Key Points
Two doses of VLDRT at 2 Gy are associated with an impressive overall response rate of 90% across diverse indolent B-cell lymphomas.
Patients with early-stage, potentially[AQ4] curable disease had outcomes similar to those treated with 4 Gy as part of a novel RT strategy.
Visual Abstract
Abstract
Radiotherapy plays an important role in managing highly radiosensitive, indolent non-Hodgkin lymphomas, such as follicular lymphoma and marginal zone lymphoma. Although the standard of care for localized indolent non-Hodgkin lymphomas remains 24 Gy, de-escalation to very-low-dose radiotherapy (VLDRT) of 4 Gy further reduces toxicities and duration of treatment. Use of VLDRT outside palliative indications remains controversial; however, we hypothesize that it may be sufficient for most lesions. We present the largest single-institution VLDRT experience of adult patients with follicular lymphoma or marginal zone lymphoma treated between 2005 and 2018 (299 lesions; 250 patients) using modern principles including positron emission tomography staging and involved site radiotherapy. Outcomes include best clinical or radiographic response between 1.5 and 6 months after VLDRT and cumulative incidence of local progression (LP) with death as the only competing risk. After VLDRT, the overall response rate was 90% for all treated sites, with 68% achieving complete response (CR). With a median follow-up of 2.4 years, the 2-year cumulative incidence of LP was 25% for the entire cohort and 9% after first-line treatment with VLDRT for potentially curable, localized disease. Lesion size >6 cm was associated with lower odds of attaining a CR and greater risk of LP. There was no suggestion of inferior outcomes for potentially curable lesions. Given the clinical versatility of VLDRT, we propose to implement a novel, incremental, adaptive involved site radiotherapy strategy in which patients will be treated initially with VLDRT, reserving full-dose treatment for those who are unable to attain a CR.
Introduction
Radiotherapy (RT) is an integral component of the management of indolent non-Hodgkin lymphomas (iNHLs) such as follicular lymphoma (FL) and marginal zone lymphoma (MZL). The foundation of treating iNHL with RT was established before noninvasive staging or effective systemic therapies, which necessitated treatment to broad fields using high doses.1 Although disease management has evolved, RT remains a highly effective tool for treatment and is supported by guidelines for curative and palliative indications.2,3
Given the long natural history of iNHL, reducing treatment-associated toxicities is paramount. For RT, this has been accomplished through better RT delivery techniques, volume reductions from extended field to involved field radiotherapy (IFRT) to involved site radiotherapy (ISRT),4,5 and through dose de-escalation. The first major systematic dose reduction occurred after the British National Lymphoma Investigation study,6 which randomly assigned 361 indolent sites (mostly in FL or MZL) to receive 24 Gy or 40 to 45 Gy. Patients treated with 24 Gy had no differences in response or local control and significantly less dermatitis, which established a new standard. Modern positron emission tomography (PET)–guided RT for localized FL is associated with excellent control of local and overall disease.7 Furthermore, most patients with localized disease who relapse after definitive RT can achieve a successful outcome with salvage therapy.8
For many, particularly those who receive palliative care, further dose reduction is reasonable. It has been established that iNHL can be remarkably radiosensitive; for example, patients with advanced-stage disease can achieve sustained responses to just 1.5 to 2.5 Gy total body irradiation.9-13 The now common very-low-dose radiotherapy (VLDRT) regimen of 2 Gy × 2 fractions (total dose of 4 Gy) matured from these observations and early clinical experience.14 The first series of 48 patients demonstrated an excellent overall response rate (ORR) of 81% and a complete response (CR) rate of 57%, with median freedom from local progression (LP) over 4 years.15 Numerous subsequent reports16-22 confirmed efficacy with CR rates of 36% to 84% across heterogeneous contexts. The benefits of VLDRT to patients are clear; fewer RT sessions reduce clinical and financial toxicities.23
Encouraging data motivated the phase 3 Follicular Radiotherapy Trial (FoRT), which randomly assigned 614 FL or MZL sites from 548 patients to receive 24 Gy or 4 Gy.24,25 Initial inclusion criteria limited the study to only those patients who required palliation, but the criteria were expanded to include curative intent. The primary outcome was time to LP using a noninferiority design. With a median follow-up of 6.2 years, VLDRT was inferior to 24 Gy (hazard ratio [HR] of LP, 3.5; P < .0001) in both the curative and palliative settings. However, there was no significant difference in overall survival (OS) and similar proportions of patients who subsequently required systemic therapy.25 The authors advocate that 24 Gy remains standard of care when durable local control is paramount, and 4 Gy should be limited to palliative treatment.
Broader use of VLDRT thus remains controversial, particularly for curative intent. Critics of an expanded role cite clearly inferior local progression-free survival. Proponents acknowledge that while 24 Gy produces more durable responses, 70% of sites treated with VLDRT remain controlled at 5 years.25 FL and MZL are indolent diseases of the elderly, and no differences in OS suggest that salvage treatment can be used successfully for patients who experience treatment failure after VLDRT.26
At Memorial Sloan Kettering Cancer Center, we support the latter interpretation, which hypothesizes that 4 Gy is sufficient for most lesions and that additional RT may constitute overtreatment. We increasingly integrate VLDRT into an adaptive RT program, in which potential patients are offered VLDRT with short-interval PET/computed tomography (CT) scanning after robust informed discussion. Early evaluation informs whether 4 Gy is sufficient or whether additional ISRT should be offered. In our experience, given the indolence of the disease, few curable patients progress in the short interval after 4 Gy and previous reassessment, which supports the suitability of an incremental strategy. Because there are no firmly established clinicodemographic predictors of improved response to VLDRT, we offer this program broadly, including to select curable patients with localized iNHL.
Following this strategy, we have analyzed what is (to the best of our knowledge) the largest single-institutional VLDRT experience. Importantly, this series incorporates expert pathology review, use of ISRT, and a large proportion of patients with PET staging, making it highly relevant for modern practice. Specific attention is paid to those treated with curative intent.
Methods
Patient selection
The study received approval from the Memorial Sloan Kettering Institutional Review Board (#16-1512) and was conducted in accordance with the Declaration of Helsinki. After Institutional Review Board approval, we retrospectively analyzed adults treated with VLDRT. Patients who received 4 Gy before transplant total body irradiation were excluded. All had biopsy-confirmed mature B-cell lymphomas reviewed by an expert hematopathologist, including FL (grade 1-3A), MZL, or low-grade disease indistinguishable from FL and MZL and not otherwise specified. Lesions that had been completely excised were excluded.
VLDRT characteristics
The VLDRT treatment approach was determined by lesion anatomy and size. Our practice generally incorporates PET-based simulation. Patients who received VLDRT were treated with ISRT principles defined by International Lymphoma Radiation Oncology Group (ILROG) guidelines for nodal,27 extranodal,28 and cutaneous29 NHL. Patients received nonstandardized surveillance after VLDRT; reassessment was typically 8 to 12 weeks after VLDRT.
Study end points and statistics
The primary end point was incidence of LP, defined as radiographic or clinical relapse within the VLDRT treatment field. The primary statistical method was cumulative incidence of LP analyzed per lesion with death as a competing risk. We elected to consider death as the only competing risk to maximize analyzable events in a real-world setting and for comparability to the primary FoRT outcomes, which did not censor subsequent systemic therapy.24,25 Given the possibility that early deaths or initiation of systemic therapy before LP might influence cumulative incidence of local failures, we compared this analysis with a Kaplan-Meier analysis of LP, censoring for death or receipt of systemic therapy after VLDRT. This additional statistical approach also aimed to assess the robustness of our findings using competing risk analysis and to allow comparability to the secondary analysis from the long-term FoRT update, which did censor sites at the time they received additional therapy before local failure.25
Outcomes were analyzed for the full cohort and stratified into 2 subgroups by VLDRT treatment intent. Potentially curative lesions were defined as localized (stage I-II) sites in patients without previous lymphoma-directed treatment, treated comprehensively with VLDRT. Other lesions, including advanced-stage or status after previous treatments (even if localized at VLDRT) were considered non-curable intent. Univariable and multivariable competing risk regressions were used to identify potential associations with LP. These results were compared with univariable and multivariable Cox regression analysis of LP censoring for death and systemic therapy. Follow-up was estimated by using a reverse Kaplan-Meier method.
