Abstract
Coagulase-negative staphylococci are commensals that are known to be prevalent in most environments, and they are also an important reservoir of antimicrobial-resistant genes. Staphylococcal infections in animal husbandry are a high economic burden. Thus, we aimed to determine the prevalence and species diversity of methicillin-resistant coagulase-negative staphylococci (MRCoNS) in poultry slaughtered for human consumption and to study the antimicrobial resistance of the isolates. Swab samples were recovered from 220 commercial chickens, homebred chickens and quails. Species identification was performed using MALDI-TOF. Antimicrobial susceptibility testing was performed by the disc diffusion method against 14 antimicrobials. The presence of antimicrobial-resistant genes was investigated by polymerase chain reaction. Totals of 11 (19.6%), 13 (20.3%), and 51 (51%) MRCoNS were isolated from commercial chickens, homebred chickens and quails, respectively. S. lentus was isolated from all homebred chickens, whereas 11 S. lentus and 2 S. urealyticus were isolated from commercial chickens. As for quails, the most prevalent MRCoNS were S. urealyticus. Almost all isolates had a multidrug-resistant profile and carried the mecA gene. Most isolates showed resistance to erythromycin, clindamycin, penicillin, tetracycline, ciprofloxacin and fusidic acid and harbored the ermA, ermB, ermC, mphC tetK, tetL, tetM and tetO genes. This study showed a frequent occurrence of multidrug resistance in MRCoNS isolated from healthy poultry in Portugal.
Keywords: coagulase-negative Staphylococcus, CoNS, antimicrobial resistance, poultry, quails, broilers
1. Introduction
Staphylococci colonize the skin and mucous membranes of humans and are considered commensals or opportunistic pathogens [1]. By 2018, 45 species and 24 subspecies of Staphylococcus had been described [2]. Staphylococci are divided into two groups, coagulase-positive (CoPS) and coagulase-negative staphylococci (CoNS), according to their ability to coagulate plasma. CoPS are pathogenic species which have the coagulase enzyme that converts plasma fibrinogen into fibrin [3]. CoNS lack this enzyme and were considered, until recently, to be minor pathogens or apathogenic [4]. CoNS possess fewer virulence factors that participate in the pathogenesis of infection when compared to CoPS, such as S. aureus, but, in the last few decades, CoNS have emerged as common causes of nosocomial infections [4]. Within the CoNS species, S. epidermidis, S. haemolyticus and S. saprophyticus are examples of the most significant types of CoNS in human infections [5]. As opportunistic pathogens, CoNS generally cause infection in colonized immunocompromised individuals, patients with catheters and prosthetic implants, dialysis and oncologic patients and neonates [6]. CoNS are responsible for a broad spectrum of infections, such as invasive endocarditis, bacteremia and bone infections [6,7]. In addition, increasing rates of antibiotic resistance have been detected in CoNS, in some cases even greater than for S. aureus, which limits the therapeutic options available [5]. Methicillin resistance in CoNS is usually due to the expression of the mecA gene, which encodes an alternative binding protein 2a (PBP2a) that has a low affinity for β-lactam antibiotics, although some studies have reported the presence the mecC gene, a homologue of mecA [8,9,10]. The mec genes are located on a mobile genetic element called the Staphylococcal Cassette Chromosome mec (SCCmec). SCCmec elements are more diverse in methicillin-resistant CoNS when compared to S. aureus, and many SCCmec elements could not be typed using multiplex PCR [10]. Tetracycline resistance is also frequently detected in different CoNS species [11].
CoNS also colonize and infect other mammals besides humans, with S. chromogenes, S. simulans and S. xylosus being the principal cause of infection [11]. CoNS are frequently responsible for arthritis, cow mastitis and, less often, systemic infections in animals [12]. The presence of CoNS has been reported in pets, livestock and wild animals [13,14,15]. It has been shown that food of animal origin can carry CoNS and other foodborne pathogens and, besides being able to cause infection, CoNS can also cause food poisoning [16]. Both CoPS and CoNS have been associated with avian pathologies such as arthritis, osteomyelitis, pododermatitis, septicemia and blepharitis [17,18]. Nevertheless, the presence of CoPS and CoNS has also been observed in healthy poultry and poultry meat, which may act as reservoirs and vehicles of zoonotic pathogens and antimicrobial resistance [16,19]. The spread of antimicrobial resistance among commensal CoNS in healthy poultry may represent a hazard for human and animal health [11]. Studies reporting the monitorization of antimicrobial-resistant pathogens in poultry and poultry meat have been published, but most studies focus only on S. aureus species [20,21,22,23,24]. The prevalence of antimicrobial-resistant pathogens in poultry, particularly staphylococci, may be due to their high consumption of antimicrobials. According to the ESVAC report, in Portugal the population-weighted mean consumption (expressed in milligrams per kilogram of estimated biomass) of antimicrobials was 175.8 mg/Kg in food-producing animals in 2020 [25]. In Portugal, the biomass-corrected consumption of third- and fourth-generation cephalosporins, quinolones, penicillin, macrolides and tetracyclines in food-producing animals was around 0.4, 7.3, 38.9, 20 and 60.4 mg/Kg [25]. Furthermore, all these antimicrobial classes were used in poultry production. Therefore, we aimed to investigate the presence of methicillin-resistant CoNS (MRCoNS) in healthy poultry for human consumption as well as the antimicrobial-resistant phenotypes and genotypes of the isolates.
2. Results
In this study, the presence of methicillin-resistant CoNS (MRCoNS) was detected in 71 (32.3%) of the 220 birds tested (Table 1). The co-carriage of two different species was identified in four animals, and 67 birds carried only one staphylococcal species. Co-carriage of MRCoNS species was identified only among quail samples, and the pattern of co-carriage was as follows: Staphylococcus sciuri/S. urealyticus (n = 2), Staphylococcus lentus/S. urealyticus and Staphylococcus lentus/Staphylococcus haemolyticus. A total of 75 MRCoNS were recovered and identified as S. lentus (n = 26), S. urealyticus (n = 21), S. sciuri (n = 15) and S. haemolyticus (n = 3). S. haemolyticus was exclusively isolated from quails. Chickens, both commercial and homebred, were mainly colonized by S. lentus, while S. urealyticus was the most frequently detected species in quails, followed by S. lentus. Quails were colonized significantly more frequently by MRCoNS than homebred chickens. Furthermore, the prevalence of S. lentus and S. urealyticus was significantly higher than that of S. haemolyticus. Results of the prevalence of each staphylococcal species are shown in Supplementary Figure S1.
