Key Points
In CD34+ TCD allogeneic-HCT, optimum post-HCT rATG exposure decreases NRM driven by faster CD4+ IR and improves survival.
Personalized rATG exposure using a PK-directed strategy may improve survival after allogeneic CD34+ TCD HCT.
Visual Abstract
Abstract
Traditional weight-based dosing results in variable rabbit antithymocyte globulin (rATG) clearance that can delay CD4+ T-cell immune reconstitution (CD4+ IR) leading to higher mortality. In a retrospective pharmacokinetic/pharmacodynamic (PK/PD) analysis of patients undergoing their first CD34+ T-cell–depleted (TCD) allogeneic hematopoietic cell transplantation (HCT) after myeloablative conditioning with rATG, we estimated post-HCT rATG exposure as area under the curve (arbitrary unit per day/milliliter [AU × day/mL]) using a validated population PK model. We related rATG exposure to nonrelapse mortality (NRM), CD4+ IR (CD4+ ≥50 cells per µL at 2 consecutive measures within 100 days after HCT), overall survival, relapse, and acute graft-versus-host disease (aGVHD) to define an optimal rATG exposure. We used Cox proportional hazard models and multistate competing risk models for analysis. In all, 554 patients were included (age range, 0.1-73 years). Median post-HCT rATG exposure was 47 AU × day/mL (range, 0-101 AU × day/mL). Low post-HCT area under the curve (<30 AU × day/mL) was associated with lower risk of NRM (P < .01) and higher probability of achieving CD4+ IR (P < .001). Patients who attained CD4+ IR had a sevenfold lower 5-year NRM (P < .0001). The probability of achieving CD4+ IR was 2.5-fold higher in the <30 AU × day/mL group compared with 30-55 AU × day/mL and threefold higher in the <30 AU × day/mL group compared with the ≥55 AU × day/mL group. In multivariable analyses, post-HCT rATG exposure ≥55 AU × day/mL was associated with an increased risk of NRM (hazard ratio, 3.42; 95% confidence interval, 1.26-9.30). In the malignancy subgroup (n = 515), a tenfold increased NRM was observed in the ≥55 AU × day/mL group, and a sevenfold increased NRM was observed in the 30-55 AU × day/mL group compared with the <30 AU × day/mL group. Post-HCT rATG exposure ≥55 AU × day/mL was associated with higher risk of a GVHD (hazard ratio, 2.28; 95% confidence interval, 1.01-5.16). High post-HCT rATG exposure is associated with higher NRM secondary to poor CD4+ IR after TCD HCT. Using personalized PK-directed rATG dosing to achieve optimal exposure may improve survival after HCT.
Introduction
There have been consistent improvements in outcomes after allogeneic hematopoietic cell transplantation (allo-HCT) over several decades, yet disease relapse and nonrelapse mortality (NRM) remain major causes of treatment failure.1 Some of the key factors in preventing these complications are robust and timely CD4+ immune reconstitution (CD4+IR) and prevention of graft-versus-host disease (GVHD).2 Early attainment of CD4+ IR has been recently identified as a reliable predictor for outcomes such as viral reactivation, NRM, GVHD, and overall survival (OS).3-6 The addition of antithymocyte globulin (ATG) or antilymphocyte globulin to standard calcineurin inhibitor–based prophylaxis helps prevent graft rejection and GVHD across several allo-HCT settings.7 However, in cord blood transplantation, the use of ATG has been associated with high rates of infections, subsequently leading to higher rates of NRM and a potentially increased risk of disease relapse because of the negative effect ATG on CD4+ IR.8 An important analysis by Admiraal et al4 demonstrated that higher exposure to ATG before HCT was not only associated with lower rates of graft failure but was also associated with lower rates of GVHD in the T-cell replete HCT setting.
Rabbit ATG (rATG) is currently dosed by patient weight, yet a recently validated rATG population pharmacokinetic (PK) model demonstrated that weight is a factor in predicting exposure only in patients who weight <40 kg. The most important factor in rATG clearance is a patient’s absolute lymphocyte count (ALC) when rATG is administered.9,10 By using these essential variables, an optimal PK-directed dose of rATG can be calculated that maintains its favorable properties while minimizing its negative effects on CD4+ IR.9,11
Several studies have demonstrated favorable outcomes using various ex vivo T-cell–depleted (TCD) techniques, including CD34 selection with low rates of GVHD after HCT (for malignant and nonmalignant hematologic diseases).12-16 However, a main limitation when compared with T-cell–replete allo-HCT is delayed CD4+ IR.17 In patients undergoing ex vivo CD34 selection, rATG is primarily used in conditioning regimens and has been proven to be effective in preventing graft rejection. We recently showed that standard weight-based dosing in adults undergoing ex vivo CD34-selected allo-HCT results in heavier patients receiving higher total doses of rATG, which consequently leads to markedly inferior OS driven by NRM.18 This analysis suggested that there is an optimal post-HCT rATG exposure for patients undergoing TCD allo-HCT. In this analysis, we used the validated population PK model (Admiraal et al)9 to estimate post-HCT rATG exposure and related the estimated rATG exposures to outcomes, such as NRM, CD4+ IR, OS, acute GVHD (aGVHD), and relapse. The goal was to identify the optimal post-TCD HCT exposure that is associated with optimal CD4+ IR and clinical outcomes. We hypothesized that lower exposure to rATG after HCT would result in lower NRM driven by improved CD4+ IR.
Methodology
Patients
We performed a retrospective PK and pharmacodynamic (PK/PD) analysis of data from pediatric and adult patients with malignant or nonmalignant hematologic diseases who underwent their first allo-HCT with ex vivo TCD allografts using the CliniMACS CD34 Reagent System (Miltenyi Biotech, Gladbach, Germany) as calcineurin inhibitor–free GVHD prophylaxis at Memorial Sloan Kettering Cancer Center (MSKCC) between 2008 and 2018. Patients received supportive care, growth factors, and antimicrobial prophylaxis according to MSKCC institutional protocols (which were similar between the pediatric and adult services). Patients with hematologic malignancies and nonmalignant hematologic diseases received either chemotherapy-based or high-dose total body irradiation (TBI)–based myeloablative conditioning.19 Patients received rATG (thymoglobulin; Sanofi, Paris, France) as part of a conditioning regimen at a dose of 2.5 mg/kg intravenously once per day beginning on days −4 to −2 in the pediatric service and days −3 to −1 in the adult service. Patients who got an HLA-mismatched allograft received rATG for 3 days (cumulative dose, 7.5 mg/kg), whereas recipients of HLA-matched allografts received rATG for 2 days (cumulative dose, 5 mg/kg). We excluded patients who switched to equine ATG or alemtuzumab because of adverse reactions to rATG. The study protocol was approved by the institutional review board at MSKCC and was conducted in accordance with the Declaration of Helsinki.
