Abstract
Repetitive low-level blast exposure is one of the major occupational health concerns among US military service members and law enforcement. This study seeks to identify gene expression using microRNA and RNA sequencing in whole-blood samples from experienced breachers and unexposed controls. We performed experimental RNA sequencing using Illumina’s HiSeq 2500 Sequencing System, and microRNA analysis using NanoString Technology nCounter miRNA expression panel in whole-blood total RNA samples from 15 experienced breachers and 14 age-, sex-, and race-matched unexposed controls. We identified 10 significantly dysregulated genes between experienced breachers and unexposed controls, with FDR corrected <0.05: One upregulated gene, LINC00996 (long intergenic non-protein coding RNA 996); and nine downregulated genes, IGLV3-16 (immunoglobulin lambda variable 3-16), CD200 (CD200 molecule), LILRB5 (leukocyte immunoglobulin-like receptor B5), ZNF667-AS1 (ZNF667 antisense RNA 1), LMOD1 (leiomodin 1), CNTNAP2 (contactin-associated protein 2), EVPL (envoplakin), DPF3 (double PHD fingers 3), and IGHV4-34 (immunoglobulin heavy variable 4-34). The dysregulated gene expressions reported here have been associated with chronic inflammation and immune response, suggesting that these pathways may relate to the risk of lasting neurological symptoms following high exposures to blast over a career.
Keywords: repetitive low-level blast, experienced breacher, traumatic brain injury
1. Introduction
Blast exposure is a prominent feature of the Iraq and Afghanistan conflicts due to the use of improvised explosive devices [1]. The prevalence of blast-exposure injury dramatically increased from 60% (2008) to 74% (2009) in the US military, which accounts for most combat-related casualties [2]. Repetitive high-blast exposure has been associated with neuronal changes as well as cognitive and affective symptoms [3,4]. In addition to these concerns from high-pressure blast exposure, repetitive low-level blast exposure is a major occupational health concern for military and law-enforcement training. Experienced breachers in military and law-enforcement training encounter more than 100 occurrences of repetitive low-level blast overpressure throughout their careers [5]. However, the underlying biological mechanism of repetitive low-level blast exposure and related neurological effects are not well-understood. The lack of understanding of repetitive low-level blast exposure on neurological functioning makes its identification and potential health intervention challenging in a military setting. Previously repetitive low levels of blast exposure have been associated with neurocognitive and neurosensory decline [6,7,8], which positively correlated with blood-based levels of tau, amyloid β (Aβ)40, and Aβ42 proteins [9,10], suggesting that there may be biological changes in the blood that result from these high levels of blast exposure over a career.
To date, a growing number of investigations have been conducted to identify biomarkers following repetitive blast exposure [5,9,10,11,12,13]. Previously we reported the effects of acute blast exposure during military training, which include acute changes in amyloid precursor protein [12], inflammatory markers [interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α)] [13], and longitudinal changes in DNA methylation [14]. It is important to understand the biological mechanism following repetitive low-level blast exposure to develop interventions in preventing short- or long-term associated symptoms that influence the health-related quality of life of US military service members. To address this critical issue, we performed transcriptome-wide analysis in whole-blood RNA sequencing to identify potential gene-expression activity across the genome in an experienced breacher population with a high number of repetitive low-level blast exposures and an unexposed control group. In addition, we used multiplexed miRNA assays to quantify the levels of microRNA expression in the whole blood of the experienced breacher population and unexposed control group.
2. Materials and Methods
2.1. Study Protocol
This study was reviewed and approved by the Naval Medical Research Center (NMRC) and the National Institutes of Health (NIH) Institutional Review Boards. All the study procedures were performed at NIH Clinical Center after obtaining written informed consent. The detailed procedure of this study protocol has been published elsewhere [5,15].
2.2. Demographic, Clinical History, and Psychometric Testing
All participants were administered demographic and clinical information. Psychometric tests were conducted to assess cognitive domains and symptomology of the participants. The groups are well-matched on age, sex, race, and ethnicity. The Immediate Post-Concussion Assessment and Cognitive Test (ImPACT 2.0) was utilized to evaluate verbal-memory composite, visual-memory composite, reaction-time composite, impulse-control composite, and total symptom composite score [16]. The Brief Symptom Inventory (BSI)-18 is an 18-item scale of psychological distress classified into somatization, depression, anxiety, and global severity index subscales [17]. The Combat Exposure Checklist (CEC) is a self-report scale used to measure the frequency of stressful events experienced during deployments. The Neurobehavioral Symptom Inventory (NSI) was used to assess post concussive symptoms. The NSI is a 22-item self-report scale and has shown both excellent internal consistency (α = 0.95) as well as the ability to differentiate veterans with TBIs from those without [18]. Post-Traumatic Stress Disorder (PTSD) Checklist-Military (PCL-M) was used to assess PTSD symptoms. The PCL-M is a 17-item self-report PTSD-symptom scale with scores ranging from 17 to 85. It has been shown to have high test–retest reliability (r = 0.96) and internal consistency (α = 0.96) in Vietnam veterans [19].