Table 1.
Number of animals sampled, frequency and diversity of CoNS species detected among healthy poultry.
| Animal | Number of Animals Sampled | Number of CoNS Carriers (%) | Isolates Recovered | S. lentus | S. urealyticus | S. sciuri | S. haemolyticus |
|---|---|---|---|---|---|---|---|
| Quails | 100 | 47 (47) | 51 | 15 | 19 | 14 | 3 |
| Commercial chickens | 50 | 13 (26) | 13 | 11 | 2 | - | - |
| Homebred chickens | 70 | 11 (15.7) | 11 | 10 | - | 1 | - |
| Total | 220 | 71 (32.3) | 75 | 36 | 21 | 15 | 3 |
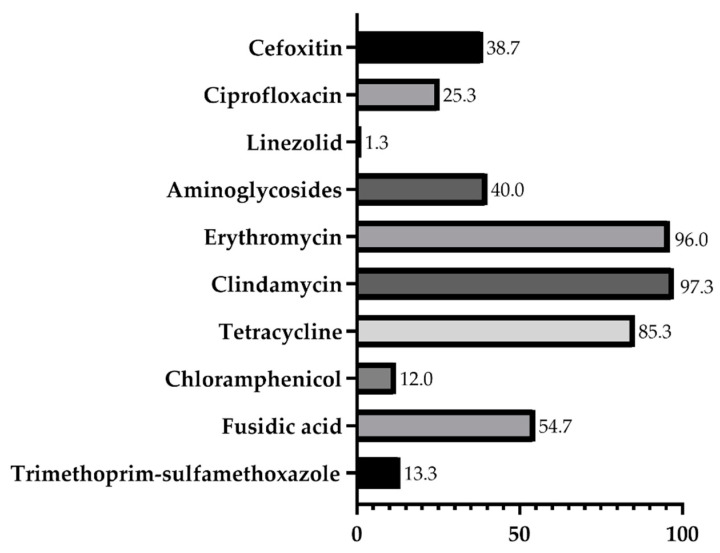
Table 2 shows the antimicrobial-resistant phenotypes and genotypes of MRCoNS, while the detailed characterization of each isolate is summarized in Supplementary Table S1. The percentage of resistance to each antibiotic is shown in Figure 1. All isolates showed phenotypic and genotypic resistance to antibiotics, with 73 (97.3%) isolates displaying a multidrug-resistant profile since they showed resistance to at least three different classes of antimicrobials. The multidrug-resistance pattern was as follows: 15 (20%) isolates were resistant to 3 classes, 27 (26%) to 4 classes, 17 (22.7%) to 5 classes, 12 (16%) to 6 classes and 2 (2.7%) to 7 classes of antimicrobials. The non-multiresistant isolates were both S. lentus and were isolated from chickens. Both isolates showing resistance to seven antimicrobial classes were isolated from quails. The mecA gene was detected in all isolates, including those that were susceptible to cefoxitin. Totals of 11 S. lentus, 21 S. urealyticus, 14 S. sciuri and 3 S. haemolyticus were phenotypically resistant to penicillin, but the mechanism of penicillin resistance could not be identified. Resistance to aminoglycosides was detected in 40% of the isolates and was mediated by the aph(3′)-IIIa, ant(4′)-Ia and str genes in different combinations. All S. lentus and S. urealyticus were resistant to macrolides and lincosamides, while 14 S. sciuri and 2 S. haemolyticus showed resistance to this antimicrobial class. Macrolide-lincosamide resistant isolates harbored the ermA, ermB, ermC and mphC genes alone or in different combinations: ermB (n = 5); ermC (n = 11); mphC (n = 3); ermC and mphC (n = 27); ermA, ermC and mphC (n = 6); ermB, ermC and mphC (n = 10); ermB and mphC (n = 8); ermA and ermC (n = 1); ermA, ermB, ermC and mphC (n = 1); and ermA, ermB and mphC (n = 1). Tetracycline resistance, which was detected in all S. urealyticus, S. sciuri and S. haemolyticus, and in 25 (69.4%) S. lentus, was mediated by the tetK, tetL, tetM and/or tetO genes. The tetL gene was the most frequent, followed by the tetK. The catp194 encoding resistance to chloramphenicol was detected in one S. lentus isolate. Resistance to trimethoprim-sulfamethoxazole was detected in 10 isolates. Some S. lentus isolates harbored a combination of dfrK and dfrD genes, while S. sciuri and S. haemolyticus carried only the dfrK. One S. sciuri exhibited resistance to linezolid, mediated by the cfr gene. None of the isolates showed resistance to vancomycin.
Table 2.
Antimicrobial-resistant genes identified among the CoNS isolated from poultry.
| Species | Number of Isolates | Antimicrobial Resistance | |
|---|---|---|---|
| Phenotype | Genotype | ||
| S. lentus | 36 | PEN11, FOX4, CIP11, CN2, TOB14, KAN9, ERY35, CD36, TET25, C4, FD12, SXT6 | mecA36, ermA8, ermB8, ermC28, mphC29, aph(3′)-IIIa9, ant(4′)-Ia12, str2, tetL19, tetK14, tetO1, tetM2, catp1941, dfrK6, dfrD2 |
| S. urealyticus | 21 | PEN21, FOX18, CIP3, CN4, TOB6, KAN5, ERY21, CD21, TET21, C3, FD17 | mecA21, ermA1, ermB7, ermC19, mphC16, aph(3′)-IIIa5, ant(4′)-Ia2, str2, tetL17, tetK18, tetO13, tetM4 |
| S. sciuri | 15 | PEN14, FOX6, LNZ1, CIP3, TOB8, KAN4, ERY14, CD14, TET15, C2, FD10, SXT2 | mecA15, cfr1, ermB9, ermC7, mphC9, aph(3′)-IIIa3, ant(4′)-Ia7, str1, tetL11, tetK12, tetO2, tetM3, dfrK1 |
| S. haemolyticus | 3 | PEN3, FOX1, CIP2, TOB2, KAN1, ERY2, CD2, TET3, FD2, SXT2 | mecA3, ermB1, ermC2, mphC2, aph(3′)-IIIa2, ant(4′)-Ia1, str1, tetL3, tetK1, dfrK1 |
Abbreviations. C: chloramphenicol; CD: clindamycin; CIP: ciprofloxacin; ERY: erythromycin; FD, fusidic acid; FOX: cefoxitin; PEN: penicillin; SXT: trimethoprim-sulfamethoxazole; TET: tetracycline; CN: gentamicin; KAN: kanamycin; TOB: tobramycin; LNZ: linezolid. Note: the superscript number after each antibiotic and gene indicates the number of strains showing resistance to that antibiotic and harboring that gene, respectively.
