Abstract
Background and Aims
Many angiosperms can secrete both floral (FN) and extrafloral (EFN) nectar. However, much remains unclear about how EFN and FN differ in secretion, composition and ecological function, especially when both FN and EFN are secreted on flowers of the same species.
Methods
Hemerocallis citrina flowers secrete both FN and EFN. The FN and EFN traits including volume, presentation pattern and temporal rhythms of secretion were compared by field observation. Sugar and amino acid contents were analysed using regular biochemical methods, whereas the proteome was investigated by combined gel-based and gel-free approaches. Animal feeders on FN and EFN were investigated by field observation. Hemerocallis citrina plants were exposed by soil drenching to two systemic insecticides, acetamiprid and imidacloprid, and the concentration of these in FN and EFN was measured by ultra-high performance liquid chromatography coupled with mass spectrometry.
Key Results
Hemerocallis citrina FN was concentrated and sucrose dominant, secreted in the mature flower tube and served as a reward for pollinators. Conversely, EFN was hexose rich, more dilute and less rich in sugar and amino acids. EFN was secreted on the outside of developing floral buds, and was likely to attract predatory animals for defence. EFN had fewer phenolics, but more pathogenesis-related components, such as chitinase and glucanase. A significantly different proteomic profile and enzymatic activities between FN and EFN suggest that they had different biosynthesis mechanisms. Both neonicotinoid insecticides examined became present in both nectar types soon after application, but in greater concentration within EFN; EFN also attracted a wider range of insect species than FN.
Conclusions
Hemerocallis citrina FN and EFN differed in production, composition and ecological function. The EFN pathway could be a significant way for neonicotinoids to enter the wild food chain, and must be considered when evaluating the risks to the environment of other systemic insecticides.
Keywords: Extrafloral nectar, floral nectar, Hemerocallis citrina Baroni, nectar proteome, systemic insecticides
INTRODUCTION
In angiosperms, nectar is a secreted sugar-rich liquid that mediates interactions between plants and mutualists such as pollinators and non-pollination visitors. Nectar can be secreted on almost any above-ground part of a plant, and usually fulfils nutritive functions for diverse nectar feeders, either encouraging pollinators to visit, or promoting the defence of plant tissues against herbivores by encouraging the presence of mutualists such as ants (Heil, 2015). Usually, nectar is classified into two categories according to the location where it is secreted, i.e. floral nectar (FN) and extrafloral nectar (EFN) (Escalante-Pérez and Heil, 2012). In this paper, unless otherwise specified, the term ‘nectar’ refers to both FN and EFN. FN is usually secreted into the inner side of the corolla and is sited at the base of the ovary, during blooming or a short time before. FN has long been discussed in the context of pollination and is hence widely thought to promote beneficial plant–pollinator interactions. However, EFN is secreted on the vegetative and less commonly on the reproductive parts of a plant, and usually its secretion does not necessarily accompany flowering (Heil, 2011). It is generally thought that EFN does not contribute to pollination but acts as a reward for predators (Lundgren, 2009) that can deliver top-down control of herbivore pests, possibly improving plant fitness (Cuautle et al., 2005; Kost and Heil, 2008; Heil, 2015).
Both FN and EFN are similar in that both contain sugars, and most are colourless liquids from a plant. In addition, both of them are deemed to have protective functions, FN for pollen by offering an alternative reward for visitors, and EFN for the tender above-ground parts by attracting predatory animals which provide indirect defence (Willmer, 2011). However, it is still largely in doubt whether FN and EFN share a common evolutionary origin, and to what extent they share similar generation and biosynthesis mechanisms, especially given that some plants secrete EFN on their flowers. Some ferns can secrete sugary liquids on the leaves in response to herbivore damage (Koptur et al., 2013) and, if this mechanism shares a common origin with angiosperm EFN, then it would follow that EFN evolution pre-dates the origin of FN, and even pollen (Lundgren, 2009). A comparison of metabolites in FN and EFN could be a good indicator to show differences in how they are generated, offering insights into whether they share a common origin, yet comparative studies between FN and EFN are still rare. However, rather few species secrete both FN and EFN, whereas comparison studies where the FN and EFN come from different plants face the problem that differences detected might be down to species differences, not functional ones. Nectar biosynthesis was shown to be conserved among floral and extrafloral nectaries in cotton (Gossypium hirsutum) using comparative nectary ultrastructure and transcriptomic analysis (Chatt et al., 2021), but no such work has been done among monocots.
In recent years, systemic insecticide contamination in floral nectar has caused global pollinator decline, leading to strong concerns (Raine, 2018). Systemic insecticides, mainly neonicotinoids, are water soluble and can be taken into a plant by any of its living parts, following which they persist inside the plant and become distributed throughout its tissues via the vascular system. Compared with other types of insecticide, e.g. organochlorine and pyrethroids, systemic insecticides have higher mobility in a plant which can lead to their presence in some unwanted places, such as pollen and nectar, thus resulting in the deaths of beneficial insects (Goulson, 2013). For nectar, a lot of attention have been paid to FN in which systemic insecticides is one the main drivers of worldwide pollinator decline (Pisa et al., 2015; Raine, 2018; Sánchez-Bayo and Wyckhuys, 2019). However, to our knowledge, studies on the risk of systemic insecticides through the EFN pathway have only looked at cotton and sunflower (Stapel et al., 2000; Moscardini et al., 2014; Bredeson and Lundgren, 2018; Jiang et al., 2018; Jones et al., 2020). Moreover, so far, there is no direct comparative study on the environmental risks of systemic insecticide in both FN and EFN from the same species.
Hemerocallis citrina Baroni (Asphodelaceae) is an ideal study system for contrasting mechanisms of FN or EFN biosynthesis and ecophysiological function (Rodriguez-Enriquez and Grant-Downton, 2013), because it secretes both FN and EFN in considerable amounts, EFN on the outside of young floral buds and FN deep inside opened flowers. Also called edible daylily or nightlily, H. citrina is endemic to the mountain areas of South China but is widely cultivated as a vegetable for its edible young flower bud (Rodriguez-Enriquez and Grant-Downton, 2013). It is a strictly outcrossing entomophilous species, which uses two kinds of rewards to attract pollinators, pollen for honey-bees and bumble-bees, and FN for lepidopterans (Rodriguez-Enriquez and Grant-Downton, 2013).
Nectar is notoriously plastic mainly due to post-secretory hydrolysis of its components, and the activities of micro-organisms inhabiting it (Parachnowitsch et al., 2019). However, such post-secretory changes can be largely ignored in both FN and EFN because H. citrina flowers are open for only one night. Furthermore, neonicotinoid insecticides such as imidacloprid (IMI) are widely used to deal with aphids and spider mites that attack H. citrina plants cultivated in China (Li et al., 2019). However, the risk of these systemic insecticides for non-target insects via the H. citrina FN and EFN pathways has not been investigated; indeed there has been very little investigation of insecticides entering the food chain via EFN from any plant. In this study, we used two systemic insecticides, IMI and acetamiprid (ACE). These are among the systemic insecticides currently used in China; IMI is banned in many western countries (Pesticide Properties DataBase, 2021a), and ACE is highly toxic to birds and earthworms but supposedly only moderately so to bees (Pesticide Properties DataBase, 2021b).
The current study therefore has two goals. First, the differences between FN and EFN from a monocot will be examined for the first time based on the chemical composition, proteome, secretion pattern and ecological function of nectar in H. citrina. Second, the presence of two systemic insecticides in H. citrina FN and EFN was quantified and compared as preliminary evidence for further environmental risk assessment.
MATERIALS AND METHODS
Hemerocallis citrina cultivation and observation of floral and extrafloral nectar secretion
Forty H. citrina plants grown since 2017 in the open experimental field at Huangshan University (29°41′N, 118°17′E; Anhui province, China) were used in this study. To determine when H. citrina starts to secret FN, floral tubes from flowers at different developmental stages were opened using razor blades, and the existence of FN was then checked by eye (Fig. 1). Because secreted EFN is presented on the outside of the H. citrina flower bud, its secretion can be directly observed without disturbing the plants. Ten individual flowers from each developmental stage, each from a separate individual (Fig. 1A), were examined for the presence of FN and EFN in this way during its peak flowering season, June to July in 2019. To determine whether H. citrina FN and EFN were reabsorbed after secretion, we selected 20 inflorescences, each containing 5–8 individual flowers at different developmental stages. Each was bagged at 08.00 h using a 40-mesh nylon net to block animal visitors, but left attached to grow normally. After bagging, flower buds within were checked by eye at noon the next day to see if any EFN remained on the outside of the flower buds. Any flowers that had wilted after opening were taken out of the bag, and opened by hand to check whether any FN remained in the tube. If no visible nectar remained, it would be deemed that reabsorption occurred.
Fig. 1.
