Abstract
In vitro pharmacodynamic studies investigating the antimicrobial properties of five fluoroquinolones, (trovafloxacin, sparfloxacin, clinafloxacin, levofloxacin, and ciprofloxacin) against Bacteroides fragilis ATCC 23745 were conducted. The times required to reduce the viable counts by 3 log units were as follows: clinafloxacin, 2.9 h; levofloxacin, 4.6 h; trovafloxacin, 6 h; and sparfloxacin, 10 h. Exposure to ciprofloxacin did not achieve a 3-log decrease in viable counts. The susceptibility of B. fragilis was determined both prior to exposure and following 24 h of exposure to each of the five fluoroquinolones tested. The MICs of clinafloxacin, levofloxacin, trovafloxacin, sparfloxacin, ciprofloxacin, metronidazole, cefoxitin, chloramphenicol, and clindamycin were determined by the broth microdilution method. The MICs for B. fragilis preexposure were as follows: clinafloxacin, 0.25 μg/ml; trovafloxacin, 0.5 μg/ml; sparfloxacin, 2 μg/ml; levofloxacin, 2 μg/ml; and ciprofloxacin, 8 μg/ml. Similar pre- and postexposure MICs were obtained for cultures exposed to trovafloxacin, clinafloxacin, levofloxacin, and ciprofloxacin. However, following 24 h of exposure to sparfloxacin, a fluoroquinolone-resistant strain emerged. The MICs for this strain were as follows: clinafloxacin, 1 μg/ml; trovafloxacin, 4 μg/ml; sparfloxacin, 16 μg/ml; levofloxacin, 16 μg/ml; and ciprofloxacin, 32 μg/ml. No changes in the susceptibility of B. fragilis pre- and postexposure to sparfloxacin were noted for metronidazole (MIC, 1 μg/ml), cefoxitin (MIC, 4 μg/ml), chloramphenicol (MIC, 4 μg/ml), and clindamycin (MIC, 0.06 μg/ml). Resistance remained stable as the organism was passaged on antibiotic-free agar for 10 consecutive days. Mutant B. fragilis strains with decreased susceptibility to clinafloxacin, trovafloxacin, sparfloxacin, levofloxacin, and ciprofloxacin were selected on brucella blood agar containing 8× the MIC of levofloxacin at a frequencies of 6.4 × 10−9, 4× the MICs of trovafloxacin and sparfloxacin at frequencies of 2.2 × 10−9 and 3.3 × 10−10, respectively, and 2× the MIC of clinafloxacin at a frequency of 5.5 × 10−11; no mutants were selected with ciprofloxacin. The susceptibilities of strains to trovafloxacin, levofloxacin, clinafloxacin, sparfloxacin, and ciprofloxacin before and after exposure to sparfloxacin were modestly affected by the presence of reserpine (20 μg/ml), an inhibitor of antibiotic efflux. The mechanism of fluoroquinolone resistance is being explored, but it is unlikely to be efflux due to a lack of cross-resistance to unrelated antimicrobial agents and to the fact that the MICs for strains before and after exposure to sparfloxacin are minimally affected by reserpine.
Bacteroides fragilis is a pathogen frequently associated with serious intra-abdominal and gynecologic infections in humans (26). Along with other species of Bacteroides, B. fragilis is the single anaerobe most often isolated from patients with intra-abdominal infections and accounts for 30 to 60% of all the anaerobic isolates from patients with such infections (17). Historically, antimicrobial regimens for the treatment of B. fragilis infections have been limited to select beta-lactams, clindamycin, chloramphenicol, or metronidazole; however, newly developed fluoroquinolones (trovafloxacin, clinafloxacin, moxifloxacin, levofloxacin, and sparfloxacin) have been demonstrated to have various degrees of in vitro and in vivo activity against B. fragilis and other anaerobic bacteria (3–5, 7–9, 12, 14, 23, 24). Intrinsic antibacterial action against aerobic gram-negative, aerobic gram-positive, and anaerobic bacteria make select fluoroquinolones attractive single-agent therapies for polymicrobial intra-abdominal infections. These select fluoroquinolones also possess favorable pharmacokinetic properties such as long half-lives and almost complete oral bioavailability which permits once-daily dosing and oral administration of these agents (26).
