Abstract
Background and Aims
The precise control of brassinosteroid (BR) homeostasis and signalling is a prerequisite for hypocotyl cell elongation in plants. Arabidopsis MYB42 and its paralogue MYB85 were previously identified to be positive regulators of secondary cell wall formation during mature stages. Here, we aim to reveal the role of MYB42 and MYB85 in hypocotyl elongation during the seedling stage and clarify how MYB42 coordinates BR homeostasis and signalling to regulate this process.
Methods
Histochemical analysis of proMYB42-GUS transgenic plants was used for determination of the MYB42 expression pattern. The MYB42, 85 overexpression, double mutant and some crossing lines were generated for phenotypic observation and transcriptome analysis. Transcription activation assays, quantitative PCR (qPCR), chromatin immunoprecipitation (ChIP)-qPCR and electrophoretic mobility shift assays (EMSAs) were conducted to determine the relationship of MYB42 and BRASSINAZOLE-RESISTANT 1 (BZR1), a master switch activating BR signalling.
Key Results
MYB42 and MYB85 redundantly and negatively regulate hypocotyl cell elongation. They function in hypocotyl elongation by mediating BR signalling. MYB42 transcription was suppressed by BR treatment or in bzr1-1D (a gain-of-function mutant of BZR1), and mutation of both MYB42 and MYB85 enhanced the dwarf phenotype of the BR receptor mutant bri1-5. BZR1 directly repressed MYB42 expression in response to BR. Consistently, hypocotyl length of bzr1-1D was increased by simultaneous mutation of MYB42 and MYB85, but was reduced by overexpression of MYB42. Expression of a number of BR-regulated BZR1 (non-)targets associated with hypocotyl elongation was suppressed by MYB42, 85. Furthermore, MYB42 enlarged its action in BR signalling through feedback repression of BR accumulation and activation of DOGT1/UGT73C5, a BR-inactivating enzyme.
Conclusions
MYB42 inhibits hypocotyl elongation by coordinating BR homeostasis and signalling during primary growth. The present study shows an MYB42, 85-mediated multilevel system that contributes to fine regulation of BR-induced hypocotyl elongation.
Keywords: Hypocotyl elongation, MYB transcription factor, BR homeostasis and signalling, feedback regulation, DOGT1/UGT73C5, Arabidopsis thaliana
INTRODUCTION
Brassinosteroids (BRs) are predominant hormones that promote hypocotyl cell elongation. Functional characterization of a subset of Arabidopsis mutants established the BR synthesis and signalling pathways (Kim and Wang, 2010; Oh et al., 2014; Nolan et al., 2020). The biologically most active BR is brassinolide (BL), which is produced from campesterol in plants. BR signalling through the membrane-localized BR INSENSITIVE1 (BRI1) receptor kinase and intracellular components induces dephosphorylation and accumulation of the central transcription factors BRASSINAZOLE-RESISTANT1 (BZR1) and BRI1-EMS SUPPRESSOR1 (BES1). The activated BZR1 and BES1 promote hypocotyl elongation by mediating regulation of a number of (non-)target genes associated with cell elongation (Sun et al., 2010; Yu et al., 2011). Also, BZR1 and BES1 directly suppress the expression of BR biosynthetic genes, including CPD, DWF4 and BR6OX2, to inhibit BR level. Currently, the mechanism for the integration of BR homeostasis and signalling remains unclear.
R2R3-MYB transcription factors (TFs) are ubiquitously found in plants, and some of its members mediate BR signalling to participate in diverse developmental processes (Dubos et al., 2010). In Arabidopsis, MYB30 is directly activated by BES1 and it also cooperates with BES1, coordinately promoting hypocotyl elongation (Li et al., 2009). BRASSINOSTEROIDS AT VASCULAR AND ORGANIZING CENTER (BRAVO) is directly suppressed by and physically interacts with BES1, forming a switch that regulates the divisions of the quiescent centre at the root stem cell niche (Vilarrasa-Blasi et al., 2014). WEREWOLF (WER, an MYB protein), GLABRA3 (GL3, a bHLH protein) and TRANSPARENT TESTA GLABRA1 (TTG1, a WD40 protein) form a transcriptional complex that is essential for BR-mediated control of root epidermal cell fate (Cheng et al., 2014). Phosphorylation of GL3 and TTG1 by the GSK3-like kinase BIN2 inhibits the activity of this complex. In soybean, overexpression of GmMYB14 reduces endogenous BR contents, increases high-density yield and improves drought tolerance (Chen et al., 2021).
Arabidopsis MYB42 and MYB85 are a pair of paralogous proteins, and they play a redundant role in regulating lignin and phenylpropanoid synthesis during secondary wall formation in stems (Zhong et al., 2008; Geng et al., 2020). In the present study, we have shown that during the young seedling stage MYB42 inhibits hypocotyl cell elongation by coordinating BR homeostasis and signalling. MYB42 functions as a negative component of the BZR1 pathway. Furthermore, MYB42 can enlarge its action in BR signalling through feedback repression of BR level and activation of DON-GLUCOSYLTRANSFERASE 1 (DOGT1), a BR-inactivating enzyme. Our results suggest that MYB42 might be a node in a transcriptional circuit by which BR regulates cell elongation-associated gene expression.
MATERIALS AND METHODS
Mutant identification and hypocotyl elongation assays
A DNA insertion mutant line for MYB42 (SALKseq_78190.1) (Geng et al., 2020) was ordered from the Arabidopsis Biological Resource Center. Plants were cultivated in a glasshouse at 23–25 °C and 65 % relative humidity, with a 16-h/8-h light/dark cycle.
For hypocotyl elongation assays, seeds were vernalizated on ½ MS medium at 4 °C and then transferred at 23 °C in darkness for 5 d or under dim light for 7 d. Thirty seedlings were measured using the ImageJ software and an independent experiment was repeated at least three times. The length of epidermal cells was quantified with an Olympus confocal laser scanning microscope (FluoView FV1000). At least 30 seedlings from three independent lines were used for analysis.
GUS staining
A 1.9-kb MYB42 promoter fragment was fused with β-glucuronidase (GUS) in the DX2181 vector. The resulting construct was transformed into wild-type (WT) plants by Agrobacterium tumefaciens (GV3101)-mediated floral-dip method. T0 transgenic seeds were screened on ½ MS containing 50 mg L–1 hygromycin and T3 homozygous seedlings were used for detection of GUS activity following our previous method (Chai et al., 2015).
