Significance
Ambient nighttime light exposure is implicated as a risk factor for adverse health outcomes, including cardiometabolic disease. However, the effects of nighttime light exposure during sleep on cardiometabolic outcomes and the related mechanisms are unclear. This laboratory study shows that, in healthy adults, one night of moderate (100 lx) light exposure during sleep increases nighttime heart rate, decreases heart rate variability (higher sympathovagal balance), and increases next-morning insulin resistance when compared to sleep in a dimly lit (<3 lx) environment. Moreover, a positive relationship between higher sympathovagal balance and insulin levels suggests that sympathetic activation may play a role in the observed light-induced changes in insulin sensitivity.
Keywords: light, sleep, metabolism, sympathetic nervous system, insulin resistance
Abstract
This study tested the hypothesis that acute exposure to light during nighttime sleep adversely affects next-morning glucose homeostasis and whether this effect occurs via reduced sleep quality, melatonin suppression, or sympathetic nervous system (SNS) activation during sleep. A total of 20 young adults participated in this parallel-group study design. The room light condition (n = 10) included one night of sleep in dim light (<3 lx) followed by one night of sleep with overhead room lighting (100 lx). The dim light condition (n = 10) included two consecutive nights of sleep in dim light. Measures of insulin resistance (morning homeostatic model assessment of insulin resistance, 30-min insulin area under the curve [AUC] from a 2-h oral glucose tolerance test) were higher in the room light versus dim light condition. Melatonin levels were similar in both conditions. In the room light condition, participants spent proportionately more time in stage N2 and less in slow wave and rapid eye movement sleep. Heart rate was higher and heart rate variability lower (higher sympathovagal balance) during sleep in the room light versus the dim light condition. Importantly, the higher sympathovagal balance during sleep was associated with higher 30-min insulin AUC, consistent with increased insulin resistance the following morning. These results demonstrate that a single night of exposure to room light during sleep can impair glucose homeostasis, potentially via increased SNS activation. Attention to avoiding exposure to light at night during sleep may be beneficial for cardiometabolic health.
Exposure to artificial light during the night is widespread globally, particularly in industrialized countries (1–3). Given that light and dark exposure patterns play a key role in the timing of many behaviors and physiological functions (4), exposure to light in the evening and night has been posited to be deleterious for human health and well-being (1, 5–10). Impacts of light exposure during sleep are not as well studied as other kinds of nighttime light exposure. However, a recent cross-sectional observation study noted that, compared to no light exposure during sleep, any self-reported artificial light exposure in the bedroom during sleep (small nightlight in room, light from outside room, or television/light in room) was associated with obesity in women (11). Furthermore, the incidence of obesity was highest in those who reported sleeping with a television or light on in the bedroom (11). These findings suggest that light in the bedroom during nighttime sleep may negatively influence metabolic regulation.
Emerging evidence indicates that light exposure plays a role in human metabolic regulation, with evening light exposure having unfavorable effects on metabolic functions including decreased glucose tolerance and decreased insulin sensitivity (12, 13). In line with these data, we have previously shown that blue-enriched light exposure in the morning and evening alters glucose metabolism, with an increase in insulin resistance compared to dim light exposure (14). In addition, evidence indicates that nighttime indoor light exposure during the habitual sleep period while awake (15), and during sleep itself (16), likely has deleterious metabolic effects. A recent study prospectively measured light exposure in the bedroom during nighttime sleep and showed that higher levels of bedroom light exposure were associated with a higher incidence of type 2 diabetes in an elderly population (16). However, the exact mechanisms by which light exposure, particularly during nighttime sleep, impacts metabolic regulation are not well understood.
A proposed pathway to explain the relationship between nighttime light exposure and altered metabolic function is via changes in sleep. Robust evidence from epidemiological and experimental studies indicates that nighttime light exposure, either from outdoor or indoor sources, has negative impacts on subjective and objective sleep quality as indicated by actigraphy or polysomnography (PSG) measures of reduced total sleep time (TST), sleep efficiency (SE), increased wake after sleep onset (WASO), reduced amount of slow wave sleep (SWS), or increased arousal index (AI) (17–20). Given the well-established contribution of sleep disruptions to metabolic dysfunction (21), it is plausible that nighttime light exposure alters glucose metabolism due to disturbances to sleep. However, nighttime light exposure also appears to have a direct effect on glucose regulation that is independent of sleep loss, as shown by a study that subjected healthy male individuals to sleep deprivation in the dark or to sleep deprivation with nighttime light exposure (22). This study showed that a full night of sleep deprivation with nighttime light exposure increased postprandial levels of insulin and glucagon-like peptide-1, increased insulin resistance, and reduced nighttime melatonin; these changes were not observed under conditions of sleep deprivation in darkness.
A second proposed mechanism to explain the impairment of glucose metabolism from nighttime light exposure is via light-induced changes to the endogenous circadian system, including suppression and phase shifting of the melatonin rhythm (23). It is well established that light exposure suppresses melatonin secretion (24, 25), and several studies have implicated suppression of nighttime melatonin with incidence of diabetes (26) and insulin resistance (27). The association between altered melatonin levels and changes in glucose regulation may be explained by evidence that melatonin plays a role in the secretion and action of insulin (28–30). In particular, lower melatonin levels resulting from light exposure during the nighttime sleep period, in a fasting condition, have been suggested to alter melatonin’s facilitation of pancreatic β-cell recovery (31). Moreover, evidence shows that light exposure, even of moderate intensity, during the nighttime sleep period can produce a phase shift of the internal circadian system (32, 33). Given the established role of the circadian system in the control of glucose metabolism, light exposure during the nighttime sleep period could facilitate the misalignment between the central clock and peripheral clocks in metabolic tissues, with consequent negative impact on glucose homeostasis (34).
A third potential mechanism is the effect of light exposure on autonomic nervous system (ANS) activity. Light exposure has an arousing effect on the sympathetic autonomic system as revealed by the increase in cortisol or heart rate (HR) associated with light exposure mainly during the morning and/or nighttime hours as compared to evening hours (35–37). Beyond the direct excitatory effect exerted by light exposure on sympathetic activity (35), alterations of the ANS characterized by a shift toward an increased sympathetic drive have also been suggested to mediate the negative effects of sleep disruption on many physiological systems such as glucose metabolism (38). Thus, it is plausible that light-induced autonomic activation, either directly and/or mediated by sleep disruption, significantly contributes to the observed relationship between nighttime light exposure and altered glucose metabolism. Notably, sympathetic overactivity has been shown to precede the development of insulin resistance and prediabetes and contribute to the development of obesity and metabolic syndrome (39–41).
Prior studies have reported that light exposure during sleep increases HR and decreases HR variability (HRV), consistent with increased sympathetic activation (42–44). These studies either examined bright light (1,000 lx) over the entire sleep period (42) or lower light levels (50 lx or dawn simulation) early or late in the sleep period (43, 44). However, the effect of a single night of moderate room light exposure across the entire nighttime sleep period on autonomic activation and its impact on metabolic function has never been fully investigated.
In the present study, we tested the hypothesis that room light exposure (100 lx) during habitual nighttime sleep is associated with increased insulin resistance as measured by the homeostatic model of insulin resistance (HOMA-IR), the Matsuda insulin sensitivity index, and impaired response to an oral glucose tolerance test (OGTT) the next morning. In addition, we hypothesized potential mechanisms of light-induced metabolic changes, such as reduced sleep quality, suppression of melatonin level, and elevated sympathetic activation (HR and HRV) during the sleep period.
Results
Participants.