Figure 1.
Percentage of resistance to each antibiotic by MRCoNS isolated from poultry.
3. Discussion
MRCoNS in livestock was first reported in healthy chickens in Japan in 1996. Despite the increasing interest in CoNS in recent years, there is very limited information on their prevalence and resistance profiles in poultry production, and information is even more limited regarding MRCoNS. In our study, we investigated the presence of MRCoNS in healthy quails and commercial and homebred chickens. Among the 220 birds tested, 71 (32.3%) carried at least one CoNS, which is in accordance with the results obtained by Marek et al. [26]. CoNS colonized 47% and 20% of the quails and chickens, respectively. This carriage frequency was higher than the one obtained by Younis et al., who found a prevalence of CoNS in quails and chickens of 8.75% and 7.14%, respectively [27]. A study conducted with turkey samples found a frequency of CoNS of 15.6%, which is also lower than the one obtained in this study [28]. Other studies found a higher frequency of CoNS in poultry [18,29]. Nevertheless, it is important to point out that in our study all samples were only screened for the presence of MRCoNS, which may have contributed to a higher frequency of CoNS. Furthermore, some studies focused only on diseased animals that would most likely have been discarded in the slaughterhouse and would not have reached the final consumer. In our study, only four different species of CoNS were detected: S. lentus (n = 26), S. urealyticus (n = 21), S. sciuri (n = 15) and S. haemolyticus (n = 3). The predominant CoNS species found in our study included those commonly found in skin microbiota in chickens [29,30]. The occurrence of the staphylococci species among poultry samples appears to vary widely. Pyzik et al. detected a high number of CoNS species in diseased broiler chickens and turkeys, with S. cohnii being the most frequent followed by S. saprophyticus and S. epidermidis [29]. In accordance with our results, Saha et al. found a higher occurrence of S. lentus in poultry samples [30]. Boamah et al. reported a frequency of 42.97% S. sciuri, 35.94% S. lentus, 4.30% S. xylosus, 3.91%, S. haemolyticus 3.91%, 1.95% S. saprophyticus and 0.39% S. cohnii [31]. A study conducted in Brazil found that most CoNS from chickens were S. gallinarum followed by S. simulans [18]. In a report by El-Nagar et al., the majority of CoNS were S. xylosus [32]. Marek et al. found a higher occurrence of S. epidermidis in poultry in Poland [26]. Finally, S. hominis followed by S. xylosus and S. lentus were the most frequently detected species in quail eggs [33]. Yet, most studies have reported the presence of S. sciuri, S. lentus and S. cohnii. It has been shown that some species of CoNS, such as S. sciuri, S. xylosus or S. cohnii, are considered important poultry pathogens, particularly when associated with antimicrobial resistance [29]. Furthermore, most of these CoNS species are considered an issue of meat safety rather than the classical poultry pathogens [29].
The most common species found among poultry in this study was S. lentus. This species is considered an animal pathogen and has been detected among livestock, pets, wild animals and retail meats [13,16,34,35]. Nevertheless, S. lentus has also been responsible for a wide range of human infections and its clinical relevance seems to be increasing [36]. S. urealyticus was the second most common CoNS species found in poultry and it was mostly detected in quail samples. This CoNS species has been regarded as a commensal organism and is not usually involved in severe infections [37]. S. urealyticus strains of animal origin were shown to have multiple phenotypic resistances and carry several antimicrobial resistance genes [38]. All CoNS isolated in this study harbored the mecA gene, and the methicillin resistance of the isolates was confirmed. However, most S. lentus and S. sciuri isolates were phenotypically susceptible to cefoxitin. It has been shown that the staphylococcal species belonging to the S. sciuri group, which include S. sciuri, S. fleurettii, S. lentus, S. stepanovicii and S. vitulinus, carry a close homologue to the mecA gene, which does not confer resistance to β-lactam antibiotics [39]. Accordantly, almost all S. urealyticus had phenotypic resistance to cefoxitin. Multidrug resistance was exhibited in almost all isolates, which is in accordance with other studies conducted with poultry samples [27,28,29]. Although the European Union banned the use of antibiotics for growth promotion in livestock in 2006, and several other measures have been taken since then, it is estimated that over 60% of all antimicrobials produced are used in livestock comprising poultry [40]. Higher resistance levels were detected among quails, including two isolates resistant to seven antimicrobial classes, which may be explained by the fact that in Portugal the legislation for antibiotics administration in quails is not as well-regulated as that for other poultry, such as chickens; thus, antibiotics may be administrated indiscriminately to quails, leading to an increase in antimicrobial resistance [20]. Only one isolate, S. sciuri, was resistant to linezolid and carried the cfr gene. This gene was first detected in a bovine S. sciuri [41]. Although uncommon, resistance to linezolid mediated by the cfr gene is worrisome, since this gene confers cross-resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins and streptogramin A antibiotics [42,43]. Studies reporting the cfr gene in poultry identified it in S. lentus, S. urealyticus, S. arlettae. sciuri and S. simulans [39,44,45]. Furthermore, a low frequency of this gene has been reported in CoNS from poultry [39]. Resistance to macrolides and lincosamides was detected in all isolates, except for one S. sciuri and one S. haemolyticus, and it was mediated by the ermA, ermB, ermC and mphC genes. Both ermC and mphC genes were carried by 56 isolates. Phosphotransferases are encoded by the mphC gene which confers resistance to erythromycin and other macrolides but not to lincosamides [46]. Nevertheless, the erm genes confer cross-resistance to macrolides, lincosamides and streptogramins B [46]. Although the ermA and ermC genes are the most frequent erm genes in staphylococci, the ermA gene was only detected in the S. lentus and S. urealyticus isolates, while ermB was identified in all MRCoNS species in this study. Other studies reported similar results for the frequency of erm genes in poultry [28,39]. A study by Syed et al. investigated the resistance of staphylococci in poultry intestines and reported a lower frequency of resistance to macrolides and lincosamides, but the ermC gene was also the most prevalent [47]. In the same study, resistance to tetracycline was detected in more than half of the isolates encoded by the tetK and tetM genes [47]. In our study, resistance to tetracycline was detected in 85.3% of the isolates, including all S. sciutri, S. urealyticus and S. haemolyticus, and in 25 out of 36 S. lentus, which was similar to the findings of other studies [28,31,48]. The high frequency of tetracycline resistance in poultry samples may be due to the fact that, according to the ECDC/EFSA/EMA report, tetracycline and penicillin were the most prescribed antibiotics for food-producing animals in 2017 [49]. Among the genes that confer resistance to tetracycline, tetL (n = 50) was the most prevalent, followed by tetK (n = 45), tetO (n = 16) and tetM (n = 9). Similar results were obtained by Lee et al. in a study that investigated the tet genes in poultry meat [16]. In contrast, in a study by Nemeghaire et al. tetM was the most common gene among S. sciuri from healthy chickens [39]. However, due to the lack of studies investigating the prevalence of resistant genes in CoNS from poultry, it is difficult to make a direct comparison. Fusidic acid was detected in 54.6% of the isolates but none of the resistance genes tested were found, which suggests the presence of other resistant genes. Indeed, in a study by Chen et al. none of the fusidic acid-resistant S. urealyticus possessed fusB, fusC or fusD genes; instead, S. urealyticus isolates carried the novel fusF gene, which seems to be an intrinsic factor in S. urealyticus and may not be conserved in another subspecies [50]. Resistance to vancomycin was not detected in this study, which was unsurprising since vancomycin-resistant staphylococci are rare and, as far as we know, in Portugal there is only one study reporting a vancomycin intermediate-resistant S. aureus isolated from a human infection [51].