In vitro pharmacodynamic studies were performed to investigate the antibacterial as well as the pharmacodynamic properties of trovafloxacin, clinafloxacin, levofloxacin, sparfloxacin, and ciprofloxacin against B. fragilis ATCC 23745. While we were performing these studies, a fluoroquinolone-resistant B. fragilis ATCC 23745 strain for which MICs increased four- to eightfold was unexpectedly produced following a single exposure to sparfloxacin. Bacterial resistance was confirmed by conducting repeat in vitro studies with sparfloxacin and performing susceptibility testing with the sparfloxacin-resistant strain of B. fragilis before and after exposure to sparfloxacin; four- to eightfold increases in MICs were detected. Following these experiments, studies were performed to determine the stability of resistance, the frequency of mutation, and the corresponding MICs in the presence of reserpine, an inhibitor of antibiotic efflux. At the time of discovery of the fluoroquinolone-resistant B. fragilis strain, no data regarding B. fragilis resistance to fluoroquinolones had been published. Recently, data suggesting that active efflux (13) and alterations in the quinolone resistance-determining region (QRDR) of gyrA (22) are possible mechanisms of resistance have been reported. The following is a report of the pharmacodynamic properties of trovafloxacin, clinafloxacin, levofloxacin, sparfloxacin, and ciprofloxacin against B. fragilis ATCC 23745 and an initial evaluation of the characteristics of the fluoroquinolone-resistant B. fragilis strain that was obtained.
(Data from this study were presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 27 September 1997 to 1 October 1997 [17a].)
MATERIALS AND METHODS
In vitro studies.
The study consisted of a series of 24-h concentration time-kill experiments performed with a one-compartment, in vitro pharmacodynamic model (28). First-order elimination pharmacokinetics were simulated in the model by displacing an equal volume of antibiotic-containing anaerobic medium with antibiotic-free medium with a peristaltic pump. Pump rates were calculated on the basis of the desired clearance (Kd · volume of distribution), which depended on the volume of the reaction vessel and preselected half-life (t1/2; t1/2 = 0.693/Kd). An anaerobic environment was created by placing the in vitro pharmacodynamic model within a Bactron IV anaerobic chamber (Anaerobe Systems, Morgan Hill, Calif.) with an anaerobic mixed gas of 5% hydrogen, 5% carbon dioxide, and 90% nitrogen.
Antibiotics were provided by their respective manufacturers, as follows: trovafloxacin, Pfizer Inc., Groton, Conn.; sparfloxacin, Rhone-Poulenc Rorer, Collegeville, Pa.; clinafloxacin, Parke-Davis, Ann Arbor, Mich.; levofloxacin, the R. W. Johnson Pharmaceutical Research Institute, Spring House, Pa.; and ciprofloxacin, Bayer Pharmaceuticals, West Haven, Conn. Compounds obtained from Sigma included clindamycin, cefoxitin, metronidazole, chloramphenicol, and reserpine. Reconstitutions were in accordance with the manufacturer’s specifications, and stock solutions were stored at −80°C.
Inocula were prepared by inoculating anaerobe broth medium (Difco Laboratories, Detroit, Mich.), which was used for all in vitro model experiments and susceptibility tests, with B. fragilis ATCC 23745 and incubating the mixture at 37°C over 24 h. Approximately 1 h prior to the start of the experiments, an overnight inoculum of 50 ml was added to 200 ml of fresh broth, and the mixture was incubated anaerobically at 37°C for 1 h to achieve logarithmic growth. The final inoculum was prepared by adding approximately 8 ml of the overnight inoculum at a 0.5 McFarland standard to each chemostat to achieve a final inoculum of 106 CFU/ml. The final inoculum was verified by serial dilution and counting of the visible colonies. All fluoroquinolones tested were administered as bolus injections into the reaction vessel to achieve the desired peak concentrations. Selected peak concentrations and t1/2s of each fluoroquinolone were chosen to emulate human pharmacokinetic parameters, and the respective values were as follows: trovafloxacin, 2 μg/ml and 10 h; clinafloxacin, 3 μg/ml and 6 h; levofloxacin, 6 μg/ml and 8 h; sparfloxacin, 2 μg/ml and 18 h; and ciprofloxacin, 5 μg/ml and 4 h.
Samples were collected at time zero and following the injection of the antibiotic at 1, 2, 3, 4, 6, 8, 12, and 24 hours and were evaluated for bacterial load (numbers of CFU per milliliter) and medium pH. Antibiotic carryover was avoided by exposing 1-ml samples containing fluoroquinolones to antimicrobial polymeric binding resin (Amberlite XAD-4; Supelco, Bellefonte, Pa.) (27). Following serial dilution, the samples were plated onto anaerobic blood agar plates, and the plates were incubated anaerobically for 48 h at 37°C. After incubation, the colonies were counted, with a lower quantitative limit of accuracy for bacterial counts of 3 × 102 CFU/ml or 30 colonies on an agar plate containing 100 μl of undiluted sample.
Susceptibility testing.