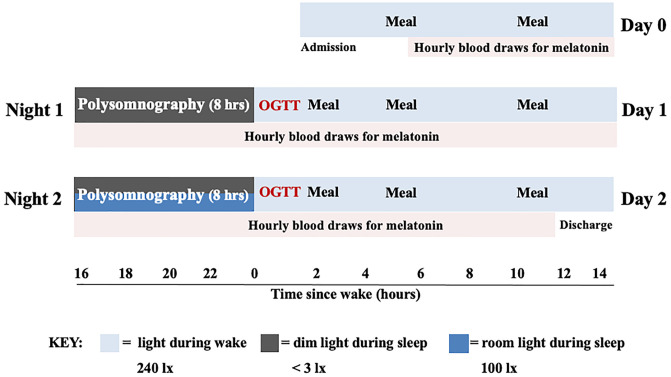
A total of 20 healthy adults were randomized into the room light condition (n = 10, one night in dim light < 3 lx followed by one night with room light at 100 lx) and the dim light condition (n = 10, two consecutive nights in dim light < 3 lx), Fig. 1. The two groups were not significantly different for age, body mass index (BMI), sex, and race (Table 1). Actigraphy in the week preceding the laboratory stay (Table 1) indicated that participants randomized to the room light and dim light conditions had similar bedtime, sleep duration, and SE. Furthermore, a measure of daytime sleepiness (Epworth Sleepiness Scale [ESS]) was similar in both groups and was within normal range at screening (room light condition: 7 ± 2, dim light condition: 6 ± 3, P = 0.49).
Fig. 1.
Schematic of laboratory protocol. OGTT: oral glucose tolerance test.
Table 1.
Participant demographics and sleep variables from 1 wk of actigraphy in the week prior to the laboratory visit
| Demographics | Room light condition | Dim light condition | p |
|---|---|---|---|
| Number | 10 | 10 | |
| Age (years) | 26.61 ± 4.34 | 26.78 ± 5.15 | 0.89a |
| BMI (kg/m2) | 23.25 ± 3.94 | 24.25 ± 3.71 | 0.39a |
| Sex (females, n) | 8 | 6 | 0.16c |
| Race | 6 White, 3 Asian, 1 African-American | 4 White, 5 Asian, 1 African-American | 0.24c |
| Actigraphy measures | |||
| Bedtime (hh:mm) | 23:05 ± 0:36 | 23:02 ± 0:37 | 0.96a |
| Sleep duration (hh:mm) | 7:13 ± 0:40 | 7:00 ± 0:27 | 0.46a |
| SE (%) | 88.07 ± 3.10 | 84.08 ± 6.21 | 0.09c |
SE = sleep efficiency. (a) two-sided Student’s t test; (b) nonparametric two-sided Wilcoxon rank-sum test; (c) χ2 test.
HOMA-IR.
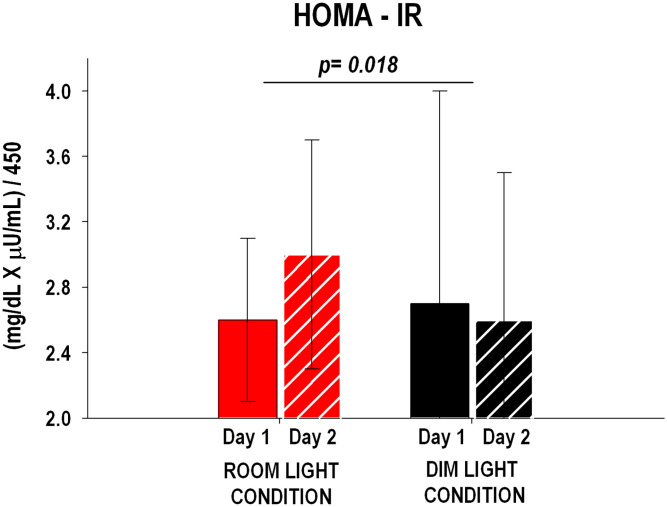
The change in HOMA-IR from Day 1 to Day 2 was significantly different (P = 0.018) between the room light and dim light conditions (Fig. 2) and was characterized by a ∼15% increase in the room light condition compared to a ∼4% decrease in the dim light condition.
Fig. 2.
HOMA-IR for room light (n = 10) and dim light (n = 10) conditions on Day 1 and Day 2. HOMA-IR was significantly higher on Day 2 compared to Day 1 in the room light condition versus the dim light condition. P values refer to the change from Day 1 to Day 2 between conditions (unpaired Student’s t test). The error bars represent SD.
OGTT.
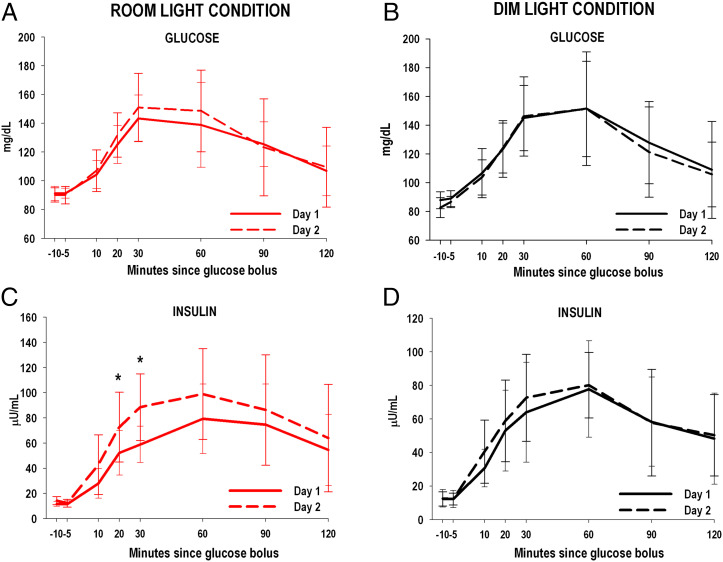
Glucose and insulin profiles from the 2-h OGTT are shown in Fig. 3. Glucose values were not different between room light and dim light conditions (condition × day P = 0.18, condition × day × time P = 0.99; Fig. 3 A and B). The difference in insulin from Day 1 to Day 2 was significantly larger for the room light compared to the dim light condition (condition × day P = 0.034) and was observed across the 2-h OGTT (condition × day × time P = 0.95). However, post hoc analysis indicated that higher insulin levels in the room light condition on Day 2 were most pronounced at 20 and 30 min post ingestion of the glucose bolus (P = 0.01 and P = 0.03, respectively; Fig. 3 C and D).
Fig. 3.
Glucose and insulin measures from 2-h OGTT for room light (n = 10) and dim light conditions (n = 10) on Day 1 and Day 2. Within group changes in glucose levels (A and B) on Days 1 and 2 were similar between room light and dim light conditions while within group changes in insulin levels (C and D) were significantly higher on Day 2 after sleeping in room lighting in the room light condition compared to the dim light condition (General Linear Model: condition × day P = 0.034). Higher insulin levels on Day 2 in the room light condition were most pronounced at 20 and 30 min post ingestion of the glucose bolus. *P < 0.05. The error bars represent SD.
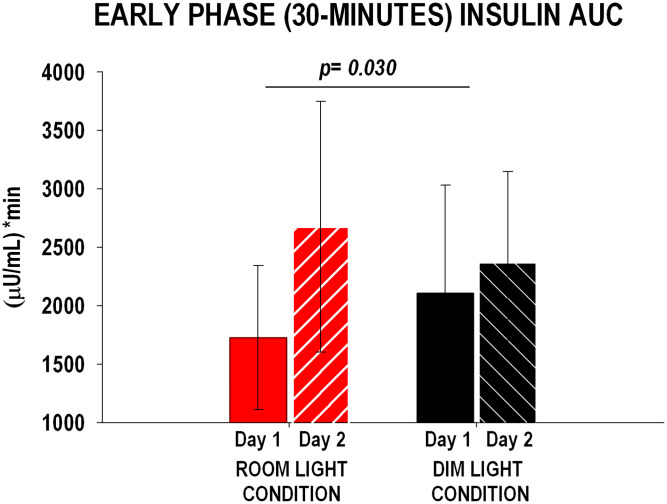
The change in 2-h AUC of glucose and insulin from Day 1 to Day 2 did not differ between conditions (glucose: room light condition: 505 ± 1,398 mg / dL × min, dim light condition: −294 ± 192 mg/dL × min, P = 0.30; insulin: room light condition: 1,999 ± 1,740 µU/mL × min, dim light condition: −726 ± 1,226 µU/mL × min, P = 0.12). However, the change in 30-min AUC of insulin (a measure of early phase of insulin secretion) from Day 1 to Day 2 was significantly larger (P = 0.030) for the room light condition compared to the dim light condition (Fig. 4).
