Key Points
Propensity analysis confirmed substantially higher ORR and a 73% reduction in risk for death with axi-cel vs standard salvage regimens.
This 2-year analysis indicates that axi-cel is associated with durable clinical benefit for patients with refractory LBCL.
Visual Abstract
Abstract
The SCHOLAR-1 international retrospective study highlighted poor clinical outcomes and survival among patients with refractory large B-cell lymphoma (LBCL) treated with conventional chemotherapy. Axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, demonstrated durable responses in patients with refractory LBCL in the pivotal phase 1/2 ZUMA-1 study (NCT02348216). Here, we compared SCHOLAR-1 with the 2-year outcomes of ZUMA-1. Prior to comparison of clinical outcomes, propensity scoring (based on a broad set of prognostic covariates) was used to create balance between ZUMA-1 and SCHOLAR-1 patients. In the pivotal phase 2 portion of ZUMA-1, 101 patients received axi-cel and were evaluable for response and survival. In SCHOLAR-1, 434 and 424 patients were evaluable for response and survival, respectively. ZUMA-1 patients were more heavily pretreated than were SCHOLAR-1 patients. The median follow-up was 27.1 months in ZUMA-1. The objective response rate (ORR) and complete response rate were 83% and 54% in ZUMA-1 vs 34% and 12% in SCHOLAR-1, respectively. The 2-year survival rate was 54% in ZUMA-1 and 20% in SCHOLAR-1, and a 73% reduction in the risk of death was observed in ZUMA-1 vs SCHOLAR-1. These results were consistent with those of an additional standardization analysis in which strata were limited to 2 prognostic factors (refractory categorization and presence/absence of stem cell transplant after refractoriness to chemotherapy) to conserve sample size. Despite the limitations of a nonrandomized analysis, these results indicate that axi-cel produces durable responses and a substantial survival benefit vs non–CAR T-cell salvage regimens for patients with refractory LBCL.
Introduction
Survival rates in large B-cell lymphoma (LBCL), the most common subtype of non-Hodgkin lymphoma, have improved over the past few decades in the United States and Europe.1-3 However, the prognosis of patients with relapsed or refractory disease remains poor, and potentially curative options are few.4,5 Of patients who progress during initial immunochemotherapy or within 1 year after first remission, only 30% to 40% will respond to salvage chemotherapy and may subsequently undergo consolidation with autologous stem cell transplant (ASCT).6-8 Approximately 50% of patients with relapsed or refractory LBCL who respond to salvage therapy and are able to undergo ASCT will ultimately relapse after transplant.9 The prognosis is particularly poor among those who have high-risk factors, such as a secondary International Prognostic Index (IPI) score >2 or relapse ≤12 months post-ASCT.8 Thus, most patients with refractory LBCL have no curative treatment options.4,5,8
The poor outcomes, including low overall survival (OS) rates, for patients with refractory LBCL were highlighted in the SCHOLAR-1 study, the largest published international retrospective analysis of outcomes in this patient population.4 Refractory status was defined as progressive disease as best response to any line of chemotherapy, stable disease as best response to ≥4 cycles of first-line therapy or ≥2 cycles of later-line therapy, or relapse <12 months after ASCT. Patient-level data were collected for patients with refractory LBCL subtypes, including diffuse LBCL, transformed follicular lymphoma, and primary mediastinal B-cell lymphoma.4 Among these patients, the objective response rate (ORR) was 26%, the complete response (CR) rate was 7%, and the median OS was 6.3 months with standard salvage therapies.4 These findings underscored the considerable unmet need for effective therapies for patients with refractory LBCL and have served as benchmarks for assessing novel therapies in this patient population. Chimeric antigen receptor (CAR) T-cell therapies have emerged as an effective treatment approach for patients with refractory aggressive lymphomas, and those targeting CD19 have shown high response rates in patients with refractory LBCL after failure of conventional therapy.10-12 ZUMA-1 (NCT02348216) is the pivotal multicenter single-arm phase 1/2 study evaluating axicabtagene ciloleucel (axi-cel), an autologous anti-CD19 CAR T-cell therapy, in patients with refractory LBCL that supported the approval of axi-cel in the United States and Europe.10,13,14 The ORR was 83%, the CR rate was 58%, and the median OS was not reached (95% confidence interval [CI], 12.8 months-not estimable) after ≥2 years of follow-up for all patients.13 In an updated analysis with a median follow-up of 39.1 months, the median OS was 25.8 months and the 3-year OS rate was 47%.15 In the absence of a randomized trial, comparison with a historical control group consisting of patients with characteristics similar to those in the ZUMA-1 trial is likely to be informative when assessing the effectiveness of axi-cel over available non–CAR T-cell salvage therapies, especially with longer follow-up. Here, we used propensity scoring to adjust for imbalances in baseline characteristics prior to comparison of 2-year clinical outcomes in SCHOLAR-1 and ZUMA-1.