The MIC, which was the lowest concentration that prevented visible growth, and the MBC, which was the lowest concentration of antibiotic that produced a 99.9% reduction of the initial inoculum, were determined prior to and following fluoroquinolone exposure. The pre- and postexposure MICs of all five fluoroquinolones (trovafloxacin, sparfloxacin, clinafloxacin, levofloxacin, and ciprofloxacin) as well as traditional anaerobic agents (clindamycin, metronidazole, cefoxitin, and chloramphenicol) were determined by using Clinical Microbiology Procedures Handbook method for broth microdilution susceptibility testing of anaerobic bacteria (10a). Susceptibilities to traditional anaerobic agents were determined only before and after exposure to sparfloxacin. MIC microtiter trays containing anaerobe broth and antibiotics were inoculated at 106 CFU/ml and were read after 48 h of incubation anaerobically in a Bactron IV anaerobic chamber with an anaerobic mixed gas of 5% hydrogen, 5% carbon dioxide, and 90% nitrogen at 37°C. The reference strain B. fragilis ATCC 25285 was used as a control in all MIC determinations.
The stability of resistance was determined by consecutively passaging the B. fragilis ATCC 23745 that had been exposed to sparfloxacin for 24 h onto antibiotic-free anaerobic blood agar plates for 10 consecutive days. MICs were determined by broth microdilution procedures by randomly selecting approximately 100 colonies on days 1, 3, 7, and 10 following an initial 48 h of anaerobic incubation at 37°C.
MIC studies were also conducted with reserpine, an inhibitor of antibiotic efflux by aerobic gram-negative and gram-positive bacteria (2, 15). Reserpine (20 μg/ml) was incorporated into the anaerobe broth used to prepare the MIC broth microtiter trays. MICs were determined by broth microdilution methods for B. fragilis ATCC 23745 (before exposure to sparfloxacin) and the fluoroquinolone-resistant mutant of B. fragilis ATCC 23745 (after exposure to sparfloxacin). The inoculum was 106 CFU/ml, and the results were read after 48 h of anaerobic incubation at 37°C. These results were directly compared to the MICs determined without reserpine.
Pharmacodynamic analysis.
Analyses of the data for possible correlations between resistance and pharmacodynamic parameters (area under the concentration-time curve from time zero to 24 h [AUC0–24] divided by the MIC, time above the MIC [T > MIC], and peak drug concentration [Cmax] divided by the MIC) were conducted because previous studies showed that relationships between resistance and pharmacodynamic parameters exist (11, 18, 25). Time-kill studies were also performed to analyze the times to 3-log killing and the presence of regrowth of bacteria at 24 h.
Frequency of mutation.
Agar dilution plating experiments were performed to determine the frequencies of mutation and whether selection of resistance was indirectly correlated with fluoroquinolone efficacy. Brucella blood agar was prepared as described in reference 9a, and each fluoroquinolone (trovafloxacin, sparfloxacin, levofloxacin, clinafloxacin, and ciprofloxacin) was incorporated into the agar at 1×, 2×, 4×, and 8× the MIC for B. fragilis ATCC 23745. B. fragilis ATCC 23745 was inoculated onto agar plates at 106, 107, 108, 109, and 1010 CFU/ml in the logarithmic growth phase following overnight anaerobic incubation at 37°C. Antibiotic-free brucella blood agar plates were also inoculated to confirm the presence of the desired inocula. After 48 h of anaerobic incubation at 37°C, the colonies were quantified and the frequencies of mutation were determined by the following calculation: number of countable colonies divided by the inoculum. The MICs for any visible colonies selected on brucella blood agar plates were determined by broth microdilution methods.
RESULTS
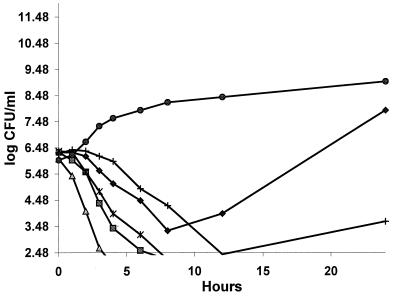
Time-kill curves for clinafloxacin, levofloxacin, trovafloxacin, sparfloxacin, and ciprofloxacin against B. fragilis ATCC 23745 are presented in Fig. 1. The associated time(s) to 3-log killing were as follows: clinafloxacin, 2.9 h; levofloxacin, 4.6 h; trovafloxacin, 6 h; and sparfloxacin, 10 h. Ciprofloxacin did not achieve a 3-log killing. Bacterial regrowth at 24 h was not observed with clinafloxacin, trovafloxacin, or levofloxacin; however, regrowth was apparent with sparfloxacin and ciprofloxacin.
FIG. 1.
