Abstract
In this study, we tested the efficacy of amphotericin B (AmB)-arabinogalactan (AmB-AG) conjugates for the treatment of experimental leishmaniasis. Chemical conjugation of AmB to a water-soluble, biodegradable, and biocompatible polymer could present many advantages over presently available AmB formulations. Two conjugates were tested, a reduced (rAmB-AG) form and an unreduced (uAmB-AG) form. In vitro, the drug concentrations which lower the values of parasites (for promastigotes) or infected macrophages (for amastigotes) to 50% of the untreated values (ED50s) of uAmB-AG and rAmB-AG were 0.19 and 0.34 μg/ml, respectively, for Leishmania major promastigotes and 0.17 and 0.31 μg/ml, respectively, for amastigotes. The effect on Leishmania infantum-infected macrophages was more marked, with ED50s of 0.035 μg/ml for rAmB-AG and 0.027 μg/ml for uAmB-AG. In in vivo experiments, BALB/c mice injected with L. major were treated from day 2 onwards on alternate days for 2 weeks. Both conjugates, as well as liposomal AmB (all at 6 mg/kg of body weight) and Fungizone (1 mg/kg), significantly delayed the appearance of lesions compared to that in untreated mice. In addition, both conjugates, but not liposomal AmB, were significantly more effective than Fungizone. Subcutaneous injection of the conjugates (6 mg/kg) was significantly more effective than liposomal AmB in delaying the appearance of lesions. Higher AmB concentrations of up to 12 mg/kg could be administered by this route. When an established infection was treated, uAmB-AG was somewhat more effective than liposomal AmB. In summary, water-soluble polymeric AmB derivatives were found effective and safe for the treatment of leishmanial infections. The conjugates, which are stable and can be produced relatively cheaply (compared to lipid formulations), can be used in the future for the treatment of leishmaniasis infections.
Amphotericin B (AmB), a polyene antibiotic, is a standard drug for the treatment of fungal infections and is currently recommended as a second-line treatment for visceral leishmaniasis (VL) and mucocutaneous leishmaniasis, especially with human immunodeficiency virus coinfection. AmB has also been proven effective as a first-line treatment for post-kala-azar dermal leishmaniasis (20) and for VL in Europe and India (4, 8, 16).
AmB is a hydrophobic molecule with negligible solubility in aqueous solution and poor solubility in most organic solvents. The marketed formulation, Fungizone, forms a micellar dispersion after the addition of water to the lyophilized sodium deoxycholate-AmB mixture. Fungizone exhibits major clinical limitations. In systemic use, both the sodium deoxycholate and AmB toxicities are not selective enough, and therefore, the therapeutic index of Fungizone is narrow. Additionally, the rather low maximal tolerated dose (1 mg/kg of body weight per day) makes the drug ineffective in many cases of leishmanial infections, especially when the immune status of the patient is compromised, and therefore larger drug doses are necessary in order to kill parasites in unusual locations (5, 14).
Lipid AmB formulations were examined in rodent models and found effective against VL. Recently, AmB-containing liposomes, administered intravenously (i.v.), have been successfully used for human VL. These liposomes allow for the use of larger doses of the drug with much lower side effects and toxicity (8, 11, 21). In addition, it has been shown that mice treated with AmB liposomes have a persistent high drug level in tissues (19). However, some problems, such as the high cost of production and necessity of repeated i.v. infections for successful treatment, prevent their widespread use.
Another approach to improving drug performance and reducing toxicity is the conjugation to a polymeric carrier (15). Conjugation of an insoluble drug such as AmB to a water-soluble biodegradable polymer may increase the water solubility of the drug, drug circulation time, and accumulation in the diseased tissue (15), resulting in an improved therapeutic effect and reduced toxicity.
Arabinogalactan (AG) is a highly branched natural polysaccharide with unusual water solubility (70% in water). It is extracted from the Larix tree and it is available in a 99.9% pure form with reproducible molecular weight and physicochemical properties (1, 18). The high solubility in water, biocompatibility, biodegradability, and ease of drug conjugation in an aqueous medium make AG an attractive drug carrier.