2.3. RNA Sequencing and Bioinformatic Analysis
Peripheral blood samples were collected in PAXgene tubes and stored at −80 °C until analyzed. Samples from 29 participants were analyzed using RNA-seq with Illumina’s HiSeq 2500 system, using paired-end sequencing. Each sample has at least 30 million reads—15 million reads for read 1 and 15 million reads for read 2. Each read has 101 bp for its read length. For bioinformatics analysis, we first performed bioinformatics quality control (QC) using FastQC, version 0.11.9. Then, we trimmed 15 bp from 5′-end, and 10 bp from 3′-end, to remove adapter contamination as well as low-quality base calls in 3′-end. We aligned to GRCh38 reference genome using STAR, version 2.7.6a. We counted number of reads mapped to genes using htseq, version 0.11.4. Finally, we found differentially expressed genes using DESeq2, version 1.30.1 with the cutoff of 0.05 on false discovery rate (FDR) adjusted by independent hypothesis weighting. R, version 4.0.3 (10 October 2020) and Bioconductor, version 3.11 was used for analysis.
2.4. MicroRNA Profiling and Bioinformatic Analysis
Analysis was performed with nCounter® Human v3 miRNA Expression Panels (NanoString Technologies, Seattle, WA, USA). The expression panel contained 798 miRNA probes; this was the maximum number of probes available for analysis in human samples. The probes were incorporated in the code sets and used for analysis along with positive and negative controls. All hybridizations took place at 18 h ± 30 min, and all counts were obtained from nCounter® Digital Analyzer. Raw miRNA data were subtracted from the geometric means of the negative control incorporated in the code sets, and top-100 normalization was performed using the nSolver analysis software (version 4.0, NanoString technologies). Normalized data were analyzed by ROSALIND® (with a HyperScale architecture developed by ROSALIND, Inc. (San Diego, CA, USA). Read-distribution violin plots, identity heatmaps, and sample MDS plots were generated as part of the QC step. The limma R library [20] was used to calculate fold changes and p-values and perform optional covariate correction.
2.5. Statistical Analysis
Statistical analysis was conducted with SPSS version 28.0 (IBM Corp., Armonk, NY, USA). Demographic and clinical characteristics were compared between the experienced breacher and control groups using chi-square and independent-samples t-test. Pearson correlation coefficient was performed to assess the association of the interested study variables. Statistical tests were two-tailed and p < 0.05 was considered a significant difference.
3. Results
3.1. Demographic and Clinical Characteristic
The participants recruited for this study were well-matched on demographic characteristics between the unexposed control (N = 14) and experienced breacher (N = 15) groups (Table 1). The majority of participants were white, military personnel, and had a mean age of 40 years. There were no differences in demographics including age, sex, and ethnicity between experienced breachers and unexposed controls. The mean values of self-reported career breachers were 4659.20 breaching blast exposures in the experienced breacher group and 5.86 breaching blast exposures in the unexposed control group over their careers.
Table 1.
Demographic characteristics of study participants.
| Unexposed Control (N = 14) |
Experienced Breacher (N = 15) | Significance | |
|---|---|---|---|
| Age, mean (SD) | 38.86 (7.81) | 41.60 (8.42) | t = 0.907, p = 0.372 |
| Sex (Male), N (%) | 14 (100) | 15 (100) | N/A |
| Race, N (%) | |||
| White | 12 (85.7) | 13 (86.7) | χ2 = 2.008, p = 0.571 |
| Black | 1 (7.1) | 0 (0.0) | |
| Asian | 1 (7.1) | 1 (6.7) | |
| American Indian/Alaskan | 0 (0) | 1 (6.7) | |
| Ethnicity (Non-Hispanic), N (%) | 13 (92.9) | 15 (100) | χ2 = 1.110, p = 0.483 |
| Type of Service, N (%) | |||
| Military | 10 (71.4) | 10 (66.7) | N/A |
| Civilian Law Enforcement | 4 (28.6) | 5 (33.3) | |
| Duration of service, mean (SD) | 13.71 (7.12) | 18.40 (6.82) | t = −1.809, p = 0.082 |
| Total blast exposures, mean (SD) | 5.86 (10.42) | 5659.20 (9649.52) | t = −2.269, p = 0.040 |
| Breaches in career, N (%) | |||
| 0 | 13 (92.9) | 0 (0.0) | N/A |
| 10–39 | 1 (7.1) | 0 (0.0) | |
| 100–199 | 0 (0.0) | 1 (6.7) | |
| 200–399 | 0 (0.0) | 1 (6.7) | |
| 400+ | 0 (0.0) | 13 (86.7) | |
| Breaches in past year, N (%) | |||
| 0 | 14 (100) | 2 (13.3) | N/A |
| 1–9 | 0 (0.0) | 2 (13.3) | |
| 10–39 | 0 (0.0) | 1 (6.7) | |
| 40–99 | 0 (0.0) | 1 (6.7) | |
| 100–199 | 0 (0.0) | 3 (20.0) | |
| 200–399 | 0 (0.0) | 3 (20.0) | |
| 400+ | 0 (0.0) | 3 (20.0) |
N/A: Not Applicable.
