Abstract
Consistent engagement in care is associated with positive health outcomes among people living with HIV (PLWH). However, traditional retention measures ignore the evolving dynamics of engagement in care. To understand the longitudinal patterns of HIV care, we analyzed medical records from 2008–2015 of PLWH >18 years-old receiving care at a public, hospital-based HIV clinic (N=2,110). Using latent class analysis, we identified five distinct care trajectory classes: (1) consistent care (N=l,281); (2) less frequent care (N=270); (3) return to care after initial attrition (N=192); (4) moderate attrition (N=163); and (5) rapid attrition (N=204). The majority of PLWH in Class 1 (73.9%) had achieved sustained viral suppression (viral load <200 copies/mL at last test and >12 months prior) by study end. Among the other care classes, there was substantial variation in sustained viral suppression (61.1% in Class 2 to 3.4% in Class 5). Care trajectories could be used to prioritize re-engagement efforts.
Keywords: HIV, retention in care, care patterns, latent class analysis, sustained viral suppression
RESUMEN
La participación constante en el cuidado médico se asocia con resultados de salud positivos entre las personas que viven con el VIH (PVVIH). Sin embargo, las medidas de retención tradicionales no tienen en cuenta los cambios constantes en la dinámica de adherencia de los pacientes en el cuidado de su salud. Para entender los patrones longitudinales de la retención en atención médica al VIH, analizamos los registros médicos de 2,110 PVVIH ≥18 años de edad que recibieron atención en una clínica de VIH de un hospital público entre 2008 y 2015. Utilizando un análisis de clases latentes, identificamos cinco clases distintas de trayectorias de retención en la atención: (1) retención constante (N=l,281); (2) retención menos frecuente (N=270); (3) retomo al cuidado médico después del abandono inicial (N=192); (4) abandono ocasional (N=163); (5) abandono rápido (N=205). La mayoría de las PVVIH en la Clase 1 (73.9%) alcanzaron supresión viral sostenida (carga viral <200 copias/mL en todas las cuentas virales disponibles entre 12 meses antes del final del seguimiento y en la última carga viral disponible. Entre las otras clases de adherencia, hubo una variación sustancial en la supresión viral sostenida (de 61.1% en Clase 2 a 3.4% en Clase 5). Las trayectorias de atención podrían usarse para priorizar los esfuerzos de re-integración a la atención médica.
INTRODUCTION
Biomedical advances have transformed HIV infection into a chronic condition for those who can access antiretroviral therapy (ART) and achieve viral suppression [1, 2], Viral suppression through ART also has the public health benefit of dramatically reducing a person’s infectivity to negligible levels [3,4], However, the promise of ART can only be realized if people living with HIV (PLWH) have regular engagement with healthcare providers. In 2015, it was estimated that in the US only 57.2% of PLWH were retained in care [5]. Inconsistent engagement in care has been associated with increased risks of death [6–8], viral non-suppression [9–11], disease progression [11, 12], and drug resistance [13]. In contrast, regular medical care is associated with a higher likelihood of being prescribed ART, higher levels of adherence to ART regimens, and higher rates of viral suppression [14, 15], Retention in care is therefore critical for both individual and public health outcomes.
While regular engagement in care consistently yields better health outcomes than irregular or infrequent care, there is no gold-standard definition of retention in care [16, 17]. A definition used by the US Centers for Disease Control and Prevention (CDC), the US National HIV/AIDS Strategy, and others is having 2 visits in a 12-month period that are at least 3 months apart [5, 16], A number of other alternative retention measures have been suggested, such as visit constancy (visits every three, four, or six months), visit adherence (proportion of scheduled visits attended), or gaps in care [17–19], Comparisons of these different retention in care measures have found that some of these alternatives, such as visit adherence and visit constancy, may be better predictors of viral suppression than the more commonly used CDC definition, though all measures are significantly associated with viral suppression outcomes [17, 18].
Having an agreed-upon standard measure of retention in care would have the advantage of facilitating comparability across different clinics and patient populations. However, regardless of the metric used, standard approaches to calculating retention in care are limited in that they only capture a patient’s care status over a limited timeframe, typically just the past 12 or 24 months. Notably, these measures may not reflect a patient’s longitudinal, evolving experience of engagement in care that often has periods of both retention and non-retention [16, 18,20–22], Attempts to extend standard measures to capture more longitudinal patterns have typically done so by calculating the proportion of intervals in which a patient meets the definition for retention in care, yielding a continuous measure between 0 and 1 [18, 19], Interpreting the findings from these types of analyses can be challenging, as patients with the same level of overall retention may still have vastly different patterns of care. For example, a retention level of 50% could reflect one long absence from care or several shorter, intermittent gaps in care. To elucidate this ambiguity, more recent longitudinal examinations of retention in care have used multi-state models to capture temporal care dynamics in terms of a number of mutually-exclusive care “states” that reflect different levels of engagement in care [21, 22], While these studies better reflect complex longitudinal dynamics of HIV patient care, they still rely on a set of pre-defined categories of care statuses through which patient may transition.
In this analysis, we use a data-driven approach to identify common longitudinal patterns of HIV care and classify PLWH who receive care at a large, public HIV clinic into different classes based on their care patterns. Unlike previous studies of retention in care, we do not presuppose criteria for classifying patients into different retention categories. Instead, we use latent class analysis to construct the relevant “care classes” based on the observed care patterns. This kind of data-driven analysis provides a tractable approach to describing the common longitudinal patterns of care exhibited by a specific clinic population. Latent class analysis has been previously applied to patterns of ART adherence [23], illicit drug use [24–26], and sexual behavior [27], This is the first application of latent class analysis to retention in HIV care.
METHODS
This study was approved by the internal review boards of the University of Minnesota and the Hennepin Healthcare Research Institute (on behalf of the Hennepin County Medical Center).
