Abstract
Human saliva contains histidine-rich proteins, histatins, which have antifungal activity in vitro. The mechanism by which histatins are able to kill Candida albicans may have clinical significance but is currently unknown. Using radiolabeled histatin 3, we show that the protein binds to C. albicans spheroplasts in a manner that is dependent on time and concentration. Binding to the spheroplasts was saturable and could be competed with unlabeled histatin 3. A single histatin 3 binding site with a Kd = 5.1 μM was detected. Histatin 3 binding resulted in potassium and magnesium efflux, predominantly within the first 30 min of incubation. Studies with fluorescent histatin 3 demonstrate that the protein is internalized by C. albicans and that translocation of histatin inside the cell is closely associated with cell death. Histatin binding, internalization, and cell death are accelerated in low-ionic-strength conditions. Indeed, a low extracellular salt concentration was essential for cell death to occur, even when histatin 3 was already bound to the cell. The interaction of histatin 3 with C. albicans, and subsequent cell death, is inhibited at low temperature. These results demonstrate that the candidacidal activity of histatin 3 is not due exclusively to binding at the cell surface but also involves subsequent interactions with the cell.
Histatins are a family of low-molecular-weight proteins found in abundance in the saliva of humans and old-world monkeys (1, 2). The histatin family consists of 12 members; the three major histatins (histatins 1, 3, and 5) constitute 70 to 80% of the total amount. Histatins demonstrate a number of biological activities in vitro, including maintenance of tooth surface integrity (8, 19), the induction of histamine release (27), inhibition of proteases (13, 18), and the potentiation of rabbit chondrocyte growth (16). However, most attention has been focused on the antimicrobial activity of histatins. These proteins exhibit fungicidal activity against several Candida species (including Candida albicans), Saccharomyces cerevisiae, and Cryptococcus neoformans at physiological concentrations (6, 10, 20, 25, 28). In addition, the histatins have modest bactericidal or inhibitory effects on Streptococcus mutans, Streptococcus mitis, Porphyromonas gingivalis, and Actinobacillus actinomycetemcomitans (14, 15).
The mechanism by which histatins can kill or inhibit the germination of fungi remains unclear. Incubation of C. albicans with histatin 5 is reported to cause structural changes in the cell wall, membrane, and cytoplasm and the release of intracellular potassium (20, 26). However, the action of histatins on the cell surface probably differs from that of larger antimicrobial peptides because a 14-amino-acid peptide corresponding to the C terminus of histatin 5 (and the middle of histatin 3) was identified previously as the active domain (21, 30). Additionally, histatins are weakly amphipathic and not likely to independently form transmembrane structures (11, 22). It has been suggested elsewhere that the tendency to form an α helix is an important functional feature of histatins, though the significance of this motif is not evident from functional studies (21, 23, 24).
A specific binding site for histatin 5 has been identified on P. gingivalis and C. albicans (7, 17). Histatins bind to the C. albicans cell membrane but not to the membrane of a mammalian cell, which is consistent with the selective killing activity of these proteins (7). Recently, it has been reported that histatin 5 is targeted to the mitochondrion and results in a loss of transmembrane potential (9). However, the process by which histatins enter the candidal cell is not known. In this study, we explore the relationship among the specific binding of histatin 3 to C. albicans, internalization of histatin, and cell death. These experiments help to clarify the effects of the extracellular environment on histatin binding and uptake by C. albicans and demonstrate that the mechanism of cell death is distal to the initial binding of histatin to the plasma membrane.
MATERIALS AND METHODS
Labeling of histatin 3.
Histatin 3 was synthesized by GeneMed Synthesis, Inc. (San Francisco, Calif.), and purified by high-pressure liquid chromatography. Composition of the protein was verified by mass spectrometry and amino acid analysis. Histatin 3 was radiolabeled by reductive methylation. Briefly, the protein was dissolved in 0.1 M HEPES buffer (pH 7.5) at a concentration of 5 mg/ml, and recrystallized sodium cyanoborohydride was added to a concentration of 20 mM. [14C]formaldehyde was first passed through a Dowex 1X-8 column to remove contaminants and then added at a 20-fold molar excess to the histatin 3. The reaction mixture was incubated at 37°C for 24 h, and then the labeled histatin 3 was separated on CL2B agarose. The specific activity of the [14C]histatin 3 preparations was from 1.4 × 105 to 2.4 × 105 cpm/μg. In order to assess the anticandidal activity of methylated histatin 3, the above reaction was performed with unlabeled formaldehyde. Methylation was confirmed by amino acid analysis. The methylated histatin 3 was used in the standard Candida killing assay (see below).