The secondary end point was best response after VLDRT (clinical or radiographic using Lugano criteria) analyzed per lesion.30 ORR includes CR or partial response (PR). A multivariable logistic regression model was constructed using lesion size, histology, extent of previous systemic therapy, lesion site, lesion type, standardized uptake value (SUV), and treatment intent to identify associations with CR between 1.5 and 6 months. After 6 months, completely responding lesions were deemed to be successfully treated, and patients would not be likely to have subsequent therapy recommended for them. Given the variability in time to response assessments, associations between best response and LP were analyzed by using a landmark analysis for 6 months after VLDRT.
Additional end points included distant progression (DP) outside the VLDRT field and composite overall progression (OP) comprising LP, DP, any additional RT, or systemic therapy. Pathological confirmation after VLDRT relapse was not required, except for establishing transformation to diffuse large B-cell lymphoma (DLBCL). OS was estimated by using the Kaplan-Meier method. Cumulative incidence of DP, OP, receipt of additional treatment, and transformation to DLBCL were analyzed per patient controlling for competing risk of death.
Given the possibility that previous exposure to systemic therapy could theoretically influence the efficacy of VLDRT, we also performed a subset analysis of the patients who received no previous systemic therapy. For this subset, we evaluated LP and OP after VLDRT response as well as cumulative incidence of LP and OP. Comparisons of cumulative incidence and Kaplan-Meier curves between lesions and patients treated with curative or palliative intent were assessed by using Gray’s test and log-rank tests, respectively. All time-to-event analyses were measured from the start of VLDRT. Statistical tests were two-sided, and P values of < .05 were considered significant. Statistics were performed using R version 4.0.1.31
Results
Patient characteristics
A total of 299 sites from 250 adult patients treated with VLDRT between 2005 and 2018 were included. Patient characteristics are summarized in Table 1. Heterogeneity among patients was reflective of a mix of limited and advanced stages, and 10% of patients received VLDRT for retrograde transformed DLBCL. Bone marrow biopsy was available for 147 patients (59%) of whom 42 (29%) were positive.
Table 1.
Patient characteristics
| Characteristic | No. (% [frequency]) |
|---|---|
| Total No. of patients | 250 |
| Sex | |
| Male | 127 (51) |
| Female | 123 (49) |
| Age at first VLDRT, y | |
| Median (IQR) | 67 (57-76) |
| Minimum-maximum | 20-94 |
| Original lymphoma stage | |
| I | 71 (29) |
| II | 30 (12) |
| III | 35 (14) |
| IV | 82 (33) |
| Localized (cutaneous) | 29 (12) |
| Multifocal (cutaneous) | 2 (0.8) |
| Unknown | 1 |
| Bone marrow biopsy at initial diagnosis | |
| Negative | 105 (71) |
| Positive | 42 (29) |
| Unknown | 103 |
| Previous diagnosis of DLBCL or aggressive lymphoma | 25 (10) |
| Exposure to systemic therapy before VLDRT | |
| No. of previous lines of systemic therapy | |
| 0 | 114 (46) |
| 1 | 57 (23) |
| 2-4 | 70 (28) |
| >4 | 9 (3.6) |
| Previous rituximab | 95 (38) |
| Previous chemotherapy or chemoimmunotherapy | 85 (34) |
| No. of previous transplantations | |
| 0 | 245 (98) |
| 1 | 4 (1.6) |
| 2 | 1 (0.4) |
IQR, interquartile range.
Characteristics of treated sites
Table 2 provides descriptive statistics per lesion overall (n = 299) and stratified by treatment intent as either non-curable (n = 247; 83%) or potentially curable (n = 52; 17%). Nearly one third (29%) received VLDRT as first-line treatment. Histologic distribution was 66% FL, 27% MZL, and 7% low-grade not otherwise specified. VLDRT was used most often for limited-stage disease, either stage I and localized (40%) or stage II (24%). Most sites (83%) were PET staged, of which 34 lesions (14%) were either non-avid or with SUV similar to background. Of the remaining 217 lesions, median pre-VLDRT maximum SUV was 7.3 (range, 0.7-27.0).
Table 2.
Lesion characteristics
| Characteristic | Overall (N = 299) |
Intent of VLDRT | |
|---|---|---|---|
| Non-curable (n = 247) |
Potentially curable (n = 52) | ||
| Previously observed site | 67 (22) | 63 (26) | 4 (7.7) |
| Previous excision to same site | |||
| No | 288 (96) | 236 (95) | 52 (100) |
| Yes | 11 (4) | 11 (5) | 0 (0) |
| VLDRT given to newly diagnosed patient | 88 (29) | 36 (15) | 52 (100) |
| Histology at VLDRT | |||
| FL | 197 (66) | 177 (72) | 20 (38) |
| MZL | 80 (27) | 57 (23) | 23 (44) |
| Low-grade indolent NOS | 22 (7.4) | 13 (5.3) | 9 (17) |
| Stage at time of VLDRT | |||
| I | 99 (33) | 73 (30) | 26 (50) |
| II | 72 (24) | 66 (27) | 6 (12) |
| III | 43 (14) | 43 (17) | 0 (0) |
| IV | 63 (21) | 63 (26) | 0 (0) |
| Localized (cutaneous) | 20 (6.7) | 0 (0) | 20 (38) |
| Multifocal (cutaneous) | 2 (0.7) | 2 (0.8) | 0 (0) |
| Modality of staging at time of VLDRT | |||
| PET/CT | 249 (83) | 199 (81) | 50 (96) |
| CT | 32 (11) | 32 (13) | 0 (0) |
| MRI | 6 (2.0) | 4 (1.6) | 2 (3.8) |
| Mammogram | 2 (0.7) | 2 (0.8) | 0 (0) |
| Clinical | 10 (3.3) | 10 (4.0) | 0 (0) |
| Median pre-VLDRT lesion size, cm* | 2.55 (1.8, 4.1) |
2.60 (1.9, 4.2) |
2.10 (1.2, 3.0) |
| Pre-VLDRT lesion size, cm † | |||
| 0-2 | 79 (30) | 63 (28) | 16 (39) |
| 2-4 | 115 (44) | 94 (42) | 21 (51) |
| 4-6 | 47 (18) | 45 (20) | 2 (4.9) |
| ≥6 | 23 (8.7) | 21 (9.4) | 2 (4.9) |
| Unknown | 35 | 24 | 11 |
| Nodal characteristics of treated fields | |||
| Extranodal | 163 (55) | 123 (50) | 40 (77) |
| Nodal | 122 (41) | 111 (45) | 11 (21) |
| Both | 14 (4.7) | 13 (5.3) | 1 (1.9) |
| Site category | |||
| Skin | 51 (17) | 31 (13) | 20 (38) |
| Head and neck (nodal) | 43 (14) | 41 (17) | 2 (3.8) |
| Eye and orbit | 29 (9.7) | 21 (8.5) | 8 (15) |
| Inguinofemoral | 29 (9.7) | 27 (11) | 2 (3.8) |
| Other | 147 (49) | 127 (51) | 20 (38) |
| Median pre-VLDRT PET highest SUV (IQR) * ‡ | 7.3 (5.0-10.5) |
7.8 (5.2-10.8) |
5.0 (3.0-8.1) |
| Pre-VLDRT PET highest SUV † | |||
| 0-5 | 53 (21) | 39 (19) | 14 (29) |
| 5-7.5 | 58 (23) | 50 (25) | 8 (16) |
| 7.5-10 | 41 (16) | 37 (18) | 4 (8.2) |
| 10-15 | 56 (22) | 53 (26) | 3 (6.1) |
| ≥15 | 9 (3.6) | 8 (4.0) | 1 (2.0) |
| Unspecified but less than liver background | 34 (14) | 15 (7.4) | 19 (39) |
| Unknown | 48 | 45 | 3 |
Data are presented as n (% [frequency]) unless otherwise stated.
MRI, magnetic resonance imaging; NOS, not otherwise specified.
Continuous variable.
Categorical variable.
A total of 82 lesions had unspecified or unknown SUV.