Population and data sources
The study population consisted of all adult HIV+ patients seen at least once between 2008–2013 by a prescribing provider (medical doctor or nurse practitioner) at the Positive Care Center (PCC), a hospital-based HIV clinic at the Hennepin County Medical Center in Minneapolis, MN. The PCC cares for a racially diverse, urban, high-poverty patient population and is the largest provider of HIV care in Minnesota. In 2013, the clinic saw 1,685 HIV+ patients, which represents 22% of all PLWH in Minnesota and 42% of PLWH who reside in the twin cities of Minneapolis and St. Paul [28], The study population consisted of both established patients (those with a history of care at the PCC prior to 2008) and patients newly initiating care at the PCC during the 2008–2013 eligibility period. Patients younger than 18 years old at their first observed PCC visit were excluded.
Electronic medical record (EMR) data from the PCC were extracted for all qualifying individuals for all clinic visits and HIV-related laboratory tests between January 1, 2008 and December 31, 2015. Using data through 2015 ensured that patients who established care at the PCC towards the end of our eligibility period (2008–2013) still had the potential for at least two years of observed care patterns. It has been shown that clinic data alone can substantially over-estimate the proportion of patients who are out of care due to unobserved deaths, transfer of care to other clinics, and relocation out of state [29, 30], We therefore merged clinic EMR data with HIV surveillance data from the Minnesota Department of Health (MDH) to account for these events. The MDH HIV surveillance unit conducts regular reviews of national mortality records to update the vital status of PLWH in the state; thus, HIV surveillance records have more complete mortality information than EMR data, which will only include a record of death if the patient died while in the care of the same healthcare system. The HIV surveillance unit also conducts data harmonization exercises to identify PLWH who have moved to other states to maintain accurate estimates of the number of PLWH in the state. Finally, the transfer of a PCC patient to another clinic was determined using HIV-related laboratory test results that are reported statewide to the enhanced HIV/AIDS Reporting System (eHARS) database. In Minnesota, voluntary reporting of viral load and CD4 count results began in 2008 and became mandatory in 2011. PLWH whose most recent laboratory results in eHARS were not reported by the PCC were classified as having transferred care to another clinic. The date of that transfer was recorded as the first laboratory test result reported by a non-PCC provider. Due to data access restrictions at the time of data extraction, MDH surveillance data were only available for 2008–2014; thus, we could not account for any patient transfers, relocations, or deaths that occurred in 2015, the final year for which we had EMR data. We explored the impact of this data limitation in a sensitivity analysis in which we repeated the analysis below restricted to the end of 2014. Personal identifiers were removed prior to analysis.
Latent class analysis
For the purposes of this analysis, we defined a care event as a clinic visit with a PCC prescribing provider or an HIV-related laboratory test result. From the EMR data, we constructed patient-specific care trajectories by dividing the time from a patient’s first observed care event until death, transfer, relocation, or the end of 2015 (whichever came first) into six-month intervals. For a patient i and interval j. we defined a variable ti (j). which we set equal to 1 if a patient had at least one care event in that interval, and 0 otherwise. The maximum trajectory length was J= 16 intervals (the number of six-month intervals from 2008 through 2015). Note that in the first interval, ti;(l) = 1 for every patient i as we constructed each patient’s trajectory starting from their first observed care event. For patients observed for fewer than the maximum number of six-months intervals (e.g., due to death, transfer, or relocation or for patients whose first observed care event occurred in the latter half of 2008 or later), we assumed that trajectory values after the end of the observation period were missing. Patients who had an observation period of less than one year were excluded from our analysis. Examples of possible care trajectories are illustrated in Figure 1.
Figure 1:
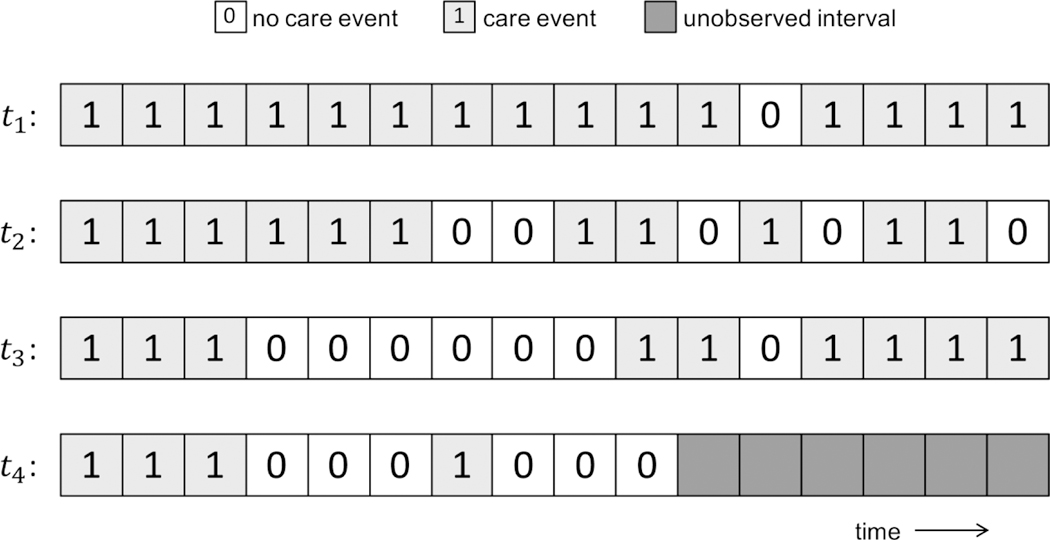
Hypothetical HIV care trajectories for four individual patients. In our latent class analysis, trajectory t1 would ultimately be classified as Class 1, t2 as Class 2, t3 as Class 3, and t4 as Class 4 or 5.
