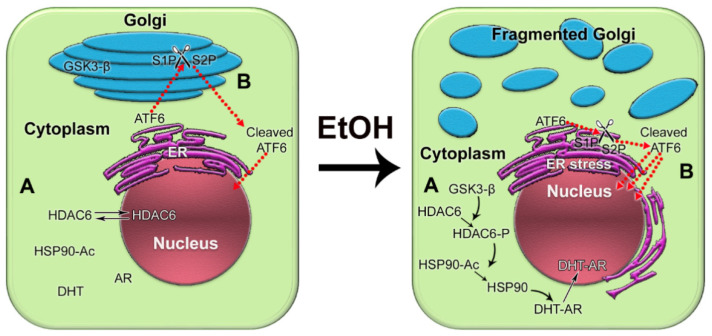
Figure 4.
The impact of alcohol-induced Golgi disorganization on prostate carcinogenesis. (A) In normal prostate cells or low aggressive PCa cells, HDAC6 is distributed in both the nucleus and the cytoplasm. Typically, the phosphorylation of HDAC6 is moderate because the enzyme that phosphorylates HDAC6, GSK3β, is sequestered primarily within the Golgi. The acetylated HSP90 has a limited binding capacity to AR. EtOH treatment results in Golgi fragmentation and translocation of GSK3β to the cytoplasm, which results in increased phosphorylation of HDAC6. HDAC6-P deacetylates HSP90, which, in turn, accelerates conformational maturation of AR, its binding to DHT, and translocation to the nucleus. (B) In normal prostate and low-aggressive PCa cells, ATF6α is cleaved sequentially in the Golgi by S1P and S2P proteases. The dimeric form of trans-golgin GCC185 is the retention partner for both S1P and S2P. Cleaved ATF6 enters the nucleus and binds to ER stress-response elements, stimulating the expression of UPR genes. EtOH and its metabolites fragment Golgi membranes, which is associated with the monomerization of GCC185 and the subsequent shift of S1P and S2P to the ER. This simplifies and accelerates ATF6 cleavage, resulting in more prominent UPR signaling to maintain tumor cell growth and proliferation.