Time-kill curves for various fluoroquinolones against B. fragilis ATCC 23745. ⧫, ciprofloxacin (5 μg/ml); ■, Levofloxacin (6 μg/ml); ▵, clinafloxacin (3 μg/ml); +, sparfloxacin (2 μg/ml); ✻, trovafloxacin (2 μg/ml); ●, growth control (B. fragilis ATCC 23745).
Analysis of MICs showed four- to eightfold increases in the MICs of all five fluoroquinolones tested following a 24-h exposure of B. fragilis ATCC 23745 to sparfloxacin (Table 1). Increases in the MICs were not observed for B. fragilis strains following exposure to the other four fluoroquinolones for 24 h. Upon observation of this phenomenon, repeat in vitro pharmacodynamic testing was performed with sparfloxacin against B. fragilis ATCC 23745. The results confirmed previous findings, with four- to eightfold increases in the MICs of all fluoroquinolones tested. Prior to exposure of B. fragilis ATCC 23745 to sparfloxacin, the MICs of the tested fluoroquinolones were similar to those previously reported in the literature (3–5, 7–9, 12, 14, 24).
TABLE 1.
Susceptibility of B. fragilis ATCC 23745 before and after exposure to sparfloxacin determined by broth microdilution method
| Drug | MIC (μg/ml)a
|
|||
|---|---|---|---|---|
| Before exposure | Before exposure, with reserpine | After exposure | After exposure, with reserpine | |
| Trovafloxacin | 0.5 | 0.25 | 4 | 2 |
| Sparfloxacin | 2 | 2 | 16 | 8 |
| Clinafloxacin | 0.25 | 0.25 | 1 | 0.5 |
| Levofloxacin | 2 | 2 | 16 | 8 |
| Ciprofloxacin | 8 | 4 | 32 | 16 |
| Metronidazole | 1 | 1 | ||
| Clindamycin | 0.06 | 0.06 | ||
| Cefoxitin | 4 | 4 | ||
| Chloramphenicol | 4 | 4 | ||
MICs are reported as mode values for six different studies in which MICs were determined.
Serial passage of resistant strains onto antibiotic-free agar plates for 10 consecutive days revealed no change in the elevated MICs and stable resistance at 10 days. Testing of the MICs for strains, both before and after exposure to sparfloxacin, of other commonly used antimicrobial agents for the treatment of anaerobes (clindamycin, metronidazole, cefoxitin, and chloramphenicol) revealed that the strains were sensitive to these agents both before and after exposure, with no increases in MICs following exposure to sparfloxacin (Table 1). Efflux as a possible mechanism of fluoroquinolone resistance was explored by determining the MICs for B. fragilis ATCC 23745 strains, both before and after exposure to sparfloxacin, of the fluoroquinolones tested in the presence of reserpine. The results were compared to the MICs obtained without reserpine (Table 1). Reserpine appeared to have little effect on susceptibility to the fluoroquinolones tested against strains before and after exposure to sparfloxacin. For clinafloxacin, levofloxacin, and sparfloxacin, there were single-dilution decreases in the MICs only for the strains after exposure. For ciprofloxacin and trovafloxacin, there were single-dilution decreases in the MICs for the strains both before and after exposure.
The frequencies of selection of mutant B. fragilis ATCC 23745 strains when the strain was exposed to trovafloxacin, levofloxacin, sparfloxacin, clinafloxacin and ciprofloxacin are presented in Table 2. Experiments were performed to determine approximate rates of mutation and whether an indirect relationship could be observed between the frequency of mutation and the efficacies of the fluoroquinolones against B. fragilis. A relationship between efficacy and rates of mutation could be established only for clinafloxacin, which at 2× the MIC selected mutants with the lowest frequency of mutation, 5.5 × 10−10, and shortest time to 3-log killing. Levofloxacin at 8× the MIC selected mutants at a frequency of 6.4 × 10−9. Trovafloxacin and sparfloxacin at 4× the MIC selected mutants at frequencies of approximately 2.2 × 10−9 and 3.3 × 10−10, respectively; of all fluoroquinolones tested, trovafloxacin selected mutants at the greatest frequency. No mutants were selected in the presence of ciprofloxacin. The corresponding MICs for the B. fragilis ATCC 23745 mutants that were selected consistently rose 4- to 16-fold.
TABLE 2.
Frequency of mutation for B. fragilis ATCC 23745 and susceptibility
| Drug (MIC [μg/ml]) | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Trovafloxacin at 4× the MICa (2.2 × 10−9)b | Sparfloxacin at 4× the MIC (3.3 × 10−10) | Clinafloxacin at 2× the MIC (5.5 × 10−11) | Levofloxacin at 8× the MIC (6.4 × 10−9) | Ciprofloxacin at 1× MIC (no mutants) | |
| Trovafloxacin (0.5) | 4 | 4 | 4 | 4 | 0.5 |
| Sparfloxacin (2) | 16 | 8 | 8 | 16 | 2 |
| Clinafloxacin (0.25) | 2 | 2 | 2 | 2 | 0.25 |
| Levofloxacin (2) | 32 | 32 | 16 | 32 | 2 |
| Ciprofloxacin (8) | 32 | 16 | 16 | 32 | 8 |
Highest drug concentration-to-MIC ratio at which B. fragilis mutants with decreased susceptibility to the fluoroquinolones were selected.