We applied latent class analysis to patient care trajectories to group patients into “care classes”. Latent class analysis is a statistical method for categorizing individuals into distinct subgroups based on observed variables [31], For a predetermined number of classes, K, class membership and class characteristics are determined such that the following likelihood function is maximized (eq. 1):
where pi (c) is the probability that patient i is a member of class c and p(ti \θ, c) is the probability of observing care trajectory ti given membership in class c. In our analysis, we modeled care trajectories as sets of J independent (conditioned on class membership), binomial random variables. Thus, we formulated the likelihood of observing a trajectory ti as (eq. 2):
where θc(j) is the probability of having a care event in interval j for a patient in class c. The parameters estimated by the latent class model were therefore pi(c) and θc(j) for c = {1, … ,K}. j = {1, …,J}. and i = {1,…N}.
We implemented the latent class analysis in R v.3.1.3 using the poLCA package [32, 33], which uses the iterative expectation-maximization (EM) algorithm to maximize the likelihood, In the poLCA implementation, missing values are handled by excluding them in the calculation of The EM algorithm requires the specification of initial values for the parameters that govern the probability that an individual is in each of the classes. The algorithm then iteratively updates these parameters and the remaining parameters in the expected value of the complete data log-likelihood until this quantity stops increasing (within some arbitrarily small tolerance) with subsequent iterations. We estimated the latent class model using 50 random starting values and took the best-fitting model (i.e., the set of parameters resulting in the largest likelihood) as the final model to avoid local maxima. Since the number of classes, K, needs to be pre-specified but is generally unknown, we varied K from 2 to 10 and selected the number of classes that minimized the Bayesian Information Criterion (BIC), a measure that balances model complexity and model fit, as our final latent class model [31, 32].
Patient outcomes
We evaluated how patient characteristics and health outcomes varied across the care classes. Patient characteristics included sex, age, race, and receipt of Ryan White health insurance benefits during the study period. Health outcomes included cumulative mortality by study end, viral suppression at last viral load, and sustained viral suppression. Viral suppression was defined as a viral load of ≤200 copies/mL, consistent with CDC definitions [5], Sustained viral suppression was defined as being virally suppressed at last viral load and having evidence of consistent viral suppression for at least 12 months prior. Thus, to be classified as having achieved sustained viral suppression, patients must have had a viral load of ≤200 copies/mL at least 12 months prior to their last viral load test, as well as suppressed status on any intervening viral load test results. Patients who were virally suppressed at last test but did not have a viral load measured more than 12 months prior were classified as not having achieved sustained viral suppression because they had not yet accumulated the evidence to make this determination.
We also evaluated how traditional measures of retention in care varied for patients in different care classes. Consistent with previous studies, we used the CDC definition of retention in care of having at least 2 care events in each 12-month period at least 3 months apart. For each patient, we calculated the proportion of fully observed 12-month periods in which they met the CDC definition. We also calculated the proportion of patients who met CDC’s retention in care criteria for all of their observed 12-month periods. Similar to the latent class analysis, these 12-month periods were defined as starting from each patient’s first observed care event.
Retention as a predictor of viral suppression
The significance of retention in care is often described in terms of its power to predict viral suppression. We compared the predictive power of the care trajectory classifications to that of traditional retention measures by constructing receiver-operating characteristic (ROC) curves. An ROC curve displays the tradeoff between the true positive rate and the false positive rate of a given predictor in detecting an outcome of interest (in our case, sustained viral suppression). The area under the curve (AUC) is a metric used to summarize the discriminatory ability of the measure, with an AUC=1.0 indicating perfect predictive power and an AUC=0.50 being equivalent to random chance [34].
We constructed an ROC curve based on care trajectory class by calculating the true and false positive rates associated with different positivity criteria (i.e., which subset of the five classes are considered “positive” indicators for sustained viral suppression). We constructed a second ROC curve using the proportion of observed 12-month periods in which a patient met CDC retention in care criteria as a predictor of sustained viral suppression. We again varied the positivity criterion, now defined as the threshold on the level of retention that would be considered a “positive” result for sustained viral suppression (e.g., retained in care for at least 50% of intervals, 60% of intervals, and so on), and calculated the resulting true and false positive rates for each threshold. We compared the AUC of each ROC curve to determine which retention in care classification resulted in better discriminatory ability in predicting sustained viral suppression. ROC curves were constructed using the pROC package in R [35], AUC confidence intervals were estimated by generating 5,000 bootstrapped datasets using the boot R package [36, 37] and constructing ROC curves for each dataset.
RESULTS
A total of 2,194 patients ≥18 years old had at least one visit from 2008–2013 at the PCC with a prescribing provider. When clinic data was merged with surveillance data, 84 patients had an observation period shorter than one year due to death (n=17) and transfer/relocation (n=67), yielding a final study population of 2,110 patients. The study population was 71.7% male with a median age of 40 (interquartile range (IQR): 31–48) at first observed care event (Table 1). The study population was 45.8% black and 37.6% white. About a third (33.7%) of patients received Ryan White insurance benefits at some point over the study period. Median baseline CD4 count was 387 (IQR: 233–552) cells/mm3 and 49.7% of the study population was virally suppressed at first observed viral load test. The median observation period was 6.5 (IQR: 3.8–7.8) years with a median of 25 (IQR: 13–38) care events per patient. Over the study period, 264 (12.5%) patients transferred to other providers or relocated out of state and 45 (2.1%) died.
