Abstract
A shock wave (SW), which carries energy and propagates through a medium, is a type of continuous transmitted sonic wave that can achieve rapid energy transformations. SWs have been applied for many fields of medical science in various treatment settings. In urology, high-energy extracorporeal SWs have been used to disintegrate urolithiasis for 30 years. However, at lower energy levels, SWs enhance the expression of vascular endothelial growth factor (VEGF), endothelial nitric oxide synthase (eNOS), proliferating cell nuclear antigen (PCNA), chemoattractant factors, and the recruitment of progenitor cells, and inhibit inflammatory molecules. Low energy extracorporeal shock wave (LESW) therapy has been used in urology for treating chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), interstitial cystitis/bladder pain syndrome (IC/BPS), overactive bladder, stress urinary incontinence, and erectile dysfunction through the mechanisms of anti-inflammation, neovascularization, and tissue regeneration. Additionally, LESW have been proven to temporarily increase tissue permeability and facilitate intravesical botulinum toxin delivery for treating overactive bladders in animal studies and in a human clinical trial. LESW assisted drug delivery was also suggested to have a synergistic effect in combination with cisplatin to improve the anti-cancer effect for treating urothelial cancer in an in vitro and in vivo study. LESW assisted drug delivery in uro-oncology is an interesting suggestion, but no comprehensive clinical trials have been conducted as of yet. Taken together, LESW is a promising method for the treatment of various diseases in urology. However, further investigation with a large scale of clinical studies is necessary to confirm the real role of LESW in clinical use. This article provides information on the basics of SW physics, mechanisms of action on biological systems, and new frontiers of SW medicine in urology.
Keywords: shock waves, cryoinjury, bladder, erectile dysfunction
1. Introduction
Extracorporeal shock wave lithotripsy (ESWL) was the first application of SW medicine in humans [1]. Weinstein et al. demonstrated that SWs have loosening effects on the bone-cement interface for revision arthroplasty in dogs [2]. Thereafter, ESWT has been widely used and studied in musculoskeletal disorders [3,4]. Additionally, cardiac SW therapy has anti-anginal effects, increases exercise capacity, and improves myocardial perfusion and clinical symptoms in the treatment of patients with stable coronary artery disease with a safety profile [5]. ESWT was also shown to enhance skin flap survival through increasing blood perfusion and neovascularization in a compromised skin flap model in rats [6]. Thereafter, ESWT was suggested as an effective and safe treatment for soft tissue wounds [7,8], and was approved for treating diabetic foot ulcers by the FDA in 2017 [9].
ESWT has been widely studied in various new fields of biomedicine [10,11]. New findings and indications of ESWT for urology have increased in recent years [12,13,14]. This paper reviews mechanisms of action and clinical results of ESWT on new frontiers in urology.
2. Characteristics of SWs
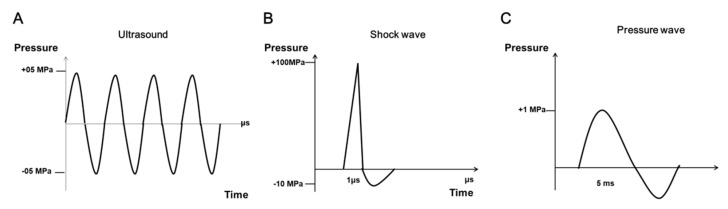
The SW is an acoustic wave, similar to the sound of thunder in nature, or the sound of a supersonic aircraft. A SW can be used to transfer energy into the human body to restore body function. The characteristics of SWs are different from those of an ultrasound wave (Figure 1A). The SW is a biphasic supersonic wave that has high pressure amplitude, a thousand times more than that of an ultrasound wave [15]. When a SW is generated, a high pressure wave is produced with a value of approximately 100 megapascals (MPa) in 10 nanoseconds, followed by a negative pressure value of approximately negative 10 MPa in microseconds (Figure 1B) [15]. Three types of generators can be used to produce SWs, including electrohydraulic, electromagnetic, and piezoelectric. Radial extracorporeal shock wave (rESW), a ballistic pressure wave (approximately with 1 MPa in microseconds), is produced by a compressed air device. The characteristics of rESWs are different from those of acoustic SWs (Figure 1C). There are four forms of SW, including focused, defocused, planar, and radial, which are generated depending on the source and the design of the devices. SWs carry energy through the human body and induce physical, chemical, and biological effects [16]. Focused, defocused, and planar SW treatments are called ESWT. The energy of LESWs used for tissue regeneration is approximately 5 Mpa, which is lower than that of ESWL (above 100 MPa) used to disintegrate kidney stones.
Figure 1.
Typical types of ultrasound, focused shock wave, and pressure wave. (A) Ultrasound waves are periodic oscillations with a limited bandwidth. (B) Focused shock wave is characterized by a single positive pressure wave (100 MPa), followed by a negative pressure wave (−10 MPa). The 1 microsecond (μs) of time interval is the positive pressure wave. (C) The pressure wave is 1 MPa positive wave and less than 1 MPa negative wave in 5 milliseconds (ms).
3. Dosage of ESWT
The energy flux density (EFD) is used as the parameter of ESWT to measure the energy per square area (mJ/mm2) that transmits the energy of the SW into tissue [15]. There is currently no consensus on the boundary values of high, medium, and low energy SWs. Different manufacturers or studies define the range of the energy into energy bands. Indeed, in 1998, high (>0.6 mJ/mm2), medium (0.08–0.28 mJ/mm2), and low energy (>0.08 mJ/mm2) of ESWT was defined by Rompe at al. to define dose-related effects of SWs on rabbit tendo Achillis [17]. A review article on ESWT for the treatment of soft tissue conditions considered low energy ESWT as EFD ≤ 0.12 mJ/mm2, and high energy as >0.12 mJ/mm2 [18]. Bannuru et al. suggested that high energy (≥0.28 mJ/mm2) SWs better relieve pain and improve function in chronic calcific shoulder tendinitis compared to low energy (<0.28 mJ/mm2) SW therapy [18]. Wang and Lue et al. defined low intensity extracorporeal SW therapy (Li ESWT) as a form of energy transfer that is <0.2 mJ/mm2 than the ESWT employed for lithotripsy and nephrolithiasis treatment [19]. Therefore, there remains no agreement to the definition of high, medium, and low energy for ESWT.
4. Biological Effects of ESWT
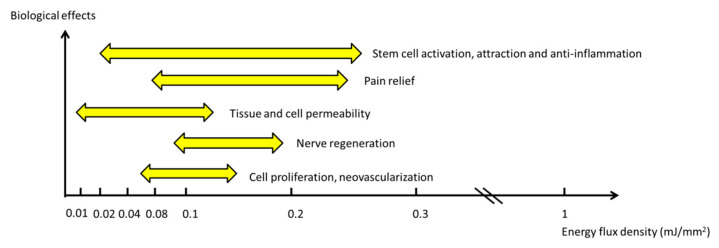
The biological effects and related energy levels of ESWT are presented in Figure 2. Haupt proposed that the mechanisms of action of ESWT have four reaction phases [20], including physical, physical-chemical, chemical, and biological phases. The generation of SWs induces a positive pressure wave, followed by a negative pressure wave to pass through the medium. A portion of the wave undergoes absorption, reflection, and refraction [15]. Additionally, a negative pressure wave produces a tensile force and induces cavitation and ionization in the liquid after a positive pressure wave. These physical forces increase cell membrane permeability and release biomolecules, such as ATP or ions, in the physical-chemical and chemical phases [21,22,23]. Finally, mechanotransduction after SW treatment induces the release of exosomes, microRNA, growth factors, cytokines, chemokines, and other molecules from different cells to produce various biological effects [16,24,25,26].
Figure 2.
The biological effects associated with related energy levels of ESWT.
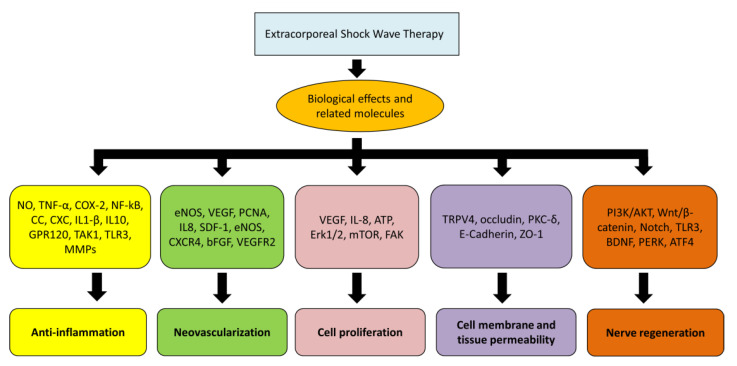
Many biological effects and related molecules of ESWT were reported, including anti-inflammation, immunomodulation, angiogenesis, cell proliferation and differentiation, stem cell attraction, exosome release, nerve regeneration, and increase in cell membrane permeability (Figure 3) [13,16,25,27,28,29,30].
Figure 3.
Biological effects and related molecules after ESWT.
5. Anti-Inflammation
ESWT reduces expression of tumor necrosis factor alpha (TNF-α), COX2, nuclear factor kappa B (NF-κB), and chemokines (CC and CXC), all of which contribute to anti-inflammation reactions [31]. ESWT induces expression of G-protein coupled receptor 120 (GPR120), which has been shown to inhibit transforming growth factor beta-activated kinase 1 (TAK1) and NF-κB signaling to modulate the inflammatory response in a cystitis model in rats [32]. Sukubo et al. demonstrated that macrophage exposure to low energy SW dampens the induction of the pro-inflammatory profile characterizing M1 macrophages and promotes the acquisition of an anti-inflammatory profile synergizing with macrophage alternative activation, which is associated with a significant increase in IL-10 and a reduction in IL-1β production [33]. Holfeld et al. revealed that ESWT modulates inflammation via the TLR3 pathway, including the interaction between interleukin (IL)-6 and IL-10 in TLR3 stimulation as a three-phase regulation over time in a study of ESWT application to human umbilical vein endothelial cells (HUVECs) [34]. ESWT also has effects on the suppression of pro-inflammatory cytokines, chemokines, and matrix metalloproteases (MMPs), as well as the attenuation of leukocyte infiltration for treating wound healing, severe cutaneous burn injury, and cystitis models in animals [35,36,37,38].