Methods
Studies compared
The SCHOLAR-1 study design and cohort details were previously described.4 Briefly, patient-level data were collected for patients with refractory LBCL from 4 cohorts (N = 636), including 2 observational institutional cohorts and 2 large phase 3, randomized controlled trials: MD Anderson Cancer Center study,16 University of Iowa/Mayo Clinic SPORE,17,18 Canadian Cancer Trials Group LY.12 phase 3 randomized study,6 and the Lymphoma Academic Research Organization CORAL randomized study.7-9 Patients must have received a prior anti-CD20 antibody and an anthracycline-containing regimen. For University of Iowa/Mayo Clinic SPORE, LY.12, and CORAL, patients were included at the first instance of meeting refractory criteria, whereas at the MD Anderson Cancer Center, patients who first met refractory criteria from second-line therapy onward were included (supplemental Table 1).4 The median follow-up from the overall SCHOLAR-1 study ranged from 7.6 to 14.8 years across cohorts.4,13 For the purpose of comparing outcomes with ZUMA-1, salvage therapy in SCHOLAR-1 was the next line of therapy after determination of refractory status (see supplemental Methods for additional details).
ZUMA-1 is a single-arm multicenter phase 1/2 study of axi-cel in refractory LBCL, including diffuse LBCL, primary mediastinal B-cell lymphoma, and transformed follicular lymphoma, that is being conducted at 22 medical cancer centers in the United States and Israel.10,13,14 Patients had refractory disease and also must have received a prior anti-CD20 antibody and an anthracycline-containing regimen. Patients with central nervous system lymphoma at screening or a history of central nervous system lymphoma were excluded. Following leukapheresis and the manufacture of CAR T cells, patients received lymphodepleting chemotherapy with fludarabine (30 mg/m2 body surface area per day) and cyclophosphamide (500 mg/m2 body surface area per day) for 3 days, followed by axi-cel infusion at a target dose of 2 × 106 CAR T cells per kilogram.10,13 The data cutoff date for this analysis was 11 August 2018, consistent with that of the 2-year long-term analysis of efficacy and safety outcomes (median follow-up, 2.3 years).13 As mentioned, the criteria for refractory disease were consistent between SCHOLAR-1 and ZUMA-1. This study was an analysis of published data and did not require Institutional Review Board or Institutional Animal Care and Use Committee approval.
Assessments
For these analyses, responses in ZUMA-1 were determined based on the revised International Working Group criteria for malignant lymphoma19 per investigator assessment.13 Assessments included ORR, CR rate, and OS. In SCHOLAR-1, response to therapy was determined based on International Working Group response criteria20 per investigator assessment for the 2 observational cohorts and per local review of computerized tomography scans for the phase 3 trial cohorts.4
Propensity scoring
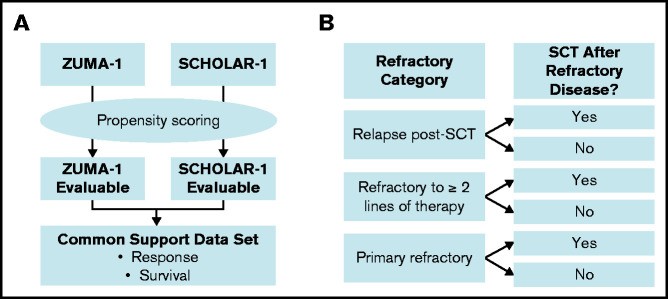
Propensity scoring was used to create balance between the ZUMA-1 and SCHOLAR-1 patient cohorts21 and was performed in accordance with the previous propensity score–balanced comparison of 1-year outcomes between ZUMA-1 and SCHOLAR-1 (Figure 1A).22 Propensity scores were calculated for each patient by combining the ZUMA-1 and SCHOLAR patients into a single dataset and calculating the probability of being in the ZUMA-1 trial based on demographics and disease characteristics. The primary common support set for response was based on the primary propensity model, which incorporated 7 covariates: age (at determination of refractory status), sex, disease type (diffuse large B-cell lymphoma, transformed follicular lymphoma, or primary mediastinal B-cell lymphoma), relapse within 12 months of ASCT, whether the patient was ever primary refractory (refractory to the initial chemotherapy regardless of refractoriness to subsequent lines of therapy) or refractory to ≥2 consecutive lines of chemotherapy, and the number of prior lines of chemotherapy. An additional sensitivity common support set for response was based on the sensitivity propensity model, which incorporated age, sex, disease type, ever primary refractory, refractory or not to ≥2 consecutive lines of chemotherapy, number of prior lines of chemotherapy, IPI score, and disease stage (when collected within 3 months of determination of refractory status). Additionally, the presence of posttreatment stem cell transplant (SCT) was added as a time-varying covariate in propensity models used to determine the primary and sensitivity common support sets for survival (supplemental Table 2). The statistician developing the common support data sets had access to the treatment status and covariate values for each patient but did not reference outcomes.