Values in parentheses are frequencies of mutation.
An analysis of the data for possible correlations between resistance and pharmacodynamic parameters such as AUC0–24/MIC, T > MIC, and/or Cmax/MIC is presented in Table 3.
TABLE 3.
Pharmacodynamic parameters of fluoroquinolones against B. fragilis ATCC 23745
| Drug | AUC0–24/MIC | Cmax/MIC | T > MIC (h) | T3Ka (h) |
|---|---|---|---|---|
| Trovafloxacin | 47 | 4 | 20 | 6 |
| Sparfloxacin | 16 | 1 | 0 | 10 |
| Clinafloxacin | 100 | 12 | 22 | 2.9 |
| Levofloxacin | 30 | 3 | 13 | 4.6 |
| Ciprofloxacin | 4 | 0.6 | 0 | NAb |
T3K, time to 3-log killing.
NA, not applicable. Ciprofloxacin did not achieve 3-log killing.
DISCUSSION
The in vitro pharmacodynamic experiments conducted to evaluate the efficacies of five fluoroquinolones (trovafloxacin, levofloxacin, sparfloxacin, clinafloxacin, and ciprofloxacin) against B. fragilis ATCC 23745 produced results similar to those reported previously (23) and further suggest that trovafloxacin, clinafloxacin, and levofloxacin are effective therapeutic options in the management of B. fragilis infections. The MICs for B. fragilis ATCC 23745 before exposure to sparfloxacin were also similar to those found in the literature (3–5, 7–9, 12, 14, 24). Therefore, the emergence of a fluoroquinolone-resistant strain of B. fragilis following a one-time exposure to sparfloxacin was a surprising finding which had not previously been reported in the literature.
Several questions arise from the results of the in vitro pharmacodynamic studies. Were differences in the magnitude of exposure of B. fragilis ATCC 23745 to the fluoroquinolones responsible for the selection of resistant strains only with sparfloxacin, and/or were properties unique to the in vitro pharmacodynamic model used in this study responsible for the selection of resistant strains? Exposure differences included Cmax/MIC and AUC/MIC ratios of 0.6 and 4, respectively, for ciprofloxacin and up to 12 and 100, respectively, for clinafloxacin. Correlations between the pharmacodynamic parameters AUC0–24/MIC and Cmax/MIC for the fluoroquinolones and the development of resistance in aerobic gram-negative organisms have been reported (11, 18, 25). In in vitro modeling studies that have examined various ciprofloxacin and ofloxacin dosing regimens against Pseudomonas aeruginosa, investigators selected subvariant bacterial populations for which increasing MICs correlated to a Cmax/MIC50 (the MIC50 is the MIC at which 50% of strains are inhibited) ratio of 7.3 and an AUC/MIC50 ratio of 95 (11). In a retrospective study that evaluated 107 acutely ill patients, 128 pathogens (89% gram-negative organisms), and five antimicrobial regimens, the selection of antimicrobial resistance appeared to be strongly associated with suboptimal antimicrobial exposure, defined as an AUC0–24/MIC ratio of less than 100 (25). This relationship was strongest for P. aeruginosa treated with ciprofloxacin but was also found for other organism groups and antibiotic treatments. To date, no information regarding the pharmacodynamics of fluoroquinolones and B. fragilis and/or their relationship to resistance have been reported with the exception of a report of in vitro pharmacodynamic studies recently conducted with trovafloxacin and levofloxacin against B. fragilis ATCC 25285 and ATCC 23745 and a clinical strain, B. fragilis M97-117 (19). Data from those studies suggest that fluoroquinolones exhibit concentration-independent killing activity against B. fragilis (19), with a direct correlation between an AUC0–24/MIC ratio of 10 to 50 and the selection of a resistant subvariant population of bacteria (18). Those studies suggest that the AUC0–24/MIC ratio may be a useful parameter that can be used to guide therapy with the goal of preventing the selection of resistance (11, 18, 25). Further analysis of the pharmacodynamic parameters AUC0–24/MIC and Cmax/MIC from the present study suggest that sparfloxacin could be selecting a subpopulation of bacteria resistant to fluoroquinolones due to a Cmax/MIC ratio of 1 and an AUC0–24/MIC ratio of 16. Other quinolones may not have produced this resistance because the AUC0–24/MIC either was outside the range of 10 to 50 or was close to its outer limits. However, further studies are required before these conclusions can be confirmed.