In general, penicillin and tetracycline are extensively used for the treatment of staphylococcal infections in poultry [52]. In our study, we also found higher levels of resistance to those antimicrobial agents. The ingestion of poultry meat contaminated with staphylococci may lead to food poisoning. Furthermore, the handling or ingesting of staphylococci contaminated meat is a potential risk factor for colonization by methicillin-resistant staphylococci [53]. Our findings show that the frequency of multidrug-resistant staphylococci in poultry is alarming and may represent a public health problem.
4. Materials and Methods
4.1. Sample Collection and Bacterial Isolates
During the month of February 2020, a total of 220 samples were collected from poultry in a Portuguese slaughterhouse. Swab samples were collected from the cloaca and trachea of 100 quails, 50 commercial chickens and 70 homebred chickens. Batches of quails, homebred and commercial chickens arrived at the slaughterhouse 3 days a week and around 36,000 quails, 3500 homebred and 8000 commercial chickens were slaughtered each day. Four samples were recovered from each batch. The swabs were inserted into tubes containing brain heart infusion (BHI) broth with 6.5% of NaCl and incubated at 37 °C under aerobic conditions for 24 h. The inoculum was then seeded onto ORSAB agar plates supplemented with 2 mg/mL of oxacillin, incubated at 37 °C and examined after 24 h to 48 h. Up to three colonies per plate with different colors and morphology were recovered and further investigated. The staphylococci species identification was performed by matrix-assisted laser desorption/ionization time-of-flight coupled to time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, Bremen, Germany) as described by Dubois et al. [54].
4.2. Phenotypic Antibiotic Resistance Testing
Antibiotic susceptibility profiles were determined for all of isolates by the Kirby–Bauer disc diffusion method on Mueller Hinton agar. The tested antibiotics included: cefoxitin (30 μg), chloramphenicol 132 (30 μg), ciprofloxacin (5 μg), clindamycin (2 μg), erythromycin (15 μg), fusidic acid (10 133 μg), gentamicin (10 μg), kanamycin (30 μg), linezolid (10 μg), mupirocin (200 μg), penicillin (1 U), tetracycline (30 μg), tobramycin (10 μg), and trimethoprim/sulfamethoxazole 135 (1.25/23.75 μg). The diameter of the inhibition zones was measured for each antibiotic disk and recorded in millimeters. The interpretation of results followed the recommendations given in the European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2019 guidelines with the exception of kanamycin that followed the Clinical and Laboratory Standards Institute (CLSI) 2017 recommendations. The minimal inhibitory concentrations (MICs) of vancomycin were determined by a standard broth microdilution method in sterile 96-well microplates according to the EUCAST guidelines. Briefly, bacterial suspension was adjusted to 0.5 McFarland standards and then diluted 1:20. Then, 50 µL of Mueller–Hinton broth, 50 μL of the antibiotic dilutions, and 5 μL of the inoculum were mixed and incubated at 37 °C for 24 h. Isolates showing a vancomycin MIC ≤ 4 µg/mL were considered susceptible and those showing an MIC > 4 µg/mL were classified as resistant. The reference strain S. aureus ATCC 25923 was used for quality control.
4.3. DNA Extraction
DNA extraction was performed as previously described. Briefly, 2 staphylococci colonies were suspended in 45 μL of Milli-Q water and 5 μL of lysostaphin (1 mg/mL) was added. The samples were incubated at 37 °C for 10 min, after which 45 μL of Milli-Q water, 150 μL of Tris-HCl (0.1 M) and 5 μL of proteinase K (2 mg/mL) were added. After 10 min of incubation at 67 °C, the samples were boiled at 100 °C for 5 min. The DNA was stored at −20 °C until use. The spectrophotometric quantification of DNA was carried out through the NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA, USA) [55].
4.4. Antimicrobial-Resistant Genes
The presence of antimicrobial-resistant genes was investigated in each isolate according to the phenotypic resistance. The detection of the following antimicrobial-resistant genes was performed in a ProFlexTM PCR system (Applied Biosystems, Waltham, MA, USA): beta-lactams (blaZ, mecA and mecC), linezolid (cfr), aminoglycosides (aac(6′)-aph(2″), aph(3′)-IIIa, ant(4′)-Ia and str), macrolides and lincosamide (ermA, ermB, ermC, ermT, msr(A/B), mphC, lnuA, lnuB, vgaA and vgaB), tetracycline (tetK, tetM, tetL and tetO), chloramphenicol (fexA, fexB, catpC194, catpC221 and catpC223), fusidic acid (fusB, fusC and fusD) and trimethoprim/sulfamethoxazole (dfrA, dfrG, dfrK and dfrD). The protocol used for DNA amplification was as follows: a final volume of 50 µL contained 39.7 µL of ultra-pure water, 5 µL 10× complete buffer (Bioron, Römerberg, Germany), 1 µL 25 mM MgCl2, 1 µL deoxynucleotides triphosphate, 1 µL of each primer, 0.3 µL DFS Taq DNA polymerase (Bioron) and 1 µL DNA sample at 10 pg/µL. Primer sequences and PCR programs for the same are given in Table S2. The concentration and purity of the extracted DNA was measured using a spectrophotometer and Nano-DropTM software (Thermo ScientificTM, Waltham, MA, USA). Positive and negative controls used in all the experiments belonged to the strain collection of the University of Trás-os-Montes and Alto Douro.
