Abstract
The effect of clindamycin (CLI) combined with autovaquone (ATO) was examined in a murine model of acute toxoplasmosis. Swiss Webster mice intraperitoneally infected with 102 or 104 tachyzoites of the RH strain of Toxoplasma gondii were perorally treated with either drug alone (for ATO, 5, 25, 50, or 100 mg/kg of body weight/day; for CLI, 25, 50, or 400 mg/kg/day) or both combined (for ATO plus CLI, respectively, 5 plus 25, 25 plus 25, 25 plus 50, 50 plus 50, or 100 plus 400 mg/kg/day) starting with day 1 for 14 days. Survival was monitored during 7 weeks. Residual infection was assessed by a bioassay of representative 4-week survivors and by parasite DNA detection by PCR for representative 7-week survivors. An effect of treatment was shown in all treatment groups compared to untreated control mice (P = 0.0000). Among mice infected with 102 parasites, ATO and CLI at any dose combination protected significantly more animals than ATO alone (P = 0.0000), but compared to CLI alone, given its good effect, the combined drugs were no more effective (P > 0.05). For mice infected with 104 parasites, the drugs combined at the lowest and highest doses (5 plus 25 and 100 plus 400 mg/kg/day) were, similarly, more effective than ATO alone (P = 0.035 and 0.000, respectively) but not than CLI alone (P > 0.05). However, treatment with ATO plus CLI at 25 plus 25, 25 plus 50, and 50 plus 50 mg/kg/day protected 20, 33, and 78% of mice, respectively, compared to virtually no survivals among those treated with either drug alone (P < 0.0005), thus demonstrating a significant synergistic effect of ATO and CLI against T. gondii. Furthermore, the dose of ATO at a given dose of CLI was shown to be critical to the effect. Moreover, the absence of residual infection in some survivors shows the potential of this drug combination to eliminate the parasite.
The inability of any presently available therapeutic regimen to eliminate Toxoplasma gondii from the infected host requires lifelong maintenance therapy for patients with human immunodeficiency virus-induced immunosuppression who develop reactivated toxoplasmosis. Standard treatment with pyrimethamine-sulfadiazine is often associated with significant toxicity, requiring discontinuation of the drugs (13, 16). The consequent need for new therapies led to evaluation of a number of known and newly developed drugs and drug combinations, which resulted in increasing recognition that combination therapies may be necessary to treat toxoplasmosis in this setting (5).
Clindamycin (CLI) is an alternative drug widely used as a single agent or combined with pyrimethamine (7, 8). It has remarkable but delayed in vitro anti-T. gondii activity, achieved at low drug concentrations (22). In addition to the well-established anti-T. gondii activity of CLI as a single agent in animal models of infection (1, 10, 19, 29), doses of CLI that were ineffective when used alone were shown to afford protection in acute murine toxoplasmosis if combined with rifabutin (5).
The impetus to combine CLI with the hydroxynaphthoquinone atovaquone (ATO) was provided by reports on the activity of ATO against both tachyzoites and bradyzoite-containing T. gondii cysts (2, 3, 12). In addition, simultaneous administration of ATO has been shown to enhance the anti-T. gondii activity of pyrimethamine and sulfadiazine (4), as well as that of rifabutin (5, 27). Moreover, it has recently been suggested that a combination of ATO and CLI could act synergistically on both asexual life stages of T. gondii (28). Based on the proposed mechanisms of action of the two drugs—ATO inhibits the parasite’s mitochondrial function (23), and CLI, which inhibits protein synthesis on prokaryotic ribosomes (11), is thought to act on the T. gondii prokaryote-type plastid-like organelle (24)—this hypothesis predicts that simultaneous administration of ATO and CLI would cause parasites resistant to ATO to differentiate to bradyzoites, which should be hypersensitive to clindamycin. An end point of this joint action may be parasite elimination.