Sites treated with VLDRT had significant anatomic diversity. Median pre-VLDRT maximum diameter was 2.6 cm (range, 0.1-12.5 cm), and 9% were ≥6 cm. Treated sites were extranodal (55%), nodal (41%), or both (5%). Common extranodal sites included cutaneous (17%) and orbit (9.7%), whereas common nodal sites included head and neck (14%) and inguinofemoral (9.7%). A complete breakdown of anatomic sites is provided in supplemental Table 1.
We identified 50 potentially curable patients with treatment to 52 sites. Lesions were treatment naïve (8% were observed before VLDRT) and were comprehensively encompassed within VLDRT fields. Of 52 lesions, 96% were PET/CT staged as stage I (50%), stage II (12%), or cutaneous with localized disease (38%) (Table 2). Like lesions in the full cohort, the majority of curable lesions were extranodal, with leading sites being cutaneous (n = 20; 38%), orbit (n = 8; 15%), and parotid (n = 6; 12%). Other extranodal sites included breast, duodenum, esophagus, and vulva. Compared with non-curable lesions, potentially curable sites had a greater nominal composition of MZL (44% vs 23%) and tended to be smaller (median maximum diameter of 2.1 cm vs 2.6 cm) and less avid (median maximum SUV of 5.0 vs 7.8).
Treatment history before VLDRT
Before VLDRT, 54% of patients had received at least 1 cycle of systemic therapy, including rituximab monotherapy (n = 95; 38%) or chemotherapy or chemoimmunotherapy (n = 85; 34%) (Table 1). We considered oral steroids (or topical or injectable preparations for cutaneous lesions) as a line of systemic therapy before VLDRT. In total, 13 sites were treated with some formulation of steroids before VLDRT. An additional 7 sites received steroids in addition to rituximab. Four percent of lesions were previously excised; all patients had residual or recurrent disease. Overall, 22% of lesions were observed before VLDRT, either after initial diagnosis or after relapse.
VLDRT response
The optimal interval for assessing response after VLDRT has not been established. Table 3 shows the best clinical and radiographic responses within the interval of 1.5 to 6 months after VLDRT. There were 227 evaluable sites with available imaging that met criteria (76%), and best overall response was recorded if there were multiple assessments.
Table 3.
Best response for lesions at 1.5 to 6 months after VLDRT
| Best response | Overall (N = 227) |
Intent of VLDRT | Lesion histology | Nodal description | Site description | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-curable (n = 181) |
Potentially curable (n = 46) |
FL (n = 144) |
MZL (n = 65) |
Low-grade indolent (n = 18) |
Nodal (n = 90) |
Extranodal or both (n = 137) |
Cutaneous (n = 41) |
Non-skin (n = 186) |
||
| CR | 154 (68) | 117 (65) | 37 (80) | 95 (66) | 47 (72) | 12 (67) | 56 (62) | 98 (72) | 35 (85) | 119 (64) |
| PR | 49 (22) | 42 (23) | 7 (15) | 34 (24) | 11 (17) | 4 (22) | 20 (22) | 29 (21) | 5 (12) | 44 (24) |
| SD | 13 (5.7) | 11 (6.1) | 2 (4.3) | 8 (5.6) | 4 (6.2) | 1 (5.6) | 7 (7.8) | 6 (4.4) | 1 (2.4) | 12 (6.5) |
| PD | 11 (4.8) | 11 (6.1) | 0 (0) | 7 (4.9) | 3 (4.6) | 1 (5.6) | 7 (7.8) | 4 (2.9) | 0 (0) | 11 (5.9) |
Data are n (%).
PD, progressive disease; SD, stable disease.
Most lesions were reassessed by PET/CT (n = 155; 68%), CT (n = 17; 7%), or clinically (n = 44; 19%), largely for cutaneous sites. Composite ORR was 90% (n = 227; CR, 68%; PR, 22%). Overall, relatively few lesions (5%) progressed through VLDRT, of which the majority (57%) had concurrent out-of-field progressive disease (PD). No significant differences in best response were observed when stratifying the cohort by histology (P > .9) or treatment intent (P = .2). Response distribution was similar using intervals of either 1.5 to 3 months or 1.5 to 12 months. Multivariable logistic regression for associations with achievement of CR identified size as significant (supplemental Table 2). Compared with lesions <6 cm, lesions ≥6 cm were less likely to achieve CR (odds ratio, 0.17; 95% confidence interval [CI], 0.04-0.65; P = .013).
Local control after VLDRT
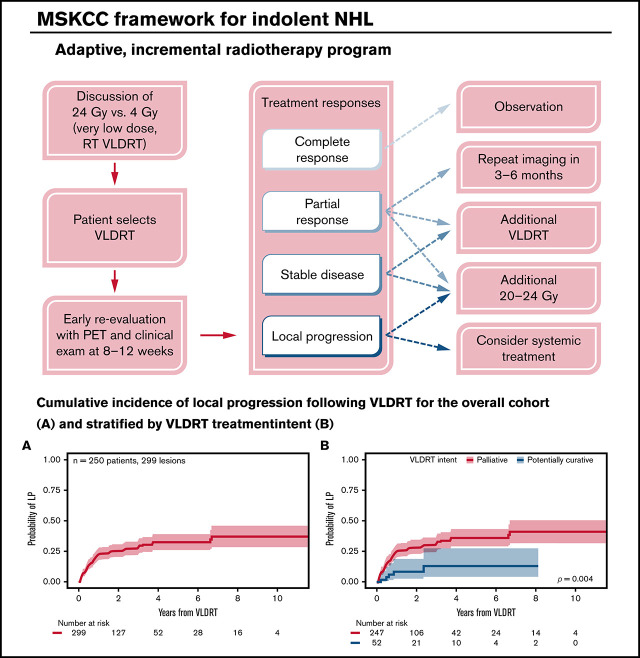
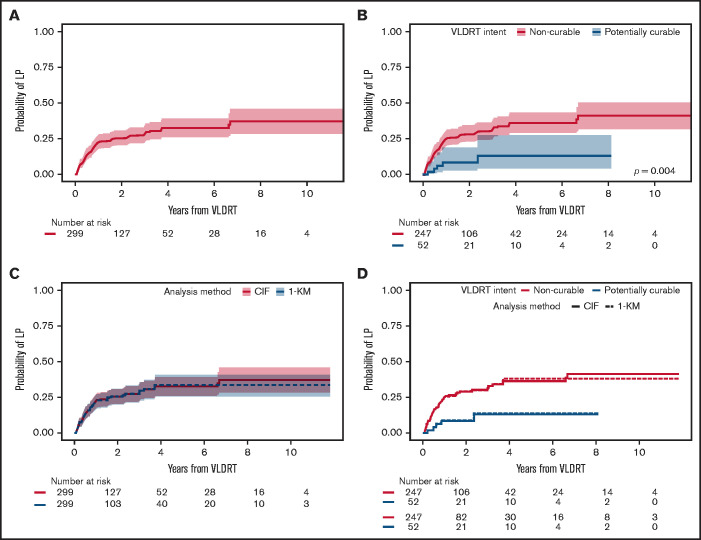
Median follow-up was 2.4 years (95% CI, 2.2-3.1 years) for the overall cohort, 2.5 years (95% CI, 2.3-3.5 years) for the non-curable subgroup, and 1.7 years (95% CI, 1.2-3.5 years) for the potentially curable subgroup. VLDRT was well-tolerated without any serious toxicities. In total, 81 (27%) of 299 sites developed LP. The 1-year cumulative incidence of LP was 22% (95% CI, 18%-27%), 2-year was 25% (95% CI, 20%-31%), and 5-year was 33% (95% CI, 26%-39%) (Figure 1A). Patients in the potentially curable subgroup had considerably lower 2-year LP of 9% (95% CI, 3%-19%) compared with 29% (95% CI, 23%-34%) for patients in the non-curable subgroup (Figure 1B). When comparing analytic methods, we found that the probabilities of LP are visually similar irrespective of the approach: cumulative incidence with death as a competing risk vs Kaplan-Meier analysis censoring death and systemic therapy (Figure 1C-D).
Figure 1.
Cumulative incidence of local progression. Cumulative incidence (CIF) of LP assuming death as competing risk after treatment with VLDRT for the (A) overall cohort and (B) stratified by treatment intent with Gray’s test P value. Comparison of analytic methods for LP: CIF vs Kaplan-Meier (KM) curve censoring for death and start of systemic therapy (1-KM) after VLDRT for (C) the entire cohort and (D) stratified by intent of VLDRT.