4.5. Statistical Analysis
Pearson’s chi-square test was used compare the carriage of S. sciuri, S. lentus, S. urealyticus and S. haemolyticus between the quails, the homebred chickens and the commercial chickens. The analyses were carried out using IBM SPSS Statistics, Version 26.0 (IBM Corp., Armonk, NY, USA) and significance was set at p ≤ 0.05.
5. Conclusions
MRCoNS are common bacteria found in healthy poultry in Portugal. S. urealyticus seems to be more prevalent in quails, while broiler chickens are more often colonized by S. lentus, indicating a separate epidemiology. The high frequency of MRCoNS isolates in this study may be due to the fact that these bacteria are colonizers of the normal skin flora of animals. However, the multidrug resistance found in almost all isolates indicates that MRCoNS in poultry may be an important reservoir of antimicrobial-resistant genes. This is of great concern for public health, since most antimicrobial resistances detected were antimicrobials commonly used in human medicine. Some measures to overcome antimicrobial resistance in poultry in Portugal should be taken into consideration, such as the education of poultry producers, limiting the availability of antibiotics and the application of strict legislation concerning antimicrobial prescription.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics11030365/s1: Table S1: Antimicrobial-resistant phenotype and genotype and SCCmec typing of CoNS isolated from poultry. Table S2: Primer pairs used for molecular typing and detection of antimicrobial resistance genes in MRSA strains. Figure S1: Prevalence of each staphylococci specie in poultry samples. References [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, V.S., M.V.-P. and P.P.; methodology, V.S.; validation, V.S., M.C. and P.P.; investigation, V.S.; resources, M.V.-P. and C.S.; data curation, V.S. and E.F.; writing—original draft preparation, V.S.; writing—review and editing, V.S., M.C. and P.P.; visualization, J.E.P.; supervision, J.L.C., G.I. and P.P.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the R&D Project CAREBIO2: Comparative assessment of antimicrobial resistance in environmental biofilms through proteomics—towards innovative theranostic biomarkers, with reference NORTE-01-0145-FEDER-030101 and PTDC/SAU-INF/30101/2017, financed by the European Regional Development Fund (ERDF) through the Northern Regional Operational Program (NORTE 2020) and the Foundation for Science and Technology (FCT). This work was supported by the Associate Laboratory for Green Chemistry-LAQV, which is financed by national funds from FCT/MCTES (UIDB/50006/2020 and UIDP/50006/2020) and by the projects UIDB/CVT/00772/2020 and LA/P/0059/2020 funded by the Portuguese Foundation for Science and Technology (FCT). Vanessa Silva is grateful to FCT (Fundacão para a Ciência e a Tecnologia) for financial support through the PhD grant SFRH/BD/137947/2018.
Institutional Review Board Statement
The study was conducted according to the Helsinki Declaration (ICH-GCP principles), compliance with Schedule Y/ICMR Guidelines, the Oviedo Convention, and approved by the Ethics Committee of the University of Trás-os-Montes e Alto Douro (EC-UTAD, 8 November 2019).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parlet C.P., Brown M.M., Horswill A.R. Commensal staphylococci influence Staphylococcus aureus skin colonization and disease. Trends Microbiol. 2019;27:497–507. doi: 10.1016/j.tim.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gherardi G., Di Bonaventura G., Savini V. Pet-to-Man Travelling Staphylococci. Academic Press; Cambridge, MA, USA: 2018. Chapter 1—Staphylococcal Taxonomy; pp. 1–10. [Google Scholar]
- 3.Smith J.T., Andam C.P. Extensive horizontal gene transfer within and between species of coagulase-negative Staphylococcus. Genome Biol. Evol. 2021;13:evab206. doi: 10.1093/gbe/evab206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heilmann C., Ziebuhr W., Becker K. Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 2019;25:1071–1080. doi: 10.1016/j.cmi.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Becker K., Heilmann C., Peters G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michalik M., Samet A., Podbielska-Kubera A., Savini V., Międzobrodzki J., Kosecka-Strojek M. Coagulase-negative staphylococci (CoNS) as a significant etiological factor of laryngological infections: A review. Ann. Clin. Microbiol. Antimicrob. 2020;19:1–10. doi: 10.1186/s12941-020-00367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noshak M.A., Rezaee M.A., Hasani A., Mirzaii M. The role of the coagulase-negative staphylococci (CoNS) in infective endocarditis; a narrative review from 2000 to 2020. Curr. Pharm. Biotechnol. 2020;21:1140–1153. doi: 10.2174/1389201021666200423110359. [DOI] [PubMed] [Google Scholar]
- 8.MacFadyen A.C., Harrison E.M., Drigo I., Parkhill J., Holmes M.A., Paterson G.K. A mecC allotype, mecC3, in the CoNS Staphylococcus caeli, encoded within a variant SCCmecC. J. Antimicrob. Chemother. 2019;74:547–552. doi: 10.1093/jac/dky502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loncaric I., Kübber-Heiss A., Posautz A., Ruppitsch W., Lepuschitz S., Schauer B., Feßler A.T., Krametter-Frötscher R., Harrison E.M., Holmes M.A., et al. Characterization of mecC gene-carrying coagulase-negative Staphylococcus spp. isolated from various animals. Vet. Microbiol. 2019;230:138–144. doi: 10.1016/j.vetmic.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Zong Z., Peng C., Lü X. Diversity of SCCmec Elements in Methicillin-Resistant Coagulase-Negative Staphylococci Clinical Isolates. PLoS ONE. 2011;6:e20191. doi: 10.1371/journal.pone.0020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chajęcka-Wierzchowska W., Zadernowska A., Nalepa B., Sierpińska M., Łaniewska-Trokenheim Ł. Coagulase-negative staphylococci (CoNS) isolated from ready-to-eat food of animal origin—Phenotypic and genotypic antibiotic resistance. Food Microbiol. 2015;46:222–226. doi: 10.1016/j.fm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Argemi X., Hansmann Y., Prola K., Prévost G. Coagulase-negative staphylococci pathogenomics. Int. J. Mol. Sci. 2019;20:1215. doi: 10.3390/ijms20051215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva V., Pereira J.E., Maltez L., Ferreira E., Manageiro V., Caniça M., Capelo J.L., Igrejas G., Poeta P. Diversity of methicillin-resistant staphylococci among wild Lepus granatensis: First detection of mecA-MRSA in hares. FEMS Microbiol. Ecol. 2019;96:fiz204. doi: 10.1093/femsec/fiz204. [DOI] [PubMed] [Google Scholar]