AmB-AG conjugates have been synthesized and tested for efficacy against common pathogenic fungi and for toxicity in mice. The conjugate containing 20% by weight of AmB was soluble in water (>100 mg/ml), was as effective as the parent drug, and possessed a 20-fold-lower toxicity. In a direct comparison between the AmB-AG conjugate and AmBisome, the most-effective and safe AmB formulation available, the conjugate was superior in safety and in efficacy against fungal infections (10). Another water-soluble polyene was also recently shown to be active against Leishmania amazonensis and Leishmania donovani in mice (2).
The advantage of the development of new antileishmanial AmB derivatives over other drugs is further stressed by the recently reported lack of parasite resistance to AmB (9). In this study, we show the effects of two AmB-AG derivatives, a reduced (rAmB-AG) form and an unreduced (uAmB-AG) form, on leishmania promastigotes and amastigotes in vitro and on Leishmania major infection in BALB/c mice.
(Part of this research was conducted by T. Ehrenfreund in partial fulfillment of the requirements for a Ph.D. from Hebrew University of Jerusalem, Jerusalem, Israel.)
MATERIALS AND METHODS
AmB-AG synthesis.
AmB (a gift from Dumex, Copenhagen, Denmark) was conjugated to AG (a gift from Larex International, St. Paul, Minn.) in two steps: first, AG was oxidized to dialdehyde AG with potassium periodate in water at 25°C and purified from the interfering oxidative anions (periodate and iodate) by passage through an ion-exchange column of Dowex-1 (Aldrich, Milwaukee, Wis.) in acetate form. Dowex-1 acetate was obtained by pretreating the commercial anion exchanger with 1 M acetic acid. The purified oxidized AG solution was dialyzed through a 12,000-molecular-weight cutoff dialysis tubing (Medicell International, London, United Kingdom) against deionized water for 48 h at 4°C and lyophilized to dryness. This purification step was critical because AmB is very sensitive to oxidation. In the next step, AmB was bound to the oxidized AG by the formation of an imine bond between the primary amine bond of AmB and the aldehydes of the oxidized AG. Conjugation occurred in 0.1 M borate buffer solution, pH 11, for 48 h under constant stirring. A clear yellow-orange solution was obtained. The imine conjugate was purified by dialysis and lyophilized to dryness. The average yield was 80%. The imine (unreduced) conjugate (uAmB-AG) was reduced to a more stable amine form by the addition of sodium borohydride powder (1.1 M NaBH4/mol of saccharide units in the polymer) to the imine conjugate solution, with overnight stirring at room temperature. The average yield of this reaction was 90%. During the reduction process, a clear change of color, from yellow-orange to light yellow, was observed. The amine (reduced) conjugate (rAmB-AG) obtained was purified by dialysis and lyophylized as described above for the imine conjugate.
Other AmB formulations used in this study.
Other AmB formulations used in this study were Fungizone (Bristol Myers Squibb), a mixed micelle formulation composed of AmB and sodium deoxycholate, and AmBisome, a unilamellar liposome formulation (Vestar, San Dimas, Calif.).
Parasites.
L. major parasites, strain WR1045, were periodically isolated from BALB/c mice (Harlan Laboratories, Jerusalem, Israel) and maintained in RPMI 1640 culture medium supplemented with 20% fetal calf serum (Biological Industries, Beth Haemek, Israel) at 28°C, by weekly passage, for no more than 2 months.
L. infantum parasites, strain LON 49 (MCAN/IL/94/LRC-639) were isolated from an infected dog near Jerusalem. The original isolate was frozen in aliquots which were thawed shortly before use. The parasites were maintained in culture under the same conditions as those of L. major.
In vitro effect on promastigotes.
Parasites, after 3 or 4 days of growth, were washed and resuspended in fresh medium containing fetal calf serum to a concentration of 15 × 104 promastigotes in 200 μl and distributed into flat-bottom wells (96-well plate; Nunc, Roskilde, Denmark). The plates were incubated at 28°C in a wet chamber in an atmosphere of 5% CO2, 5% O2, and 90% N2. After 24 h, 0.5 μCi of [3H]thymidine (2 μCi/mmol; Nuclear Research Centre, Neger, Israel) was added to each well, the parasites were incubated for another 24 h, and then harvested on glass-microfiber filters. Radioactivity was measured in a liquid scintillation counter. Each treatment was performed in triplicate; the results are expressed as percent growth inhibition. [(100 − cpm of wells with drug)/cpm of untreated wells] × 100 (cpm stands for counts per minute).
