Abstract
It has been postulated that a mutation 11 bp 3′ to the −10 motif of the norA promoter is involved in the increased expression of the gene observed in some strains of Staphylococcus aureus exhibiting efflux-related fluoroquinolone resistance. Introduction of this mutation into the chromosome of a fluoroquinolone-susceptible strain by plasmid integration resulted in the minimum inhibitory concentrations of NorA substrates being increased, fluoroquinolone uptake being reduced, and norA expression being enhanced. Diffuse hybridization of norA and integrating vector probes at a similar molecular weight range, higher than that of the norA transcript, was observed in the integrant, suggesting the possibility of a plasmid-based promoter contributing to norA expression. The ratio of the quantity of this transcript, which was also observed in the parent strain of the integrant, to the quantity of primary norA transcript was 0.14, demonstrating that it was unlikely that this mRNA species contributed significantly to the results observed. It is more likely that the introduced promoter region mutation does affect the expression of norA.
A number of mechanisms by which Staphylococcus aureus develops resistance to fluoroquinolone antimicrobial agents have been described. Selected mutations in the grlA and gyrA genes, encoding the A subunits of topoisomerase IV and DNA gyrase, respectively, and in the gyrB gene (encoding the B subunit of DNA gyrase) correlate with fluoroquinolone resistance (3–6, 16, 20, 21). Up regulation of norA, a naturally occurring gene which encodes a membrane-based multidrug efflux transporter (NorA), also results in raised fluoroquinolone minimum inhibitory concentrations (MICs) (7). These mechanisms can occur singly or in combination, with fluoroquinolone susceptibility generally decreasing as mutations accumulate (4, 9).
It has been shown that some strains that have increased expression of norA have a point mutation in the promoter region of that gene (7, 15). It has been postulated that this mutation, which lies 11 bp 3′ to the −10 motif, is responsible for up regulating norA expression. We investigated this possibility by using plasmid integration to introduce such a mutation into the chromosome of a fluoroquinolone-susceptible S. aureus strain.
MATERIALS AND METHODS
Bacterial strains and plasmids.
SA-1199 is a methicillin- and fluoroquinolone-susceptible clinical isolate of S. aureus. SA-1199B is a fluoroquinolone-resistant mutant of this strain, recovered during ciprofloxacin therapy of experimental endocarditis, that has been shown to possess a grlA mutation in addition to having increased norA expression (7, 9). SA-RN4220 is a fluoroquinolone-susceptible restriction-deficient mutant of S. aureus 8325-4 that readily accepts DNA propagated in Escherichia coli (10). E. coli DH10B was used as the host organism for the propagation of plasmids (12). The plasmid vector utilized for chromosomal integration was pG+Host6, a 6.7-kb construct that possesses erythromycin (EM) and ampicillin resistance markers and can replicate in both S. aureus and E. coli (13). The S. aureus replicon in pG+Host6 is temperature sensitive.
Determination of antimicrobial susceptibilities.
Unless otherwise noted, all antimicrobial agents and other reagents used were of the highest grade available and were obtained from their respective manufacturers or were purchased from Sigma Chemical Co., St. Louis, Mo. MIC testing was performed with a microdilution technique and cation-adjusted Mueller-Hinton II broth (BBL Microbiology Systems, Cockeysville, Md.) according to the guidelines of the National Committee for Clinical Laboratory Standards (14). The effect of reserpine (final concentration, 20 μg/ml) on selected MICs was also determined.
PCR procedures.
We have shown that SA-1199B has a T→A transversion 11 bp 3′ to the −10 motif of the norA promoter (7). PCR was employed to amplify a fragment that included this position. The primers used were 5′-TACATTCAACGGTACCTTCGCCTT-3′ (forward), which introduced an artificial KpnI site (underlined), and 5′-TAACGTACCACCGAATGGCG-3′ (reverse). Parameters for PCR were 94°C (1 min), 55°C (1 min), and 72°C (0.5 min) for 30 cycles. This produced a 461-bp product which then was digested with KpnI and HindII, leaving a 367-bp fragment. This fragment was cloned into pG+Host6 digested with KpnI and SmaI, producing pK120 (7.1 kb), which then was transformed into E. coli DH10B by standard techniques (1).