Fig. 4.
Early phase insulin response (30-min AUC) during the OGTT for room light (n = 10) and dim light (n = 10) conditions on Day 1 and Day 2. Insulin 30-min AUC and HOMA-IR were significantly higher on Day 2 compared to Day 1 in the room light condition versus the dim light condition. P values refer to the change from Day 1 to Day 2 between conditions (unpaired Student’s t test). The error bars represent SD.
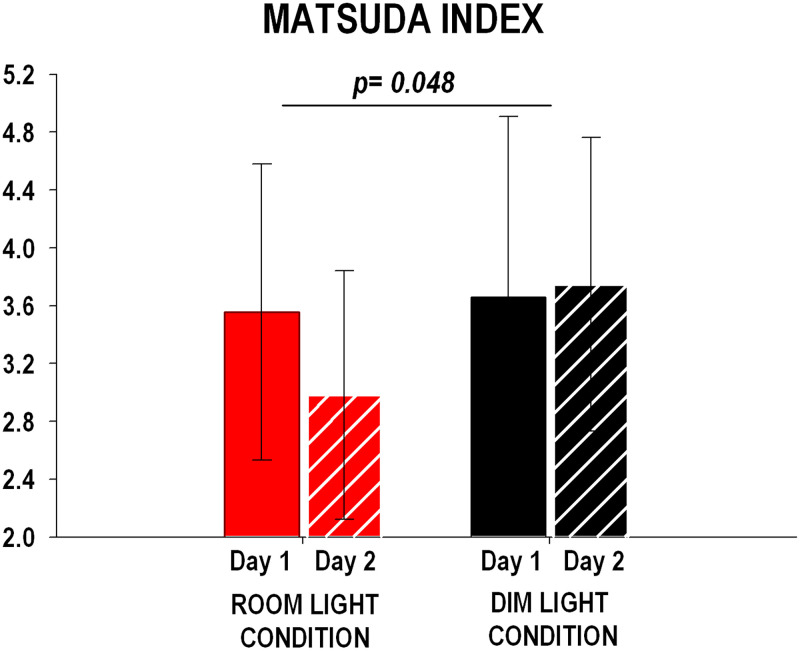
Matsuda Insulin Sensitivity Index.
The change in Matsuda index from Day 1 to Day 2 was significantly different (P = 0.048) between the room light and dim light conditions (Fig. 5) and was characterized by a ∼16% decrease in the room light condition compared to a ∼3% increase in the dim light condition.
Fig. 5.
Matsuda insulin sensitivity index for room light (n = 10) and dim light (n = 10) conditions on Day 1 and Day 2. Matsuda index (y-axis) was calculated as 10,000/square root of [fasting glucose × fasting insulin] × [mean glucose × mean insulin during OGTT]. Matsuda index was significantly lower on Day 2 compared to Day 1 in the room light condition versus the dim light condition. P values refer to the change from Day 1 to Day 2 between conditions (unpaired Student’s t test). The error bars represent SD.
Sleep.
Macrostructure.
The change in PSG parameters from Night 1 to Night 2 showed a greater percentage of TST spent in stage N2 (P = 0.004) and a lower percentage of TST spent in SWS (P = 0.017) and rapid eye movement sleep (REM, P = 0.033) in the room light condition compared to the dim light condition (Table 2). There was no difference between the two conditions in the change from Night 1 to Night 2 for PSG-derived measures of sleep fragmentation, number, and averaged duration of the wake episodes during the sleep period, and sleep–wake stage stability (SI Appendix, Table S1).
Table 2.
Polysomnographic features of sleep macrostructure
| Room light condition | Dim light condition | |||||||
|---|---|---|---|---|---|---|---|---|
| n = 10 | n = 10 | p | ||||||
| Night 1 | Night 2 | △ (Night 2) − (Night 1) | Night 1 | Night 2 | △ (Night 2) − (Night 1) | |||
| TST (min) | 428.05 ± 47.79 | 440.25 ± 37.91 | 12.20 ± 59.33 | 427.20 ± 23.15 | 451.15 ± 13.85 | 23.95 ± 22.38 | 0.344b | |
| Stage N1 (%) | 6.81 ± 4.38 | 5.07 ± 2.45 | −1.73 ± 4.53 | 7.16 ± 3.62 | 3.88 ± 1.54 | −3.28 ± 3.08 | 0.141b | |
| Stage N2 (%) | 51.51 ± 7.18 | 51.44 ± 8.66 | −0.08 ± 4.69 | 53.66 ± 5.73 | 46.28 ± 4.57 | −7.39 ± 5.30 | 0.004a | |
| SWS (%) | 20.64 ± 7.33 | 23.58 ± 7.84 | 2.94 ± 2.16 | 19.98 ± 5.62 | 26.31 ± 4.62 | 6.33 ± 3.46 | 0.017a | |
| REM sleep (%) | 21.04 ± 5.13 | 19.91 ± 5.09 | −1.13 ± 5.61 | 19.20 ± 2.65 | 23.53 ± 3.25 | 4.34 ± 4.95 | 0.033a | |
| SOL (min) | 11.20 ± 16.80 | 6.15 ± 4.09 | −5.05 ± 17.60 | 9.30 ± 6.46 | 7.75 ± 5.11 | −1.55 ± 7.17 | 0.969b | |
| SE (%) | 89.42 ± 10.07 | 91.90 ± 7.95 | 2.47 ± 12.44 | 89.04 ± 5.10 | 93.99 ± 2.83 | 4.94 ± 4.80 | 0.345b | |
| WASO (min) | 39.50 ± 32.18 | 32.70 ± 36.12 | −6.80 ± 47.56 | 43.35 ± 25.45 | 21.20 ± 12.13 | −22.25 ± 21.44 | 0.361a | |
| AI | 16.49 ± 5.35 | 13.82 ± 4.03 | −2.67 ± 6.10 | 17.46 ± 6.37 | 12.13 ± 4.12 | −5.32 ± 5.44 | 0.212b | |
SWS = slow wave sleep; REM = rapid eye movement; SOL = sleep onset latency; SE = sleep efficiency; WASO = wake after sleep onset. P values refer to differences in △ (Night 2) − (Night 1) between room light and dim light conditions. (a) two-sided Student’s t test; (b) nonparametric two-sided Wilcoxon rank-sum test.
Relationship between the change in sleep macrostructure and metabolic measures.
There was no association between the change in sleep macrostructure (percentage of N2, SWS, or REM sleep) from Night 1 to Night 2 and the change from Day 1 to Day 2 in 30-min AUC of insulin, HOMA-IR, or Matsuda index (all P > 0.05 when analyzing participants from both groups together or by condition).
Microstructure.
There were no differences in the change from Night 1 to Night 2 between the room light and the dim light conditions for slow wave activity (SWA: 0.5 to 4 Hz) or slow oscillatory (SO) activity (0.5 to 1 Hz) across sleep cycles (SWA: condition × night P = 0.68, condition × night × cycle P = 0.86; SO activity: condition × night P = 0.96, condition × night × cycle P = 0.86; SI Appendix, Fig. S1).
Subjective sleepiness.
There were no differences in Karolinska Sleepiness Scale (KSS) from Day 1 to Day 2 between the room light and dim light conditions across the wake period (condition × day P = 0.61, condition × day × time P = 0.99).
Plasma Melatonin.