Construction of recombinant vector and generation of transgenic plants
The coding regions of MYB42, MYB85 and DOGT1 were individually inserted into the pEARLY100 vector (Invitrogen) to create the overexpression constructs. A 359-bp cDNA fragment of MYB85 was inserted into pUCCRNAi in an inverted repeat orientation, forming a stem-loop structure. The MYB85 RNA interference (RNAi) construct was generated by introducing this stem-loop fragment into pCAMBIA 1300. The overexpression and RNAi constructs were transformed into WT or myb42 plants. The MYB85 RNAi;myb42 plants were crossed with bri1-5 (a loss-of-function mutant of BRI1), bzr1-1D (a gain-of-function mutant of BZR1) or DOGT1 overexpression lines (female parent). MYB42 overexpression plants were introduced into bzr1-1D or MYB85 RNAi;myb42 plants. Homozygous lines from F2 segregating populations were determined by sequencing, PCR and morphological observation.
Transcription activation assays
Transcription activation assays (TAAs) were carried out in Arabidopsis leaf protoplasts as described previously (Chai et al., 2015). A 1782-bp MYB42 promoter fragment was fused with the GUS gene in the modified pBI221 vector to generate the reporter construct. The coding region of BZR1 was inserted into the modified pBI221 vector to generate the effector construct. In each experiment, the 35S promoter-driven LUCIFERASE (LUC) reporter gene was used as a control to normalize the data. TAAs were conducted more than three times independently, and an average value was determined.
Electrophoretic mobility shift assays (EMSAs)
EMSAs were conducted as described by Chai et al. (2014). The coding region of BZR1 was fused to the MBP tag in pMAL-c4X and the recombinant protein was purified with amylose resin beads. A biotin-labelled synthetic oligonucleotide covering an E-box site (CAAGTG) in the MYB42 promoter was used as the probe (BGI, Supplementary Data Table S1). The E-box site is known to be recognized by BZR1 protein (Sun et al., 2010). Biotin-labelled DNA was detected by chemiluminescence according to the instructions provided by the manufacturer of the LightShift Chemiluminescent EMSA Kit (Thermo Fisher). EMSAs were performed at least three times independently.
Chromatin immunoprecipitation (ChIP)-qPCR
The ChIP samples were prepared from 10-d-old WT (control) and mx3 (pBZR1:mBZR-CFP) plants following the manufacturer’s instruction for the EZ CHIP Kit (Millipore). The amounts of precipitated DNA fragments were detected by quantitative PCR (qPCR) using gene-specific primers (Supplementary Data Table S1). ChIP enrichment was calculated as the ratio between BR-treated and control samples. ChIP-qPCR assays were conducted at least three times independently.
RNA-seq
WT and MYB85RNAi;myb42 seedlings were grown on ½ MS containing 0 or 0.02 µm propiconazole (PCZ, an alternative to the BR inhibitor brassinazole) for 5 d in darkness. Total RNAs were isolated from the hypocotyls using TRIzol reagent (Invitrogen). Three biological repeats were set. Each sample includes three independent lines with at least 100 plants. mRNA libraries were sequenced on an Illumina HiSequation 2500 platform (Annoroad Gene Technology). The reads were aligned to the Arabidopsis genome (version TAIR10) using TopHat2 with default parameters (Kim et al., 2013). Those genes with fragment per kilobase of exon per million (FPKM) < 1 were excluded from the analysis. Differentially expressed genes (DEGs) were determined based on the criterion of the |log2fold change| > 1 and false discovery rate (FDR) < 0.05.
qRT-PCR
qRT-PCR assay was completed with a SYBR Green Realtime PCR kit (Takara). The reaction was performed with a LightCycler 480 system (Roche) following the method described by Chai et al. (2015). ACTIN2 was used as a reference gene. The relative changes in gene expression were analysed using the 2−ΔΔCT method (Livak and Schmittgen, 2001). For each genotype, three biological replicates were used.
Quantification of BR
Approximately 6 g aerial plant parts per repeat were harvested from 4-week-old WT and six MYB42 overexpression lines grown in soil. The contents of BL and castasterone (CS) were quantified by liquid chromatography tandem mass spectrometry (LC-MS/MS) following a previously reported method (Ding et al., 2013) (Wuhan Greensword Creation Technology Company). For each genotype, three biological replicates were set.
RESULTS
MYB42 is highly expressed in the vascular tissues of seedling
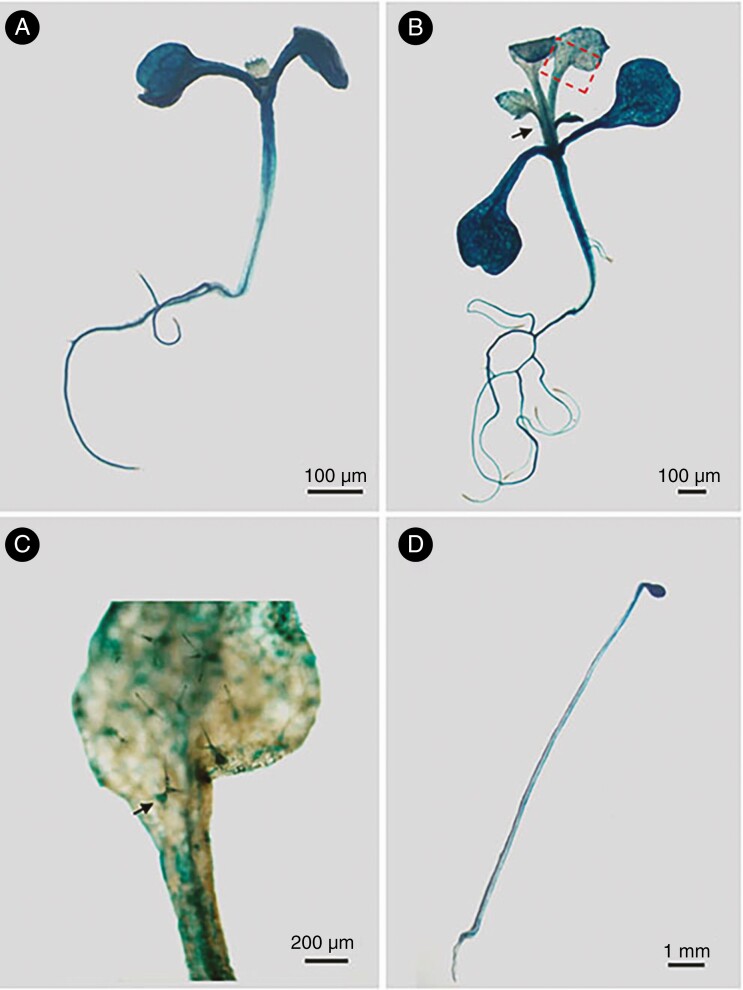
To investigate the tissue expression pattern of MYB42 during primary growth, we detected GUS activity in transgenic plants expressing proMYB42:GUS at the seedling stage. Strong GUS staining was visible in the cotyledons, hypocotyls and roots of 7-d-old transgenic seedlings (Fig. 1A). After 14 d of growth, GUS signals were also detected in the vasculature and trichome of true leaves, in addition to the above tissues (Fig. 1B, C). In darkness, strong GUS signals were observed in the elongated hypocotyls, unopened cotyledons and shortened roots of etiolated seedlings (Fig. 1D). These results revealed a potential role of MYB42 in the vascular tissues during the seedling stage.