Fluorescent histatin 3 (F-histatin 3) was made by using a fluorescein conjugate at the N terminus of the protein. The protein was purified by high-pressure liquid chromatography, and its composition was verified by mass spectrometry.
C. albicans spheroplast preparation.
A clinical isolate of C. albicans, CA8621, was used in this study (29). The MICs of amphotericin B, fluconazole, and flucytosine for CA8621 are 0.125, 0.5, and 1.0 mg/ml, respectively. Sabouraud dextrose broth (50 ml) was inoculated with a colony of CA8621 and grown overnight at 37°C. Cells were then centrifuged at 1,500 × g for 10 min, and the pellet was washed with normal saline. Cells were resuspended in 8 ml of spheroplast buffer (1 M sorbitol, 50 mM sodium phosphate, 0.1% [vol/vol] 2-mercaptoethanol, 100 μg of lyticase per ml) and incubated at 30°C for 45 min. The spheroplasts were washed twice with normal saline and once with sodium phosphate buffer (10 mM sodium phosphate, 0.5 M sodium chloride, pH 7.0). Typically, >90% of cells were lysed by 5% sodium dodecyl sulfate following lyticase treatment.
Binding of histatin 3 to spheroplasts.
Freshly made spheroplasts were resuspended at 1.7 × 107 cells/ml in phosphate-buffered saline (PBS), pH 7.0, and added to 1.5-ml microcentrifuge tubes that were precoated with 1% (wt/vol) bovine serum albumin overnight. The spheroplasts were centrifuged at 1,500 × g for 10 min, and the supernatant was aspirated. Radiolabeled histatin 3 was added to the spheroplasts in a total volume of 200 μl of either PBS or PBS diluted to 35 mM sodium chloride (35 mM PBS). In competition assays, a 100-fold excess of unlabeled histatin 3 was included in the binding reaction mixture. The tubes were incubated with shaking at either 0 or 30°C, for up to 120 min. After the appropriate incubation time, the cells were washed three times with 400 μl of PBS and once with spheroplast buffer. The cells were resuspended in 200 μl of 10 mM sodium phosphate, which was then added to 4 ml of liquid scintillation fluid. The tubes were rinsed twice more to remove all of the spheroplasts, and the radioactivity associated with the spheroplasts was determined. In order to calculate the unbound histatin 3, the total counts per minute added to the tube was measured, and the background level of histatin binding to the tube was also determined.
Visualization of F-histatin 3 binding to C. albicans was assessed by using freshly prepared spheroplasts. In coated microcentrifuge tubes, 3.4 × 106 cells were incubated with 12 nmol of F-histatin 3 per ml in a total volume of 1.1 ml of PBS or 35 mM PBS. The cells were incubated at either 0 or 30°C for 10, 45, or 90 min. The cells were then pelleted, and all the supernatant was aspirated. The spheroplast pellets were resuspended in 100 μl of water, which was spotted directly onto glass slides. The slides were dried briefly at 37°C, flamed quickly three times, and covered with glass coverslips. The slides were kept in the dark at 4°C until they were examined by plain or confocal microscopy. Plain micrographs were made at standardized exposures on a Nikon Optiphot microscope, with a fluorescent light source. Confocal images were made on a Leica TCS microscope, equipped with an argon-krypton laser. Images in each experiment were made at the same instrument settings.
Candidacidal assay of histatin 3.
Freshly grown colonies of CA8621 were used to inoculate 50-ml cultures in 2% (vol/vol) Sabouraud dextrose broth. After overnight growth at 37°C, the cells were pelleted at 1,500 × g and washed once with normal saline. The CA8621 cells were resuspended in normal saline to a concentration of 105 cells/ml. For the Candida killing assay, there were 104 cells in a total of 2 ml of PBS or 35 mM PBS, as well as the test concentration of histatin 3 (0 to 200 nmol/ml). After incubation at 0 or 37°C, the cells were serially diluted in normal saline and plated onto Sabouraud dextrose agar. Colonies were counted after 48 h, and the percent killing was calculated as [1 − (number with histatin/number without histatin)] × 100.
Determination of potassium and magnesium efflux from C. albicans.