Self-reports of several symptoms were different between the experienced breacher and unexposed control groups (Table 2). A total of 10 out of 15 experienced breachers reported having memory problems and ringing in the ears, whereas only 4 out of 14 reported these symptoms in the unexposed control group (p = 0.04). A total of 8 out of 15 experienced breachers reported having irritability problems, and only 2 out of 14 unexposed controls reported irritability (p = 0.027). A total of 9 out of 15 experienced breachers reported having concentration problems, whereas only 2 were reported in unexposed controls (p = 0.011). Although it did not reach significance, sleep problems were reported in 9 experienced breachers as compared to 5 unexposed controls. Self-reports of headaches and depression were not different between experienced breachers and unexposed controls.
Table 2.
Clinical symptoms of study participants.
| Unexposed Control (N = 14) |
Experienced Breacher (N = 15) | Significance | |
|---|---|---|---|
| Headaches, Yes, N (%) | 2 (14.3) | 2 (13.3) | χ2 = 0.006, p = 0.941 |
| Memory problem, Yes, N (%) | 4 (28.6) | 10 (66.7) | χ2 = 4.209, p = 0.040 |
| Ringing in ears, Yes, N (%) | 4 (28.6) | 10 (66.7) | χ2 = 4.209, p = 0.040 |
| Sleep problems, Yes, N (%) | 5 (35.7) | 9 (60.0) | χ2 = 1.710, p = 0.191 |
| Irritability, Yes, N (%) | 2 (14.3) | 8 (53.3) | χ2 = 4.887, p = 0.027 |
| Depression, Yes, N (%) | 3 (21.4) | 3 (20.0) | χ2 = 0.009, p = 0.924 |
| Concentration problems, Yes, N (%) | 2 (14.3) | 9 (60.0) | χ2 = 6.428, p = 0.011 |
| PCL-M, mean (SD) | 20.64 (4.48) | 26.07 (7.69) | t = −2.338, p = 0.029 |
| NSI, mean (SD) | 16.86 (5.29) | 16.80 (6.70) | t = −0.025, p = 0.980 |
| BSI subscale, mean (SD) | |||
| Somatization | 45.29 (4.41) | 49.33 (7.46) | t = −1.762, p = 0.089 |
| Depression | 45.21 (6.40) | 46.00 (7.05) | t = −0.313, p = 0.756 |
| Anxiety | 44.07 (5.81) | 44.60 (8.95) | t = −0.187, p = 0.853 |
| Global severity index | 43.50 (6.40) | 46.40 (9.15) | t = −0.986, p = 0.333 |
| ImPACT, mean (SD) | |||
| Verbal memory | 92.21 (6.33) | 92.13 (6.53) | t = 0.034, p = 0.973 |
| Visual memory | 68.00 (9.12) | 78.60 (11.06) | t = −2.804, p = 0.009 |
| Visual motor speed | 27.83 (3.25) | 27.33 (4.57) | t = 0.337, p = 0.738 |
| Reaction time | 0.57 (0.07) | 0.64 (0.10) | t = −2.213, p = 0.034 |
| Impulse control | 0.07 (0.28) | 0.33 (0.62) | t = −1.500, p = 0.150 |
| Total symptom | 4.71 (6.75) | 12.40 (17.59) | t = −1.573, p = 0.133 |
PCL-M, Post-Traumatic Stress Disorder Checklist-Military; NSI, Neurobehavioral Symptom Inventory; BSI, Brief Symptom Inventory.
PCL-M scores were higher in experienced breachers when compared with unexposed controls (p = 0.029), indicating increased PTSD-related symptoms, although these levels do not meet clinical criteria for PTSD diagnosis (>44 PTSD cutoff) (Table 2). There was no difference in BSI subscale score, including somatization, depression, anxiety, and global severity index scores in experienced breachers when compared with unexposed controls. There was a significant difference between the groups in visual memory (p = 0.009) and reaction time (p = 0.034).
The results of Pearson correlation coefficients between behavioral symptoms and number of blast exposures are shown in Table 3. The number of blast exposures was positively correlated with BSI subscale, including somatization (ρ = 0.399, p = 0.032), depression (ρ = 0.430, p = 0.020), anxiety (ρ = 0.496, p = 0.006), and global severity index (ρ = 0.413, p = 0.026).
Table 3.
Pearson Correlation Coefficients of Study Variables.
| Number of Blast Exposures | CEC Total Score | |||
|---|---|---|---|---|
| ρ | p | ρ | p | |
| BSI-somatization | 0.399 | 0.032 | 0.377 | 0.044 |
| BSI-depression | 0.430 | 0.020 | −0.003 | 0.990 |
| BSI-anxiety | 0.496 | 0.006 | −0.056 | 0.773 |
| BSI-global severity index | 0.413 | 0.026 | 0.162 | 0.402 |
| ImPACT-verbal memory | −0.055 | 0.776 | −0.114 | 0.557 |
| ImPACT-visual memory | 0.053 | 0.784 | 0.508 | 0.005 |
| ImPACT-visual motor speed | −0.324 | 0.087 | 0.133 | 0.491 |
| ImPACT-reaction time | 0.448 | 0.015 | 0.264 | 0.166 |
| ImPACT-impulse control | 0.202 | 0.293 | −0.069 | 0.722 |
| ImPACT-total symptom | 0.243 | 0.204 | 0.223 | 0.244 |
BSI, Brief Symptom Inventory; CEC, Combat Exposure Checklist; ImPACT, Immediate Post-Concussion Assessment and Cognitive Test.