In vitro effect on amastigotes.
Peritoneal macrophages were obtained from 10- to 12-week-old outbred mice previously stimulated for 4 or 5 days with 3% thioglycolate. The macrophages were distributed into 8-well slides (Nunc) to 2.5 × 105 cells in 300 μl per well and incubated at 37°C and 5% CO2 for 3 h in order to allow for cell adhesion. The supernatant was discarded, and promastigotes (8 × 105 to 10 × 105 per well) were added. After overnight incubation, fresh medium containing the AmB derivatives was added for an additional 24 to 48 h. At the end of this period, the wells were washed, fixed with methanol, and stained with Giemsa. Each treatment was done in duplicate wells; in each well, 100 macrophages and the number of amastigotes they contained were counted.
In vivo experiments.
Female BALB/c mice (Harlan Laboratories) 6 to 10 weeks old, were injected subcutaneously (s.c.) in the base of the tail with 2 × 104 stationary-phase promastigotes, six to eight mice per experimental group. Drug treatment was initiated 2 days after parasite injection or after the appearance of lesions (1 month after parasite injection) and was administered six times on alternate days. The drugs were given i.v. or s.c. in the abdominal area, as detailed for each experiment. Once a week, mice were checked for appearance of lesions, and lesion size was measured. To determine the diameter, the average was taken between the longest distance across the lesion and the length of the line bisecting this distance at a 90° angle. Mice were kept in a sterile pathogen-free animal facility throughout the experiments. In most cases, results are presented as number of mice with lesions at a given time, since the appearance of lesions is usually followed by a steady increase in lesion size.
Statistical analyses.
Statistical evaluations were performed by Kaplan-Meier survival analysis. The log rank test was used to compare groups. Multiple comparisons were performed using Holm’s correction to the significance level. The SPSS program was used to run the analyses.
RESULTS
Synthesis.
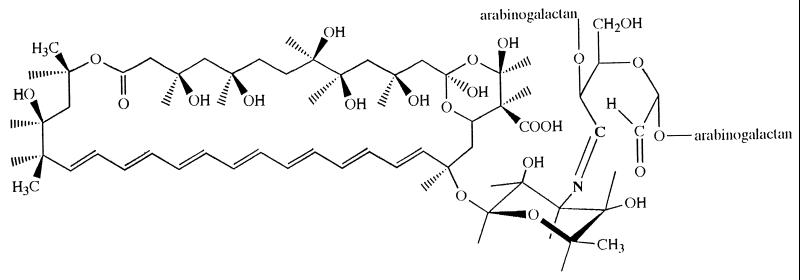
AmB was conjugated to oxidized AG in borate buffer solution, pH 11, for 24 h at room temperature with an overall yield of 85%. Lower pH resulted in a very low yield, while higher pH resulted in increased toxicity. Pure water-soluble conjugates containing 20% by weight of AmB were obtained as lyophilized powder. The reduced conjugate where the drug is attached by a stable amine bond was prepared by reducing the imine bond of the derivative using sodium borohydride. The structure of the imine conjugate is shown in Fig. 1.
FIG. 1.
Structure of uAmB-AG conjugate. rAmB-AG is obtained by reduction of the double bond N⩵C between AmB and the sugar.
Effects of AmB-AG conjugates on parasites in vitro (Table 1).
TABLE 1.
Effects of AmB-AG conjugates and Fungizone on L. major and L. infantum promastigotes and amastigotes in vitroa
| Drug | ED50 (μg/ml) for in vitro activity against:
|
|||
|---|---|---|---|---|
|
L. major
|
L. infantum
|
|||
| Promastigotes | Amastigotes | Promastigotes | Amastigotes | |
| Fungizone | 0.02 | NDb | 0.01 | ND |
| rAmB-AG | 0.34 | 0.31 | 0.06 | 0.035 |
| uAmB-AG | 0.19 | 0.17 | 0.03 | 0.027 |
The effect on promastigotes was evaluated by [3H]thymidine incorporation, and the effect on amastigotes was evaluated by microscopic counting of macrophages and intracellular amastigotes.
ND, not done.