6. Angiogenesis
Wang et al. investigated the effect of ESWT on neovascularization at the tendon–bone junction in a rabbit model [24]. The results demonstrated that ESWT produces a significantly higher number of neo-vessels and angiogenesis-related markers, including eNOS, VEGF, and PCNA, compared to the control. Another study showed that treatment with multiple sessions of ESWT significantly enhanced diabetic wound healing, which was associated with increased neovascularization and tissue regeneration, correlating with VEGF and the mitogen-activated protein kinase-mediated pathway [39]. It was also demonstrated that ESWT enhanced the expression of various angiogenesis-related factors, including VEGF, IL8, stromal cell-derived factor 1 (SDF-1), eNOS, CXC motif chemokine 4 (CXCR4), and basic fibroblast growth factor (bFGF), improving tissue perfusion in clinical trials and animal models [40,41,42]. Huang et al. suggested that ESWT enhanced angiogenesis via ligand-independent activation of VEGFR2, and further prolonged angiogenesis through endosome-to-plasma membrane recycling in HUVECs [43].
7. Cell Proliferation
Cell proliferation is a major necessity for tissue regeneration. ESWT induces up-regulation of VEGF and IL-8, promotes the proliferation of endothelial progenitor cells, and improves myocardial function in patients with refractory coronary artery disease [42]. ESWT was proven to trigger the release of cellular ATP, which subsequently activates purinergic receptors and finally enhances proliferation in vitro and in vivo via downstream of Erk1/2 signaling [22]. Additionally, ESWT activates the mTOR-FAK mechanotransduction signaling axis and enhances the proliferation of mesenchymal stem cells (MSCs) [44].
8. Cell Membrane Permeability
ESWT, inducing a shear force and cavitation through the liquid on cells, can temporarily increase the cell membrane and tissue permeability, and facilitate drug delivery or gene therapy [45,46]. Kung et al. reported that ESWT transiently opens the blood-brain barrier and delivers the genes into brain with no detrimental effects [47]. Our previous study demonstrated that ESWT induces significant down regulation of E-cadherin and ZO-1, increases the permeability of the bladder urothelium, and facilitates intravesical botulinum toxin delivery in a rat model [48]. Moreover, Ito et al. evaluated the interferometric and fluorescence analysis of SW effects on the cell membrane, the results of which suggested a possible mechanism of membrane permeation where sharp pressure gradients create pores on the membrane, as evidenced by the transport of fluorescent dye molecules from outside to inside the cells following shock-induced membrane permeability [30]. Additionally, LESW-assisted drug delivery was suggested to have a synergistic effect in combination with cisplatin, improving the anti-cancer effect by improving chemo-agent tissue infiltration and decreasing cisplatin efflux membranous protein expression for treating urothelial cancer in an in vitro and in vivo study [49].
9. Nerve Regeneration
Hausner et al. demonstrated that ESWT improves the rate of axonal regeneration in a sciatic nerve injury model in rats, likely via faster Wallerian degeneration and improved removal of degenerated axons and regenerated injured axons with greater capacity [27]. Lee et al. demonstrated that ESWT significantly increased the sciatic functional index score, reduced the level of muscle atrophy, reordered the injured nerves, and activated the conjunction of the muscle and neurons in a sciatic nerve-crushing damage model in rats [50]. The rESW enhances neural stem cell proliferation and differentiation by activation of the PI3K/AKT, Wnt/β-catenin, and Notch pathway [51]. Sensing of tissue damage and subsequent orchestration of the inflammatory response via TLR3 were suggested to represent an innate mechanism of tissue regeneration, which was proven to be involved in neuroprotection and spinal cord repair in a mice spinal cord contusion model treated with ESWT [52]. ESWT was also shown to increase the expression of brain-derived neurotrophic factor (BDNF) and activate the PERK/ATF4 signaling pathway to induce nerve regeneration after nerve injury [53]. However, the detailed mechanism and more evidence-based results and clinical studies should be investigated in the future.
10. Application of Low Energy Shock Wave (LESW) in Urology
10.1. Chronic Prostatitis/Chronic Pelvic Pain Syndrome
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is an inflammatory disease, with an estimated prevalence from 8.4% to 25% in different studies [54,55]. According to the National Institutes of Health (NIH) categories of prostatitis, type III chronic abacterial prostatitis, including type IIIa–inflammatory CP/CPPS and type IIIb—noninflammatory CP/CPPS, and type IV—asymptomatic inflammatory prostatitis, meet the definition of CP/CPPS. It was suggested that the pathophysiology of CP/CPPS involves nerve growth factor (NGF) and IL-10, which directly correlated with pain severity and the inflammatory process [56,57]. As no single modality was proven to be effective with long-term durability for treatment of CP/CPPS, ESWT was investigated as a promising alternative in recent decades. All the studies are summarized in the Table 1.
Table 1.
LESW for chronic prostatitis/chronic pelvic pain syndrome.
| Study Design | N | Treatment Setting Treatment Course (mJouls/mm2) | Following Duration (Weeks) | Treatment Effect | |
|---|---|---|---|---|---|
| Zimmermann et al., 2008 [58] | Cohort | 34 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. | 1, 4, 12 | Improvements in pain and QoL. |
| Zimmermann et al., 2009 [59] | Double-blind. RCT | 60 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. | 1,4,12 | Improved QoL, IIEF, CPSI, VAS, and IPSS at 1, 4, and 12 weeks. |
| Zeng et al., 2012 [60] |
RCT | 80 | 3000 impulses, 5 times a week, 2 weeks. 0.06-maximum tolerated dose. |
4, 12 | Decreased NIH-CPSI score, improved QOL, 71.1% vs. 28.9% at week 2. |
| Vahdatpour et al., 2013 [61] |
RCT | 40 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz, increased to 0.3, 0.35, 0.4. |
1,2,3, 12 | Pain scores decreases at 2, 3, and 12 weeks. Urinary scores improved in weeks 3 and 12. |
| Moayednia et al., 2014 [62] |
RCT | 19 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. |
16, 20, 24 | NIH-CPSI, VAS, IPSS decreased. Not statistically different at week 24. |
| Pajovic et al., 2016 [63] |
RCT | 30 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. |
12, 24, 36 | Improved NIH-CPSI. No change for PVR or QMAX. |
| Al Edwan et al., 2017 [64] |
Cohort | 41 | 2500 impulses per week, 4 weeks. 0.25, 3 Hz. |
2, 24, 48 | Improved NIH-CPSI, IIEF, IPSS. |
| Guu et al., 2018 [65] |
Cohort | 33 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. |
1, 4, 12 | Improved NIH-CPSI, IIEF, IPSS. |
| Salama et al., 2018 [66] | RCT | 40 | 3000 pulses, 12 Hz at 3 to 5 bar, twice a week for 4 weeks. | 1, 4, 8 | Four domains of the NIH-CPSI decreased at weeks 1, 4, and 8. |
| Zhang et al., 2019 [67] |
nRCT | 40 | 3000 pulses per week, 8 weeks. 1.8–2.0 bar; 10 Hz. |
4, 8, 12 | Both groups improved NIH-CPSI, QoL, VAS, IPSS, and IIEF-5. Only 8 weeks CPSI. IIEF statistically significant. |
| Skaudickas et al., 2020 [68] | Cohort | 40 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. |
0, 4, 12 | NIH-CPSI, IPSS, VAS, and IIEF-5 showed greatest improvement at week 4; VAS and IPSS, improvement at week 12. |
| Li et al., 2020 [69] | Cohort | 32 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. |
1, 2, 4, 12 | VAS and the NIH-CPSI showed substantial improvement at week 4 and 12. |
| Kim et al., 2021 [70] | Double-blind RCT |
34 | 3000 impulses per week, 8 weeks. 0.25, 3 Hz. |
0, 4 | NIH-CPSI, QoL, IIEF, and VAS decrease at Week 0 and 4. |
| Mykoniatis et al., 2021 [71] | Double-blind RCT |
45 | 5000 shockwaves per week, 6 weeks, 0.1. | 4, 12, 24 | NIH-CPSI, pain, and QoL improved, persisted at 24 weeks. No improvement of NIH-CPSI urinary subdomain and IPSS. |
| Sakr et al., 2021 [72] |
RCT | 155 | 3000 impulses per week, 4 weeks. 0.25, 3 Hz. |
4, 12, 24, 48 | NIH-CPSI, IPSS, VAS, and IIEF-5. |
| Wu et al., 2021 [73] |
Cohort | 215 | 3000 impulses per week, 6 weeks. 0.25, 4 Hz. |
4, 12, 24, 48 | Improved NIH-CPSI, IIEF, IPSS, and AUA QoL_US at 4, 12, 24, and 48 weeks |
CPSI = Chronic Prostatitis Symptom Index; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; VAS = Visual Analog Scale; AUA QoL_US = American Urological Association Quality of Life due to Urinary Symptom.
Jeon et al. reported that ESWT improved CP/CPPS and reduced inflammation by degrading COX-2 in the microenvironment through the TLR4-NFκB-inhibiting pathway in lipopolysaccharide-induced inflammation in RWPE-2 cells and a 17 β-estradiol and dihydrotestosterone-induced prostatitis rat model [74].
Wang et al. demonstrated that LESW significantly suppressed the expression of IL-1β, COX-2, caspase-1, and NGF on day 3, and IL-1β, TNF-α, COX-2, NALP1, caspase-1, and NGF expression on day 7, in a dose-dependent fashion in a capsaicin-induced prostatitis model in rats [75]. A recent study revealed that ESWT decreased the number of total and degranulated mast cells and alleviated pelvic pain in a rat model of prostatitis induced by intraprostatic injection of 1% carrageenan [76]. Taken together, these findings suggest that ESWT can inhibit prostate inflammation and prostatic pain through different mechanisms of action, according to various CP/CPPS animal models.