Methods
We estimated the exposure of rATG after day 0 (post-HCT) as the area under the curve (arbitrary unit per day/milliliter [AU × day/mL]) based on an established PK model (Admiraal et al9) assessable via the InsightRx platform (San Francisco, CA). This model was developed and validated in a large cohort of patients and included all cell sources, T-cell–replete and TCD transplants in which T-cell lymphocytes in the graft were found not to be a predictor for clearance. The following parameters were used to estimate rATG exposure using the population PK model: date of HCT, weight, date of first dose of rATG, actual daily dose of rATG administered, length of rATG infusion, number of days of infusion, and ALC on the first day of rATG. For patients in whom the ALC was not available on the day of administration of rATG, the ALC closest to the previous day of rATG infusion was considered (supplemental Figure 1). Exposure for the patients who did not receive rATG was considered 0 AU × day/mL.
Outcomes
The main outcome of interest was NRM, defined as the time from HCT to death as a result of causes other than relapse of hematologic malignancies. Patients who were still alive were censored at their date of last follow-up, and relapse was treated as a competing event. CD4+ IR was defined as described previously3,5: CD4+ count ≥50 cells per µL at 2 consecutive measures within 100 days after HCT. Time to CD4+ IR was defined as the time from HCT to the time of first day that CD4+ level reached ≥50 cells per µL. Patients who did not reach CD4+ IR were censored at 100 days, whereas deaths without reaching CD4+ IR (including missing CD4+ IR) occurring in the first 100 days were treated as competing events.
Other outcomes of interest were relapse (in patients with hematologic malignancies), aGVHD at day +180, and OS. OS was defined as the time from HCT to death as a result of any cause. Surviving patients were censored at their date of last contact. Time-to-relapse was defined as the time from HCT to disease relapse. Patients without relapses were censored at their date of last follow-up, whereas deaths before relapse were treated as competing events. aGVHD was graded and staged according to national and international guidelines (staging by Center for International Blood and Marrow Transplant Research (CIBMTR) guidelines with grading by modified Keystone criteria).20,21 Primary graft failure was defined as failure to achieve neutrophil engraftment by day 30 after allo-HCT.
Statistical analyses
Time-to-event end points were as defined above. Survival rates were estimated by using a Kaplan-Meier estimator or by using an Aalen-Johansen estimator when competing risks were present.22 The impact of ATG exposure and other factors (sex, malignant disease, donor type, receipt of TBI-based conditioning regimen, patient and donor cytomegalovirus [CMV] serostatus, Hematopoietic Cell Transplantation-Specific Comorbidity Index [HCT Comorbidity Index]) in all patients and Disease Risk Index (DRI) in patients with hematologic malignancies only were assessed by using Cox proportional models.23-26 A multistate approach was used (impact on cause-specific hazards) when competing risks were present. Models were stratified by pediatric service vs adult service and were further adjusted on age. The results are presented as hazard ratios (HRs), 95% confidence intervals (95% CIs), and log-likelihood test P values. Factors were assessed in univariable models first and entered into multivariable models if P ≤ .10. A backward variable selection was performed, and variables with P ≤ .05 were kept in the final model. For variables entered into the multivariable model but not kept in the final model, HRs of the variables were added one by one to the final model to ensure that no effect was missed. To define the optimal post-HCT estimated rATG exposure, univariable models stratified on pediatric service vs adult service and donor type were used with P splines. HR plots were drawn and thresholds were found graphically as the point where the CI crosses 1.27 Thereafter, the impact of estimated rATG exposure as a categorical variable (using the previously defined thresholds) was assessed.
Results
Patient characteristics
We identified 569 patients who underwent first allo-HCT with ex vivo TCD (by CD34+ selection using the CliniMacs CD34 Reagent System (Miltenyi Biotech, Gladbach, Germany) between 2008 and 2018 at MSKCC. Of these 569 patients, 15 were excluded because they received equine ATG alone or because rATG was switched to equine ATG or alemtuzumab because of adverse reactions to rATG (supplemental Figure 1). Therefore, 554 patients (pediatric, n = 139; adult, n = 415) were included in the analyses. The ALC values on day of ATG dosing were 0 to 0.1 in 92% of the patients (irrespective of conditioning). Table 1 provides details regarding patient and HCT characteristics.
Table 1.