Univariable competing risk regression identified several factors associated with LP (Table 4). To assess association between best response and subsequent LP, we performed a landmark analysis at 6 months after VLDRT. With 235 evaluable lesions, we found that not achieving CR by 6 months after VLDRT increased risk of LP (HR, 2.8; 95% CI, 1.5-5.1; P = .001). Next, we fit a multivariable competing risk regression model with stage at VLDRT and all statistically significant factors from univariable models. By multivariable modeling, larger lesion size and best response were the only significant factors (Table 5); lesions ≥6 vs <6 cm had significantly greater LP risk (HR, 4.7; 95% CI, 1.8-12.6; P = .002) as did a non-CR response (HR, 2.3; 95% CI, 1.1-5.1; P = .036). The estimated HR for extranodal vs nodal lesions was 0.57 (95% CI, 0.3-1.2). After adjusting for other factors, curable intent was not associated with a lower risk of LP. As a sensitivity analysis, we compared the univariable and multivariable competing risk regression analysis to the Cox regression analysis, which censored death and subsequent systemic therapy. The statistically significant factors in both univariable and multivariable models were largely the same, irrespective of approach (supplemental Tables 3 and 4). These findings suggest little difference between the 2 analytical methods.
Table 4.
Univariable competing risk regression models for LP
| Characteristic | No. | HR | 95% CI | P |
|---|---|---|---|---|
| Age at VLDRT (y) | 299 | 1.01 | 0.99-1.02 | .5 |
| Sex | 299 | |||
| Male | — | — | ||
| Female | 0.89 | 0.57-1.37 | .6 | |
| Histology at VLDRT | 299 | |||
| FL | — | — | ||
| MZL | 0.65 | 0.39-1.11 | .12 | |
| Low-grade indolent | 0.80 | 0.32-1.97 | .6 | |
| Best overall response* | 235 | |||
| CR | — | — | ||
| PR, SD, or PD | 2.75 | 1.48-5.10 | .001 | |
| Maximum SUV before VLDRT | 251 | |||
| 0-5 | — | — | ||
| 5-7.5 | 0.82 | 0.36-1.84 | .6 | |
| 7.5-10 | 0.68 | 0.27-1.73 | .4 | |
| 10-15 | 1.59 | 0.78-3.24 | .2 | |
| ≥15 | 1.30 | 0.36-4.74 | .7 | |
| Unspecified but less than liver background | 0.73 | 0.28-1.88 | .5 | |
| Stage at VLDRT | 299 | |||
| Early | — | — | ||
| Advanced | 1.20 | 0.77-1.87 | .4 | |
| Nodal status | 299 | |||
| Nodal | — | — | ||
| Extranodal | 0.59 | 0.38-0.94 | .024 | |
| Both | 1.36 | 0.57-3.26 | .5 | |
| Site category | 299 | |||
| Cutaneous | — | — | ||
| Non-cutaneous | 1.29 | 0.69-2.40 | .4 | |
| Pre-VLDRT lesion size (cm) | 264 | |||
| 0-6 | — | — | ||
| ≥6 | 5.53 | 3.23-9.48 | <.001 | |
| Intent of VLDRT | 299 | |||
| Non-curable | — | — | ||
| Potentially curable | 0.30 | 0.12-0.73 | .008 | |
| Previous No. of lines of systemic therapy | 299 | |||
| 0 | — | — | ||
| 1 | 1.95 | 1.06-3.57 | .031 | |
| 2-4 | 2.23 | 1.29-3.84 | .004 | |
| > 4 | 3.73 | 1.47-9.47 | .006 | |
| Previous rituximab | 299 | 1.71 | 1.10-2.65 | .017 |
Given the variability in time of best response assessment, this factor was assessed using a landmark analysis with landmark time at 6 months after VLDRT.
Table 5.
Multivariable competing risk regression model for LP
| Characteristic | HR | 95% CI | P |
|---|---|---|---|
| Nodal status | |||
| Nodal | — | — | |
| Extranodal | 0.57 | 0.28-1.16 | .12 |
| Both | 0.72 | 0.17-3.30 | .7 |
| Pre-VLDRT lesion size (cm) | |||
| 0-6 | — | — | |
| ≥6 | 4.69 | 1.75-12.62 | .002 |
| Intent of VLDRT | |||
| Non-curable | — | — | |
| Potentially curable | 0.73 | 0.17-3.11 | .7 |
| Previous No. of lines of systemic therapy | |||
| 0 | — | — | |
| 1 | 1.76 | 0.54-5.77 | .3 |
| 2-4 | 1.71 | 0.53-5.51 | .4 |
| > 4 | 1.15 | 0.11-12.04 | >.9 |
| Previous rituximab | 1.04 | 0.39-2.76 | >.9 |
| Stage at VLDRT | |||
| Limited | — | — | |
| Advanced | 0.78 | 0.35-1.75 | .6 |
| Best overall response | |||
| CR | |||
| PR, SD, or PD | 2.32 | 1.05-5.09 | .036 |
Risk of DP and OP
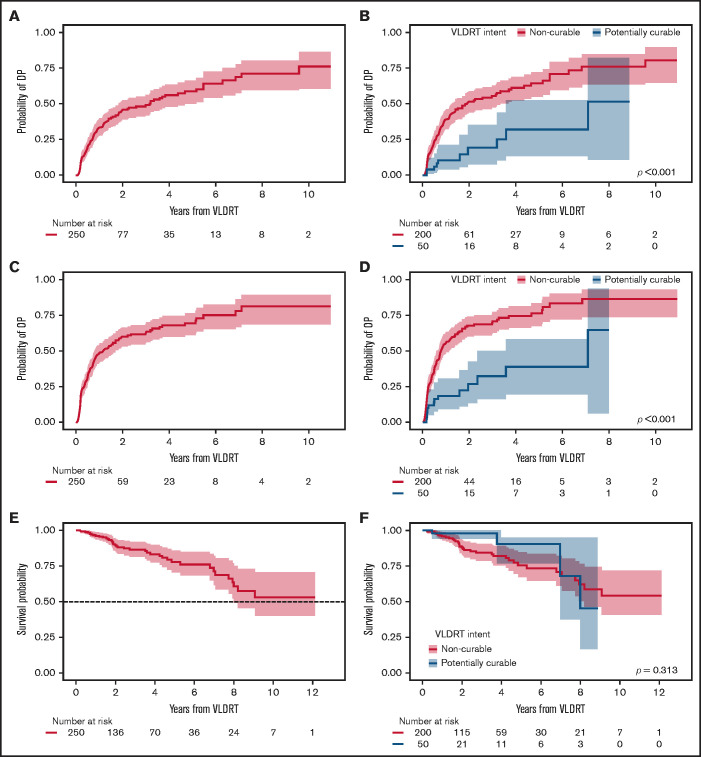
The cumulative incidence of DP increased most rapidly in the first year after VLDRT (34% [95% CI, 28%-40%] at 1 year and 46% [95% CI, 39%-53%] at 2 years) (Figure 2A). We observed significantly lower cumulative incidence of DP for potentially curable patients (P < .001; Figure 2B) with a 1-year probability of 11% (95% CI, 4%-21%) vs 39% (95% CI, 32%-46%) for patients in the non-curable subgroup.
Figure 2.
Additional post-VLDRT outcomes. Cumulative incidence of DP after VLDRT for (A) the overall cohort and (B) stratified by treatment intent and of OP after VLDRT for (C) the overall cohort and (D) stratified by treatment intent with Gray’s test P value. Kaplan-Meier estimates of OS after VLDRT for (E) the overall cohort and (F) stratified by treatment intent with log-rank test P value. Shading is 95% CI.
We next considered composite OP and found that 150 patients (60%) had an event and 6 died with no PD. Median OP-free survival was 1.0 years (95% CI, 0.8-1.7 years); cumulative OP incidence at 1 year after VLDRT was 49% (95% CI, 42%-55%); at 2 years, it was 60% (95% CI, 53%-66%) (Figure 2C). Just over half the patients (52%) had DP as their first OP event, followed by 37% with LP. Again, patients in the potentially curable group had significantly lower OP vs those in the non-curative group (P < .001; Figure 2D) with a 1-year rate of 19% (95% CI, 9%-31%) and a 2-year rate of 27% (95% CI, 13%-42%). The 2-year estimate of OS was 89% (95% CI, 85%-94%) and the 5-year estimate was 78% (95% CI, 70%-86%) (Figure 2E). OS distributions for patients with potentially curable vs non-curable disease were not significantly different (P = .3; Figure 2F), possibly owing to fewer definitively treated patients with sufficient follow-up.