3.2. Differential microRNA Expression Differences between Experienced Breacher vs. Unexposed Control
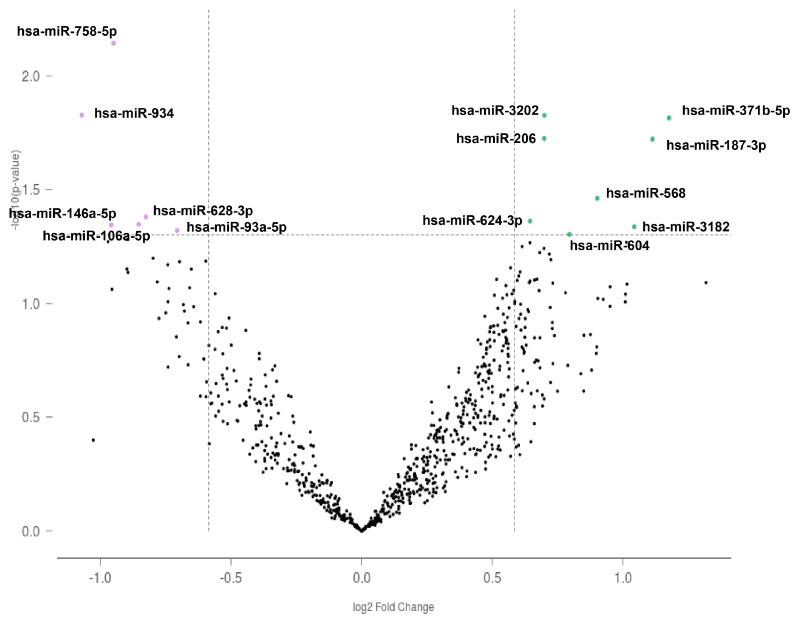
We identified 14 miRNAs differentially expressed in experienced breachers compared to unexposed controls (p < 0.05). Among them, eight miRNAs were upregulated and six miRNAs were downregulated in the experienced breachers compared with the unexposed controls. These microRNAs were not significantly different after FDR correction. The volcano plot of differentially expressed miRNAs is shown in Figure 1 and the fold change of each probe is presented in Table 4.
Figure 1.
Volcano plot for differentially expressed microRNAs between experienced breacher vs. unexposed control. Green dots indicate genes that are upregulated, and purple dots indicate genes that are downregulated.
Table 4.
MicroRNA differential expression between experienced breacher vs unexposed control.
| Probe Name | Log2FC | p-Value | FDR |
|---|---|---|---|
| hsa-miR-371b-5p | 1.177 | 0.015 | 0.848 |
| hsa-miR-187-3p | 1.113 | 0.019 | 0.848 |
| hsa-miR-3182 | 1.043 | 0.046 | 0.848 |
| hsa-miR-568 | 0.901 | 0.035 | 0.848 |
| hsa-miR-604 | 0.795 | 0.049 | 0.848 |
| hsa-miR-3202 | 0.699 | 0.015 | 0.848 |
| hsa-miR-206 | 0.699 | 0.019 | 0.848 |
| hsa-miR-624-3p | 0.644 | 0.043 | 0.848 |
| hsa-miR-93-5p | −0.706 | 0.048 | 0.848 |
| hsa-miR-628-3p | −0.826 | 0.042 | 0.848 |
| hsa-miR-106a-5p | −0.853 | 0.045 | 0.848 |
| hsa-miR-758-5p | −0.950 | 0.007 | 0.848 |
| hsa-miR-146a-5p | −0.959 | 0.045 | 0.848 |
| hsa-miR-934 | −1.071 | 0.015 | 0.848 |
3.3. Differential Gene Expression between Experienced Breacher vs. Unexposed Control
We performed whole-blood RNA-seq in experienced breacher and unexposed control individuals. The comparison between experienced breacher vs. control shows one upregulated gene and nine downregulated genes. We identified one upregulated gene, long intergenic non-protein coding RNA 996 (LINC00996), and 9 downregulated genes, namely immunoglobulin lambda variable 3-16 (IGLV3-16), CD200 molecule (CD200), Leukocyte immunoglobulin-like receptor B5 (LILRB5), ZNF667 antisense RNA 1 (ZNF667-AS1), leiomodin 1 (LMOD1), contactin-associated protein 2 (CNTNAP2), envoplakin (EVPL), double PHD fingers 3 (DPF3), and immunoglobulin heavy variable 4-34 (IGHV4-34) in the experienced breacher group compared to control with the multiple corrected threshold of FDR < 0.05. Differential gene expression with fold changes and adjusted p-values are shown in Table 5.