Figure 1.
Overview of analysis populations for propensity score–balanced and standardized comparisons. (A) Propensity scores were derived to generate a primary and sensitivity common support data set for ZUMA-1 and SCHOLAR-1, which were then used to estimate the average treatment differences in response to and survival for anti-CD19 CAR T-cell therapy and historical standard of care (non–CAR T-cell therapy). (B) Strata by refractory category and postrefractory SCT for standardization are depicted. Standardized analyses were conducted that equally weighted the proportions of patients by refractory categorization and presence of autologous or allogeneic SCT after establishing refractoriness to salvage therapy (postrefractory SCT) in each study.
Within the common support data sets, treatment differences in response and survival for axi-cel vs the historical standard of care (ie, non–CAR T-cell therapy) were evaluated. Estimators utilizing stratification with regression adjustment were used to estimate the average treatment effect (ATE) of axi-cel for the ORR and CR end points. ATE was the difference in the response rates that would be expected if both groups of patients had been treated with axi-cel in ZUMA-1 vs if both groups had received salvage chemotherapy in SCHOLAR-1.23 Augmented inverse-probability weighted complete-case estimators24 were used to adjust for the effects of confounding covariates and censoring in the calculation of the survival functions. The difference between these treatment-specific survival functions were calculated: this difference function is the survival-function analog of the ATE. From the treatment-specific survival functions, the treatment-specific median OS times and their differences were calculated, along with treatment-specific OS rates at 3, 6, and 12 months. Among patients in the primary common support set for survival, stratification with regression-adjustment hazard ratio estimator was used to estimate the hazard ratio between treatments. Bootstrap 95% CIs were calculated for all quantities.
Standardization analysis
An additional standardized analysis was performed to minimize loss of patients due to missing covariate data. Figure 1 compares the derivation of analysis populations in the standardization analysis with the aforementioned propensity score–balanced analysis. To address potential imbalances in refractory status that could affect outcomes, the standardization analyses25 equally weighted proportions of patients by refractory categorization (ie, primary refractory, refractory to ≥2 lines of therapy, or relapse within 1 year after SCT), as well as the presence of ASCT or allogeneic SCT after establishing refractoriness to chemotherapy (postrefractory SCT) in each study (supplemental Figure 1). The response rates, survival rates, or median OS estimates from these strata within SCHOLAR-1 were weighted by the proportion of patients in those strata within ZUMA-1 to calculate an overall estimate that reflected the distribution across those strata within ZUMA-1. Strata were limited to these 2 factors (ie, refractory categorization and presence of postrefractory SCT), because an increased number of prognostic factors would lead to a sparsity of patients across additional strata. Statistical methods are described in supplemental Methods.
Results
Demographics and baseline characteristics
Demographics and baseline characteristics for ZUMA-1 and SCHOLAR-1 patients were published previously.4,10 The primary and sensitivity common support sets were based on patient subsetting (supplemental Table 2) and then propensity scoring, as described in supplemental Methods. Complete patient accountability is summarized in supplemental Table 3.
Baseline characteristics for the primary common support sets for response (ZUMA-1, N = 80; SCHOLAR-1, N = 340) and survival (ZUMA-1, N = 81; SCHOLAR-1, N = 331) are presented in Table 1. The sensitivity common support set for response and survival included substantially fewer patients (ZUMA-1, N = 30; SCHOLAR-1, N = 78); thus, the primary common support set was our focus.