Simple time-kill experiments also suggest that the ability to select for fluoroquinolone-resistant strains of B. fragilis at an inoculum of 106 CFU/ml could be due to the pharmacodynamic properties of the in vitro model used in the present study, in which fresh medium was continuously pumped into the chemostat and replaced the used medium at a rate consistent with the rate of elimination of the fluoroquinolone from the human body. Simple time-kill experiments performed anaerobically by exposing B. fragilis ATCC 23745 at an inoculum of 106 CFU/ml to sparfloxacin at 1 and 2 μg/ml in 50 ml of Anerobe Broth MIC over 24 h revealed no resistant strains at 24 h.
Studies of the selection of mutant B. fragilis strains showed a correlation between the frequency of mutation and the fluoroquinolone’s efficacy only for clinafloxacin. The fact that no relationship was found could be due to the similarities in the efficacy profiles of the fluoroquinolones studied, as demonstrated by similar times to reductions of the viable count by 3 log units, which then produced similar rates of mutation. Replicate studies need to be performed to provide an average frequency of mutation for each fluoroquinolone before final conclusions can be made. However, frequencies of mutation did coincide with those in another report (22) which showed selection of mutant B. fragilis NCTC 9343 with decreased susceptibility to ciprofloxacin, moxifloxacin, and trovafloxacin on Wilkins-Chalgren agar containing 2× the MIC of each agent at a frequency of approximately 2 × 10−10.
Thus far, little information is available regarding B. fragilis and possible mechanisms of fluoroquinolone resistance (1, 21). A recently published paper by Miyamae et al. (13) described the possibility that active efflux of norfloxacin by norfloxacin-resistant B. fragilis ATCC 25285 plays a role in fluoroquinolone resistance. Those investigators suggest that the phenotype involved in the fluoroquinolone efflux process in B. fragilis resembles that produced by the NorA- and Bmr-type transporter in gram-positive bacteria, which confers strong resistance to fluoroquinolones, chloramphenicol, cationic dyes, and puromycin but no increased resistance to β-lactams, erythromycin, or tetracycline (2, 15, 16). The norfloxacin-resistant strain B. fragilis ATCC 25285 was resistant to norfloxacin, ofloxacin, ethidium bromide, puromycin, and cetyltrimethylammonium bromide, but no changes in the MICs of sparfloxacin, cefoxitin, chloramphenicol, tetracycline, and erythromycin were detected (13). Since the NorA and Bmr multidrug efflux pump is known to be inhibited by reserpine (15, 16), the investigators also examined its effect on the susceptibility of B. fragilis to norfloxacin, and an increase in susceptibility (the MIC went from 16 to 8 μg/ml) was reported for the three strains tested (13). In the present study, the role of active fluoroquinolone efflux by sparfloxacin-resistant B. fragilis was investigated by performing susceptibility tests in the presence of reserpine. Results for mutant B. fragilis revealed single-dilution decreases in the MICs of clinafloxacin, levofloxacin, and sparfloxacin for the strain after exposure to sparfloxacin and single-dilution decreases in the MICs of trovafloxacin and ciprofloxacin in strains of B. fragilis both before and after exposure to sparfloxacin. These small increases in the susceptibility of B. fragilis following the addition of reserpine suggest that efflux may play a small role in resistance but cannot account for the four- to eightfold increases in the MICs of fluoroquinolones following a one-time exposure of B. fragilis ATCC 23745 to sparfloxacin. Since all changes in the MICs were within a single dilution, which is not considered significant when interpreting the results, repeat studies are needed before definitive conclusions can be made. However, Ricci and Piddock (22) also showed that reserpine has no effect on the activity of trovafloxacin, clinafloxacin, moxifloxacin, gatifloxacin, tetracycline, chloramphenicol, or cefoxitin against B. fragilis NCTC 9343. The mechanism of fluoroquinolone resistance also appears unlikely to be efflux because no cross-resistance was observed when the susceptibilities to unrelated antimicrobial agents (metronidazole, cefoxitin, chloramphenicol, and clindamycin) were determined for B. fragilis ATCC 23745 after exposure to sparfloxacin.