Thus, we examined in an in vivo experimental model of acute toxoplasmosis whether the addition of ATO to CLI would be beneficial for its anti-T. gondii effect and, moreover, if this combination is capable of eliminating the parasite, as suggested.
(The results of this study were presented in part at the 9th European Congress of Clinical Microbiology and Infectious Diseases [ECCMID] in Berlin, Germany, 21 to 24 March 1999.)
MATERIALS AND METHODS
Mice.
Female Swiss Webster mice (Medical Military Academy Animal Research Facility, Belgrade, Yugoslavia) weighing 18 to 20 g at the beginning of each experiment were used. Mice were housed six to a cage and offered drinking water ad libitum.
T. gondii.
Tachyzoites of the virulent RH strain maintained through serial intraperitoneal (i.p.) passages were used. For experimental infections, tachyzoites were harvested from mouse peritoneal fluids 72 h postinfection and purified by centrifugation, cotton wool filtration, and needle extraction. The parasites were counted in a hemocytometer, and their numbers were adjusted to 2 × 106/ml with saline. Suspensions were serially 10-fold diluted, and 0.5-ml aliquots of 2 × 104/ml and 2 × 102/ml dilutions were inoculated i.p. into fresh mice.
Drugs.
CLI (CLI hydrochloride powder, lot 353YS; Upjohn Co.; supplied by Yusapharm, Belgrade, Yugoslavia) was administered at 25, 50, and 400 mg per kg of body weight per day.
ATO (micronized powder, lot 291604A; Glaxo Wellcome, Stevenage, United Kingdom) was administered at 5, 25, 50, and 100 mg/kg/day.
These doses were selected on the basis of previous work (1, 2, 19, 27, 29) as effective (400 mg of CLI/kg/day and 100 mg of ATO/kg/day) or, to better reveal the effects of the combined therapy, as suboptimal (all lower doses of both drugs).
Based on the observation that mice consume 4 g of food per day (10, 19), the desired doses were obtained by adding 0.125, 0.25, or 2.0 mg of CLI and 0.02, 0.1, 0.2, or 0.4 mg of ATO, respectively, per 1 g of ground mouse feed. The mixture was then repelleted to conform to normal rodent dietary habits. Fresh food was supplied daily.
To control for drug side effects, separate groups of animals were given CLI (400 mg/kg/day) and ATO (100 mg/kg/day) for 3 weeks, as well as ATO plus CLI (100 plus 400 mg/kg/day) for 2 weeks; no clinically significant toxicity (piloerection, lethargy, or weight loss) was observed over 3 months.
Experimental design.
Mice injected i.p. with 102 or 104 parasites were arbitrarily assigned to one of the 12 treatment groups according to the treatment given, as follows: CLI alone at 25, 50, or 400 mg/kg/day, ATO alone at 5, 25, 50, or 100 mg/kg/day, or ATO plus CLI at 5 plus 25, 25 plus 25, 25 plus 50, 50 plus 50, and 100 plus 400 mg/kg/day. Each treatment group comprised 6 to 12 animals, depending on the experiment. Treatment was initiated 24 h following parasite inoculation and was continued for 14 consecutive days. A group of untreated animals served as the negative control. Since a preliminary experiment showed lethal events in mice treated with the combined drugs beyond the usual observation period for acute toxoplasmosis models of 4 weeks, we chose to continue the observation period to 7 weeks postinfection. Mouse survival was monitored daily. Peritoneal exudates of mice that succumbed were examined at random for the presence of T. gondii. All experiments were performed three times, with similar results, and the data shown represent their cumulative results.
To assess residual infection in mice treated with CLI plus ATO, groups of two arbitrarily chosen survivors were sacrificed (i) 4 weeks postinfection, for subinoculation of brains into fresh mice to attempt reisolation of T. gondii (bioassay), and (ii) 7 weeks postinfection, for the detection of T. gondii DNA in brains and lungs by PCR. Brains and lungs were chosen as likely reservoirs of parasites following treatment of murine infection (25).