First, AmB-AG was added to promastigote cultures, and its leishmanicidal effect was compared to that of Fungizone. The results using L. major show that the drug concentration which lowers the value of parasites (for promastigotes) or infected macrophages (for amastigotes) to 50% of the untreated values (ED50) of Fungizone was lowest (0.02 μg/ml), while the ED50s of uAmB-AG and rAmB-AG were 0.19 and 0.34 μg/ml, respectively. The leishmanicidal effect on L. infantum was considerably higher: 0.01 μg/ml for Fungizone, 0.03 μg/ml for uAmB-AG, and 0.06 μg/ml for rAmB-AG.
Second, the effect of the derivatives on intracellular L. major amastigotes was determined in infected macrophage cultures. The percentage of infected macrophages was reduced by both conjugates (ED50s of 0.17 μg/ml for uAmB-AG and 0.31 μg/ml for rAmB-AG). The effect on L. infantum-infected macrophages was even more marked, with an ED50 of 0.035 μg/ml for rAmB-AG and 0.027 μg/ml for uAmB-AG.
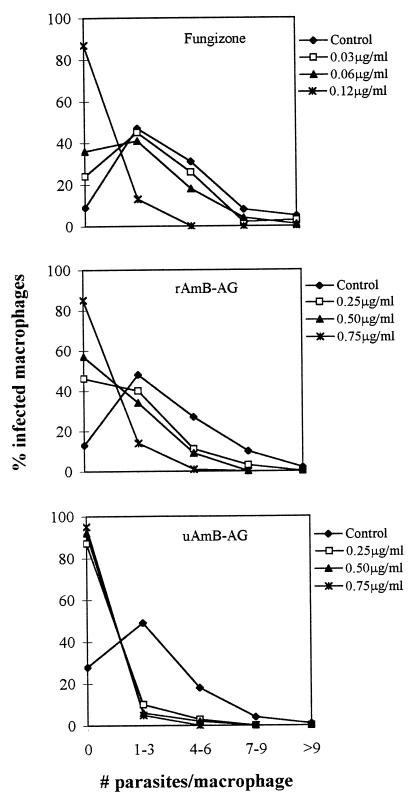
Incubation of infected macrophages in the presence of the conjugates also reduced the number of amastigotes per macrophage: while most untreated macrophages contained one to six amastigotes, 85% of the macrophages treated with 0.25 μg of uAmB-AG per ml and 45% of the macrophages treated with 0.25 μg of rAmB-AG per ml contained no parasites (Fig. 2).
FIG. 2.
Effects of AmB conjugates and Fungizone on the number of amastigotes in infected macrophages. The conjugates were added for 48 h to macrophages containing L. major amastigotes. The number of amastigotes per macrophage was counted by microscopic evaluation.
Effects of AmB-AG derivatives on the course of infection.
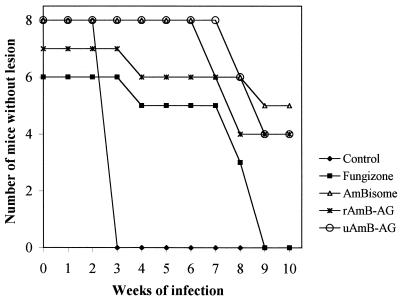
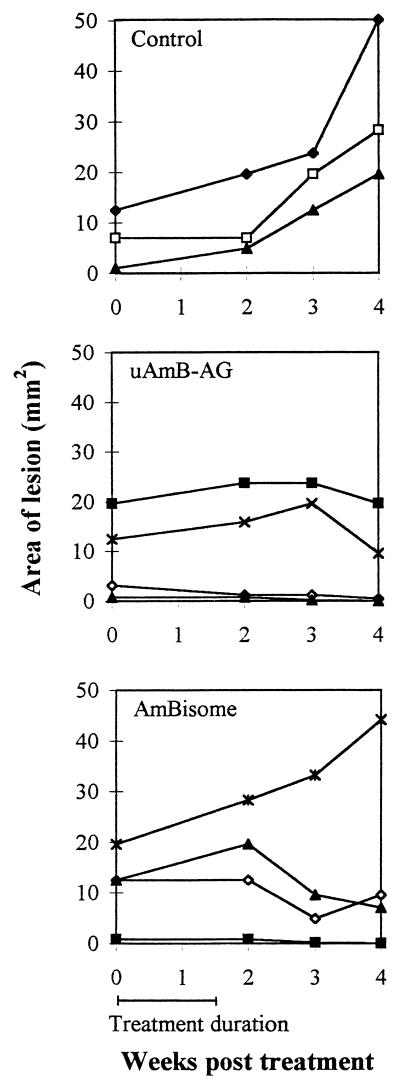
BALB/c mice were injected in the rump with L. major promastigotes and treated on alternate days with 6 i.v. injections of Fungizone (1 mg/kg/day), AmBisome, or the AmB-AG conjugates (6 mg/kg/day), starting on day 2 after parasite injection. Mice were checked weekly for the appearance of lesions. Figure 3 presents the results of one typical experiment (of four performed, although not every experiment included all groups). Cumulative data of all experiments show that rAmB-AG (n = 23), uAmB-AG (n = 11), AmBisome (n = 16), and Fungizone (n = 14) significantly delayed the appearance of lesions (P < 0.0000) from that of untreated mice. In addition, rAmB-AG and uAmB-AG, but not AmBisome, were significantly more effective than Fungizone (P < 0.03 and 0.0001, respectively).
