration of mature blood vessels. Acta Biomater. 2012;8:2659–69. [DOI] [PubMed] [Google Scholar]
- 107. Raftery R, O'Brien FJ, Cryan SA. Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules. 2013;18:5611–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Raftery RM, Tierney EG, Curtin CM, Cryan SA, O'Brien FJ. Development of a gene-activated scaffold platform for tissue engineering applications using chitosan-pDNA nanoparticles on collagen-based scaffolds. J Control Release. 2015;210:84–94. [DOI] [PubMed] [Google Scholar]
- 109. Hwang BW, Kim SJ, Park KM, Kim H, Yeom J, Yang JA, et al. Genetically engineered mesenchymal stem cell therapy using self-assembling supramolecular hydrogels. J Control Release. 2015;220:119–29. [DOI] [PubMed] [Google Scholar]
- 110. Chen X, Nomani A, Patel N, Nouri FS, Hatefi A. Bioengineering a non-genotoxic vector for genetic modification of mesenchymal stem cells. Biomaterials. 2018;152:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chen J, Wang Q, Zhou J, Deng W, Yu Q, Cao X, et al. Porphyra polysaccharide-derived carbon dots for non-viral co-delivery of different gene combinations and neuronal differentiation of ectodermal mesenchymal stem cells. Nanoscale. 2017;9:10820–31. [DOI] [PubMed] [Google Scholar]
- 112. Lee YH, Wu HC, Yeh CW, Kuan CH, Liao HT, Hsu HC, et al. Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration. Acta Biomater. 2017;63:210–26. [DOI] [PubMed] [Google Scholar]
- 113. Peng B, Chen Y, Leong KW. MicroRNA delivery for regenerative medicine. Adv Drug Deliv Rev. 2015;88:108–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kowalczewski CJ, Saul JM. Surface-mediated delivery of siRNA from fibrin hydrogels for knockdown of the BMP-2 binding antagonist noggin. Acta Biomater. 2015;25:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ma W, Shao X, Zhao D, Li Q, Liu M, Zhou T, et al. Self-assembled tetrahedral DNA nanostructures promote neural stem cell proliferation and neuronal differentiation. ACS Appl Mater Interfaces. 2018;10:7892–900. [DOI] [PubMed] [Google Scholar]
- 116. Zhang Y, Ma W, Zhu Y, Shi S, Li Q, Mao C, et al. Inhibiting methicillin-resistant Staphylococcus aureus by tetrahedral DNA nanostructure-enabled antisense peptide nucleic acid delivery. Nano Lett. 2018;18:5652–9. [DOI] [PubMed] [Google Scholar]
- 117. Ge Y, Tian T, Shao X, Lin S, Zhang T, Lin Y, et al. PEGylated protamine-based adsorbing improves the biological properties and stability of tetrahedral framework nucleic acids. ACS Appl Mater Interfaces. 2019;11:27588–97. [DOI] [PubMed] [Google Scholar]
- 118. Ma W, Zhan Y, Zhang Y, Xie X, Mao C, Lin Y. Enhanced neural regeneration with a concomitant treatment of framework nucleic acid and stem cells in spinal cord injury. ACS Appl Mater Interfaces. 2020;12:2095–106. [DOI] [PubMed] [Google Scholar]
- 119. Fu W, You C, Ma L, Li H, Ju Y, Guo X, et al. Enhanced efficacy of Temozolomide loaded by a tetrahedral framework DNA nanoparticle in the therapy for glioblastoma. ACS Appl Mater Interfaces. 2019;11:39525–33. [DOI] [PubMed] [Google Scholar]
- 120. Zhang M, Zhu J, Qin X, Zhou M, Zhang X, Gao Y, et al. Cardioprotection of tetrahedral DNA nanostructures in myocardial ischemia-reperfusion injury. ACS Appl Mater Interfaces. 2019;11:30631–9. [DOI] [PubMed] [Google Scholar]
- 121. Cui W, Zhan Y, Shao X, Fu W, Xiao D, Zhu J, et al. Neuroprotective and Neurotherapeutic effects of tetrahedral framework nucleic acids on Parkinson's disease in vitro. ACS Appl Mater Interfaces. 2019;11:32787–97. [DOI] [PubMed] [Google Scholar]
- 122. Liu N, Zhang X, Li N, Zhou M, Zhang T, Li S, et al. Tetrahedral framework nucleic acids promote corneal epithelial wound healing in vitro and in vivo. Small. 2019; 15:1907–21. [DOI] [PubMed] [Google Scholar]
- 123. Qin X, Li N, Zhang M, Lin S, Zhu J, Xiao D, et al. Tetrahedral framework nucleic acids prevent retina ischemia-reperfusion injury from oxidative stress via activating the Akt/Nrf2 pathway. Nanoscale. 2019;11:20667–75. [DOI] [PubMed] [Google Scholar]
- 124. Zhu J, Zhang M, Gao Y, Qin X, Zhang T, Cui W, et al. Tetrahedral framework nucleic acids promote scarless healing of cutaneous wounds via the AKT-signaling pathway. Signal Transduct Target Ther. 2020;5:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhao D, Liu M, Li J, Xiao D, Peng S, He Q, et al. Angiogenic Aptamer-modified tetrahedral framework nucleic acid promotes angiogenesis in vitro and in vivo. ACS Appl Mater Interfaces. 2021;13:29439–49. [DOI] [PubMed] [Google Scholar]
- 126. Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech. 2014;7:1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med. 2008;25:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Boniakowski AE, Kimball AS, Jacobs BN, Kunkel SL, Gallagher KA. Macrophage-mediated inflammation in normal and diabetic wound healing. J Immunol. 2017;199:17–24. [DOI] [PubMed] [Google Scholar]
- 130. Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Li N, Luo HC, Yang C, Deng JJ, Ren M, Xie XY, et al. Cationic star-shaped polymer as an siRNA carrier for reducing MMP-9 expression in skin fibroblast cells and promoting wound healing in diabetic rats. Int J Nanomedicine. 2014;9:3377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data or related information supporting the conclusions of the review is included in the article.