Bioassay.
Brains were homogenized, and 0.5-ml saline suspensions were inoculated into two fresh mice per sample, one each by the i.p. and intraesophageal routes. Mice were monitored daily over 4 weeks; peritoneal fluids of those succumbing were examined for the presence of T. gondii.
PCR.
For DNA extraction, mouse brains and lungs were dilacerated and suspended in a lysis buffer containing 200 mM Tris-HCl, 1 mM EDTA, 0.5 M NaCl, 1% sodium dodecyl sulfate, and 5 mg of proteinase K/ml. The extraction procedure was conducted according to a classical protocol (18) including two purification steps with phenol-chloroform-isoamyl alcohol (25:24:1). The DNA obtained was precipitated with cold ethanol, and after centrifugation, DNA pellets were washed in 70% alcohol, centrifuged, and resuspended in sterile water. The DNA concentration was determined by spectroscopy (Pharmacia Biotech, Saint-Quentin en Yvelines, France), and 1 μg of DNA was used for PCR. The gene target was a 129-bp sequence from the 35-fold-repeated B1 gene (6), amplified by using the primers 5′-CCGCCTCCTTCGTCCGTCGTA and 5′-TGAAGAGGAAACAGGTGGTCG. The amplification was carried out in a 50-μl final volume by using the PCR enzyme-linked immunosorbent assay (ELISA) DIG Labeling Plus kit (Boehringer Mannheim, Meylan, France) yielding digoxigenin (DIG)-labeled PCR products. Negative controls were brain DNA of a noninfected mouse and leukocyte DNA of a noninfected patient, and positive controls were brain DNA of a mouse infected with a chronic strain of T. gondii isolated from the amniotic fluid of a congenitally infected human fetus and two quantitated controls (i.e., low [1 to 10 parasites] and high [100 parasites]) consisting of T. gondii RH strain DNA from tachyzoites obtained by peritoneal lavage of a mouse infected 4 days earlier. Following 35 amplification cycles, PCR products were detected by an adapted protocol supplied with the PCR ELISA DIG Detection kit (Boehringer Mannheim) by using a biotin-labeled probe (5′-GCAAGAGAAGTATTTGAGGTC) and a detection system with streptavidin-coated microwells. Hybridized PCR products were revealed with an anti-DIG conjugate labeled with peroxidase, then incubated with the specific substrate. Appropriate technical controls were included (probe and substrate blank), according to the manufacturer’s recommendations. The optical density (OD) was determined on an ELISA reader, and the threshold was obtained by a threefold multiplication of the OD of a negative patient. The technique was validated when all the controls were correct. In particular, special attention was given to the positivity of the low DNA control, corresponding to 1 to 10 parasites. The sensitivity was therefore estimated as between 1 and 10 parasites per sample. The specificity of this PCR technique was previously evaluated and determined to be 100% (26). Carryover contamination was prevented by use of uracil DNA glycosylase (17).
Statistical analysis.
The rates of survival in particular treatment groups were estimated by the Kaplan-Meier product limit method and compared by the log-rank test (two curves) and multiple sample (three or more curves) tests. The level of statistical significance was 0.05.
RESULTS
The effects of treatment with ATO and CLI combined in five different doses on the survival of mice infected with 102 or 104 RH strain T. gondii tachyzoites were compared to those of the same drugs given alone. Peritoneal exudates of mice dying during the 7-week observation period were examined at random; T. gondii tachyzoites were seen in all.
Drug activity in infection model with 102 parasites.