Plasmid integration.
pG+Host6 and pK120 were recovered from E. coli DH10B and were purified by cesium chloride density-gradient centrifugation (1). These plasmids were electrotransformed into SA-RN4220, with propagation of recipient strains at 28°C on Luria-Bertani (LB) agar plates containing 10 μg of erythromycin (LB-EM) per ml. Selected EM-resistant colonies were shown to contain either pG+Host6 or pK120 by using a miniprep procedure (17). Randomly selected transformants then were grown in 10 ml of LB broth containing 10 μg of EM/ml at 28°C overnight. A 1:100 dilution of this culture was plated onto the same medium and was grown for 2.5 h at 28°C, followed by a shift in the incubation temperature to 39°C for 3 h. The higher temperature inhibits independent plasmid replication and favors integration of pK120 via a single crossover event into the site of homology within the chromosome. Serial dilutions were made onto LB-EM, and the plates were incubated at 37°C overnight. No EM-resistant colonies were recovered from SA-RN4220 transformed with pG+Host6 following exposure to the higher temperature, indicating that nonspecific plasmid integration did not occur. The absence of pK120 in EM-resistant colonies of SA-RN4220 originally transformed with this plasmid was verified by miniprep analysis. One such isolate (SA-K1606) was chosen for further study.
Southern and Northern blotting.
Chromosomal DNA from SA-RN4220 and SA-K1606 was isolated and digested with EcoRV, for which there are no sites in either norA or pK120. Fragments were separated in an agarose gel and transferred to a nylon membrane. Southern hybridization was carried out with probes specific for norA and pG+Host6 (19).
Protoplasts of SA-RN4220 and SA-K1606 were produced by exposing organisms to lysostaphin (30 μg/ml) in SMM buffer (0.5 M sucrose, 0.014 M sodium maleate, 0.02 M MgCl2, pH 6.8) for 45 min on ice (9). Total RNA was isolated by the method of Chomczynski (2). RNA (30 μg) from each strain was applied to and separated in a formaldehyde-containing agarose gel. The RNA was transferred to a nylon membrane, and hybridization with a norA probe produced by PCR from SA-RN4220 was carried out under high-stringency conditions (42°C, 50% formamide) (19). The procedure was repeated with a 341-bp AseI fragment of pG+Host6, which originates just proximal to the integration site, as a probe.
DNA sequence determination.
The nucleotide sequence of the norA region of SA-K1606 was determined by using the dideoxy chain-termination method with primers located within pG+Host6 and norA, but outside of the presumed site of recombination in norA (18).
Transcription initiation and mRNA quantification.
The 5′ termini of the norA mRNAs of all strains examined in this study were mapped by primer extension with a commercially available kit (1) (Promega Corp., Madison, Wis.). The quantity of transcript present was estimated by using a phosphorimaging system according to the manufacturer’s guidelines (Molecular Dynamics, Inc., Sunnyvale, Calif.).
Uptake of [14C]enoxacin.
Uptake studies were performed in quadruplicate with whole cells as described previously (8). [14C]enoxacin (specific activity, 15.9 μCi/mg) was provided by Parke-Davis Pharmaceutical Research, Ann Arbor, Mich. Carbonyl cyanide m-chlorophenylhydrazone (CCCP) (final concentration, 100 μM) was used to dissipate the proton motive force across the cytoplasmic membrane.
Statistics.
Comparison of [14C]enoxacin uptake data was performed by using the rank sum test. A p value of less than 0.05 was considered significant.
RESULTS AND DISCUSSION
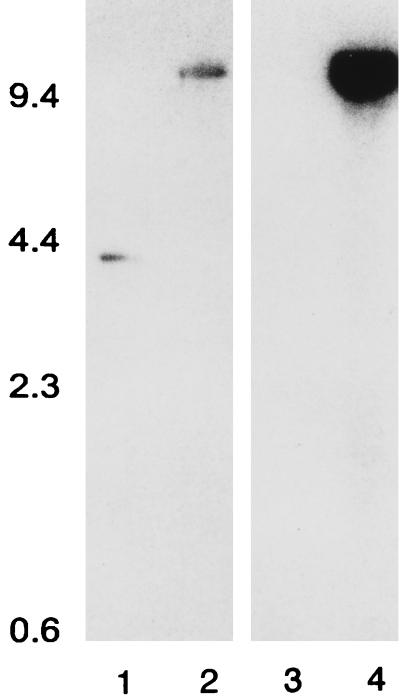
Southern hybridization revealed that the norA and pG+Host6 probes hybridized with an EcoRV fragment of the same size in SA-K1606 (∼10.1 kb) (Fig. 1). This fragment is larger than that observed with the norA probe and native SA-RN4220 DNA by an amount approximately equal to the size of the integrating plasmid. Only the norA probe hybridized with SA-RN4220 DNA (3.4-kb fragment). These data indicated that the vector did integrate into the targeted region of the chromosome. A determination of the nucleotide sequence of the norA region of SA-K1606 confirmed that integration had occurred as expected. The norA promoter of SA-1199B that had been cloned into pG+Host6 was found to be present in place of the wild-type SA-RN4220 norA promoter, and pG+Host6 was upstream of this. The norA gene itself was completely intact. The difference in the intensity of the hybridization signals generated by the norA and pG+Host6 probes is likely due to the fact that the norA probe used in this experiment was produced from SA-1199B by PCR. There is a moderate degree of sequence variance in the region targeted by the probe between SA-RN4220 and SA-1199B (68 mismatches with the 790-bp probe).