Table 1.
Baseline patient characteristics of primary common support sets for response and survival
| Characteristics | Common support set for response | Common support set for survival | ||
|---|---|---|---|---|
| ZUMA-1 (N = 80) | SCHOLAR-1 (N = 340) | ZUMA-1 (N = 81) | SCHOLAR-1 (N = 331) | |
| Male sex | 53 (66) | 231 (68) | 54 (67) | 225 (68) |
| Age ≥65 y | 19 (24) | 56 (16) | 19 (23) | 51 (15) |
| ≥3 lines of chemotherapy and ASCT* | 49 (61) | 98 (29) | 50 (62) | 93 (28) |
| Ever primary refractory† | 23 (29) | 126 (37) | 23 (28) | 125 (38) |
| Refractory to ≥2 consecutive lines of therapy | 43 (54) | 170 (50) | 43 (53) | 165 (50) |
| SCT any time after refractory disease | 14 (18) | 126 (37) | 14 (17) | 125 (38) |
| Relapse within 12 mo of ASCT | 16 (20) | 74 (22) | 16 (20) | 71 (21) |
| ECOG PS 0-1‡ | 80 (100) | 126/126 (100)§ | 81 (100) | 126/126 (100)§ |
| Disease stage III-IV‡ | 67 (84) | 80/124 (65)§ | 68 (84) | 80/124 (65)§ |
| IPI score ≥3‡ | 34 (43) | 33/119 (28)§ | 35 (43) | 33/119 (28)§ |
Unless otherwise noted, data are n (%).
ECOG PS, Eastern Cooperative Oncology Group Performance Status.
Either prior to ZUMA-1 or prior to and including the qualifying line of therapy (ie, the next-to-last treatment a SCHOLAR-1 patient received that was used to determine the most recent refractory status) in SCHOLAR-1.
Refractory to initial therapy; patients may or may not have been refractory to subsequent therapies.
Assessed at baseline for ZUMA-1 and within 3 months of determination of refractory status and prior to salvage therapy in SCHOLAR-1. Disease stage, IPI score, and ECOG PS were not available for all SCHOLAR-1 patients.
Data are n/N (%).
Among the primary common support sets for response and survival, similar proportions were male in ZUMA-1 and SCHOLAR-1; more patients were ≥65 years old in ZUMA-1 (Table 1). In general, ZUMA-1 patients were more heavily pretreated, and more patients received ≥3 lines of therapy (chemotherapy or ASCT) in ZUMA-1. A greater proportion of ZUMA-1 patients had stage III-IV disease and high-intermediate or high-risk IPI classification (score ≥3). Comparatively, fewer ZUMA-1 patients received SCT any time after refractory disease compared with SCHOLAR-1 patients. Demographics and baseline characteristics for the standardization analysis are summarized in supplemental Table 4.
Response rates
Among the primary common support set, the ORR and CR rates in ZUMA-1 vs SCHOLAR-1 were 83% and 54% vs 34% and 12%, respectively (Table 2). The respective ATEs of axi-cel for ORR and CR were 49% (95% CI, 34-63%) and 42% (95% CI, 26-59%). In the sensitivity common support set, ATEs were 36% (95% CI, 15-61%) and 33% (95% CI, 3-51%) for ORR and CR, respectively. A standardized comparison of ORR and CR rates also strongly favored axi-cel (supplemental Table 5).
Table 2.
Comparison of ORR and CR rate
| ZUMA-1 | SCHOLAR-1 | ATE | |
|---|---|---|---|
| ORR, % * | 83 | 34 | |
| Treatment difference (95% CI), % | 49 (34-63) | ||
| CR, % * | 54 | 12 | |
| Treatment difference (95% CI), % | 42 (26-59) |
Stratified with regression adjustment estimator on the primary common support data set for response (ZUMA-1, N = 80; SCHOLAR-1, N = 340).
Response was evaluated by investigator assessment according to International Working Group response criteria.