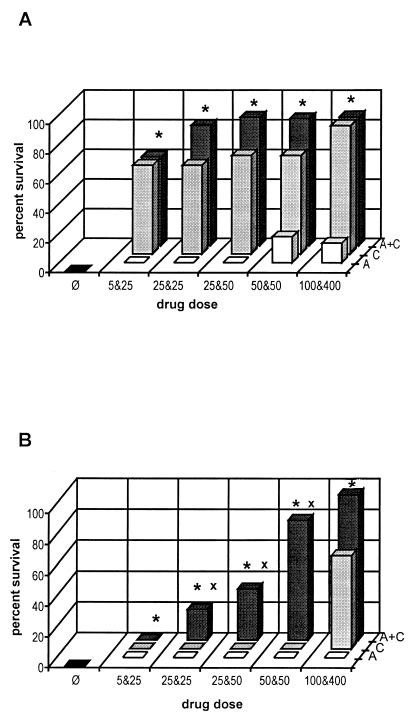
Untreated infected mice died between days 8 and 11 (mean ± standard deviation [SD], 9.4 ± 0.9 days), compared to which an effect of treatment was shown in all treatment groups (Fig. 1A). ATO alone was effective compared with no treatment, since as little as 5 mg/kg/day significantly prolonged time to death (P = 0.0000), and when it was given at 50 or 100 mg/kg/day, it resulted in 7-week survival of 18 or 13% of mice, respectively. CLI alone at 25, 50, or 400 mg/kg/day led to survival of 60, 67, or 86% of mice, respectively.
FIG. 1.
Survival rates of mice treated with ATO (5, 25, 50, or 100 mg/kg/day) and CLI (25, 50, or 400 mg/kg/day) alone or combined (5 plus 25, 25 plus 25, 25 plus 50, 50 plus 50, or 100 plus 400 mg/kg/day) in infection models induced by i.p. inoculation of 102 (A) or 104 (B) T. gondii RH strain tachyzoites. ■, control mice; □, ATO;  , CLI;
, CLI;  , ATO plus CLI; ∗, P < 0.05 for ATO plus CLI versus ATO alone; x, P < 0.05 for ATO plus CLI versus CLI alone.
, ATO plus CLI; ∗, P < 0.05 for ATO plus CLI versus ATO alone; x, P < 0.05 for ATO plus CLI versus CLI alone.
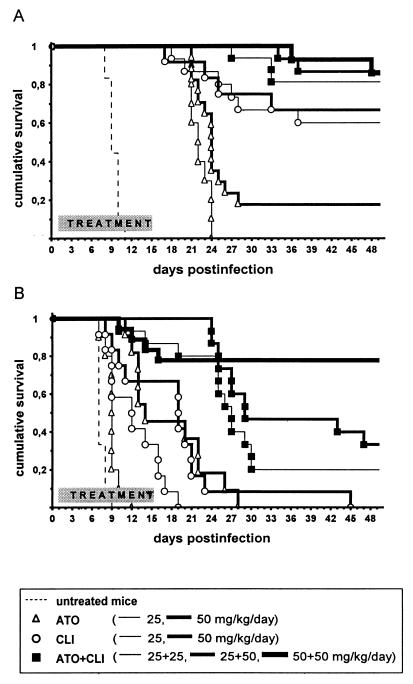
Treatment with ATO and CLI combined at 5 plus 25, 25 plus 25, 25 plus 50, 50 plus 50, or 100 plus 400 mg/kg/day protected 60, 81, 87, 86, or 87% of mice, respectively, with no significant variation among particular groups (P = 0.371 by a multiple sample test). However, all drug combinations were significantly more effective than any dose of ATO alone (P = 0.022 for ATO plus CLI at 5 plus 25 mg/kg/day versus ATO at 100 mg/kg/day). This was not the case when the two drugs combined were compared with CLI alone. Given the efficacy of CLI alone, the addition of ATO to any given dose of CLI, including those at which the effect of the combined drugs was apparently better (Fig. 1A), did not significantly enhance its activity (Fig. 2A) (P > 0.05 by the log-rank test). Thus, the combined drugs exhibited a simple additive effect.
FIG. 2.