FIG. 1.
Southern analysis of chromosomal DNA digested with EcoRV. Lanes 1 and 3, SA-RN4220; lanes 2 and 4, SA-K1606. The norA probe was used in lanes 1 and 2, and the pG+Host6 probe was used in lanes 3 and 4. The positions of molecular mass markers are indicated, with size in kDa given on the left.
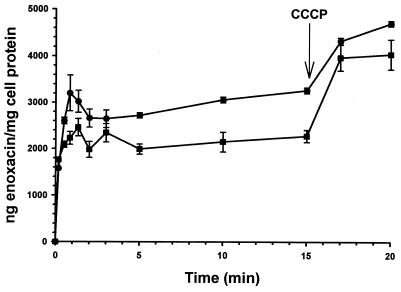
The MICs of various NorA substrates for SA-RN4220 and SA-K1606 are shown in Table 1. At the concentration employed, reserpine did not inhibit the growth of either organism (MIC of reserpine for each organism was greater than 100 μg/ml). The presence of the integrated plasmid resulted in a two- to fourfold rise in MICs, which for enoxacin, norfloxacin, and ethidium bromide was reversible by reserpine. The uptake of [14C]enoxacin was significantly reduced in SA-K1606 between 0.5 and 15 min, with the exception of the 1.3- and 3-min time points (Fig. 2). The addition of CCCP disrupted drug efflux and eliminated the uptake difference between the strains.
TABLE 1.
MICs for study strains
| Compounda | MIC of compound against (μg/ml)b:
|
|
|---|---|---|
| SA-RN4220 | SA-K1606 | |
| Enoxacin (+R) | 1.6 (0.8) | 3.1 (0.8) |
| Norfloxacin (+R) | 0.8 (≤0.2) | 3.1 (0.4) |
| EtBr (+R) | 3.1 (0.4) | 12.5 (1.6) |
| Acriflavine | 12.5 | 50.0 |
| Benzalkonium chloride | 1.6 | 3.1 |
| Cetrimide | 1.6 | 3.1 |
| TPP | 12.5 | 25.0 |
R, reserpine (20 μg/ml); EtBr, ethidium bromide; TPP, tetraphenylphosphonium bromide.
Values in parentheses indicate MICs in the presence of reserpine.
FIG. 2.
[14C]enoxacin uptake profiles (mean ± standard error). CCCP (final concentration, 100 μM) was added at the indicated time. ●, SA-RN4220; ■, SA-K1606.
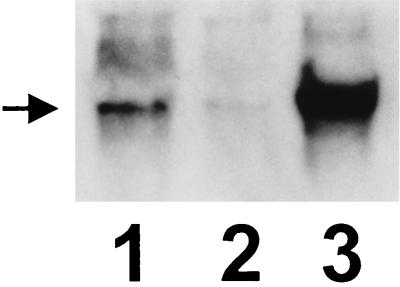
By using the norA probe it can be seen that, compared to SA-RN4220, SA-K1606 produces an increased quantity of norA transcript (Fig. 3). The identity of the more-defined signal of the highest molecular weight that is observed in all lanes (including the SA-1199B lane) is unknown. This signal is always observed in analyses of the norA mRNA of SA-1199 and SA-1199B (8).
FIG. 3.
Northern analysis. Lane 1, SA-K1606; lane 2, SA-RN4220; lane 3, SA-1199B. The position of norA mRNA is indicated.
The diffuse signal seen just above that of the norA transcript in SA-K1606 is also observed with SA-RN4220 (Fig. 3, lane 2; more visible on the original autoradiogram) and in approximately the same location (but less diffuse) with SA-1199B RNA (Fig. 3, lane 3). These signals, as well as the more-defined signal noted above, may represent larger transcripts including norA. Such transcripts may originate upstream of norA or may begin with norA and include downstream sequences. Diffuse hybridization in this region was also observed by using the pG+Host6 probe in SA-K1606 (data not shown), suggesting the possibility of a plasmid-based promoter contributing to the increased expression of norA observed in this organism.