- 14.Roberts M.C., Garland-Lewis G., Trufan S., Meschke S.J., Fowler H., Shean R.C., Greninger A.L., Rabinowitz P.M. Distribution of Staphylococcus species in dairy cows, workers and shared farm environments. FEMS Microbiol. Lett. 2018;365:fny146. doi: 10.1093/femsle/fny146. [DOI] [PubMed] [Google Scholar]
- 15.Suepaul S., Georges K., Unakal C., Boyen F., Sookhoo J., Ashraph K., Yusuf A., Butaye P. Determination of the frequency, species distribution and antimicrobial resistance of staphylococci isolated from dogs and their owners in Trinidad. PLoS ONE. 2021;16:e0254048. doi: 10.1371/journal.pone.0254048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S.I., Kim S.D., Park J.H., Yang S.-J. Species Distribution, Antimicrobial Resistance, and Enterotoxigenicity of Non-aureus Staphylococci in Retail Chicken Meat. Antibiotics. 2020;9:809. doi: 10.3390/antibiotics9110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huynh M., Carnaccini S., Driggers T., Shivaprasad H.L. Ulcerative Dermatitis and Valvular Endocarditis Associated with Staphylococcus aureus in a Hyacinth Macaw (Anadorhynchus hyacinthinus) Avian Dis. 2014;58:223–227. doi: 10.1637/10690-101413-Reg.1. [DOI] [PubMed] [Google Scholar]
- 18.Pimenta R.L., de Melo D.A., Bronzato G.F., Souza V.R.d.S., Holmström T.C.N., de Mattos de Oliveira Coelho S., de Silva Coelho I., de Souza M.M.S. Characterization of Staphylococcus spp. isolates and β-lactam resistance in broiler chicken production. Braz. J. Vet. Med. 2021;43:e00720. doi: 10.29374/2527-2179.bjvm000720. [DOI] [Google Scholar]
- 19.Bhargava K., Zhang Y. Characterization of methicillin-resistant coagulase-negative staphylococci (MRCoNS) in retail meat. Food Microbiol. 2014;42:56–60. doi: 10.1016/j.fm.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Silva V., Vieira-Pinto M., Saraiva C., Manageiro V., Reis L., Ferreira E., Caniça M., Capelo J.L., Igrejas G., Poeta P. Prevalence and Characteristics of Multidrug-Resistant Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) CC398 Isolated from Quails (Coturnix Coturnix Japonica) Slaughtered for Human Consumption. Animals. 2021;11:2038. doi: 10.3390/ani11072038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernier-Lachance J., Arsenault J., Usongo V., Parent É., Labrie J., Jacques M., Malouin F., Archambault M. Prevalence and characteristics of Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) isolated from chicken meat in the province of Quebec, Canada. PLoS ONE. 2020;15:e0227183. doi: 10.1371/journal.pone.0227183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y., Larsen J., Kjeldgaard J., Andersen P.S., Skov R., Ingmer H. Methicillin-resistant and -susceptible Staphylococcus aureus from retail meat in Denmark. Int. J. Food Microbiol. 2017;249:72–76. doi: 10.1016/j.ijfoodmicro.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Okorie-Kanu O.J., Anyanwu M.U., Ezenduka E.V., Mgbeahuruike A.C., Thapaliya D., Gerbig G., Ugwuijem E.E., Okorie-Kanu C.O., Agbowo P., Olorunleke S., et al. Molecular epidemiology, genetic diversity and antimicrobial resistance of Staphylococcus aureus isolated from chicken and pig carcasses, and carcass handlers. PLoS ONE. 2020;15:e0232913. doi: 10.1371/journal.pone.0232913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bala H.K., Igwe J.C., Olayinka B.O., Olonitola O.S., Onaolapo J.A., Okafo C.N. Antibiotic susceptibility profile of Staphylococcus aureus isolated from healthy chickens in poultry farms in Kano state, Nigeria. Sky J. Microbiol. Res. 2016;4:42–46. [Google Scholar]
- 25.European Medicines Agency European Surveillance of Veterinary Antimicrobial Consumption . ‘Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020′ EMA/58183/2021. Publications Office of the European Union; Luxembourg: 2021. [Google Scholar]
- 26.Marek A., Pyzik E., Stępień-Pyśniak D., Dec M., Jarosz Ł.S., Nowaczek A., Sulikowska M. Biofilm-Formation Ability and the Presence of Adhesion Genes in Coagulase-Negative Staphylococci Isolates from Chicken Broilers. Animals. 2021;11:728. doi: 10.3390/ani11030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Younis W., Sabra M., Sayed H.H. Occurrence and characterization of coagulase positive and negative Staphylococci isolated from Japanese quails and broiler chickens at Qena Governorate, Egypt. SVU-Int. J. Vet. Sci. 2021;4:1–15. doi: 10.21608/svu.2021.92987.1146. [DOI] [Google Scholar]
- 28.Moawad A.A., Hotzel H., Awad O., Roesler U., Hafez H.M., Tomaso H., Neubauer H., El-Adawy H. Evolution of Antibiotic Resistance of Coagulase-Negative Staphylococci Isolated from Healthy Turkeys in Egypt: First Report of Linezolid Resistance. Microorganisms. 2019;7:476. doi: 10.3390/microorganisms7100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pyzik E., Marek A., Stępień-Pyśniak D., Urban-Chmiel R., Jarosz Ł.S., Jagiełło-Podębska I. Detection of antibiotic resistance and classical enterotoxin genes in coagulase-negative staphylococci isolated from poultry in Poland. J. Vet. Res. 2019;63:183. doi: 10.2478/jvetres-2019-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saha O., Rakhi N.N., Istiaq A., Islam I., Sultana M., Hossain M.A., Rahaman M.M. Evaluation of Commercial Disinfectants against Staphylococcus lentus and Micrococcus spp. of Poultry Origin. Vet. Med. Int. 2020;2020:8811540. doi: 10.1155/2020/8811540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boamah V.E., Agyare C., Odoi H., Adu F., Gbedema S.Y., Dalsgaard A. Prevalence and antibiotic resistance of coagulase-negative Staphylococci isolated from poultry farms in three regions of Ghana. Infect. Drug Resist. 2017;10:175–183. doi: 10.2147/IDR.S136349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Nagar S., Abd El-Azeem M.W., Nasef S.A., Sultan S. Prevalence of toxigenic and methicillin resistant staphylococci in poultry chain production. J. Adv. Vet. Res. 2017;7:33–38. [Google Scholar]