Hemerocallis citrina flower and presentation of FN and EFN. (A) The development of the H. citrina flower is divided into different stages based on day. (B) EFN on a H. citrina flower bud, indicated by an arrow. (C) FN in H. citrina floral tube, indicated by an arrow.
H. citrina FN and EFN collection and measurements of physicochemical character
Raw FN and EFN samples were collected using a pipette and autoclaved tips between 07.00 and 08 00 h during June to July 2019. Because H. citrina FN sits deep inside of the H. citrina flower tubes, the flower tube was cut using a razor blade just above the ovary then FN was pipetted out from the cut end (Fig. 1C). The FN from all flowers of each individual H. citrina plant on the same day was pooled and formed a single sample. The same was done with EFN, pooling samples from different development stages from the same plant and day. All nectar samples were centrifuged at 12 000 g for 5 min to remove any dirt and pollen granules, and stored at –20 °C prior to use.
The pH of fresh FN and EFN was tested using narrow range pH test strips (Supelco MQuant® pH 4.0–7.0, Merck). The total dissolved solids in nectar was measured using a handheld refractometer (MASTER-500, Atago, Tokyo, Japan) as its refraction index (°Brix). The sugar composition of FN and EFN was determined using an EClassical 3100 high-performance liquid chromatograph (HPLC) (Elite, Dalian, China) equipped with a refractive index detector (RI-201H, Shodex, Shoko Science, Tokyo, Japan) as described in Zhou et al. (2018). The concentration of total free amino acids in the nectar samples was measured using leucine as a standard according to Rosen (1957). The protein content in the H. citrina FN and EFN samples was determined according to Bradford (1976). The Folin–Ciocalteu method (Meda et al., 2005) was used to measure total phenolic content in the nectar samples. Gallic acid was taken as a standard, and the content was expressed in micrograms of gallic acid equivalents (GAE) of fresh H. citrina FN or EFN mL–1. The level of hydrogen peroxide (H2O2) in H. citrina FN or EFN was analysed using a commercially available kit (Sangon Biotech Co, Shanghai, China), according to the manufacturer’s instructions.
Ultraviolet–visible (UV–VIS) absorbance spectra for wavelengths from 200 to 400 nm of FN and EFN were measured in triplicate using a spectrophotometer (Model TU1901; Pgeneral, Beijing, China) within a quartz cuvette.
Nectar chitinase and glucanase activity assay
The most common enzymes detected so far in EFN, FN and pollination drops from Gymnosperms are chitinase and glucanase, both typical pathogenesis-related (PR) proteins (Gonzalez-Teuber et al., 2009; Roy et al., 2017; von Aderkas et al., 2018). Profiling of H. citrina FN and EFN chitinolytic activity in-gel after SDS–PAGE was performed according to Song et al. (2019). Fresh H. citrina FN or EFN (20 μL per well) was loaded in gels without boiling beforehand. The clear lytic zones of chitinase isoforms were visualized as dark bands against a fluorescent background under the UV transilluminator, and then photographed. Chitinase activity in H. citrina FN and EFN was also determined using a fluorimetric chitinase assay kit (CS1030, Sigma-Aldrich) following the manufacturer’s instructions with minor modification according to Song et al. (2019) using a SpectraMax i3x microplate reader (Molecular Devices, CA, USA). One unit of chitinase activity was defined as the amount of enzyme that liberated 1 nmol of 4-methylumbelliferone (MU) from the substrate per minute at pH 5.0 and 30 °C.
Endo-β-1,3-glucanase activity of H. citrina FN and EFN was measured using Azurine-cross-linked curdlan (AZCL–curdlan, Megazyme, Ireland) as the substrate according to the method of Morohashi and Matsushima (2000) with minor modifications. In brief, the assay mixture contained in a total volume of 200 μL, 25 μL of FN or EFN, 1 mg of AZCL–curdlan and 175 μL water, and was incubated at 30 °C for 2 h. The amount of soluble dyed fragments released from AZCL–curdlan was determined colorimetrically at 590 nm using a SpectraMax i3x microplate reader. One unit of enzyme activity represents an increase in 0.1 absorbance units under the conditions used.
H. citrina FN and EFN proteomic analysis
To profile the proteome of H. citrina FN and EFN, 10 mL of each type of nectar was examined. Protein content was first concentrated 30-fold via ultracentrifugation using Amicon Ultra centrifugal filters (10 kDa cut-off; EMD Millipore). Tricine-SDS–PAGE (Schägger, 2006) was conducted to separate the nectarins from FN or EFN. Each well contained 5 μg of total FN or EFN nectarins, and samples were run in triplicate. After electrophoresis, nectarins were visualized by Coomassie Brilliant Blue (CBB) G-250 staining. Visible protein bands were manually excised from gels and subjected to in-gel digestion using trypsin as the protease. The samples were analysed in a MALDI-TOF/TOF (matrix-assisted laser desorption/ionization-tandem time of flight) mass spectrometer (Model 5800, Applied Biosystems-Sciex). The combined mass spectrometry (MS) and tandem MS (MS/MS) peak lists were analysed using Global Proteome Server (GPS) Explorer Software 3.6 (Applied Biosystems) with a Mascot search engine (MASCOT version 2.3; Matrix Science, London, UK). The following settings were selected for searching: cysteine carbamidomethylation as fixed modifications; methionine oxidation as variable modifications; peptide mass tolerance of 300 ppm at the most; and a general fragment mass tolerance of 0.5 Da. Protein identifications were accepted if they contained at least two identified peptides. According to the search engine, a score of 56 represents a significant identification (P < 0.05) when the database is restricted to the Viridiplantae taxonomy (NCBInr 20191120).
Because only one nectarin from EFN and none from FN had been successfully identified by the above approach, we next used a gel-free-based analysis with liquid chromatography coupled to high-definition MS (LC-MS/MS). For this, 20 μg each of H. citrina FN and EFN nectarin isolate were sent to the proteomic facility of Institute of Microbiology (Chinese Academy of Sciences) for identification. Trypsin-digested protein samples were analysed using an EASY-nLC 1000 liquid chromatograph that was connected in-line with an Orbitrap Fusion Tribrid mass spectrometer equipped with a nanoelectrospray ionization (nanoESI) source (Thermo Fisher Scientific, Waltham, MA, USA). The mass spectrometric data were analysed using the Mascot database search engine. Peptide sequences were interpreted from the MS/MS spectra by searching across all plants in the NCBI protein database. Carbamidomethylation of cysteines was set as a fixed modification, and methionine oxidation was set as a variable modification. The peptide mass tolerance was set at 15 ppm and the fragment mass tolerance at 0.6 Da. Trypsin was specified as the proteolytic enzyme, and two missed cleavage events were allowed. The MS proteomics data including experimental details have been deposited at the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository (Perez-Riverol et al., 2019) with the dataset identifier PXD025892.
H. citrina FN and EFN feeder observation
All observations of nectar-feeding animals were done during the peak H. citrina flowering season (mid to late June), on a 2 m2 land area with 12 H. citrina plants, within the open experimental field in Huangshan University. Because H. citrina secretes both FN and EFN between dawn and dusk (Hong-Guang Zha, pers. obs.), observations were conducted during three time periods during the day: morning (06.00–08.00 h; 20 h in 2019 and 10 h in 2020), evening (18.00–20.00 h; 20 h in 2019 and 10 h in 2020) and night (21.00–22.00 h; 10 h in 2020). A red headlamp was used in the night observations to avoid deterring nocturnal feeding arthropods. A ‘visit’ is defined here as an insect making some form of contact with an inflorescence, hence if it contacts more than one flower it is still classed as a single visit. For flying visitors, the number of visits were counted, whereas for non-flying insects (mostly EFN feeders) it was possible to count the number of individuals of each species that visited a plant with the assumption that only one plant was visited per individual. Predatory arthropods that were present on the H. citrina inflorescence and preyed on visiting FN or EFN feeders, but were not observed to feed on nectar themselves, were recorded as indirect feeders. Arthropods that landed on H. citrina flowers or plants without feeding on nectar, such as sap-suckers, were not counted in this study. Because the sole focus here is on nectar feeders, visitors were also excluded if they collected pollen but not nectar; these were bees such as Apis mellifera, A. cerana and Bombus spp.
Only those visitors that appeared to have successfully fed on FN were deemed as FN feeders. These were either those with long (≥3 cm) mouthparts that were observed reaching into the H. citrina floral tube, or those with tough mouthparts that were observed biting through the end of the floral tube where FN is stored inside, or very small insects such as thrips that can enter H. citrina floral tubes to feed on nectar. These last were checked for by opening by hand 30 fully open H. citrina flowers between 07.00 and 08.00 h, in 2019 and 2020, respectively. Visitors were deemed EFN feeders if their mouthparts were observed contacting extrafloral nectars. Where possible, visiting species were identified by eye; otherwise photos or collected specimens were used.
