Key Points
Three-year survival is similar after PTCy haplo- and UCB transplant.
Lower relapse but higher nonrelapse mortality in ≤5/8 matched UCB as compared with haplo- and 6-8/8 UCB transplant.
Visual Abstract
Abstract
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has emerged as an important treatment modality. Most reports comparing haplo-HSCT with posttransplant cyclophosphamide (PTCy) and other donor sources have focused on outcomes in older adults treated with reduced intensity conditioning. Therefore, in the current study, we evaluated outcomes in patients with hematological malignancy treated with myeloablative conditioning prior to haplo- (n = 375) or umbilical cord blood (UCB; n = 333) HSCT. All haplo recipients received a 4 of 8 HLA-matched graft, whereas recipients of UCB were matched at 6-8/8 (n = 145) or ≤5/8 (n = 188) HLA antigens. Recipients of 6-8/8 UCB transplants were younger (14 years vs 21 and 29 years) and more likely to have lower comorbidity scores compared with recipients of ≤5/8 UCB and haplo-HSCT (81% vs 69% and 63%, respectively). UCB recipients were more likely to have acute lymphoblastic leukemia and transplanted in second complete remission (CR), whereas haplo-HSCT recipients were more likely to have acute myeloid leukemia in the first CR. Other characteristics, including cytogenetic risk, were similar. Survival at 3 years was similar for the donor sources (66% haplo- and 61% after ≤5/8 and 58% after 6-8/8 UCB). Notably, relapse at 3 years was lower in recipients of ≤5/8 UCB (21%, P = .03) compared with haplo- (36%) and 6-8/8 UCB (30%). However, nonrelapse mortality was higher in ≤5/8 UCB (21%) compared with other groups (P < .0001). These data suggest that haplo-HSCT with PTCy after myeloablative conditioning provides an overall survival outcome comparable to that after UCB regardless HLA match group.
Introduction
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has emerged as an important alternative donor source.1,2 Historically, the successful use of haplo-HSCT was limited by high risks of severe graft-versus-host disease (GVHD), graft failure, and mortality.3-5 In order to ameliorate these risks, more intensive conditioning regimens were often coupled with complex T-cell depletion procedures,6-13 often leading to increased risks of opportunistic infections because of delayed immune recovery as well as conditioning-related toxicities. However, the seminal work by Santos and Owens14 and later by Luznik et al15 laid the foundation for exploring the potential of high-dose posttransplant cyclophosphamide (PTCy). Today, haplo-HSCT with PTCy represents ∼15% of all allogeneic transplants worldwide. The PTCy platform is relatively inexpensive and readily available. PTCy is easy to incorporate into existing treatment plans and does not require more toxic conditioning or ex vivo T-cell depletion of the allograft. It provides significant protection from GVHD and is associated with a low incidence of nonrelapse mortality particularly in adults with hematological malignancies treated with in a reduced intensity conditioning.1,2,16,17 In the absence of an HLA-matched related or unrelated donor, haploidentical relatives and banked umbilical cord blood (UCB) offer access to a potentially life-saving treatment as well as rapid availability of a donor and greater flexibility in timing of transplantation.18
To date, most comparisons between haplo- and UCB HSCT for hematologic malignancy have been restricted to patients treated with either nonmyeloablative or reduced intensity conditioning regimens.19-21 These studies have failed to show a difference in disease-free survival between haplo- and UCB HSCT. In a comparative study of haplo- and UCB HSCT for lymphoma, progression-free and overall survival were higher after haplo-HSCT.22 In this study, we compared the outcomes of patients with acute leukemia in remission who underwent a haplo- and UCB HSCT with myeloablative conditioning regimens for acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) in remission at transplantation .
Methods
Patients
Data were reported prospectively to the Center for International Blood and Marrow Transplant Research, a voluntary working group of >450 transplant centers worldwide that contribute data on allogeneic and autologous hematopoietic cell transplant. Participating centers report consecutive transplants, and compliance is monitored by on-site audits. All patients are followed longitudinally until death or lost to follow-up. For this study, eligible patients were <50 years of age and received either a haploidentical (≥2 HLA-loci mismatch) or partially HLA-matched UCB (2 to 8 of 8 HLA match) graft for AML or ALL between 2012 and 2017 in the United States. All patients were transplanted in first or second complete remission (CR). Recipients of UCB HSCT received a single UCB unit (n = 69) or 2 UCB units (n = 113). All patients regardless of donor type received myeloablative conditioning regimens.23 Recipients of haplo-HSCT received pharmacological immune suppression with a calcineurin inhibitor and mycophenolate mofetil in addition to PTCy for GVHD prophylaxis. Recipients of UCB HSCT received a calcineurin inhibitor and mycophenolate mofetil. None of the patients received in vivo T-cell depletion. Excluded were patients who were not in remission at transplantation or received a reduced intensity or nonmyeloablative conditioning regimens. Other exclusion criteria included transplantations with graft manipulation (eg, expansion of UCB HSC or ex vivo T-cell depletion of haplo-HSC). Patients or their legal guardian provided written consent for research. The study was approved by the Institutional Review Board of the National Marrow Donor Program.
