Key Points
Allo-HCT and auto-HCT produce durable remissions in patients with DLBCL-RS.
Outcomes after allo-HCT are associated with remission status at HCT but not with receipt of prior novel agents or 17p deletion status.
Visual Abstract
Abstract
Richter syndrome (RS) represents a transformation from chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) to aggressive lymphoma, most commonly diffuse large B-cell lymphoma (DLBCL), which is associated with a dismal prognosis. Patients with DLBCL-RS have poor outcomes with DLBCL-directed therapy; thus, consolidation with hematopoietic cell transplantation (HCT) has been used, with durable remissions observed. Studies reporting HCT outcomes in patients with DLBCL-RS have been small, have not evaluated the prognostic impact of cytogenetic risk factors, and were conducted prior to the era of novel targeted therapy of CLL/SLL. We performed a Center for International Blood and Transplant Research registry study evaluating outcomes after autologous HCT (auto-HCT; n = 53) and allogeneic HCT (allo-HCT; n = 118) in patients with DLBCL-RS treated in the modern era. More auto-HCT recipients were in complete response (CR) at HCT relative to allo-HCT recipients (66% vs 34%), whereas a higher proportion of allo-HCT recipients had 17p deletion (33% vs 7%) and had previously received novel agents (39% vs 10%). In the auto-HCT cohort, the 3-year relapse incidence, progression-free survival (PFS), and overall survival (OS) were 37%, 48%, and 57%, respectively. Among allo-HCT recipients, the 3-year relapse incidence, PFS, and OS were 30%, 43%, and 52%, respectively. In the allo-HCT cohort, deeper response at HCT was associated with outcomes (3-year PFS/OS, 66%/77% CR vs 43%/57% partial response vs 5%/15% resistant; P < .0001 for both), whereas cytogenetic abnormalities and prior novel therapy did not impact outcomes. In our study, HCT resulted in durable remissions in therapy-sensitive patients with DLBCL-RS treated in the era of targeted CLL/SLL therapy, including patients with high-risk features.
Introduction
About 2% to 10% of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) will ultimately develop Richter syndrome (RS), which is characterized by transformation to an aggressive lymphoma, most commonly diffuse large B-cell lymphoma (DLBCL).1–7 DLBCL-RS is associated with a poor prognosis, with estimated overall survival (OS) measured in months and a median OS ≤ 1 year.2–4,8–11 Prognostic factors for outcome after the development of RS include clinical features at the time of transformation (eg, performance status, tumor size, number of prior lines of therapy) and whether the RS is clonally related to the preceding CLL/SLL.4,5,8–11 Patients with DLBCL that is clonally unrelated to the preceding CLL/SLL (∼20% of RS) have been reported to have favorable outcomes relative to patients with clonally related DLBCL-RS.5 Patients with DLBCL-RS are typically treated with DLBCL-directed chemotherapy, but response rates and long-term outcomes are lower than observed with de novo DLBCL.3,6,8,10–14 Because of the poor outcomes after standard DLBCL-directed therapy, consolidative hematopoietic cell transplantation (HCT) has been studied in patients who are chemotherapy sensitive and has produced durable remissions in a subset of patients.8,10,11,15–18 Autologous HCT (auto-HCT) and allogeneic HCT (allo-HCT) have been used for RS, with the largest study being a registry analysis from the European Society for Blood and Marrow Transplantation reporting 3-year relapse-free survival of 27% after allo-HCT (n =25) and 45% after auto-HCT (n =34).15 Although multiple studies have suggested improved outcomes for DLBCL-RS patients who undergo HCT, a small minority of patients with DLBCL-RS respond sufficiently to therapy to proceed to HCT. Nevertheless, based on these limited data, it is generally recommended that patients with DLBCL-RS who are responsive to RS-directed therapy, particularly clonally related DLBCL-RS, undergo consolidative HCT.16
The therapeutic landscape of CLL/SLL has shifted dramatically in the past decade. A range of effective targeted therapies, such as Bruton’s tyrosine kinase inhibitors, BCL-2 inhibitors, and phosphatidylinositol 3-kinase inhibitors, produce durable responses in a large proportion of patients, including traditionally high-risk patients with chromosome 17p deletion (del17p) or TP53 aberration.19–31 Of note, the use of novel agents for CLL/SLL does not appear to impact the incidence of RS.19,21,24,32,33 Multiple studies have recently demonstrated that the development of RS in a patient who previously received a novel agent for CLL/SLL tends to be highly aggressive and associated with short survival.23,34,35 Targeted agents have been evaluated as a treatment for DLBCL-RS with objective responses observed, although the median duration of response tends to be short with single-agent therapies.2,11,36,37
Although the modern treatment for CLL/SLL and DLBCL-RS has evolved, no study has comprehensively evaluated HCT outcomes for patients with RS in the modern era. Although the impact of prior exposure to novel therapies on allo-HCT outcomes has been studied in CLL/SLL,38 no study has evaluated the relationship between prior novel therapy exposure and HCT outcomes in patients with RS. In the limited studies performed to date, the primary indicator of outcome in patients with RS who undergo HCT was disease status at the time of transplantation, with patients in response to RS-directed therapy having more favorable outcomes.10,15 However, there has not been an analysis of the impact of cytogenetic risk factors on HCT outcomes in patients with RS. Because RS is rare and prospective data on the topic are not forthcoming, we used the Center for International Blood and Marrow Transplant Research (CIBMTR) registry to evaluate the outcomes after HCT in patients with RS-DLBCL. We included patients who had undergone auto-HCT or allo-HCT prior to and since the availability of novel agents and sought to evaluate the impact of clinical prognostic factors, cytogenetic abnormalities, and the use of novel agents on HCT outcomes.
Methods
This study used data from the CIBMTR, a working group of >380 transplantation centers worldwide that contributed detailed data on HCT to a statistical center at the Medical College of Wisconsin. Participating centers are required to report all HCTs consecutively, and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensured data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Patients provided written informed consent for research. The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Patients
Eligible patients included adults ≥18 years of age with DLBCL-RS (DLBCL transformed from underlying CLL/SLL) who underwent their first auto-HCT or allo-HCT between January of 2007 and December of 2017. Patients with prior auto-HCT were eligible for inclusion in the allo-HCT cohort; in these cases, data from only the first allo-HCT procedure were eligible for inclusion. Patients with DLBCL transformed from other subtypes of lymphoma or patients with non-DLBCL subtype RS (ie, Hodgkin lymphoma or peripheral T-cell lymphoma) were not eligible for this analysis. In eligible patients identified from the registry, additional data were collected on the type and number of therapies (including novel agents) received for antecedent CLL/SLL vs DLBCL-RS, the presence of cytogenetic abnormalities (patients classified according to highest-risk abnormality as any time point), and IGHV mutation testing. Because IGHV mutation data were not available for >95% of patients, these data are not presented.