Systemic insecticides (acetamiprid and imidacloprid) in H. citrina FN and EFN
Acetamiprid (ACE) and imidacloprid (IMI) were purchased from Sichuan Guoguang Agrochemical Co., Ltd, JianYan, China. A 2 g aliquot each of ACE and IMI was dissolved in 10 L of water and, at 08.00 h on 7 July 2019, this was applied as a soil drench to an 8 m2 open field plot, where 30 H. citrina plants grew, avoiding getting any insecticide directly onto the shoots. Ten H. citrina plants on a separate plot without any insecticide treatment, 2 m away from the treated plot, were used as a control.
Nectar was sampled from each H. citrina plant at 1, 6 and 11 d after insecticide application, always between 07.00 and 08.00 h. FN was collected only from fully opened flowers, and EFN from the outside of young buds using a pipette and autoclaved tips. Where an individual had more than one flower open on a given day, all FN was pooled, and all EFN was pooled from that plant. When a sample of FN or EFN from a single plant was <150 μL, then two or more samples were combined to form a sample containing ≥150 μL for all subsequent tests. Six samples of each nectar type, from each of the treated and control plots, and from each time period, were examined. Each nectar sample was filtered through 0.22 μm syringe filters (Millipore) to remove any dirt or pollen grains therein, and stored at –20 °C prior to use, then directly analysed without any extraction or clean-up steps. The contents of the nectar were separated on a UPLC-MS/MS (ACQUITY ultra-performance liquid chromatograph; Waters, Milford, MA, USA) with a BEH Shield RP C18 column (100 mm × 2.1 mm internal diameter, particle size 1.7 µm) following Jiang et al. (2018). Raw chromatographs and mass spectrogram data were processed with MassLynx 4.1 Software (Waters). The peak area ratio of ACE and IMI to external standards was used for quantification.
RESULTS
Hemerocallis citrina FN and EFN showed different secretion patterns
In the area where our study was conducted, H. citrina flowered in summer from June to July. Its flowers started to open at dusk (approx. 19.00 h), fully opened by approx. 21.00 h, remained open overnight and then wilted duting the following morning whether pollinated or not (Supplementary data Fig. S1). From an early stage where buds are approx.1 cm long to full opening takes nearly 10 d (Fig. 1A). Hemerocallis citrina EFN was exclusively secreted on the middle area of the outer surface of the young flower bud, lasting for 8 d from when a flower bud reached 1 cm long until it reached half its final length and turned yellowish (1 d before opening) without any externally visible structure associated with EFN exudate (Fig. 1A, B). Hemerocallis citrina EFN secretion also showed a very clear circadian rhythm, starting from dusk (approx. 19.00 h) and ending at dawn (approx. 07.00 h) each day during our investigation (Fig. 1B). An individual flower daily produced up to about 50 µL of EFN, but the amount varied dramatically depending on the age of the bud and the weather conditions, such as humidity. Usually, younger and smaller flower buds (<3 cm long) secreted more EFN than the bigger ones (Hong-Xia Zhou, pers. obs.). Because no EFN residue could be seen left on bagged H. citrina flower buds in the middle of the day, reabsorption apparently did happen even though evaporation might also play a role in the disappearance of EFN.
Hemerocallis citrina has deep-tubed flowers with a gynoecial nectary which is situated at the base of the ovary (Fig. 1C). Individual H. citrina flowers produce 10–40 µL (22.5 ± 9.5 µL, mean ± s.d., n = 30) of FN which sits in the deep end of the floral tube. The H. citrina floral tube was 3–4 cm long and its inside diameter was <3 mm. Hemerocallis citrina FN was not visible from outside and only accessible to feeders with a long proboscis, or to very small insects such as thrips that can enter the floral tube (Fig. 1C). Hemerocallis citrina flowers begin to secrete FN about 1 d before they fully open (Fig. 1A). FN was never present in wilted flowers by about 10.00 h after they opened, including in bagged flowers that received no insect visits, indicating that FN was completely reabsorbed. Therefore, FN is present in each H. citrina flower for <2 d, from when secretion starts to full reabsorption (Fig. 1C).
Therefore, even though H. citrina plants were rich in both FN and EFN every night during its flowering season, the timing of EFN and FN secretion on an individual flower never overlapped (Fig. 1A) which also indicated a shift from EFN production to FN production during flower development.
H. citrina FN and EFN have different physiochemical characters
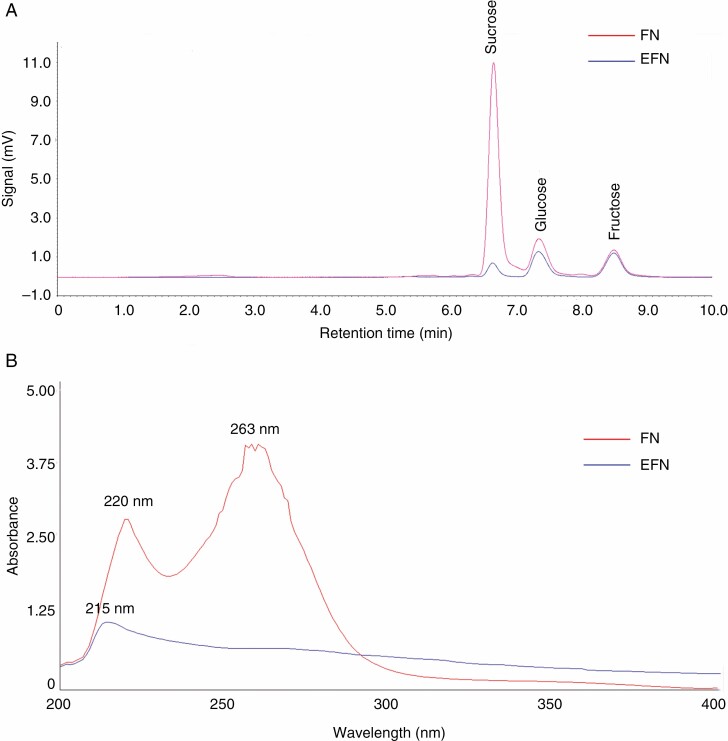
Both H. citrina FN and EFN were colourless liquids and slightly acidic, with the pH value of 5.4 ± 0.2 and 5.4 ± 0.1, respectively (mean ± s.d., n = 16) (Table 1). FN contained more than five times more solutes than EFN, with Brix values of 17.0 ± 0.33 and 3.3 ± 0.35, respectively (mean ± s.d., n = 8) (Table 1). Both FN and EFN contained glucose, fructose and sucrose, but FN was sucrose dominant whereas EFN was hexose rich (Table 1; Fig. 2A). This supports the hypothesis that sucrose is usually dominant in deep hidden nectars which are preferred by longer tongued bees, hummingbirds and moths (Willmer, 2011). Hemerocallis citrina FN contained a significantly higher amount of free amino acids and total phenolics than did EFN (Table 1). The average concentration of H2O2 in H. citrina FN was 2.5 μm, which was much lower than detected in tobacco FN (approx. 1 mm) in which H2O2 was reported to limit microbial growth (Carter and Thornburg, 2004). No H2O2 was detected in EFN samples. Chitinase and glucanase were deemed important enzymes for protecting FN or EFN from infection by micro-organisms (Gonzalez-Teuber et al., 2009; Roy et al., 2017). In our study, H. citrina FN contained lower chitinase activity than EFN (FN, 0.04 ± 0.03; EFN, 1.46 ± 0.31, mean ± s.d., n = 8) and no glucanase activity, unlike EFN (45.7 ± 15.1 U mL–1, mean ± s.d., n = 8) (Table 1). Hemerocallis citrina FN and EFN were very different in absorbance spectra in the wavelength range 200–400 nm (Fig. 2B). EFN only had one absorbance peak at 215 nm, whereas FN had two absorbance peaks at 220 and 263 nm; furthermore, FN had higher absorbance in the wavelength range 200–300 nm (UV region) than did EFN. Distinct UV absorption spectra between FN and EFN indicated that they contained different UV-absorbing substances. Both H. citrina FN and EFN had no absorbance at wavelengths >400 nm in the visible region (data not shown).
Table 1.
The chemical traits of H. citrina FN and EFN (means ± s.d., n = 8).
| Test | FN | EFN |
|---|---|---|
| pH (n = 16) | 5.4 ± 0.2 | 5.4 ± 0.1 |
| Total soluble solids (Brix) | 17.0 ± 0.33 | 3.3 ± 0.35** |
| Sucrose (%) | 12.9 ± 1.6 | 0.8 ± 0.5** |
| Glucose (%) | 3.0 ± 0.3 | 1.7 ± 0.3** |
| Fructose (%) | 2.4 ± 0.4 | 1.8 ± 0.3** |
| Class of nectar† | Sucrose dominant | Hexose rich |
| Total free amino acids (µg mL–1) | 0.37 ± 0.12 | 0.08 ± 0.01** |
| Total protein (µg mL–1) | 15.1 ± 8.5 | 15.8 ± 2.6 |
| Total phenolics (µg GAE mL–1) | 114.0 ± 44.4 | 14.5 ± 4.5** |
| Hydrogen peroxide (µm) | 2.5 ± 1.9 | nd‡ |
| Chitinolytic activity (U mL–1) | 0.04 ± 0.03 | 1.46 ± 0.31** |
| Endo-β-1,3-glucanase activity (U mL–1) | nd | 45.7 ± 15.1 |
Values are means ± s.d.