OS
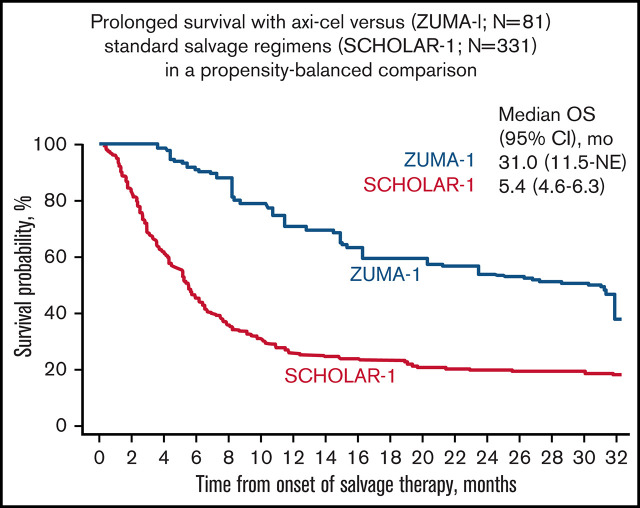
Median follow-up in ZUMA-1 was 27.1 months. In the primary common support set, the median OS was 31.0 months (95% CI, 11.5 months-not estimable) in ZUMA-1 and 5.4 months (95% CI, 4.6-6.3 months) in SCHOLAR-1 (Figure 2). The treatment difference suggested a 73% reduction in the risk of death in ZUMA-1 vs SCHOLAR-1 (Table 3). In the sensitivity common support set, the treatment difference suggested a 77% reduction in the risk of death in ZUMA-1. The 2-year survival rate after propensity score analysis was 54% (95% CI, 30-80%) in ZUMA-1 vs 20% (95% CI, 16-26%) in SCHOLAR-1.
Figure 2.
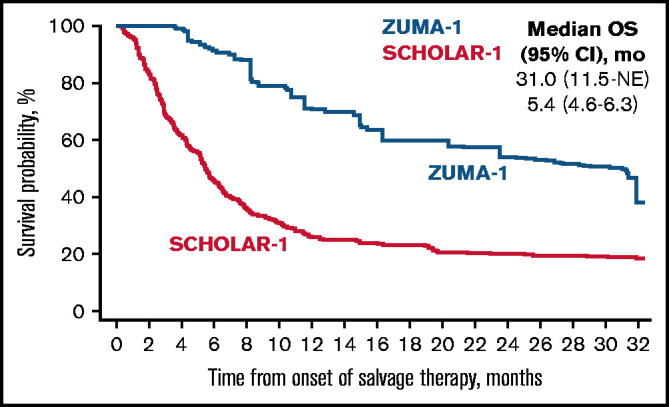
Comparison of confounder-adjusted OS. To control for confounding, the treatment-specific survival functions were obtained using augmented inverse-probability weighted complete-case estimators24 on the primary common support data set for survival (ZUMA-1, N = 81; SCHOLAR-1, N = 331). mo, month; NE, not estimable.
Table 3.
Comparison of OS rates and median OS
| ZUMA-1 | SCHOLAR-1 | Ratio | |
|---|---|---|---|
| 12-mo OS rate (95% CI), %* | 71 (46-91) | 26 (22-32) | 2.7 (1.7-3.8) |
| 18-mo OS rate (95% CI), %* | 60 (37-83) | 23 (19-29) | 2.6 (1.5-3.9) |
| 24-mo OS rate (95% CI), %* | 54 (30-80) | 20 (16-26) | 2.7 (1.4-4.3) |
| Median OS (95% CI), mo* | 31.0 (11.5-NE) | 5.4 (4.6-6.3) | 25.6 (6.0-NE)† |
| Treatment difference, HR (95% CI)‡ | 0.27 (0.00-0.38) (73% reduction in the risk of death) |
||
HR, hazard ratio; NE, not estimable.
To control for confounding, the treatment-specific survival functions were obtained using augmented inverse-probability weighted complete-case estimators24 on the common support set for survival (ZUMA-1, N = 81; SCHOLAR-1, N = 331).
Difference between the 2 studies.
Stratification with regression-adjustment hazard ratio estimator on common support set for survival (ZUMA-1, N = 81; SCHOLAR-1, N = 331). This estimator was applied to the sensitivity common support set at 1 year and produced with similar results to the 1-year propensity–balanced analysis (data on file); however, it was not applied to the sensitivity common support set at 2 years.