Study end points and definitions
Disease response at the time of HCT was determined with the standard criteria in use at the time of HCT, including the International Working Group criteria39 or the Lugano Classification.40 Using consensus criteria, the intensity of allo-HCT conditioning regimens was categorized as nonmyeloablative, reduced intensity, or myeloablative.41 Baseline and transplant characteristics are reported descriptively. The primary end point was OS, defined as death from any cause. Secondary end points included progression-free survival (PFS), nonrelapse mortality (NRM), and relapse/progression. For PFS, an event was defined as progression/relapse or death from any cause. NRM was defined as death without preceding lymphoma relapse/progression, with relapse considered a competing risk. Progression/relapse was defined as progressive lymphoma after HCT or lymphoma recurrence after a complete remission, with NRM considered a competing risk. Data regarding histology at relapse (ie, CLL/SLL vs DLBCL) were not available; therefore, any relapse was considered an event. Surviving patients were censored at the date of last follow-up. PFS and OS probabilities were calculated using Kaplan-Meier estimates.
Acute and chronic graft-versus-host disease (GVHD) were graded using established clinical criteria.42,43 Neutrophil recovery was defined as the first of 3 successive days with an absolute neutrophil count ≥500 per microliter after post-HCT nadir. Platelet recovery was defined as the first of 3 consecutive days with a platelet count ≥20 000 per microliter in the absence of platelet transfusion for 7 consecutive days. For neutrophil and platelet recovery, death without the event was considered a competing risk.
Statistical analyses
Baseline patient and HCT characteristics were described. Because there were notable differences in the characteristics of patients who received auto-HCT compared with patients who received allo-HCT (ie, more auto-HCT patients in a complete response [CR] at HCT, very few auto-HCT patients had received prior novel agents, and few patients with high-risk cytogenetics in the auto-HCT cohort) and because of the potential for selection bias in utilizing 1 transplant approach vs the other, we did not compare outcomes between the auto-HCT and allo-HCT cohorts. Cumulative incidences of hematopoietic recovery, GVHD, relapse, and NRM were calculated to accommodate for competing risks. Cox proportional-hazard analysis for PFS and OS and the proportional cause-specific hazards model for relapse and NRM were used to identify prognostic factors, via forward stepwise selection, for the allo-HCT cohort. The proportional hazard assumption for each variable was examined by testing whether its coefficient is constant over time. The small sample size of the auto-HCT cohort precluded multivariate analysis. Center effect was tested using the score test of homogeneity.22 Covariates with a P value < .05 were considered significant. The variables considered in the multivariable regression analysis are shown in supplemental Table 1. Because the regression models for relapse, NRM, PFS, and OS identified 1 significant covariate for each outcome, Kaplan-Meier estimates for PFS and OS and cumulative incidences for relapse and NRM were calculated. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
One hundred and seventy-one patients with DLBCL-RS who underwent their first HCT between 2007 and 2017 with data reported to the CIBMTR were included. One hundred and eighteen patients received allo-HCT and 53 patients received auto-HCT. Baseline and HCT characteristics for all patients, organized according to type of HCT, are shown in Tables 1 and 2. Among auto-HCT patients, 7% had del17p aberration (although nearly half did not have available cytogenetic data); 5 of 53 (9%) had received a novel agent prior to HCT (all Bruton’s tyrosine kinase inhibitors), including 2 prior to and 3 after the RS; the median number of lines of therapy for the antecedent CLL/SLL was 2 (range, 1-5); the median number of lines of therapy for DLBCL-RS was 2 (range, 1-6); and 66% were in a CR prior to HCT. Six patients who underwent auto-HCT as their first HCT procedure subsequently underwent allo-HCT. Among allo-HCT patients, 33% had del17p; 46 of 118 (39%) had received a novel agent prior to HCT, including 11% prior to RS, 15% after RS, and 13% before and after RS; and 34% were in a CR prior to HCT. The majority of allo-HCT recipients received reduced-intensity/nonmyeloablative conditioning (77%), received calcineurin inhibitor-based GVHD prophylaxis without posttransplant cyclophosphamide (85%), and had a fully matched related or unrelated donor (63%).The median follow-up time in surviving patients in the auto-HCT and allo-HCT cohorts was 48 months (range, 4-98) and 49 months (range, 3-75), respectively.
Table 1.
Baseline characteristics according to type of HCT
| Characteristics | Allo-HCT (n =118) | Auto-HCT (n =53) |
|---|---|---|
| Age, median (range), y | 61 (26-73) | 65 (30-79) |
| Males | 81 (69) | 37 (70) |
| White race | 96 (81) | 47 (89) |
| KPS | ||
| ≥90 | 69 (58) | 28 (53) |
| Missing | 2 (2) | 1 (2) |
| HCT-CI | ||
| 3+ | 41 (35) | 32 (60) |
| Missing | 8 (7) | 2 (4) |
| Prior auto-HCT | 4 (3) | N/A |
| Cytogenetic abnormalities | ||
| 17p ± others | 39 (33) | 4 (7) |
| 11q deletion ± others (except 17p) | 10 (9) | 0 |
| Trisomy 12 ± others (except 17p and/or 11q) | 13 (11) | 3 (6) |
| None | 13 (11) | 13 (24) |
| Deletion of 13q alone | 4 (3) | 6 (11) |
| Others | 15 (13) | 5 (9) |
| Missing | 24 (20) | 22 (42) |
| Received novel agents before HCT* | ||
| Bruton’s tyrosine kinase inhibitor | 45 (38) | 5 (9) |
| Venetoclax | 7 (6) | 0 |
| PI3K inhibitor | 6 (5) | 0 |
| None | 69 (59) | 46 (87) |
| Missing | 3 (2) | 2 (4) |
| Novel agent | ||
| Yes, before RS | 13 (11) | 2 (4) |
| Yes, after RS | 18 (15) | 3 (6) |
| Both | 15 (13) | 0 |
| Neither | 69 (58) | 46 (87) |
| Missing | 3 (3) | 2 (4) |
| Response to first line RS therapy | ||
| CR | 31 (26) | 24 (45) |
| PR | 25 (21) | 10 (19) |
| Refractory disease | 41 (35) | 8 (15) |
| Not assessed | 21 (18) | 11 (21) |
| Prior therapy regimens received for CLL/SLL before RS, median (range), n | 2 (1-10) | 2 (1-5) |
| Prior therapy regimens received for RS, median (range), n | 2 (1-8) | 2 (1-6) |
| Time from CLL diagnosis to RS, median (range), mo | 30 (0-296) | 32 (0-166) |
Unless otherwise indicated, data are n (%).