FIG. 3.
Effect of i.v. treatment with AmB derivatives on the appearance of lesions caused by L. major in BALB/c mice. Mice infected with L. major were treated with 6 i.v. injections of Fungizone (1 mg/kg/day), AmBisome, or AmB-AG conjugates (6 mg/kg/day) on alternate days. Lesion appearance was monitored weekly.
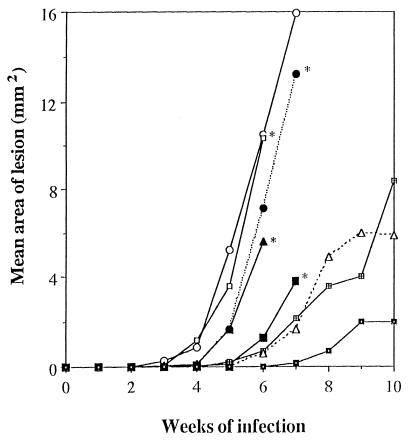
In another experiment, treatment by s.c. injection of the drugs was compared to i.v. treatment. AmBisome at 6 mg/kg had no significant effect when injected s.c. or i.v. However, s.c. injection of 12 mg/kg was more effective than the control (P < 0.0008) or than 6 mg/kg (P < 0.0017). uAmB-AG (6 mg/kg, i.v. or s.c.) was significantly more effective than the controls (0.0013 and 0.0003, respectively) or AmBisome given i.v. (0.007 and 0.0023, respectively) and s.c. (0.0023 and 0.0006, respectively) (Table 2). Figure 4 summarizes the mean lesion area data for the mice from this experiment. The data show that, after 10 weeks, there is a marked difference not only in the number of mice with lesions but also in lesion size. It should be stressed that all the mice received the same parasite inoculum. The effects of treatment of an established infection with uAmB-AG and with AmBisome were examined. Treatment was started after lesions appeared, 1 month after parasite injection. While lesion size continued to increase in the control group, lesion size remained stable or decreased in the treated groups. The conjugate was slightly more effective than AmBisome (Fig. 5). However, the number of animals was too small to draw definite conclusions from this experiment.
TABLE 2.
Effects of AmB-AG conjugates and AmBisome administered i.v. or s.c. on the time of lesion appearance in BALB/c micea
| Treatment (n) | No. of mice with lesions at the following time (wks) after infection:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Control (8) | 0 | 5 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| AmBisome | |||||||||
| i.v., 6 mg/kg (8) | 0 | 3 | 5 | 6 | 8 | 8 | 8 | 8 | 8 |
| s.c., 6 mg/kg (8) | 0 | 3 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| s.c., 12 mg/kg (8) | 0 | 0 | 2 | 5 | 6 | 6 | 7 | 7 | 7 |
| uAmB-AG | |||||||||
| i.v., 6 mg/kg (6) | 0 | 0 | 1 | 1 | 2 | 3 | 5 | 5 | 5 |
| s.c., 6 mg/kg (8) | 0 | 0 | 1 | 2 | 2 | 5 | 5 | 5 | 5 |
| s.c., 12 mg/kg (8) | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 2 | 2 |
| rAmB-AG, s.c., 12 mg/kg (8) | 0 | 0 | 0 | 0 | 1 | 2 | 4 | 4 | 5 |
Treatment was started 2 days after injection of parasites.