Table 5.
Gene-expression differences between experienced breacher vs unexposed control.
| Gene Symbol | Gene Name | Log2FC | FDR |
|---|---|---|---|
| IGLV3-16 | Immunoglobulin lambda variable 3-16 | −1.880 | 0.002 |
| CD200 | CD200 molecule | −0.879 | 0.024 |
| LILRB5 | Leukocyte immunoglobulin-like receptor B5 | −1.689 | 0.024 |
| ZNF667-AS1 | ZNF667 antisense RNA 1 | −0.974 | 0.024 |
| LMOD1 | Leiomodin 1 | −2.688 | 0.025 |
| CNTNAP2 | Contactin-associated protein 2 | −1.715 | 0.030 |
| EVPL | Envoplakin | −2.263 | 0.030 |
| DPF3 | Double PHD fingers 3 | −1.542 | 0.039 |
| LINC00996 | Long intergenic non-protein coding RNA 996 | 0.866 | 0.039 |
| IGHV4-34 | Immunoglobulin heavy variable 4-34 | −1.250 | 0.043 |
4. Discussion
In this study, we report significant transcriptome differences in whole-blood associated with repetitive low-level blast exposures compared to unexposed controls. Differentially expressed genes reported are related to inflammation and immune-response process. In addition, the number of blast exposures are strongly correlated with clinical symptoms of BSI-somatization, BSI-anxiety, BSI-depression, and BSI-global severity scores. Dysregulation of these genes may associate with persistent clinical symptoms following repetitive blast exposures. These findings provide some initial insights into the biological changes related to repetitive low-level blast exposure.
Our finding of downregulated CD200 may play a role in chronic inflammation within the CNS by releasing inflammatory cytokines after exposure to blast. CD200 is an immune inhibitory molecule which is highly expressed in neurons and plays a critical role in inhibiting microglia activation [21]. The downregulation of CD200 expression has been observed in both chronic active and inactive multiple sclerosis lesions from postmortem brains in patients [22]. Downregulation of CD200 in our study may reflect the chronic neuroinflammation activity by microglia activation in individuals exposed to repetitive low levels of blast. Activated microglia is a neuroinflammatory process that affects the astrocytes, leading to astrogliosis, which was observed in the elevation of glial fibrillary acidic protein reported in the preclinical model of repetitive low-level blast exposure [11]. The ongoing inflammatory activity in the CNS can be detected in the peripheral circulation. In support of this, we also report the downregulation of IGLV3-16, IGHV4-34, and LILRB5 genes, which are linked to immune-system response and may play an important role in proinflammatory cytokine production [23,24]. Previously, we observed the elevation of plasma IL-6 and TNF-α proteins in a military training population with >5 psi blast exposure compared with low-level <2 psi blast exposure [13]. More recently, analysis of inflammatory proteins in this population of experienced breachers and control subjects showed increases in brain-derived extracellular vesicles (EVs) for IL-6 and TNF-a with a corresponding decrease in IL-10 EVs (unpublished data). These findings further support the important role of CD200 in the peripheral and CNS activity in response to inflammation after exposure to a high number of blasts during a career.
In addition, we reported a downregulation of CNTNAP2 gene expression in this cohort. CNTNAP2 encodes CASPR2, a transmembrane protein associated with voltage potassium channels and a neurexin superfamily protein that plays a critical role in neurodevelopment [25,26]. The CNTNAP2 gene, located on chromosome 7q35, is one of the largest genes in the human genome [27,28]. Multiple mutations within the gene have been identified and are characterized with a set of neurologically related phenotypes that include intellectual disability, seizures, and language impairment. Additionally, mutations have been clinically associated with neurological disorders such as autism spectrum disorder and Pitt Hopkins-like Syndrome 1 [29,30,31]. Most identified mutations within CNTNAP2 were heterozygous, indicating that a single allele disruption could be sufficient to cause disorder and deficit. Preclinical models examining homozygous disruption and complete loss of function in the CNTNAP2 gene have demonstrated an exacerbated and severe neurodevelopmental and neurocognitive deficit outcome [32].
Currently, the majority of investigations revolve around CNTNAP2 gene mutations with large deletions, which likely lead to a nonfunctional protein product. However, our results demonstrate that in our cohort of experienced breachers, who have repeated low-level blast exposure, CNTNAP2 gene expression is downregulated. Interestingly, studies have identified individuals with deletions predicted to not interfere with the protein product, or with a deletion to an upstream promoter [33,34,35]. Unlike the majority of deletions that result in a loss of function, these mutations resulted in decreased expression [36], reduced protein function, and displayed more moderate phenotypes including epilepsy, schizophrenia, obsessive compulsive disorder, Tourette syndrome, and attention deficit hyperactivity disorder [33,35,37,38]. Furthermore, downregulation of CNTNAP2 has been clinically implicated in neurodegeneration, and found significantly downregulated in a cohort of Alzheimer’s patients [39]. Thus, our findings suggest that dysregulation of this gene may be implicated in neurocognitive declines in repetitive low-level blast exposure. Longitudinal follow-up and further analysis of our cohort’s psychiatric health could potentially elucidate an association of blast-related CNTNAP2 downregulation with other psychological disorders. Additionally, future investigations should examine if differential CNTNAP2 expression is acutely impacted by blast exposures and possible roles in symptom development in a more lasting manner.