Endpoint
Neutrophil recovery was defined as achieving a count of ≥0.5 × 109/L for 3 consecutive days and platelet recovery, as achieving a count of ≥20 × 109/L without transfusions for 7 consecutive days. Grades II to IV acute GVHD, grade III to IV acute GVHD, and chronic GVHD were defined using standard definitions.23,24 Morphologic, cytogenetic, or molecular recurrence of leukemia was considered as relapse and death in remission, nonrelapse mortality. Leukemia-free survival was defined as being alive in continuous remission. Death from any cause was an event, and surviving patients were censored at last follow-up.
Statistical analysis
Patient-, disease-, and transplant-related characteristics were compared between treatment groups using the χ2 test for categorical variables. The probability of neutrophil and platelet recovery and acute and chronic GVHD was calculated using the cumulative incidence estimator to accommodate competing risks.25 Cox regression models were built to identify risk factors for acute and chronic GVHD, nonrelapse mortality, relapse, overall, and leukemia-free survival.26 Donor type (haplo- vs ≤5/8 UCB vs 6-8/8 UCB) was held in all steps of model building and the final model, and other covariates were retained in the final model if a significance level of <0.05 was achieved. Forward stepwise selection was used to identify significant covariates. All covariates met the assumption for proportionality, and there were no first-order interactions between donor type and other covariates held in the final model. The probabilities of overall and leukemia-free survival and cumulative incidence probabilities for nonrelapse mortality and relapse were generated from the final Cox models.27,28 An effect of transplant center on overall survival was tested using the frailty test and center volume.29 All analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
Patient, disease, and transplant characteristics
Seven hundred eight patients were eligible, and their characteristics are shown by donor type in Table 1. Compared with recipients of haplo-HSCT, UCB recipients regardless of HLA-match group were more likely to have a better performance score and less likely to have AML or be in first CR. However, recipients of UCB matched at 6-8/8 also tended to be younger and have a lower comorbidity index relative to recipients of haplo-HSCT; recipients of more mismatched (≤5/8) UCB were more likely to be male relative to recipients of either haplo- or 6-8/8 UCB HSCT. The median age and comorbidity index of recipients of haploidentical relative and ≤5/8 UCB HLA-matched transplants were not different. All recipients of UCBs were conditioned with a combination of total body irradiation (TBI), cyclophosphamide (Cy), and fludarabine (Flu), whereas recipients of haplo-HSCT received either a TBI (TBI/Cy or TBI/Flu; N = 225 of 375, 60%) or busulfan-based (Flu/Bu or Bu/Cy; N = 150 of 375, 40%) myeloablative regimen. All recipients received a calcineurin inhibitor and mycophenolate as GVHD prophylaxis with posttransplant Cy (PTCy) only administered for haplo-HSCTs. Recipients of haplo-HSCT received a peripheral blood (n = 249; 66%) or bone marrow (n = 126; 34%) graft. Although UCB HSCTs were fairly well distributed between the transplant periods, haplo-HSCT was more likely after 2014. Consequently, the median follow-up after haplo-HSCT was 24 months as compared with 46 months and 36 months in recipients of ≤5/8 and 6-8/8 UCB HLA-matched transplants, respectively.
Table 1.
Patient and disease characteristics
| Variables | Haplo-HSCT | ≤5/8 HLA-matched UCB | 6-8/8 HLA-matched UCB | P value comparing haplo- to UCB | |
|---|---|---|---|---|---|
| ≤5/8 | 6-8/8 | ||||
| Number of subjects | 375 | 188 | 145 | ||