†Nectar categorization was based on sugar type ratios, defined as the ratio by weight of sucrose to the combined hexose sugars, S/(G + F). Nectar was defined as ‘hexose rich’ if the sugar ratio was between 0.1 and 0.5, or ‘sucrose dominant’ if it was >1.0 (Nicolson and Thornburg 2007).
‡nd = not detected.
**P < 0.01, significant differences (using the Student’s test).
Fig. 2.
HPLC chromatogram (A) and UV–VIS absorption spectrogram (B) of H. citrina FN and EFN.
Nectarins of H. citrina FN and EFN
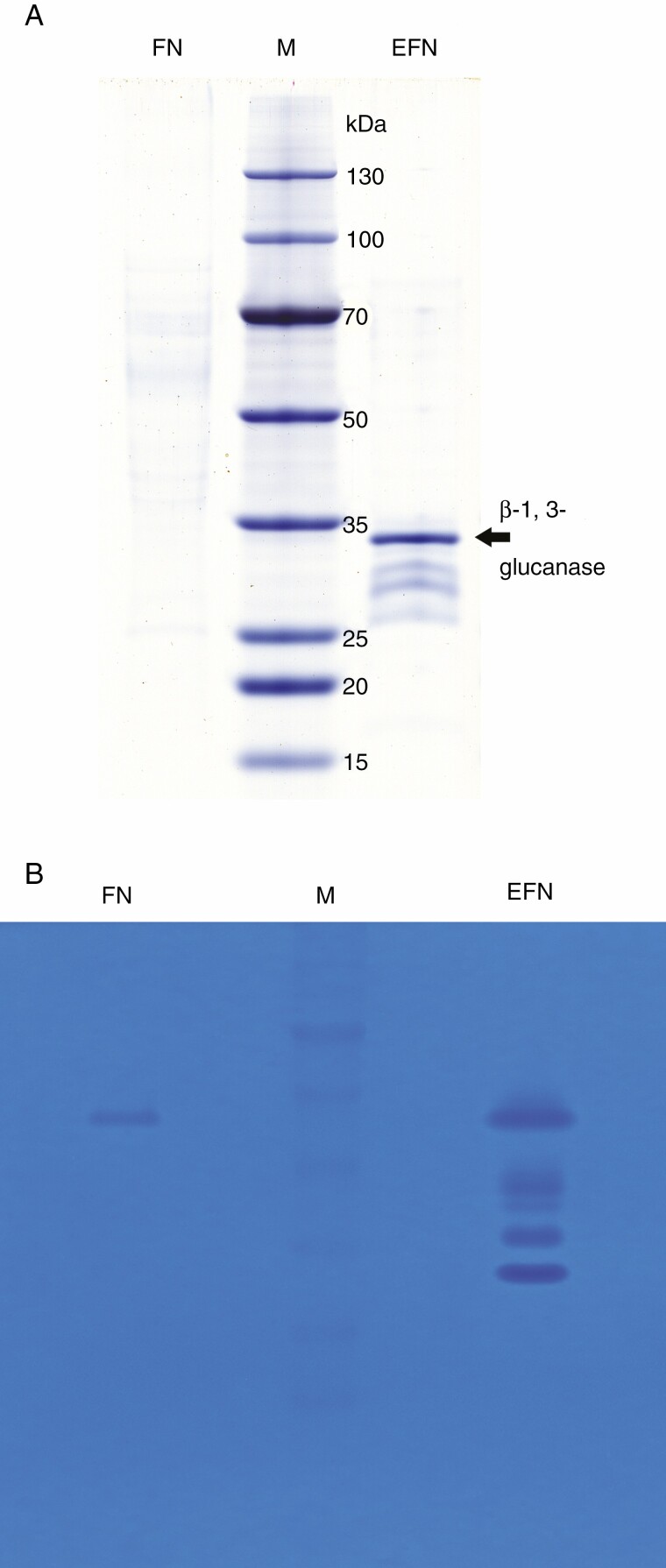
Most reported nectarins are enzymes, and these are the only components in nectar with catalytic activity, playing important roles in nectar biosynthesis and post-secretory modification (Heil, 2011; Roy et al., 2017; Ma et al., 2019). In this study, both H. citrina FN and EFN contained a low but almost equal concentration of nectarins, 15.1 ± 8.5 and 15.8 ± 2.6 µg mL–1, respectively (Table 1), which were lower than the mean value of nectarin concentration in some other floral nectars (approx. 100 µg mL–1) (Nicolson and Thornburg, 2007). Staining with CBB G-250 in a Tricine-SDS–PAGE gel visualized completely different proteome profiles between FN and EFN (Fig. 3A). Unlike many reported nectar proteome profiles (Park and Thornburg, 2009; Roy et al., 2017), H. citrina FN presented weakly, forming a smear area across the lane (Fig. 3A), indicating that it comprised diverse proteins at low concentration with different molecular weights, among which none predominated. We excised ten bands from the top to the bottom across the lane which were slightly CBB stained, but none could be identified by MALDI-TOF/TOF (data not shown). In contrast to FN, EFN yielded four distinct bands visualized by CBB G-250 staining, ranging in size from 30 to 35 kDa with a predominant band separated at approx. 33 kDa (Fig. 3A), from which two peptides were identified: ‘AIETYLFAMFDENQK’ and ‘QPEVEK’. Both of these matched the identity of a β-1,3-glucanase from Hevea brasiliensis (ACZ74626) or Sesamum indicum (XP_011083775) with a score of 134. No other nectarins from EFN were successfully identified by this approach. Chitinase zymograms (Fig. 3B) revealed that both H. citrina FN and EFN contained chitinolytic activity but in different profiles. FN showed only one faint band whereas EFN showed four bands with different mobility across the lane. This indicated that EFN had multiple nectarins with chitinolytic activity but FN only had one.
Fig. 3.
SDS–PAGE of H. citrina FN and EFN nectarins (A) and zymography of chitinase (B). From left to right: FN, floral nectar; M, molecular weight marker; EFN, extrafloral nectar. The predominant nectarin (β-1,3 glucanase) in EFN is indicated by an arrow.
Gel-free MS (LC-ESI-MS/MS) analysis revealed that H. citrina FN and EFN had significantly different protein compositions (Supplementary data Tables S1 and S2). Hemerocallis citrina EFN contained 11 unique proteins (Table 2), six of which belonged to the glycoside hydrolase (GH) family and probably played roles in carbohydrate metabolic processes. The major nectarin in EFN, a β-1,3-glucanase that was recognized by gel-based MS, was also identified in this approach, and is a member of PR family 2. Three other PR proteins were also identified in EFN, endochitinase (PR-3), peptidase S8 (PR-7) and an uncharacterized protein with cysteine-type endopeptidase inhibitor activity (PR-6). This indicates that these PR proteins might function in antimicrobial processes in EFN, consistent with previous findings from other species (Gonzalez-Teuber et al., 2009; Park and Thornburg, 2009; Roy et al., 2017).
Table 2.
Proteins identified in EFN from Hemerocallis citrina
| No. | Protein identified | Accession number (GenBank) | Organism | Protein Mascot score/count of distinct sequences | Biological process* | PR protein family† | GH family* | Others |
|---|---|---|---|---|---|---|---|---|
| 1 | Beta-d-xylosidase | ONK73945 | Asparagus officinalis | 243/6 | Carbohydrate metabolic | 3 | ||
| ACL53913 | Zea mays | 79/5 | 3 | Also detected in FN | ||||
| PKA56762 | Apostasia shenzhenica | 61/5 | 3 | |||||
| SPT20554 | Triticum aestivum | 133/4 | 3 | |||||
| OIV96210 | Lupinus angustifolius | 147/3 | 3 | |||||
| NP_196535 | Arabidopsis thaliana | 146/2 | 3 | |||||
| 2 | Beta-galactosidase | KVH90786 | Cynara cardunculus var. scolymus | 74/4 | Carbohydrate metabolic | 35 | ||
| 3 | Alpha-amylase | CDP01359 | Coffea canephora | 62/2 | Carbohydrate metabolic | 13 | ||
| 4 | Polygalacturonase | RCV19800 | Setaria italica | 65/2 | Carbohydrate metabolic | 28 | ||
| 5 | Endochitinase | NP_181890 | Arabidopsis thaliana | 68/2 | Carbohydrate metabolic | 3 | 19 | |
| 6 | Endo-1,3-beta-glucanase | XP_020700743 | Dendrobium catenatum | 57/2 | Carbohydrate metabolic | 2 | 17 | |
| 7 | Peptidase S8 | OVA17070 | Macleaya cordata | 64/2 | Proteolysis | 7 | – | |
| 8 | Cobalamin-independent methionine synthase | KJB09184 | Gossypium raimondii | 50/2 | Cellular amino acid biosynthetic | – | – | Also detected in FN |
| 9 | Uncharacterized protein (with cysteine-type endopeptidase inhibitor activity) | RRT32118 | Ensete ventricosum | 122/2 | Unknown | 6 | – | |
| 10 | Fasciclin-like arabinogalactan protein | KNA25811 | Spinacia oleracea | 63/2 | Unknown | – | – | |
| 11 | 14-3-3 h-1 protein | EPS73301 | Genlisea aurea | 51/2 | Unknown | – | – | Also detected in FN |
*Source: InterPro- https://www.ebi.ac.uk/interpro.