Table 1:
Study population characteristics, summarized overall and stratified by care trajectory class.
| Overall | Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | |
|---|---|---|---|---|---|---|
| N (%) | 2,110 (100.0) |
1,281 (60.7) |
270 (12.8) | 192 (9.1) | 163 (7.7) | 204 (9.7) |
| Patient characteristics – N (% of class) | ||||||
| Sex | ||||||
| Male | 1,512 (71.7) |
899 (70.2) | 194 (71.9) | 145 (75.5) | 117 (71.8) | 157 (77.0) |
| Female | 598 (28.3) | 382 (29.8) | 76 (28.1) | 47 (24.5) | 46 (28.2) | 47 (23.0) |
| Age at first observed care event | ||||||
| <30 | 429 (20.3) | 212 (16.5) | 73 (27.0) | 54 (28.1) | 35 (21.5) | 55 (27.0) |
| 30–50 | 1,330 (63.0) |
815 (63.6) | 175 (64.8) | 116 (60.4) | 106 (65.0) | 118 (57.8) |
| >50 | 351 (16.6) | 254 (19.8) | 22 (8.1) | 22 (11.5) | 22 (13.5) | 31 (15.2) |
| Race | ||||||
| Black | 967 (45.8) | 586 (45.7) | 125 (46.3) | 80 (41.7) | 84 (51.5) | 92 (45.1) |
| White | 793 (37.6) | 486 (37.9) | 91 (33.7) | 90 (46.9) | 58 (35.6) | 68 (33.3) |
| Hispanic | 162 (7.7) | 102 (8.0) | 26 (9.6) | 5 (2.6) | 13 (8.0) | 16 (7.8) |
| Other | 170 (8.1) | 102 (8.0) | 23 (8.5) | 15 (7.8) | 7 (4.3) | 23 (11.3) |
| Missing | 18 (0.9) | 5 (0.4) | 5(1.9) | 2(1.0) | 1 (0.6) | 5 (2.5) |
| Ever received Ryan White health insurance benefits 2008–2015 | ||||||
| Yes | 712 (33.7) | 442 (34.5) | 114 (42.2) | 63 (32.8) | 54 (33.1) | 39 (19.1) |
| No | 1398 (66.3) | 839 (65.5) | 156 (57.8) | 129 (67.2) | 109 (66.9) | 165 (80.9) |
| Virally suppressed at baseline † | ||||||
| Yes | 1048 (49.7) | 672 (52.5) | 135 (50.0) | 63 (32.8) | 83 (50.9) | 95 (46.6) |
| No | 1052 (49.9) | 609 (47.5) | 135 (50.0) | 128 (66.7) | 80 (49.1) | 100 (49.0) |
| Unknown | 10 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 9 (4.4) |
| Baseline CD4 count (cells/mm 3 ) - median (IQR) | 387 (233– 552) | 383 (229– 555) | 397 (243– 564) | 395 (240– 551) | 401 (249– 544) | 389 (224– 556) |
A viral load result ≤ 200 cells/mL at first observed test was considered virally suppressed at baseline.
The study population was optimally divided into five care classes, which was the number of classes that minimized the BIC. The probability of a care event in each six-month interval for each of the five classes is presented in Figure 2. The care classes can be broadly characterized as “consistently in care” (Class 1); “decreasing in frequency of care” (Class 2); “returning to care after initial attrition” (Class 3); “moderate attrition” (Class 4); and “rapid attrition” (Class 5). Assigning patients to their most likely class resulted in the majority (60.7%) of patient belonging to the consistent care class (Class 1). Class 1 was characterized as having a consistently high (>90%) probability of having at least one care event in each six-month interval. The next largest class was Class 2 (decreasing frequency of care), accounting for 12.8% of patients. In this class, the probability of having a care event decreased over time, from 92.5% in the second six-month interval down to 50.9% by the twelfth interval. After this point, the probability of a care event stabilized at around 45%, though confidence intervals increased over time as fewer patients contributed observations to later trajectory intervals. Remaining patients were roughly equally distributed across the last three classes, with 9.1%, 7.7%, and 9.7% of patients assigned to Classes 3, 4 and, 5, respectively. Each of these three classes was characterized by an initial decrease in the probability of a care event. In Class 3, this trend reversed in the fourth six-month interval, with the probability of a care event stabilizing at >75% by the tenth interval. In Classes 4 and 5, the probability of a care event continued to decrease over time, dropping below 10% by the third and eighth intervals, respectively.
Figure 2:
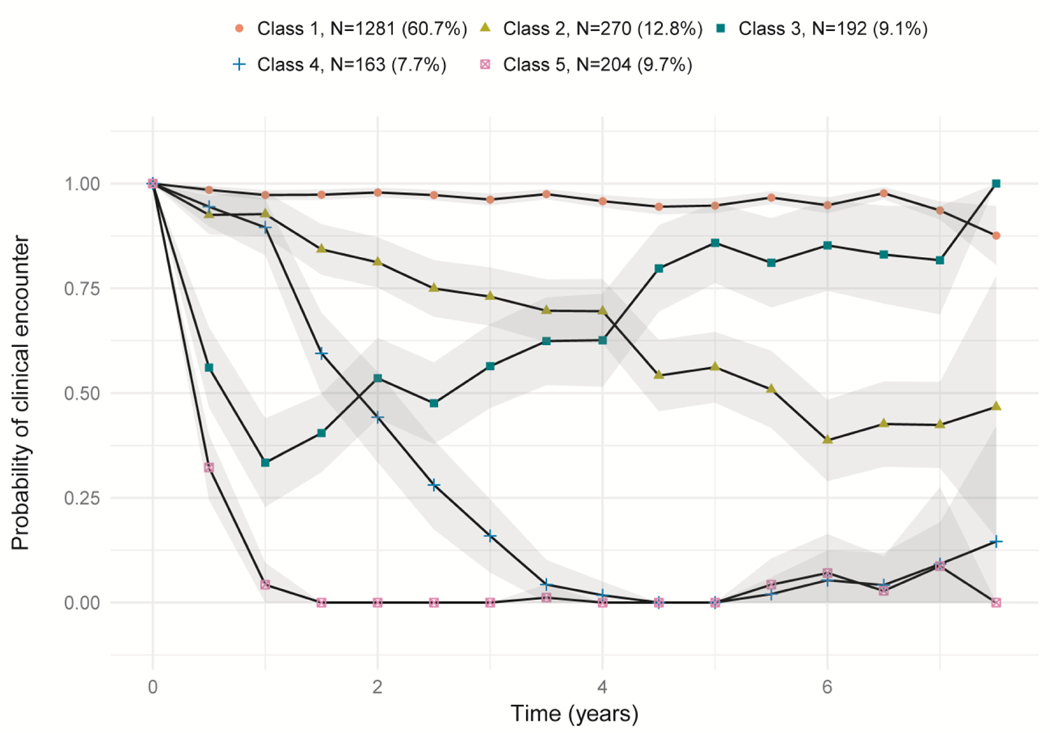
The probability of a care event in each six-month interval for the five care trajectory classes identified through latent class analysis of HIV patient care patterns. Shading indicates 95% confidence intervals around point estimates of the probability of a care event in each interval. Care classes can be broadly characterized as “consistently in care” (Class 1); “decreasing in frequency of care” (Class 2); “returning to care after initial attrition” (Class 3); “moderate attrition” (Class 4); and “rapid attrition” (Class 5).