There was no difference in the change of 24-h melatonin levels from Day 1 to Day 2 between the room light and dim light conditions (condition × day P = 0.96, condition × day × time P = 0.99, SI Appendix, Fig. S2A). In addition, there was no difference in the change of timing of melatonin onset from Night 1 to Night 2 between the room light and dim light conditions (room light condition: 0 ± 1.1 h and dim light condition: 0 ± 0.9 h, P = 0.79). The change in the timing of melatonin offset from Night 1 to Night 2 showed a trend toward an advance in the room light versus the dim light conditions (room light condition: −0.4 ± 1.1 h and dim light condition: 0.9 ± 1.3 h, P = 0.07).
There was no difference in the AUC of melatonin levels during the sleep opportunity from Night 1 to Night 2 between the room light and dim light conditions (Δ in the room light condition: −155 ± 177 pg/mL × min and Δ in the dim light condition: −156 ± 167 pg/mL × min, P = 0.49, SI Appendix, Fig. S2B).
HR, HRV, and Blood Pressure (BP).
HR and HRV during the sleep period.
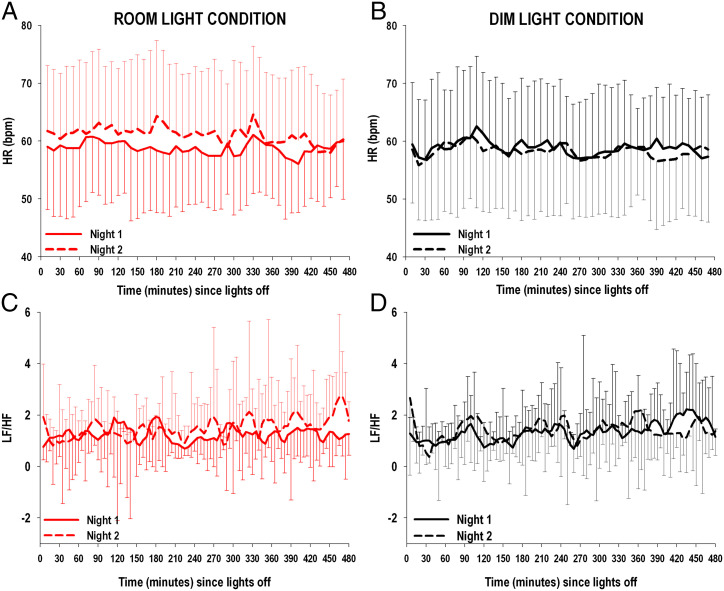
There was a significant increase in HR during the sleep period from Night 1 to Night 2 (condition × night P < 0.0001) in the room light compared to the dim light condition, which was maintained across the entire night (condition × night × time P = 0.98) (Fig. 6 A and B).
Fig. 6.
(Top, A and B): HR during the sleep period for room light (n = 10) and dim light (n = 10) conditions on Night 1 and Night 2. (Lower, C and D): LF/HF derived from HRV analysis during the sleep period for room light (n = 10) and dim light (n = 10) conditions on Night 1 and Night 2. Beat-to-beat HR during the sleep period was averaged every 10 min across the sleep period, starting from the time at lights off. Change in HR from Night 1 to Night 2 was significantly larger in participants randomized to the room light condition (A) compared to those randomized to the dim light condition (B) (General Linear Model: condition × night P < 0.001). LF/HF was calculated on time windows with a stable signal of at least 5 min for the entire duration of the sleep period, starting from lights off. Change in LF/HF from Night 1 to Night 2 was significantly larger in participants randomized to the room light condition (C) compared to those randomized to the dim light condition (D) (General Linear Model: condition × night P < 0.019). The error bars represent SD.
HRV analysis showed that the change in HF relative power from Night 1 to Night 2 was not different between the room light and dim light conditions (condition × night P = 0.86, condition × night × time P = 0.48, SI Appendix, Fig. S3 A and B), while the change in LF relative power from Night 1 to Night 2 was significantly higher in the room light compared to the dim light condition across the 8-h sleep period (condition × night P < 0.0001, condition × night × time P = 0.49, SI Appendix, Fig. S3 C and D). In addition, the change from Night 1 to Night 2 of low-frequency to high-frequency ratio (LF/HF) was significantly higher in the room light compared to the dim light condition across the 8-h sleep period (condition × night P = 0.019, condition × night × time P = 0.11, Fig. 6 C and D).
Relationship between the change in HR and HRV during the sleep period and metabolic measures.
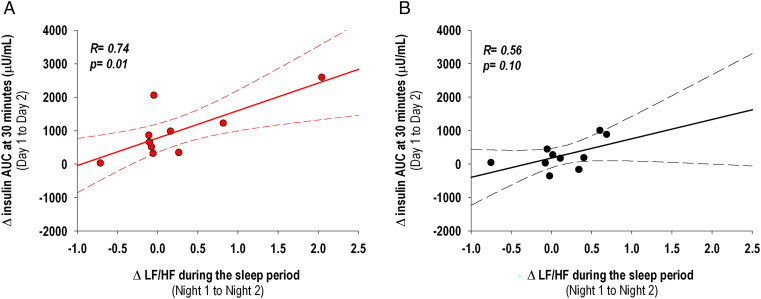
When considering participants from both conditions together, the increase in the average HR and LF/HF during the sleep period from Night 1 to Night 2 were positively correlated with the increase in the initial 30-min AUC of insulin from the OGTT (HR: R = 0.47, P = 0.037; LF/HF: R = 0.65, P = 0.0019). When analyzing the two conditions separately, the positive association between the change in HR during the sleep period and the change in the early phase of insulin response on the following morning was not significant in both conditions, although it appeared to be stronger in participants randomized to the room light condition (R = 0.45, P = 0.19) compared to those randomized to the dim light condition (R = 0.16, P = 0.64). However, when analyzing the two conditions separately, the positive association between the change in LF/HF during the sleep period and the change in the early phase of insulin response was significant in the room light condition (R = 0.74, P = 0.013) but not in the dim light condition (R = 0.56, P = 0.10), Fig. 7.
Fig. 7.
Pairwise correlations between the change in LF/HF obtained from HRV and averaged across the sleep period (Night 1 to Night 2) and the change in the AUC of insulin at 30 min from OGTT (Day 1 to Day 2). A significant positive correlation between LF/HF change and the change in the AUC of insulin at 30 min was observed in participants randomized to the room light condition (A, n = 10), and not in those randomized to the dim light condition (B, n = 10). The dotted lines represent 95% CIs.
There was no significant association between the change in HR and LF/HF during the sleep period from Night 1 to Night 2 with the change in HOMA-IR or Matsuda index from Day 1 to Day 2 (P > 0.05 when analyzing participants from both groups together or by condition).
Daytime BP and HR.
There were no differences in HR and systolic or diastolic BP across the wake period from Day 1 to Day 2 between the room light and dim light conditions (HR: condition × day P = 0.91, condition × day × time P = 0.86; systolic BP: condition × day P = 0.26, condition × day × time P = 0.56; diastolic BP: condition × day P = 0.90, condition × day × time P = 0.71).
Visual Analog Scale (VAS).
There were no differences in the change from Day 1 to Day 2 of subjective hunger (VAS-H) or vigor and affect (VAS-GVA) across the wake period between the room light and dim light conditions (VAS-H: condition × day P = 0.82, condition × day × time P = 0.32; VAS-GVA: condition × day P = 0.19, condition × day × time P = 0.77).
Discussion
This study provides insight into the physiological mechanisms underlying the relationship between nighttime light exposure, specifically during sleep, with cardiometabolic function. The primary finding of this study is that exposure to a single night of room light (100 lx) during sleep increased measures of insulin resistance the next morning. Interestingly, the effect of nighttime light exposure on metabolic function was correlated with an increase in sympathovagal balance during sleep.