In the standardization analysis, the median OS was not reached in ZUMA-1 (27.1 months of follow-up; 95% CI, 11.5 months-not estimable) but was 4.1 months (95% CI, 3.5-5.1 months) in SCHOLAR-1 (supplemental Table 6). Similar to the findings in the primary and sensitivity common support sets, standardized comparison resulted in a 73% reduction in the risk of death in ZUMA-1 vs SCHOLAR-1 (supplemental Table 6). Two-year OS rates were 50% and 12%, respectively (supplemental Figure 2; supplemental Table 6). Notably, for patients who relapsed post-SCT and underwent postrefractory SCT, the 2-year OS rate was greater in ZUMA-1 vs SCHOLAR-1 (50% vs 22%), although ZUMA-1 patient numbers were small (supplemental Table 6). Similar 2-year OS rates were observed in ZUMA-1 vs SCHOLAR-1 for patients who were refractory to ≥2 lines of therapy and later underwent SCT (40% vs 42%). Complete OS results from the standardization analysis are presented in supplemental Table 6.
Discussion
Evaluation of the benefit of a novel therapy in LBCL cannot rely solely on response rates; it must also include long-term survival. CAR T-cell therapy is considered a major breakthrough in the treatment of refractory LBCL, but its approval and potential benefit vs risks, which include manageable toxicities, were based on single-arm phase 2 trials.10,11 These nonrandomized designs were appropriate given the poor outcomes with salvage chemotherapy in the refractory setting.4 Still, comparisons of single-arm trials with historical controls may be difficult to interpret or prone to bias. Statistical methods, such as the weighting or matching of patients based on baseline characteristics, can reduce bias and provide quantitative validation of such comparisons.
Prior to comparing 2-year clinical outcomes, we used propensity scoring to adjust for potential imbalances in a range of covariates between ZUMA-1 and SCHOLAR-1. Although conventional propensity scoring often uses direct patient-level matching methodology, the propensity and standardization analyses conducted here, and the generation of the common support sets, allowed for robust comparison between the ZUMA-1 and SCHOLAR-1 populations by minimizing the number of patients who needed to be excluded from the analysis.21,23-25 Our results indicate a substantial benefit in response rates and OS with axi-cel compared with non-CAR T-cell salvage regimens. More than 50% of ZUMA-1 patients were still alive at 2 years, highlighting the ability of axi-cel to provide long-term disease control for patients who lack other curative treatment options. Analyses based on a sensitivity common support set, as well as a standardization analysis that limited strata to 2 prognostic factors, also favored ZUMA-1 for response and survival end points.
Patients with LBCL who relapse after ASCT have very poor prognoses, although long-term survival may be improved for some by allogeneic SCT.26 However, because of a number of clinical and economic factors, few patients who relapse after ASCT can receive allogeneic SCT.13,26 Our standardization analysis evaluated patients who relapsed post-SCT. Although ZUMA-1 patient numbers were small, 2-year OS rates favored ZUMA-1 over SCHOLAR-1, regardless of whether patients underwent further SCT.
The SCHOLAR-1 study has become the standard comparator used in reporting results of anti-CD19 CAR T-cell therapies for refractory LBCL,10-12 as well as in the regulatory assessment of these products.27,28 However, ours is the first comparison of long-term results of CAR T-cell therapy with a historical control group. A similar comparison of CAR T-cell therapy with recently approved polatuzumab vedotin (in combination with rituximab and bendamustine) may be possible once long-term data are available in patients with relapsed/refractory LBCL ineligible for ASCT.29 Furthermore, results of the ongoing ZUMA-7 trial will determine whether axi-cel can replace ASCT as second-line standard of care in this patient population.30
We acknowledge the limitations inherent in retrospective analysis and cross-study comparisons. Furthermore, propensity score-based methods do not provide the same level of evidence as do randomized controlled trials and cannot control for unmeasured confounding. Thus, other sources of bias may exist as a result of differences between the populations that were not controlled for. For example, patients in SCHOLAR-1 underwent salvage therapy in earlier years compared with patients in ZUMA-1. The results of our propensity score–balanced analyses and standardized analyses indicate that axi-cel is a highly effective treatment option for patients with refractory LBCL compared with available salvage chemoimmunotherapies. Axi-cel produced durable meaningful responses and long-term disease control in patients who otherwise lack curative treatment options. In summary, our results indicate that axi-cel addresses, in part, the considerable unmet need for effective therapies in refractory aggressive LBCL that were identified by the SCHOLAR-1 global retrospective study.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients who participated in the study and their family, friends, and caregivers, as well as the study staff and health care providers.