Zimmermann et al. conducted the first randomized double-blind, placebo-controlled study using ESWT on chronic nonbacterial prostatitis [59]. Thirty patients in the study group received 3000 impulses at 0.25 mJouls/mm2, 3 Hz once per week for 4 weeks. The study group demonstrated significant improvement in pain, quality of life (QoL), and voiding function, compared to the placebo group. The various treatment settings of ESWT for treating CP/CPPS are described in Table 1. Taken together, ESWT is an efficient and safe method to decrease the NIH-CPSI score, with therapeutic effects sustained from 12 weeks to 12 months [60,61,65,68,69]. In a recent study including 155 patients, the results showed that 82.8% patients had a ≥6-point decrease in the NIH-Chronic Prostatitis Symptom Index (NIH-CPSI) total score at 1 month; and other functional scores, such as IPSS, VAS, and IIEF-5, were also improved [72]. Wu et al. reported the largest cohort study with long-term follow up [73], which demonstrated that, apart from pain alleviation, ESWT ameliorated the severity of other prostatitis symptoms in a CP/CPPS cohort, with a 53.6% decrease in NIH-CPSI, 17.9% increase in IIEF-5, 6.8% increase in EHS, and 50.9% decrease in IPSS 12 months after ESWT. Although these studies provided clinical evidence for the efficacy of ESWT, the mechanisms of its action in human prostate specimens and biomarkers remained to be determined.
10.2. Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS), Ketamine Cystitis, and Radiation Cystitis
IC/BPS is defined as “an unpleasant sensation (pain, pressure, or discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than six weeks duration, in the absence of infection or other identifiable causes” [77]. Many treatments have been used for IC/BPS based on the different pathophysiology; however, there is currently no established optimal treatment that is considered to be effective long-term without side effects.
In an animal study, LESW showed anti-inflammatory effects in the down regulation of IL-6, COX-2, and NGF expression, and suppressed the bladder overactivity and pain behavior in cyclophosphamide (CYP)-induced cystitis in rats [78]. Chen et al. demonstrated that LESW eased inflammation and oxidative stress by decreasing IL-12, MMP9, TNF-a, nuclear factor-kB, NADPH oxidase 1 (NOX-1) and NOX-2, and iNOS expression in another cyclophosphamide (CYP)-induced cystitis model in rats [36].
Chuang et al. demonstrated the first randomized double-blind, placebo-controlled study using ESWT for 54 patients with IC/BPS. The treatment groups received 2000 shocks, with an energy level up to 0.25 mJ/mm2 and frequency of 3 Hz once a week for 4 weeks at the suprapubic bladder area. The patients who received ESWT showed improvements in pain scale and O’Leary-Sant symptom (OSS) scores from baseline to 4 weeks, but only the VAS score reached statistical significance, compared to the placebo group [79]. Shen et al. analyzed 25 eligible patients with IC/BPS from a single center and found that the ESWT group exhibited a significant reduction in the OSS and VAS, compared to the placebo group 4 weeks post-treatment (p < 0.05), and the effects were persistent at 12 weeks. The change in the difference of urinary markers in ESWT versus placebo was p = 0.054 for IL-4, p = 0.013 for VEGF, and p = 0.039 for IL-9 at 4 weeks [80].
Chen et al. illustrated a radiotherapy-induced chronic cystitis rat model that was treated with 0.1 mJ/mm2/120 impulses every 3 days for a total of 20 times. By day 60, the detrusor contraction was significantly better for the rats receiving LESW. The protein expression of oxidative stress markers, apoptosis markers, DNA-damage markers, inflammatory signaling markers, and fibrosis markers were significantly reduced after LESW treatment [81,82]. The researchers further conducted a ketamine-induced cystitis model treated with 0.12 or 0.16 mJ/mm2/120 impulses/at 3 h and 3, 7, 14, 21, and 28 days after ketamine administration. The study demonstrated that LESW effectively attenuated ketamine-induced bladder damage in both molecular and histopathological findings [83]. However, the beneficial effects of ESWT on pre-clinical studies of radiation cystitis and ketamine cystitis warrant that larger multi-institutional studies with placebo control are needed.
10.3. Overactive Bladder (OAB)
OAB is a symptom characterized by “urgency with or without urge incontinence, usually with frequency and nocturia” in ICS terminology [82]. Therapies for OAB include behavioral therapies/lifestyle modifications, monotherapy, combinations of antimuscarinics and β3-adrenoceptor agonist, and intradetrusor injection of onaBoNTA, or neuromodulation [84]. However, some patients remain refractory to the above therapies.
LESWs have been studied for treatment of OAB. Lee et al. conducted the first clinical cohort of 82 patients with OAB who received 3000 pulses, 0.25 mJ/mm2 and 3 pulses/s per week over an 8-week treatment period. The subjects showed an improvement in OAB symptoms, QoL, and urodynamic parameters during the 3-month follow-up period [85]. Lu et al. reported that the therapeutic effects on OAB symptoms persisted for as long as 6 months. However, further investigation is warranted to confirm the efficacy of ESWT for treating OAB [86].
10.4. Erectile Dysfunction (ED)
The pathophysiology of ED is considered multifaceted, with complex mechanisms, including neural, vascular, and hormonal signaling problems. Many chronic diseases, such as hypertension, diabetes, hyperlipidemia, and metabolic syndrome, have been suggested to be associated with ED [87]. Therefore, many studies of ED are important to have solutions to improve the ED after ESWT (Table 2). In the era of phosphodiesterase type 5 inhibitor (PDE5i), oral medication has become the first-line treatment for ED. However, there remain approximately 30–35 % PDE5i non-responders [88,89].
Table 2.
LESW for erectile dysfunction.
| Study Design | N | Treatment Setting Treatment Course (mJouls/mm2) | Following Duration (Months) | Treatment Effect | |
|---|---|---|---|---|---|
| Skolarikos et al., 2005 [90] |
Cohort | 40 | 3000 impulses, 6 weeks. |
3, 12 | 64.2% improve IIEF Improvement in penile angulation, with a mean reduction of 35 degrees No significant change in plaque size |
| Poulakis et al., 2006 [91] |
RCT | 68 | 2000 impulses per week, 5 weeks, 0.25. | 1, 3, 6 | Improvement in pain, IIEF-5 score and plaque size, but no difference compared to another group. |
| Zimmermann et al., 2009 [59] | RCT | 60 | 3000 impulses per week, 4 weeks, 0.25, 3 Hz. |
1, 3 | Improvement of pain, QoL, and voiding conditions IIEF-5. |
| Chitale et al., 2010 [92] |
RCT | 36 | 3000 impulses per week, 6 weeks. | 3, 6 | Improved IIEF-5 and VAS score. No significant change in Peyronie’s disease. |
| Gruenwald et al., 2012 [93] | Cohort | 29 | 1500 impulses twice per week, 3 weeks, 0.09. | 1, 2 | DE-5i poor responders. Improved EHS and IIEF-ED. |
| Vardi et al., 2012 [94] |
RCT | 67 | 1500 impulses twice per week, 9 weeks, 0.09. | 1 | Improved IIEF-5, EHS, and penile blood flow. |
| Palmieri et al., 2012 [95] |
Cohort | 50 | 2000 impulses per week, 4 weeks, 0.25. | 3, 6 | Improved IIEF-5 and quality of life. |
| Yee et al., 2014 [96] |
RCT | 70 | 1500 impulses twice per week, 9 weeks, 0.09. | 1 | Clinical improvement in IIEF-ED and EHS, but no significant difference between two groups. |
| Srini et al., 2015 [97] |
RCT | 135 | NA | 1, 3, 6, 9, 12 | Improvement in IIEF-EF, EHS, and CGIC. |
| Pelayo-Nieto et al., 2015 [98] |
Cohort | 15 | 5000 impulses per week, 4 weeks, 0.09. | 1, 6 | Improvement in IIEF, SEP, and GAQ. |
| Chung and Cartmill 2015 [99] |
Cohort | 30 | 3000 impulses twice per week, 6 weeks, 0.25. | 1, 4 | PDE5i non-responders; 18 (60%) patients ≥ 5 points improvement in IIEF-5 score; 21 (70%) patients >50% in EDITS index score, last 4-months. |
| Bechara et al., 2015 [100] |
Cohort | 25 | 5000 impulses once per week, 4 weeks, 0.09. | 3 | PDE-5i non-responders. Improved IIEF-6, SEP2, SEP3, and GAQ. Restoring PDE5i response in >50% of patients. |
| Frey et al., 2015 [101] |
Cohort | 18 | 3000 impulses twice per week, 6 weeks. 20, 15, and 12. | 1, 12 | post-prostatectomy erectile dysfunction. Improved IIEF-5 scores at 1 and 12 months. |
| Olsen et al., 2015 [102] |
RCT | 112 | 3000 impulses per week, 5 weeks, 0.15. | 1, 3, 6 | Improved IIEF-5 and EHS. |
| Hisasue 2016 [103] |
Cohort | 57 | 1500 impulses twice per week, 9 weeks, 0.09. | 1, 3, 6 | Improvement in IIEF, EHS, and MPCC. |
| Kalyvianakis et al., 2018 [104] | RCT | 44 | 5000 impulses once/twice per week, 6 weeks, 2 phase treatment, 0.05. | 1,3, 6 | Vasculogenic ED, PDE5 responders. Improvement in IIEF-EF score, MCID, and SEP3 score. 12 sessions twice per week were better than 6 sessions once a week. |
| Vinay et al., 2021 [105] |
RCT | 76 | 5000 impulses once per week, 4 weeks, 0.09. | 1, 3, 6 | *PDE5I refractory patients. Improved IIEF-EF in 3 and 6 months. |
| Oliveira et al., 2019 [106] |
Cohort | 25 | 2000 impulses on perineum + 2000 on dorsum penis once per week, 6 weeks, 0.16. | 1.5, 3 | Improved PSV and EDV. Disease duration dose not negative impact on treatment outcomes. |
| Huang et al., 2020 [107] |
Cohort | 35 | NA | 1, | Improved IIEF-5, EHS, erectile rigidity, and nocturnal erection frequency. |
| Sramkova et al., 2020 [108] |
RCT | 60 | 6000 impulses twice per week, 2 weeks, 0.160. | 1, 3 | Improve IIEF-5, EHS, GAQ, SEP 2, and SEP 3. |
| Palmieri et al., 2021 [109] |
RCT | 106 | 3000 impulses twice per week, 3 weeks, 0.25. | 1 | vasculogenic ED PDE5i non-responders. Improved IIEF-EF, PSV, and EDV. |
| Shendy et al., 2021 [110] |
RCT | 42 | 3 | Improved IIEF-5 and PSV. |
GAQ = Global Assessment Questionnaire EHS = Erectile Hardness Score, CGIC = Clinical Global Impression of Change, MPCC = maximal penile circumferential change, NA = not available, SEP3 = Sexual Encounter Profile question 3 score, MCIDs = minimally clinical important differences.