HCT-CI, HCT Comorbidity Index; IGHV, immunoglobulin variable region heavy chain, PI3K, phosphatidylinositol 3-kinase; PS, Karnofsky Performance Status; ±, with or without.
Total adds up to 130 rather than 118 because some patients received >1 type of novel therapy.
Table 2.
HCT characteristics according to type of HCT
| Characteristic | Allo-HCT (n =118) | Auto-HCT (n =53) |
|---|---|---|
| Time from CLL diagnosis to HCT, median (range), mo | 44 (4-303) | 46 (5-172) |
| Time from RS diagnosis to HCT, median (range), mo | 11 (1-213) | 11 (3-172) |
| DLBCL-RS disease status (at HCT) | ||
| CR | 40 (34) | 35 (66) |
| PR | 51 (43) | 15 (28) |
| Resistant | 20 (17) | 2 (4) |
| Unknown | 7 (6) | 1 (2) |
| CLL/SLL disease status (at HCT) | ||
| CR | 27 (23) | 19 (36) |
| PR | 39 (33) | 15 (28) |
| Stable/untreated | 13 (11) | 5 (9) |
| Progressive | 17 (14) | 5 (9) |
| Missing/not assessed | 22 (19) | 9 (17) |
| Conditioning regimen (allo-HCT only) | ||
| MAC | 27 (23) | N/A |
| NMA/RIC | 91 (77) | N/A |
| TBI-containing conditioning regimen | 44 (37) | 1 (2) |
| GVHD prophylaxis | ||
| CNI ± others (except PTCY) | 100 (85) | N/A |
| PTCY ± CNI ± MMF | 14 (12) | N/A |
| Others* | 3 (3) | N/A |
| Missing | 1 (1) | N/A |
| Received ATG/alemtuzumab | 28 (24) | N/A |
| Donor type | ||
| HLA-matched related donor | 27 (23) | N/A |
| HLA-mismatched related donor | 18 (15) | N/A |
| Matched unrelated donor (8/8) | 47 (40) | N/A |
| Mismatched unrelated donor (≤7/8) | 7 (6) | N/A |
| Unrelated donor, matching unknown | 9 (8) | N/A |
| Cord blood | 10 (9) | N/A |
| Graft source | ||
| Bone marrow | 8 (7) | 0 |
| Peripheral blood | 103 (87) | 53 (100) |
| Cord blood | 7 (6) | 0 |
| Year of transplant | ||
| 2007-2013 | 31 (26) | 13 (25) |
| 2014-2017 | 87 (74) | 40 (75) |
| Follow-up after HCT, median (range), mo | 48 (4-98) | 49 (3-75) |
Unless otherwise noted, data are n (%).
ATG, anti-thymocyte globulin; CNI, calcineurin inhibitor; MAC, myeloablative conditioning; MMF, mycophenolate mofetil; NMA, nonmyeloablative conditioning; PR, partial response; PTCY, posttransplant cyclophosphamide; RIC, reduced-intensity conditioning; TBI, total body irradiation; ±, with or without.
CD34 selection (n =1), ex vivo T-cell depletion (n =1), sirolimus (n =1).
Hematopoietic recovery, GVHD, NRM, and relapse
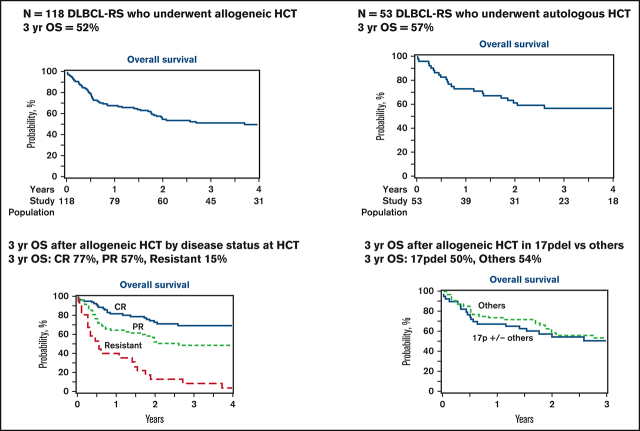
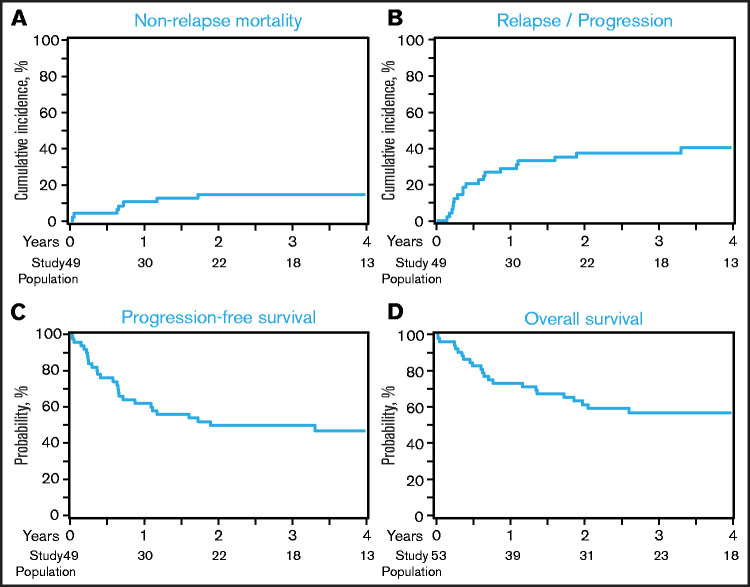
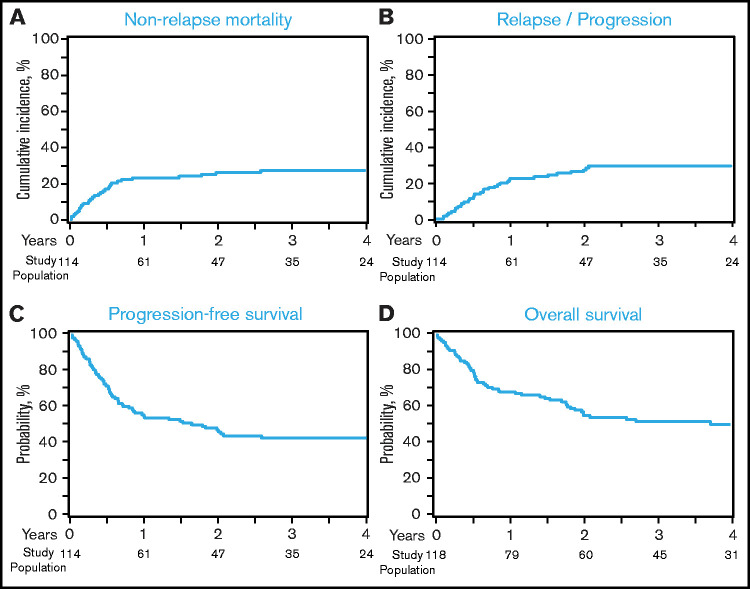
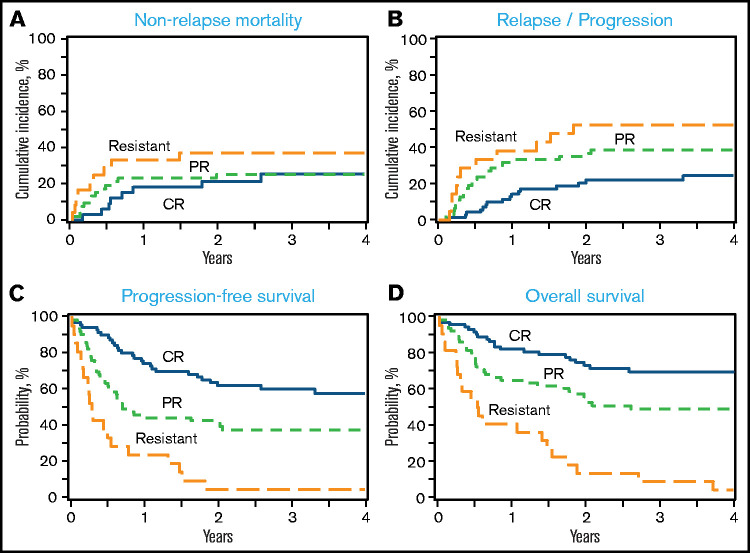