Primer extension analysis revealed two transcripts that included norA in all strains and which were present in varying quantities. The 5′ terminus of the most abundant of the two mapped to 93 bp upstream of the norA initiation codon and 7 bp downstream of the −10 promoter motif (data not shown). Such a start site is typical, and this species likely represents the primary norA transcript (11). This transcript was 10- and 12-fold more abundant in SA-K1606 and SA-1199B than in the respective parent strains. The 5′ terminus of the second species mapped to 194 bp upstream of the norA coding region. In SA-K1606 and SA-1199B, the quantity of this transcript was only 14 and 2% that of the primary norA transcript, respectively. Computer-based analysis of the region immediately upstream from the start site of this mRNA species, which included no pG+Host6 sequences, revealed no promoter-like motifs.
Our data do not provide conclusive evidence that the quantity of the secondary transcript is increased due to the activity of a plasmid-based promoter, but the possibility cannot be dismissed based on the Northern analysis results observed with the norA and pG+Host6 probes. However, the fact that this mRNA species is detected in SA-RN4220 and SA-1199 and accounts for only a minor proportion of the norA-containing transcripts in SA-K1606 makes it unlikely that it is a factor that contributes significantly to the results observed. Rather, the data presented here strongly suggest that the T→A norA promoter region mutation found in SA-1199B does play a role in efflux-mediated multidrug resistance. There is an 8-bp, perfectly inverted repeat encompassing the −10 motif of the norA promoter which may serve as a binding site for a protein regulating the expression of the gene (7, 9). We have preliminary evidence that a protein(s) recognizing the norA promoter region does exist (data not shown). It is conceivable that promoter region mutations may affect the binding of a regulatory protein to its recognition site, leading to altered norA expression. However, such a mutation is not required for increased expression of norA. We have described an S. aureus strain (SA-1199-3) displaying inducible up regulation of norA and a wild-type promoter region nucleotide sequence (8). It is possible that in this situation a mutational alteration of the putative regulatory protein itself has occurred, affecting norA expression. Clearly, further work is necessary to more completely characterize the processes involved in norA regulation.
ACKNOWLEDGMENTS
This study was supported by VA Medical Research funds.
We thank E. Manguin for providing pG+Host6.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1995. [Google Scholar]
- 2.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA, and proteins from cell and tissue samples. BioTechniques. 1993;15:532–535. [PubMed] [Google Scholar]
- 3.Ferrero L, Cameron B, Manse B, Lagneaux D, Crouzet J, Famechon A, Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: a primary target of fluoroquinolones. Mol Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferrero L, Cameron B, Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:1554–1558. doi: 10.1128/aac.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goswitz J J, Willard K E, Fasching C E, Peterson L R. Detection of gyrA gene mutations in methicillin-resistant Staphylococcus aureus: analysis by polymerase chain reaction and automated direct DNA sequencing. Antimicrob Agents Chemother. 1992;36:1166–1169. doi: 10.1128/aac.36.5.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito H, Yoshida H, Bogaki-Shonai M, Niga T, Hattori H, Nakamura S. Quinolone resistance mutations in the DNA gyrase gyrA and gyrB genes of Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:2014–2023. doi: 10.1128/aac.38.9.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaatz G W, Seo S M, Ruble C A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaatz G W, Seo S M. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:2650–2655. doi: 10.1128/aac.39.12.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaatz G W, Seo S M. Mechanisms of fluoroquinolone resistance in genetically related strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2733–2737. doi: 10.1128/aac.41.12.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreiswirth B N, Lofdahl S, Betley M J, O’Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 11.Lewin B. Genes. IV. New York, N.Y: Oxford University Press; 1990. [Google Scholar]
- 12.Lorow D, Jessee J. Focus. 1990;12:19. [Google Scholar]
- 13.Maguin E, Prevost H, Dusko Ehrlich S, Gruss A. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J Bacteriol. 1996;178:931–935. doi: 10.1128/jb.178.3.931-935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Approved standard M7-A4. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 15.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mediated by norA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother. 1994;38:1345–1355. doi: 10.1128/aac.38.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 18.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 20.Sreedharan S, Oram M, Jensen B, Peterson L R, Fisher L M. DNA gyrase gyrA mutations in ciprofloxacin-resistant strains of Staphylococcus aureus: close similarity with quinolone resistance mutations in Escherichia coli. J Bacteriol. 1990;172:7260–7262. doi: 10.1128/jb.172.12.7260-7262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamagishi J, Kojima T, Oyamada Y, Fujimoto K, Hattori H, Nakamura S, Inoue M. Alterations in the DNA topoisomerase IV grlA gene responsible for fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1157–1163. doi: 10.1128/aac.40.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]