This work was supported by Kite, a Gilead Company. Medical writing support was provided by Ashley Skorusa (Nexus Global Group Science, LLC) and funded by Kite, a Gilead Company.
Authorship
Contributions: S.S.N., L.N., J.J.K., and C.G. designed the study; L.N. and V.D. performed statistical analyses; S.S.N., L.N., J.J.K., C.G., and V.D. analyzed and interpreted data; and all authors collected data and drafted, reviewed, and revised the manuscript and approved its final version.
Conflict-of-interest disclosure: S.S.N. has received personal fees from Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, and Unum Therapeutics; has received research support from Kite, a Gilead Company, Bristol Myers Squibb, Merck, Poseida Therapeutics, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics, Allogene Therapeutics, Precision Biosciences, and Acerta; has received royalties from Takeda Pharmaceuticals; and holds intellectual property related to cell therapy. F.L.L. has served as an advisor for Kite, a Gilead Company, Novartis, Celgene/Bristol Myers Squibb, Amgen, Calibr, GammaDelta Therapeutics, Wugen, and Allogene; has served as a consultant with grant options for Cellular Biomedicine Group, Inc; and has received research funding from Kite, a Gilead Company. His employer holds patents in his name with regard to improvements to CAR T-cell therapy. N.L.B. has served as an advisor for Acerta, ADC Therapeutics, BTG, Seattle Genetics, and Roche/Genentech and has received research funding from Affimed, Bristol Myers Squibb, Celgene, Forty Seven, Genentech, Janssen, Immune Design, Kite, a Gilead Company, Merck, Millennium, Pharmacyclics, Pfizer, and Seattle Genetics. P.M.R. has served as a consultant or advisor for Kite, a Gilead Company, and Curis and has received research funding from Seattle Genetics. D.B.M. has served as a consultant or advisor for Kite-Gilead, Novartis, Juno-Celgene-Bristol Myers Squibb, Adaptive Biotech, Pharmacyclics, and Janssen; has received research funding from Kite-Gilead, Novartis, Juno-Celgene-Bristol Myers Squibb, Adaptive Biotech, and Pharmacyclics; has received patents, royalties, or other intellectual property from Pharmacyclics; and has received travel support from Kite-Gilead, Novartis, Juno-Celgene-Bristol Myers Squibb, Adaptive Biotech, Pharmacyclics, and Janssen. C.A.J. has received honoraria from Kite, a Gilead Company, Novartis, Celgene, Bristol Myers Squibb, Humanigen, Precision Biosciences, and Nkarta; has served as a consultant or advisor for Kite, a Gilead Company, Novartis, Celgene, Bristol Myers Squibb, Humanigen, Precision Biosciences, and Nkarta; has participated in the speakers’ bureau for AXIS and Clinical Care Options; has received research funding from Pfizer; and has received travel support from Kite, a Gilead Company, Novartis, Celgene, Precision Biosciences, and Lonza. O.O.O. has received honoraria from Kite, a Gilead Company; has served as a consultant or advisor for Kite, a Gilead Company, Pfizer, Spectrum Pharmaceuticals, and Bayer; and has received research funding from Kite, a Gilead Company. T.S. has served as a consultant or advisor for Juno, Pharmacyclics, and AstraZeneca; has participated in the speakers’ bureau for AstraZeneca, Pharmacyclics, Janssen, and Seattle Genetics; has received research funding from Pharmacyclics, Juno, Kite, a Gilead Company, AstraZeneca, TG Therapeutics, Celgene, and BeiGene; and has received travel support from Juno. Y.L. has served as a consultant or advisor for Kite, a Gilead Company, Janssen, Novartis, Celgene, bluebird bio, Juno, Legend, Sorrento, Gamida Cells, and Vineti and has received research funding from Kite, a Gilead Company, Janssen, Celgene, bluebird bio, Merck, and Takeda. M.C. has served as a consultant or advisor for Servier and Janssen Ortho and has received research funding from Roche. J.K. has received honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Gilead, Janssen, Karyopharm, Merck, Novartis, Roche, and Seattle Genetics; has served as a consultant or advisor for AbbVie, Bristol Myers Squibb, Gilead, Karyopharm, Merck, Roche, and Seattle Genetics; and has received research funding from Roche and Janssen. E.V.D.N. has received travel support from Gilead. U.F. has received honoraria from Kite, a Gilead Company. L.N. was formerly employed by Kite, a Gilead Company, is a current employee of Arcutis Biotherapeutics, and has stock or other ownership in Amgen, bluebird bio, Juno, Kite, a Gilead Company, and Arcutis Biotherapeutics. V.D. has served as a consultant or advisor for and has received travel support from Kite, a Gilead Company. J.J.K. is employed by Kite, a Gilead Company, and has stock or other ownership in the company. The remaining authors declare no competing financial interests.