C. albicans was incubated overnight in Sabouraud glucose broth, rinsed, and suspended in saline at a final concentration of 5 × 105 CFU/ml. Cells were incubated with 100 μM histatin 3 at 37°C for 5, 15, 30, 60, and 120 min in sterile deionized double-distilled (SDDS) water. Following this incubation, tubes were spun at 1,100 × g for 10 min; the supernatants were collected and analyzed. Supernatants from cells with and without histatin 3 were collected and analyzed at time zero in order to establish a baseline. The supernatants were quantitated for potassium and magnesium concentrations by using an atomic absorption spectrophotometer (Perkin-Elmer model 2380). Samples were diluted 1:2 with SDDS water before testing. Potassium (40 and 80 mM) and magnesium (5.0 and 20 mM) standards were run before every five samples to ensure accuracy. Controls containing SDDS water alone, or with histatin 3, revealed no detectable potassium or magnesium. C. albicans in this assay is the only source of potassium and magnesium in the extracellular fluid.
RESULTS
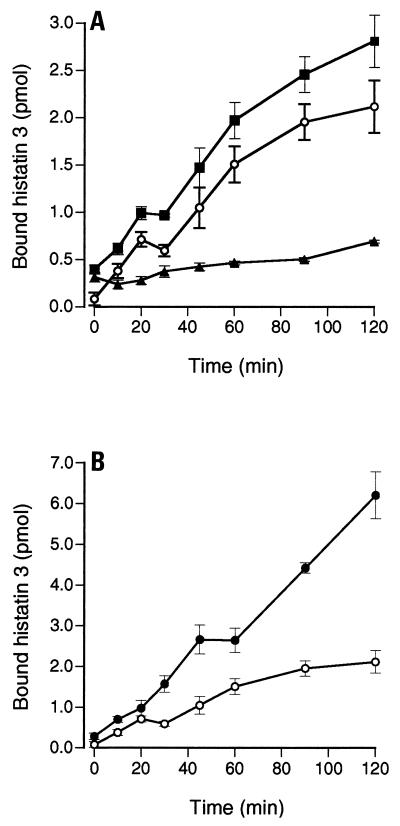
Synthetic histatin 3 was radiolabeled by reductive methylation, since this method causes minimal chemical changes to the protein (12). The functional integrity of the protein was verified by demonstrating that methylated histatin 3 had the same anticandidal activity as the unmodified protein (data not shown). The labeling reaction yielded specific activities of 568 to 1,480 cpm of [14C]histatin 3 per pmol. Radiolabeled histatin 3 bound to spheroplasts of C. albicans (CA8621), and most of the binding could be eliminated by competition with a 100-fold excess of unlabeled histatin 3 (Fig. 1A). It is known that the killing of C. albicans by histatins is highly dependent on the ionic composition and strength of the assay mixture (31). In these studies, we used ionic strengths that reflect two possible environments encountered by histatins: PBS, which is similar to the subsurface tissue interface, and PBS diluted to 35 mM sodium chloride (35 mM PBS), which is in the range of human saliva (4). The binding of [14C]histatin 3 to spheroplasts increased over 120 min of incubation; however, there was much higher total binding in 35 mM PBS than in PBS (Fig. 1B). Moreover, at the higher salt concentration [14C]histatin 3 binding to C. albicans spheroplasts appeared to proceed in two phases, an initial association over approximately 20 min followed by a second phase where the increase in binding was slower.
FIG. 1.
Time course of [14C]histatin 3 binding to C. albicans in PBS and 35 mM PBS. (A) A total of 2 × 105 spheroplasts (CA8621) were incubated in 2 μM labeled histatin in PBS (pH 7.0), at 30°C, for up to 120 min. After the cells were washed, the total amount of bound [14C]histatin 3 was calculated (■). In order to measure the nonspecific binding of histatin 3 to C. albicans spheroplasts, the same reaction mixtures were prepared with the addition of 400 μM unlabeled histatin 3 protein (▴). Hence, the specific binding of [14C]histatin 3 was calculated to be the total binding minus the nonspecific binding (○). (B) Specific binding of [14C]histatin 3 in 35 mM PBS (●) and in PBS (○). The data shown are the means of at least two experiments performed in triplicate ± standard errors of the means.