The fundamental mechanisms of LESW for treating ED are still incompletely understood. LESW activates focal adhesion kinase (FAK), extracellular-signal-regulated kinase (ERK), and Wnt signaling pathways, induces cell proliferation, endothelial and smooth muscle restoration, and increases VEGF and eNOS expression [88]. It has been suggested that LESW regenerates penile smooth muscle via the PERK pathway and ATP/P2X7 pathway. Moreover, LESW mediates BDNF activation in penis tissue to sustain neurotrophic healing and induces MSCs to express VEGF.
Muller et al., in a rat model using three sessions of 2000 SWs at 2 BAR and 1000 SWs at 1 BAR on erectile tissue, revealed increased apoptosis and corporal smooth muscle collagenization [111]. On the contrary, the other studies revealed tissue restoration, neuronal nitric oxide synthase (nNOS)-positive nerve generation, and angiogenesis of penile tissue in the diabetes mellitus-associated ED rat model [112,113].
Vardi et al. conducted the first clinical randomized-controlled trial delivering SWs with 300 shocks at 0.09 mJ/mm2 on the distal, middle, and proximal penile shaft, and bilateral crura. After a 9-week treatment course, the treatment group revealed significant improvement on the International Index of Erectile Function (IIEF; 6.7 points increase) score, with no significant side effect [94]. Many cohort studies and double-blind, sham controlled studies have been published, which revealed encouraging results. We have summarized the clinical studies in Table 2. However, LESWs have no established definitive recommendations from the EAU guideline for ED treatment due to lack of longer-term follow-up data and unequivocal evidence.
10.5. Stress Urinary Incontinence
Stress urinary incontinence (SUI) is currently defined as involuntary loss of urine on abdominal effort, physical exertion, sneezing, or coughing. Treatment modalities include conservative measurement, pelvic floor muscle training, biofeedback, pharmacological, and surgery therapy [114].
Wu et al. described a rat model using vaginal balloon dilation-induced stress urinary incontinence, followed by treatment with 0.06 mJ/mm2/300 shocks/3 Hz of SWs. The results showed that LESW eased SUI by promoting angiogenesis, progenitor cell recruitment, and urethral sphincter regeneration [115]. Zhang et al. developed a delayed treatment with LESW in an irreversible rat model of SUI. They found that LESW appeared to increase the smooth muscle content in the urethra and vagina, increase the thickness of the urethral wall, improve striated muscle content and neuromuscular junctions, restore the integrity of the urothelium, increase the number of EdU-retaining progenitor cells in the urethral wall, and improve the continent function [116].
A multicenter, single-blind, randomized-controlled trial study was developed from 60 female patients with SUI who were randomly assigned to receive LESW with 0.25 mJ/mm2 intensity, 3000 pulses, and 3 pulses/s, once weekly for a 4-week (W4) or 8-week (W8) period, or an identical sham LESW treatment without energy transmission. They reported that 8 weeks of LESW attenuated SUI symptoms upon physical activity, induced urine leakage, and alleviated OAB symptoms, which implied that LESWT significantly improved QoL [117]. However, a larger scale of the clinical study is necessary to elucidate the real role of LESW for the treatment of SUI.
10.6. Detrusor Underactivity/Underactive Bladder
Detrusor underactivity (DU) represents a contraction of reduced strength and/or duration during voiding, resulting in prolonged bladder emptying and/or failure to empty the bladder within a normal timespan [118,119]. The estimated prevalence of DU is 9–28% of men < 50 years, 48% in older men, and 12–45% in elderly women [120]. The pathophysiology of DU is not well understood, but chronic ischemia and atherosclerosis of the microcirculation were mentioned in the literature [121,122]. There is currently no effective treatment for DU.
LESWs have been proven to induce cell proliferation, angiogenesis, and facilitate tissue regeneration. Chuang et al. investigated the effects of LESW in a DU model induced by cryoinjury in female Sprague Dawley rats. The results showed that LESW downregulated IL-6 and COX-2 expression, upregulated α-SMA, induced cell proliferation (evidenced by increasing CD31 and Ki67), increased the contraction amplitude, and improved voiding function [123], suggesting the potential for a non-invasive modality for myogenic DU. Wang et al. treated streptozotocin-induced diabetic rats with LESW 0.02 mJ/mm2 at 3 Hz for 400 pulses once per week [124]. The study revealed improved detrusor contractility and reduced post-void residual urine, in association with increasing muscle proportion of the bladder, higher smooth muscle actin (SMA) expression, and neuronal reinnervation. Moreover, Lee et al. investigated the therapeutic effect and possible mechanisms of LESW on diabetic bladder dysfunction in a rat model. They found that LESW eased bladder dysfunction and urinary continence, reflected by the restoration of the nerve expression of the urethra and the vascularization of the bladder [123]. However, a clinical report of LESW on patients with DU is still lacking.
10.7. Drug Delivery
Codama et al. reported that SWs produced a shear force, increased the tissue permeability, and facilitated transportation of pharmaceutical molecules into cells [28]. OnabotulinumtoxinA (onaBoNTA) intradetrusor injection is the third line treatment for OAB. Due to the large size of onaBoNTA (150 KDa), direct bladder instillation was shown to have no effect with an intact urothelium [125]. Chuang et al. used magnetic resonance imaging to demonstrated bladder urothelial leakage of Gd-DTPA after low energy SWs, which was not seen in controls [48]. Moreover, rats pretreated with botulinum toxin A plus low energy SWs showed a decreased inflammatory reaction and decreased expression of SNAP-23, SNAP-25, and COX-2, compared to the control group, in an acetic acid-induced bladder hyperactivity rat model.
A single arm prospective clinical study was conducted in 15 patients with refractory OAB receiving intravesical instillation of 100 IU of BoNT-A, followed by LESW (3000 shocks greater than 10 min) exposure to the suprapubic area. The results showed significant improvements in OABSS after 1 and 2 months, without compromising voiding function [126]. These results inspire clinical experience for following studies.
Luo et al. evaluated the synergistic effects of LESW and cisplatin in upper urinary tract urothelial carcinoma (UTUC). They reported that LESW increased tissue permeability and enhanced cisplatin cytotoxicity in UTUC cells, and suppressed tumor cell proliferation both in vitro and in vivo. Furthermore, the anti-tumor effect was enhanced in the human-derived organoid model, by interfering with ZO-1 and E-cadherin to increase the permeability of cisplatin into cancer cells [49]. Elkashef et al. also reported that LESW enhanced intravesical epirubicin penetration in a rat model of bladder cancer with decreasing p53, IL-6, miR-210, HIF-1α, and TNF-α expression, and better histopathological results [127]. Taken together, ESWT might be applied as a tool for drug delivery for bladder dysfunction and urothelial tumors. LESWs assisted drug delivery in uro-oncology is an interesting suggestion, but no comprehensive clinical trials have been conducted as of yet.
11. Conclusions
SW medicine has been gaining attention for urological applications in which shock induces various biological effects and might assist with restoration of body function. However, the mechanisms and molecular changes after LESW remain mostly unknown, and large clinical studies are still lacking. The applications of LESW on new frontiers of urology are promising, but more evidence is necessary before LESW can be widely used in daily practice.
Acknowledgments
We thank the Center for Shockwave Medicine and Tissue Engineering, Department of Urology, Department of Medical Research, Kaohsiung Chang Gung Memorial Hospital for supporting this work.