| Age, y | <.001 | <.001 | |||
| ≤16 | 67 (18%) | 70 (37%) | 84 (58%) | ||
| 17 to 29 | 132 (35%) | 61 (32%) | 26 (18%) | ||
| 30 to 39 | 75 (20%) | 34 (18%) | 21 (15%) | ||
| 40 to 49 | 101 (27%) | 23 (12%) | 14 (10%) | ||
| Sex | .13 | .99 | |||
| Male | 202 (54%) | 114 (61%) | 78 (54%) | ||
| Female | 173 (46%) | 74 (39%) | 67 (46%) | ||
| Race | .84 | .06 | |||
| Caucasian | 235 (63%) | 113 (60%) | 100 (69%) | ||
| Non-Caucasian | 109 (29%) | 58 (31%) | 28 (19%) | ||
| Not reported | 31 (8%) | 17 (9%) | 17 (12%) | ||
| Performance score | <.0001 | .31 | |||
| 90 to 100 | 235 (63%) | 150 (80%) | 101 (70%) | ||
| ≤80 | 132 (35%) | 37 (20%) | 42 (29%) | ||
| Not reported | 8 (2%) | 1 (<1%) | 2 (1%) | ||
| Comorbidity index | .20 | <.0001 | |||
| ≤2 | 237 (63%) | 129 (69%) | 117 (81%) | ||
| ≥3 | 138 (37%) | 59 (31%) | 28 (19%) | ||
| Recipient CMV serostatus | .74 | .65 | |||
| Negative | 119 (32%) | 57 (30%) | 52 (36%) | ||
| Positive | 254 (68%) | 129 (69%) | 92 (63%) | ||
| Not reported | 2 (<1%) | 2 (1%) | 1 (<1%) | ||
| Disease | .003 | .002 | |||
| AML | 193 (52%) | 72 (38%) | 53 (37%) | ||
| ALL | 182 (49%) | 116 (62%) | 92 (63%) | ||
| Disease status | .02 | .002 | |||
| 1st CR | 233 (62%) | 98 (52%) | 68 (47%) | ||
| 2nd CR | 142 (38%) | 90 (48%) | 77 (53%) | ||
| Disease risk index | .17 | .003 | |||
| Low/intermediate | 280 (75%) | 130 (70%) | 89 (62%) | ||
| High/very high | 95 (25%) | 57 (30%) | 56 (39%) | ||
| Cytogenetic risk | .002 | .06 | |||
| Favorable | 79 (21%) | 61 (32%) | 44 (30%) | ||
| Intermediate | 116 (31%) | 55 (29%) | 48 (33%) | ||
| Poor | 148 (40%) | 68 (36%) | 46 (32%) | ||
| Not reported | 32 (9%) | 4 (2%) | 7 (5%) | ||
| Transplant period | <.0001 | <.0001 | |||
| 2012-2014 | 64 (19%) | 105 (58%) | 67 (48%) | ||
| 2015-2017 | 272 (81%) | 77 (42%) | 73 (52%) | ||
Hematopoietic recovery
The median time to neutrophil recovery was 16 days (interquartile range [IQR] 14-19) after haplo-HSCT compared with 22 days (IQR, 17-26) and 19 days (IQR, 17-26) after ≤5/8 and 6-8/8 UCB HLA-matched transplants, respectively (P < .001). Consequently, the day 28 incidence of neutrophil recovery was higher after haplo-HSCT (93%, 95% confidence interval [CI], 91-96) compared with UCB regardless of HLA match (74%, 95% CI, 68-80 for ≤5/8 UCB and 81%, 95% CI, 74-87 for 6-8/8 UCB; P < .001). The corresponding time to platelet recovery was 27 days (IQR, 18-30), 43 days (IQR, 37-55), and 40 days (IQR, 37-52) (P < .001) with the day 100 incidence of platelet recovery also higher after haplo-HSCT (90%, 95% CI, 87-93) compared with UCB (73%, 95% CI, 66-79 for ≤5/8 UCB and 89%, 95% CI, 83-94 for 6-8/8 UCB; P < .001).
Acute and chronic GVHD
Compared with haplo-HSCT, grade II to IV acute GVHD risk was higher after ≤5/8 and 6-8/8 UCB (Table 2). The day 90 incidence of grade II to IV acute GVHD was 26% (95% CI, 22-31), 63% (95% CI, 56-69), and 48% (95% CI, 40-56) after haplo-HSCT, ≤5/8 and 6-8/8 UCB, respectively (P < .0001 and P < .0001 comparing haplo-HSCT to each UCB cohort). No other factor was associated with acute grade II to IV GVHD risk. Similarly, grade III to IV acute GVHD risk was also higher after ≤5/8 and 6-8/8 UCB HLA-matched transplants (Table 2) with the day 90 incidence of grade III to IV acute GVHD being 6% (95% CI, 4-9), 30% (95% CI, 24-37), and 18% (95% CI, 12-25) after haplo-HSCT, ≤5/8 and 6-8/8 UCB, respectively (P < .0001 and P = .0005). Separate analysis of pediatric (age ≤16 years) and adult cohorts also showed higher acute GVHD after UCB HSCT (supplemental Tables 1 and 2). In a subset analysis limited to recipients of haploidentical HSCT, grade II to IV but not grade III to IV acute GVHD was higher after transplantation of peripheral blood compared with bone marrow grafts (Table 3).
Table 2.