Fig. 1.
Histochemical analysis of MYB42 expression during the seedling stage. (A–D) The expression patterns of MYB42 in 7-d-old (A) or 14-d-old (B and C) proMYB42-GUS plants under light, or in 5-d-old plants in darkness (D), detected by GUS reporter gene. The partial leaf region (red box) is enlarged in C. A stained trichome is shown by a black arrow.
MYB42 negatively controls hypocotyl elongation
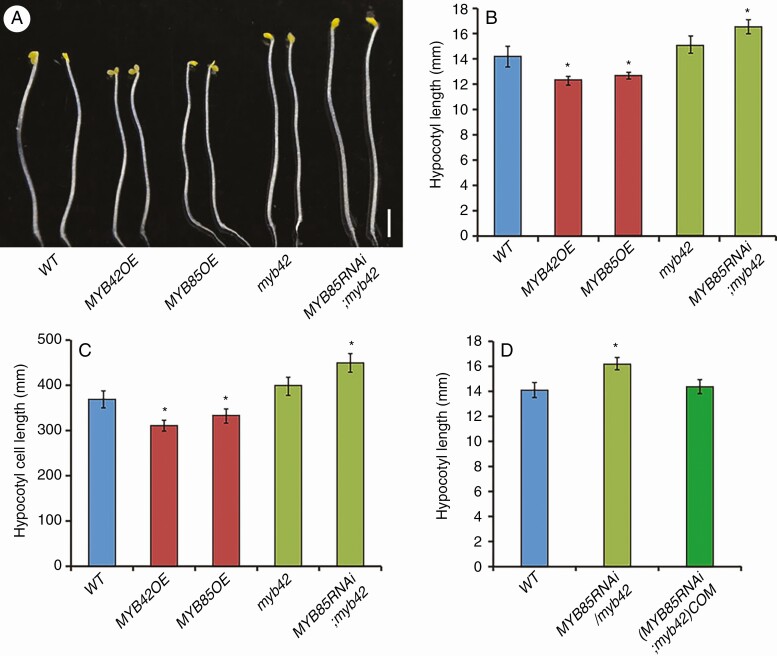
To identify the biological function of MYB42 during primary growth, we generated transgenic lines over-expressing MYB42 or its paralogue MYB85. MYB85 was used as a control because MYB42 and MYB85 redundantly regulate secondary wall formation in Arabidopsis stems (Zhong et al., 2008; Geng et al., 2020). At least 30 overexpression lines were obtained for each gene (Supplementary Data Fig. S1A, B). All these overexpression lines exhibited shorter hypocotyls and shortened hypocotyl epidermal cells in darkness, relative to WT seedlings (Fig. 2A–C; Fig. S2). This indicated the possible negative roles of MYB42 and MYB85 in regulating hypocotyl elongation.
Fig. 2.
MYB42 and its paralogue MYB85 redundantly inhibit hypocotyl cell elongation. (A–C) Hypocotyls of 5-d-old dark-grown wild-type (WT), MYB42/MYB85 overexpression, myb42 and MYB85 RNAi;myb42 plants. Similar phenotypes were observed among different lines of each genotype, and a representative line is shown. Bar = 50 μm. (D) Hypocotyl length of MYB85RNAi;myb42 plants was restored to the WT-like level by MYB42 overexpression. Statistical analysis was conducted from more than three lines for each transgenic genotype. Data are the means ± s.d. of 30–40 plants in each line. Student’s t-test, *P < 0.05.
To examine whether MYB42 is required for hypocotyl elongation, we identified a MYB42 T-DNA insertion line that was used previously (Geng et al., 2020). RT-PCR analysis of MYB42 transcription indicated that this mutant line is a knock-out allele (Supplementary Data Fig. S1C). Statistical analysis of hypocotyl length revealed no apparent difference between dark-grown myb42 and WT seedlings (Fig. 2A–C). To overcome the functional redundancy of MYB42 and MYB85, we generated RNAi transgenic plants of MYB85 in an myb42 background (Fig. S1D). Five-day-old MYB85RNAi;myb42 seedlings displayed longer hypocotyls and epidermal cells than WT seedlings in darkness (Fig. 2A–C; Fig. S2). This further confirmed the redundant and essential roles of MYB42/85 in controlling hypocotyl elongation. The hypocotyl length of MYB85RNAi;myb42 plants was restored to the WT-like level by overexpression of MYB42 (Fig. 2D), indicating that MYB42 overexpression is sufficient to inhibit hypocotyl elongation.
MYB42 functions as a positive component of the BR pathway
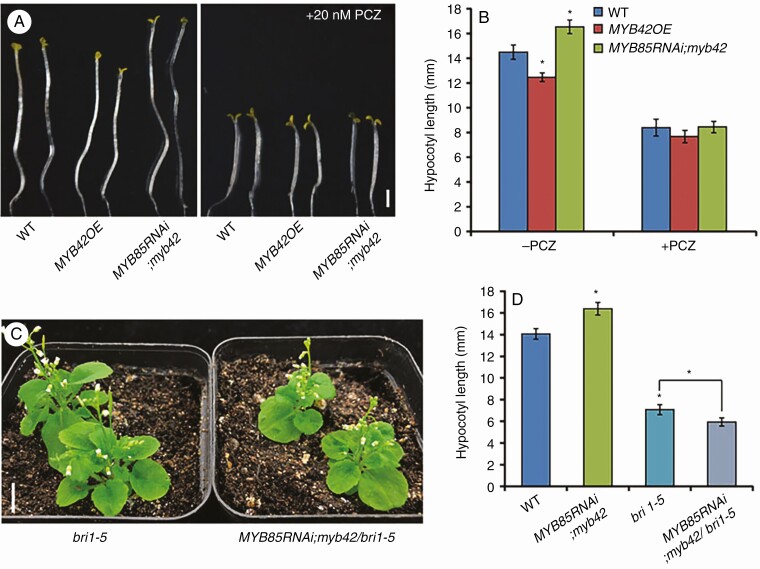
BRs are a group of predominant hormones promoting plant cell elongation (Nolan et al., 2020). To examine the association of MYB42-mediated inhibition of cell elongation with BR response, we first investigated whether 2,4-epibrassinolide (2,4-eBL) treatment affects MYB42 (MYB85 as control) transcription. The expression level of MYB42 in WT plants was rapidly decreased to 5 % of basal level at 1 h, and remained stable until 72 h (Supplementary Data Fig. S3A). A similar expression pattern was observed for MYB85 in response to BL treatment. We further detected the responses of WT, MYB42OE and MYB85RNAi;myb42 seedlings to PCZ, a BR biosynthesis inhibitor. With PCZ treatment, hypocotyl elongation of the three genotypes was inhibited to varying degrees (Fig. 3A, B). The strongest inhibition was observed in MYB85RNAi;myb42 plants (51.9 %), followed by WT plants (42.3 %) and the weakest inhibition in MYB42OE plants (37.5 %). All these results indicated that MYB42 may inhibit hypocotyl elongation by mediating BR signalling.