Impact of previous systemic therapy on VLDRT efficacy
Given the heterogeneity among patients in exposure to systemic therapies before VLDRT and to better isolate the efficacy of VLDRT alone, we performed a subset analysis of the 114 patients (46%) who received no previous systemic therapy. This subset included 128 lesions (43%) with a greater share of potentially curable sites compared with the full cohort (41% vs 17%). Overall, the subset with no previous systemic therapy (ORR, 94% [77% CR]) seemed to have a response pattern similar to that for the full cohort (ORR, 90%). Acknowledging that the subset of patients with no previous systemic therapy had relatively few LP events (n = 21), the cumulative incidence curves seemed to show improvement compared with those for the full cohort (supplemental Figure 1). In addition, cumulative incidence of OP for the subset of patients with no previous systemic therapy was similar to that for the full cohort (supplemental Figure 2).
Additional treatments after VLDRT
Overall, 97 patients (39%) had additional RT and/or subsequent systemic therapy after VLDRT. Patients with potentially curable lesions were significantly less likely to receive additional therapy (P < .001). In total, 34 lesions (11%) were treated with systemic therapy after patients received VLDRT without first experiencing LP. Nine lesions (3%) treated with systemic therapy after VLDRT was received for DP subsequently developed LP.
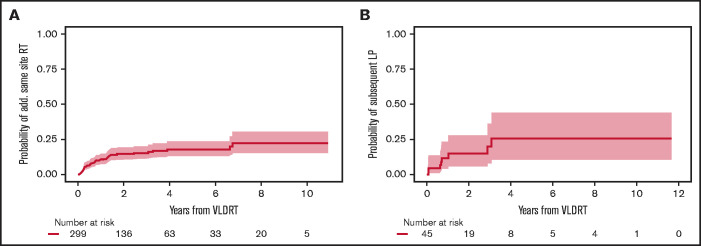
Additional RT was administered to 45 lesions (15%) treated with VLDRT for suboptimal response without intercurrent LP in the 6 months after VLDRT (n = 12; 4%), salvage therapy after LP (n = 30; 10%), or more delayed retreatment for marginal recurrence with overlap of the field previously treated with VLDRT (n = 3; 1%). Additional doses ranged from 4 to 50 Gy with the most common regimens being 4 Gy (n = 13; 29%), 24 Gy (n = 11; 24%), and 30 Gy (n = 11; 24%). The probability of receiving additional RT to the same site 2 years after VLDRT was 14.6% (95% CI, 10.6%-19.2%; Figure 3A). Subsequent response after additional RT was 68% CR (n = 30), 18% PR (n = 8), and 14% nonresponse (n = 6) with 1 lesion lost to follow-up. There were 8 lesions that progressed locally after subsequent RT; all had previously documented LP after VLDRT. The 2-year probability of subsequent LP was 15.0% (95% CI, 5.9%-28.0%; Figure 3B).
Figure 3.
Additional same site RT. Cumulative incidence of (A) receipt of additional RT and (B) subsequent LP after additional RT to same site after VLDRT. Shading is 95% CI.
There was a trend toward potentially curable lesions having a lower probability of receiving additional RT to a site previously treated with VLDRT (P = .06). For curable lesions that had suboptimal response or LP after VLDRT, re-treatment with additional RT was effective; 3 lesions (6%) received additional RT, and all resolved completely without documented LP.
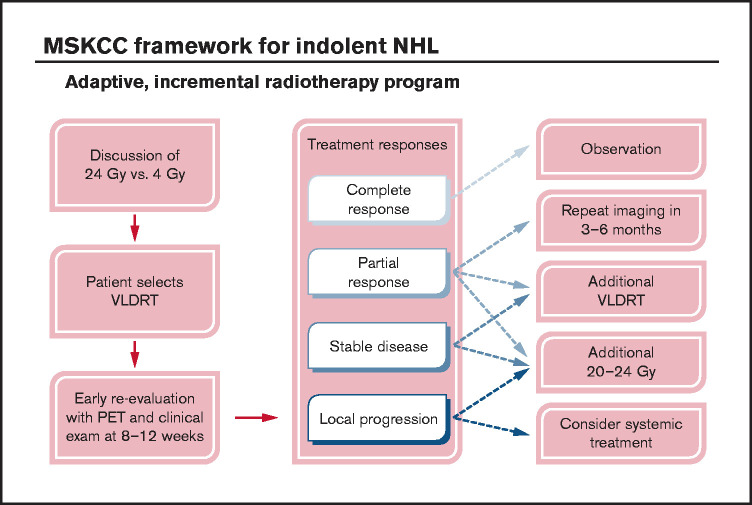
At our institution, we pioneered an adaptive RT strategy that allowed us to offer VLDRT more broadly to patients who agreed to close observation (Figure 4). To follow our program, patients with iNHL started with VLDRT followed by short-interval reassessment (typically 2-3 months) with consideration of additional VLDRT or possibly escalation to full-dose RT (ie, 20-24 Gy) contingent on the response to VLDRT. For this analysis, we defined the program as receipt of additional RT to the VLDRT field within 6 months without intercurrent LP.
Figure 4.
Schematic framework for adaptive treatment of patients with iNHL using VLDRT and early PET guidance. MSKCC, Memorial Sloan Kettering Cancer Center.
In total, 12 lesions (4%) received additional in-field RT per the program, of which 3 (25%) received additional 4 Gy and the remainder received 20 to 30 Gy. Patients who received additional VLDRT had treatment to the vulva, bilateral orbits/periorbita, bilateral neck, and Waldeyer’s ring. Although the median SUV for lesions that required additional RT was nominally higher than that for the overall cohort (10.1 vs 7.3), we lacked the power to better identify predictors of a requirement for subsequent RT. After the program, there were 2 LPs and 7 DPs.
Impact of best response on early clinical events
Our landmark analyses at 6 months did not capture the full impact of early progression because LP often occurs in the first year (Figure 1). Thus, we performed an exploratory analysis of association between best response by 6 months and risk of either LP or the composite end point of LP, additional in-field/out-of-field RT, systemic therapy, or death at 6 months. Patients who did not achieve CR had higher proportions of both events, which suggests that early responses have clinical importance (P < .001; Table 6). Overall, 68 lesions (23%) had PR to VLDRT, 27 (40%) subsequently developed LP, 4 (6%) received systemic therapy without LP, and the remaining 37 (54%) had no recorded LP.
Table 6.
Association between best response and LP or composite event at 6 months after VLDRT
| Characteristic | Overall (N = 276) |
Best overall response determined ≤6 mo after VLDRT | P * | ||
|---|---|---|---|---|---|
| CR (n = 1721) |
PR (n = 651) |
SD or PD (n = 391) |
|||
| LP at 6 mo after VLDRT | <.001 | ||||
| LP | 36 (13) | 5 (2.9) | 7 (11) | 24 (62) | |
| No LP | 240 (87) | 167 (97) | 58 (89) | 15 (38) | |
| Event at 6 mo after VLDRT | <.001 | ||||
| Composite | 65 (24) | 16 (9.3) | 19 (29) | 30 (77) | |
| None | 211 (76) | 156 (91) | 46 (71) | 9 (23) | |
Data are presented as n (% [frequency]). Composite event includes LP, additional local RT, systemic therapy, or death following VLDRT.
χ2 test of independence.
Risk of histologic transformation
Before treatment with VLDRT, 25 patients (10%) had a history of DLBCL and received VLDRT for relapse of an indolent component (ie, retrograde transformation). Of these 25, 10 (40%) developed recurrent DLBCL after VLDRT. Of the 225 patients without previously diagnosed DLBCL, 9 (4%) had DLBCL transformation, and none were potentially curable. All cases of transformed DLBCL were diagnosed distant to the VLDRT fields. The 2-year cumulative incidence of transformation was 1.7% (95% CI, 0.5%-4.5%) and the 5-year incidence was 8.5% (95% CI, 3.6%-15.9%) (Figure 5).
Figure 5.