Patient characteristics
| Characteristic | Adult program (n = 415) |
Pediatric program (n = 139) |
Overall (N = 554) |
|---|---|---|---|
| Median age at transplant, years | 56 (45-64; 20-73) | 12 (7-19; 0-44) | 49 (25-62; 0-73) |
| Age group, years | |||
| <20 | 1 (0.2) | 105 (76) | 106 (19) |
| 20-40 | 77 (19) | 33 (24) | 110 (20) |
| 40-60 | 173 (42) | 1 (0.7) | 174 (31) |
| and ≥ 60 | 164 (40) | 0 (0) | 164 (30) |
| Sex | |||
| Female | 187 (45) | 52 (37) | 239 (43) |
| Male | 228 (55) | 87 (63) | 315 (57) |
| Disease | |||
| Leukemia | 274 (66) | 80 (58) | 354 (64) |
| Leukemia (second primary) | 1 (0.2) | 2 (1.3) | 3 (0.5) |
| Myelodysplastic syndrome | 112 (27) | 17 (12) | 129 (23.3) |
| Myeloproliferative disorder | 23 (5.5) | 0 (0) | 23 (4.2) |
| Non-Hodgkin lymphoma | 5 (1.2) | 1 (0.7) | 6 (1.1) |
| Nonmalignant hematologic disorders | 0 (0) | 39 (28) | 39 (7) |
| Malignancy | |||
| Malignant | 415 (100) | 100 (72) | 515 (93) |
| Nonmalignant | 0 (0) | 39 (28) | 39 (7.0) |
| TBI-based conditioning regimen | 136 (33) | 41 (41) | 177 (34) |
| Donor type | |||
| MMRD | 2 (0.5) | 15 (11) | 17 (3.1) |
| MMUD | 61 (15) | 51 (37) | 112 (20) |
| MRD | 150 (36) | 24 (17) | 174 (31) |
| MUD | 202 (49) | 49 (35) | 251 (45) |
| CMV match | |||
| R–/D– | 118 (29) | 40 (29) | 158 (29) |
| R–/D+ | 43 (11) | 19 (14) | 62 (11) |
| R+/D– | 101 (25) | 25 (18) | 126 (23) |
| R+/D+ | 142 (35) | 55 (40) | 197 (36) |
| Unknown | 11 | 0 | 11 |
| HCT Comorbidity Index score | |||
| 0-1 | 162 (39) | 74 (53) | 236 (43) |
| 2-3 | 160 (39) | 45 (32) | 205 (37) |
| 4-10 | 93 (22) | 20 (14) | 113 (20) |
| DRI | |||
| High | 47 (13) | 16 (18) | 63 (14) |
| Intermediate | 284 (80) | 57 (63) | 341 (77) |
| Low | 22 (6) | 18 (20) | 40 (9) |
| Unknown | 62 | 48 | 110 |
| Post-HCT rATG exposure | 48 (43-53; 17-101) | 35 (21-48; 0-87) | 47 (40-52; 0-101) |
Statistics presented are No. (%) for categorical variables, and median (interquartile range, range) for the continuous variables.
D, donor; MMRD, mismatched related donor; MMUD, mismatched unrelated donor; MRD, matched related donor; MUD, matched unrelated donor; R, recipient; DRI, Disease Related Index; CMV, Cytomegalovirus; rATG, rabbit antithymocyte globulin.
Outcomes
Among all patients, 540 (97%) engrafted, with similar engraftment rates in both pediatric and adult patients. Median follow-up among survivors was 4 years (range, 1 day-11.5 years). Median post-HCT rATG exposure among all patients was 47 AU × day/mL (range, 0-101 AU × day/mL), with median post-HCT rATG exposure being lower in the pediatric group (35 AU × d/L; range, 0-87 AU × day/mL) than in the adult group (48 AU × day/mL; range, 17-101 AU × day/mL). Among the 14 (2.5%) who failed to engraft, there was no correlation between estimated rATG exposure and rejection, although this was limited by low numbers of patients. Among the 515 patients who underwent allo-HCT for hematologic malignancies, median rATG exposure post-HCT was 47 AU × day/mL (range, 0-101 AU × day/mL).
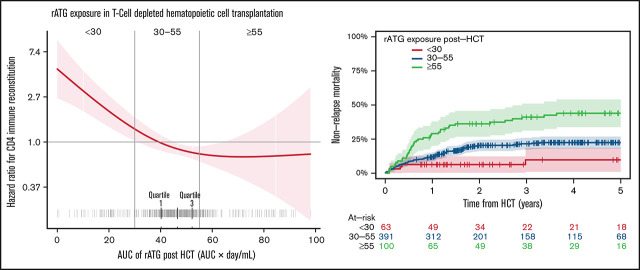
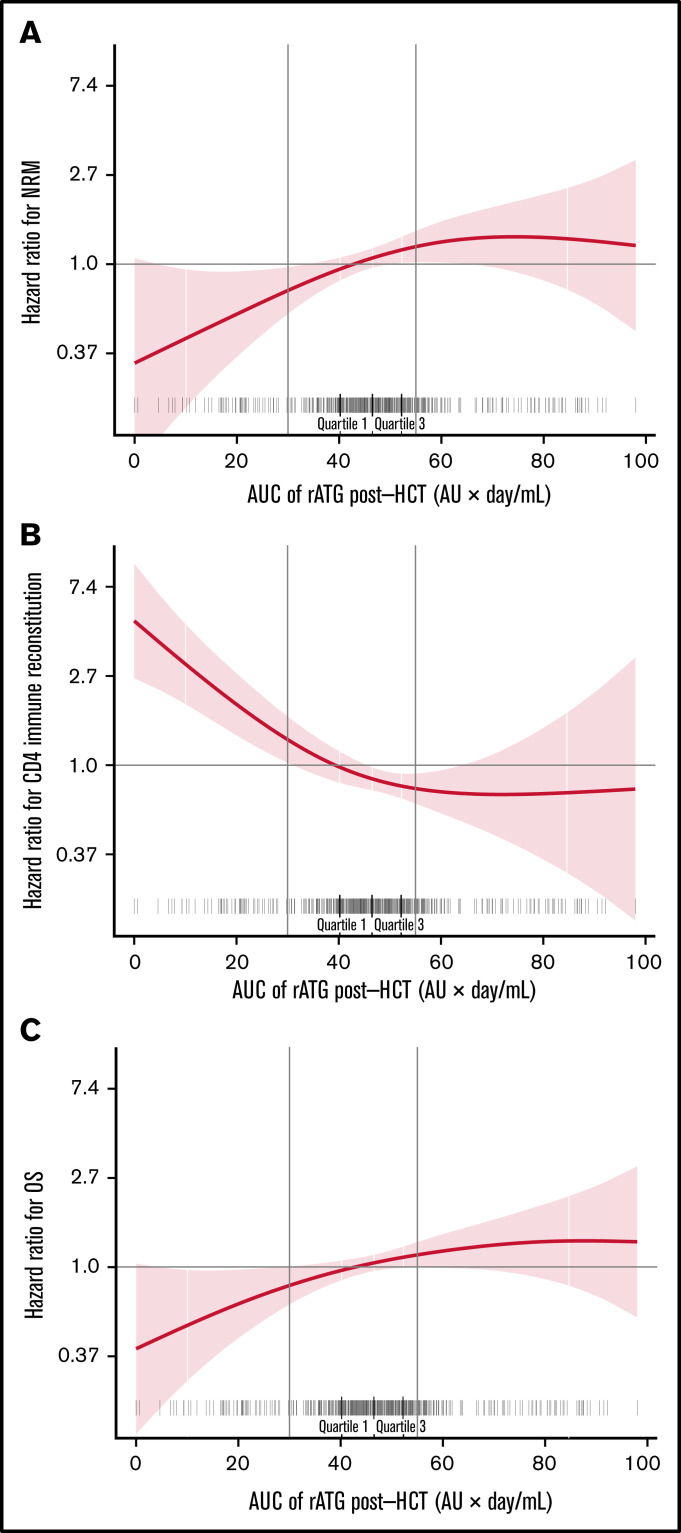
We evaluated the association of post-HCT rATG exposures by relating them with log-HRs for NRM, CD4+ IR, and OS. Post-HCT rATG exposure <30 AU × day/mL was associated with lower risk of NRM (P = .03; Figure 1A), higher probability of CD4+ IR (P < .0001; Figure 1B), and increased probability of OS (P = .05; Figure 1C). For the 3 outcomes, the threshold found graphically was <30 AU × day/mL. For subsequent analyses, we stratified post-HCT rATG exposure into 3 groups (<30 AU × day/mL, 30-55 AU × day/mL, and ≥55 AU × day/mL) to relate to the outcomes of interest.