Patient characteristics (sex, age, race) were similar across the care classes (Table 1). The proportion of patients ever receiving Ryan White insurance benefits over the study period was similar across Classes 1, 3, and 4, but was somewhat higher in Class 2 (42.2%) and was markedly lower in Class 5 (19.1%). Baseline clinical characteristics were similar across the care classes, except for patients in Class 3, who had lower levels of baseline viral suppression (32.8%) than patients in other care classes. Patients in Classes 1 and 2 had longer median observation periods (7.1 and 7.2 years, respectively) and were more likely to have been established patients prior to 2008 (52.4% and 50.5%, respectively) than patients in other classes (Table 2). Patients in Class 5 were the most likely to have transferred care or relocated out of state (25.4%), while very few patients in Class 1 had done so (8.7%). Mortality was highest among patients in Class 1 (2.9%), although the absolute number of deaths was small over the study period.
Table 2:
Patient care dynamics and health outcomes overall and stratified by care trajectory class. A viral load of ≤ 200 cells/mL was considered virally suppressed. Viral suppression status was unknown for patients with no viral load test results over the study period.
| Overall | Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | |
|---|---|---|---|---|---|---|
| Patient status at first observed care event - N (%) | ||||||
| Established patient prior to 2008 | 993 (47.1) | 667 (52.1) | 142 (52.6) | 76 (39.6) | 72 (44.2) | 36 (17.6) |
| New patient | 1,117 (52.9) | 614 (47.9) | 128 (47.4) | 116 (60.4) | 91 (55.8) | 168 (82.4) |
| Length of observation period (years) - median (IQR) | 6.5 (3.8–7.8) | 7.1 (3.9–7.9) | 7.2 (5.3–7.8) | 5.6 (3.8–7.6) | 6.0 (3.7–7.8) | 4.0 (2.4–6.2) |
| Study end events - N (%) | ||||||
| Relocation or provider transfer | 264 (12.5) | 109 (8.5) | 34 (12.6) | 31 (16.1) | 35 (21.5) | 55 (27.0) |
| Mortality | 45 (2.1) | 37 (2.9) | 1 (0.4) | 4(2.1) | 1 (0.6) | 2(1.0) |
| Viral suppression at last test - N (%) | ||||||
| Yes | 1,737 (82.3) | 1,137 (88.8) | 210 (77.8) | 146 (76.0) | 121 (74.2) | 123 (60.3) |
| No | 363 (17.2) | 144 (11.2) | 60 (22.2) | 45 (23.4) | 42 (25.8) | 72 (35.3) |
| Unknown | 10 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.50 | 0 (0.0) | 9 (4.4) |
| Sustained viral suppression - N (%) | ||||||
| Yes | 1,278 (60.6) | 947 (73.9) | 165 (61.1) | 81 (42.2) | 78 (47.9) | 7 (3.4) |
| No | 822 (39.0) | 334 (26.1) | 105 (38.9) | 110 (57.3) | 85 (52.1) | 188 (92.2) |
| Unknown | 10 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 9 (4.4) |
| Retention in care | ||||||
| Proportion of 12-month periods retained in care - median (IQR) | 1.0 (0.57–1.0) | 1.0 (1.0–1.0) | 0.62 (0.50–0.75) | 0.50 (0.33–0.67) | 0.37 (0.29–0.50) | 0.14 (0.0–0.30) |
| Patients retained in care every observed 12-month period - N (%) | 1,063 (50.4) | 1016 (79.3) | 16 (5.9) | 13 (6.8) | 12 (7.4) | 6 (2.9) |
Overall, retention was quite high in the study population, with half of patients meeting the CDC retention in care criteria for all observed 12-month periods (Table 2). This high level of retention was driven by patients in Class 1, 79.3% of whom had full retention over their entire observation periods. Fewer than 10% of patients in other classes were retained in care for every observed 12-month period. Median retention was 62% (IQR: 50–75%) of observed 12-month periods for patients in Class 2, decreasing to 50% (IQR: 33–67%) and 37% (IQR: 29–50%) in Classes 3 and 4. Median retention was lowest in Class 5 at 14% (IQR: 0–30%).
Viral suppression was also relatively high in the study population, with 82.3% of patients having a viral load of ≤200 copies/mL at last viral load test and 60.6% patients having achieved sustained viral suppression in the preceding 12 months. Stratified by class, viral suppression at last test varied from 88.8% (in Class 1) to 60. 3% (in Class 5). Sustained viral suppression had even greater variation across classes, from 73.9% in Class 1 to 42.2% in Class 3. Sustained viral suppression was extremely low in Class 5 (3.4%); however, this was largely driven by patients lacking sufficient viral load histories rather than evidence of detectable viral loads.
We constructed ROC curves to summarize the ability of care trajectory class and the proportion of years retained in care according to CDC’s definition, respectively, to predict sustained viral suppression for different positivity criteria (Figure 3). The two ROC curves had very similar AUCs, with a care trajectory class ROC curve yielding an AUC of 0.70 (95% confidence interval [Cl]: 0.68–0.72) compared to an AUC of 0.69 (95% Cl: 0.67–0.71) using the CDC retention in care definition.