Estimated probabilities of survival (Kaplan-Meier product limit method) for mice treated with ATO (25 or 50 mg/kg/day) or CLI (25 or 50 mg/kg/day) alone or combined (25 plus 25, 25 plus 50, or 50 plus 50 mg/kg/day) in infection induced by i.p. inoculation of 102 (A) or 104 (B) T. gondii RH strain tachyzoites. Peroral treatment was initiated 24 h following challenge and continued for 14 days.
Drug activity in infection model with 104 parasites.
All untreated control mice infected with 104 parasites died between days 7 and 9 (mean ± SD, 7.4 ± 0.6 days). As in infection caused with 102 parasites, an effect of treatment was shown in all treatment groups infected with this higher parasite load relative to the control animals (Fig. 1B), e.g., treatment with 5 mg of ATO/kg/day significantly prolonged survival (P = 0.0000). However, in contrast with results for animals infected with fewer parasites, treatment with both drugs combined was much more effective than treatment with either drug given alone.
The rate of survival of mice treated with 5 plus 25, 25 plus 25, 25 plus 50, 50 plus 50, or 100 plus 400 mg of ATO plus CLI/kg/day was 0, 20, 33, 78, or 94%, respectively. In sharp contrast, no dose of either ATO or CLI alone prevented mortality, except for 400 mg of CLI/kg/day, which protected 61% of animals.
The combination of the lowest doses (5 plus 25 mg/kg/day) was obviously too low to prevent mortality. Still, it significantly prolonged time to death compared with 5 mg of ATO/kg/day (P = 0.035) but not compared with 25 mg of CLI/kg/day (P = 0.141). Similarly, the combination of the highest doses (100 plus 400 mg/kg) was significantly more effective than 100 mg of ATO/kg/day (P = 0.0000) but not more effective than 400 mg of CLI/kg/day (P = 0.097), since the latter was very effective itself.
However, the effects of the drugs combined at all but the highest and lowest doses, i.e., 25 plus 25, 25 plus 50, and 50 plus 50 mg/kg/day, highly significantly (P < 0.0005 for all pairs) surpassed the added effects of the respective doses of both drugs given alone (Fig. 2B), thus showing a remarkable synergistic activity. Moreover, ATO plus CLI at 25 plus 50 and 50 plus 50 mg/kg/day were both as effective as CLI at 400 mg/kg/day (P > 0.05).
The effects of ATO plus CLI differed significantly among particular dose groups (P = 0.0000 by the multiple sample test). However, at a specific CLI dose, the dose of ATO appeared critical to the effect. At 25 mg of CLI/kg/day, an increase in the dose of ATO from 5 to 25 mg/kg/day enhanced protection from 0 to 20% (P = 0.0015 by the log-rank test), and at 50 mg of CLI/kg/day, an increase in ATO from 25 to 50 mg/kg/day augmented protection from 33 to 78% (P = 0.0191). In contrast, at 25 mg of ATO/kg/day, an increase in CLI from 25 to 50 mg/kg/day failed to enhance protection (P = 0.131).
Detection of residual infection.
The results of the procedures performed to assess the presence of residual infection are presented in Table 1. Subinoculation in fresh mice of brain tissue of 4-week survivors infected with 102 and 104 parasites treated with the 100-plus-400-mg/kg/day combination resulted in mortality due to acute toxoplasmosis of 75% (mean survival ± SD, 14.3 ± 4.2 days) and 50% (mean survival ± SD, 10 ± 2.8 days) of mice, respectively. Interestingly, lethal events were recorded following both i.p. and peroral inoculation of brain tissue, suggesting the presence of both the tachyzoite and bradyzoite life stages of the parasite in some survivors at that time. However, residual infection was harbored by fewer long-term survivors from the respective groups of animals, since parasite DNA was detected in 50 and 0% of brains 7 weeks postinfection. At this time, parasite DNA was detected in 50 and 100% of animals treated with lower doses of the combined drugs. No parasite DNA was detected in any of the lungs examined.
TABLE 1.