- 33.Pyzik E., Marek A. Characterization of bacteria of the genus Staphylococcus isolated from the eggs of Japanese quail (Coturnix japonica) Pol. J. Vet. Sci. 2012;15:791–792. doi: 10.2478/v10181-012-0116-1. [DOI] [PubMed] [Google Scholar]
- 34.Ruzauskas M., Couto N., Kerziene S., Siugzdiniene R., Klimiene I., Virgailis M., Pomba C. Prevalence, species distribution and antimicrobial resistance patterns of methicillin-resistant staphylococci in Lithuanian pet animals. Acta Vet. Scand. 2015;57:27. doi: 10.1186/s13028-015-0117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhaouadi S., Soufi L., Campanile F., Dhaouadi F., Sociale M., Lazzaro L., Cherif A., Stefani S., Elandoulsi R.B. Prevalence of meticillin-resistant and -susceptible coagulase-negative staphylococci with the first detection of the mecC gene among cows, humans and manure in Tunisia. Int. J. Antimicrob. Agents. 2020;55:105826. doi: 10.1016/j.ijantimicag.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Wu C., Zhang X., Liang J., Li Q., Lin H., Lin C., Liu H., Zhou D., Lu W., Sun Z., et al. Characterization of florfenicol resistance genes in the coagulase-negative Staphylococcus (CoNS) isolates and genomic features of a multidrug-resistant Staphylococcus lentus strain H29. Antimicrob. Resist. Infect. Control. 2021;10:9. doi: 10.1186/s13756-020-00869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seni J., Mshana S.E., Msigwa F., Iddi S., Mazigo H., Parkhill J., Holmes M.A., Paterson G.K. Draft genome sequence of a multidrug-resistant caprine isolate of Staphylococcus cohnii subsp. urealyticus from Tanzania encoding ermB, tet(K), dfrG, fusF and fosD. J. Glob. Antimicrob. Resist. 2019;18:163–165. doi: 10.1016/j.jgar.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Lienen T., Schnitt A., Hammerl J.A., Marino S.F., Maurischat S., Tenhagen B.-A. Multidrug-resistant Staphylococcus cohnii and Staphylococcus urealyticus isolates from German dairy farms exhibit resistance to beta-lactam antibiotics and divergent penicillin-binding proteins. Sci. Rep. 2021;11:6075. doi: 10.1038/s41598-021-85461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemeghaire S., Argudín M.A., Haesebrouck F., Butaye P. Molecular epidemiology of methicillin-resistant Staphylococcus sciuri in healthy chickens. Vet. Microbiol. 2014;171:357–363. doi: 10.1016/j.vetmic.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 40.Agyare C., Boamah V.E., Zumbi C.N., Osei F.B. Antimicrobial Resistance—A Global Threat. IntechOpen; London, UK: 2018. Antibiotic use in poultry production and its effects on bacterial resistance; pp. 33–50. [Google Scholar]
- 41.Schwarz S., Werckenthin C., Kehrenberg C. Identification of a plasmid-borne chloramphenicol-florfenicol resistance gene in Staphylococcus sciuri. Antimicrob. Agents Chemother. 2000;44:2530–2533. doi: 10.1128/AAC.44.9.2530-2533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wendlandt S., Shen J., Kadlec K., Wang Y., Li B., Zhang W.-J., Feßler A.T., Wu C., Schwarz S. Multidrug resistance genes in staphylococci from animals that confer resistance to critically and highly important antimicrobial agents in human medicine. Trends Microbiol. 2015;23:44–54. doi: 10.1016/j.tim.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Schoenfelder S.M.K., Dong Y., Feßler A.T., Schwarz S., Schoen C., Köck R., Ziebuhr W. Antibiotic resistance profiles of coagulase-negative staphylococci in livestock environments. Vet. Microbiol. 2017;200:79–87. doi: 10.1016/j.vetmic.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., He T., Schwarz S., Zhao Q., Shen Z., Wu C., Shen J. Multidrug resistance gene cfr in methicillin-resistant coagulase-negative staphylococci from chickens, ducks, and pigs in China. Int. J. Med. Microbiol. 2013;303:84–87. doi: 10.1016/j.ijmm.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 45.He T., Wang Y., Schwarz S., Zhao Q., Shen J., Wu C. Genetic environment of the multi-resistance gene cfr in methicillin-resistant coagulase-negative staphylococci from chickens, ducks, and pigs in China. Int. J. Med. Microbiol. 2014;304:257–261. doi: 10.1016/j.ijmm.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Petinaki E. Resistance of Staphylococci to Macrolides-Lincosamides-Streptogramins B (MLSB): Epidemiology and Mechanisms of Resistance. In: Hemeg H., Ozbak H., Afrin F., editors. Staphylococcus Aureus. IntechOpen; Rijeka, Croatia: 2019. [Google Scholar]
- 47.Syed M.A., Ullah H., Tabassum S., Fatima B., Woodley T.A., Ramadan H., Jackson C.R. Staphylococci in poultry intestines: A comparison between farmed and household chickens1. Poult. Sci. 2020;99:4549–4557. doi: 10.1016/j.psj.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamed E.A., Abdelaty M.F., Sorour H.K., Roshdy H., AbdelRahman M.A.A., Magdy O., Ibrahim W.A., Sayed A., Mohamed H., Youssef M.I., et al. Monitoring of Antimicrobial Susceptibility of Bacteria Isolated from Poultry Farms from 2014 to 2018. Vet. Med. Int. 2021;2021:6739220. doi: 10.1155/2021/6739220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.European Centre for Disease Prevention and Control (ECDC) European Food Safety Authority (EFSA) European Medicines Agency (EMA) Third joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicro. EFSA J. 2021;19:e06712. doi: 10.2903/j.efsa.2021.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H.-J., Hung W.-C., Lin Y.-T., Tsai J.-C., Chiu H.-C., Hsueh P.-R., Teng L.-J. A novel fusidic acid resistance determinant, fusF, in Staphylococcus cohnii. J. Antimicrob. Chemother. 2015;70:416–419. doi: 10.1093/jac/dku408. [DOI] [PubMed] [Google Scholar]
- 51.Gardete S., Aires-De-Sousa M., Faustino A., Ludovice A.M., de Lencastre H. Identification of the First Vancomycin Intermediate-Resistant Staphylococcus aureus (VISA) Isolate from a Hospital in Portugal. Microb. Drug Resist. 2008;14:1–6. doi: 10.1089/mdr.2008.0816. [DOI] [PubMed] [Google Scholar]