Multivariate analyses
| Outcomes | Hazard ratio (95% CI) |
P |
|---|---|---|
| Grade II to IV acute GVHD | ||
| Donor type | ||
| Haplo-HSCT | 1.00 | |
| ≤5/8 HLA-matched UCB | 2.90 (2.25-3.74) | <.0001 |
| 6-8/8 HLA-matched UCB | 1.80 (1.35-2.39) | <.0001 |
| Grade III to IV acute GVHD | ||
| Donor type | ||
| Haplo-HSCT | 1.00 | |
| ≤5/8 HLA-matched UCB | 4.95 (3.09-7.93) | <.0001 |
| 6-8/8 HLA-matched UCB | 2.74 (1.59-4.72) | .0003 |
| Chronic GVHD | ||
| Donor type | ||
| Haplo-HSCT | 1.00 | |
| ≤5/8 HLA-matched UCB | 1.28 (0.95-1.73) | .10 |
| 6-8/8 HLA-matched UCB | 1.18 (0.84-1.67) | .34 |
| Nonrelapse mortality | ||
| Donor type | ||
| Haplo-HSCT | 1.00 | |
| ≤5/8 HLA-matched UCB | 3.05 (2.00-4.67) | <.0001 |
| 6-8/8 HLA-matched UCB | 2.28 (1.35-3.86) | .002 |
| Age, y | ||
| ≤16 | 1.00 | |
| >16 | 2.98 (1.79-4.96) | <.0001 |
| Relapse | ||
| Donor type | ||
| Haplo-HSCT | 1.00 | |
| ≤5/8 HLA-matched UCB | 0.65 (0.45-0.91) | .026 |
| 6-8/8 HLA-matched UCB | 0.95 (0.66-1.36) | .79 |
| Disease status at transplantation | ||
| 1st CR | 1.00 | |
| 2nd CR | 1.63 (1.22-2.18) | .001 |
| Overall survival | ||
| Donor type | ||
| Haplo-HSCT | 1.00 | |
| ≤5/8 HLA-matched UCB | 1.38 (1.02-1.87) | .037 |
| 6-8/8 HLA-matched UCB | 1.34 (0.94-1.92) | .10 |
| Age, y | ||
| ≤16 | 1.00 | |
| >16 | 1.50 (1.09-2.07) | .014 |
| Disease status at transplantation | ||
| 1st CR | 1.00 | |
| 2nd CR | 1.45 (1.12-1.89) | .005 |
| HCT comorbidity score | ||
| ≤2 | 1.00 | |
| ≥3 | 1.36 (1.04-1.79) | .030 |
| Leukemia-free survival | ||
| Donor type | ||
| Haplo-HSCT | 1.00 | |
| ≤5/8 HLA-matched UCB | 1.17 (0.89-1.53) | .26 |
| 6-8/8 HLA-matched UCB | 1.17 (0.87-1.58) | .28 |
| Disease status at transplantation | ||
| 1st CR | 1.00 | |
| 2nd CR | 1.42 (1.13-1.79) | .003 |
| HCT comorbidity score | ||
| ≤2 | 1.00 | |
| ≥3 | 1.39 (1.09-1.77) | .007 |
Table 3.
Haploidentical transplant: comparison of graft source
| Bone marrow | Peripheral blood | P | |||
|---|---|---|---|---|---|
| Outcomes | N evaluable | Cumulative incidence (95% CI), % |
N evaluable | Cumulative incidence (95% CI), % |
|
| Day 100 grade II to IV acute GVHD | 126 | 21 (14-28) | 247 | 30 (25-26) | .04 |
| Day 100 grade III to IV acute GVHD | 119 | 6 (2-11) | 233 | 6 (4-10) | .84 |
| Chronic GVHD at 3 y | 124 | 22 (15-30) | 244 | 48 (41-56) | <.001 |
| Nonrelapse mortality at 3 y | 126 | 6 (2-10) | 247 | 14 (10-19) | .03 |
| Relapse at 3 y | 126 | 35 (26-45) | 247 | 35 (27-43) | .51 |
| Overall survival at 3 y | 126 | 71 (62-80) | 249 | 60 (52-68) | .39 |
| Leukemia-free survival at 3 y | 126 | 59 (49-69) | 247 | 51 (43-59) | .40 |
Chronic GVHD, however, was similar between the donor sources (Table 2). The 3-year incidence of chronic GVHD was 38% (95% CI, 32-44), 40% (95% CI, 32-48), and 43% (95% CI, 33-52) after haplo-HSCT, ≤5/8 and 6-8/8 UCB, respectively (P = .65 and P = .42). Patients >16 years had significantly higher risk for chronic GVHD compared with younger patients (HR, 1.61; 95% CI, 1.19-2.19; P = .0020). A separate analysis of pediatric and adult cohorts did not show differences in chronic GVHD risks between donor types (supplemental Tables 1 and 2). Among haploidentical HSCT recipients, chronic GVHD was higher after transplantation of peripheral blood (Table 3).
Nonrelapse mortality and relapse
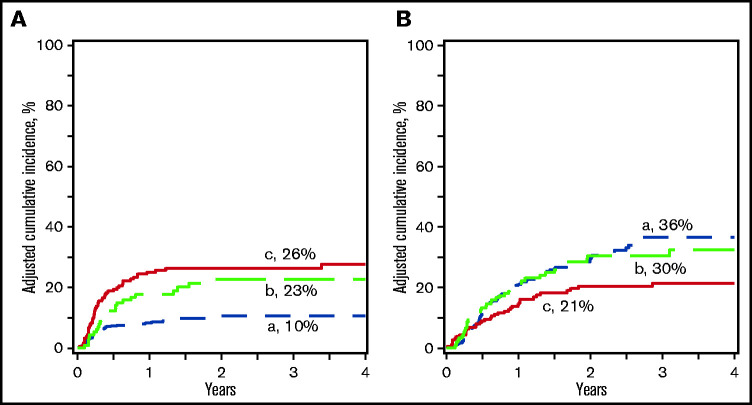
Compared with haplo-HSCT, nonrelapse mortality was higher after UCB regardless of HLA match (Table 2; Figure 1A). The 3-year incidence of nonrelapse mortality was 10% (95% CI, 7-14), 26% (95% CI, 20-33), and 23% (95% CI, 15-31) after haplo-HSCT, ≤5/8 and 6-8/8 UCB HLA-matched transplants, respectively (P < .0001 and P = .007). Regardless of donor type, patients >16 years had higher risk for nonrelapse mortality compared with those who were younger (Table 2). A separate analysis of the adult cohort confirmed higher nonrelapse mortality after ≤5/8 and 6-8/8 UCB HSCT (supplemental Table 2). In those aged <16 years, the risk for nonrelapse mortality was twofold higher after ≤5/8 UCB HSCT but overlapping CIs from a modest sample size meant level of significance was >0.05 (supplemental Table 1). The 3-month incidence of systemic infections (bacterial, viral, or fungal) was higher after ≤5/8 (15%, 95% CI, 10-20; P = .003) UCB compared with haplo- or 6-8/8 (5%, 95% CI, 2-9 and 6%, 95% CI, 2-10, respectively). Similarly, the corresponding 6-month incidence of systemic infection was 22% (95% CI, 16-28), 11% (95% CI, 7-17), and 11% (95% CI, 6-17) (P = .010).