The 1-month cumulative incidence of neutrophil recovery among auto-HCT and allo-HCT recipients was 98% (95% confidence interval [CI], 89-100) and 96% (95% CI, 91-99), respectively, and the 100-day cumulative incidence of platelet recovery was 96% (95% CI, 86- 100) and 93% (95% CI, 87-97), respectively (Table 3). Among allo-HCT recipients, the 180-day cumulative incidence of grade 2-4 and grade 3-4 acute GVHD was 39% (95% CI, 29-49) and 13% (95% CI, 7-21), respectively, and the 1-year incidence of cGVHD was 37% (95% CI, 27-46). The 1-year NRM and incidence of relapse among auto-HCT recipients were 10% (95% CI, 3-21) and 29% (95% CI, 17-42), respectively (Figure 1A-B; Table 3); they were 23% (95% CI, 16-31) and 23% (95% CI, 16-31) among allo-HCT recipients, respectively (Figure 2A-B; Table 3). Disease status at the time of allo-HCT was associated with 3-year adjusted incidence of relapse: 16% (95% CI, 6-29) among patients in CR at allo-HCT compared with 31% (95% CI, 18-44) in patients with partial response (PR) and 47% (95% CI, 22-69) in patients with resistant disease (Figure 3B; Table 4; P =.05). There was not a significant difference in the 3-year adjusted incidence of relapse among allo-HCT recipients with del17p (37%; 95% CI, 22-51) vs those without del17p (32%; 95% CI, 19-44; P =.62; supplemental Table 2).
Table 3.
Outcomes of patients with DLBCL-RS undergoing auto-HCT or allo-HCT
| Outcomes | Allo-HCT (n =118) | Auto-HCT (n =53) | ||
|---|---|---|---|---|
| n | Prob, % (95% CI) | n | Prob, % (95% CI) | |
| Neutrophil recovery | 117 | 52 | ||
| 1 mo | 96 (91-99) | 98 (89-100) | ||
| Platelet recovery | 114 | 51 | ||
| 100 d | 93 (87-97) | 96 (86-100) | ||
| Grade 2-4 acute GVHD | 91 | N/A | N/A | |
| 100 d | 34 (25-44) | |||
| 6 mo | 39 (29-49) | |||
| Grade 3-4 acute GVHD | 91 | N/A | N/A | |
| 100 d | 12 (6-20) | |||
| 6 mo | 13 (7-21) | |||
| Chronic GVHD | 100 | N/A | N/A | |
| 1 y | 37 (27-46) | |||
| 2 y | 41 (31-51) | |||
| NRM | 114 | 49 | ||
| 100 d | 11 (6-17) | 4 (0-11) | ||
| 1 y | 23 (16-31) | 10 (3-21) | ||
| 3 y | 27 (19-36) | 15 (6-26) | ||
| Relapse/progression * | 114 | 49 | ||
| 1 y | 23 (16-31) | 29 (17-42) | ||
| 3 y | 30 (22-39) | 37 (24-51) | ||
| PFS | 114 | 49 | ||
| 1 y | 54 (45-63) | 61 (47-74) | ||
| 3 y | 43 (34-52) | 48 (34-62) | ||
| OS | 118 | 53 | ||
| 1 y | 69 (60-77) | 73 (61-84) | ||
| 3 y | 52 (43-61) | 57 (43-70) | ||
N/A, not applicable; Prob, probability.
Histology at relapse (CLL/SLL vs DLBCL-RS) was not available; therefore, any relapse was considered an event.
Figure 1.
Outcomes after auto-HCT. (A) NRM. (B) Relapse/progression. (C) PFS. (D) OS. Histology at relapse (CLL/SLL vs DLBCL-RS) was not available; therefore, any relapse was considered a relapse and PFS event.
Figure 2.
Outcomes after allo-HCT. (A) NRM. (B) Relapse/progression. (C) PFS. (D) OS. Histology at relapse (CLL/SLL vs DLBCL-RS) was not available; therefore, any relapse was considered a relapse and PFS event.