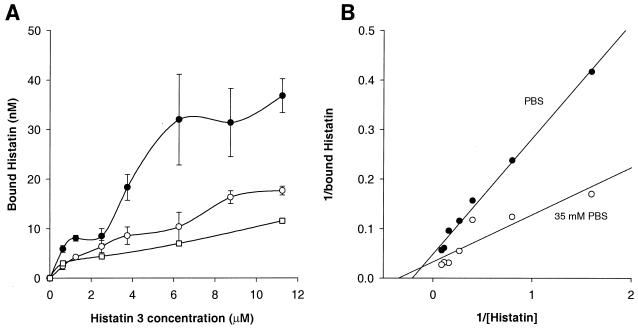
To further characterize the binding of histatin to the C. albicans spheroplasts, we determined the kinetic parameters of [14C]histatin 3 binding under three different conditions, 30°C in PBS, 30°C in 35 mM PBS, and 4°C in PBS (Fig. 2). In these experiments, the initial binding of histatin following a 10-min incubation was measured. Figure 2A shows the binding of [14C]histatin 3 to the spheroplasts as a function of the concentration of histatin in the assay medium. A concentration-dependent increase in binding, which appeared to saturate around 6 μM when the incubation was carried out at 30°C in the hypo-osmotic medium, was seen. Further, the level of binding at each histatin 3 concentration was higher in this medium than in normal PBS at 30°C or in normal PBS at 4°C. In the latter two conditions, the binding did not appear to saturate within the concentration range tested (up to 12 μM; higher concentrations could not be used due to the relatively low specific activity of the [14C]histatin). The data were plotted as Lineweaver-Burke plots (1/bound versus 1/[histatin]). Figure 2B shows the plots of the data obtained at 30°C with PBS and 35 mM PBS. The plot of the data at 4°C was similar to that of the 30°C-PBS condition and is not shown. Linear regression of both sets of data demonstrated a dramatic difference in the slopes of the lines (P < 0.05). The dissociation constant (Kd) of histatin was significantly lower in the 35 mM PBS medium than in normal PBS, 2.52 compared with 5.1 μM. The maximal binding of histatin was not significantly affected by the ionic strength of the medium, 22.85 and 28.9 nM in PBS and 35 mM PBS, respectively. Thus, the apparent affinity for the binding of histatin to spheroplasts is increased under hypo-osmotic conditions. This increased affinity is consistent with the higher level of binding seen in the 35 mM PBS medium (Fig. 1 and Fig. 2A).
FIG. 2.
Characteristics of initial [14C]histatin 3 binding to C. albicans spheroplasts. A total of 2 × 105 spheroplasts were incubated for 10 min with 0 to 11.25 μM [14C]histatin 3. The assays were performed in PBS at 4°C (□) and 30°C (○) and in 35 mM PBS at 30°C (●). Nonspecific binding was determined by the addition of a 100-fold excess concentration of unlabeled histatin 3. (A) Specific binding under these conditions (means of three experiments ± standard errors of the means). (B) Data collected at 30°C are shown as Lineweaver-Burke plots, from which the dissociation constant and maximal binding were calculated. The Kd of histatin 3 in PBS and 35 mM PBS was 5.1 and 2.52 μM, respectively. The maximal binding (Vmax) of histatin 3 was 22.85 and 28.9 nM, respectively.
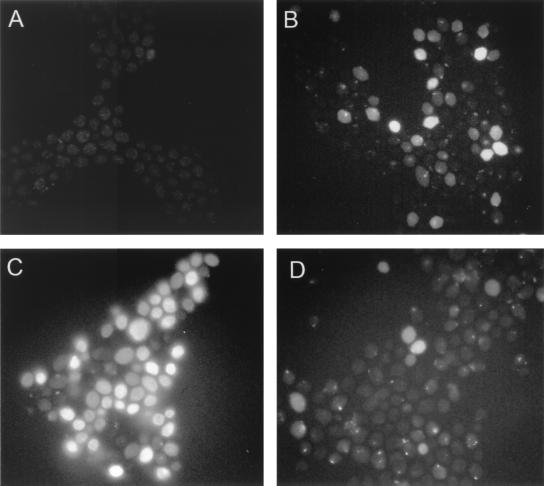
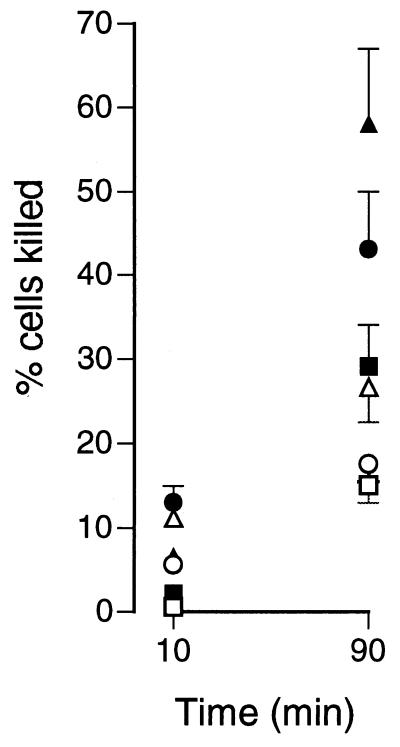
The use of F-histatin 3 facilitated the visualization of histatin binding to C. albicans. Incorporation of fluorescein at the N terminus of F-histatin 3 reduced the killing activity of the protein by less than 10% (data not shown). After 10 min of incubation with C. albicans spheroplasts, there was little visible binding of F-histatin 3 (Fig. 3). By 45 min in 35 mM PBS, F-histatin 3 could be seen binding to the spheroplasts with a patchy distribution, and a few cells appeared to be full of the protein. In 35 mM PBS, almost all of the spheroplasts contained abundant F-histatin 3 by 90 min, while those incubated in PBS showed weak patches of F-histatin 3 binding (Fig. 3). Visualization of F-histatin 3 with spheroplasts demonstrated that binding is dependent on time and extracellular ion concentration, which is consistent with the binding of [14C]histatin 3. Additionally, it seems that F-histatin 3 is internalized by C. albicans only after a period of binding to the surface. Exposure of C. albicans cells to histatin 3 in either PBS or 35 mM PBS for 10 min resulted in little loss of viability (Fig. 4). Extending the exposure time to 90 min increased cell killing, and the effect was much more marked in 35 mM PBS (Fig. 4).