Figure 1.
Nonrelapse mortality and relapse. (A) The incidence of nonrelapse mortality after haploidentical relative (a), ≤5/8 (b), and 6-8/8 (c) UCB transplant. (B) The incidence of relapse after haploidentical relative (a), ≤5/8 (b), and 6-8/8 (c) UCB transplant.
Compared with haplo-HSCT, relapse risk was lowest after ≤5/8 UCB (Table 2; Figure 1B). The 3-year incidence of relapse was 21% (95% CI, 15-28) after ≤5/8 UCB compared with 36% (95% CI, 30-43) after haplo-HSCT (P = .0009) and 30% (95% CI, 23-38) after 6-8/8 UCB (P = .23). Regardless of donor type, relapse risk was higher for recipients transplanted second CR as compared with first CR. Although the relapse risk was lower after ≤5/8 UCB compared with haplo-HSCT in patients aged ≤16 years, this did not reach statistical significance (supplemental Table 1). However, in those who were older, relapse risk was lower after ≤5/8 and 6-8/8 UCB compared with haplo-HSCT (supplemental Table 2). In subset analysis limited to recipients of haplo-HSCT, nonrelapse mortality was higher after transplantation of peripheral blood, but relapse risks did not differ by graft type (Table 3).
Overall and leukemia-free survival
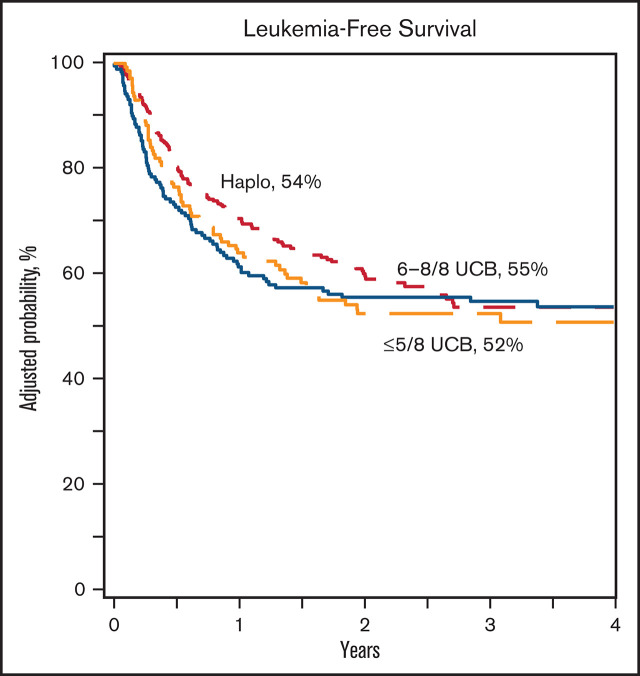
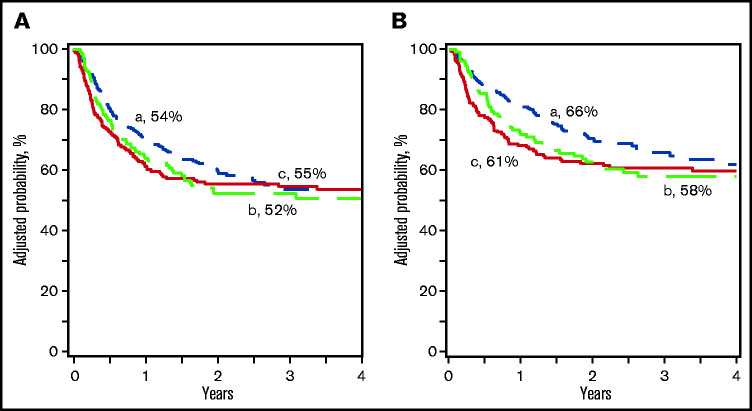
Compared with haplo-HSCT, overall survival was lower after ≤5/8 UCB and comparable to 6-8/8 UCB (Table 2; Figure 2A). The 3-year probability of survival adjusted for age, HCT comorbidity score, and disease status was 66% (95% CI, 60-71), 61% (95% CI, 53-67), and 58% (95% CI, 48-67) after haplo-HSCT, ≤5/8 and 6-8/8 UCB, respectively (P = .28 and P = .17). Overall survival was lower for patients >16 years, transplanted in CR2, and a comorbidity score ≥3. Overall survival was not associated with performance score or disease type (supplemental Table 3). A separate analysis of the pediatric and adult cohorts confirmed higher mortality after ≤5/8 and 6-8/8 UCB HSCT in adults but not in children (supplemental Tables 1 and 2). There were no differences in leukemia-free survival by treatment groups (Table 2; Figure 2B). The 3-year probability of leukemia-free survival adjusted for HCT comorbidity score and disease status was 54% (95% CI, 47-60), 55% (95% CI, 47-62), and 52% (95% CI, 44-60) after haplo-HSCT, ≤5/8 and 6-8/8 UCB HLA-matched transplants, respectively (P = .82 and P = .82). Leukemia-free survival was lower for patients transplanted in CR2 and comorbidity score ≥3. Leukemia-free survival was not associated with performance score or disease type (supplemental Table 3). Separate analysis of the pediatric and adult cohorts did not show differences by donor type (supplemental Tables 1 and 2). Among recipients of haploidentical HSCT, leukemia-free and overall survival did not differ by graft type (Table 3).