Figure 3.
Outcomes after allo-HCT according to disease status at allo-HCT. (A) NRM. (B) Relapse/progression. (C) PFS. (D) OS. Histology at relapse (CLL/SLL vs DLBCL-RS) was not available; therefore, any relapse was considered a relapse and PFS event.
Table 4.
Outcomes after allo-HCT according to disease status at HCT
| Outcomes | CR (n = 40) | PR (n = 51) | Resistant (n = 20) | P | |||
|---|---|---|---|---|---|---|---|
| n | Prob, % (95% CI) | n | Prob, % (95% CI) | n | Prob, % (95% CI) | ||
| Adjusted relapse * | 40 | 49 | 19 | ||||
| 1 y | 13 (5-25) | 29 (17-42) | 32 (12-53) | .11 | |||
| 3 y | 16 (6-29) | 31 (18-44) | 47 (22-69) | .05 | |||
| Adjusted NRM | 40 | 49 | 19 | ||||
| 1 y | 18 (5-31) | 23 (12-34) | 33 (14-52) | .47 | |||
| 3 y | 25 (10-41) | 25 (14-36) | 37 (16-58) | .61 | |||
| Adjusted PFS * | 40 | 49 | 19 | ||||
| 1 y | 72 (58-86) | 47 (33-61) | 26 (7-46) | <.0001 | |||
| 3 y | 66 (51-81) | 43 (29-57) | 5 (0-15) | <.0001 | |||
| Adjusted OS | 40 | 51 | 20 | ||||
| 1 y | 80 (67-92) | 67 (54-80) | 45 (23-67) | .02 | |||
Prob, probability.
Histology at relapse (CLL/SLL vs DLBCL-RS) was not available; therefore, any relapse was considered an event.
PFS and OS
The 1-year and 3-year PFS among auto-HCT recipients was 61% (95% CI, 47-74) and 48% (95% CI, 34-62), respectively, and the 1-year and 3-year OS was 73% (95% CI, 61-84) and 57% (95% CI, 43-70), respectively (Figure 1C-D; Table 3). Among allo-HCT recipients, the 1-year and 3-year PFS was 54% (95% CI, 45-63) and 43% (95% CI, 34-52), respectively, and the 1-year and 3-year OS was 69% (95% CI, 60-77) and 52% (95% CI, 43-61), respectively (Figure 2C-D; Table 3). Disease status at the time of allo-HCT was significantly associated with 3-year adjusted PFS: 66% (95% CI, 51-81) among patients in CR at allo-HCT compared with 43% (95% CI, 29-57) in patients with PR and 5% (95% CI, 0-15) in patients with resistant disease (P < .0001; Figure 3C; Table 4). Similarly, disease status at allo-HCT was associated with 3-year adjusted OS (P < .0001; Figure 3D; Table 3). However, the presence of del17p was not associated with adjusted PFS or OS (supplemental Table 2).
Multivariable analysis
A multivariable regression model was constructed to evaluate for covariates associated with NRM, relapse, PFS, and OS in the larger allo-HCT cohort. Covariates are listed in supplemental Table 1). Receipt of >3 regimens for DLBCL-RS was associated with an increased risk for NRM (hazard ratio [HR], 4.05, 95CI, 1.75-9.39, P =.001, Table 5). Disease status at allo-HCT was associated with an increased risk for relapse (HR for PR, 2.65; 95% CI, 1.10-6.38; P =.03 and HR for resistant, 6.81; 95% CI, 2.49-18.59; P < .001), poorer PFS (HR for PR, 1.97; 95% CI, 1.07-3.61; P =.03 and HR for resistant, 5.42; 95% CI, 2.73-10.79; P < .001), and poorer OS (HR for PR, 2.05; 95% CI, 1.04-4.06; P =.04 and HR for resistant, 6.63; 95% CI, 3.16-13.92; P < .001; Table 5). Other covariates were not significantly associated with outcomes, including presence of del17p, conditioning intensity, receipt of prior novel agents (supplemental Table 3), and time between diagnosis of CLL/SLL and diagnosis of DLBCL-RS. No center effect on outcomes was observed.
Table 5.
Multivariable regression analysis in allo-HCT cohort
| n | HR | 95% CI, lower limit | 95% CI, upper limit | P | Overall P | |
|---|---|---|---|---|---|---|
| NRM | ||||||
| Prior therapy regimens received, n | ||||||
| 1 | 51 | 1 | .01 | |||
| 2 | 27 | 1.38 | 0.52 | 3.63 | .52 | |
| ≥3 | 30 | 4.05 | 1.75 | 9.39 | <.001 | |
| Missing | 6 | 0.96 | 0.12 | 7.59 | .97 | |
| Relapse* | ||||||
| Disease status | ||||||
| CR | 40 | 1.00 | <.001 | |||
| PR | 49 | 2.65 | 1.10 | 6.38 | .03 | |
| Resistant | 19 | 6.81 | 2.49 | 18.59 | <.001 | |
| Missing | 6 | 0.87 | 0.11 | 7.07 | .90 | |
| PFS* | ||||||
| Disease status | ||||||
| CR | 40 | 1.00 | <.001 | |||
| PR | 49 | 1.97 | 1.07 | 3.61 | .03 | |
| Resistant | 19 | 5.42 | 2.73 | 10.79 | <.001 | |
| Missing | 6 | 0.78 | 0.18 | 3.39 | .74 | |
| OS | ||||||
| Disease status | ||||||
| CR | 40 | 1.00 | <.001 | |||
| PR | 51 | 2.05 | 1.04 | 4.06 | .04 | |
| Resistant | 20 | 6.63 | 3.16 | 13.92 | <.001 | |
| Missing | 7 | 1.05 | 0.23 | 4.70 | .95 |
Histology at relapse (CLL/SLL vs DLBCL-RS) was not available; therefore, any relapse was considered an event.
Causes of death
Disease relapse/progression was the most common cause of death after auto-HCT (48%) and allo-HCT (39%); causes of death by HCT type are listed in supplemental Table 4. Other frequent causes of death included GVHD (21%) and infection (11%) after allo-HCT, as well as organ failure (9%) after auto-HCT.