Figure 1.
Post-HCT rATG exposure <30 AU × day/mL was associated with lower NRM, faster CD4+ IR, and lower overall all-cause mortality and thus higher OS. The correlation between post-HCT rATG exposure and NRM (A) and CD4+ IR (B). CD4 + IR was defined as CD4+ levels twice above 50 cells per µL at 2 consecutive measures within 100 days. (C) The correlation between post-HCT rATG exposure and all-cause mortality and OS.
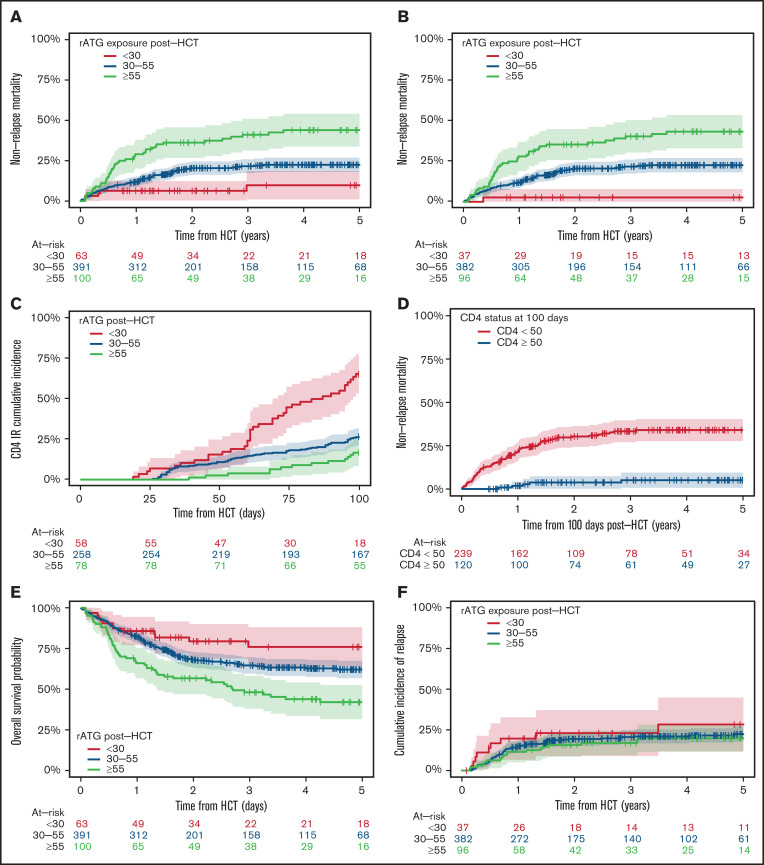
The 5-year NRM was 10% (95% CI, 1%-19%) for the <30 AU × day/mL exposure group, 23% (95% CI, 18% to 27%) for the 30-55 AU × day/mL exposure group, and 44% (95% CI, 34%-54%) for the ≥55 AU × day/mL exposure group (Figure 2A). The trend for higher NRM in rATG groups with high exposure was also seen when stratifying by matched and mismatched donor groups (supplemental Figure 2A-B). In multivariable analyses, the risk of NRM was 3.4 times higher in the ≥55 AU × day/mL exposure group compared with the <30 AU × day/mL group (HR, 3.42; 95% CI, 1.26-9.30) (Table 2). In patients who underwent allo-HCT for malignant indications, the probability for risk of developing NRM was tenfold higher (HR, 9.88; 95% CI, 1.31-74.70) in the ≥55 AU × day/mL exposure group and was already sevenfold higher in the 30-55 AU × day/mL exposure group compared with the <30 AU × day/mL group (Table 2; Figure 2B). In addition, in multivariable analysis, other variables such as donor- or recipient-positive CMV serostatus, receiving an HLA-mismatched allograft, and HCT Comorbidity Index ≥2 were associated with a higher risk of NRM.
Figure 2.
Post-HCT rATG exposure <30 AU × day/mL was associated with lower NRM (overall and in patients who underwent HCT for malignant disorders), faster CD4+ IR, and higher OS rates. Post-HCT rATG exposure was not associated with risk of relapse in patients who underwent HCT for malignant disorders. The correlation between different levels of post-HCT rATG exposure and NRM in all patients (A), NRM in patients who underwent HCT for malignant disorders (B), and CD4+ IR (C). CD4+ IR was defined as CD4+ levels twice above 50 cells per µL at 2 consecutive measures within 100 days. (D) Correlation between CD4+ IR and NRM in all patients, demonstrating that patients who reconstitute CD4+ earlier have lower NRM. Note that this figure represents a landmark analysis starting 100 days after HCT. Therefore, only patients alive at 100 days after HCT were included in this specific analysis, which is 359 patients alive with known CD4+ IR status. (E-F) Correlation between different levels of post-HCT rATG exposure and OS (E) and relapse rates (F) in patients who underwent HCT for malignant disorders.