Figure 3:
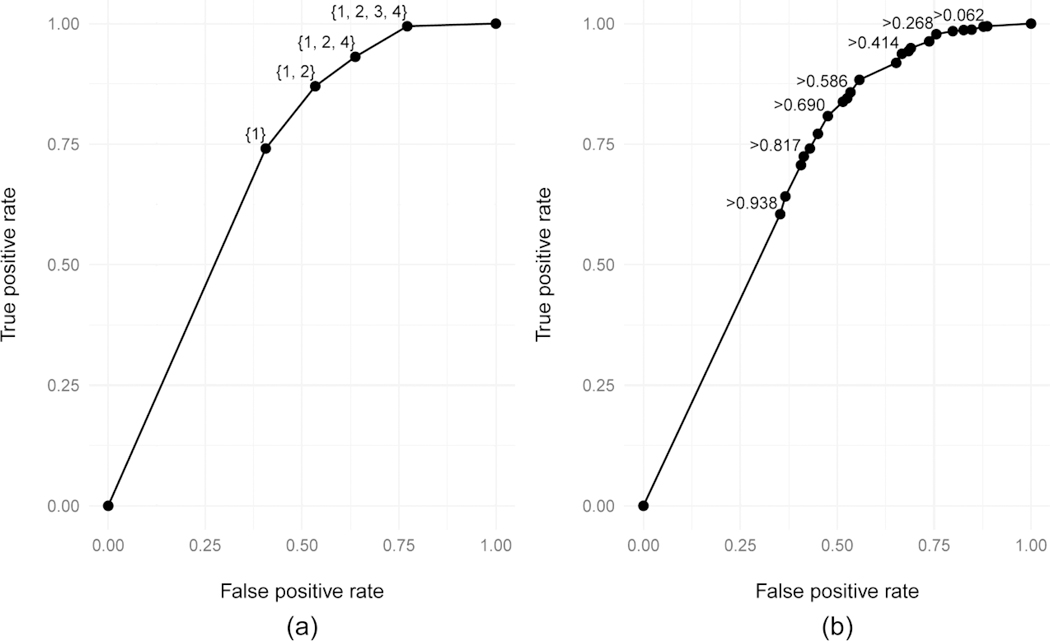
Receiver-operating characteristic (ROC) curves showing the true positive and false positive rates achieved by using (a) care trajectory class membership or (b) the proportion of 12-months periods satisfying CDC retention in care criteria to predict sustained viral suppression status. Select points are labeled with the positivity criterion used to generate that specific true and false positive rate pair. For the care trajectory classes, the label is the subset of classes considered to be a positive result in predicting sustained viral suppression. For the CDC retention in care measure, the label is the lowest retention level which would be considered a positive result in the prediction.
In sensitivity analysis, trajectory class definitions and class sizes were similar regardless of inclusion of the 2015 clinic EMR data (Supplemental Figure 1). Care trajectory classes were also similar when care patterns among patients who had established care at the PCC prior to 2008 were fit separately from patients newly initiating care during the study period, though uncertainty around the estimated care event probabilities for each class was larger due to the smaller sample size in fitting each model (Supplemental Figures 2 & 3). Uncertainty was particularly high in the new patient model at later care intervals, as only a small number of patients who initiated care during the eligibility period were observed for the entire study period. Consistent with patient distributions across classes in the primary latent class model, when new and existing patients were fit in separate models, the consistent care class was larger in established patients while the moderate attrition class was larger in new patients (Supplemental Table 1).
DISCUSSION
Using a longitudinal, data-driven approach, we identified five distinct classes of care patterns among a population of PLWH receiving care at a public, hospital-based HIV clinic. Encouragingly, we saw that the largest care class was one of consistent engagement in care over time (Class 1), consisting of 60.7% of our study population. Patients in Class 1 had over a 90% probability of having a care event in each six-month interval; accordingly, they also had high retention in care as measured by traditional metrics, with 79.3% of patients meeting the CDC criteria for being retained in care in every 12-month period for which they were observed. Patients in Class 1 also had much higher levels of sustained viral suppression than patients in other classes. When used as a predictor of sustained viral suppression, care trajectory class had similar discriminatory power as CDC’s retention in care measure.
The advantages of our analytic approach are most apparent when we consider the 40% of patients not assigned to Class 1. While a traditional analysis would have noted that levels of retention in care were much lower among this group of patients, by using latent class analysis, we were able to identify four distinct patterns of sub-optimal care (Classes 2–5). Notably, these care patterns differed in the temporal trend of care event probabilities, with Classes 4 and 5 having decreasing care probabilities; Class 3 having an initial decline followed by an increase; and Class 2 exhibiting a slower decline and then a plateau at a little less than a 50% probability of care event per six-month interval. These different trajectories may have implication for the severity of the sub-optimal retention. For example, patients in Class 2 may still be having a visit every year even if they are not satisfying the CDC criteria for retention in care in each 12-month period. Intuitively, having regular contact, even if less frequent than would be ideal, seems preferable over having no contact at all, as occurs with patients in Class 4 or 5 as time progresses. We see this reflected in the much high level of viral suppression among patients in Class 2.
Class 3 represents a group of patients who may have had a gap in care in the past and then returned to care. These patients are not necessarily of current concern, as they have a high level of engagement in care at the end of their observation periods. However, identifying the existence of this class of patients is still valuable; if we hypothesize that these trajectories represent typical patterns of care for a cohort of patients, it would be reasonable to think about how to prevent this initial attrition or how to facilitate a timelier return to care. Furthermore, the strategies necessary to address the factors leading to attrition in patients ultimately in Class 3 may be different than for patients with a pattern that evolves according to Class 4 or 5. While our analysis is descriptive in nature, it is unique in the retention in care literature in providing a data-driven process to generate an easily-interpreted characterization of how patients are currently engaging in care. Before sub-optimal retention in care can be fully addressed, these care patterns must be described and understood.