Fig. 3.
MYB42 is a positive component of the BR pathway. (A, B) Hypocotyls of 5-d-old WT, MYB42OE and MYB85RNAi;myb42 plants in darkness in the presence or absence of 0.02 µm propiconazole (PCZ, a BR biosynthesis inhibitor). (C) Five-week-old bri1-5 (a BRI1 mutant allele) and MYB85RNAi;myb42/bri1-5 plants in soil. (D) Hypocotyl length measurement of 5-d-old WT, MYB85RNAi;myb42, bri1-5 and MYB85RNAi;myb42/bri1-5 plants. Statistical analysis was conducted from more than three lines for each transgenic genotype. Data are the means ± s.d. of 30 plants in each line. t-test, *P < 0.05. Bar = 1 mm in A and 1 cm in C.
To determine the genetic interaction between MYB42 and the receptor BRI1 of BRs, we crossed MYB85RNAi;myb42 plants with the bri1-5 mutant that shows a semi-dwarf phenotype (Noguchi et al., 1999). Mutation of both MYB42 and MYB85 slightly enhanced the phenotype of bri1-5 on ½ MS or in soil (Fig. 3C, D; Supplementary Data Fig. S3B). MYB85RNAi;myb42/bri1-5 plants exhibited downward-curling cotyledons, shorter inflorescence stems and smaller leaves compared with bri1-5 under light, and they displayed visibly shorter hypocotyls in darkness. These results, combined with the PCZ responses of MYB42OE and MYB85RNAi;myb42 plants (Fig. 3B), indicated that MYB42 may function as a positive component of the BR pathway.
BR promotes BZR1 targeted MYB42 to control hypocotyl elongation
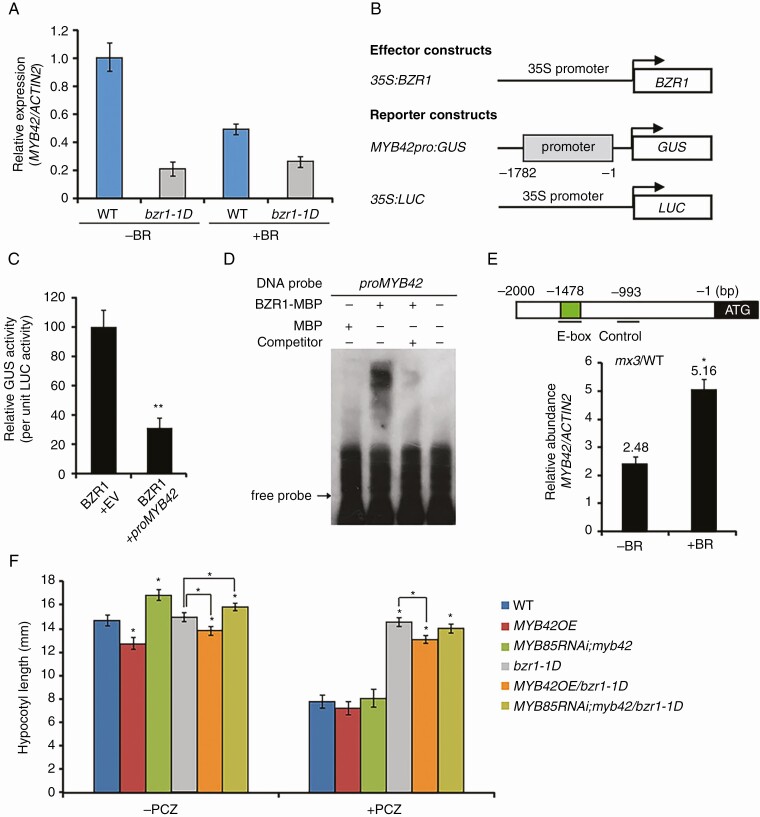
BZR1 promotes hypocotyl elongation by activating or suppressing the expression of a huge number of cell elongation-associated genes (Oh et al., 2014). To examine the relationship of BZR1 and MYB42, we first detected MYB42 transcription in WT (control) and bzr1-1D plants in the absence or presence of BR. The MYB42 expression level was 79 % lower in bzr1-1D than that in WT plants (Fig. 4A). When treated with 2,4-eBL, MYB42 expression was inhibited by 50 % in WT plants, but was not significantly altered in bzr1-1D. This was consistent with a previous description that bzr1-1D is insensitive to BRZ treatment in darkness (Wang et al., 2002). To validate the suppression of MYB42 expression by BZR1, transient expression assays were conducted in Arabidopsis leaf mesophyll protoplasts. Lower GUS activity was observed in the protoplasts transfected with 35S:BZR1 and MYB42pro:GUS than that in the protoplasts with 35S:BZR1 and empty vector (Fig. 4B, C). All these results indicated that BZR1 may repress MYB42 expression in BR signalling.
Fig. 4.
MYB42 expression is directly repressed by BRZ1 in BR signalling. (A) qRT-PCR showing MYB42 expression in 10-d-old WT and bzr1-1D plants in the absence or presence of 1 µm 24-eBL. (B, C) Transcription activity assays showing that BZR1 represses the MYB42 promoter-driven GUS expression in Arabidopsis leaf protoplasts. GUS activity in the protoplasts co-transformed with BZR1 and empty vector (EV) was set to 1. The expression of LUC was used as a control for normalization. Data are means ± s.d. of three independent experiments. (D) EMSA showing the specific binding of BZR1 to an E-box site (CAAGTG) in the MYB42 promoter in vitro. (E) ChIP-qPCR on 10-d-old WT and pBZR1:mBZR1-CFP (mx3) plants with or without 24-eBL showing that BR induction increased the binding of BZR1 to the E-box site in the MYB42 promoter in vivo. A non-binding site of BZR1 in the MYB42 promoter was used as a control for normalization. Data are the means ± s.d. of triplicate experiments. (F) Hypocotyl length measurement of 5-d-old dark-grown WT, MYB42OE, MYB85RNAi;myb42, bzr1-1D, MYB42OE/bzr1-1D and MYB85RNAi;myb42/bzr1-1D plants with or without PCZ treatment. Statistical analysis was conducted from more than three lines for each transgenic genotype. Data are the means ± s.d. of 30 seedlings in each line. t-test, *P < 0.05.