Discussion
DLBCL-RS is a rare and aggressive lymphoma with limited treatment options; it is associated with dismal outcomes. In this registry study, the largest study of HCT in RS and the first analysis to include a significant number of patients treated with novel targeted therapies, we demonstrate that auto-HCT or allo-HCT resulted in durable remission in nearly half of patients with DLBCL-RS. In the larger allo-HCT cohort, disease response status at the time of HCT was associated with incidence of relapse, PFS, and OS, whereas a higher number of RS-directed lines of therapy was associated with NRM. Among allo-HCT recipients, the presence of del17p or the receipt of a prior novel agent was not associated with outcomes. We did not compare outcomes after auto-HCT against outcomes after allo-HCT because of the differences in the characteristics of the study cohorts (ie, more patients in CR, very few patients had received prior novel agents, and few had high-risk cytogenetics in the auto-HCT cohort, including nearly half with missing cytogenetic data), as well as the possible biases in selecting 1 transplant approach vs the other; however, auto-HCT and allo-HCT appear to provide a proportion of treated patients with a durable remission.
Prior studies of HCT in patients with RS have been limited to small retrospective analyses, mostly single-center experiences of outcomes in all patients with RS, of which a small proportion underwent HCT.10,11,15,17 Our study confirmed the important finding from previous studies, including an European Society for Blood and Marrow Transplantation multicenter registry study from Cwynarski et al that disease status at the time of allo-HCT is associated with survival.15 The larger number of patients in our study allowed us to compare outcomes in patients with CR at allo-HCT against patients in PR or with resistant disease; indeed, patients with CR had decreased incidence of relapse and improved PFS and OS (3-year PFS and OS of 66% and 77%, respectively) compared with PR and resistant patients. Patients in PR at allo-HCT also had significantly better outcomes than did resistant patients; the 3-year PFS and OS of 43% and 57%, respectively, suggest that allo-HCT can provide durable remission in a sizable proportion of patients with PR to pre-HCT therapy. Similar to what was observed in some studies of allo-HCT in patients with CLL/SLL,38,44–46 del17p did not have a significant impact on outcomes after allo-HCT in patients with DLBCL-RS. As was observed in other studies of allo-HCT in patients with aggressive non-Hodgkin lymphomas, conditioning intensity did not impact outcomes; higher-intensity conditioning should be avoided to mitigate the risk of NRM.47–51 Although the development of DLBCL-RS following the receipt of CLL/SLL-directed novel therapies has been associated with dismal outcomes, in our study, in which 39% of the allo-HCT cohort had received prior novel agents and 24% had received the novel agent prior to transformation to DLBCL-RS, receipt of a novel agent was not associated with outcome after allo-HCT. This parallels the observation that the type of prior novel agent received, the number of prior novel agents received, and the receipt of only novel agents vs receipt of novel agents and chemoimmunotherapy prior to allo-HCT are not associated with PFS or OS after allo-HCT in patients with CLL.38 There were too few patients who received prior novel agents (9%) or had del17p (10%) to assess their prognostic impact in the auto-HCT cohort, which may reflect a bias in the patients selected for auto-HCT.
Our study has several limitations, including the inherent limitations of potential selection bias and center-specific transplantation practices with a multicenter registry analysis. Because a prospective study in this population is not forthcoming, our registry study is important for understanding the outcomes and risk factors for treatment failure after HCT in DLBCL-RS. Any study of HCT in DLBCL-RS is subject to the reality that a selected minority of patients diagnosed with DLBCL-RS undergo HCT (10-12% in studies that report HCT as a proportion of all diagnosed RS patients10,11), as well as that these patients may represent a biologically more favorable group. We did not have information about the clonal relationship between the DLBCL-RS and antecedent CLL/SLL, which has been identified as an important prognostic factor of outcome with DLBCL-RS.5 Clonally unrelated DLBCL-RS is associated with more favorable outcomes, and we cannot exclude that there may be patients with clonally unrelated disease included in this study. This especially may have been true in the auto-HCT cohort, given the low proportion of confirmed del17p and high proportion of CR at HCT in that group. TP53 disruption/del17p are less common in clonally unrelated DLBCL-RS.5 About 40% of patients in the allo-HCT cohort in this study had del17p, and outcomes were similar regardless of del17p status, suggesting that our results are applicable across DLBCL-RS patients undergoing allo-HCT. Information regarding the histology at relapse after HCT (DLBCL-RS vs the underlying CLL/SLL) was not available because of the limitations of the registry data. This limits the precision of our relapse and PFS estimates because some patients may have had relapse of CLL/SLL rather than DLBCL-RS. Post-HCT progression of CLL/SLL vs DLBCL-RS has starkly different clinical implications: relapsed DLBCL-RS would be expected to be more difficult to control and more likely to impact survival. Still, our results represent the most conservative “worst-case” estimate of outcomes specific to DLBCL-RS because all DLBCL-RS relapses were included.
Although our study evaluated patients with DLBCL-RS who were diagnosed and treated in the era of novel targeted therapy of CLL/SLL, there are newer immunotherapies that are being studied in DLBCL-RS and were not reflected in our study, including checkpoint inhibitors,52,53 bispecific antibodies,54 and chimeric antigen receptor–modified T cells.55 In future studies, it will be important to study the impact of these therapies on HCT outcomes in patients with DLBCL-RS. Future work should also address the optimal timing of HCT in relation to these newer immunotherapies, including chimeric antigen receptor–modified T cells. Our study provides important data on observed outcomes after HCT in the modern era that will hopefully guide decision making for transplant-eligible patients with DLBCL-RS who are responsive to therapy.