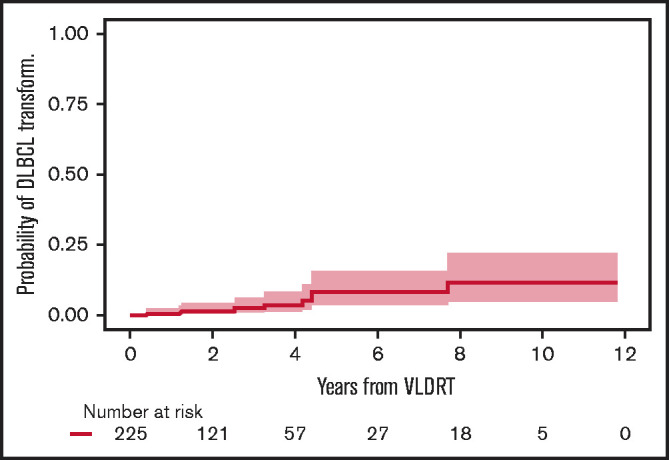
Cumulative incidence of DLBCL transformation or diagnosis after VLDRT.
Discussion
VLDRT is a versatile, effective strategy for treating iNHL. With a median follow-up of more than 2 years, we demonstrated that modern VLDRT approaches using PET staging and ISRT results in excellent ORR and nearly 70% CR. Importantly, most lesions remain controlled with low probability of progression at 2 years after VLDRT. We also report very low probability of transformation and none within the VLDRT field. Although additional follow-up is required, the LP cumulative incidence curve starts to plateau, which suggests the possibility of durable response. Overall, median OP-free survival was 1 year, suggesting that VLDRT can offer meaningful remission and stability.
Our composite ORR is concordant with other published VLDRT series (Table 7). Our reported CR rate is higher than the historical median (59%), which may be the result of rigorous pathology review or inclusion of cutaneous lymphomas. Furthermore, we had greater use of PET for response assessment compared with older series that relied on CT or clinical assessments. For FL, a significant proportion of PR by CT will be metabolically CR.32 Similar ORR and LP outcomes for VLDRT across our heterogeneous population highlight its versatility. We have used a large cohort to confirm findings from numerous centers that VLDRT works well in diverse clinical contexts: FL or MZL histology, early or advanced stage, nodal or extranodal lesions, or heavily pretreated or treatment naïve patients.14,15,17-19,21,22,24,33,34 We performed a subset analysis of patients who received no previous systemic therapy, and the similarity of the cumulative incidence curves of LP and OP compared with those of the full cohort suggest that the direct efficacy of VLDRT is robust.
Table 7.
Selected prospective and retrospective series investigating VLDRT for iNHL
| Reference | Year | Design | RT fields | Pathology summary | No. of patients | No. of sites | Median follow-up (mo) |
ORR (%) |
CR (%) |
PR (%) |
Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| This study | 2021 | Retrospective | ISRT | 66% FL, 27% MZL, and 7% low-grade NOS | 250 | 299 | 26 | 90 | 68 | 22 | 2-y cumulative incidence of LP for overall, 25%; non-curable, 29%; potentially curable, 9% |
| 24,25 | 2014, 2021 | Randomized, phase 3, noninferiority of 4 Gy vs 24 Gy (FoRT) | IFRT | 56% FL, 7% MZL, 6% other (ie, CLL, DLBCL, HL), 12% no diagnosis possible, 21% with no central review | 281 | 315 | 74 | 81 | 49 | 32 | LPFS at 5 y: 89.9% after 24 Gy and 74.4% after 4 Gy (P < .001) |
| 21 | 2008 | Phase 2 | IFRT | 62% indolent (mostly FL) and 36% aggressive lymphoma | 36 | 47 | 5 | 86* | 48* | 38* | Median time to LP for the entire group, 15 mo |
| 19 | 2005 | Phase 2 | IFRT | SLL and CLL (n = 23), MZL (n = 18), MCL (n = 17), DLBCL (n = 13) | 71 | 177 | 9 | 93* | 56 | 37 | Median time to LP for iNHL, 23 mo |
| 18 | 2003 | Phase 2 (HORA-1) | IFRT | FL 90%, MZL 8%, lymphoplasmacytoid lymphoma 2% | 109 | 304 | 7 | 92 | 61 | 31 | Median time to LP, 25 mo |
| 17 | 2002 | Phase 2 | IFRT | 68% iNHL, 32% CLL | 22 | 31 | 8 | 87* | 74* | 13* | Median time to LP for full group, 22 mo |
| 35 | 2018 | Retrospective | 57% FL, 43% MZL | 47 | 50 | 21 | 90 | LPFS at 2 y for all patients, 91.1%; curative, 96.7%; palliative, 83.8% | |||
| 34 | 2013 | Retrospective | IFRT | 66% FL, 9% CLL or SLL, 10% MZL, 6% MCL, 8% other | 127 | 187 | 23 | 82 | 57 | 25 | Median time to first recurrence, 13.6 mo |
| 22 | 2011 | Retrospective | 56% indolent, 28% CLL, 13% aggressive, 2% of sites were other | 54 | 85 | 16 | 88* | 71 | 17 | LPFS at 2 years, 50% | |
| 20 | 2008 | Retrospective | IFRT | FL 85%, MZL 6%, MCL 6%, CLL 3% | 33 | 43 | 14 | 95 | 84 | 12 | Median time to LP, 9 mo |
| 15 | 2001 | Retrospective | IFRT | 100% low-grade lymphomas | 48 | 135 | 54 | 81 | 57 | 24 | LPFS at 2 y, 56% |
LPFS, local progression-f ree survival.
Outcomes of only the indolent subgroup of studied patients.
To guide patient selection for treatment with VLDRT, researchers have attempted to identify predictive characteristics for durable response. In our series, only the pretreatment size of the lesion was associated with greater risk of suboptimal response and LP. The observation that larger lesions may be suboptimally treated with VLDRT has been suggested, but a specific bulky disease threshold remains uncertain. In our work, lesions ≥6 cm have poorer relative outcomes. Girinsky et al15 and König et al35 both reported that diameter >5 cm had significantly lower ORR and greater LP. Haas et al19 noted similar ORR but significantly lower CR rates for bulky (>5 cm) vs smaller sites (P = .006). Others report a significant CR difference between lesions ≤4 cm and those >4 cm (90% vs 56%; P = .04), although it was unclear whether this was related to proportional differences of head and neck (vs pelvic) sites.20
The current role of VLDRT has been shaped by the phase 3 FoRT study, which found that 4 Gy had inferior time to LP compared with 24 Gy.24,25 Critics of VLDRT argue that inferior local control makes it unsuitable outside of palliation. We and others support a more positive interpretation of the data; for lesions treated with VLDRT, median time to LP was not reached, which suggests that most lesions will be controlled with 4 Gy. Furthermore, given that there were no differences in OS between the arms, patients treated with VLDRT can successfully receive salvage therapy or face death as a result of unrelated causes in similar proportions.
Furthermore, focusing purely on local control likely fails to capture the bigger picture of the iNHL clinical trajectory. Patterns of treatment failure for this cohort demonstrate that after VLDRT, patients are more likely to experience out-of-field DP. The 2-year cumulative incidence estimate of DP was 46% vs 25% for LP. This observation aligns with well-understood iNHL behavior and previous trends from series using extended fields or IFRT. For example, in the Dutch experience, median time to overall progression was 14 months and 25 months for LP.18 Although it may be attractive to consider higher doses of RT to improve the durability of local control, it is unlikely that dose escalation will meaningfully change the broader disease course. A recently published ILROG multicenter analysis of curative dose RT (>24 Gy) for newly diagnosed, localized, PET-staged FL found that although in-field recurrence was extremely low (1.8%), 5-year freedom from progression was 69% overall (74% for stage I and 49% for stage II).7
Use of VLDRT with curative intent remains particularly controversial. The FoRT study originally focused on palliatively treated patients but expanded to include patients with curable lesions. Roughly 40% of lesions were curable, and ORR was significantly better for 24 Gy (82% vs 95%; P = .005) as was the durability of local control. In our series, 52 lesions were potentially curable, and we did not detect any signals suggestive of inferior outcomes; we observed significantly lower incidence of DP and OP without DLBCL transformation. There are a number of possible reasons why the outcomes for sites in the potentially curable subgroup are improved compared with the palliatively treated sites. First, early-stage disease not exposed to chemoimmunotherapy might have more indolent biology. Second, the potentially curable sites were slightly smaller and had a greater proportion of extranodal lesions and MZL histology, both of which have better overall outcomes. Of note, the potentially curable subgroup included a moderate proportion of cutaneous disease which is known to have excellent outcomes with distinct clinical course. Given these caveats, for now, we would caution against using VLDRT as a curative strategy for certain populations, including those with bulky tumors (>6 cm) or patients unable to return for close follow-up.