Analysis of the 2000-bp MYB42 promoter sequence showed an E-box site (CAAGTG) that is recognized by BZR1 (Sun et al., 2010), 1478 bp upstream of the translation initiation site. This promoted us to investigate whether BZR1 could recognize this E-box site in the MYB42 promoter. EMSA data revealed that MBP-BZR1 protein bound to a fragment from the MYB42 promoter that contained the E-box (Fig. 4D). Addition of the unlabelled promoter fragment efficiently competed with labelled probe, indicating a specific binding of BZR1 to this fragment in vitro. Using ChIP-qPCR assays, we demonstrated that BZR1 was significantly enriched at the E-box (−1478 bp) but not at control (−274 bp) in the promoter of MYB42 in mx3 (pBZR1:mBZR1-CFP) seedlings (Fig. 4E). BR treatment further enhanced the binding of BZR1 to this E-box site in the MYB42 promoter (Fig. 4E). These results indicated that MYB42 may be a negative target of BZR1 in BR signalling.
To determine whether BZR1-targeted MYB42 affects hypocotyl elongation in response to BR, we generated MYB42OE/bzr1-1D and MYB85 RNAi;myb42/bzr1-1D plants. No apparent difference in hypocotyl length was observed between bzr1-1D and the WT in darkness (Fig. 4F, left), which is consistent with the description by Wang et al. (2002). However, the hypocotyl length of bzr1-1D was reduced by overexpression of MYB42, and was increased by simultaneous mutation of MYB42 and MYB85 (Fig. 4F, left). The insensitivity of bzr1-1D to PCZ treatment was also weakened in the MYB85 RNAi;myb42 background (Fig. 4F, right). These results indicated that BZR1 promotes hypocotyl elongation probably by MYB42 and MYB85 in the BR pathway.
A number of BR-regulated BZR1 (non-)targets show altered expression levels in MYB85RNAi/myb42 plants with PCZ treatment
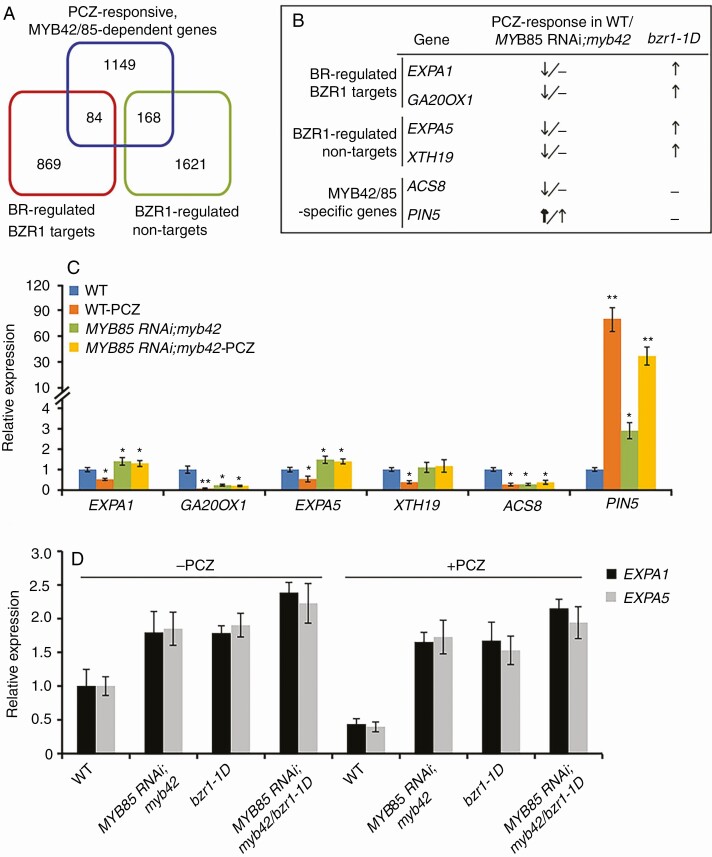
To explore the MYB42-mediated regulatory network in BR signalling, we performed RNA-sequencing (RNA-seq) analysis with 5-d-old dark-grown WT and MYB85RNAi;myb42 plants in the absence or presence of PCZ. Using the stringent criteria |log2FC| > 1 and FDR < 0.05, we obtained 1943 PCZ-responsive genes in WT plants and 1273 PCZ-responsive genes in MYB85RNAi;myb42 plants (Supplementary Data Table S2). A total of 1401 genes were defined as PCZ-responsive, MYB42/85-dependent genes based on the classification as follows: Group I, 1275 PCZ-responsive genes that were upregulated or downregulated in WT plants but not in MYB85RNAi;myb42 plants; and Group II, 126 genes with at least 1.5-fold higher or lower expression in MYB85RNAi;myb42 plants than in WT plants with PCZ treatment (Table S3).
Genes regulated by BR and BRZ (a BR biosynthetic inhibitor) show a strong inverse correlation (Li et al., 2009). To investigate how MYB42 mediates BZR1 signalling to regulate hypocotyl elongation, we compared 1401 PCZ-responsive, MYB42/85-dependent genes with 953 BR-regulated BZR1 targets or 1789 BZR1-regulated non-targets (Sun et al., 2010; Oh et al., 2012). In total, 8.8 % (84/953) of BZR1 targets and 9.4 % (168/1789) of BZR1-regulated non-targets were affected by MYB42/85 in BR signalling in darkness (Fig. 5A; Supplementary Data Table S4). Of these, we further analysed two BR-regulated BZR1 targets [EXPANSIN A1 (EXPA1) and GA20OX1], two BZR1-regulated non-targets [EXPA5 and XYLOGLUCAN ENDOTRANSGLUCOSYLASE/HYDROLASE 19 (XTH19)] and two MYB42/85-regulated specific genes [1-AMINOCYCLOPROPANE-1-CARBOXYLATE SYNTHASE (ACS8) and PIN-FORMED 5 (PIN5)] (Fig. 5B). EXPA5 and XTH19 participate in BR-induced hypocotyl elongation, and EXPA1 is essential for hypocotyl elongation (Park et al., 2010; Miedes et al., 2013; Ilias et al., 2017; Xu et al., 2020). PIN5 is an auxin efflux carrier, and GA20OX1 and ACS8 are responsive for the biosynthesis of gibberellin (GA) and ethylene, respectively. Auxin, GA and ethylene are known to interact with BRs to regulate hypocotyl cell elongation (Peres et al., 2019). In our RNA-seq data, the expression levels of EXPA1, EXPA5, XTH19, GA20OX1 and ACS8 were markedly reduced and PIN5 expression level was increased in WT plants when treated with PCZ (Fig. 5B). This is consistent with a previous description that expression levels of EXPA1, EXPA5, XTH19 or GA20OX1 are higher in bzr1-1D than in WT plants (Oh et al., 2012). Moreover, in the MYB85RNAi;myb42 background, PCZ-repressed expression of EXPA1, EXPA5, XTH19, GA20OX1 and ACS8 and PCZ-activated expression of PIN5 were largely attenuated, as was further confirmed by qRT-PCR assays (Fig. 5B, C). Collectively, these results indicated that MYB42 and BZR1 coordinately control hypocotyl elongation probably by regulating expression of a number of common genes.