In conclusion, we observed that a sizable proportion of patients with DLBCL-RS who were transplanted in the modern era achieved a durable remission with auto-HCT or allo-HCT. Among recipients of allo-HCT, outcomes were independent of receipt of prior novel agents or cytogenetic risk, and deeper response at the time of transplantation was associated with decreased relapse and improved PFS and OS. Allo-HCT outcomes were dismal among treatment-resistant patients; these patients should be considered for alternative management approaches. Although a minority of patients with DLBCL-RS will respond to treatment and be considered for HCT, our data suggest that HCT should be considered for responding patients with DLBCL-RS, regardless of whether they have del17p or have received prior novel therapies, and, especially, for those in CR.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Institutes of Health National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases. It is also supported by the Health Resources and Services Administration (HHSH250201700006C and HHSH250201700007C) and the Office of Naval Research (N00014-20-1-2705 and N00014-20-1-2832). Additional federal support is provided by the National Institutes of Health National Cancer Institute (R01CA215134), the National Institutes of Health National Institute of Allergy and Infectious Diseases (R01AI128775 U01AI126612), the National Institutes of Health National Heart, Lung, and Blood Institute (R01HL130388), the National Institutes of Health National Eye Institute (UG1HL06924), and the Biomedical Advanced Research and Development Authority. Support is also provided by Be the Match Foundation, Boston Children’s Hospital, Dana Farber, St. Baldrick’s Foundation, Stanford University, the Medical College of Wisconsin the National Marrow Donor Program, and the following commercial entities: Actinium Pharmaceuticals, Inc., Adienne SA, AlloVir, Inc., Amgen, Inc., Angiocrine Bioscience, Astellas Pharma US, bluebird bio, Inc., Bristol Myers Squibb Co., Celgene Corp., CSL Behring, CytoSen Therapeutics, Inc., Daiichi Sankyo Co., Ltd., ExCellThera, Fate Therapeutics, Gamida-Cell, Ltd., Genentech Inc., Incyte Corporation, Janssen/Johnson & Johnson, Jazz Pharmaceuticals, Inc., Kiadis Pharma, Kite, a Gilead Company, Kyowa Kirin, Legend Biotech, Magenta Therapeutics, Merck Sharp & Dohme Corp., Millennium, the Takeda Oncology Co., Miltenyi Biotec, Inc., Novartis Pharmaceuticals Corporation, Omeros Corporation, OncoImmune, Inc., Orca Biosystems, Inc., Pfizer, Inc., Pharmacyclics, LLC, Sanofi Genzyme, StemCyte, Takeda Pharma, Vor Biopharma, and Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration, or any other agency of the US government.
Authorship
Contribution: M. Hamadani, A.F.H., M.A.K., and C.S.S. conceived and designed the study; Y.C., K.W.A., and C.L. collected and assembled data; Y.C., K.W.A., C.L., and M. Hamadani analyzed data; A.F.H. and M. Hamadani wrote the manuscript; and all authors interpreted data and revised the manuscript.
Conflict-of-interest disclosure: A.F.H. has received research funding from Bristol Myers Squibb, Merck, Genentech, Inc./F. Hoffmann-La Roche Ltd., Gilead Sciences, Seattle Genetics, AstraZeneca, and ADC Therapeutics; has acted as a consultancy for Bristol Myers Squibb, Merck, Genentech, Inc./F. Hoffmann-La Roche Ltd., Gilead Sciences, Seattle Genetics, and Karyopharm and has had travel, accommodations, and expenses paid by Bristol Myers Squibb. M. Hamadani has received research support/funding from Takeda Pharmaceutical Company, Spectrum Pharmaceuticals, and Astellas Pharma; has acted as a consultant for Janssen, Incyte Corporation, ADC Therapeutics, Celgene Corporation, Omeros, Verastem, and MorphoSys; and has been a member of the Speaker’s Bureau for Sanofi Genzyme, AstraZeneca, and BeiGene. A.A. has received grants from Incyte Corporation, outside of the submitted work. J.M. reports other from AlloVir HCP, Juno Therapeutics, Inc., Gilead-Kite Pharmaceuticals, and Magenta Therapeutics, outside of the submitted work. M.A.K.-D. reports consultancy for Daiichi Sankyo, outside of the submitted work. C.S.S, has received grants from Juno Therapeutics, Celgene, Bristol-Myers Squibb, Sanofi Genzyme, and Precision Biosciences and has received personal fees from Precision Biosciences, Sanofi Genzyme, Juno Therapeutics, Spectrum Pharmaceuticals, Novartis, Genmab, Kite, a Gilead Company, Celgene, Gamida Cell, Karyopharm Therapeutics, and GlaxoSmithKline, outside of the submitted work. R.T.M. has received grants, nonfinancial support, and other from Novartis and Bristol Myers Squibb; nonfinancial support and other from Kite Therapeutics; and other from Artiva Biotherapeutics, outside the submitted work. K.R. reports personal fees from Seattle Genetics, outside the submitted work. C.J. has received personal fees from Kite, Novartis, BMS/Celgene, Precision Biosciences, Nkarta, Lonza, AbbVie, and bluebird bio, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Mehdi Hamadani, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, 9200 W. Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; e-mail: mhamadani@mcw.edu.
References
- 1.Ben-Dali Y, Hleuhel MH, da Cunha-Bang C, et al. Richter’s transformation in patients with chronic lymphocytic leukaemia: a nationwide epidemiological study. Leuk Lymphoma. 2020;61(6):1435-1444. [DOI] [PubMed] [Google Scholar]
- 2.Ding W. Richter transformation in the era of novel agents. Hematology (Am Soc Hematol Educ Program). 2018;2018(1):256-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parikh SA, Kay NE, Shanafelt TD.. How we treat Richter syndrome. Blood. 2014;123(11):1647-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh SA, Rabe KG, Call TG, et al. Diffuse large B-cell lymphoma (Richter syndrome) in patients with chronic lymphocytic leukaemia (CLL): a cohort study of newly diagnosed patients. Br J Haematol. 2013;162(6):774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi D, Spina V, Deambrogi C, et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood. 2011;117(12):3391-3401. [DOI] [PubMed] [Google Scholar]
- 6.Rossi D, Spina V, Gaidano G.. Biology and treatment of Richter syndrome. Blood. 2018;131(25):2761-2772. [DOI] [PubMed] [Google Scholar]
- 7.Tsimberidou AM, Keating MJ.. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer. 2005;103(2):216-228. [DOI] [PubMed] [Google Scholar]
- 8.Abrisqueta P, Delgado J, Alcoceba M, et al. Clinical outcome and prognostic factors of patients with Richter syndrome: real-world study of the Spanish Chronic Lymphocytic Leukemia Study Group (GELLC). Br J Haematol. 2020;190(6):854-863. [DOI] [PubMed] [Google Scholar]
- 9.Al-Sawaf O, Robrecht S, Bahlo J, et al. Richter transformation in chronic lymphocytic leukemia (CLL)-a pooled analysis of German CLL Study Group (GCLLSG) front line treatment trials. Leukemia. 2021;35(1):169-176. [DOI] [PubMed] [Google Scholar]