Figure 2.
Leukemia-free and overall survival. (A) The probability of leukemia-free survival after haploidentical relative (a), ≤5/8 (b), and 6-8/8 (c) UCB transplant. (B) The probability of overall survival after haploidentical relative (a), ≤5/8 (b), and 6-8/8 (c) UCB transplant.
Recurrent disease was the most frequent cause of death after haplo-HSCT (60 of 110, 55%) and 6-8/8 HLA-matched UCB (25 of 51, 49%) in contrast to ≤5/8 HLA-matched UCB (21 of 73, 29%). Infection was the second most frequent cause of death after haplo-HSCT (16 of 110, 15%). Multiorgan failure (15 of 73, 21%), GVHD (14 of 73, 19%), and infection (12 of 73, 16%) were common causes of death after ≤5/8 HLA-matched UCB transplants. Multiorgan failure (6 of 51, 12%) and interstitial pneumonitis (6 of 51, 12%) were also common causes of death after 6-8/8 HLA-matched USB. Other causes of death did not differ by donor type.
Transplant center effect
An effect of transplant center on overall survival was examined using the frailty test, and we did not observe an effect (P = .24). Consistent with the main analyses (Table 2), compared with haplo-HSCT, survival was lower after ≤5/8 UCB (HR, 1.41; 95% CI, 1.02-1.95; P = .041) but not after 6-8/8 UCB HSCT (HR, 1.33; 95% CI, 0.91-1.93; P = .14). One hundred fifteen centers contributed patients to the study. Of that, 73 centers contributed 1 to 5 cases, 24 centers contributed 6 to 10 cases, and 18 centers contributed >10 cases. We did not observe differences in survival comparing centers that contributed >10 cases vs 6 to 10 cases (HR, 1.00; 95% CI, 0.73-1.38; P = .98) and 1 to 5 cases (HR, 0.89; 95% CI, 0.66-1.21; P = .47). An examination of center volume separately by haplo-HSCT (>10 vs 6-10 [P = .17] and 1-5 [P = .51] cases) and UCB HSCT (>10 vs 6-10 [P = .19] and 1-5 [P = .56]) also failed to show an effect on survival.
Discussion
The primary aim of this analysis was to determine the relative risks of relapse and mortality in recipients of haplo-HSCT and UCB HSCT that was HLA-matched at ≤5/8 or 6-8/8 in children, adolescents, and adults with AML and ALL in first or second CR treated with myeloablative transplant conditioning regimens. After adjusting for HCT comorbidity score, performance score disease type, and disease status at transplantation, we did not observe significant differences in leukemia-free survival after haplo- and UCB HSCT. An examination of the pediatric (age ≤16 years) and adult cohorts separately also failed to show significant differences in leukemia-free survival between donor types. Those >16 years were at higher risk for nonrelapse mortality and lower survival. This was particularly evident when the analysis included only adults but consistent with the main analysis that leukemia-free survival did not differ by donor type. The pattern of treatment failure differed by donor type. After adjusting for age at transplantation, nonrelapse mortality was higher after ≤5/8 and 6-8/8 UCB HSCT, but relapse risk was lower especially after ≤5/8 UCB HSCT and thought to be mediated by graft-versus-leukemia effect. This negated any potential adverse effect on leukemia-free survival after UCB compared with haplo-HSCT. Our findings are consistent with that of an earlier report from Europe that did not find a difference in leukemia-free survival between haplo- and UCB HSCT.19 However, that report19 included both myeloablative and reduced intensity conditioning regimens, and only about half of the haplo-HSCTs received PTCy containing GVHD prophylaxis. In contrast, 2 recent reports from Europe on patients with acute leukemia who received a thiotepa-containing non-irradiation-containing myeloablative regimen found higher survival after haplo- compared with UCB HSCT.30,31 We searched carefully for a transplant center on survival and found none. Thus, the available data support using haplo or UCB as alternate donor sources to increase access to HSCT when an HLA-matched relative or unrelated adult donor is not available.