Fig. 5.
MYB42 influences BZR1 regulation of cell elongation-associated targets and non-targets. (A) Venn diagram of PCZ-responsive MYB42/85-dependent genes and BR-regulated BZR1 targets or BZR1-regulated non-targets. PCZ-responsive MYB42/85-dependent genes are shown in Supplementary Data Table S3. (B) Partial cell elongation-associated genes from A. (C) Expression of six genes (B) in 5-d-old WT and MYB85RNAi;myb42 plants in darkness with or without PCZ treatment. t-test, *P < 0.05; **P < 0.01. (D) Expression of EXPA1 and EXPA5 in 5-d-old WT, MYB85RNAi;myb42, bzr1-1D and MYB85RNAi;myb42/bzr1-1D plants in darkness with or without PCZ treatment. For qRT-PCR, data are expressed as the means ± s.d. from three biological replicates.
To determine how MYB42 affects BZR1-mediated regulation of hypocotyl elongation, we detected the expression levels of EXPA1 and EXPA5, two BZR1-induced genes, in PCZ-treated or untreated WT, MYB85RNAi;myb42, bzr1-1D and MYB85RNAi;myb42/bzr1-1D plants. As shown in Fig. 5D, expression levels of EXPA1 and EXPA5 were higher in bzr1-1D or MYB85RNAi;myb42 plants than in WT plants, and such expression in MYB85RNAi;myb42 plants was enhanced by introduction into bzr1-1D. With PCZ treatment, expression levels of EXPA1 and EXPA5 were reduced by 50 % in WT plants, but showed slight reductions in bzr1-1D, MYB85RNAi;myb42 and MYB85RNAi;myb42/bzr1-1D plants. These results suggested that MYB42 inhibits hypocotyl elongation possibly by partially inhibiting BZR signalling.
MYB42 involves feedback regulation of BR homeostasis
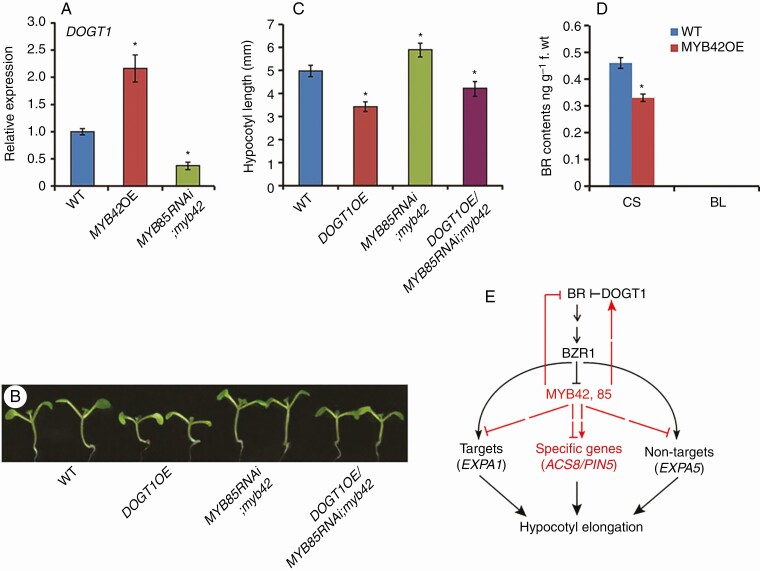
PCZ-responsive, MYB42/85-dependent genes contained DOGT1/UGT73C5 (Supplementary Data Table S2), which encodes an enzyme glucosylating BRs and reducing the activity of BRs (Poppenberger et al., 2005). This promoted us to investigate whether MYB42 involves feedback regulation of BR activity during hypocotyl growth. Compared with WT plants, the expression level of DOGT1 was reduced in MYB85 RNAi;myb42 plants but increased in MYB42OE plants (Fig. 6A). This indicated that MYB42 may up-regulate DOGT1 expression. To test the association of MYB42-inhibited hypocotyl elongation with DOGT1, we generated transgenic plants over-expressing DOGT1 (DOGT1OE) and then introduced the representative overexpression lines into MYB85 RNAi;myb42 plants. The DOGT1OE plants, like BR-deficient mutants, showed shortened stems and hypocotyls, small leaves, and partial fertility (Fig. 6B, C; Fig. S4). These growth defects of DOGT1 overexpression plants were also described previously (Poppenberger et al., 2005). Statistical analysis of hypocotyl length in DOGT1OE seedlings was performed on experiments under dim light, because overexpression of DOGT1 inhibits hypocotyl elongation under light, but not in darkness (Poppenberger et al., 2005). The results revealed that mutation of both MYB42 and MYB85 in DOGT1OE plants resulted in an apparent increase in both sterility and length of stems and hypocotyls (Fig. 6B, C; Fig. S4). These results indicated that MYB42/85 inhibits hypocotyl elongation possibly by activation of the BR-inactivating enzyme DOGT1.
Fig. 6.
MYB42 involves feedback regulation of BR homeostasis. (A) qRT-PCR showing DOGT1 expression levels in 10-d-old WT, MYB42OE and MYB85RNAi;myb42 plants. (B, C) Hypocotyl length of 7-d-old WT, DOGT1OE, MYB85 RNAi;myb42 and DOGT1OE/MYB85RNAi;myb42 plants under dim light. Statistical analysis was conducted from more than three lines for each transgenic genotype. Data are the means ± s.d. of 30 plants in each line. (D) Levels of BRs (CS, castasterone; BL, brassinolide) in WT and MYB42OE plants detected by LC-MS/MS analysis; f.wt, fresh weight. BL was not detected in either genotype. (E) A model of MYB42 and MYB85 function in BR-induced cell elongation. MYB42, 85 may enlarge their action in BR signalling and cell elongation by a feedback loop. The pathway identified in this study is shown with red. In A, C and D, t test, *P < 0.05.