- 10.Tsimberidou AM, O’Brien S, Khouri I, et al. Clinical outcomes and prognostic factors in patients with Richter’s syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006;24(15):2343-2351. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Tschautscher MA, Rabe KG, et al. Clinical characteristics and outcomes of Richter transformation: experience of 204 patients from a single center. Haematologica. 2020;105(3):765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabaja BS, O’Brien SM, Kantarjian HM, et al. Fractionated cyclophosphamide, vincristine, liposomal daunorubicin (daunoXome), and dexamethasone (hyperCVXD) regimen in Richter’s syndrome. Leuk Lymphoma. 2001;42(3):329-337. [DOI] [PubMed] [Google Scholar]
- 13.Rogers KA, Huang Y, Ruppert AS, et al. A single-institution retrospective cohort study of first-line R-EPOCH chemoimmunotherapy for Richter syndrome demonstrating complex chronic lymphocytic leukaemia karyotype as an adverse prognostic factor. Br J Haematol. 2018;180(2):259-266. [DOI] [PubMed] [Google Scholar]
- 14.Tsimberidou AM, Kantarjian HM, Cortes J, et al. Fractionated cyclophosphamide, vincristine, liposomal daunorubicin, and dexamethasone plus rituximab and granulocyte-macrophage-colony stimulating factor (GM-CSF) alternating with methotrexate and cytarabine plus rituximab and GM-CSF in patients with Richter syndrome or fludarabine-refractory chronic lymphocytic leukemia. Cancer. 2003;97(7):1711-1720. [DOI] [PubMed] [Google Scholar]
- 15.Cwynarski K, van Biezen A, de Wreede L, et al. Autologous and allogeneic stem-cell transplantation for transformed chronic lymphocytic leukemia (Richter’s syndrome): a retrospective analysis from the chronic lymphocytic leukemia subcommittee of the chronic leukemia working party and lymphoma working party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30(18):2211-2217. [DOI] [PubMed] [Google Scholar]
- 16.Kharfan-Dabaja MA, Kumar A, Hamadani M, et al. Clinical practice recommendations for use of allogeneic hematopoietic cell transplantation in chronic lymphocytic leukemia on behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2016;22(12):2117-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kharfan-Dabaja MA, Kumar A, Stingo FE, et al. Allogeneic hematopoietic cell transplantation for Richter syndrome: a single-center experience. Clin Lymphoma Myeloma Leuk. 2018;18(1):e35-e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aulakh S, Reljic T, Yassine F, et al. Allogeneic hematopoietic cell transplantation is an effective treatment for patients with Richter syndrome: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther. 2021;14(1):33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32(1):83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019; 380(23):2225-2236. [DOI] [PubMed] [Google Scholar]
- 22.Lin VS, Lew TE, Handunnetti SM, et al. BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax. Blood. 2020;135(25):2266-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mato AR, Nabhan C, Barr PM, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood. 2016;128(18):2199-2205. [DOI] [PubMed] [Google Scholar]
- 24.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coutre S, Choi M, Furman RR, et al. Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood. 2018;131(15):1704-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flinn IW, Hillmen P, Montillo M, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23): 2446-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4): 311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davids MS, Fisher DC, Tyekucheva S, et al. A phase 1b/2 study of duvelisib in combination with FCR (DFCR) for frontline therapy for younger CLL patients. Leukemia. 2021;35(4):1064-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849-2861. [DOI] [PubMed] [Google Scholar]
- 33.Kater AP, Wu JQ, Kipps T, et al. Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO Phase III Study. J Clin Oncol. 2020;38(34):4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1(1):80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain P, Thompson PA, Keating M, et al. Long-term outcomes for patients with chronic lymphocytic leukemia who discontinue ibrutinib. Cancer. 2017;123(12):2268-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsang M, Shanafelt TD, Call TG, et al. The efficacy of ibrutinib in the treatment of Richter syndrome. Blood. 2015;125(10):1676-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roeker LE, Dreger P, Brown JR, et al. Allogeneic stem cell transplantation for chronic lymphocytic leukemia in the era of novel agents. Blood Adv. 2020;4(16):3977-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 40.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 43.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204-217. [DOI] [PubMed] [Google Scholar]
- 44.Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol. 2008;26(31):5094-5100. [DOI] [PubMed] [Google Scholar]
- 45.Dreger P, Döhner H, Ritgen M, et al. ; German CLL Study Group . Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116(14):2438-2447. [DOI] [PubMed] [Google Scholar]
- 46.Krämer I, Stilgenbauer S, Dietrich S, et al. Allogeneic hematopoietic cell transplantation for high-risk CLL: 10-year follow-up of the GCLLSG CLL3X trial. Blood. 2017;130(12):1477-1480. [DOI] [PubMed] [Google Scholar]
- 47.Epperla N, Ahn KW, Khanal M, et al. Impact of reduced-intensity conditioning regimens on outcomes in diffuse large B cell lymphoma undergoing allogeneic transplantation. Biol Blood Marrow Transplant. 021;27(1):58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghosh N, Ahmed S, Ahn KW, et al. Association of reduced-intensity conditioning regimens with overall survival among patients with non-Hodgkin lymphoma undergoing allogeneic transplant. JAMA Oncol. 2020;6(7):1011-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamadani M, Saber W, Ahn KW, et al. Impact of pretransplantation conditioning regimens on outcomes of allogeneic transplantation for chemotherapy-unresponsive diffuse large B cell lymphoma and grade III follicular lymphoma. Biol Blood Marrow Transplant. 2013;19(5):746-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah NN, Hamadani M.. Is there still a role for allogeneic transplantation in the management of lymphoma? J Clin Oncol. 2021;39(5):487-498. [DOI] [PubMed] [Google Scholar]
- 51.Kharfan-Dabaja MA, Moukalled N, Reljic T, El-Asmar J, Kumar A.. Reduced intensity is preferred over myeloablative conditioning allogeneic HCT in chronic lymphocytic leukemia whenever indicated: a systematic review/meta-analysis. Hematol Oncol Stem Cell Ther. 2018;11(2):53-64. [DOI] [PubMed] [Google Scholar]
- 52.Armand P, Murawski N, Molin D, et al. Pembrolizumab in relapsed or refractory Richter syndrome. Br J Haematol. 2020;190(2):e117-e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129(26): 3419-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alderuccio JP, Mackrides N, Chapman JR, Vega F, Lossos IS.. Rapid complete response to blinatumomab as a successful bridge to allogeneic stem cell transplantation in a case of refractory Richter syndrome. Leuk Lymphoma. 2019;60(1):230-233. [DOI] [PubMed] [Google Scholar]
- 55.Kittai AS, Bond DA, William B, et al. Clinical activity of axicabtagene ciloleucel in adult patients with Richter syndrome. Blood Adv. 2020;4(19): 4648-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.