Table 2.
Multivariable models for prognostic analyses
| Prognostic analysis | HR | 95% CI | P |
|---|---|---|---|
| NRM * | |||
| Age group, y | .13 | ||
| <20 | Ref. | ||
| 20-40 | 1.20 | 0.54-2.67 | |
| 40-60 | 1.07 | 0.39-2.94 | |
| ≥60 | 1.76 | 0.65-4.77 | |
| Sex | .02† | ||
| Female | Ref. | ||
| Male | 0.64 | 0.44-0.92 | |
| Donor type | .007† | ||
| MRD | Ref. | ||
| MUD | 0.98 | 0.62-1.53 | |
| MMD | 2.14 | 1.23-3.72 | |
| rATG exposure groups, AU × day/mL | .01† | ||
| <30 | Ref. | ||
| 30-55 | 1.93 | 0.72-5.17 | |
| ≥55 AU × day/ml | 3.42 | 1.26-9.30 | |
| CMV match | .03† | ||
| R–/D– | Ref. | ||
| R–/D+ | 1.70 | 0.92-3.15 | |
| R+/D– | 1.39 | 0.81-2.38 | |
| R+/D+ | 1.94 | 1.22-3.08 | |
| HCT Comorbidity Index score | <.001† | ||
| 0-1 | Ref. | ||
| 2-3 | 2.16 | 1.40-3.33 | |
| 4-10 | 2.79 | 1.72-4.53 | |
| TBI-based conditioning regimen‡ | .47 | ||
| No | Ref. | ||
| Yes | 0.83 | 0.50-1.38 | |
| NRM in patients with hematologic malignant disorders§ | |||
| Age group, y | .18 | ||
| <20 | Ref. | ||
| 20-40 | 1.17 | 0.48-2.87 | |
| 40-60 | 1.10 | 0.37-3.25 | |
| ≥60 | 1.72 | 0.59-5.02 | |
| Donor type | .002† | ||
| MRD | Ref. | ||
| MUD | 0.90 | 0.57-1.41 | |
| MMD | 2.29 | 1.30-4.02 | |
| rATG exposure groups, AU × day/mL | .007† | ||
| <30 | Ref. | ||
| 30-55 | 7.11 | 0.96-52.6 | |
| ≥55 | 9.88 | 1.31-74.70 | |
| HCT Comorbidity Index score | <.001† | ||
| 0-1 | Ref. | ||
| 2-3 | 2.07 | 1.33-3.21 | |
| 4-10 | 2.43 | 1.49-3.94 | |
| CMV match‡ | .08 | ||
| R–/D– | Ref. | ||
| R–/D+ | 1.68 | 0.91-3.14 | |
| R+/D– | 1.42 | 0.82-2.45 | |
| R+/D+ | 1.84 | 1.14-2.97 | |
| TBI-based conditioning regimen‡ | .58 | ||
| No | Ref. | ||
| Yes | 0.86 | 0.52-1.45 | |
| CD4+IR | |||
| Age groups, y | .19 | ||
| <20 | Ref. | ||
| 20-40 | 0.83 | 0.44-1.57 | |
| 40-60 | 1.98 | 0.67-5.80 | |
| ≥60 | 1.68 | 0.55-5.10 | |
| Donor type | .003† | ||
| MRD | Ref. | ||
| MUD | 0.59 | 0.38-0.91 | |
| MMD | 0.39 | 0.22-0.67 | |
| rATG exposure groups, AU × day/mL | <.001† | ||
| <30 | Ref. | ||
| 30-55 | 0.41 | 0.25-0.66 | |
| ≥55 | 0.35 | 0.17-0.71 | |
HR, Hazard Ratio, CI, Confidence Interval, MUD, matched unrelated donor, CMV, cytomegalovirus, TBI, total body irradiation, NRM, Non- Relapse Mortality, CD4+IR, CD4+immune reconstitution, rATG , rabbit antithymocyte globulin
Variables with P < .05 were kept in the final model (with age as an adjustment factor); results of the other variables (at the bottom in italic) are results of the variables added one by one in the final model.
Indicates significance.
Results of the variables are added one by one in the final model.
Multivariable Cox model is adjusted on age; variables with P < .05 are kept in the final model (with age as an adjustment factor); results of the other variables (at the bottom in italic) are results of the variables added one by one in the final model.
Among all 554 patients, 160 were excluded from the analysis of time to CD4+ IR because of missing CD4+ IR status. The cumulative incidence of CD4+ IR at 100 days was 66% (95% CI, 53%-78%) for the <30 AU × day/mL exposure group, 27% (95% CI, 21%-32%) for the 30-55 AU xday/mL exposure group, and 17% (95% CI, 8%-25%) for the ≥55 AU × day/mL exposure group. Lower post-HCT rATG exposure (<30 AU × day/mL) was associated with a cumulative incidence ∼3 times higher (HRs, 0.35 and 0.41) of CD4+ IR (P < .0001; Figure 2C; Table 2), with similar patterns noted in matched and mismatched donor groups (supplemental Figure 3A-B) and in patients who underwent allo-HCT for hematologic malignancies (P < .001). In multivariable analyses, in addition to post-HCT rATG exposure, having a mismatched unrelated donor or a matched unrelated donor was associated with lower probability of attaining CD4+ IR (Table 2). Incidence of NRM at 5 years in patients who attained CD4+ IR 100 days after HCT was 5% (range, 1%-10%) compared with 36% (range, 29%-43%) in patients who did not attain CD4+ IR at 100 days after HCT (P < .0001; Figure 2D). In patients who underwent allo-HCT for a hematologic malignancy, NRM was sevenfold lower in patients who attained CD4+ IR (supplemental Figure 3C).