We further investigated whether overexpression of MYB42 affects endogenous BR levels. BR contents were detected in aerial plant parts of six MYB42OE lines (WT as a control) by LC-MS/MS according to the method described previously (Poppenberger et al., 2005; Husar et al., 2011). As shown in Fig. 6D, the level of CS (the direct precursor of BL) was reduced in MYB42OE plants in comparison to WT plants. BL was below the limit of detection in both genotypes, similar to a previous description (Husar et al., 2011). Together, these data indicated that MYB42 may inhibit BR homeostasis by a feedback loop.
DISCUSSION
MYB42 and MYB85 play dual roles in primary and secondary cell wall formation
Primary cell walls (capable of expanding in area) and secondary cell walls (incapable of expanding) have different cellular locations and polysaccharide compositions (Srivastava et al., 2017). Primary cell walls are more easily deconstructed due to their flexible features, while secondary cell walls are largely recalcitrant to microbial degradation. Generally, the formation of primary and secondary walls is regulated by different systems. Several recent studies in Arabidopsis have shown the dual roles of TFs in regulating the two types of cell walls. Transgenic plants overexpressing the group IIId and IIIe AP2/ERF TFs in mutants lacking secondary walls show thickened cell wall characteristics of primary cell walls (Sakamoto et al., 2018). The homeodomain TF KNAT7 mediates the regulation of primary and secondary wall formation in seeds and stems, respectively (Li et al., 2011; Qin et al., 2020; Wang et al., 2020a, b).
In this study, we identified MYB42 and MYB85 as another regulator of primary and secondary wall formation. During mature stages, MYB42 and MYB85 are down-regulated by SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN1 (SND1), a first-layer master switch activating secondary wall biosynthesis in Arabidopsis stems (Zhong et al., 2008). The dominant repressor of MYB85 has thinner fibre cell walls, while overexpression of MYB85 causes ectopic accumulation of lignin in cortical and epidermal cells (Zhong et al., 2008). Furthermore, MYB42, 85 and other two homologues MYB20, 43 redundantly regulate lignin biosynthesis and they also directly activate the expression of MYB4, specifically inhibiting flavonoid biosynthesis (Geng et al., 2020).
During seedling stages, MYB42 and MYB85 redundantly inhibit hypocotyl elongation. The myb42 single mutant exhibited a similar phenotype to WT plants, but a reduced level of MYB85 in myb42 resulted in a significant increase in hypocotyl length compared with WT plants. MYB42 overexpression was sufficient to inhibit hypocotyl cell elongation in WT plants and to restore the hypocotyl length of MYB85 RNAi;myb42 to the WT level. Therefore, MYB42 and MYB85, like KNAT7, may regulate hypocotyl elongation and secondary wall biosynthesis in different pathways at different developmental stages.
MYB42 inhibits hypocotyl elongation by coordinating BR homeostasis and signalling
BRs are steroidal phytohormones that are essential for hypocotyl cell elongation (Oh et al., 2014; Nolan et al., 2020). Currently, it remains unclear about the transcriptional regulation of BR homeostasis and signalling. Here, we have demonstrated that MYB42 is capable of partially inhibiting BZR1 signalling and promoting BR inactivation. Genetic and biochemical data revealed that MYB42 was a negative target of BZR1 and acted as a positive component of the BR pathway. Consistently, the hypocotyl length of bzr1-1D was increased in MYB85 RNAi;myb42 plants, but reduced in MYB42 overexpression plants in terms of genetics. Comparison of transcriptome data showed that 18.2 % of BZR1-regulated genes were affected by MYB42/85 in BR signalling, of which some cell elongation-associated genes were down-regulated by MYB42, implying a partial inhibition of BZR1-induced hypocotyl elongation by MYB42.
In contrast to our understanding of feedback repression of BR biosynthetic genes by BZR1, the mechanisms underlying BR inactivation are largely unclear. It is known that DOGT1/UGT73C5 mediates the glucosylation of BL and CS at the 23-OH position and causes BR-deficient phenotypes (Poppenberger et al., 2005). Our current results revealed that MYB42 activated DOGT1 expression and mutation of both MYB42 and MYB85 attenuated DOGT1-mediated inhibition of hypocotyl elongation. Furthermore, the CS level was reduced in MYB42 overexpression lines compared with WT plants. Thus, it is possible that MYB42, 85 enlarge their action in BR signalling through feedback activation of DOGT1 expression and repression of BR accumulation. Based on our observations, we propose that MYB42 and MYB85 might function in hypocotyl elongation by coordinating BR homeostasis and signalling (Fig. 6D). These results provide novel insight into the gene network of BR-induced hypocotyl elongation.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Fig. S1: Identification of MYB42 or MYB85 overexpression, myb42, and MYB85 RNAi;myb42 plants. Fig. S2: Hypocotyl epidermal cells of 5-d-old dark-grown WT, MYB42 or MYB85 overexpression, and MYB85RNAi;myb42 plants. Fig. S3: MYB42 and MYB85 are associated with BR signalling. Fig. S4: Phenotypes of 8-week-old WT, DOGT1OE and DOGT1OE/MYB85RNAi;myb42 plants. Table S1: Primers used in this study. Table S2: Differentially expressed genes in wild-type or MYB85RNAi/myb42 plants in response to PCZ. Table S3: 1401 PCZ-responsive MYB42/85-dependent genes. Table S4: Overlap between PCZ-responsive MYB42/85-dependent genes and BR-regulated BZR1 targets.
ACKNOWLEDGMENTS
We thank Professor Ming-yi Bai (Shandong University, China) for providing the bri1-5, bzr1-1D and mx3 seeds.
Contributor Information
Yamei Zhuang, College of Resources and Environment, Qingdao Agricultural University, Qingdao, China; Qingdao Institute of BioEnergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao, China.
Wenjun Lian, College of Resources and Environment, Qingdao Agricultural University, Qingdao, China.
Xianfeng Tang, Qingdao Institute of BioEnergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao, China.
Guang Qi, State Key Laboratory of Wheat and Maize Crop Science and College of Agronomy, Henan Agricultural University, Zhengzhou, China.
Dian Wang, College of Agronomy, Qingdao Agricultural University, Qingdao, China.
Guohua Chai, College of Resources and Environment, Qingdao Agricultural University, Qingdao, China.