†Pathogenesis-related (PR) proteins classification according to Sels et al. (2008).
In FN, 1333 proteins were identified by LC-ESI-MS/MS, but only three of them were the same as identified in EFN (Table 2; Supplementary data Table S1). These were β-d-xylosidase (ONK73945), cobalamin-independent methionine synthase (KJB09184) and 14-3-3 h-1 protein (EPS73301). Even though β-1,3-glucanase was the major nectarin in H. citrina EFN and was frequently identified in FN or EFN from different plant species (Roy et al., 2017), it was not detected in H. citrina FN. This was consistent with no glucanase activity having been detected in H. citrina FN by our enzymatic analysis in this study.
No chitinase was identified in FN by this sequence-based proteomic analysis; however, weak chitinolytic activity was detected in FN by enzymatic methods with chitin substrates. This suggests that H. citrina FN might contain a chitinase whose sequence was different from known chitinases. One-tenth of proteins identified by proteomics analysis were heat shock proteins (HSPs) – in particular HSP70 – but also including HSP81, HSP82, HSP83 and HSP90 (Supplementary data Table S1). It is known that HSP70 is required for flower opening under normal temperatures or mild heat stress (Chen et al., 2019), and in Nicotiana FN it is thought to protect other nectarins from oxidative damage caused by high levels of H2O2 (Silva et al., 2020). However, in this study, neither HSPs nor H2O2 was detected in EFN. We did detect some non-secretory proteins present in H. citrina FN, e.g. proteasome subunit alpha type-5 protein (PHU25788; nucleus or cytoplasm located) and 2,3-bisphosphoglycerate-independent phosphoglycerate mutase (NP_001311960; cytoplasm located). Because the FN is sited deeply in the end of the long H. citrina floral tube, we had to cut the tube to collect it and could not make it totally free of contaminants from floral tissue. These non-secretory proteins in the FN proteome could be collection-related contaminations, but they were present in very small amounts and would thus have little effect on the comparative proteomic analysis between EFN and FN.
H. citrina FN and EFN attracted different types of arthropods to visit and feed
Even though both FN and EFN presented simultaneously during the night, they attracted different types of animal nectar feeders (Table 3). Three hawkmoths, Agrius convolvuli, Theretra suffuse and Ampelophaga rubiginosa (Sphingidae), were the most frequently observed FN feeders, probing into the narrow floral tube and sucking FN using their long mouthparts during the evening and night (Supplementary data Video S1 and S2), during which their bodies touched the stigma and anther. Therefore, hawkmoths were observed to be the dominant H. citrina FN feeders and pollinators (Table 2). Thrips (Frankliniella intonsa) also fed on FN, by entering the floral tube (they are <2 mm long). We found thrips in 20 out of 60 opened H. citrina flowers examined. Thrips are well known to feed on both FN and pollen (Ananthakrishnan, 1993). However, in this study, no contact was observed between thrips and H. citrina anthers or stigma, and, furthermore, their small size makes them poor pollen vectors, hence thrips appear to be FN feeders but not pollinators for H. citrina. Xylocopa nasalis and X. appendiculata (Apidae) had been observed piercing through the end of the H. citrina floral tube with their tough mouthparts and sucking out FN, but these were not observed collecting H. citrina pollen (Supplementary data Fig. S2).
Table 3.
Hemerocallis citrina FN and EFN feeders in the 2 years of study
| Nectar feeder type | Order/Species | Visits or number of FN and EFN feeders* | |
|---|---|---|---|
| 2019 | 2020 | ||
| FN | Thysanoptera | ||
| Frankliniella intonsa | 12† | 8† | |
| Hymenoptera | |||
| Xylocopa nasalis | 5 | 2 | |
| Xylocopa appendiculata | 8 | 3 | |
| Lepidoptera | |||
| Agrius convolvuli | 5 | 11 | |
| Ampelophaga rubiginosa | 4 | 13 | |
| Theretra suffusa | 12 | 26 | |
| EFN‡ | Scutigeromorpha | ||
| Scutigera coleoptrata | 45 | 13 | |
| Blattodea | |||
| Blattella bisignata | 25 | 9 | |
| Coleoptera | |||
| Chauliognathus sp. | 9 | 7 | |
| Hymenoptera | |||
| Camponotus japonicus | 135 | 74 | |
| Monomorium pharaonis | 51 | 16 | |
| Parapolybia varia | 114 | 62 | |
| Polistes jokahamae | 9 | 5 | |
| Polistes snelleni | 5 | 2 | |
| Vespa affinis | 32 | 14 | |
| Diptera | |||
| Drosophila melanogaster | 74 | 33 | |
| Episyrphus balteatus | 19 | 11 | |
| Lucilia sericata | 17 | 23 | |
| Musca domestica | 15 | 18 | |
| Rivellia nigroapicalis | 15 | 6 | |
| Sarcophaga peregrina | 10 | 7 | |
| Indirect feeding on FN or EFN§ | |||
| Araneae | |||
| Argiope bruennichi | 17 | 4 | |
| Ebrechtella pseudovatia | 44 | 27 | |
| Tetragnatha praedonia | 18 | 13 | |
| Orthoptera | |||
| Ducetia japonica | 12 | 4 | |
| Mantodea | |||
| Hierodula patellifera | 6 | 2 | |
| Coleoptera | |||
| Harmonia axyridis | 33 | 18 |
*Arthropods that visited H. citrina flowers but were not seen feeding on FN or EFN, e.g. honey-bees and aphids, were not counted in this investigation.
†Numbers of flowers out of 30 adult flowers checked which had Frankliniella intonsa found within them.
‡For flightless or weakly mobile arthropods, numbers of individuals observed was treated as number of visits, for each species counted during our observation.
§Predatory arthropods which had not been observed directly feeding on FN or EFN but preying on FN or EFN feeders.
Compared with FN, EFN was completely exposed and accessible to more diverse animals. Hemerocallis citrina EFN feeders were mainly flies (Rivellia nigroapicalis, Sarcophaga peregrine, Lucilia sericata, Drosophila melanogaster, Musca domestica and Episyrphus balteatus), ants (Camponotus japonicas and Monomorium pharaonis) and wasps (Vespa affinis, Polistes jokahamae, Polistes snelleni and Parapolybia varia) (Table 2). Notably, wasps are well-known predatory insects, but we frequently watched these wasps feeding on EFN on H. citrina flower buds in the early morning instead of hunting their prey, such as flies or aphids, on H. citrina (Supplementary data Video S3). Ants could be seen on H. citrina flower buds almost throughout the whole day, especially in the morning before sunrise because of ample EFN on H. citrina floral buds at that time. However, we seldom observed the ants entering the opened flower for FN (Supplementary data Video S4).
Consumption of EFN by some regular predatory animals has been reported, e.g. crab spiders (Taylor and Foster, 1996). On H. citrina flowers, spiders (Ebrechtella pseudovatia, Tetragnatha praedonia and Argiope bruennichi), katydid (Ducetia japonica) and mantis (Hierodula patellifera) were observed preying on flies or other insects on H. citrina flowers (Table 2; Supplementary data Fig. S2), but none of these was observed directly feeding on FN or EFN. Hence they may only be regarded as indirect feeders of H. citrina FN and especially EFN.
No animals other than arthropods were observed feeding on H. citrina FN or EFN during our investigation. Furthermore, no animal species was observed to feed on both H. citrina FN and EFN during our investigation. Honey-bees and bumble-bees were observed collecting pollen on H. citrina flowers, but none of these was observed to feed on H. citrina FN or EFN.