Receipt of Ryan White benefits was notably low in Class 5 at 19.1% compared to 33.7% overall. This may be due to the short amount of time spent at the PCC by many of the patients in Class 5. Patients in Class 5 had the shortest median observation time (4.0 years vs. 6.5 years overall) and the largest proportion of patients transferring to other clinics or relocating out of state (27% vs. 12.5% overall) of any care class. Rather than reflecting differences in eligibility, it may be that many patients in Class 5 simply did not spend a sufficient amount of time at the PCC to enroll in Ryan White benefits programs.
Our analysis was retrospective. An important next step is to develop tools to predict a patients’ future care trajectory that could guide early and real-time intervention strategies to address emerging sub-optimal engagement patterns. We did not find demographic factors to differ substantially across classes, though patients newly establishing care were more likely to fall into Class 4 and less likely into Class 1 than established patients. Following patients prospectively over time and establishing an evolving care classification as more care behavior is observed could be one way to identify patients at risk of reduced engagement in care. Incorporating other co-evolving clinical features, such as viral load, CD4 count, and co-occurring diagnoses, may also be important to account for the influence of a patient’s health status on the appropriate frequency of care. For example, it might be appropriate for virally suppressed patients to decrease their frequency of care, while patients with progressive HIV disease or worsening health status due to other conditions may be advised to increase their frequency of care. Viewing engagement in care as an evolving process for each patient introduces the possibility of applying the same lens to the criteria by which that care is judged. Recognizing that patient needs evolve throughout the HIV life course, it may be appropriate to consider patient-specific retention in care metrics that change over time as a function of a patient’s clinical history and current medical needs.
Our analysis has several limitations. We used state surveillance data to account for death, transfer to other providers, and relocation out-of-state. While surveillance data provide a statewide picture of HIV care, the reporting of this information to state health departments can be delayed or incomplete. These factors may mean that some patients who were assumed to be out of care at the end of their trajectory in the analysis (and assigned a trajectory value of 0) were in fact receiving care elsewhere and should instead have had their PCC care trajectory terminated. This would result in an over-representation of care patterns like those of Classes 4 and 5. Furthermore, we did not have access to any surveillance data for the final year of our analysis; however, repeating the analysis only for years in which we had access to both clinic and surveillance data (2008–2014) did not result in qualitatively different conclusions. In the latent class analysis, we made a number of assumptions in constructing the care trajectories. In particular, we assumed that care patterns were time-homogeneous over the study period, meaning that the care patterns among patients who started care at the PCC in one year (e.g., 2008) were compared to patients who started care in any other included year (e.g., 2012). This assumption is implicit in aligning care trajectories starting from a patient’s first observed care event rather than analyzing trajectories in absolute time. For patients whose observation time was shorter than the maximal eight years, we assumed that the end of their care event trajectory was simply missing after their last observed six-month interval. In the poLCA package implementation, these missing observations are excluded when estimating the care trajectory class parameters. This resulted in widening confidence bounds around the care event probabilities at later time points as there are fewer observations to inform those estimates. Similarly, when estimating individual posterior class probabilities, only observed intervals are used in this calculation. Thus, individuals with shorter observation periods had greater uncertainty in their assigned class membership.
In summary, we developed a novel, data-driven approach for identifying typical patterns of care among HIV patients. Traditional measures of retention in care can be useful quality metrics that facilitate comparability of across clinical settings; our analysis complements these measures by providing a more nuanced characterization of sub-optimal patterns of care that could inform interventions to improve engagement in care or prevent disengagement from care. While the specific care patterns identified in our analysis may not be generalizable to other HIV patient populations, our approach could be readily applied to other HIV clinics seeking to understand the specific care patterns of their patient populations.
Supplementary Material
ACKNOWLEDGEMENTS
Funding for this study was provided by the National Institute for Allergy and Infectious Disease of the National Institutes of Health under award no. K25AI118476 (PI: Enns). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors wish to acknowledge Allison La Pointe and Jessica Munroe at the Minnesota Department of Health for facilitating the merging of clinic data with state surveillance data and Dr. Tenko Raykov at Michigan State University for early discussions on the application of latent class methods in this analysis.
Funding: This study was funded by the National Institute for Allergy and Infectious Disease of the National Institutes of Health under award no. K25AI118476 (PI: Enns).
Disclosures
Funding for this study was provided by the National Institute for Allergy and Infectious Disease of the National Institutes of Health under award no. K25AI118476 (PI: Enns). Portions of this analysis were presented at the 38th Annual Meeting of the Society for Medical Decision Making, Oct. 23–24, 2016 and at the 2018 Conference on Retroviruses and Opportunistic Infections (CROI), March 4–7, 2018.
Footnotes
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Conflict of Interest: Dr. Enns received an honorarium from ViiV Healthcare for participation in a technical advisory meeting unrelated to this work. Dr. Reilly declares that he has no conflict of interest. Dr. Horvath declares that he has no conflict of interest. Ms. Baker-James declares that she has no conflict of interest. Dr. Henry has received research funding unrelated to this work from Merck, Janssen, ViiV/GSK, and Gilead.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- [1].Deeks SG; Lewin SR; Havlir D The End of AIDS: HIV Infection as a Chronic Disease. Lancet 2013;382: 1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Samji H, Cescon A, Hogg RS, et al. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8: 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Attia S, Egger M, Müller M, et al. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS2009; 23: 1397–404. [DOI] [PubMed] [Google Scholar]
- [4].Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Centers for Disease Control and Prevention. Selected National HIV Prevention and Care Outcomes in the United States. 2018; Atlanta, GA, https://www.cdc.gov/hiv/pdf/library/factsheets/cdc-hiv-national-hiv-care-outcomes.pdf (accessed 22 February 2019).