Gongke Zhou, College of Resources and Environment, Qingdao Agricultural University, Qingdao, China.
FUNDING
Financial support was obtained from the National Natural Science Foundation of China (31770315, 31972955, 31972860 and 32101549), Taishan Scholar Program of Shandong (tsqn202103092) and ‘First Class Grassland Science Discipline’ Program of Shandong Province.
CONFLICT OF INTEREST
The authors declare that they have no no competing interests.
LITERATURE CITED
- Chai G, Qi G, Cao Y, et al. 2014. Poplar PdC3H17 and PdC3H18 are direct targets of PdMYB3 and PdMYB21, and positively regulate secondary wall formation in Arabidopsis and poplar. The New Phytologist 203: 520–534. [DOI] [PubMed] [Google Scholar]
- Chai G, Kong Y, Zhu M, et al. 2015. Arabidopsis C3H14 and C3H15 have overlapping roles in the regulation of secondary wall thickening and anther development. Journal of Experimental Botany 66: 2595–2609. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang H, Fang Y, et al. 2021. Overexpression of GmMYB14 improves high-density yield and drought tolerance of soybean through regulating plant architecture mediated by the brassinosteroid pathway. Plant Biotechnology Journal 19: 702–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Zhu W, Chen Y, Ito S, Asami T, Wang X. 2014. Brassinosteroids control root epidermal cell fate via direct regulation of a MYB-bHLH-WD40 complex by GSK3-like kinases. Elife 3: e02525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Mao LJ, Wang ST, Yuan BF, Feng YQ. 2013. Determination of endogenous brassinosteroids in plant tissues using solid-phase extraction with double layered cartridge followed by high-performance liquid chromatography-tandem mass spectrometry. Phytochemical Analysis: PCA 24: 386–394. [DOI] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis. Trends in Plant Science 15: 573–581. [DOI] [PubMed] [Google Scholar]
- Geng P, Zhang S, Liu J, et al. 2020. MYB20, MYB42, MYB43, and MYB85 regulate phenylalanine and lignin biosynthesis during secondary cell wall formation. Plant Physiology 182: 1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husar S, Berthiller F, Fujioka S, et al. 2011. Overexpression of the UGT73C6 alters brassinosteroid glucoside formation in Arabidopsis thaliana. BMC Plant Biology 11: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilias IA, Airianah OB, Baharum SN, Goh HH. 2017. Transcriptomic data of Arabidopsis thaliana hypocotyl upon suppression of expansin genes. Genomics Data 12: 132–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Wang ZY. 2010. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annual Review of Plant Biology 61: 681–704. [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. 2013. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biology 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Wang S, Liu Y, Chen JG, Douglas CJ. 2011. OVATE FAMILY PROTEIN4 (OFP4) interaction with KNAT7 regulates secondary cell wall formation in Arabidopsis thaliana. The Plant Journal: for Cell and Molecular Biology 67: 328–341. [DOI] [PubMed] [Google Scholar]
- Li L, Yu X, Thompson A, et al. 2009. Arabidopsis MYB30 is a direct target of BES1 and cooperates with BES1 to regulate brassinosteroid-induced gene expression. The Plant Journal: for Cell and Molecular Biology 58: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedes E, Suslov D, Vandenbussche F, et al. 2013. Xyloglucan endotransglucosylase/hydrolase (XTH) overexpression affects growth and cell wall mechanics in etiolated Arabidopsis hypocotyls. Journal of Experimental Botany 64: 2481–2497. [DOI] [PubMed] [Google Scholar]
- Nolan TM, Vukašinović N, Liu D, Russinova E, Yin Y. 2020. Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. The Plant Cell 32: 295–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Choe S, et al. 1999. Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiology 121: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu JY, Wang ZY. 2012. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nature Cell Biology 14: 802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu J, Bai M, Arenhart RA, Sun Y, Wang Z. 2014. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife 3: e03031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Kim TW, Son SH, et al. 2010. Brassinosteroids control AtEXPA5 gene expression in Arabidopsis thaliana. Phytochemistry 71: 380–387. [DOI] [PubMed] [Google Scholar]
- Peres A, Soares JS, Tavares RG, et al. 2019. Brassinosteroids, the sixth class of phytohormones: a molecular view from the discovery to hormonal interactions in plant development and stress adaptation. International Journal of Molecular Sciences 20: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, Calif.) 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Poppenberger B, Fujioka S, Soeno K, et al. 2005. The UGT73C5 of Arabidopsis thaliana glucosylates brassinosteroids. Proceedings of the National Academy of Sciences of the United States of America 102: 15253–15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yin Q, Chen J, et al. 2020. The class II KNOX transcription factors KNAT3 and KNAT7 synergistically regulate monolignol biosynthesis in Arabidopsis. Journal of Experimental Botany 71: 5469–5483. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Somssich M, Nakata MT, et al. 2018. Complete substitution of a secondary cell wall with a primary cell wall in Arabidopsis. Nature Plants 4: 777–783. [DOI] [PubMed] [Google Scholar]
- Srivastava V, McKee LS, Bulone V. 2017. Plant cell walls. Chichester: eLS. Wiley. [Google Scholar]
- Sun Y, Fan XY, Cao DM, et al. 2010. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Developmental Cell 19: 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarrasa-Blasi J, González-García MP, Frigola D, et al. 2014. Regulation of plant stem cell quiescence by a brassinosteroid signaling module. Developmental Cell 30: 36–47. [DOI] [PubMed] [Google Scholar]
- Wang S, Yamaguchi M, Grienenberger E, Martone PT, Samuels AL, Mansfield SD. 2020. The Class II KNOX genes KNAT3 and KNAT7 work cooperatively to influence deposition of secondary cell walls that provide mechanical support to Arabidopsis stems. The Plant Journal: for Cell and Molecular Biology 101: 293–309. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu Y, Pei S, et al. 2020. KNAT7 regulates xylan biosynthesis in Arabidopsis seed-coat mucilage. Journal of Experimental Botany 71: 4125–4139. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Nakano T, Gendron J, et al. 2002. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Developmental Cell 2: 505–513. [DOI] [PubMed] [Google Scholar]
- Xu P, Fang S, Chen H, Cai W. 2020. The brassinosteroid-responsive xyloglucan endotransglucosylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. The Plant Journal: for Cell and Molecular Biology 104: 59–75. [DOI] [PubMed] [Google Scholar]
- Yu X, Li L, Zola J, et al. 2011. A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. The Plant Journal: for Cell and Molecular Biology 65: 634–646. [DOI] [PubMed] [Google Scholar]
- Zhong R, Lee C, Zhou J, McCarthy RL, Ye ZH. 2008. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. The Plant Cell 20: 2763–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.