Both acetamiprid and imidacloprid accumulated more in EFN than FN
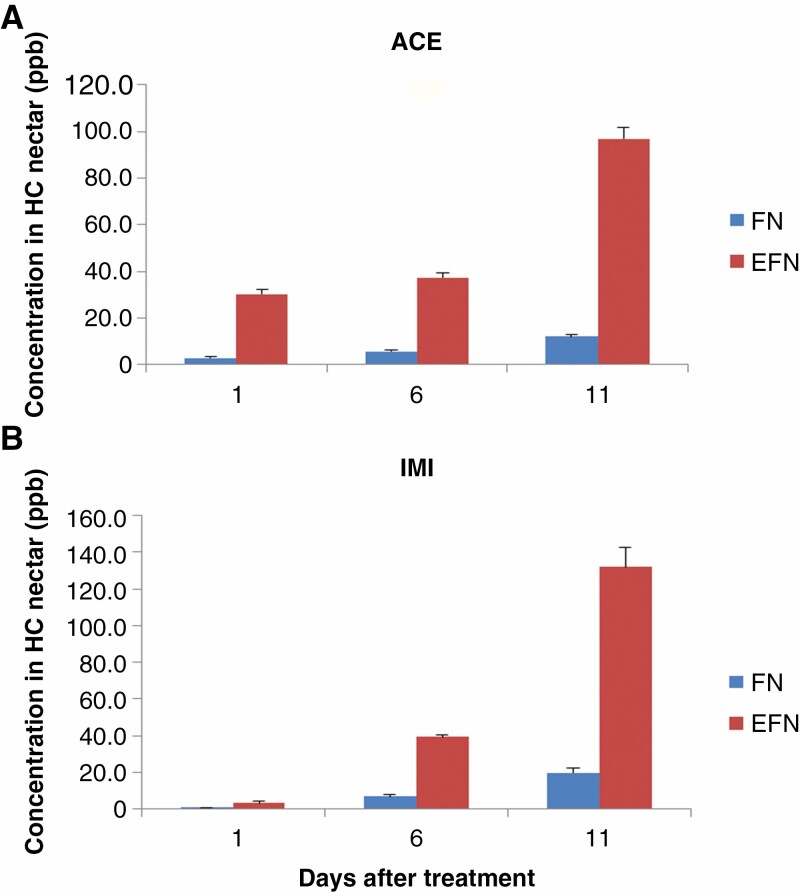
Both ACE and IMI compounds were detected in H. citrina FN and EFN at a time 24 h after their application into the soil, and the concentration of both in FN and EFN continuously increased over 11 d from application (Fig. 4). Both ACE and IMI concentrations in EFN were significantly higher than in FN at the three checked time points (Fig. 4). After 1 d, ACE concentration was 30.2 ± 2.0 ppb in EFN and 3.0 ± 0.3 ppb in FN; after 11 d it was 97.0 ± 4.7 and 12.2 ± 0.9 ppb, respectively (Fig. 4A). The IMI concentration was 3.4 ± 0.7 ppb in EFN and 0.8 ± 0.1 ppb in FN; after 11 d it was 131.8 ± 10.7 and 19.9 ± 2.5 ppb, respectively (Fig. 4B). Metabolites, e.g. 6-chloronicotinic acid from the breakdown of IMI and 6-chloro-pyridilmethyl alcohol from ACE (Malev et al., 2012), could not be detected in the EFN or FN samples using MS (data not shown) which indicates that H. citrina could not degrade or detoxify either compound during any stage of nectar production. Hence there are different generation processes or pathways for EFN vs. FN, which ACE and IMI become involved in. Compared with IMI, ACE reached significantly higher concentrations in FN and EFN, which might be due to ACE having higher water solubility (2950 mg L−1 at 20 °C, compared with 610 mg L−1 at 20 °C for IMI; Pesticide Properties DataBase, PPDB https://sitem.herts.ac.uk/aeru/ppdb/en/index.htm). No morphological changes between treated and untreated groups or phytotoxicity caused by IMI and ACE application were observed.
Fig. 4.
Imidacloprid (IMI) and acetamiprid (ACE) residues in H. citrina FN and EFN. (A) ACE; (B) IMI. Error bars represent the standard error.
DISCUSSION
Similarities and differences between Hemerocallis citrina FN and EFN biosynthesis, composition and secretion
The similarities between H. citrina FN and EFN were as expected, in that both are acidic and have sugars as their major components, with smaller amounts of amino acids, proteins, phenolics, etc. Both FN and EFN were secreted on flowers between dusk and dawn, and attracted a lot of animal visitors. However, many differences were detected between H. citrina FN and EFN; for example, FN had sucrose as the dominant sugar, while EFN had hexose. Hexose in nectar is believed to result from sucrose hydrolysis by a cell wall-bound invertase in the nectary (Nicolson and Thornburg, 2007; Zhou et al., 2018), but if so this process was important in H. citrina EFN generation but not FN generation. Sugar type in nectar is considered to be a determinant for which visitors are attracted to a flower (Baker and Baker, 1983; Nicolson and Thornburg, 2007). For example, sucrose-dominant nectar can be less viscous than a hexose-dominant nectar of equivalent caloric value, making it more suitable for lepidopterans sucking nectar from deep-tubed flowers with a long proboscis (Willmer, 2011). Given that hawkmoths were the most commonly observed pollinators for H. citrina, sucrose-dominant FN appears to be an ecological adaptation to its native pollinators and flower shape. Conversely, because EFNs are always presented on the plant surface, viscosity is not a serious limitation for visitors to feed on, and furthermore higher viscosity makes it less likely that externally presented nectar will drip from the flower.
The comparative proteomic analysis showed further differences between FN and EFN. The EFN proteome of H. citrina was quite simple, with only 11 detected proteins, of which four belonged to four different PR protein families, PR-2, PR-3, PR-6 and PR-7. This strongly supports the idea that nectarins in EFN, as well as FN, mainly serve to protect the nectar, and surrounding tissues, from microbial infections (Park and Thornburg, 2009; Heil, 2011). A chitinase was the predominant protein in H. citrina EFN, and both chitinase and glucanase activity were detected in EFN; both of these enzymes also have antimicrobial properties (Gonzalez-Teuber et al., 2009; Ma et al., 2017). However, neither enzyme could be detected in FN, even using high-resolution MS-based proteomic analysis. Furthermore, the FN proteome was significantly lacking in PR proteins, despite being far more complex than that of EFN, comprising mainly HSPs with hundreds of other proteinaceous components. These differences probably reflect the different roles and situations of the two nectars. Microbial defences are not obligate in nectars (Ma et al., 2017), and there may be little selection pressure on H. citrina FN to develop such defences, because the FN is deeply concealed, accessible to only a selection of visitors and only present for half a day from when it first becomes accessible, all of which limits opportunities for pathogen establishment. Conversely, H. citrina EFN is continuously secreted on the outside of flower buds for at least 1 week and is completely exposed to any visitors or wind, giving ample time for microbes to arrive and initiate infection, making an antimicrobial mechanism essential. In this context, it is surprising that H2O2 was detected in H. citrina FN but not EFN, given that this compound has an antimicrobial role in Nicotiana sp. (Carter and Thornburg, 2004). However, the H2O2 concentration in H. citrina FN was very low, only around 2.5 µm, >1000 times less than the 4 mm detected in Nicotiana sp. (Carter and Thornburg, 2004), making it quite possible that H2O2 does not play any antimicrobial roles in H. citrina FN.
In addition, we observed that there was almost no EFN present on the flowers when it was about to rain, whereas, on such days, no difference in FN production was detected. Higher sensitivity to weather conditions in EFN secretion, relative to that of FN, indicated that they have different secretion regulation mechanisms.
EFN is secreted on flowers but does not function directly in encouraging pollination
Hemerocallis citrina is a strict outcrosser, and provides pollen and FN as a reward for its animal pollinators, which are mainly hawkmoths, although honey-bees and bumble-bees also pollinate the flowers. However, during our 2 year observation, these bees were only observed to collect pollen but never nectar (FN or EFN). The likely reason is that the relatively short mouthparts of bees cannot reach the FN deep within the corolla tube. The EFN of H. citrina is very dilute (<4 % sugars), making it an unsuitable energy source for flying pollinators, especially bees (Parachnowitsch et al., 2019). Consistent with this, across 2 years of observation, we never once saw any of the insects that pollinate H. citrina (e.g. honey-bees or bumble-bees) feeding on the EFN. Furthermore, we found that H. citrina EFN secretion stops on each flower at least 2 d before it opens, so it cannot help the flower it is on attract pollinators. EFN secretion on Bixa orellana similarly reaches a peak on the mature floral buds, and ceases by the time the flowers open (Bentley, 1977; de Miranda et al., 2017). Conversely, EFN is attractive to various insects that do not pollinate H. citrina, such as wasps, ants, mantises and flies. To these it offers water and nutrients, as well as limited amounts of sugar. For example, it has been shown that on summer days, solitary wasps are more likely to choose flowers with diluted nectar which can give them safe water as well as sugar (Willmer, 1985). The question therefore becomes, how are visits from these insects, that are attracted by EFN, beneficial to H. citrina? Because many are predatory, they might serve a defensive role for the flower, discouraging herbivores and sap-suckers, although this has yet to be tested.