Residual infection in representative treated survivors
| Inoculum (no. of parasites) | ATO + CLI dose (mg/kg/day) | No. positive/no. studieda by:
|
|||
|---|---|---|---|---|---|
| Bioassay (4 wk p.i.)
|
PCR (7 wk p.i.)
|
||||
| p.o. | i.p. | Brain | Lungs | ||
| 102 | 5 + 25 | ND | ND | 1/2 | 0/1 |
| 100 + 400 | 2/2 | 1/2 | 1/2 | 0/1 | |
| 104 | 25 + 50 | ND | ND | 2/2 | 0/1 |
| 100 + 400 | 1/2 | 1/2 | 0/2 | 0/1 | |
p.i., postinfection; p.o., peroral infection; i.p., i.p. infection; ND, not done.
DISCUSSION
This is the first report to date on the anti-T. gondii activity of CLI combined with ATO. The results presented demonstrate that the combination of CLI and ATO acts synergistically against T. gondii, since the combined drugs exhibit a significantly higher activity than that expected from the simple additive effects of both drugs. In our murine model of RH strain-induced acute toxoplasmosis, the effect of CLI and ATO was both drug dose and parasite inoculum dependent. The synergistic effect was remarkable in infections with 104 parasites, in which 14-day treatment with combinations of 25 and 50 mg of CLI and ATO/kg/day protected 20 to 78% of animals by week 7, whereas the same doses of either CLI or ATO alone could not prevent mortality. Moreover, the effect of a combination of 50 mg of CLI/kg/day with either 25 or 50 mg of ATO/kg/day was similar to the effect of 400 mg of CLI/kg/day alone. On the other hand, in infections with 102 parasites, which were well controlled by any dose of CLI used, the combinations of ATO and CLI exhibited an additive effect compared to that of either drug alone.
It is difficult to put these results in perspective by comparing them with the efficacy of these or other drugs, alone or in combination, obtained in other animal models, since the infection and treatment protocol characteristics vary widely. One important characteristic of our model is the length of the observation period, since, having observed lethal events following the standard observation period of 4 weeks, we continued to monitor survival to 7 weeks following infection.
This late mortality (between weeks 4 and 7 postinfection) may explain why less residual infection is detected in 7-week than in 4-week survivors. The absence of residual infection, determined by a sensitive PCR method, in the brains of four of eight 7-week survivors (and in the lungs of four of four) treated with ATO and CLI at various dose combinations demonstrates the potential of the combined drugs to eliminate the parasite. This potential is emphasized by the fact that the mere presence of T. gondii DNA does not necessarily indicate the presence of viable parasites able to induce infection. If it does, it implies immune control of infection. Thus, in the treated, apparently healthy mice in which T. gondii DNA was detected, drug treatment, by reducing the initial parasite burden, may have provided the time for protective immunity against an otherwise lethal parasite infection to develop; a delayed specific T-cell response has been associated with treatment (20).
The results presented show ATO and CLI to be a promising drug combination with the potential to eliminate the parasite, therefore warranting further investigation. The low doses used to obtain high protection and cure rates may allow prolonged administration; 4- and 8-week courses of ATO were shown to be effective in chronic murine toxoplasmosis (3, 9), and we have previously shown that the effect of CLI critically depends on treatment duration (21). Furthermore, adverse side effects of CLI (7, 8) may be reduced by the use of lower doses, and ATO is generally well tolerated (14, 15). However, to appreciate the potential of the combination of CLI and ATO in the treatment of human disease, studies of the levels of the drugs achieved in serum and tissues with the regimens used, relative to the doses feasible in human therapy, are needed. Nevertheless, while one should bear in mind the limitations of extrapolating data from animal models to the human situation, the observation that at both 25 and 50 mg of CLI/kg/day an increase in the dose of ATO significantly enhanced the effect of the combined drugs may offer direction for future clinical trials of the anti-T. gondii potential of this drug combination.