The 5-year OS rate was 76% (95% CI, 64%-88%) for the <30 AU × day/mL exposure group, 62% (95% CI, 57%-67%) for the 30-55 AU × day/mL exposure group, and 42% (95% CI, 32%-53%) for the ≥55 AU × day/mL exposure group (Figure 2E). The trend toward poorer OS with higher exposure to rATG was similar when stratifying by matched and mismatched donor group (supplemental Figure 4A-B). In multivariable analyses, donor- and recipient-positive CMV serostatus, donor matching (receiving mismatched donor allograft), and HCT Comorbidity Index >2 were associated with higher all-cause mortality (supplemental Table 1). High DRI was also a risk factor for OS in 515 patients who underwent allo-HCT for hematologic malignancies. Among those 515 patients, 111 had disease relapse, and rATG exposure after HCT was not associated with the risk of disease relapse (Figure 2F). In multivariable analysis for patients with hematologic malignancies, high DRI, male sex, and TBI-based conditioning were predictors for a higher risk of relapse (supplemental Table 2).
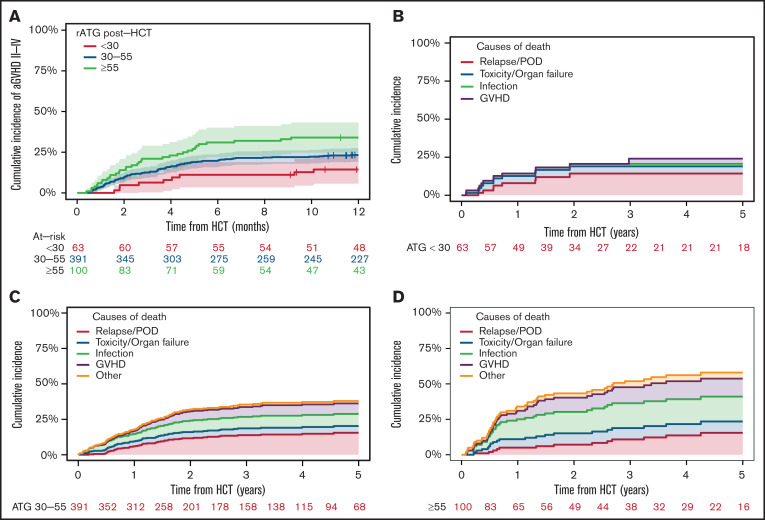
The post-HCT high rATG exposure group (≥55 AU × day/mL) was associated with up to double the incidence of grade ≥2 aGVHD (HR, 2.28; 95% CI, 1.01-5.16 after adjustment on donor type) compared with the low rATG exposure group (<30 AU × day/mL; Figure 3A) and was similar in matched and mismatched donor groups (supplemental Figure 5A-B). Attainment of CD4+ IR (as a time-dependent variable) was associated with lower risk of aGVHD, even after adjusting for the effect of rATG exposure (HR, 0.29; 95% CI, 0.14-0.61; P < .001). The results were adjusted on donor type, and matching was found not to be a predictor in multivariable analyses (P = .70). Chronic GVHD (cGVHD) rate in these analyses was relatively low: ∼8% only (all grades). We did not find an association between ATG exposure and cGVHD (P = .4).
Figure 3.
aGVHD rATG exposure and mortality. Post-HCT high rATG exposure group (≥55 AU × day/mL) was associated with up to double the incidence of grade ≥2 aGVHD (HR, 2.28; 95% CI, 1.01-5.16 after adjustment for donor type) compared with the low rATG exposure group (<30 AU × day/mL). The proportion of deaths attributed to aGVHD and infection increased with higher post-HCT rATG exposures. (A) Correlation between different levels of post-HCT rATG exposures and aGVHD. Post-HCT rATG exposure of <30 AU × day/mL was associated with lower rates of GVHD. (B-D) Causes of mortality in the post-HCT rATG <30 AU × day/mL exposure group (B), 30-55 AU × day/mL exposure group (C), and ≥55 AU × day/mL exposure group (D). POD, progression of disease.
Among all patients, 204 died as a result of disease relapse or disease progression (n = 73; 36%), GVHD (n = 40; 20%), infection (n = 51; 25%), toxicity or organ failure (n = 29; 14%), or other causes (n = 11; 5%). The proportion of deaths attributed to aGVHD and infection increased with higher post-HCT rATG exposures (Figure 3B-D).
Discussion
Previous PK/PD analyses have demonstrated that weight-based dosing results in highly variable post-HCT rATG exposure, leading to unpredictable CD4+ IR and thus higher NRM and worse OS in patients undergoing T-cell–replete allo-HCT. Here, using a validated population PK model, we found that rATG exposure is also a major driver of outcomes in the ex vivo TCD HCT setting.9 To our knowledge, this is the first study to define an optimal post-HCT rATG exposure in this setting. Optimal rATG exposure after HCT was found to be <30 AU × day/mL, which was associated with a lower NRM, higher probability of CD4+ IR, and superior OS. We also confirmed that attainment of CD4+ IR is a sensitive and powerful early predictor for outcomes, specifically NRM and OS, although it was not a predictor for risk of relapse. Our findings suggest that individualized rATG dosing to target optimal post-HCT exposures may enhance IR and subsequently improve survival, and they support the results of the PARACHUTE trial NL4836 (NTR4960), a prospective validation trial in pediatric patients undergoing T-cell–replete allo-HCT.28
Interestingly, overall rates of GVHD were low in our analysis, but we observed that patients with the highest post-HCT rATG exposures (≥55 AU × day/mL) had an increased incidence of aGVHD and GHVD-related mortality. Many studies have demonstrated low rates of acute and chronic GVHD when rATG is incorporated into the conditioning regimen. Although our study was not designed to specifically evaluate this finding, we offer 2 potential hypotheses that will be evaluated in future analyses: post-HCT overexposure to rATG leads to a higher risk of infections, including viral reactivations that may trigger GVHD. This is supported, in part, by CMV seropositivity being a significant risk factor for NRM and all-cause mortality and by the finding that infections and GVHD accounted for most causes of death in the highest rATG exposure group. Others have also shown that viral reactivations are associated with increased rates of GVHD.3,29,30 Alternatively (or additionally), post-HCT overexposure to rATG leads to delayed CD4+ IR, which includes depletion of regulatory CD4+ T cells that are considered important in preventing and/or mitigating severe GVHD.31-33 Unfortunately, we were not able to analyze this with the IR data currently available. In addition, the very low T-cell counts (median CD4+ cell count, 0) in the 2 higher ATG exposure groups complicates these analyses.