That EFN secretion always stopped before FN secretion began suggests a trade-off between herbivore defence and pollination attraction (Fig. 1A). This could be due to the cost of nectar production: it is estimated that 4–37 % of daily photosynthate assimilated during blossoming is secreted as nectar sugar (Southwick, 1984), and H. citrina might not have the resources to produce both FN and EFN on the same flower at once. However, both are produced on the same inflorescence when flowers are in different development stages. An alternative explanation is that EFN ceases to be advantageous, and even becomes disadvantageous, as the flower opens. If the EFN function is to attract predatory arthropods, then these might discourage pollinators from visiting, making it necessary to cease EFN production before the flower opens.
Neonicotinoid insecticides in EFN are a potential threat to non-target animals
The use of systemic insecticides, especially neonicotinoids, is well documented as causing global pollinator decline (Goulson, 2013). ACE and IMI are the two most frequently detected neonicotinoids in agricultural products and honey in China (Wang et al., 2020). Both are currently legal in China, despite being highly toxic to a broad range of insects and other animals, especially to birds and earthworms (Pisa et al., 2015).
Thus far, most of the attention to the problems caused by insecticides has focused on poisoning pollinators through FN and pollen (Goulson, 2013; Raine, 2018). Here, we demonstrate that both ACE and IMI can become components of EFN, as well as FN, at least in H. citrina. The presence of insecticides in EFN indicates they will hence kill a further range of non-target animals more diverse than those that feed on FN, because EFN is more accessible and because, being hexose rich, it is more digestible (Heil et al., 2005). In this case, both ACE and IMI became more concentrated in H. citrina EFN than in FN. It is predictable that the insecticides will also be passed on to the predators of all these EFN feeders. Moreover, because EFN appears to have evolved to attract those arthropods that prey on herbivorous insects, the effect of killing these insects will be to reduce natural predation on the very pest species that the insecticides are supposed to control, hence worsening the exact problem the chemicals are supposed to be dealing with.
The EFN is always ignored in environmental risk evaluation because of its usually small amount, not always adjacent to flowers and easily cleaned by feeders. Our results show that it is important and must be included. Thus far, 4017 angiosperm species across 110 families are known to secrete EFN, and the numbers keep growing (http://www.extrafloralnectaries.org/). Though the number is small compared with FN-bearing species, it includes several major crops, such as castor oil (Ricinus communis), beans (Phaseolus vulgaris and P. lunatus), sunflower (Helianthus annuus) and cotton (Gossypium hirsutum). Moreover cotton, like H. citrina, secretes more EFN than it does FN (Jones et al., 2020; Chatt et al., 2021), and the same is true of the widespread and common genus Acacia (Heil, 2015). Hemerocallis citrina itself is a common vegetable in China with an annual yield of 1.25 Mt fresh weight in 2020 (Zhi-Xin Qin, Hunan Agriculture University; pers. commun.). Furthermore, it generally flowers in June and July, a time when relatively few species are flowering in China relative to spring or late summer, making it a significant food source for pollinating insects. Systemic pesticides, including both IMI and ACE, are commonly used on farmed H. citrina (Li et al., 2019) and, because flowers are always harvested before they have opened, EFN but not FN provides a route for these chemicals to enter the wild food chain, and might have a major ecological impact. Our work demonstrates that EFN is a potential pathway for these chemicals to enter the wild food chain, and must be considered when evaluating the ecological risk of any such chemical.
CONCLUSIONS
This study demonstrates that FN and EFN differ in timing of production and function, within H. citrina, with FN being concentrated and sucrose dominant, secreted in the mature flower tube, and serving as reward for pollinators. In contrast, EFN is dilute and hexose rich, secreted on the outside of the developing floral bud, and is likely to attract predatory animals for defence. There were also significant physiochemical differences between FN and EFN, especially concerning the proteins they contained, with microbial defence proteins only evident in EFN. The two neonicotinoid insecticides examined, IMI and ACE, became present in both nectar types soon after application, but in greater concentration within EFN. This, plus the fact that a wider range of insect species were seen to feed on EFN, means that EFN is a more significant pathway for these chemicals to enter the wild food chain. Therefore, the EFN pathway must be considered when evaluating the risks to the environment of any systemic insecticide.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: H. citrina flower in the daytime at 14.00 h and at night at 20.00 h. Figure S2: some H. citrina flower visitors. Table S1: summary of proteins identified in H. citrina FN. Table S2: summary of proteins identified in H. citrina EFN. Video S1: hawkmoth fed on H. citrina FN-1. Video S2: hawkmoth fed on H. citrina FN-2. Video S3: a wasp patrolling on an H. citrina flower bud and searching for EFN. Video S4: ants patrolling on H. citrina flower buds.
ACKNOWLEDGEMENTS
We thank Mr Hong-Cao Wang (Nanjing Forestry University) for arthropod identification, and Qiu-Yuan Zha for his help in field work. Author contributions: H.G.Z., H.X.Z. and Y.Q.S. conceived and designed the experiments. P.C., M.F.H., X.Y.L. and W.J.G. performed the experiments. H.G.Z., R.I.M. and J.C. analysed the data. H.G.Z. and R.I.M. wrote the paper.
Contributor Information
Hong-Xia Zhou, College of Life and Environment Sciences, Huangshan University, Huangshan 245041, China.
Richard I Milne, Institute of Molecular Plant Sciences, University of Edinburgh, Edinburgh EH9 3JH, UK.
Peng Cui, Instrumental Analysis Centre, Huangshan University, Huangshan 245041, China.
Wen-Jing Gu, College of Life and Environment Sciences, Huangshan University, Huangshan 245041, China.
Meng-Fang Hu, College of Life and Environment Sciences, Huangshan University, Huangshan 245041, China.
Xin-Yue Liu, College of Life and Environment Sciences, Huangshan University, Huangshan 245041, China.
Yue-Qin Song, College of Life and Environment Sciences, Huangshan University, Huangshan 245041, China.
Jun Cao, Yunnan Key Laboratory of Plant Reproductive Adaption and Evolutionary Ecology and School of Ecology and Environmental Science, Yunnan University, Kunming 650500, China.
Hong-Guang Zha, College of Life and Environment Sciences, Huangshan University, Huangshan 245041, China.
FUNDING
This study was supported by the Collaborative Innovation Centre of Plant Physiology and Active Ingredient Research of Huangshan University (to H.G.Z.); National Natural Science Foundation of China Science (32070258 to H.G.Z.); Key Research Projects of Natural Science in Universities in Anhui Province (KJ2016A680 to Y.Q.S. and KJ2020A0689 to H.X.Z.); National College Students’ Innovation and Entrepreneurship Training Program (S202010375056 to H.G.Z. and S202010375048 to H.X.Z.); the Open Fund of Laboratory of Ecology & Evolutionary Biology, Yunnan University (to H.G.Z.); and Science and Technology program of Yunnan province (2019FY003026 to J.C.).