In summary, this preliminary study suggests that occupational exposure to repetitive low-level blasts is associated with dysregulation of gene expression in whole-blood samples. The differentially expressed genes are largely associated with the chronic inflammation process, and linked to various neurological disorders. A major strength of this study is that it is the first to analyze differential gene expression in the whole blood of a unique population with similar occupational factors between experienced breacher and unexposed control groups. Although this is the first study to provide molecular insight into repetitive low level blast exposure, it was constrained by a small sample size and only one timepoint. In addition, the experienced breachers group had significantly higher PCL-M scores compared to unexposed controls, indicating increased PTSD-related symptoms. Higher scores of PTSD-related symptoms may have a significant role on these genes’ expression levels, although these levels do not meet clinical criteria for PTSD diagnosis. Our findings suggest that need for future studies to be undertaken in larger cohorts over time.
Despite these limitations, this is the first study to the best of our knowledge to quantify microRNAs and mRNAs, and this provides initial insights into the pathophysiological mechanism of repetitive low-level blast exposure. Replicating these findings in a larger cohort may provide potential biomarkers and therapeutic targets for experienced breacher populations.
Author Contributions
Conceptualization, R.V. and J.M.G.; methodology, R.V., H.-S.K. and C.L.; software, R.V. and S.Y.; formal analysis, R.V. and S.Y.; investigation, W.C., A.M.Y., M.L.L., P.W., S.T.A., J.R.S., E.P. and K.C.D.; writing—original draft preparation, R.V.; writing—review and editing, K.A.E., J.H., S.Y., H.-S.K., C.L., C.D., W.C., A.M.Y., M.L.L., P.W., E.P., K.C.D., S.T.A., J.R.S. and J.M.G.; visualization, R.V.; supervision, J.M.G.; project administration, W.C., A.M.Y., M.L.L., P.W., E.P. and K.C.D.; funding acquisition, J.M.G. and S.T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Institute of Nursing Research (NINR) Intramural Research Programs. This work was supported in part by an appointment to the Research Participation Program at the Walter Reed Army Institute of Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Army Medical Research and Development Command. This work was supported/funded by the Joint Program Committee-5 Development of Exposure Standards to Repeated Blast Exposure program, work unit #603115HP-3730-001-A1118.
Institutional Review Board Statement
The Institutional Review Boards at the Naval Medical Research Center (NMRC) and the National Institutes of Health (NIH) reviewed and approved all study procedures, and all methods were performed in accordance with guidelines and regulations (NMRC Protocol No. NMRC.2011.002 and NIH Protocol No. 12N0065, approval on 29 December 2011.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this study can be found in online repositories at Gene Expression Omnibus with the access number GSE193952. The anonymized clinical data that support the findings of this study are available upon reasonable request from any qualified investigator to the corresponding author.
Conflicts of Interest
The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army, Department of the Navy, Department of Defense, or the U.S. Government. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70-25. Several of the authors are military service members or federal civil service employees. This work was prepared as a part of their official duties. Title 17 U.S.C. § 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. § 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ritenour A.E., Baskin T.W. Primary blast injury: Update on diagnosis and treatment. Crit. Care Med. 2008;36:S311–S317. doi: 10.1097/CCM.0b013e31817e2a8c. [DOI] [PubMed] [Google Scholar]
- 2.Belmont P.J.J., McCriskin B.J., Sieg R.N., Burks R., Schoenfeld A.J. Combat wounds in Iraq and Afghanistan from 2005 to 2009. J. Trauma Acute Care Surg. 2012;73:3–12. doi: 10.1097/TA.0b013e318250bfb4. [DOI] [PubMed] [Google Scholar]