As hypothesized, participants randomized to the room light condition showed increased insulin resistance in the morning (i.e., higher fasting HOMA-IR and lower Matsuda index from the OGTT) when compared to participants in the dim light condition. As expected for these healthy, normal weight participants, similar glucose levels were maintained in both conditions during the OGTT. However, in the room light condition, there were higher insulin levels during the OGTT in the morning after sleeping with overnight room light exposure when compared to the dim light condition, which is indicative of a compensatory insulin response to maintain euglycemia under a condition of increased insulin resistance (45). Interestingly, while there were no differences in the overall AUC of insulin during the OGTT, there were higher insulin levels in the early phase of insulin secretion (i.e., AUC of insulin response within the first 30 min of the OGTT) following the night of room light exposure for participants in the room light condition when compared to those in the dim light condition. This early phase of insulin secretion, or acute insulin response, is a measure of pancreatic β-cell function and plays a physiological role in the maintenance of postprandial glucose homeostasis (46). Higher acute insulin response has been shown to be a predictor of the onset of type 2 diabetes in individuals with both normal fasting and 2-h OGTT plasma glucose concentrations (47). Increased insulin resistance and altered β-cell function play a key role in the pathogenesis of diabetes (48). As such, our findings might have implications for individuals who are frequently exposed to nighttime light during sleep and who are at increased risk for type 2 diabetes. The long-term (10 wk) negative effect of constant light exposure on glucose homeostasis has been shown in a rodent model (48); long-term effects in humans of evening and nighttime light exposure on obesity and metabolic disorders remain unknown.
Our initial hypothesis was that room light exposure would impact sleep and that the changes in sleep could be a primary mechanism to explain altered glucose regulation. In our study, nighttime light exposure was accompanied by changes in sleep macrostructure but not in sleep microstructure (i.e., SWA and SO activity across sleep cycles) nor subjective sleep quality. In line with existing literature (20), there was a greater percentage of stage N2 and a lower percentage of SWS and REM sleep during the night with room light exposure compared to during the dim light condition. Sleep did not appear to be more fragmented in response to the room light exposure as there were no significant changes in PSG-derived measures of cortical arousal, sleep fragmentation, or wake–sleep stage stability between the room light and dim light conditions. It has been postulated that the association between sleep disruption with metabolic function is via ANS activation, which has been reported in association with measures of sleep fragmentation (49). The lack of increased sleep fragmentation or arousals with room light exposure during sleep might explain why we did not observe an association between changes in sleep macrostructure with changes in glucose metabolism.
The lack of difference in melatonin between the room light and dim light conditions is in accordance with previous studies reporting mixed findings on the effect of light exposure during nighttime sleep on the level and timing of melatonin (19, 43, 50,). We chose 100 lx for the room light condition as this level is within the range of illuminance that has been employed in previous experimental studies as typical indoor illumination (23, 51–53). Although there are some data showing that exposure to light levels >65 lx can suppress melatonin secretion and delay biological rhythms (23, 54), only 5 to 9% of light is estimated to be transmitted through the closed eyelids to the eyes and subsequently to the retinohypothalamic pathway that mediates melatonin suppression (55, 56). In addition, the known interindividual variability in melatonin suppression to light exposure might account for the negative findings in this study (57). However, due to our relatively small sample size and since melatonin samples during wake were not collected under dim light conditions, we are unable to rule out the possible effect of light-induced changes of melatonin level or phase on metabolic parameters.
A finding from this study is the sustained increase in HR during the sleep period in the room light condition. In addition, and consistent with the observed increase in HR, increase in LF/HF derived from HRV during the sleep period was seen in the room light condition, indicative of an increased sympathovagal balance. The increase in LF/HF in response to light exposure during the nighttime sleep period has been reported by a previous study that exposed participants to much brighter light levels (1,000 lx) (42) than used in the present study. Other studies utilizing 50 lx or dawn simulation early or late in the sleep period have also reported increases in HR and decreases in HRV, consistent with increased sympathetic activation (43, 44). It is unknown whether light exposure levels similar to those used in the current study would have any or similar impact in awake individuals during the biological night.
Moreover, the significant positive association between the change (Night 1 to Night 2) in LF/HF during the sleep period and the change (Day 1 to Day 2) in the early phase of insulin secretion, which was observed in participants in the room light condition but not in those in the dim light condition, provides a potential mechanism underlying the metabolic changes seen following nighttime light exposure during sleep. While we are unable to definitively conclude that nighttime light exposure caused sympathetic activation, these results provide further support for this possibility.
There are multiple pathways through which light can impact the ANS. Increases in HR and LF/HF in response to room light exposure during sleep are consistent with existing data that indicate that light exposure can stimulate cardiac activity by eliciting a sympathoexcitatory response (36, 58). An important pathway proposed to mediate the effect of light on HR is via the retinohypothalamic tract to the suprachiasmatic nucleus (SCN) and then to the paraventricular nucleus, brainstem, and spinal cord to the heart (36, 59, 60). Other than via the SCN, evidence from animal models indicates that light can also directly affect brain regions with sympathoexcitatory effects (e.g., orexinergic system) (61), which might, in part, explain the differences we observed between the changes in melatonin and HR in response to light. Finally, given the state-dependent regulation of the ANS, it is possible that the observed changes in sleep macrostructure associated with nighttime light exposure may have also contributed to the increase in sympathetic activity. While there may be multiple pathways by which light may increase sympathetic activity, the relationship between sympathetic activation and glucose homeostasis are well established. Specifically, in response to increased sympathetic outflow to skeletal muscle, postprandial glucose uptake from skeletal muscles is reduced, resulting in an increase of plasma glucose levels which, in turn, stimulate additional insulin production by the pancreas and promote insulin resistance (40).
The effects of light exposure at night, particularly during sleep, on cardiometabolic function could have implications for those living in modern societies where indoor and outdoor nighttime light exposure is increasingly widespread and where concerns regarding cardiometabolic health are also on the rise. Thus, it is plausible that decreasing exposure to indoor nighttime light during sleep could have beneficial effects on cardiometabolic health. Future studies using a larger sample size and a randomized cross-over design to study the effects of varying light wavelengths, duration, and intensities are needed to confirm our findings and potential ecological translatability. Follow-up studies are warranted in sectors of our society who are more likely to be affected by nighttime light exposure during the sleep period, including those living in long-term care facilities. Larger studies powered to assess the role of sex are also warranted given the known effect of sex on ANS activity. Moreover, research is needed to determine how nighttime light exposure during sleep may interact with daytime light exposure history during wake and to establish whether chronic nighttime light exposure has long-term effects on cardiometabolic function.
Methods
The Northwestern University Institutional Review Board approved this study protocol, and all participants provided written informed consent.
Participants.
Healthy adults were recruited from the Chicago area. Initial eligibility was determined via a survey taken online (REDCap) or administered over the telephone. Participants who passed initial screening had an in-person screening visit where details of the study were reviewed, informed consent was obtained, and questionnaires were completed. Participants then completed 1 wk of at-home monitoring using actigraphy (Actiwatch Spectrum, Philips Respironics) and sleep diary for eligibility screening purposes.
Requirements for study participation included age of 18 to 40 y, habitual sleep duration of 6.5 to 8.5 h, and habitual sleep onset of 9:00 PM to 1:00 AM. Participants were excluded from the study if they had the following: 1) any sleep disorder as assessed by history and screening questionnaires for obstructive sleep apnea (Berlin) and excessive daytime sleepiness (ESS >12) and by PSG to exclude sleep apnea (apnea hypopnea index ≥ 15), periodic leg movements (movement AI ≥ 15), or REM sleep behavior disorder; 2) history of a cognitive or neurological disorder; 3) history of a major psychiatric disorder, including but not limited to mood/anxiety, eating, and alcohol/substance abuse disorders; 4) depressed mood (Beck Depression Inventory II score ≥ 20); 5) diabetes or other endocrine disorders; 6) any gastrointestinal disease requiring dietary adjustment; 7) blindness or significant vision loss; 8) any unstable or serious medical conditions; 9) current or recent (within the past month) use of psychoactive, hypnotic, stimulant, or analgesic medications; 10) shift work or other types of self-imposed irregular sleep schedules; 11) obesity (BMI > 30 kg/m2); 12) history of habitual smoking (six or more cigarettes per wk) or drinking (seven or more alcoholic beverages per week) or caffeine consumption greater than 300 mg per day; 13) current use of light therapy; 14) use of any other legal or illicit substance that may affect sleep and/or appetite; and 15) allergy to heparin. Due to the metabolic stress associated with pregnancy and breastfeeding, patients who were pregnant or breastfeeding were also excluded. Female participants were asked about the timing of their menstrual periods in the past 3 mo, and laboratory stays were scheduled to coincide with the follicular phase so as not to overlap with menstruation and ovulation phases, which have known associations with sleep and metabolism.