LITERATURE CITED
- von Aderkas P, Prior NA, Little SA. 2018. The evolution of sexual fluids in gymnosperms from pollination drops to nectar. Frontiers in Plant Science 9: 1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananthakrishnan TN. 1993. The role of thrips in pollination. Current Science 65: 262–264. [Google Scholar]
- Baker HG, Baker I. 1983. Floral nectar sugar constituents in relation to pollinator type. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold Co., 117–141. [Google Scholar]
- Bentley BL. 1977. The protective function of ants visiting the extrafloral nectaries of Bixa orellana (Bixaceae). Journal of Ecology 65: 27–38. [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Bredeson MM, Lundgren JG. 2018. Thiamethoxam seed treatments reduce foliar predator and pollinator populations in sunflowers (Helianthus annuus), and extra-floral nectaries as a route of exposure for seed treatments to affect the predator, Coleomegilla maculata (Coleoptera: Coccinellidae). Crop Protection 106: 86–92. [Google Scholar]
- Carter C, Thornburg RW. 2004. Is the nectar redox cycle a floral defense against microbial attack? Trends in Plant Science 9: 320–324. [DOI] [PubMed] [Google Scholar]
- Chatt EC, Mahalim SN, Mohd-Fadzil NA, et al. 2021. Nectar biosynthesis is conserved among floral and extrafloral nectaries. Plant Physiology 185: 1595–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shi L, Chen Y, et al. 2019. Arabidopsis HSP70-16 is required for flower opening under normal or mild heat stress temperatures. Plant, Cell & Environment 42: 1190–1204. [DOI] [PubMed] [Google Scholar]
- Cuautle M, Rico-Gray V, Diaz-Castelazo C. 2005. Effects of ant behaviour and presence of extrafloral nectaries on seed dispersal of the Neotropical myrmecochore Turnera ulmifolia L. (Turneraceae). Biological Journal of the Linnean Society 86: 67–77. [Google Scholar]
- Escalante-Pérez M, Heil M. 2012. Nectar secretion: its ecological context and physiological regulation. In: Vivanco JM, Baluška F, eds. Secretions and exudates in biological systems. Berlin, Heidelberg: Springer Berlin Heidelberg, 187–220. [Google Scholar]
- Gonzalez-Teuber M, Eilmus S, Muck A, Svatos A, Heil M. 2009. Pathogenesis-related proteins protect extrafloral nectar from microbial infestation. The Plant Journal 58: 464–473. [DOI] [PubMed] [Google Scholar]
- Goulson D. 2013. An overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology 50: 977–987. [Google Scholar]
- Heil M. 2011. Nectar: generation, regulation and ecological functions. Trends in Plant Science 16: 191–200. [DOI] [PubMed] [Google Scholar]
- Heil M. 2015. Extrafloral nectar at the plant–insect interface: a spotlight on chemical ecology, phenotypic plasticity, and food webs. Annual Review of Entomology 60: 213–232. [DOI] [PubMed] [Google Scholar]
- Heil M, Rattke J, Boland W. 2005. Postsecretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science 308: 560–563. [DOI] [PubMed] [Google Scholar]
- Jiang J, Ma D, Zou N, et al. 2018. Concentrations of imidacloprid and thiamethoxam in pollen, nectar and leaves from seed-dressed cotton crops and their potential risk to honeybees (Apis mellifera L.). Chemosphere 201: 159–167. [DOI] [PubMed] [Google Scholar]
- Jones AG, Hoover K, Pearsons K, Tooker JF, Felton GW. 2020. Potential impacts of translocation of neonicotinoid insecticides to cotton (Gossypium hirsutum (Malvales: Malvaceae)) extrafloral nectar on parasitoids. Environmental Entomology 49: 159–168. [DOI] [PubMed] [Google Scholar]
- Koptur S, Palacios-Rios M, Díaz-Castelazo C, Mackay WP, Rico-Gray V. 2013. Nectar secretion on fern fronds associated with lower levels of herbivore damage: field experiments with a widespread epiphyte of Mexican cloud forest remnants. Annals of Botany 111: 1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost C, Heil M. 2008. The defensive role of volatile emission and extrafloral nectar secretion for lima bean in nature. Journal of Chemical Ecology 34: 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Han ZP, Li YQ, Zhang HX. 2019. Review on biological characteristics and higher production culture technique of datong daylily. Horticulture & Seed 5: 5–10. (In Chinese with English abstract.) [Google Scholar]
- Lundgren JG. 2009. Extrafloral nectar. In: Relationships of natural enemies and non-prey foods. Dordrecht: Springer Netherlands, 61–71. [Google Scholar]
- Ma XL, Milne RI, Zhou HX, Fang JY, Zha HG. 2017. Floral nectar of the obligate outcrossing Canavalia gladiata (Jacq.) DC. (Fabaceae) contains only one predominant protein, a class III acidic chitinase. Plant Biology 19: 749–759. [DOI] [PubMed] [Google Scholar]
- Ma XL, Milne RI, Zhou HX, Song YQ, Fang JY, Zha HG. 2019. Proteomics and post-secretory content adjustment of Nicotiana tabacum nectar. Planta 250: 1703–1715. [DOI] [PubMed] [Google Scholar]
- Malev O, Klobučar RS, Fabbretti E, Trebše P. 2012. Comparative toxicity of imidacloprid and its transformation product 6-chloronicotinic acid to non-target aquatic organisms: microalgae Desmodesmus subspicatus and amphipod Gammarus fossarum. Pesticide Biochemistry and Physiology 104: 178–186. [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. 2005. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry 91: 571–577. [Google Scholar]
- de Miranda RM, Nery LA, Ventrella MC. 2017. Extrafloral nectaries of annatto (Bixa orellana L.): anatomy, nectar composition and activity during organ development. Acta Botanica Brasilica 31: 468–476. [Google Scholar]
- Morohashi Y, Matsushima H. 2000. Development of β-1,3-glucanase activity in germinated tomato seeds. Journal of Experimental Botany 51: 1381–1387. [PubMed] [Google Scholar]
- Moscardini VF, Gontijo PC, Michaud JP, Carvalho GA. 2014. Sublethal effects of chlorantraniliprole and thiamethoxam seed treatments when Lysiphlebus testaceipes feed on sunflower extrafloral nectar. Biocontrol 59: 503–511. [Google Scholar]
- Nicolson SW, Thornburg RW. 2007. Nectar chemistry. In: Nicolson SW, Nepi M, Pacini E, eds. Nectaries and nectar. Dordrecht: Springer Netherlands, 215–264. [Google Scholar]
- Parachnowitsch AL, Manson JS, Sletvold N. 2019. Evolutionary ecology of nectar. Annals of Botany 123: 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Thornburg RW. 2009. Biochemistry of nectar proteins. Journal of Plant Biology 52: 27–34. [Google Scholar]
- Perez-Riverol Y, Csordas A, Bai J, et al. 2019. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Research 47: D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa LW, Amaral-Rogers V, Belzunces LP, et al. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environmental Science and Pollution Research 22: 68–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesticide Properties DataBase . 2021a. Imidacloprid (Ref: BAY NTN 33893). https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/397.htm, accessed 11 May 2021.
- Pesticide Properties DataBase . 2021b. Acetamiprid. https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/11.htm, accessed 11 May 2021.
- Raine NE. 2018. A systemic problem with pesticides. Nature 561: 40–41. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Enriquez MJ, Grant-Downton RT. 2013. A new day dawning: Hemerocallis (daylily) as a future model organism. AoB PLANTS 5: pls055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H. 1957. A modified ninhydrin colorimetric analysis for amino acids. Archives of Biochemistry and Biophysics 67: 10–15. [DOI] [PubMed] [Google Scholar]
- Roy R, Schmitt AJ, Thomas JB, Carter CJ. 2017. Review: nectar biology: from molecules to ecosystems. Plant Science 262: 148–164. [DOI] [PubMed] [Google Scholar]
- Sánchez-Bayo F, Wyckhuys KAG. 2019. Worldwide decline of the entomofauna: a review of its drivers. Biological Conservation 232: 8–27. [Google Scholar]
- Schägger H. 2006. Tricine–SDS-PAGE. Nature Protocols 1: 16–22. [DOI] [PubMed] [Google Scholar]
- Sels J, Mathys J, De Coninck BMA, Cammue BPA, De Bolle MFC. 2008. Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiology and Biochemistry 46: 941–950. [DOI] [PubMed] [Google Scholar]
- Silva F, Guirgis A, von Aderkas P, Borchers CH, Thornburg R. 2020. LC-MS/MS based comparative proteomics of floral nectars reveal different mechanisms involved in floral defense of Nicotiana spp., Petunia hybrida and Datura stramonium. Journal of Proteomics 213: 103618. [DOI] [PubMed] [Google Scholar]
- Song YQ, Milne RI, Zhou HX, Ma XL, Fang JY, Zha HG. 2019. Floral nectar chitinase is a potential marker for monofloral honey botanical origin authentication: a case study from loquat (Eriobotrya japonica Lindl.). Food Chemistry 282: 76–83. [DOI] [PubMed] [Google Scholar]
- Southwick EE. 1984. Photosynthate allocation to floral nectar: a neglected energy investment. Ecology 65: 1775–1779. [Google Scholar]
- Stapel JO, Cortesero AM, Lewis WJ. 2000. Disruptive sublethal effects of insecticides on biological control: altered foraging ability and life span of a parasitoid after feeding on extrafloral nectar of cotton treated with systemic insecticides. Biological Control 17: 243–249. [Google Scholar]
- Taylor RM, Foster WA. 1996. Spider nectarivory. American Entomologist 42: 82–86. [Google Scholar]
- Wang XR, Goulson D, Chen LZ, et al. 2020. Occurrence of neonicotinoids in Chinese apiculture and a corresponding risk exposure assessment. Environmental Science & Technology 54: 5021–5030. [DOI] [PubMed] [Google Scholar]
- Willmer PG. 1985. Size effects on the hygrothermal balance and foraging patterns of a sphecid wasp, Cerceris arenaria. Ecological Entomology 10: 469–479. [Google Scholar]
- Willmer P. 2011. Pollination and floral ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- Zhou HX, Milne RI, Ma XL, et al. 2018. Characterization of a l-gulono-1,4-lactone oxidase like protein in the floral nectar of Mucuna sempervirens, Fabaceae. Frontiers in Plant Science 9: 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.