Since the bactericidal activities of fluoroquinolones have been attributed to the inhibition of DNA gyrase (6, 10) and alterations in gyrA have continued to be the most reported cause of resistance of gram-negative and gram-positive organisms to fluoroquinolones (20), a mutation in gyrA with or without a mutation in parC is suspected to be responsible for the four- to eightfold increases in the MICs of fluoroquinolones for B. fragilis ATCC 23745 following exposure to sparfloxacin. Preliminary results by Ricci and Piddock (22) suggest that a mutation in gyrA plays a role in the resistance of B. fragilis to fluoroquinolones. Although, Ricci and Piddock (22) have not shown that fluoroquinolone resistance in B. fragilis is caused solely by the observed mutation in gyrA, the identity between the gyrA sequences of Escherichia coli and B. fragilis over the QRDR of the gene and the similar substitutions observed in the resistant mutants strongly suggest that the mechanisms of quinolone resistance for the two strains are identical (22). Only through further exploration into the determination of the DNA sequences of the gyrA QRDRs of both B. fragilis ATCC 23745 before exposure to sparfloxacin and B. fragilis ATCC 23745 after exposure to sparfloxacin can these predicted findings be confirmed.
An in vitro pharmacodynamic evaluation of the factors associated with the development of resistant B. fragilis is being completed to determine if a relationship between pharmacodynamic outcome parameters such as AUC0–24/MIC or Cmax/MIC and resistance exists. These findings may provide information regarding whether underdosing of fluoroquinolones in the treatment of intra-abdominal or gynecologic infections may predispose patients to the development of infections with resistant B. fragilis. Ultimately, the significance of this phenomenon and the future potential for the development of resistance will become clearer when fluoroquinolones are more routinely used to treat anaerobic infections.
ACKNOWLEDGMENTS
This work was supported by a grant from Pfizer Inc., Groton, Conn.
We thank Laura J. V. Piddock and Vito Ricci for assistance.
REFERENCES
- 1.Acar J F, Goldstein F W. Trends in bacterial resistance to fluoroquinolones. Clin Infect Dis. 1997;24(Suppl. 1):S67–S73. doi: 10.1093/clinids/24.supplement_1.s67. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed M, Borsch C M, Neyfakh A A, Schuldiner S. Mutants of the Bacillus subtilis multidrug transporter Bmr with altered sensitivity to the antihypertensive alkaloid reserpine. J Biol Chem. 1993;268:11086–11089. [PubMed] [Google Scholar]
- 3.Aldridge K E. Increased activity of a new chlorofluoroquinolone, BAY y3118, compared with activities of ciprofloxacin, sparfloxacin, and other antimicrobial agents against anaerobic bacteria. Antimicrob Agents Chemother. 1994;38:1671–1674. doi: 10.1128/aac.38.7.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borobio M V, Conejo M D C, Ramirez E, Suarez A I, Parea E J. Comparative activities of eight quinolones against members of the Bacteriodes fragilis group. Antimicrob Agents Chemother. 1994;38:1442–1445. doi: 10.1128/aac.38.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Citron D M, Appleman M D. Comparative in vitro activities of trovafloxacin (CP-99,219) against 211 aerobic and 217 anaerobic bacteria strains from patients with intra-abdominal infections. Antimicrob Agents Chemother. 1997;41:2312–2316. doi: 10.1128/aac.41.10.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gellert M, Mizuuchi K, O’Dea M H, Itoh T, Tomizawa J. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci USA. 1977;74:4722–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein E J. Possible role for the new fluoroquinolones (levofloxacin, grepafloxacin, trovafloxacin, clinafloxacin, sparfloxacin, and DU-6859a) in the treatment of anaerobic infections: review of current information on efficacy and safety. Clin Infect Dis. 1996;23(Suppl. 1):S25–S30. doi: 10.1093/clinids/23.supplement_1.s25. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein E J. Patterns of susceptibility to fluoroquinolones among anaerobic bacterial strains in the United States. Clin Infect Dis. 1993;16(Suppl. 4):S377–S381. doi: 10.1093/clinids/16.supplement_4.s377. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein E J. Comparative activity of ciprofloxacin, ofloxacin, sparfloxacin, temafloxacin, CI-960, CI-990, and WIN 57273 against anaerobic bacteria. Antimicrob Agents Chemother. 1992;36:1158–1162. doi: 10.1128/aac.36.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Hoffman S, editor. Wadsworth anaerobic bacteriology manual. 5th ed. Belmont, Calif: Star Publishing Co.; 1993. [Google Scholar]
- 10.Hooper D C, Wolfson J S, Ng E Y, Swartz M N. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987;82(Suppl. 4A):12–20. [PubMed] [Google Scholar]