ACKNOWLEDGMENTS
T. Nikolić was an ECCMID Young Investigator Award recipient.
ATOvaquone was provided to O. Djurković-Djaković as a GlaxoWellcome external investigator. The expert technical assistance of Jordanka Djurović is acknowledged.
REFERENCES
- 1.Araujo F G, Remington J S. Effect of clindamycin on acute and chronic toxoplasmosis in mice. Antimicrob Agents Chemother. 1974;5:647–651. doi: 10.1128/aac.5.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo F G, Huskinson J, Remington J S. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone, 566C80, against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991;35:293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo F G, Huskinson-Mark J, Gutteridge W E, Remington J S. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob Agents Chemother. 1992;36:326–330. doi: 10.1128/aac.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo F G, Lin T, Remington J S. The activity of atovaquone (566C80) in murine toxoplasmosis is markedly augmented when used in combination with pyrimethamine or sulfadiazine. J Infect Dis. 1993;167:494–497. doi: 10.1093/infdis/167.2.494. [DOI] [PubMed] [Google Scholar]
- 5.Araujo F G, Slifer T, Remington J S. Rifabutin is active in murine models of toxoplasmosis. Antimicrob Agents Chemother. 1994;38:570–575. doi: 10.1128/aac.38.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burg J L, Grover C M, Pouletty P, Boothroyd J C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989;27:1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dannemann B, McCutchan J A, Israelski D, Antoniskis D, Leport C, Luft B, Nussbaum J, Clumeck N, Morlat P, Chiu J, Vilde J-L, Orellana M, Feigal D, Bartok A, Heseltine P, Leedom J, Remington J S the California Collaborative Treatment Group. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. Ann Intern Med. 1992;116:33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- 8.Dannemann B R, Israelski D M, Remington J S. Treatment of toxoplasmic encephalitis with intravenous clindamycin. Arch Intern Med. 1988;148:2477–2482. [PubMed] [Google Scholar]
- 9.Ferguson D J, Huskinson-Mark J, Araujo F G, Remington J S. An ultrastructural study of the effect of treatment with atovaquone in brains of mice chronically infected with the ME49 strain of Toxoplasma gondii. Int J Exp Pathol. 1994;75:111–116. [PMC free article] [PubMed] [Google Scholar]
- 10.Filice G A, Pomeroy C. Effect of clindamycin on pneumonia from reactivation of Toxoplasma gondii infection in mice. Antimicrob Agents Chemother. 1991;35:780–782. doi: 10.1128/aac.35.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzhugh A L. Antibiotic inhibitors of the peptidyl transferase center. 1. Clindamycin as a composite analogue of the transfer RNA fragments l-Pro-Met and the d-ribosyl ring of adenosine. Bioorg Med Chem Lett. 1998;8:87–92. doi: 10.1016/s0960-894x(97)10196-2. [DOI] [PubMed] [Google Scholar]
- 12.Gormley P D, Pavesio C E, Minnasian D, Lightman S. Effects of drug therapy on toxoplasma cysts in an animal model of acute and chronic disease. Investig Ophthalmol Vis Sci. 1998;39:1171–1175. [PubMed] [Google Scholar]
- 13.Haverkos H W the TE study group. Assessment of therapy for toxoplasma encephalitis. Am J Med. 1987;82:907–914. doi: 10.1016/0002-9343(87)90151-3. [DOI] [PubMed] [Google Scholar]
- 14.Katlama C, Mouthon B, Gourdon D, Lapierre D, Rousseau F the Atovaquone Expanded Access Group. Atovaquone as long-term suppressive therapy for toxoplasmic encephalitis in patients with AIDS and multiple drug intolerance. AIDS. 1996;10:1107–1112. [PubMed] [Google Scholar]