Author Contributions
Conceptualization, P.-Y.C., J.-H.C. and Y.-C.C.; resources, P.-Y.C., Z.-S.W. and J.-H.C.; data curation, P.-Y.C. and J.-H.C.; writing—original draft preparation, P.-Y.C. and J.-H.C.; writing—review and editing, Y.-C.C.; project administration, Y.-C.C.; funding acquisition, Y.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Kaohsiung Chang Gung Memorial Hospital CRRPG8J0163; MOST, Taiwan, 109-2314-B-182A-135-MY3 (NMRPG8K6102).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chaussy C.H., Brendel W., Schmiedt E. Extracorporeally Induced Destruction of Kidney Stones by Shock Waves. Lancet. 1980;316:1265–1268. doi: 10.1016/S0140-6736(80)92335-1. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein J.N., Oster D.M., Park J.B., Park S.H., Loening S. The effect of the extracorporeal shock wave lithotriptor on the bone-cement interface in dogs. Clin. Orthop. Relat. Res. 1988;235:261–267. doi: 10.1097/00003086-198810000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Wang C.J. Extracorporeal shockwave therapy in musculoskeletal disorders. J. Orthop. Surg. Res. 2012;7:11–20. doi: 10.1186/1749-799X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moya D., Ramon S., Schaden W., Wang C.J., Guiloff L., Cheng J.H. The Role of Extracorporeal Shockwave Treatment in Musculoskeletal Disorders. J. Bone Jt. Surg. Am. 2018;100:251–263. doi: 10.2106/JBJS.17.00661. [DOI] [PubMed] [Google Scholar]
- 5.Burneikaite G., Shkolnik E., Celutkiene J., Zuoziene G., Butkuviene I., Petrauskiene B., Serpytis P., Laucevicius A., Lerman A. Cardiac shock-wave therapy in the treatment of coronary artery disease: Systematic review and meta-analysis. Cardiovasc. Ultrasound. 2017;15:11. doi: 10.1186/s12947-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meirer R., Kamelger F.S., Huemer G.M., Wanner S., Piza-Katzer H. Extracorporal shock wave may enhance skin flap survival in an animal model. Br. J. Plast. Surg. 2005;58:53–57. doi: 10.1016/j.bjps.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Fu X.-B., Chen S., Zhao Z.-B., Schmitz C., Weng C.-S. Efficacy and safety of extracorporeal shock wave therapy for acute and chronic soft tissue wounds: A systematic review and meta-analysis. Int. Wound J. 2018;15:590–599. doi: 10.1111/iwj.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hitchman L.H., Totty J.P., Raza A., Cai P., Smith G.E., Carradice D., Wallace T., Harwood A.E., Chetter I.C. Extracorporeal Shockwave Therapy for Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2019;56:330–339. doi: 10.1016/j.avsg.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration . FDA Permits Marketing of Device to Treat Diabetic Foot Ulcers [Press Release] U.S. Food and Drug Administration; Silver Spring, MD, USA: 2017. [Google Scholar]
- 10.Wang C.-J.K., Schaden W.V., Ko J.-Y.K. Translational Research in Biomedicine. Volume 6. Karger; Basel, Switzerland: 2018. Shockwave Medicine. [DOI] [Google Scholar]
- 11.Beisteiner R., Matt E., Fan C., Baldysiak H., Schönfeld M., Philippi Novak T., Amini A., Aslan T., Reinecke R., Lehrner J., et al. Transcranial Pulse Stimulation with Ultrasound in Alzheimer’s Disease—A New Navigated Focal Brain Therapy. Adv. Sci. 2019;7:1902583–1902593. doi: 10.1002/advs.201902583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porst H. Review of the Current Status of Low Intensity Extracorporeal Shockwave Therapy (Li-ESWT) in Erectile Dysfunction (ED), Peyronie’s Disease (PD), and Sexual Rehabilitation After Radical Prostatectomy With Special Focus on Technical Aspects of the Different Marketed ESWT Devices Including Personal Experiences in 350 Patients. Sex. Med. Rev. 2021;9:93–122. doi: 10.1016/j.sxmr.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Fojecki G.L., Tiessen S., Osther P.J.S. Extracorporeal shock wave therapy (ESWT) in urology: A systematic review of outcome in Peyronie’s disease, erectile dysfunction and chronic pelvic pain. World J. Urol. 2016;35:1–9. doi: 10.1007/s00345-016-1834-2. [DOI] [PubMed] [Google Scholar]
- 14.Wang H.-J., Cheng J.-H., Chuang Y.-C. Potential applications of low-energy shock waves in functional urology. Int. J. Urol. 2017;24:573–581. doi: 10.1111/iju.13403. [DOI] [PubMed] [Google Scholar]
- 15.Ogden J.A., Tóth-Kischkat A., Schultheiss R. Principles of Shock Wave Therapy. Clin. Orthop. Relat. Res. 2001;387:8–17. doi: 10.1097/00003086-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 16.D’Agostino M.C., Craig K., Tibalt E., Respizzi S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int. J. Surg. 2015;24:147–153. doi: 10.1016/j.ijsu.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Rompe J.D., Kirkpatrick C.J., Küllmer K., Schwitalle M., Krischek O. Dose-related effects of shock waves on rabbit tendo Achillis: A sonographic and histological study. J. Bone Jt. Surg. 1998;80:546–552. doi: 10.1302/0301-620X.80B3.0800546. [DOI] [PubMed] [Google Scholar]
- 18.Bannuru R.R., Flavin N.E., Vaysbrot E., Harvey W., McAlindon T. High-Energy Extracorporeal Shock-Wave Therapy for Treating Chronic Calcific Tendinitis of the Shoulder. Ann. Intern. Med. 2014;160:542. doi: 10.7326/M13-1982. [DOI] [PubMed] [Google Scholar]
- 19.Wang B., Reed-Maldonado A.B., Ly K., Lin G., Lue T.F. Potential Applications of Low-Intensity Extracorporeal Shock Wave Therapy in Urological Diseases via Activation of Tissue Resident Stem Cells. Urol. Sci. 2022;33:3–8. doi: 10.4103/UROS.UROS_56_21. [DOI] [Google Scholar]
- 20.Haupt G. Use of Extracorporeal Shock Waves in the Treatment of Pseudarthrosis, Tendinopathy and Other Orthopedic Diseases. J. Urol. 1997;158:4–11. doi: 10.1097/00005392-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Yu T., Junger W.G., Yuan C., Jin A., Zhao Y., Zheng X., Zeng Y., Liu J. Shockwaves increase T-cell proliferation and IL-2 expression through ATP release, P2X7 receptors, and FAK activation. Am. J. Physiol.-Cell Physiol. 2010;298:C457–C464. doi: 10.1152/ajpcell.00342.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weihs A.M., Fuchs C., Teuschl A.H., Hartinger J., Slezak P., Mittermayr R., Redl H., Junger W.G., Sitte H.H., Rünzler D. Shock Wave Treatment Enhances Cell Proliferation and Improves Wound Healing by ATP Release-coupled Extracellular Signal-regulated Kinase (ERK) Activation. J. Biol. Chem. 2014;289:27090–27104. doi: 10.1074/jbc.M114.580936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jan C.-R., Huang J.-K., Tseng C.-J. High-Energy Shock Waves Alter Cytosolic Calcium Mobilization in Single MDCK Cells. Nephron. 1998;78:187–194. doi: 10.1159/000044909. [DOI] [PubMed] [Google Scholar]
- 24.Wang C.-J., Wang F.-S., Yang K.D., Weng L.-H., Hsu C.-C., Huang C.-S., Yang L.-C. Shock wave therapy induces neovascularization at the tendon–bone junction. A study in rabbits. J. Orthop. Res. 2003;21:984–989. doi: 10.1016/S0736-0266(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 25.Pölzl L., Nägele F., Hirsch J., Graber M., Grimm M., Gollmann-Tepeköylü C., Holfeld J. Exosome Isolation after in vitro Shock Wave Therapy. J. Vis. Exp. 2020;163:e61508. doi: 10.3791/61508. [DOI] [PubMed] [Google Scholar]
- 26.Cheng J.-H., Wang C.-J., Su S.-H., Huang C.-Y., Hsu S.-L. Next-generation sequencing identifies articular cartilage and subchondral bone miRNAs after ESWT on early osteoarthritis knee. Oncotarget. 2016;7:84398–84407. doi: 10.18632/oncotarget.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hausner T., Pajer K., Halat G., Hopf R., Schmidhammer R., Redl H., Nógrádi A. Improved rate of peripheral nerve regeneration induced by extracorporeal shock wave treatment in the rat. Exp. Neurol. 2012;236:363–370. doi: 10.1016/j.expneurol.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Kodama T., Doukas A.G., Hamblin M.R. Shock wave-mediated molecular delivery into cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2002;1542:186–194. doi: 10.1016/S0167-4889(01)00177-X. [DOI] [PubMed] [Google Scholar]
- 29.Alshihri A., Niu W., Kämmerer P.W., Al-Askar M., Yamashita A., Kurisawa M., Spector M. The effects of shock wave stimulation of mesenchymal stem cells on proliferation, migration, and differentiation in an injectable gelatin matrix for osteogenic regeneration. J. Tissue Eng. Regen. Med. 2020;14:1630–1640. doi: 10.1002/term.3126. [DOI] [PubMed] [Google Scholar]
- 30.Ito Y., Veysset D., Kooi S.E., Martynowych D., Nakagawa K., Nelson K.A. Interferometric and fluorescence analysis of shock wave effects on cell membrane. Commun. Phys. 2020;3:124–130. doi: 10.1038/s42005-020-0394-3. [DOI] [Google Scholar]
- 31.Leu S., Huang T.-H., Chen Y.-L., Yip H.-K. Effect of Extracorporeal Shockwave on Angiogenesis and Anti-Inflammation: Molecular-Cellular Signaling Pathways. Shock. Med. 2018;6:109–116. doi: 10.1159/000485068. [DOI] [Google Scholar]
- 32.Chen Y.-L., Lin Y.-P., Sun C.-K., Huang T.-H., Yip H.-K., Chen Y.-T. Extracorporeal shockwave against inflammation mediated by GPR120 receptor in cyclophosphamide-induced rat cystitis model. Mol. Med. 2018;24:60–73. doi: 10.1186/s10020-018-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sukubo N.G., Tibalt E., Respizzi S., Locati M., d’Agostino M.C. Effect of shock waves on macrophages: A possible role in tissue regeneration and remodeling. Int. J. Surg. 2015;24:124–130. doi: 10.1016/j.ijsu.2015.07.719. [DOI] [PubMed] [Google Scholar]