Study Design.
Eligible participants were scheduled for a 3-d and two-night laboratory stay, which was preceded by 1 wk of actigraphy and sleep diary to determine habitual bedtime for the laboratory stay. Participants were randomized to take part in one of two conditions that were run in parallel: the room light condition or the dim light condition.
During the laboratory stay, participants had an 8-h sleep opportunity each night starting at habitual bedtime. Participants in the room light condition slept in dim light (<3 lx) on Night 1 and slept in overhead room lighting (100 lx; four 60-W incandescent overhead ceiling light bulbs [AERO-TECH Light Bulb Co., item No. 60A19/CL]) on Night 2. Participants in the dim light condition slept in dim light (<3 lx) on both Nights 1 and 2. Dim light < 3 lx was chosen as it is unlikely to suppress and phase shift melatonin levels (23); room lighting of 100 lx was chosen as this is within the range of light level reported in previous experimental work as typical ambient indoor room illumination (23, 51–53), and this light level has been shown able to elicit melatonin suppression and phase shifts (23, 54). Wake time room lighting was controlled at 240 lx. Meals were given 2.5, 5, and 11 h after wake, and participants had 30 min to consume each meal. Snacking and caffeine were not permitted during the study. On admission day (Day 0), a sterile, heparin-lock catheter was inserted into the nondominant forearm with the intravenous line kept patent by a slow drip of heparinized saline, for serial blood sampling. On the mornings of Day 1 and 2, a 2-h OGTT was performed.
Participants consumed standard hospital breakfast, lunch, and dinner meals (∼1,900 Kcal/day providing 100 grams protein, 235 grams carbohydrates, and 65 grams fat). Physical activity was controlled during the in-laboratory protocol. Participants stayed in their individual rooms for the entire duration of the protocol and were instructed to sit in the chair during testing and to remain seated or could stand when not involved in study-specific procedures. Participants were not allowed to engage in physical exercise during the in-laboratory protocol. Compliance was ensured via monitoring by study staff, nursing staff, and closed-circuit television.
In-Laboratory Measures.
Glucose and insulin.
On Day 1 and 2, fasting blood samples were taken 20 min after wake. Then 10 min later, a 75-g glucose bolus (Trutol) was ingested at the start of the OGTT. Blood samples were taken 10, 20, 30, 60, 90, and 120 min after ingestion of the glucose bolus. Glucose was measured using the glucose oxidase method on a Beckman CX3D Analyzer. Insulin was measured by chemiluminescent immunoassay on a Siemens Immulite 2000 with sensitivity limited to 14.4 pmol/L.
PSG.
Overnight PSG was recorded on both nights (Neurofax, EEG-1100 Digital EEG Acquisition System, Nihon-Kohden 8.0) with a sample frequency of 500 Hz. Electroencephalographic (EEG) recordings were obtained from frontal (F3, F4), central (C3, C4), and occipital (O1, O2) channels referenced to the contralateral mastoid. In addition, right and left electro-oculograms, two submental electromyograms, and two electrocardiogram (ECG) leads placed one above the collarbone on the right side and the other below the ribs on the left side, were obtained. On Night 1, nasal/oral airflow, abdominal and chest belts, and bilateral tibialis anterioris leads were also placed to determine the presence of sleep disordered breathing and/or periodic limb movements during sleep.
Subjective sleepiness.
Subjective sleepiness was measured via the KSS every 2 h during the wake period (62).
Plasma melatonin.
Blood samples were collected hourly during wake and sleep. Melatonin was measured by radioimmunoassay (IBL RE29301). The standard range of sensitivity for this assay is from 3 to 300 pg/mL
Daytime BP and HR.
BP was measured hourly during wake by trained clinical staff, following at least 5 min with participants in a seated position. HR was collected by a nurse every 4 h as part of vitals.
VAS.
VAS was given every 2 h during wake to assess subjective hunger (VAS-H) and subjective global vigor and affect (VAS-GVA) (63, 64).
Data Analysis
Actigraphy.
Actiwatches were set with 30-s epoch length and medium sensitivity. Actigraphy data were scored in conjunction with sleep diaries using Actiware (version 5, Philips Respironics). The following sleep parameters were then calculated using default settings: average sleep start and end time, sleep duration (the amount of time between sleep start and sleep end scored as “sleep”), and SE (the proportion of time from rest start to rest end scored as “sleep”) (65).
Glucose and insulin.
HOMA-IR was calculated as [(glucose (mg/dL)) × (insulin (µU/mL)) / 405] using fasting glucose and insulin samples taken 20 min after wake. Area under the curve (AUC) analyses of glucose and insulin were conducted using the trapezoidal method (66) (PRISM 4.0 software, GraphPad) for the duration of the OGTT and additionally for the first 30 min post ingestion of the glucose bolus for insulin to examine the early phase of insulin secretion (67). Additionally, Matsuda insulin sensitivity index was calculated as 10,000/square root of [fasting glucose × fasting insulin] × [mean glucose × mean insulin during OGTT]) (68).
Sleep macrostructure.
PSG recordings were visually scored according to the American Academy of Sleep Medicine scoring criteria (69) by an experienced scorer (D.G.) blinded to the participants’ condition (room light versus dim light). PSG measures were calculated for: TST (duration in minutes of time spent asleep), SE (% of time spent asleep over the entire recording period), time (duration in minutes and % of the time spent asleep) spent in each stage of sleep N1, N2, N3 (also known as SWS), and REM sleep, sleep onset latency (time from lights off to the first 30-s epoch scored as N1 or N2), WASO (time in minutes spent awake after sleep onset and before lights on), and AI (number of arousals lasting at least 3 s in duration per hour of sleep) (70). PSG recordings were also used to derive measures of sleep fragmentation and sleep–wake stage stability (as detailed in the SI Appendix).
Sleep microstructure.
EEG spectral analysis was performed on the C3 derivation using a spectral analysis software package (PRANA, PhiTools). A Fast Fourier Transform (4-s window, 50% overlap) was applied, and mean power spectral estimates were extracted in 30-s epochs by sleep stage. Spectral power in the SWA (0.5 to 4 Hz) and SO activity (0.5 to 1 Hz) were extracted and calculated for the entire duration of NREM sleep. Cycle analysis was performed to examine the time-course of SWA and SO activity changes across the night. Sleep cycles were defined using modified Feinberg and Floyd criteria (71). The first four sleep cycles were included in the analysis.
Melatonin.
Analysis of 24-h melatonin levels was conducted on two 24-h periods for each participant. The first 24-h period was from 10 h post wake on Day 0 to 10 h post wake on Day 1 (including Night 1). The second 24-h period was from 10 h post wake on Day 1 to 10 h post wake on Day 2 (including Night 2) (Fig. 1). For each 24-h melatonin profile, a threshold was calculated as the mean of three low consecutive daytime values plus twice the SD of these points. Melatonin onset was defined as the time of the first value to rise and remain above the threshold. Melatonin offset was defined as the time of the first value to fall and remain below the threshold (72).
The AUC relative to the 8-h sleep period was calculated for each night of the study using the trapezoidal method (66) (PRISM 4.0 software, GraphPad).
HR and HRV during the sleep period.