VLDRT is attractive to patients because of its logistical convenience and minimal toxicity risk (particularly when compared with systemic therapy). In the FoRT trial, patients who received 4 Gy had a <1% chance of severe toxicities. We feel comfortable treating appropriately counseled patients who have curable disease with VLDRT, given our adaptive program (Figure 4). Our experience suggests that relatively few patients will require subsequent RT, but one important caveat is that patients were treated off-protocol; this series included a highly selected, potentially lower-risk group, and recommendations for subsequent RT were not standardized. With close follow-up and indolent disease course, patients receiving first-line VLDRT are unlikely to face a significant risk of rapid progression or lose a window of curability. VLDRT with curative intent has already been proposed for orbital and salivary gland MZL in which higher doses are associated with increased toxicity.36-38 Patients must be appropriately counseled regarding a likely greater LP risk, and they should also understand that most lesions can be effectively re-treated. We confirm the observations of other researchers,19,39 that re-irradiation is safe and maintains similar efficacy after VLDRT.
PET-adapted RT already plays an important role across lymphoma indications,40,41 but its predictive power to determine control after VLDRT should be prospectively validated. Our data suggest that higher doses could be considered for bulk, but this remains a crude and imperfect guide, and we have relatively little ability to predict which patients will have a durable response. Attempts to identify radiogenomic predictors to better discriminate optimal candidates are under way at our institution and at others. Moving forward, it may be sensible to design a randomized trial that compares patients who receive 4 Gy or adaptive 24 Gy before LP with those who receive 24 Gy a priori with consideration of a broader constellation of clinical, radiogenomic, and quality-of-life outcomes.
We see numerous opportunities to expand VLDRT indications. Because VLDRT may induce local and systemic immunomodulation, there are natural opportunities to consider combinations with diverse systemic therapies.33 The TROG 99.03 study found that patients with early-stage FL who received 30 Gy IFRT with rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP) chemoimmunotherapy had significantly improved progression-free survival when compared with IFRT alone.42 Patients with greater risk of treatment failure after VLDRT (eg, those with bulky lesions) could be considered for adjuvant systemic therapy. Building on these concepts, the GAZAI study aims to use response-adapted ISRT initially with 4 Gy plus obinutuzumab for early-stage grade 1 to 2 FL.43 Excellent tolerability, even when irradiating large fields, means that VLDRT can be considered for patients with significant disease burden as a bridge to next-line systemic therapies, even for aggressive lymphomas.44
Strengths of this analysis include a large number of sites, use of PET, consideration of numerous end points using competing risk methodology (given the epidemiology of the disease), and rigorous pathology review. Of note, FoRT did include a moderate number of patients without central pathologic confirmation or non-iNHL histologies.24 We also performed a subset analysis to isolate the efficacy of VLDRT directly without potential confounding from exposure to previous systemic therapy.
Limitations include the typical biases of retrospective analysis, a nonstandardized follow-up schedule, imaging approach, and lack of objective criteria for recommending additional RT. This series includes several indolent subtypes, including cutaneous, which have different natural histories but represent real-life institutional use of VLDRT. Our follow-up was longer than that in most previous VLDRT series, but it remains relatively short (given the epidemiology), particularly for the patients with potentially curable disease. In addition, the only competing event considered in the LP analysis was death, which may not capture how receipt of previous VLDRT systemic therapy confounds the probability of LP. However, a comparison analysis shows that the cumulative incidence curves overlap nearly completely with inverse Kaplan-Meier curves censoring for death and start of systemic therapy.
In conclusion, we find that VLDRT that uses modern approaches is an effective and versatile strategy for iNHL. Future efforts will focus on refining our understanding of how to optimally incorporate this modality into evolving lymphoma treatment paradigms that are increasingly reliant on novel systemic agents.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was funded in part by a grant from the National Cancer Institute, National Institutes of Health Cancer Center (P30 CA008748) and by the Connecticut Cancer Foundation.
Authorship
Contribution: B.S.I. helped develop the project concept, collect and analyze data, and write the manuscript; K.W.C. collected and analyzed the data and helped write the manuscript; Jisun Lee helped collect and analyze data; Jasme Lee and Z.Z. performed biostatistical analysis; N.A.W. helped analyze the data and write the manuscript; J.Y. developed the project concept, provided overall project supervision, and helped write the manuscript; and all authors reviewed and edited the final version of manuscript and agreed with the submission.
Conflict-of-interest disclosure: E.J. served as an advisor for AstraZeneca and Epizyme. P.A.H. received research funding from Portola/Alexion, Janssen, Seattle Genetics, and Incyte and has consulted for AstraZeneca, Juno Therapeutics, Sandoz, Celgene, Seattle Genetics, and Karyopharm. M.L.P. has had consulting or advisory roles for Kite Pharma, Novartis, PCYC, and BeiGene (outside of the submitted work); a family member has received honoraria from Seres, Magenta, WindMIL, Rheos, Nektar, Notch, Priothera, Ceramedix, Lygenesis, and Pluto; and a family member holds stock in Seres Therapeutics and Notch as well as patents or other intellectual property for Juno Therapeutics and Seres Therapeutics. A.D.Z. has received grants and/or personal consulting fees from MEI Pharmaceutical, Gilead/Kite, Bristol Myers Squibb/Celgene/Juno, Novartis, Roche/Genentech, BeiGene, AbbVie, AstraZeneca/Acerta, Amgen, Adaptive Biotechnology, MorphoSys, and Janssen (outside the submitted work). G.A.S. has received financial compensations for participating in advisory boards or consulting from AbbVie, Beigene, Bristol Myers Squibb/Celgene, Debiopharm, Genentech/Roche, Genmab, Incyte, Ipsen, Kite/Gilead, Miltenyi Biotek, Morphosys, Novartis, and Velosbio. The remaining authors declare no competing financial interests.
Correspondence: Joachim Yahalom, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, 530 E. 74th St, New York, NY 10021; e-mail: yahalomj@mskcc.org.