The relative risks of GVHD comparing haplo- and UCB HSCT, however, have been inconsistent. In the recently reported randomized trial of haplo- vs UCB HSCT with reduced intensity conditioning, incidences of acute and chronic GVHD were similar.21 In the current analyses in which the intensity of the conditioning regimen was myeloablative and all recipients of haplo-HSCT received PTCy for GVHD prophylaxis, grade II to IV and grade III to IV acute GVHD but not chronic GVHD were higher after UCB HSCT and consistent with that reported by others.19 PTCy induces early immune tolerance mediated by destruction of alloreactive donor and recipient T cell, and any remaining alloreactivity is counterbalanced by increasing the number of regulatory T cells.32 A delayed but long-lasting intrathymic clonal deletion of antihost cells maintains long-term immune tolerance.32 Bone marrow and peripheral blood grafts were used for haplo-HSCT in the current analyses. Consistent with other reports, we observed higher chronic GVHD after transplantation of peripheral blood but without an adverse effect on survival.33 Others have examined use of PTCy containing GVHD prophylaxis for UCB HSCT in a limited number of patients.34 This strategy for GVHD prophylaxis has not been adopted widely considering the importance of lymphocyte recovery as a predictor of outcomes after UCB HSCT.35 In addition to tackling delayed immune reconstitution that uniformly occurs after allogeneic HSCT regardless of HSC source,35,36 higher risks of graft failure and slower hematopoietic recovery have long been recognized as a limitation of UCB due to the relatively low number of HSC infused, including CD34 dose and a greater HLA disparity between the UCB graft and recipient.37-39 As expected, in this analysis, hematopoietic recovery was also slower after UCB transplant in the setting of UCB. Although engraftment rates have improved over time because of the concerted effort of cord blood banks to store only units with higher cell doses, recent advances in ex vivo expansion culture have led to marked improvements in engraftment and rates of recovery after UCB transplant, increasingly comparable to that observed after peripheral blood and bone marrow transplants.40-42
In summary, our results demonstrate that both haplo- and UCB HSCT are efficacious. The effect of a higher risk of nonrelapse mortality after UCB HSCT is tempered by lower risk of relapse (although not always statistically significant) negating an adverse effect on leukemia-free survival. Access to alternative donor HSCT is especially important as the general population becomes increasingly diverse, making it more difficult to identify an HLA-matched unrelated donor when an HLA-matched sibling donor is not available. However, for those patients at risk of rapid disease progression, rapid access and transplant flexibility are advantages favoring haplo- and UCB HSCT over unrelated donors where donor attrition and prolonged search times can be obstacles.43
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported primarily by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Institutes of Health, National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases grant HHSH250201200016C with the Health Resources and Services Administration grant P01-CA065493 (J.E.W. and C.G.B.)
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: J.E.W., K.K.B., and M.E. designed and coordinated the study; M.-J.Z. performed the statistical analyses; J.E.W., C.G.B., and M.E. wrote the manuscript; and J.E.W., K.K.B., M.-J.Z. M.A.-J., C.K., F.M., M.R.V., M.E, and C.G.B. reviewed the manuscript and contributed to the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudio G. Brunstein, Blood and Marrow Transplant Program, Mayo Mail Code 480, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: bruns072@umn.edu.
References
- 1.Al Malki M, Jones R, Ma Q, et al. Proceedings from the Fourth Haploidentical Stem Cell Transplantation Symposium - HAPLO2016, San Diego, California, December 1, 2016. Biol Blood Marrow Transplant. 2018;24(5):895-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passweg JR, Baldomero H, Bader P, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52(6):811-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersdorf EW. Risk assessment in haematopoietic stem cell transplantation: histocompatibility. Best Pract Res Clin Haematol. 2007;20(2): 155-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313(13):765-771. [DOI] [PubMed] [Google Scholar]
- 5.Anasetti C, Beatty PG, Storb R, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29(2):79-91. [DOI] [PubMed] [Google Scholar]
- 6.Ash RC, Horowitz MM, Gale RP, et al. Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant. 1991;7(6):443-452. [PubMed] [Google Scholar]
- 7.Kernan NA, Flomenberg N, Dupont B, O’Reilly RJ.. Graft rejection in recipients of T-cell-depleted HLA-nonidentical marrow transplants for leukemia. Identification of host-derived antidonor allocytotoxic T lymphocytes. Transplantation. 1987;43(6):842-847. [PubMed] [Google Scholar]
- 8.Henslee-Downey PJ, Parrish RS, MacDonald JS, et al. Combined in vitro and in vivo T lymphocyte depletion for the control of graft-versus-host disease following haploidentical marrow transplant. Transplantation. 1996;61(5):738-745. [DOI] [PubMed] [Google Scholar]
- 9.Soiffer RJ, Mauch P, Fairclough D, et al. CD6+ T cell depleted allogeneic bone marrow transplantation from genotypically HLA nonidentical related donors. Biol Blood Marrow Transplant. 1997;3(1):11-17. [PubMed] [Google Scholar]
- 10.Henslee-Downey PJ, Abhyankar SH, Parrish RS, et al. Use of partially mismatched related donors extends access to allogeneic marrow transplant. Blood. 1997;89(10):3864-3872. [PubMed] [Google Scholar]
- 11.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339(17):1186-1193. [DOI] [PubMed] [Google Scholar]
- 12.Handgretinger R, Klingebiel T, Lang P, et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant. 2001;27(8):777-783. [DOI] [PubMed] [Google Scholar]