- 34.Holfeld J., Tepeköylü C., Kozaryn R., Urbschat A., Zacharowski K., Grimm M., Paulus P. Shockwave Therapy Differentially Stimulates Endothelial Cells: Implications on the Control of Inflammation via Toll-Like Receptor 3. Inflammation. 2013;37:65–70. doi: 10.1007/s10753-013-9712-1. [DOI] [PubMed] [Google Scholar]
- 35.Stojadinovic A., Elster E.A., Anam K., Tadaki D., Amare M., Zins S., Davis T.A. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis. 2008;11:369–380. doi: 10.1007/s10456-008-9120-6. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y.T., Yang C.C., Sun C.K., Chiang H.J., Chen Y.L., Sung P.H., Zhen Y.Y., Huang T.H., Chang C.L., Chen H.H., et al. Extracorporeal shock wave therapy ameliorates cyclophosphamide-induced rat acute interstitial cystitis though inhibiting inflammation and oxidative stress-in vitro and in vivo experiment studies. Am. J. Transl. Res. 2014;6:631–648. [PMC free article] [PubMed] [Google Scholar]
- 37.Davis T.A., Stojadinovic A., Anam K., Amare M., Naik S., Peoples G.E., Tadaki D., Elster E.A. Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int. Wound J. 2009;6:11–21. doi: 10.1111/j.1742-481X.2008.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo Y.-R., Wang C.-T., Wang F.-S., Yang K.D., Chiang Y.-C., Wang C.-J. Extracorporeal shock wave treatment modulates skin fibroblast recruitment and leukocyte infiltration for enhancing extended skin-flap survival. Wound Repair Regen. 2009;17:80–87. doi: 10.1111/j.1524-475X.2008.00444.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen R.F., Chang C.H., Wang C.T., Yang M.Y., Wang C.J., Kuo Y.R. Modulation of vascular endothelial growth factor and mitogen-activated protein kinase-related pathway involved in extracorporeal shockwave therapy accelerate diabetic wound healing. Wound Repair Regen. 2018;27:69–79. doi: 10.1111/wrr.12686. [DOI] [PubMed] [Google Scholar]
- 40.Ping Y., Tao G., Yun-zhu P., Yu W., Hong-yan C. GW24-e0232 Effects of cardiac shock wave therapy on the angiogenesis related cytokine of CAD patients. Heart. 2013;99:A155–A156. doi: 10.1136/heartjnl-2013-304613.429. [DOI] [Google Scholar]
- 41.Biondi-Zoccai G., Fu M., Sun C.-K., Lin Y.-C., Wang C.-J., Wu C.-J., Ko S.-F., Chua S., Sheu J.-J., Chiang C.-H., et al. Extracorporeal Shock Wave Therapy Reverses Ischemia-Related Left Ventricular Dysfunction and Remodeling: Molecular-Cellular and Functional Assessment. PLoS ONE. 2011;6:e24342. doi: 10.1371/journal.pone.0024342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai H.-Y., Li L.I.N., Guo T.A.O., Wang Y.U., Ma T.-K., Xiao J.-M., Zhao L., Fang Y.I.N., Yang P., Zhao H.U. Cardiac shockwave therapy improves myocardial function in patients with refractory coronary artery disease by promoting VEGF and IL-8 secretion to mediate the proliferation of endothelial progenitor cells. Exp. Ther. Med. 2015;10:2410–2416. doi: 10.3892/etm.2015.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang T.-H., Sun C.-K., Chen Y.-L., Wang C.-J., Yin T.-C., Lee M.S., Yip H.-K. Shock Wave Therapy Enhances Angiogenesis through VEGFR2 Activation and Recycling. Mol. Med. 2016;22:850–862. doi: 10.2119/molmed.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee F.Y., Zhen Y.Y., Yuen C.M., Fan R., Chen Y.T., Sheu J.J., Chen Y.L., Wang C.J., Sun C.K., Yip H.K. The mTOR-FAK mechanotransduction signaling axis for focal adhesion maturation and cell proliferation. Am. J. Transl. Res. 2017;9:1603–1617. [PMC free article] [PubMed] [Google Scholar]
- 45.Luh J.-J., Huang W.-T., Lin K.-H., Huang Y.-Y., Kuo P.-L., Chen W.-S. Effects of Extracorporeal Shock Wave-Mediated Transdermal Local Anesthetic Drug Delivery on Rat Caudal Nerves. Ultrasound Med. Biol. 2018;44:214–222. doi: 10.1016/j.ultrasmedbio.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Hoon Ha C., Cheol Lee S., Kim S., Chung J., Bae H., Kwon K. Novel mechanism of gene transfection by low-energy shock wave. Sci. Rep. 2015;5:12843–12856. doi: 10.1038/srep12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kung Y., Lan C., Hsiao M.-Y., Sun M.-K., Hsu Y.-H., Huang A.P.H., Liao W.-H., Liu H.-L., Inserra C., Chen W.-S. Focused shockwave induced blood-brain barrier opening and transfection. Sci. Rep. 2018;8:2218–2229. doi: 10.1038/s41598-018-20672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chuang Y.-C., Huang T.-L., Tyagi P., Huang C.-C. Urodynamic and Immunohistochemical Evaluation of Intravesical Botulinum Toxin A Delivery Using Low Energy Shock Waves. J. Urol. 2016;196:599–608. doi: 10.1016/j.juro.2015.12.078. [DOI] [PubMed] [Google Scholar]
- 49.Luo H.-L., Liu H.-Y., Chang Y.-L., Su Y.-L., Huang C.-C., Lin X.-J., Chuang Y.-C. Extracorporeal Shock Wave Enhances the Cisplatin Efficacy by Improving Tissue Infiltration and Cellular Uptake in an Upper Urinary Tract Cancer Animal and Human-Derived Organoid Model. Cancers. 2021;13:4558. doi: 10.3390/cancers13184558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J.-H., Cho S.-H. Effect of Extracorporeal Shock Wave Therapy on Denervation Atrophy and Function Caused by Sciatic Nerve Injury. J. Phys. Ther. Sci. 2013;25:1067–1069. doi: 10.1589/jpts.25.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J., Kang N., Yu X., Ma Y., Pang X. Radial Extracorporeal Shock Wave Therapy Enhances the Proliferation and Differentiation of Neural Stem Cells by Notch, PI3K/AKT, and Wnt/β-catenin Signaling. Sci. Rep. 2017;7:15321–15331. doi: 10.1038/s41598-017-15662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gollmann-Tepeköylü C., Nägele F., Graber M., Pölzl L., Lobenwein D., Hirsch J., An A., Irschick R., Röhrs B., Kremser C., et al. Shock waves promote spinal cord repair via TLR3. JCI Insight. 2020;5:e134552. doi: 10.1172/jci.insight.134552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang B., Ning H., Reed-Maldonado A., Zhou J., Ruan Y., Zhou T., Wang H., Oh B., Banie L., Lin G., et al. Low-Intensity Extracorporeal Shock Wave Therapy Enhances Brain-Derived Neurotrophic Factor Expression through PERK/ATF4 Signaling Pathway. Int. J. Mol. Sci. 2017;18:433. doi: 10.3390/ijms18020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Habermacher G.M., Chason J.T., Schaeffer A.J. Prostatitis/Chronic Pelvic Pain Syndrome. Annu. Rev. Med. 2006;57:195–206. doi: 10.1146/annurev.med.57.011205.135654. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J., Zhang X., Cai Z., Li N., Li H. The Lifetime Risk and Prognosis of Chronic Prostatitis/Chronic Pelvic Pain Syndrome in the Middle-Aged Chinese Males. Am. J. Men’s Health. 2019;13:155798831986538. doi: 10.1177/1557988319865380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller L.J., Fischer K.A., Goralnick S.J., Litt M., Burleson J.A., Albertsen P., Kreutzer D.L. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology. 2002;59:603–608. doi: 10.1016/S0090-4295(01)01597-7. [DOI] [PubMed] [Google Scholar]
- 57.Miller L.J., Fischer K.A., Goralnick S.J., Litt M., Burleson J.A., Albertsen P., Kreutzer D.L. Interleukin-10 levels in seminal plasma: Implications for chronic prostatitis-chronic pelvic pain syndrome. J. Urol. 2002;167:753–756. doi: 10.1016/S0022-5347(01)69139-0. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann R., Cumpanas A., Hoeltl L., Janetschek G., Stenzl A., Miclea F. Extracorporeal shock-wave therapy for treating chronic pelvic pain syndrome: A feasibility study and the first clinical results. BJU Int. 2008;102:976–980. doi: 10.1111/j.1464-410X.2008.07742.x. [DOI] [PubMed] [Google Scholar]
- 59.Zimmermann R., Cumpanas A., Miclea F., Janetschek G. Extracorporeal Shock Wave Therapy for the Treatment of Chronic Pelvic Pain Syndrome in Males: A Randomised, Double-Blind, Placebo-Controlled Study. Eur. Urol. 2009;56:418–424. doi: 10.1016/j.eururo.2009.03.043. [DOI] [PubMed] [Google Scholar]
- 60.Zeng X.Y., Liang C., Ye Z.Q. Extracorporeal shock wave treatment for non-inflammatory chronic pelvic pain syndrome: A prospective, randomized and sham-controlled study. Chin. Med. J. 2012;125:114–118. doi: 10.1016/S0022-5347(09)60352-9. [DOI] [PubMed] [Google Scholar]
- 61.Vahdatpour B., Alizadeh F., Moayednia A., Emadi M., Khorami M.H., Haghdani S. Efficacy of Extracorporeal Shock Wave Therapy for the Treatment of Chronic Pelvic Pain Syndrome: A Randomized, Controlled Trial. ISRN Urol. 2013;2013:972601. doi: 10.1155/2013/972601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moayednia A., Haghdani S., Khosrawi S., Yousefi E., Vahdatpour B. Long-term effect of extracorporeal shock wave therapy on the treatment of chronic pelvic pain syndrome due to non bacterial prostatitis. J. Res. Med. Sci. 2014;19:293–296. [PMC free article] [PubMed] [Google Scholar]
- 63.Pajovic B., Radojevic N., Dimitrovski A., Vukovic M. Comparison of the efficiency of combined extracorporeal shock-wave therapy and triple therapy versus triple therapy itself in Category III B chronic pelvic pain syndrome (CPPS) Aging Male. 2016;19:202–207. doi: 10.1080/13685538.2016.1197899. [DOI] [PubMed] [Google Scholar]