FIG. 4.
Comparison of s.c. and i.v. treatment of L. major-infected mice with AmB derivatives. Mice infected with L. major were treated, starting 2 days after infection, with 6 i.v. or s.c. injections of AmBisome or AmB-AG conjugates (6 or 12 mg/kg/day) on alternate days. Lesion size was measured weekly. Symbols: ○, control; ▴, AmBisome, i.v., 6 mg/kg; □, AmBisome, s.c., 6 mg/kg; ●, AmBisome, s.c., 12 mg/kg; ■, uAmB-AG, i.v., 6 mg/kg; ⊞, uAmB-AG, s.c., 6 mg/kg; ▵, uAmB-AG, s.c., 12 mg/kg; ◘, rAmB-AG, s.c., 12 mg/kg.
FIG. 5.
Effects of uAmB-AG and AmBisome on an established L. major infection. BALB/c mice were treated with uAmB-AG or AmBisome after the appearance of lesions (1 month after parasite injection). Lesion size was measured weekly, starting with the initiation of treatment. Each line represents lesion changes in an individual mouse.
DISCUSSION
In recent years, AmB lipid formulations have been developed and have been shown to be effective and significantly less toxic than Fungizone. Three lipid formulations are now marketed for clinical use in many countries worldwide (12). The efficacy of these formulations has been evaluated in mouse models of VL. Liposomal AmB was about three times more active against L. infantum than Fungizone and was not toxic (19). In L. donovani-infected BALB/c mice, the overall efficacy of AmBisome was equal to that of Amphocil and higher than that of Abelcet. Fungizone was the least effective AmB compound tested (17). In a murine L. major model for VL in which mice were treated starting 3 to 4 weeks after parasite injection, 25 and 12.5 mg of AmBisome per kg induced a reduction in lesion size, but 6.25 mg/kg was ineffective. In this model, 25 mg/kg, when injected s.c. close to the lesion, caused a small insignificant reduction in lesion size. Fungizone (1 mg/kg) was inactive both i.v. and s.c. (21).
In all studies with mice, as well as with humans (8), all lipid formulations must be repeatedly administered i.v. in order to be effective. An additional disadvantage of the lipid formulations, which may prevent their widespread use, is their high cost to the patient (7).
In this study, we have evaluated the efficacy of a new type of water-soluble AmB compound for the treatment of experimental leishmaniasis based on the conjugation of AmB to a highly branched polysaccharide, which may overcome some of the disadvantages of lipid AmB formulations.
The main difference between the conjugated drug and the lipid-based formulations is that the latter are particulate systems that may be unstable physically and chemically and they are difficult to sterilize by filtration. The conjugated AmB, however, is water soluble, easily sterilized by filtration, and physically and chemically stable as lyophilized powder and as reconstituted solution. The conjugated AmB-AG, loaded with up to 40% drug by weight, has a molecular weight of 20,000, is highly water soluble (>10% [wt/vol] in water for injection) and biodegradable, and is thus expected to be safely eliminated from the body.
The preparation of the conjugate involves two independent steps which are conducted at room temperature using water solutions with no organic solvents involved. There is essentially one reagent, potassium periodate, that is used besides AG and AmB. The first step is the formation of pure oxidized AG which is conducted by dissolving AG into a solution of the oxidizing agent, periodate, and allowing the oxidation to occur for a few hours at room temperature. The oxidized AG with about 38% of its saccharide units oxidized to dialdehydes is purified by ion-exchange chromatography to remove excess periodate and lyophilized to a powder that is stable at refrigeration for at least 1 year. The conjugation step is conducted at room temperature in borate buffer solution, pH 11, at which the drug has some solubility. The reaction solution is filtered and dialyzed to remove unreacted drug and salts and lyophilized to form a yellow powder which is easily reconstituted prior to use. If the reduced conjugate is desired, the conjugate solution is reacted with excess sodium borohydride prior to dialysis and lyophilization. As described, the method of preparation and reagents are simple and inexpensive, which may make this product available for treating leishmania infections in poor countries.