The primary limitation of this analysis is its retrospective nature. In addition, for the CD4+ IR outcome, we had insufficient data in 160 patients (29%), mainly because early lympho-phenotyping was not included in standard monitoring for all patients. Furthermore, patients receiving mismatched donor allo-HCT received 3 rather than 2 doses of rATG; thus, it is difficult to determine with certainty whether poorer outcomes in this group were related to HLA mismatch or rATG overexposure. However, in multivariable analyses, both variables were found to be independent predictors of outcomes, and we found similar trends for the various outcomes between the HLA-matched and -mismatched groups with favorable overall outcomes in the low rATG exposure group. Moreover, rATG exposure, and not HLA-mismatch, was associated with an increased risk of aGVHD, suggesting that the effect is mainly driven by rATG exposure and not the HLA mismatch. In addition, the original HCT Comorbidity Index score was used for all patients, although a newly updated and validated version that reduces the score for pediatric and young adult patients is underway.
These findings are relevant considering the early reported results of the PROGRESS II trial, which showed inferior survival with ex vivo TCD (using weight-based dosing of rATG) because of increased NRM. We hypothesize that one of the potential reasons for this finding may be related to post-HCT overexposure to rATG and poorer CD4+ IR, and we believe that our current analyses provide a path forward by using personalized PK-directed rATG dosing to reduce NRM and improve survival with low rates of GVHD.
In conclusion, in an ex vivo TCD setting, rATG exposure after HCT is an important predictor for CD4+ IR and clinical outcomes such as NRM and OS. Individualizing rATG dosing to an optimal exposure may lead to enhanced and more predictable CD4+ IR that will subsequently improve survival chances. These findings and the strategy to target the rATG to lower exposure after HCT, while preserving the cumulative rATG to maintain high engraftment rates (starting rATG earlier), need to be confirmed in a prospective clinical trial. If successful, this personalized rATG ex vivo TCD allo-HCT can be used as a low toxicity, predictable HCT platform to study novel post-HCT maintenance and cellular therapies to prevent disease relapse.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Joseph Olechnowicz for editorial support.
This work was supported by a grant from the National Institutes of Health, National Cancer Institute (P30 CA008748).
Authorship
Contribution: M.L., M.S., and J.J.B. substantially contributed to conception and design of the study; M.L., M.S., A.M., C.C., S.D., J.D.R., E.K., S.T.A., F.B., M.I.C., K.J.C., A.A.J., N.A.K., A.L.K., R.J.O., E.B.P., S.P., I.v.R., A.S., B.C.S., G.S., B.S., R.T., S.A.G., M.-A.P., and J.J.B. helped acquire, analyze, and interpret data; M.L., M.S., A.M., C.C., S.D., J.D.R., E.K., S.T.A., F.B., M.I.C., K.J.C., A.A.J., N.A.K., A.L.K., R.J.O., E.B.P., S.P., I.v.R., A.S., B.C.S., G.S., B.S., R.T., S.A.G., M.-A.P., and J.J.B. helped draft the manuscript and critically reviewed and revised it; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: M.S. received research support from Angiocrine Bioscience and Omeros; served as a consultant for Angiocrine Bioscience, Omeros, and McKinsey; was a member of an ad hoc advisory board for Kite Pharma; and had a one-time speaking commitment for i3Health. K.J.C. served as a consultant for Mesoblast, served as a consultant and received research funding from Novartis, and received research funding from Celgene. N.A.K. holds equity in Amgen, Johnson & Johnson, Pfizer, and Merck. R.J.O. served as a consultant for, holds patents with, and receives royalties from an EBV-specific T-cell bank and received research funding from Atara Biotherapeutics. S.P. received support for conducting clinical trials from Atara Biotherapeutics, Jasper Pharmaceuticals, and AlloVir; served on advisory boards for Mesoblast and Neovii; and is an inventor of intellectual property licensed to Atara Biotherapeutics by MSKCC, has assigned all rights to MSKCC, and has no financial interest in Atara Biotherapeutics. A.S. served as a member of the Board of Directors and advisory committees for Excellthera. S.A.G. served as a consultant for and received honoraria and research funding from Celgene and Novartis; served as a consultant for and received research funding from Amgen, Actinuum, and Miltenyi Biotech; served as a consultant for and received honoraria from Jazz Pharmaceuticals and Omeros; received research funding from Takeda; and served as a consultant for Kite Pharma. M.-A.P. has received honoraria from AbbVie, Astellas, Bristol Myers Squibb, Celgene, Equilium, Incyte, Karyopharm, Kite Pharma/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, Takeda, VectivBio AG, and Vor Biopharma; served on data and safety monitoring boards for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier; served on a scientific advisory board for NexImmune; has ownership interests in NexImmune and Omeros; has received research support for clinical trials from Incyte, Kite Pharma/Gilead, Miltenyi Biotec, and Novartis; serves as a volunteer member of the Board of Directors of the American Society for Transplantation and Cellular Therapy and BeTheMatch (National Marrow Donor Program); and serves on the Cellular Immunotherapy Data Resource Executive Committee for the Center for International Blood and Marrow Transplant Research. J.J.B. served as a consultant for Race Oncology, Omeros, Avrobio, Takeda, Advanced Clinical, and Bluerock. The remaining authors declare no competing financial interests.
Correspondence: Jaap Jan Boelens, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Scholar 417, New York, NY 10065; e-mail: boelensj@mskcc.org.