- 64.Al Edwan G.M., Muheilan M.M., Atta O.N.M. Long term efficacy of extracorporeal shock wave therapy [ESWT] for treatment of refractory chronic abacterial prostatitis. Ann. Med. Surg. 2017;14:12–17. doi: 10.1016/j.amsu.2016.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guu S.-J., Geng J.-H., Chao I.T., Lin H.-T., Lee Y.-C., Juan Y.-S., Liu C.-C., Wang C.-J., Tsai C.-C. Efficacy of Low-Intensity Extracorporeal Shock Wave Therapy on Men With Chronic Pelvic Pain Syndrome Refractory to 3-As Therapy. Am. J. Men’s Health. 2017;12:441–452. doi: 10.1177/1557988317736585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salama A.B., Abouelnaga W.A. Effect of radial shock wave on chronic pelvic pain syndrome/chronic prostatitis. J. Phys. Ther. Sci. 2018;30:1145–1149. doi: 10.1589/jpts.30.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z.-X., Zhang D., Yu X.-T., Ma Y.-W. Efficacy of Radial Extracorporeal Shock Wave Therapy for Chronic Pelvic Pain Syndrome: A Nonrandomized Controlled Trial. Am. J. Men’s Health. 2018;13:155798831881466. doi: 10.1177/1557988318814663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skaudickas D., Telksnys T., Veikutis V., Aniulis P., Jievaltas M. Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome. Open Med. 2020;15:580–585. doi: 10.1515/med-2020-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li G., Man L. Low-intensity extracorporeal shock wave therapy for III B chronic pelvic pain syndrome. Transl. Androl. Urol. 2020;9:1323–1328. doi: 10.21037/tau.2020.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim K.S., Choi Y.S., Bae W.J., Cho H.J., Ha U.S., Hong S.-H., Lee J.Y., Ahn S.T., Moon D.G., Kim S.W. Efficacy of Low-Intensity Extracorporeal Shock Wave Therapy for the Treatment of Chronic Pelvic Pain Syndrome IIIb: A Prospective-Randomized, Double-Blind, Placebo-Controlled Study. World J. Men’s Health. 2021;39:e40. doi: 10.5534/wjmh.210010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mykoniatis I., Kalyvianakis D., Zilotis F., Kapoteli P., Fournaraki A., Poulios E., Hatzichristou D. Evaluation of a low-intensity shockwave therapy for chronic prostatitis type IIIb/chronic pelvic pain syndrome: A double-blind randomized sham-controlled clinical trial. Prostate Cancer Prostatic Dis. 2021;24:370–379. doi: 10.1038/s41391-020-00284-2. [DOI] [PubMed] [Google Scholar]
- 72.Sakr A.M., Fawzi A.M., Kamel M., Ali M.M. Outcomes and clinical predictors of extracorporeal shock wave therapy in the treatment of chronic prostatitis/chronic pelvic pain syndrome: A prospective randomized double-blind placebo-controlled clinical trial. Prostate Cancer Prostatic Dis. 2021 doi: 10.1038/s41391-021-00464-8. [DOI] [PubMed] [Google Scholar]
- 73.Wu W.-L., Bamodu O.A., Wang Y.-H., Hu S.-W., Tzou K.-Y., Yeh C.-T., Wu C.-C. Extracorporeal Shockwave Therapy (ESWT) Alleviates Pain, Enhances Erectile Function and Improves Quality of Life in Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. J. Clin. Med. 2021;10:3602. doi: 10.3390/jcm10163602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeon S.H., Zhu G.Q., Kwon E.B., Lee K.W., Cho H.J., Ha U.S., Hong S.H., Lee J.Y., Bae W.J., Kim S.W. Extracorporeal shock wave therapy decreases COX-2 by inhibiting TLR4-NFκB pathway in a prostatitis rat model. Prostate. 2019;79:1498–1504. doi: 10.1002/pros.23880. [DOI] [PubMed] [Google Scholar]
- 75.Wang H.-J., Tyagi P., Chen Y.-M., Chancellor M.B., Chuang Y.-C. Low Energy Shock Wave Therapy Inhibits Inflammatory Molecules and Suppresses Prostatic Pain and Hypersensitivity in a Capsaicin Induced Prostatitis Model in Rats. Int. J. Mol. Sci. 2019;20:4777. doi: 10.3390/ijms20194777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song Z., Jin C., Bian Z., Liang C. Extracorporeal shock wave therapy decreases the number of total and degranulated mast cells and alleviates pelvic pain in a rat model of prostatitis. Mol. Cell. Biochem. 2021;476:1905–1913. doi: 10.1007/s11010-020-04009-w. [DOI] [PubMed] [Google Scholar]
- 77.Hanno P., Dmochowski R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol. Urodyn. 2009;28:274–286. doi: 10.1002/nau.20687. [DOI] [PubMed] [Google Scholar]
- 78.Wang H.-J., Lee W.-C., Tyagi P., Huang C.-C., Chuang Y.-C. Effects of low energy shock wave therapy on inflammatory moleculars, bladder pain, and bladder function in a rat cystitis model. Neurourol. Urodyn. 2017;36:1440–1447. doi: 10.1002/nau.23141. [DOI] [PubMed] [Google Scholar]
- 79.Chuang Y.C., Meng E., Chancellor M., Kuo H.C. Pain reduction realized with extracorporeal shock wave therapy for the treatment of symptoms associated with interstitial cystitis/bladder pain syndrome—A prospective, multicenter, randomized, double-blind, placebo-controlled study. Neurourol. Urodyn. 2020;39:1505–1514. doi: 10.1002/nau.24382. [DOI] [PubMed] [Google Scholar]
- 80.Shen Y.-C., Tyagi P., Lee W.-C., Chancellor M., Chuang Y.-C. Improves symptoms and urinary biomarkers in refractory interstitial cystitis/bladder pain syndrome patients randomized to extracorporeal shock wave therapy versus placebo. Sci. Rep. 2021;11:7558–7567. doi: 10.1038/s41598-021-87040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y.T., Chen K.H., Sung P.H., Yang C.C., Cheng B.C., Chen C.H., Chang C.L., Sheu J.J., Lee F.Y., Shao P.L., et al. Extracorporeal shock wave markedly alleviates radiation-induced chronic cystitis in rat. Am. J. Transl. Res. 2018;10:1036–1052. [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y.T., Yang C.C., Sung P.H., Lin K.C., Chiang J.Y., Huang C.R., Huang K.H., Chuang F.C., Chu Y.C., Huang E.Y., et al. Long-term effect of extracorporeal shock wave therapy on attenuating radiation-induced chronic cystitis in rat. Am. J. Transl. Res. 2020;12:999–1015. [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y.-T., Huang K.-H., Chiang J.Y., Sung P.-H., Huang C.-R., Chu Y.-C., Chuang F.-C., Yip H.-K. Extracorporeal Shock Wave Therapy Protected the Functional and Architectural Integrity of Rodent Urinary Bladder against Ketamine-Induced Damage. Biomedicines. 2021;9:1391. doi: 10.3390/biomedicines9101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lightner D.J., Gomelsky A., Souter L., Vasavada S.P. Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline Amendment 2019. J. Urol. 2019;202:558–563. doi: 10.1097/JU.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 85.Lee Y.-C., Chuang S.-M., Lin K.-L., Chen W.-C., Lu J.-H., Chueh K.-S., Shen M.-C., Liu L.-W., Long C.-Y., Juan Y.-S. Low-Intensity Extracorporeal Shock Wave Therapy Ameliorates the Overactive Bladder: A Prospective Pilot Study. BioMed Res. Int. 2020;2020:9175676. doi: 10.1155/2020/9175676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu J.-H., Chueh K.-S., Chuang S.-M., Wu Y.-H., Lin K.-L., Long C.-Y., Lee Y.-C., Shen M.-C., Sun T.-W., Juan Y.-S. Low Intensity Extracorporeal Shock Wave Therapy as a Potential Treatment for Overactive Bladder Syndrome. Biology. 2021;10:540. doi: 10.3390/biology10060540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gratzke C., Angulo J., Chitaley K., Dai Y.-T., Kim N.N., Paick J.-S., Simonsen U., Ückert S., Wespes E., Andersson K.E., et al. Anatomy, Physiology, and Pathophysiology of Erectile Dysfunction. J. Sex. Med. 2010;7:445–475. doi: 10.1111/j.1743-6109.2009.01624.x. [DOI] [PubMed] [Google Scholar]
- 88.Mykoniatis I., Pyrgidis N., Sokolakis I., Ouranidis A., Sountoulides P., Haidich A.-B., van Renterghem K., Hatzichristodoulou G., Hatzichristou D. Assessment of Combination Therapies vs Monotherapy for Erectile Dysfunction. JAMA Netw. Open. 2021;4:e2036337. doi: 10.1001/jamanetworkopen.2020.36337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McMahon C.N., Smith C.J., Shabsigh R. Treating erectile dysfunction when PDE5 inhibitors fail. bmj. 2006;332:589–592. doi: 10.1136/bmj.332.7541.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Skolarikos A., Alargof E., Rigas A., Deliveliotis C., Konstantinidis E. Shockwave Therapy as First-Line Treatment for Peyronie’s Disease: A Prospective Study. J. Endourol. 2005;19:11–14. doi: 10.1089/end.2005.19.11. [DOI] [PubMed] [Google Scholar]
- 91.Poulakis V., Skriapas K., Vries R., Dillenburg W., Ferakis N., Witzsch U., Melekos M., Becht E. Extracorporeal shockwave therapy for Peyronie’s disease: An alternative treatment? Asian J. Androl. 2006;8:361–366. doi: 10.1111/j.1745-7262.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 92.Chitale S., Morsey M., Swift L., Sethia K. Limited shock wave therapy vs sham treatment in men with Peyronie’s disease: Results of a prospective randomized controlled double-blind trial. BJU Int. 2010;106:1352–1356. doi: 10.1111/j.1464-410X.2010.09331.x. [DOI] [PubMed] [Google Scholar]
- 93.Gruenwald I., Appel B., Vardi Y. Low-Intensity Extracorporeal Shock Wave Therapy—A Novel Effective Treatment for Erectile Dysfunction in Severe ED Patients Who Respond Poorly to PDE5 Inhibitor Therapy. J. Sex. Med. 2012;9:259–264. doi: 10.1111/j.1743-6109.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 94.Vardi Y., Appel B., Kilchevsky A., Gruenwald I. Does Low Intensity Extracorporeal Shock Wave Therapy Have a Physiological Effect on Erectile Function? Short-Term Results of a Randomized, Double-Blind, Sham Controlled Study. J. Urol. 2012;187:1769–1775. doi: 10.1016/j.juro.2011.12.117. [DOI] [PubMed] [Google Scholar]
- 95.Palmieri A., Imbimbo C., Creta M., Verze P., Fusco F., Mirone V. Tadalafil once daily and extracorporeal shock wave therapy in the management of patients with Peyronie’s disease and erectile dysfunction: Results from a prospective randomized trial. Int. J. Androl. 2012;35:190–195. doi: 10.1111/j.1365-2605.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- 96.Yee C.-H., Chan E.S.Y., Hou S.S.-M., Ng C.-F. Extracorporeal shockwave therapy in the treatment of erectile dysfunction: A prospective, randomized, double-blinded, placebo controlled study. Int. J. Urol. 2014;21:1041–1045. doi: 10.1111/iju.12506. [DOI] [PubMed] [Google Scholar]