Both AmB-AG conjugates were initially prepared and investigated for treating fungal infections. Both were found very effective against candidiasis and Cryptococcus infections in mice with the unreduced conjugate being more effective, as described elsewhere (10). Studies conducted on mice indicated that both conjugates are at least as safe as AmBisome with maximal tolerated doses in the range of 50 mg of AmB equivalents per kg or 250 mg of the conjugated product per kg (10).
The two AmB-AG derivatives evaluated in this study, the reduced (rAmB-AG) and unreduced (uAmB-AG) forms were consistently more effective than Fungizone and often also more effective than AmBisome for the treatment of leishmania infection, when treatment was started 2 days after parasite injection (Fig. 3 and 4). The unreduced conjugate was also more effective than AmBisome for the treatment of an established leishmanial infection (Fig. 5).
In both in vivo and in vitro experiments, uAmB-AG was more effective than rAmB-AG against L. major. The higher efficacy of this conjugate may be related to the fact that the imine bond of the unreduced form is less hydrolytically stable than the amine bond of the reduced form, thus making AmB more easily released from the carrier molecule. If this is the case, the data presented here suggest that the performance of the imine-conjugated AmB-AG may be affected by similar delivery systems such as amphiphile-based assemblies containing AmB (6, 13).
Upon comparison of the effects of AmB-AG on L. major and L. infantum, it appears that the ED50 for L. infantum is lower than the ED50 for L. major for both promastigotes and amastigotes. Since the same is true for Fungizone (Table 1 and results not shown), and for pentavalent antimonial drugs (3), this is probably due to an intrinsic difference between L. major and L. infantum rather than to a characteristic of the AmB-AG conjugate. Regarding the effects of AmB-AG on amastigotes and promastigotes, the ED50 for amastigotes was lower than for promastigotes. Similar results were reported for Fungizone and AmBisome (21). Taken together, these data suggest that AmB administered as a polysaccharide conjugate does not interfere with the known mechanism of AmB administered as an amphiphile-based assembly.
We have shown here that higher doses of AmB-AG can be administered by the s.c. as well as by the i.v. route. The fact that fewer mice developed lesions after s.c. injection with higher doses of uAmB-AG stresses the advantage of s.c. injections. In addition to allowing for the administration of higher drug dosages, the s.c. route represents a method of slow drug release, thus lowering the toxicity of AmB.
In summary, water-soluble polymeric AmB derivatives were found effective and safe for treating leishmanial infections in mice. Such treatment could also be used for animal and human infections.
ACKNOWLEDGMENTS
This work was supported in part by the Leslie Nicholas Fund and the Gras Center for Pharmaceutical Research. A. Domb is affiliated with the David Bloom Center for Pharmacy, Hebrew University of Jerusalem.
We thank Leah Rosen for excellent statistical advice. We thank the Dumex Company for the gift of AmB.
REFERENCES
- 1.Adams M F, Douglas C. Arabinogalactan—a review of the literature. TAPPI. 1963;46:544–558. [Google Scholar]
- 2.Al-Abdely H M, Graybill J R, Bocanegra R, Najvar L, Montalbo E, Regen S L, Melby P C. Efficacies of KY62 against Leishmania amazonensis and Leishmania donovani in experimental murine cutaneous leishmaniasis and visceral leishmaniasis. Antimicrob Agents Chemother. 1998;42:2542–2548. doi: 10.1128/aac.42.10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen S, Neal R A. The in vitro susceptibility of macrophages infected with amastigotes of Leishmania spp. to pentavalent antimonial drugs and other compounds with special relevance to cutaneous isolates. In: Hart D T, editor. Leishmaniasis. London, United Kingdom: Plenum Publishing Corporation; 1989. p. 711. [Google Scholar]
- 4.Berman J D. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 5.Chabot G G, Pazdur R, Valeriote F A, Baker L H. Pharmacokinetics and toxicity of continuous infusion of amphotericin B in cancer patients. J Pharm Sci. 1989;78:307–310. doi: 10.1002/jps.2600780409. [DOI] [PubMed] [Google Scholar]