References
- 1.McDonald GB, Sandmaier BM, Mielcarek M, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: Comparing 2003-2007 versus 2013-2017 cohorts. Ann Intern Med. 2020;172(4):229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhry MS, Velardi E, Malard F, van den Brink MR. Immune reconstitution after allogeneic hematopoietic stem cell transplantation: time to T up the thymus. J Immunol. 2017;198(1):40-46. [DOI] [PubMed] [Google Scholar]
- 3.Admiraal R, de Koning CCH, Lindemans CA, et al. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol. 2017;140(6):1643-1650.e9. [DOI] [PubMed] [Google Scholar]
- 4.Admiraal R, van Kesteren C, Jol-van der Zijde CM, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015;2(5): e194-e203. [DOI] [PubMed] [Google Scholar]
- 5.Van Roessel I, Prockop SE, Klein E, et al. Early CD4+ T cell reconstruction as predictor for outcomes after allogeneic hematopoietic cell transplantation in pediatric and young adult patients: a validation cohort analyses [abstract]. Biol Blood Marrow Transplant. 2020;26(3):S302-S303. Abstract 462. [Google Scholar]
- 6.de Koning C, Prockop SE, Van Roessel I, et al. Early CD4+ T-cell reconstitution is an excellent predictor for survival and non-relapse mortality in pediatric and young adult patients who develop moderate to severe acute graft-versus-host-disease; A dual center validation [abstract]. Biol Blood Marrow Transplant. 2020;26(3):S188-S189. Abstract 277. [Google Scholar]
- 7.Bonifazi F, Rubio MT, Bacigalupo A, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel. Bone Marrow Transplant. 2020;55(6):1093-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Koning C, Admiraal R, Nierkens S, Boelens JJ. Immune reconstitution and outcomes after conditioning with anti-thymocyte-globulin in unrelated cord blood transplantation; the good, the bad, and the ugly. Stem Cell Investig. 2017;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Admiraal R, Nierkens S, de Witte MA, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017;4(4):e183-e191. [DOI] [PubMed] [Google Scholar]
- 10.Admiraal R, van Kesteren C, Jol-van der Zijde CM, et al. Population pharmacokinetic modeling of Thymoglobulin(®) in children receiving allogeneic-hematopoietic cell transplantation: towards improved survival through individualized dosing. Clin Pharmacokinet. 2015;54(4):435-446. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy VE, Chen H, Savani BN, et al. Optimizing antithymocyte globulin dosing for unrelated donor allogeneic hematopoietic cell transplantation based on recipient absolute lymphocyte count. Biol Blood Marrow Transplant. 2018;24(1):150-155. [DOI] [PubMed] [Google Scholar]
- 12.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17(9):1343-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer B, Jakubowski AA, Papadopoulos EB, et al. A chemotherapy-only regimen of busulfan, melphalan, and fludarabine, and rabbit antithymocyte globulin followed by allogeneic T-cell depleted hematopoietic stem cell transplantations for the treatment of myeloid malignancies. Biol Blood Marrow Transplant. 2017;23(12):2088-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barba P, Hilden P, Devlin SM, et al. Ex vivo CD34+-selected T cell-depleted peripheral blood stem cell grafts for allogeneic hematopoietic stem cell transplantation in acute leukemia and myelodysplastic syndrome is associated with low incidence of acute and chronic graft-versus-host disease and high treatment response. Biol Blood Marrow Transplant. 2017;23(3):452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montoro J, Ceberio I, Hilden P, et al. Ex vivo T cell-depleted hematopoietic stem cell transplantation for adult patients with acute myelogenous leukemia in first and second remission: long-term disease-free survival with a significantly reduced risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2020;26(2):323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho C, Perales MA. Expanding therapeutic opportunities for hematopoietic stem cell transplantation: T cell depletion as a model for the targeted allograft. Annu Rev Med. 2019;70(1):381-393. [DOI] [PubMed] [Google Scholar]
- 18.Scordo M, Bhatt V, Hilden P, et al. Standard antithymocyte globulin dosing results in poorer outcomes in overexposed patients after ex vivo CD34+ selected allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(8):1526-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg JD, Zheng J, Ratan R, et al. Early recovery of T-cell function predicts improved survival after T-cell depleted allogeneic transplant. Leuk Lymphoma. 2017;58(8):1859-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945-956. [DOI] [PubMed] [Google Scholar]
- 21.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR severity index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855-864. [DOI] [PubMed] [Google Scholar]
- 22.Gerds TA. prodlim: Product-Limit Estimation for Censored Event History Analysis. R package. 2018. https://cran.r-project.org/web/packages/prodlim/index.html
- 23.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qayed M, Kitko CL, Ahn KW, et al. Development and validation of a pediatric disease risk index for allogeneic hematopoietic cell transplantation [abstract]. J Clin Oncol. 2020;38(15_suppl). Abstract 7503. [Google Scholar]
- 25.Sorror ML, Logan BR, Zhu X, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant. 2015;21(8):1479-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Therneau TM. survival: Survival Analysis. R package. 2015. https://CRAN.R-project.org/package=survival
- 27.Gordon M, Seifert R. Greg: Regression Helper Functions. R package. 2019. https://CRAN.R-project.org/package=Greg
- 28.Admiraal R, Nierkens S, Bredius R, et al. Prospective open-label phase II trial of individualized anti-thymocyte globulin for improved T-cell reconstitution after pediatric allogeneic hematopoietic cell transplantation: The Parachute-Study [abstract]. Biol Blood Marrow Transplant. 2020;26(3):S33-S34. Abstract 41. [Google Scholar]
- 29.de Pagter PJ, Schuurman R, Meijer E, van Baarle D, Sanders EA, Boelens JJ. Human herpesvirus type 6 reactivation after haematopoietic stem cell transplantation. J Clin Virol. 2008;43(4):361-366. [DOI] [PubMed] [Google Scholar]
- 30.de Pagter PJ, Schuurman R, Visscher H, et al. Human herpes virus 6 plasma DNA positivity after hematopoietic stem cell transplantation in children: an important risk factor for clinical outcome. Biol Blood Marrow Transplant. 2008;14(7):831-839. [DOI] [PubMed] [Google Scholar]
- 31.Schneidawind D, Pierini A, Negrin RS. Regulatory T cells and natural killer T cells for modulation of GVHD following allogeneic hematopoietic cell transplantation. Blood. 2013;122(18):3116-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elias S, Rudensky AY. Therapeutic use of regulatory T cells for graft-versus-host disease. Br J Haematol. 2019;187(1):25-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odak I, Raha S, Schultze-Florey C, et al. Focusing of the regulatory T-cell repertoire after allogeneic stem cell transplantation indicates protection from graft- versus-host disease. Haematologica. 2019;104(12):e577-e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.