- 10a.Isenberg H D (ed. in chief). Clinical microbiology procedures handbook. Washington, D.C: American Society for Microbiology; 1992. [Google Scholar]
- 11.Kelley-Madaras K J, Ostergaard B E, Hovde L B, Rotschafer J C. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1996;40:627–632. doi: 10.1128/aac.40.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacGowan A P, Bowker K E, Holt H A, Wootton M, Reeves D S. Bay 12-8039, a new 8-methoxy-quinolone: comparative in-vitro activity with nine other antimicrobials against anaerobic bacteria. J Antimicrob Chemother. 1997;40:503–509. doi: 10.1093/jac/40.4.503. [DOI] [PubMed] [Google Scholar]
- 13.Miyamae S, Nikaido H, Yoshinobu T, Yoshimura F. Active efflux of norfloxacin by Bacteroides fragilis. Antimicrob Agents Chemother. 1998;42:2119–2121. doi: 10.1128/aac.42.8.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neu H C, Chin N X. In vitro activity of the new fluoroquinolone CP-99,219. Antimicrob Agents Chemother. 1994;38:2615–2622. doi: 10.1128/aac.38.11.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neyfakh A A. The multidrug efflux transporter of Bacillus subtilis is a structural and functional homolog of the Staphylococcus NorA protein. Antimicrob Agents Chemother. 1992;36:484–485. doi: 10.1128/aac.36.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neyfakh A A, Bidnenko V E, Chen L B. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc Natl Acad Sci USA. 1991;88:4781–4785. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols R L, Smith J W. Wound and intraabdominal infections: microbiological considerations and approaches to treatment. Clin Infect Dis. 1993;16:S266–S272. doi: 10.1093/clinids/16.supplement_4.s266. [DOI] [PubMed] [Google Scholar]
- 17a.Peterson M L, Hoang A D, Raddatz J-K, Horde L B, Rotschafer J C. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Stable fluoroquinolone (FQ) class resistance seen with Bacteroides fragilis (BF) after exposure to sparfloxacin (S), abstr. E-169; p. 144. [Google Scholar]
- 18.Peterson M L, Hovde L B, Wright D H, Hoang A D, Rotschafer J C. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Trovafloxacin and levofloxacin resistance in Bacteroides fragilis, abstr. E32; p. 176. [Google Scholar]
- 19.Peterson M L, Hovde L B, Wright D H, Hoang A D, Rotschafer J C. Program of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Pharmacodynamic outcome parameters as predictors for fluoroquinolones in the treatment of anaerobic infections, abstr. A-85; p. 52. [Google Scholar]
- 20.Piddock L J V. Mechanisms of resistance to fluoroquinolones: state-of-the-art 1992–1994. Drugs. 1995;49(Suppl. 2):29–35. doi: 10.2165/00003495-199500492-00006. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen B A, Bush K, Tally F P. Antimicrobial resistance in anaerobes. Clin Infect Dis. 1997;24(Suppl. 1):S110–S120. doi: 10.1093/clinids/24.supplement_1.s110. [DOI] [PubMed] [Google Scholar]
- 22.Ricci V, Piddock L J V. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Characterization of the quinolone resistance determining regions of gyrA of Bacteroides fragilis and their role in fluoroquinolone resistance, abstr. C180; p. 121. [Google Scholar]
- 23.Spangler S K, Jacobs M R, Appelbaum P C. Time-kill study of the activity of trovafloxacin compared with ciprofloxacin, sparfloxacin, metronidazole, cefoxitin, piperacillin/tazobactam against six anaerobes. J Antimicrob Chemother. 1997;39(Suppl. B):23–27. doi: 10.1093/jac/39.suppl_2.23. [DOI] [PubMed] [Google Scholar]
- 24.Spangler S K, Jacobs M R, Appelbaum P C. Activity of CP 99,219 compared with those of ciprofloxacin, grepafloxacin, metronidazole, cefoxitin, piperacillin, and piperacillin-tazobactam against 489 anaerobes. Antimicrob Agents Chemother. 1994;38:2471–2476. doi: 10.1128/aac.38.10.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas J K, Forrest A, Bhavnani S M, Hyatt J M, Cheng A, Ballow C H, Schentag J J. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother. 1998;42:521–527. doi: 10.1128/aac.42.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weigelt J A. Proceedings of the 8th International Congress on Infectious Diseases. 1998. Role of fluoroquinolones in surgical infections, abstr. 81.004. [Google Scholar]
- 27.Zabinski R A, Larsson A J, Walker K J, Gilliland S S, Rotschafer J C. Elimination of quinolone antibiotic carryover through use of antibiotic-removal beads. Antimicrob Agents Chemother. 1993;37:1377–1379. doi: 10.1128/aac.37.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zabinski R A, Vance-Bryan K, Krinke A J, Walker K J, Moody J A, Rotschafer J C. Evaluation of the activity of temafloxacin versus Bacteroides fragilis using an in vitro pharmacodynamic system. Antimicrob Agents Chemother. 1993;37:2454–2458. doi: 10.1128/aac.37.11.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]