References
- 1.Yahalom J. Radiotherapy of follicular lymphoma: updated role and new rules. Curr Treat Options Oncol. 2014;15(2):262-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zelenetz AD, Gordon LI, Abramson JS, et al. NCCN Guidelines Insights: B-Cell Lymphomas, Version 3.2019. J Natl Compr Canc Netw. 2019;17(6):650-661. [DOI] [PubMed] [Google Scholar]
- 3.Dreyling M, Ghielmini M, Rule S, Salles G, Vitolo U, Ladetto M; ESMO Guidelines Committee .Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v83-v90. [DOI] [PubMed] [Google Scholar]
- 4.Specht L, Yahalom J.. The concept and evolution of involved site radiation therapy for lymphoma. Int J Clin Oncol. 2015;20(5):849-854. [DOI] [PubMed] [Google Scholar]
- 5.Wirth A, Mikhaeel NG, Aleman BMP, et al. Involved site radiation therapy in adult lymphomas: an overview of International Lymphoma Radiation Oncology Group Guidelines. Int J Radiat Oncol Biol Phys. 2020;107(5):909-933. [DOI] [PubMed] [Google Scholar]
- 6.Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol. 2011;100(1):86-92. [DOI] [PubMed] [Google Scholar]
- 7.Brady JL, Binkley MS, Hajj C, et al. Definitive radiotherapy for localized follicular lymphoma staged by 18F-FDG PET-CT: a collaborative study by ILROG. Blood. 2019;133(3):237-245. [DOI] [PubMed] [Google Scholar]
- 8.Binkley MS, Brady JL, Hajj C, et al. Salvage treatment and survival for relapsed follicular lymphoma following primary radiation therapy: a collaborative study on behalf of ILROG. Int J Radiat Oncol Biol Phys. 2019;104(3):522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson RE. Management of generalized malignant lymphomata with “systemic” radiotherapy. Br J Cancer Suppl. 1975;2:450-455. [PMC free article] [PubMed] [Google Scholar]
- 10.Hoppe RT, Kushlan P, Kaplan HS, Rosenberg SA, Brown BW.. The treatment of advanced stage favorable histology non-Hodgkin’s lymphoma: a preliminary report of a randomized trial comparing single agent chemotherapy, combination chemotherapy, and whole body irradiation. Blood. 1981;58(3):592-598. [PubMed] [Google Scholar]
- 11.Lybeert MLM, Meerwaldt JH, Deneve W.. Long-term results of low dose total body irradiation for advanced non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 1987;13(8):1167-1172. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs P, King HS.. A randomized prospective comparison of chemotherapy to total body irradiation as initial treatment for the indolent lymphoproliferative diseases. Blood. 1987;69(6):1642-1646. [PubMed] [Google Scholar]
- 13.Meerwaldt JH, Carde P, Burgers JMV, et al. Low-dose total body irradiation versus combination chemotherapy for lymphomas with follicular growth pattern. Int J Radiat Oncol Biol Phys. 1991;21(5):1167-1172. [DOI] [PubMed] [Google Scholar]
- 14.Ganem G, Lambin P, Socié G, et al. Potential role for low dose limited-field radiation therapy (2 x 2 grays) in advanced low-grade non-Hodgkin’s lymphomas. Hematol Oncol. 1994;12(1):1-8. [DOI] [PubMed] [Google Scholar]
- 15.Girinsky T, Guillot-Vals D, Koscielny S, et al. A high and sustained response rate in refractory or relapsing low-grade lymphoma masses after low-dose radiation: analysis of predictive parameters of response to treatment. Int J Radiat Oncol Biol Phys. 2001;51(1):148-155. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer EJ, Timothy AR.. Low dose palliative radiotherapy in low grade non-Hodgkin’s lymphoma. Radiother Oncol. 1997;42(1):49-51. [DOI] [PubMed] [Google Scholar]
- 17.Jóhannsson J, Specht L, Mejer J, Jensen BA.. Phase II study of palliative low-dose local radiotherapy in disseminated indolent non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Int J Radiat Oncol Biol Phys. 2002;54(5):1466-1470. [DOI] [PubMed] [Google Scholar]
- 18.Haas RLM, Poortmans P, de Jong D, et al. High response rates and lasting remissions after low-dose involved field radiotherapy in indolent lymphomas. J Clin Oncol. 2003;21(13):2474-2480. [DOI] [PubMed] [Google Scholar]
- 19.Haas RLM, Poortmans P, de Jong D, et al. Effective palliation by low dose local radiotherapy for recurrent and/or chemotherapy refractory non-follicular lymphoma patients. Eur J Cancer. 2005;41(12):1724-1730. [DOI] [PubMed] [Google Scholar]
- 20.Luthy SK, Ng AK, Silver B, et al. Response to low-dose involved-field radiotherapy in patients with non-Hodgkin’s lymphoma. Ann Oncol. 2008;19(12):2043-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy V, Thomas K, Foo K, et al. Efficacy of palliative low-dose involved-field radiation therapy in advanced lymphoma: a phase II study. Clin Lymphoma Myeloma. 2008;8(4):241-245. [DOI] [PubMed] [Google Scholar]
- 22.Chan EK, Fung S, Gospodarowicz M, et al. Palliation by low-dose local radiation therapy for indolent non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2011;81(5):e781-e786. [DOI] [PubMed] [Google Scholar]
- 23.Zafar SY, Abernethy AP.. Financial toxicity, Part I: a new name for a growing problem. Oncology (Williston Park). 2013;27(2):80-81. [PMC free article] [PubMed] [Google Scholar]
- 24.Hoskin PJ, Kirkwood AA, Popova B, et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised phase 3 non-inferiority trial. Lancet Oncol. 2014;15(4):457-463. [DOI] [PubMed] [Google Scholar]
- 25.Hoskin P, Popova B, Schofield O, et al. 4 Gy versus 24 Gy radiotherapy for follicular and marginal zone lymphoma (FoRT): long-term follow-up of a multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2021;22(3):332-340. [DOI] [PubMed] [Google Scholar]
- 26.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR.. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459. [DOI] [PubMed] [Google Scholar]
- 27.Illidge T, Specht L, Yahalom J, et al. Modern radiation therapy for nodal non-Hodgkin lymphoma—target definition and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2014;89(1):49-58. [DOI] [PubMed] [Google Scholar]
- 28.Yahalom J, Illidge T, Specht L, et al. ; International Lymphoma Radiation Oncology Group . Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92(1):11-31. [DOI] [PubMed] [Google Scholar]
- 29.Specht L, Dabaja B, Illidge T, Wilson LD, Hoppe RT; International Lymphoma Radiation Oncology Group .Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015; 92(1):32-39. [DOI] [PubMed] [Google Scholar]
- 30.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 32.Trotman J, Luminari S, Boussetta S, et al. Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol. 2014;1(1):e17-e27. [DOI] [PubMed] [Google Scholar]
- 33.Ganem G, Cartron G, Girinsky T, et al. Localized low-dose radiotherapy for follicular lymphoma: history, clinical results, mechanisms of action, and future outlooks. Int J Radiat Oncol Biol Phys. 2010;78(4):975-982. [DOI] [PubMed] [Google Scholar]
- 34.Russo AL, Chen Y-H, Martin NE, et al. Low-dose involved-field radiation in the treatment of non-hodgkin lymphoma: predictors of response and treatment failure. Int J Radiat Oncol Biol Phys. 2013;86(1):121-127. [DOI] [PubMed] [Google Scholar]
- 35.König L, Hörner-Rieber J, Bernhardt D, et al. Response rates and recurrence patterns after low-dose radiotherapy with 4 Gy in patients with low-grade lymphomas. Strahlenther Onkol. 2018;194(5):454-461. [DOI] [PubMed] [Google Scholar]
- 36.Fasola CE, Jones JC, Huang DD, Le QT, Hoppe RT, Donaldson SS.. Low-dose radiation therapy (2 Gy × 2) in the treatment of orbital lymphoma. Int J Radiat Oncol Biol Phys. 2013;86(5):930-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinnix CC, Dabaja BS, Milgrom SA, et al. Ultra-low-dose radiotherapy for definitive management of ocular adnexal B-cell lymphoma. Head Neck. 2017;39(6):1095-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunther JR, Park C, Dabaja BS, et al. Radiation therapy for salivary gland MALT lymphoma: ultra-low dose treatment achieves encouraging early outcomes and spares salivary function. Leuk Lymphoma. 2020;61(1):171-175. [DOI] [PubMed] [Google Scholar]
- 39.Saleh K, Michot JM, Schernberg A, et al. Repeated courses of low-dose 2 × 2 Gy radiation therapy in patients with indolent B-cell non-Hodgkin lymphomas. Cancer Med. 2020;9(11):3725-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrington SF, Johnson PWM.. 18F-FDG PET/CT in lymphoma: has imaging-directed personalized medicine become a reality? J Nucl Med. 2017; 58(10):1539-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikhaeel NG, Milgrom SA, Terezakis S, et al. The optimal use of imaging in radiation therapy for lymphoma: guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2019;104(3):501-512. [DOI] [PubMed] [Google Scholar]
- 42.MacManus M, Fisher R, Roos D, et al. Randomized trial of systemic therapy after involved-field radiotherapy in patients with early-stage follicular lymphoma: TROG 99.03. J Clin Oncol. 2018;36(29):2918-2925. [DOI] [PubMed] [Google Scholar]
- 43.König L, Dreyling M, Dürig J, et al. Therapy of nodal follicular lymphoma (WHO grade 1/2) in clinical stage I/II using response adapted involved site radiotherapy in combination with obinutuzumab (Gazyvaro) - GAZAI Trial (GAZyvaro and response adapted involved-site radiotherapy): a study protocol for a single-arm, non-randomized, open, national, multi-center phase II trial. Trials. 2019;20(1):544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ning MS, Pinnix CC, Chapman BV, et al. Low-dose radiation (4 Gy) with/without concurrent chemotherapy is highly effective for relapsed, refractory mantle cell lymphoma. Blood Adv. 2019;3(13):2035-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.