- 3.McKee A.C., Robinson M.E. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10:S242–S253. doi: 10.1016/j.jalz.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz D.I., Bernick C., Dodick D.W., Mez J., Mariani M.L., Adler C.H., Alosco M.L., Balcer L.J., Banks S.J., Barr W.B., et al. National Institute of Neurological Disorders and Stroke Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome. Neurology. 2021;96:848–863. doi: 10.1212/WNL.0000000000011850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone J.R., Avants B.B., Tustison N.J., Wassermann E.M., Gill J., Polejaeva E., Dell K.C., Carr W., Yarnell A.M., Lopresti M.L., et al. Functional and Structural Neuroimaging Correlates of Repetitive Low-Level Blast Exposure in Career Breachers. J. Neurotrauma. 2020;37:2468–2481. doi: 10.1089/neu.2020.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaValle C.R., Carr W.S., Egnoto M.J., Misistia A.C., Salib J.E., Ramos A.N., Kamimori G.H. Neurocognitive Performance Deficits Related to Immediate and Acute Blast Overpressure Exposure. Front. Neurol. 2019;10:949. doi: 10.3389/fneur.2019.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belding J.N., Fitzmaurice S., Englert R.M., Koenig H.G., Thomsen C.J., Olaghere da Silva L.U. Self-Reported Concussion Symptomology during Deployment: Differences as a Function of Injury Mechanism and Low-Level Blast Exposure. J. Neurotrauma. 2020;37:2219–2226. doi: 10.1089/neu.2020.6997. [DOI] [PubMed] [Google Scholar]
- 8.Belding J.N., Fitzmaurice S., Englert R.M., Lee I., Kowitz B., Highfill-McRoy R.M., Thomsen C.J., da Silva U. Blast Exposure and Risk of Recurrent Occupational Overpressure Exposure Predict Deployment TBIs. Mil. Med. 2019;185:e538–e544. doi: 10.1093/milmed/usz289. [DOI] [PubMed] [Google Scholar]
- 9.Boutté A.M., Thangavelu B., Nemes J., Lavalle C.R., Egnoto M., Carr W., Kamimori G.H. Neurotrauma Biomarker Levels and Adverse Symptoms among Military and Law Enforcement Personnel Exposed to Occupational Overpressure without Diagnosed Traumatic Brain Injury. JAMA Netw. Open. 2021;4:e216445. doi: 10.1001/jamanetworkopen.2021.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boutté A.M., Thangavelu B., LaValle C.R., Nemes J., Gilsdorf J., Shear D.A., Kamimori G.H. Brain-related proteins as serum biomarkers of acute, subconcussive blast overpressure exposure: A cohort study of military personnel. PLoS ONE. 2019;14:e0221036. doi: 10.1371/journal.pone.0221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heyburn L., Abutarboush R., Goodrich S., Urioste R., Batuure A., Wheel J., Wilder D.M., Arun P., Ahlers S.T., Long J.B., et al. Repeated Low-Level Blast Acutely Alters Brain Cytokines, Neurovascular Proteins, Mechanotransduction, and Neurodegenerative Markers in a Rat Model. Front. Cell. Neurosci. 2021;15:636707. doi: 10.3389/fncel.2021.636707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill J., Cashion A., Osier N., Arcurio L., Motamedi V., Dell K.C., Carr W., Kim H.-S., Yun S., Walker P., et al. Moderate blast exposure alters gene expression and levels of amyloid precursor protein. Neurol. Genet. 2017;3:e186. doi: 10.1212/NXG.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill J., Motamedi V., Osier N., Dell K., Arcurio L., Carr W., Walker P., Ahlers S., Lopresti M., Yarnell A. Moderate blast exposure results in increased IL-6 and TNFalpha in peripheral blood. Brain Behav. Immun. 2017;65:90–94. doi: 10.1016/j.bbi.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z., Wilson C., Mendelev N., Ge Y., Galfalvy H., Elder G., Ahlers S., Yarnell A.M., LoPresti M.L., Kamimori G., et al. Acute and Chronic Molecular Signatures and Associated Symptoms of Blast Exposure in Military Breachers. J. Neurotrauma. 2019;37:1221–1232. doi: 10.1089/neu.2019.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards K.A., Greer K., Leete J., Lai C., Devoto C., Qu B.X., Yarnell A.M., Polejaeva E., Dell K.C., LoPresti M.L., et al. Neuronally-derived tau is increased in experienced breachers and is associated with neurobehavioral symptoms. Sci. Rep. 2021;11:19527. doi: 10.1038/s41598-021-97913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iverson G.L., Lovell M.R., Collins M.W. Interpreting Change on ImPACT Following Sport Concussion. Clin. Neuropsychol. 2003;17:460–467. doi: 10.1076/clin.17.4.460.27934. [DOI] [PubMed] [Google Scholar]
- 17.Franke G.H., Jaeger S., Glaesmer H., Barkmann C., Petrowski K., Braehler E. Psychometric analysis of the brief symptom inventory 18 (BSI-18) in a representative German sample. BMC Med. Res. Methodol. 2017;17:14. doi: 10.1186/s12874-016-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King P.R., Donnelly K.T., Donnelly J.P., Dunnam M., Warner G., Kittleson C.J., Bradshaw C.B., Alt M., Meier S.T. Psychometric study of the Neurobehavioral Symptom Inventory. J. Rehabil. Res. Dev. 2012;49:879–888. doi: 10.1682/JRRD.2011.03.0051. [DOI] [PubMed] [Google Scholar]
- 19.Weathers F., Litz B., Herman D., Huska J.A., Keane T. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility; Proceedings of the Annual Convention of the International Society for Traumatic Stress Studies; San Antonio, TX, USA. 25 October 1993. [Google Scholar]
- 20.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koning N., Uitdehaag B.M.J., Huitinga I., Hoek R.M. Restoring immune suppression in the multiple sclerosis brain. Prog. Neurobiol. 2009;89:359–368. doi: 10.1016/j.pneurobio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Koning N., Bö L., Hoek R.M., Huitinga I. Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Ann. Neurol. 2007;62:504–514. doi: 10.1002/ana.21220. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder H.W., Cavacini L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010;125((Suppl. 2)):S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda K., Nakamura A. Regulation of immune and neural function via leukocyte Ig-like receptors. J. Biochem. 2017;162:73–80. doi: 10.1093/jb/mvx036. [DOI] [PubMed] [Google Scholar]