- [6].Giordano TP, Gifford AL, White AC, et al. Retention in Care: A Challenge to Survival with HIV Infection. Clin Infect Dis 2007; 44: 1493–99. [DOI] [PubMed] [Google Scholar]
- [7].Mugavero MJ, Lin H, Willig JH, et al. Missed Visits and Mortality among Patients Establishing Initial Outpatient HIV Treatment. Clin Infect Dis 2009; 48: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Horberg MA, Hurley LB, Silverberg MJ, et al. Missed Office Visits and Risk of Mortality Among HIV-Infected Subjects in a Large Healthcare System in the United States. AIDS Patient Care STDS 2013; 27: 442–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Valdez H, Lederman MM, Woolley I, et al. Human immunodeficiency virus 1 protease inhibitors in clinical practice: predictors of virological outcome. Arch Intern Med 2014; 159: 1771–6. [DOI] [PubMed] [Google Scholar]
- [10].Rastegar DA, Fingerhood MI, Jasinski DR. Highly active antiretroviral therapy outcomes in a primary care clinic. AIDS Care 2003; 15: 231–37. [DOI] [PubMed] [Google Scholar]
- [11].Berg MB, Safren SA, Mimiaga MJ, et al. Nonadherence to medical appointments is associated with increased plasma HIV RNA and decreased CD4 cell counts in a community-based HIV primary care clinic. AIDS Care 2005; 17: 902–07. [DOI] [PubMed] [Google Scholar]
- [12].Park WB, Choe PG, Kim SH, et al. One-year adherence to clinic visits after highly active antiretroviral therapy: A predictor of clinical progress in HIV patients. J Intern Med 2007; 261: 268–75. [DOI] [PubMed] [Google Scholar]
- [13].Sethi AK, Celentano DD, Gange SJ, et al. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis 2003; 37: 1112–18. [DOI] [PubMed] [Google Scholar]
- [14].Horstmann E, Brown J, Islam F, et al. Retaining HIV- Infected Patients in Care: Where Are We? Where Do We Go from Here? Clin Infect Dis 2010; 50: 752–61. [DOI] [PubMed] [Google Scholar]
- [15].Yehia BR, French B, Fleishman JA, et al. Retention in care is more strongly associated with viral suppression in HIV-infected patients with lower versus higher CD4 counts. J Acquir Immune Deflc Syndr 2014; 65: 333–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kay ES, Batey DS, Mugavero MJ. The HIV treatment cascade and care continuum: Updates, goals, and recommendations for the future. AIDS Res Ther 2016; 13: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mugavero MJ, Westfall AO, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr 2012; 61: 574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Crawford TN, Sanderson WT, Thornton A. A comparison study of methods for measuring retention in HIV medical care. AIDS Behav 2013; 17: 3145–51. [DOI] [PubMed] [Google Scholar]
- [19].Yehia BR, Fleishman JA, Metlay JP, et al. Comparing different measures of retention in outpatient HIV care. AIDS 2012; 26: 1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. J Acquir Immune Deflc Syndr 2013; 62: 356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yehia BR, Stephens-Shields AJ, Fleishman JA, et al. The HIV care continuum: Changes over time in retention in care and viral suppression. PLoS One 2015; 10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee H, Wu XK, Genberg BL, et al. Beyond binary retention in HIV care: predictors of the dynamic processes of patient engagement, disengagement, and re-entry into care in a US clinical cohort. AIDS 2018; 32: 2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Glass TR, Battegay M, Cavassini M, et al. Longitudinal analysis of patterns and predictors of changes in self-reported adherence to antiretroviral therapy: Swiss HIV Cohort Study. J Acquir Immune Defic Syndr 2010; 54: 197–203. [DOI] [PubMed] [Google Scholar]
- [24].Watson C-A, Weng CX, French T, et al. Substance abuse treatment utilization, HIV risk behaviors, and recruitment among suburban injection drug users in Long Island, New York. AIDS Behav 2014; 18 Suppl 3: 305–15. [DOI] [PubMed] [Google Scholar]
- [25].Green TC, Kershaw T, Lin H, et al. Patterns of drug use and abuse among aging adults with and without HIV: A latent class analysis of a US Veteran cohort. Drug Alcohol Depend 2010; 110: 208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Parsons JT, Starks TJ, Millar BM, et al. Patterns of substance use among HIV-positive adults over 50: Implications for treatment and medication adherence. Drug Alcohol Depend 2014; 139: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vasilenko SA, Kugler KC, Butera NM, et al. Patterns of adolescent sexual behavior predicting young adult sexually transmitted infections: a latent class analysis approach. Arch Sex Behav 2015; 44: 705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Minnesota Department of Health. HIV/AIDS Prevalence and Mortality Tables - 2013. 2014; St. Paul, MN, www.health.state.mn.us/divs/idepc/diseases/hiv/stats/2013 (accessed 28 February 2018). [Google Scholar]
- [29].Enns EA, Reilly CS, Vimig BA, et al. Potential Impact of Integrating HIV Surveillance and Clinic Data on Retention-in-Care Estimates and Re-Engagement Efforts. AIDS Patient Care STDS 2016; 30: 409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dombrowski JC, Buskin SE, Bennett A, et al. Use of multiple data sources and individual case investigation to refine surveillance-based estimates of the HIV care continuum. J Acquir Immune Deflc Syndr 2014; 67: 323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Collins LM, Lanza ST. Latent Class and Latent Transition Analysis. Hoboken, NJ: John Wiley and Sons, Inc., 2010. [Google Scholar]
- [32].Linzer DA, Lewis JB. poLCA: An R Package for Polytomous Variable Latent Class Analysis. J Stat Softw 2011; 42: 1–29. [Google Scholar]
- [33].R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2018; http://www.R-project.org/. [Google Scholar]
- [34].Hunink MGM, Weinstein MC, Wittenberg E, et al. Decision Making in Health and Medicine: Integrating Evidence and Values. 2nd ed. Cambridge University Press, 2014. [Google Scholar]
- [35].Robin X, Turck N, Hainard A, et al. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Canty A, Ripley B. boot: Boostrap R (S-Plus) Functions. R package version 1.3–20, 2017. [Google Scholar]
- [37].Davison AC, Hinkley DV. Boostrap methods and their applications. Cambridge, UK: Cambridge University Press, 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