HR measurements obtained from PSG were analyzed using a dedicated software (PRANA, PhiTools). ECG artifacts were first automatically detected and then visually inspected before being removed from the analysis. Finally, HR was averaged every 10 min across the night (73). For the analysis of HRV, interbeat intervals time series were obtained using PRANA software in accordance with standard guidelines (74), and verification was done to ensure that only “normal-to-normal” R waves times were included (75). Power spectra were calculated using fast Fourier transform on time windows with a stable signal of at least 5 min for the entire duration of the sleep period. Spectral power was calculated in the high-frequency band (HF: 0.15 to 0.40 Hz) reflecting mostly parasympathetic activity, and in the low-frequency band (LF: 0.04 to 0.14 Hz) reflecting a combination of vagal and sympathetic activities. HF and LF of HRV were analyzed as relative power (percentage of total HRV power). The LF/HF ratio was calculated as an indicator of sympathovagal balance (74).
Statistics.
Comparison between room light and dim light conditions for demographics and baseline actigraphy characteristics were performed using Student’s t tests or nonparametric and Wilcoxon rank sum tests as necessary due to significant nonnormality of the observations. Normality assumptions for pairwise differences were checked using the Shapiro–Wilk test. Differences in glucose and insulin (HOMA-IR, AUC at 120 and 30 min, Matsuda index), PSG measures, and melatonin (AUC of the 8-h sleep period, melatonin onset and offset) from Day/Night 2 to Day/Night 1 between room light and dim light conditions were analyzed using Student’s t tests.
Generalized linear models (GLM) for repeated measures were used to assess differences between room light and dim light conditions from Day/Night 2 to Day/Night 1 in time series measurements of glucose and insulin from OGTT, SWA, and SO activity changes across sleep cycles, daytime BP and HR, HR, and HRV during sleep, KSS, VAS, and 24-h melatonin. The GLM included time (time of day or night or sleep cycle) and day (Day 1 and Day 2, or Night 1 and Night 2) as within-subject factors and condition (room light versus dim light) as between-subject factors. In GLM analysis of HRV, HR was introduced in the model as a covariate. P values were adjusted using the Bonferroni–Dunn method to account for the multiplicity of tests across time. Pairwise correlation coefficients were computed (76) to analyze the relationship between changes in sleep macrostructure, HR, and HRV with metabolic parameters from the OGTT.
For plasma melatonin, two participants in the room light condition and three participants in the dim light condition were excluded from the analysis as more than 25% of the time points were missing due to poor sample quality. For EEG spectral data, two participants, one in each condition, were excluded from the analysis as more than 20% of the EEG recording in one of the two nights had artifacts.
All statistical calculations were performed using JMP 14.0 software (SAS Institute Inc.). Statistical significance was set at P < 0.05. Data are presented as mean ± SDs unless otherwise specified.
Supplementary Material
Acknowledgments
We acknowledge the research participants for taking part in the study and the staff at Northwestern Memorial Hospital’s Clinical Research Unit. Research reported in this publication was supported, in part, by funds from the Center for Circadian and Sleep Medicine at Northwestern University and the NIH's National Center for Advancing Translational Sciences, Grant No. UL1TR001422. I.C.M. was supported, in part, by the NIH Grant Nos. T32HL7909-17, T32HL7901-20, and 8UL1TR000150-05 and the American Heart Association Grant No. 19POST34380188. D.G., S.M.A., K.J.R., and P.C.Z. were funded, in part, by NIH Grant Nos. R01 HL140580 and P01 AG011412. C.D.W. was funded, in part, by T32HL007909. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2113290119/-/DCSupplemental.
Data Availability
Deidentified data to support these findings are available in Arch, the open access Northwestern University Institutional Repository (https://doi.org/10.21985/n2-9zrx-ev05) (77).
References
- 1.Chepesiuk R., Missing the dark: Health effects of light pollution. Environ. Health Perspect. 117, A20–A27 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogard P., The End of Night: Searching for Natural Darkness in an Age of Artificial Light (Hachette, 2013). [Google Scholar]
- 3.Kyba C. C. M., et al. , Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 3, e1701528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prayag A. S., Münch M., Aeschbach D., Chellappa S. L., Gronfier C., Light modulation of human clocks, wake, and sleep. Clocks Sleep 1, 193–208 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fonken L. K., Nelson R. J., Illuminating the deleterious effects of light at night. F1000 Med. Rep. 3, 18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navara K. J., Nelson R. J., The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 43, 215–224 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Cho Y., et al. , Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 32, 1294–1310 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Lunn R. M., et al. , Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 607–608, 1073–1084 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason I. C., et al. , Circadian health and light: A report on the national heart, lung, and blood institute’s workshop. J. Biol. Rhythms 33, 451–457 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin A., The dark side of light: How artificial lighting is harming the natural world. Nature 553, 268–270 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Park Y. M., White A. J., Jackson C. L., Weinberg C. R., Sandler D. P., Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern. Med. 179, 1061–1071 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonken L. K., Nelson R. J., The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 35, 648–670 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Ursino G., Coppari R., Insulin under the influence of light. Swiss Med. Wkly. 150, w20273 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Cheung I. N., et al. , Morning and evening blue-enriched light exposure alters metabolic function in normal weight adults. PLoS One 11, e0155601 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albreiki M. S., Middleton B., Hampton S. M., A single night light exposure acutely alters hormonal and metabolic responses in healthy participants. Endocr. Connect. 6, 100–110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obayashi K., Yamagami Y., Kurumatani N., Saeki K., Bedroom lighting environment and incident diabetes mellitus: A longitudinal study of the HEIJO-KYO cohort. Sleep Med. 65, 1–3 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Obayashi K., Yamagami Y., Kurumatani N., Saeki K., Pre-awake light exposure and sleep disturbances: Findings from the HEIJO-KYO cohort. Sleep Med. 54, 121–125 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Ohayon M. M., Milesi C., Artificial outdoor nighttime lights associate with altered sleep behavior in the American general population. Sleep (Basel) 39, 1311–1320 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho C. H., et al. , Impact of exposure to dim light at night on sleep in female and comparison with male subjects. Psychiatry Investig. 15, 520–530 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho J. R., Joo E. Y., Koo D. L., Hong S. B., Let there be no light: The effect of bedside light on sleep quality and background electroencephalographic rhythms. Sleep Med. 14, 1422–1425 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Van Cauter E., Spiegel K., Tasali E., Leproult R., Metabolic consequences of sleep and sleep loss. Sleep Med. 9 (suppl. 1), S23–S28 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gil-Lozano M., et al. , Short-term sleep deprivation with nocturnal light exposure alters time-dependent glucagon-like peptide-1 and insulin secretion in male volunteers. Am. J. Physiol. Endocrinol. Metab. 310, E41–E50 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Zeitzer J. M., Dijk D. J., Kronauer R., Brown E., Czeisler C., Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J. Physiol. 526, 695–702 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewy A. J., Wehr T. A., Goodwin F. K., Newsome D. A., Markey S. P., Light suppresses melatonin secretion in humans. Science 210, 1267–1269 (1980). [DOI] [PubMed] [Google Scholar]