- 25.Poliak S., Gollan L., Martinez R., Custer A., Einheber S., Salzer J.L., Trimmer J.S., Shrager P., Peles E. Caspr2, a new member of the Neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–1047. doi: 10.1016/S0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- 26.Mikhail F.M., Lose E.J., Robin N.H., Descartes M.D., Rutledge K.D., Rutledge S.L., Korf B.R., Carroll A.J. Clinically relevant single gene or intragenic deletions encompassing critical neurodevelopmental genes in patients with developmental delay, mental retardation, and/or autism spectrum disorders. Am. J. Med. Genet. Part A. 2011;155:2386–2396. doi: 10.1002/ajmg.a.34177. [DOI] [PubMed] [Google Scholar]
- 27.Ashley E.A. Towards precision medicine. Nat. Rev. Genet. 2016;17:507–522. doi: 10.1038/nrg.2016.86. [DOI] [PubMed] [Google Scholar]
- 28.Nakabayashi K., Scherer S.W. The human contactin-associated protein-like 2 gene (CNTNAP2) spans over 2 Mb of DNA at chromosome 7q35. Genomics. 2001;73:108–112. doi: 10.1006/geno.2001.6517. [DOI] [PubMed] [Google Scholar]
- 29.Zweier C., de Jong E.K., Zweier M., Orrico A., Ousager L.B., Collins A.L., Bijlsma E.K., Oortveld M.A.W., Ekici A.B., Reis A., et al. CNTNAP2 and NRXN1 Are Mutated in Autosomal-Recessive Pitt-Hopkins-like Mental Retardation and Determine the Level of a Common Synaptic Protein in Drosophila. Am. J. Hum. Genet. 2009;85:655–666. doi: 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi E., Verri A.P., Patricelli M.G., Destefani V., Ricca I., Vetro A., Ciccone R., Giorda R., Toniolo D., Maraschio P., et al. A 12 Mb deletion at 7q33-q35 associated with autism spectrum disorders and primary amenorrhea. Eur. J. Med. Genet. 2008;51:631–638. doi: 10.1016/j.ejmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Bakkaloglu B., O’Roak B.J., Louvi A., Gupta A.R., Abelson J.F., Morgan T.M., Chawarska K., Klin A., Ercan-Sencicek A.G., Stillman A.A., et al. Molecular Cytogenetic Analysis and Resequencing of Contactin Associated Protein-Like 2 in Autism Spectrum Disorders. Am. J. Hum. Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peñagarikano O., Abrahams B.S., Herman E.I., Winden K.D., Gdalyahu A., Dong H., Sonnenblick L.I., Gruver R., Almajano J., Bragin A., et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman J.I., Vrijenhoek T., Markx S., Janssen I.M., Van Der Vliet W.A., Faas B.H.W., Knoers N.V., Cahn W., Kahn R.S., Edelmann L., et al. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol. Psychiatry. 2008;13:261–266. doi: 10.1038/sj.mp.4002049. [DOI] [PubMed] [Google Scholar]
- 34.Alarcón M., Abrahams B.S., Stone J.L., Duvall J.A., Perederiy J.V., Bomar J.M., Sebat J., Wigler M., Martin C.L., Ledbetter D.H., et al. Linkage, Association, and Gene-Expression Analyses Identify CNTNAP2 as an Autism-Susceptibility Gene. Am. J. Hum. Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elia J., Gai X., Xie H.M., Perin J.C., Geiger E., Glessner J.T., D’Arcy M., Deberardinis R., Frackelton E., Kim C., et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol. Psychiatry. 2010;15:637–646. doi: 10.1038/mp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nord A.S., Roeb W., Dickel D.E., Walsh T., Kusenda M., O’Connor K.L., Malhotra D., McCarthy S.E., Stray S.M., Taylor S.M., et al. Reduced transcript expression of genes affected by inherited and de novo CNVs in autism. Eur. J. Hum. Genet. 2011;19:727–731. doi: 10.1038/ejhg.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verkerk A.J.M.H., Mathews C.A., Joosse M., Eussen B.H.J., Heutink P., Oostra B.A. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 2003;82:1–9. doi: 10.1016/S0888-7543(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 38.Mefford H.C., Muhle H., Ostertag P., von Spiczak S., Buysse K., Baker C., Franke A., Malafosse A., Genton P., Thomas P., et al. Genome-wide copy number variation in epilepsy: Novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010;6:e1000962. doi: 10.1371/journal.pgen.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Abel D., Michel O., Veerhuis R., Jacobs M., Van Dijk M., Oudejans C.B.M. Direct downregulation of CNTNAP2 by STOX1A is associated with Alzheimer’s disease. J. Alzheimers Dis. 2012;31:793–800. doi: 10.3233/JAD-2012-120472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories at Gene Expression Omnibus with the access number GSE193952. The anonymized clinical data that support the findings of this study are available upon reasonable request from any qualified investigator to the corresponding author.