Correspondence: Sattva S. Neelapu, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: sneelapu@mdanderson.org.
References
- 1.Surveillance, Epidemiology and End Results Program. SEER Cancer Statistics Review, 1975-2013. Available at: https://seer.cancer.gov/archive/csr/1975_2013/. Accessed 4 May 2021.
- 2.Sant M, Minicozzi P, Mounier M, et al. ; EUROCARE-5 Working Group . Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 2014;15(9):931-942. [DOI] [PubMed] [Google Scholar]
- 3.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR.. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459. [DOI] [PubMed] [Google Scholar]
- 4.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study [published correction appears in Blood. 2018;131(5):587–588]. Blood. 2017;130(16):1800-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer JN, Rosenberg SA.. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013; 10(5):267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32(31):3490-3496. [DOI] [PubMed] [Google Scholar]
- 7.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51(1):51-57. [DOI] [PubMed] [Google Scholar]
- 9.Gisselbrecht C, Schmitz N, Mounier N, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30(36): 4462-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 12.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852. [DOI] [PubMed] [Google Scholar]
- 13.Locke FL, Go WY, Neelapu SS.. Development and use of the anti-CD19 chimeric antigen receptor T-cell therapy axicabtagene ciloleucel in large B-cell lymphoma: a review. JAMA Oncol. 2020;6(2):281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed MA, Chihara D, Vargas N, et al. Outcome of relapsed/refractory diffuse large B-cell lymphoma after second salvage therapy: MD Anderson experience. Hematol Oncol. 2015;33:279-280. [Google Scholar]
- 17.Thompson CA, Ghesquieres H, Maurer MJ, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol. 2014;32(31):3506-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Horning SJ, Coiffier B, et al. ; NCI Sponsored International Working Group . Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17(4):1244. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Zaslavsky AM, Landrum MB.. Propensity score weighting with multilevel data. Stat Med. 2013;32(19):3373-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neelapu SS, Locke FL, Bartlett NL, et al. A comparison of one year outcomes in ZUMA-1 (axicabtagene ciloleucel) and SCHOLAR-1 in patients with refractory, aggressive non-Hodgkin lymphoma (NHL). Blood. 2017;130(suppl 1):579. [Google Scholar]
- 23.Lunceford JK, Davidian M.. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study [published correction appears in Stat Med. 2017;36(14):2320]. Stat Med. 2004;23(19):2937-2960. [DOI] [PubMed] [Google Scholar]
- 24.Bai X, Tsiatis AA, O’Brien SM.. Doubly-robust estimators of treatment-specific survival distributions in observational studies with stratified sampling. Biometrics. 2013;69(4):830-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothman KJ, Greenland S, Lash TL. Introduction to Regression Modeling. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins;2008:442–451. [Google Scholar]
- 26.Rigacci L, Puccini B, Dodero A, et al. ; Gruppo Italiano Trapianto di Midollo Osseo (GITMO) . Allogeneic hematopoietic stem cell transplantation in patients with diffuse large B cell lymphoma relapsed after autologous stem cell transplantation: a GITMO study. Ann Hematol. 2012;91(6):931-939. [DOI] [PubMed] [Google Scholar]
- 27.Papadouli I, Mueller-Berghaus J, Beuneu C, et al. EMA review of axicabtagene ciloleucel (Yescarta) for the treatment of diffuse large B-cell lymphoma. Oncologist. 2020;25(10):894-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali S, Kjeken R, Niederlaender C, et al. The European Medicines Agency review of kymriah (tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Oncologist. 2020;25(2):e321-e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-CELL LYMPhoma. J Clin Oncol. 2020;38(2): 155-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oluwole OO, Bishop MR, Gisselbrecht C, et al. ZUMA-7: a phase 3 randomized trial of axicabtagene ciloleucel (Axi-Cel) versus standard-of-care (SOC) therapy in patients with relapsed/refractory diffuse large B cell lymphoma (R/R DLBCL). J Clin Oncol. 2018;36(15 suppl):TPS7585. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.