- 13.Drobyski WR, Klein J, Flomenberg N, et al. Superior survival associated with transplantation of matched unrelated versus one-antigen-mismatched unrelated or highly human leukocyte antigen-disparate haploidentical family donor marrow grafts for the treatment of hematologic malignancies: establishing a treatment algorithm for recipients of alternative donor grafts. Blood. 2002;99(3):806-814. [DOI] [PubMed] [Google Scholar]
- 14.Santos GW, Owens AH.. Production of graft-versus-host disease in the rat and its treatment with cytotoxic agents. Nature. 1966;210(5032): 139-140. [DOI] [PubMed] [Google Scholar]
- 15.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ.. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98(12):3456-3464. [DOI] [PubMed] [Google Scholar]
- 16.Luznik L, Engstrom LW, Iannone R, Fuchs EJ.. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8(3):131-138. [DOI] [PubMed] [Google Scholar]
- 17.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruggeri A, Ciceri F, Gluckman E, Labopin M, Rocha V; Eurocord and Acute Leukemia Working Party of the European Blood and Marrow Transplant Group . Alternative donors hematopoietic stem cells transplantation for adults with acute myeloid leukemia: umbilical cord blood or haploidentical donors? Best Pract Res Clin Haematol. 2010;23(2):207-216. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri A, Labopin M, Sanz G, et al. ; ALWP-EBMT study . Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(9):1891-1900. [DOI] [PubMed] [Google Scholar]
- 20.Brunstein CG, Fuchs EJ, Carter SL, et al. ; Blood and Marrow Transplant Clinical Trials Network . Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs EJ, O’Donnell PV, Eapen M, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood. 2021;137(3):420-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fatobene G, Rocha V, St Martin A, et al. Nonmyeloablative alternative donor transplantation for Hodgkin and non-Hodgkin lymphoma: from the LWP-EBMT, Eurocord, and CIBMTR [published correction appears in J Clin Oncol. 2020;38(34):4130]. J Clin Oncol. 2020;38(14):1518-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 24.Atkinson K, Horowitz MM, Gale RP, Lee MB, Rimm AA, Bortin MM. Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft-versus-host disease. Committee of the International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1989; 4(3):247-254. [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 26.Cox DR. Regression models and life-tables. J R Stat Soc [Ser A]. 1972;34(2):187-200. [Google Scholar]
- 27.Zhang X, Loberiza FR, Klein JP, Zhang MJ.. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95-101. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Zhang MJ.. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen PK, Klein JP, Zhang M-J.. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18(12):1489-1500. [DOI] [PubMed] [Google Scholar]
- 30.Giannotti F, Labopin M, Shouval R, et al. Haploidentical transplantation is associated with better overall survival when compared to single cord blood transplantation: an EBMT-Eurocord study of acute leukemia patients conditioned with thiotepa, busulfan, and fludarabine. J Hematol Oncol. 2018;11(1):110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanz J, Montoro J, Solano C, et al. Prospective randomized study comparing myeloablative unrelated umbilical cord blood transplantation versus HLA-haploidentical related stem cell transplantation for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2020;26(2):358-366. [DOI] [PubMed] [Google Scholar]
- 32.Al-Homsi AS, Roy TS, Cole K, Feng Y, Duffner U.. Post-transplant high-dose cyclophosphamide for the prevention of graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(4):604-611. [DOI] [PubMed] [Google Scholar]
- 33.Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide [published correction appears in J Clin Oncol. 2019;37(6):528]. J Clin Oncol. 2017;35(26):3002-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacigalupo A, Sica S, Laurenti L, et al. Unrelated cord blood transplantation and post-transplant cyclophosphamide. Haematologica. 2019;104(2):e77-e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciurea SO, Mulanovich V, Jiang Y, et al. Lymphocyte recovery predicts outcomes in cord blood and T cell-depleted haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(8):1169-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogonek J, Kralj Juric M, Ghimire S, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7(7):507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611-1618. [DOI] [PubMed] [Google Scholar]
- 38.Eapen M, Klein JP, Ruggeri A, et al. ; Center for International Blood and Marrow Transplant Research, Netcord, Eurocord, and the European Group for Blood and Marrow Transplantation . Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purtill D, Smith K, Devlin S, et al. Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124(19):2905-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner JE Jr, Brunstein CG, Boitano AE, et al. Phase I/II trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell. 2016;18(1):144-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horwitz ME, Wease S, Blackwell B, et al. Phase I/II study of stem-cell transplantation using a single cord blood unit expanded ex vivo with nicotinamide. J Clin Oncol. 2019;37(5):367-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen S, Roy J, Lachance S, et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1-2 safety and feasibility study. Lancet Haematol. 2020;7(2):e134-e145. [DOI] [PubMed] [Google Scholar]
- 43.Kekre N, Antin JH.. Hematopoietic stem cell transplantation donor sources in the 21st century: choosing the ideal donor when a perfect match does not exist[published correction appears in Blood. 2015;125(3):1048]. Blood. 2014;124(3):334-343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.