- 6.Cohen R, Polacheck I, Benita S, Levi M, Barenholz Y. Program and Abstracts of the Conference on Liposome Advances: Progress in Drug Delivery and Vaccine Delivery. London, United Kingdom: Centre for Drug Delivery Research, School of Pharmacy, University of London; 1996. Comparative evaluation of five types of amphiphile-based assemblies containing amphotericin B. [Google Scholar]
- 7.Coukell A J, Brogden R N. Liposomal amphotericin B. Therapeutic use in the management of fungal infections and visceral leishmaniasis. Drugs. 1998;55:585–612. doi: 10.2165/00003495-199855040-00008. [DOI] [PubMed] [Google Scholar]
- 8.di Martino L, Davidson R N, Giacchino R, Scotti S, Raimondi F, Casstagnola E, Tasso L, Cascio A, Gradoni L, Gramiccia M, Pettoellomantovani M, Bryceson A D M. Treatment of visceral leishmaniasis in children with liposomal amphotericin B. J Pediatr. 1997;131:271–277. doi: 10.1016/s0022-3476(97)70165-3. [DOI] [PubMed] [Google Scholar]
- 9.Durand R, Paul M, Pratlong F, Rivollet D, Dubreuil-Lemaire M L, Houin R, Astier A, Deniau M. Leishmania infantum: lack of parasite resistance to amphotericin B in a clinically resistant visceral leishmaniasis. Antimicrob Agents Chemother. 1998;42:2141–2143. doi: 10.1128/aac.42.8.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falk R, Domb A J, Polacheck I. A novel injectable water-soluble amphotericin B-arabinogalactan conjugate. Antimicrob Agents Chemother. 1999;43:1975–1981. doi: 10.1128/aac.43.8.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangneux J P, Sulahian A, Garin Y J F, Derouin F. Lipid formulations of amphotericin B in the treatment of experimental visceral leishmaniasis due to Leishmania infantum. Trans R Soc Trop Med Hyg. 1996;90:574–577. doi: 10.1016/s0035-9203(96)90330-2. [DOI] [PubMed] [Google Scholar]
- 12.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis. 1996;2:S133–S144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 13.Legrand P, Cheron M, Leroy L, Bolard J. Release of amphotericin B from delivery systems and its action against fungal and mammalian cells. J Drug Target. 1997;4:311–319. doi: 10.3109/10611869708995847. [DOI] [PubMed] [Google Scholar]
- 14.Maddux M S, Barriere S L. A review of complications of amphotericin B therapy: recommendation for prevention and management. Drug Intell Clin Pharm. 1980;14:177–181. [Google Scholar]
- 15.Maeda H. Polymer-conjugated macromolecular drugs for tumor-specific targetting. In: Domb A, editor. Polymeric site-specific pharmacotherapy. Chichester, United Kingdom: John Wiley and Sons; 1994. pp. 95–116. [Google Scholar]
- 16.Mishra M, Biswas U K, Jha A M, Khan A B. Amphotericin versus sodium stibogluconate in first-line treatment of Indian kala-azar. Lancet. 1994;344:1599–1600. doi: 10.1016/s0140-6736(94)90406-5. [DOI] [PubMed] [Google Scholar]
- 17.Mullen A B, Carter K C, Baillie A J. Comparison of the efficacies of various formulations of amphotericin B against murine visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:2089–2092. doi: 10.1128/aac.41.10.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nazareth M R, Narayanan V L, Bhatia V A. Studies on the larch arabogalactan II. J Pharm Sci. 1961;50:564–567. [Google Scholar]
- 19.Paul M, Durand R, Fessi H, Rivollet D, Houin R, Astier A, Deniau M. Activity of a new liposomal formulation of amphotericin B against two strains of Leishmania infantum in a murine model. Antimicrob Agents Chemother. 1997;41:1731–1734. doi: 10.1128/aac.41.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakur C P, Narain S, Kumar N, Hassan S M, Jha D K, Kumar A. Amphotericin B is superior to sodium antimony gluconate in the treatment of Indian post kala azar dermal leishmaniasis. Ann Trop Med Parasitol. 1997;91:611–616. doi: 10.1080/00034989760707. [DOI] [PubMed] [Google Scholar]
- 21.Yardley V, Croft S. Activity of liposomal amphotericin B against experimental cutaneous leishmaniasis. Antimicrob Agents Chemother. 1997;41:752–756. doi: 10.1128/aac.41.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]