- 97.Srini V.S., Reddy R.K., Shultz T., Denes B. Low intensity extracorporeal shockwave therapy for erectile dysfunction: A study in an Indian population. Can. J. Urol. 2015;22:7614–7622. [PubMed] [Google Scholar]
- 98.Pelayo-Nieto M., Linden-Castro E., Alias-Melgar A., Espinosa-Pérez Grovas D., Carreño-de la Rosa F., Bertrand-Noriega F., Cortez-Betancourt R. Terapia de ondas de choque lineales en el tratamiento de la disfunción eréctil. Actas Urológicas Españolas. 2015;39:456–459. doi: 10.1016/j.acuro.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 99.Chung E., Cartmill R. Evaluation of clinical efficacy, safety and patient satisfaction rate after low-intensity extracorporeal shockwave therapy for the treatment of male erectile dysfunction: An Australian first open-label single-arm prospective clinical trial. BJU Int. 2015;115:46–49. doi: 10.1111/bju.13035. [DOI] [PubMed] [Google Scholar]
- 100.Bechara A., Casabe A., De Bonis W., Nazar J. Effectiveness of low-intensity extracorporeal shock wave therapy on patients with Erectile Dysfunction (ED) who have failed to respond to PDE5i therapy. A pilot study. Arch. Esp. Urol. 2015;68:152–160. [PubMed] [Google Scholar]
- 101.Frey A., Sønksen J., Fode M. Low-intensity extracorporeal shockwave therapy in the treatment of postprostatectomy erectile dysfunction: A pilot study. Scand. J. Urol. 2015;50:123–127. doi: 10.3109/21681805.2015.1100675. [DOI] [PubMed] [Google Scholar]
- 102.Olsen A.B., Persiani M., Boie S., Hanna M., Lund L. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand. J. Urol. 2014;49:329–333. doi: 10.3109/21681805.2014.984326. [DOI] [PubMed] [Google Scholar]
- 103.Hisasue S.-I., China T., Horiuchi A., Kimura M., Saito K., Isotani S., Ide H., Muto S., Yamaguchi R., Horie S. Impact of aging and comorbidity on the efficacy of low-intensity shock wave therapy for erectile dysfunction. Int. J. Urol. 2016;23:80–84. doi: 10.1111/iju.12955. [DOI] [PubMed] [Google Scholar]
- 104.Kalyvianakis D., Memmos E., Mykoniatis I., Kapoteli P., Memmos D., Hatzichristou D. Low-Intensity Shockwave Therapy for Erectile Dysfunction: A Randomized Clinical Trial Comparing 2 Treatment Protocols and the Impact of Repeating Treatment. J. Sex. Med. 2018;15:334–345. doi: 10.1016/j.jsxm.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Vinay J., Moreno D., Rajmil O., Ruiz-Castañe E., Sanchez-Curbelo J. Penile low intensity shock wave treatment for PDE5I refractory erectile dysfunction: A randomized double-blind sham-controlled clinical trial. World J. Urol. 2020;39:2217–2222. doi: 10.1007/s00345-020-03373-y. [DOI] [PubMed] [Google Scholar]
- 106.De Oliveira P.S., De Oliveira T.R., Nunes Á., Martins F., Lopes T. Low-intensity shock wave therapy for erectile dysfunction and the influence of disease duration. Arch. Ital. Urol. Androl. 2019;90:276–282. doi: 10.4081/aiua.2018.4.276. [DOI] [PubMed] [Google Scholar]
- 107.Huang Y.P., Liu W., Liu Y.D., Zhang M., Xu S.R., Lu M.J. Effect of low-intensity extracorporeal shockwave therapy on nocturnal penile tumescence and rigidity and penile haemodynamics. Andrologia. 2020;52:e13745. doi: 10.1111/and.13745. [DOI] [PubMed] [Google Scholar]
- 108.Sramkova T., Motil I., Jarkovsky J., Sramkova K. Erectile Dysfunction Treatment Using Focused Linear Low-Intensity Extracorporeal Shockwaves: Single-Blind, Sham-Controlled, Randomized Clinical Trial. Urol. Int. 2020;104:417–424. doi: 10.1159/000504788. [DOI] [PubMed] [Google Scholar]
- 109.Palmieri A., Arcaniolo D., Palumbo F., Verze P., Liguori G., Mondaini N., Falcone M., Scroppo F.I., Salonia A., Cai T., et al. Low intensity shockwave therapy in combination with phosphodiesterase-5 inhibitors is an effective and safe treatment option in patients with vasculogenic ED who are PDE5i non-responders: A multicenter single-arm clinical trial. Int. J. Impot. Res. 2021;33:634–640. doi: 10.1038/s41443-020-0332-7. [DOI] [PubMed] [Google Scholar]
- 110.Shendy W.S., Elsoghier O.M., El Semary M.M., Ahmed A.A., Ali A.F., Saber-Khalaf M. Effect of low-intensity extracorporeal shock wave therapy on diabetic erectile dysfunction: Randomised control trial. Andrologia. 2021;53:e13997. doi: 10.1111/and.13997. [DOI] [PubMed] [Google Scholar]
- 111.Müller A., Akin-Olugbade Y., Deveci S., Donohue J.F., Tal R., Kobylarz K.A., Palese M., Mulhall J.P. The Impact of Shock Wave Therapy at Varied Energy and Dose Levels on Functional and Structural Changes in Erectile Tissue. Eur. Urol. 2008;53:635–643. doi: 10.1016/j.eururo.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 112.Behr-Roussel D., Giuliano F. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. Transl. Androl. Urol. 2016;5:977–979. doi: 10.21037/tau.2016.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qiu X., Lin G., Xin Z., Ferretti L., Zhang H., Lue T.F., Lin C.S. Effects of Low-Energy Shockwave Therapy on the Erectile Function and Tissue of a Diabetic Rat Model. J. Sex. Med. 2013;10:738–746. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burkhard F.C.C., Bosch J.L.H.R., Cruz F., Lemack G.E., Nambiar A.K.N., Thiruchelvam N., Tubaro A. Guidelines Associates. In: Ambühl D., Bedretdinova D., Farag F., Lombardo R., Schneider M.P., editors. Urinary Incontinence in Adults. European Association of Urology; Arnhem, The Netherlands: 2020. [Google Scholar]
- 115.Wu A.K., Zhang X., Wang J., Ning H., Zaid U., Villalta J.D., Wang G., Banie L., Lin G., Lue T.F. Treatment of stress urinary incontinence with low-intensity extracorporeal shock wave therapy in a vaginal balloon dilation induced rat model. Transl. Androl. Urol. 2018;7:S7–S16. doi: 10.21037/tau.2017.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X., Ruan Y., Wu A.K., Zaid U., Villalta J.D., Wang G., Banie L., Reed-Maldonado A.B., Lin G., Lue T.F. Delayed Treatment With Low-intensity Extracorporeal Shock Wave Therapy in an Irreversible Rat Model of Stress Urinary Incontinence. Urology. 2020;141:187.e181–187.e187. doi: 10.1016/j.urology.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Long C.-Y., Lin K.-L., Lee Y.-C., Chuang S.-M., Lu J.-H., Wu B.-N., Chueh K.-S., Ker C.-R., Shen M.-C., Juan Y.-S. Therapeutic effects of Low intensity extracorporeal low energy shock wave therapy (LiESWT) on stress urinary incontinence. Sci. Rep. 2020;10:5818–5828. doi: 10.1038/s41598-020-62471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abrams P., Cardozo L., Fall M., Griffiths D., Rosier P., Ulmsten U., Van Kerrebroeck P., Victor A., Wein A. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/S0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 119.Gammie A., Kaper M., Dorrepaal C., Kos T., Abrams P. Signs and Symptoms of Detrusor Underactivity: An Analysis of Clinical Presentation and Urodynamic Tests From a Large Group of Patients Undergoing Pressure Flow Studies. Eur. Urol. 2016;69:361–369. doi: 10.1016/j.eururo.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 120.Osman N.I., Chapple C.R., Abrams P., Dmochowski R., Haab F., Nitti V., Koelbl H., van Kerrebroeck P., Wein A.J. Detrusor Underactivity and the Underactive Bladder: A New Clinical Entity? A Review of Current Terminology, Definitions, Epidemiology, Aetiology, and Diagnosis. Eur. Urol. 2014;65:389–398. doi: 10.1016/j.eururo.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 121.Yamaguchi O., Nomiya M., Andersson K.-E. Functional consequences of chronic bladder ischemia. Neurourol. Urodyn. 2014;33:54–58. doi: 10.1002/nau.22517. [DOI] [PubMed] [Google Scholar]
- 122.van Koeveringe G.A., Rademakers K.L.J., Birder L.A., Korstanje C., Daneshgari F., Ruggieri M.R., Igawa Y., Fry C., Wagg A. Detrusor underactivity: Pathophysiological considerations, models and proposals for future research. ICI-RS 2013. Neurourol. Urodyn. 2014;33:591–596. doi: 10.1002/nau.22590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chuang Y.-C., Tyagi P., Wang H.-J., Huang C.-C., Lin C.-C., Chancellor M.B. Urodynamic and molecular characteristics of detrusor underactivity in a rat cryoinjury model and effects of low energy shock wave therapy. Neurourol. Urodyn. 2018;37:708–715. doi: 10.1002/nau.23381. [DOI] [PubMed] [Google Scholar]
- 124.Wang H.S., Oh B.S., Wang B., Ruan Y., Zhou J., Banie L., Lee Y.C., Tamaddon A., Zhou T., Wang G., et al. Low-intensity extracorporeal shockwave therapy ameliorates diabetic underactive bladder in streptozotocin-induced diabetic rats. BJU Int. 2018;122:490–500. doi: 10.1111/bju.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Coelho A., Cruz F., Cruz C.D., Avelino A. Spread of OnabotulinumtoxinA After Bladder Injection. Experimental Study Using the Distribution of Cleaved SNAP-25 as the Marker of the Toxin Action. Eur. Urol. 2012;61:1178–1184. doi: 10.1016/j.eururo.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 126.Nageib M., El-Hefnawy A.S., Zahran M.H., El-Tabey N.A., Sheir K.Z., Shokeir A.A. Delivery of intravesical botulinum toxin A using low-energy shockwaves in the treatment of overactive bladder: A preliminary clinical study. Arab J. Urol. 2019;17:216–220. doi: 10.1080/2090598X.2019.1605676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elkashef A., Barakat N., Khater S.M., Awadalla A., Belal F., El-Assmy A.M., Sheir K.Z., Shokeir A.A. Effect of low-energy shock wave therapy on intravesical epirubicin delivery in a rat model of bladder cancer. BJU Int. 2020;127:80–89. doi: 10.1111/bju.15173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.