- 52.Ali Y., Islam M.A., Muzahid N.H., Sikder M.O.F., Hossain M.A., Marzan L.W. Characterization, prevalence and antibiogram study of Staphylococcus aureus in poultry. Asian Pac. J. Trop. Biomed. 2017;7:253–256. doi: 10.1016/j.apjtb.2016.12.001. [DOI] [Google Scholar]
- 53.Bortolaia V., Espinosa-Gongora C., Guardabassi L. Human health risks associated with antimicrobial-resistant enterococci and Staphylococcus aureus on poultry meat. Clin. Microbiol. Infect. 2016;22:130–140. doi: 10.1016/j.cmi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Damien D., David L., Paul C.J., Markus K., Olivier S.P., Régine T., Richard B., Julien D. Identification of a Variety of Staphylococcus Species by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2010;48:941–945. doi: 10.1128/JCM.00413-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silva V., Almeida F., Carvalho J.A., Castro A.P., Ferreira E., Manageiro V., Tejedor-Junco M.T., Caniça M., Igrejas G., Poeta P. Emergence of community-acquired methicillin-resistant Staphylococcus aureus EMRSA-15 clone as the predominant cause of diabetic foot ulcer infections in Portugal. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:179–186. doi: 10.1007/s10096-019-03709-6. [DOI] [PubMed] [Google Scholar]
- 56.Zhang K., Sparling J., Chow B.L., Elsayed S., Hussain Z., Church D.L., Gregson D.B., Louie T., Conly J.M. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J. Clin. Microbiol. 2004;42:4947–4955. doi: 10.1128/JCM.42.11.4947-4955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnellmann C., Gerber V., Rossano A., Jaquier V., Panchaud Y., Doherr M.G., Thomann A., Straub R., Perreten V. Presence of new mecA and mph(C) variants conferring antibiotic resistance in Staphylococcus spp. isolated from the skin of horses before and after clinic admission. J. Clin. Microbiol. 2006;44:4444–4454. doi: 10.1128/JCM.00868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sutcliffe J., Grebe T., Tait-Kamradt A., Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 1996;40:2562–2566. doi: 10.1128/AAC.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shopsin B., Mathema B., Alcabes P., Said-Salim B., Lina G., Matsuka A., Martinez J., Kreiswirth B.N. Prevalence of agr specificity groups among Staphylococcus aureus strains colonizing children and their guardians. J. Clin. Microbiol. 2003;41:456–459. doi: 10.1128/JCM.41.1.456-459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomez-Sanz E., Torres C., Lozano C., Fernandez-Perez R., Aspiroz C., Ruiz-Larrea F., Zarazaga M. Detection, molecular characterization, and clonal diversity of methicillin-resistant Staphylococcus aureus CC398 and CC97 in Spanish slaughter pigs of different age groups. Foodborne Pathog. Dis. 2010;7:1269–1277. doi: 10.1089/fpd.2010.0610. [DOI] [PubMed] [Google Scholar]
- 61.Wondrack L., Massa M., Yang B.V., Sutcliffe J. Clinical strain of Staphylococcus aureus inactivates and causes efflux of macrolides. Antimicrob. Agents Chemother. 1996;40:992–998. doi: 10.1128/AAC.40.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lina G., Quaglia A., Reverdy M.E., Leclercq R., Vandenesch F., Etienne J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob. Agents Chemother. 1999;43:1062–1066. doi: 10.1128/AAC.43.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bozdogan B., Berrezouga L., Kou M.S., Yurek D.A., Farley K.A., Stockman B.J., Leclercq R. A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025. Antimicrob. Agents Chemother. 1999;43:925–929. doi: 10.1128/AAC.43.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lozano C., Aspiroz C., Rezusta A., Gómez-Sanz E., Simon C., Gómez P., Ortega C., Revillo M.J., Zarazaga M., Torres C. Identification of novel vga(A)-carrying plasmids and a Tn5406-like transposon in meticillin-resistant Staphylococcus aureus and Staphylococcus epidermidis of human and animal origin. Int. J. Antimicrob. Agents. 2012;40:306–312. doi: 10.1016/j.ijantimicag.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Hammerum A.M., Jensen L.B., Aarestrup F.M. Detection of the satA gene and transferability of virginiamycin resistance in Enterococcus faecium from food- animals. FEMS Microbiol. Lett. 1998;168:145–151. doi: 10.1111/j.1574-6968.1998.tb13267.x. [DOI] [PubMed] [Google Scholar]
- 66.Aarestrup F.M., Agers L.Y., Ahrens P., JŁrgensen J.L., Madsen M., Jensen L.B. Antimicrobial susceptibility and presence of resistance genes in staphylococci from poultry. Vet. Microbiol. 2020;74:353–364. doi: 10.1016/S0378-1135(00)00197-8. [DOI] [PubMed] [Google Scholar]
- 67.Van de Klundert J.A.M., Vliegenthart J.S. PCR detection of genes coding for aminoglycoside-modifying enzymes. In: Persing D.H., Smith T.F., Tenover F.C., White T.J., editors. Diagnostic Molecular Microbiology: Principles and Applications. American Society for Microbiology; Washington, DC, USA: 1993. pp. 547–552. [Google Scholar]
- 68.Kehrenberg C., Schwarz S. Distribution of Florfenicol Resistance Genes fexA and cfr among Chloramphenicol-Resistant Staphylococcus Isolates. Antimicrob. Agents Chemother. 2006;50:1156–1163. doi: 10.1128/AAC.50.4.1156-1163.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu H., Wang Y., Wu C., Schwarz S., Shen Z., Jeon B., Ding S., Zhang Q., Shen J. A novel phenicol exporter gene, fexB, found in enterococci of animal origin. J. Antimicrob. Chemother. 2012;67:322–325. doi: 10.1093/jac/dkr481. [DOI] [PubMed] [Google Scholar]
- 70.Mclaws F., Chopra I., O’Neill A.J. High prevalence of resistance to fusidic acid in clinical isolates of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2008;61:1040–1043. doi: 10.1093/jac/dkn071. [DOI] [PubMed] [Google Scholar]
- 71.Chen H.J., Hung W.C., Tseng S.P., Tsai J.C., Hsueh P.R., Teng L.J. Fusidic acid resistance determinants in Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 2010;54:4985–4991. doi: 10.1128/AAC.00523-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.