- 25.Gooley J. J., et al. , Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 96, E463–E472 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMullan C. J., Schernhammer E. S., Rimm E. B., Hu F. B., Forman J. P., Melatonin secretion and the incidence of type 2 diabetes. JAMA 309, 1388–1396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMullan C. J., Curhan G. C., Schernhammer E. S., Forman J. P., Association of nocturnal melatonin secretion with insulin resistance in nondiabetic young women. Am. J. Epidemiol. 178, 231–238 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S., Singh H., Ahmad N., Mishra P., Tiwari A., The role of melatonin in diabetes: Therapeutic implications. Arch. Endocrinol. Metab. 59, 391–399 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Mulder H., Nagorny C. L. F., Lyssenko V., Groop L., Melatonin receptors in pancreatic islets: Good morning to a novel type 2 diabetes gene. Diabetologia 52, 1240–1249 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Tuomi T., et al. , Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 23, 1067–1077 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Garaulet M., et al. , Melatonin effects on glucose metabolism: Time to unlock the controversy. Trends Endocrinol. Metab. 31, 192–204 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheuermaier K. D., Lee J. H., Duffy J. F., Phase shifts to a moderate intensity light exposure in older adults: A preliminary report. J. Biol. Rhythms 34, 98–104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeitzer J. M., Ruby N. F., Fisicaro R. A., Heller H. C., Response of the human circadian system to millisecond flashes of light. PLoS One 6, e22078 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian J., Scheer F. A. J. L., Circadian system and glucose metabolism: Implications for physiology and disease. Trends Endocrinol. Metab. 27, 282–293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheer F. A. J. L., Van Doornen L. J. P., Buijs R. M., Light and diurnal cycle affect autonomic cardiac balance in human; Possible role for the biological clock. Auton. Neurosci. 110, 44–48 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Scheer F. A. J. L., van Doornen L. J. P., Buijs R. M., Light and diurnal cycle affect human heart rate: Possible role for the circadian pacemaker. J. Biol. Rhythms 14, 202–212 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Scheer F. A. J. L., Buijs R. M., Light affects morning salivary cortisol in humans. J. Clin. Endocrinol. Metab. 84, 3395–3398 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Dijk D. J., Slow-wave sleep, diabetes, and the sympathetic nervous system. Proc. Natl. Acad. Sci. U.S.A. 105, 1107–1108 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreira M. C. S., et al. , Does the sympathetic nervous system contribute to the pathophysiology of metabolic syndrome? Front. Physiol. 6, 234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorp A. A., Schlaich M. P., Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J. Diabetes Res. 2015, 341583 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esler M., et al. , Sympathetic nervous system and insulin resistance: From obesity to diabetes. Am. J. Hypertens. 14, 304S–309S (2001). [DOI] [PubMed] [Google Scholar]
- 42.Yamauchi M., et al. , Effects of environment light during sleep on autonomic functions of heart rate and breathing. Sleep Breath. 18, 829–835 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Hasegawa-Ohira M., Kato Y., Nomura S., Effects of LED lighting exposure during sleep on endocrine and autonomic nervous system activity. IEEJ Trans. Electr. Electron. Eng. 14, 894–898 (2019). [Google Scholar]
- 44.Gabel V., Miglis M., Zeitzer J. M., Effect of artificial dawn light on cardiovascular function, alertness, and balance in middle-aged and older adults. Sleep (Basel) 43, zsaa082 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Weir G. C., Bonner-Weir S., Five stages of evolving β-cell dysfunction during progression to diabetes. Diabetes 53 (suppl. 3), S16–S21 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Kahn S. E., The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46, 3–19 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Bunt J. C., Krakoff J., Ortega E., Knowler W. C., Bogardus C., Acute insulin response is an independent predictor of type 2 diabetes mellitus in individuals with both normal fasting and 2-h plasma glucose concentrations. Diabetes Metab. Res. Rev. 23, 304–310 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian J., Block G. D., Colwell C. S., Matveyenko A. V., Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes 62, 3469–3478 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor K. S., et al. , Arousal from sleep and sympathetic excitation during wakefulness. Hypertension 68, 1467–1474 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Vethe D., et al. , The evening light environment in hospitals can be designed to produce less disruptive effects on the circadian system and improve sleep. Sleep (Basel) 44, zsaa194 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang A. M., Scheer F. A., Czeisler C. A., The human circadian system adapts to prior photic history. J. Physiol. 589, 1095–1102 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cajochen C., Zeitzer J. M., Czeisler C. A., Dijk D. J., Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav. Brain Res. 115, 75–83 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Boivin D. B., Czeisler C. A., Resetting of circadian melatonin and cortisol rhythms in humans by ordinary room light. Neuroreport 9, 779–782 (1998). [DOI] [PubMed] [Google Scholar]
- 54.Burgess H. J., Molina T. A., Home lighting before usual bedtime impacts circadian timing: A field study. Photochem. Photobiol. 90, 723–726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ando K., Kripke D. F., Light attenuation by the human eyelid. Biol. Psychiatry 39, 22–25 (1996). [DOI] [PubMed] [Google Scholar]
- 56.Bierman A., Figueiro M. G., Rea M. S., Measuring and predicting eyelid spectral transmittance. J. Biomed. Opt. 16, 067011 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Phillips A. J. K., et al. , High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl. Acad. Sci. U.S.A. 116, 12019–12024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grassi G., et al. , Heart rate as marker of sympathetic activity. J. Hypertens. 16, 1635–1639 (1998). [DOI] [PubMed] [Google Scholar]
- 59.Saito Y., et al. , Effect of bright light exposure on muscle sympathetic nerve activity in human. Neurosci. Lett. 219, 135–137 (1996). [DOI] [PubMed] [Google Scholar]
- 60.Niijima A., Nagai K., Nagai N., Nakagawa H., Light enhances sympathetic and suppresses vagal outflows and lesions including the suprachiasmatic nucleus eliminate these changes in rats. J. Auton. Nerv. Syst. 40, 155–160 (1992). [DOI] [PubMed] [Google Scholar]
- 61.Szabadi E., Functional organization of the sympathetic pathways controlling the pupil: Light-inhibited and light-stimulated pathways. Front. Neurol. 9, 1069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akerstedt T., Hume K., Minors D., Waterhouse J., The subjective meaning of good sleep, an intraindividual approach using the Karolinska Sleep Diary. Percept. Mot. Skills 79, 287–296 (1994). [DOI] [PubMed] [Google Scholar]
- 63.Monk T. H., A visual analogue scale technique to measure global vigor and affect. Psychiatry Res. 27, 89–99 (1989). [DOI] [PubMed] [Google Scholar]
- 64.Parker B. A., et al. , Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur. J. Clin. Nutr. 58, 212–218 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Reid K. J., et al. , Sleep during pregnancy: The nuMoM2b pregnancy and sleep duration and continuity study. Sleep (Basel) 40, zsx045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salas S., Hille E., Calculus: One and Several Variables with Analytic Geometry (John Wiley and Sons Inc., 1982). [Google Scholar]
- 67.Radziuk J., Homeostastic model assessment and insulin sensitivity/resistance. Diabetes 63, 1850–1854 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Matsuda M., DeFronzo R. A., Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999). [DOI] [PubMed] [Google Scholar]
- 69.American Academy of Sleep Medicine, International Classification of Sleep Disorders (American Academy of Sleep Medicine, ed. 2, 2005). [Google Scholar]
- 70.Iber C., Ancoli-Israel S., Chesson A. L., Quan S. F.; American Academy of Sleep Medicine, The AASM Manual for the Scoring of Sleep and Associated Events: Rules Terminology and Technical Specifications (American Academy of Sleep Medicine, ed. 1, 2007). [Google Scholar]
- 71.Feinberg I., Floyd T. C., Systematic trends across the night in human sleep cycles. Psychophysiology 16, 283–291 (1979). [DOI] [PubMed] [Google Scholar]
- 72.Voultsios A., Kennaway D. J., Dawson D., Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. J. Biol. Rhythms 12, 457–466 (1997). [DOI] [PubMed] [Google Scholar]
- 73.Reid K. J., et al. , Effects of manipulating body temperature on sleep in postmenopausal women. Sleep Med. 81, 109–115 (2021). [DOI] [PubMed] [Google Scholar]
- 74.M. Malik et al., Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the European society of cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–1065 (1996). [PubMed] [Google Scholar]
- 75.Fraden J., Neuman M. R., QRS wave detection. Med. Biol. Eng. Comput. 18, 125–132 (1980). [DOI] [PubMed] [Google Scholar]
- 76.Rousseeuw P. J., van Zomeren B. C., Unmasking multivariate outliers and leverage points. J. Am. Stat. Assoc. 85, 633–639 (1990). [Google Scholar]
- 77.I. C. Mason et al., Light exposure during nighttime sleep. Northwestern University ARCH. 10.21985/n2-9zrx-ev05. Deposited 27 October 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data to support these findings are available in Arch, the open access Northwestern University Institutional Repository (https://doi.org/10.21985/n2-9zrx-ev05) (77).