- 15.Kovacs J. Efficacy of atovaquone in treatment of toxoplasmosis in patients with AIDS. Lancet. 1992;340:637–638. doi: 10.1016/0140-6736(92)92172-c. [DOI] [PubMed] [Google Scholar]
- 16.Leport C, Raffi F, Matheron S, Katlama C, Regnier B, Saimot A G, Marche C, Vedrenne C, Vilde J L. Treatment of central nervous system toxoplasmosis with pyrimethamine/sulfadiazine combination in 35 patients with the acquired immunodeficiency syndrome. Efficacy of long-term continuous therapy. Am J Med. 1988;84:94–100. doi: 10.1016/0002-9343(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 17.Longo M C, Berninger M S, Hartley J L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene. 1990;93:125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- 18.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 19.McMaster P R B, Powers K G, Finerty J F, Lunde M N. The effect of two chlorinated lincomycin analogues against acute toxoplasmosis in mice. Am J Trop Med Hyg. 1973;22:14–17. doi: 10.4269/ajtmh.1973.22.14. [DOI] [PubMed] [Google Scholar]
- 20.Murray H W, Teitelbaum R, Hariprashad J. Response to treatment for an intracellular infection in a T-cell-deficient host: toxoplasmosis in nude mice. J Infect Dis. 1993;167:1173–1177. doi: 10.1093/infdis/167.5.1173. [DOI] [PubMed] [Google Scholar]
- 21.Nikolić T, Djurković-Djaković O, Bobić B, Nikolić A, Babić D. Treatment protocol determines the efficacy of clindamycin in acute murine toxoplasmosis. Int J Antimicrob Agents. 1999;11:145–149. doi: 10.1016/s0924-8579(98)00064-8. [DOI] [PubMed] [Google Scholar]
- 22.Pfefferkorn E R, Nothnagel R F, Borotz S E. Parasiticidal effect of clindamycin on Toxoplasma gondii grown in cultured cells and selection of a drug-resistant mutant. Antimicrob Agents Chemother. 1992;36:1091–1096. doi: 10.1128/aac.36.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfefferkorn E R, Borotz S E, Nothnagel R F. Mutants of Toxoplasma gondii resistant to atovaquone (566C80) or decoquinate. J Parasitol. 1993;79:559–564. [PubMed] [Google Scholar]
- 24.Pfefferkorn E R, Borotz S E. Comparison of mutants of Toxoplasma gondii selected for resistance to azithromycin, spiramycin, or clindamycin. Antimicrob Agents Chemother. 1994;38:31–37. doi: 10.1128/aac.38.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piketty C, Derouin F, Rouveix B, Pocidalo J-J. In vivo assessment of antimicrobial agents against Toxoplasma gondii by quantification of parasites in the blood, lungs, and brain of infected mice. Antimicrob Agents Chemother. 1990;34:1467–1472. doi: 10.1128/aac.34.8.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert-Gangneux F, Gavinet M-F, Ancelle T, Raymond J, Tourte-Schaefer C, Dupouy-Camet J. Value of prenatal diagnosis and early postnatal diagnosis of congenital toxoplasmosis: retrospective study of 110 cases. J Clin Microbiol. 1999;37:2893–2898. doi: 10.1128/jcm.37.9.2893-2898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romand S, Della Bruna C, Farinotti R, Derouin F. In vitro and in vivo effects of rifabutin alone or combined with atovaquone against Toxoplasma gondii. Antimicrob Agents Chemother. 1996;40:2015–2020. doi: 10.1128/aac.40.9.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomavo S, Boothroyd J C. Interconnection between organellar functions, development and drug resistance in the protozoan parasite, Toxoplasma gondii. Int J Parasitol. 1995;25:1293–1299. doi: 10.1016/0020-7519(95)00066-b. [DOI] [PubMed] [Google Scholar]
- 29.Vuković D, Djurković-Djaković O, Kovaĉević S, Bobić B, Nikolić A, Todorović V, Babić D. Effect of clindamycin in a murine model of acute toxoplasmosis. Clin Microbiol Infect. 1997;3:89–94. doi: 10.1111/j.1469-0691.1997.tb00256.x. [DOI] [PubMed] [Google Scholar]