FIG. 3.
Binding of fluorescently labeled histatin 3 to C. albicans. Freshly prepared C. albicans spheroplasts were incubated at 30°C in the presence of 12 μM F-histatin 3. Progressive binding of histatin to the cells was seen in 35 mM PBS at 10 min (A), 45 min (B), and 90 min (C). Little binding of histatin 3 to Candida was observed in PBS by the 90-min point (D). The binding of F-histatin 3 to the cells could be eliminated by competition with unlabeled histatin 3 (data not shown). The same film exposure was used for each panel of the figure.
FIG. 4.
Histatin 3-induced killing of C. albicans. Whole C. albicans cells were treated with increasing concentrations of histatin 3 in PBS or 35 mM PBS, for 10 or 90 min, at 37°C. The viability of Candida after treatment was determined by plating the cells onto Sabouraud dextrose agar and then counting the number of colonies after 48 h. The percentage of cells killed was calculated as [1 − (number with histatin/number without histatin)] × 100. Results are shown for cells incubated in PBS plus 12.5 (□), 25 (○), and 50 (▵) μM histatin 3 and for cells incubated in 35 mM PBS plus 12.5 (■), 25 (●), and 50 (▴) μM histatin 3. The data are from a representative experiment performed in triplicate (means ± standard errors of the means).
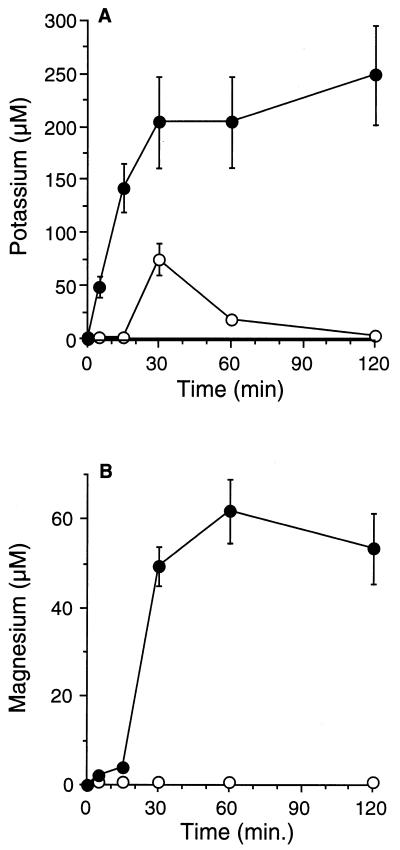
In order to further understand the mechanism of action of histatin 3 on C. albicans, we investigated the kinetics of potassium and magnesium efflux from the cells (Fig. 5). Following incubation of the cells with histatin 3, the potassium and magnesium concentrations in the extracellular medium were significantly increased above baseline and controls (P ≤ 0.0001 [analysis of variance]). The largest amount of potassium was released within the first 30 min of incubation with histatin 3. Extracellular potassium levels remained significantly elevated at ≥200 μM for the remaining incubation period of 120 min. Similar findings were observed for the effect of histatin 3 on magnesium efflux. The largest amount of magnesium also was released into the extracellular fluid within the first 30 min of incubation. Extracellular magnesium levels remained significantly elevated at ≥40 μM for the remaining incubation period of 120 min.
FIG. 5.
Kinetics of potassium and magnesium efflux from C. albicans. Efflux of potassium (A) and magnesium (B) from C. albicans during incubation with 100 μM histatin 3. Potassium and magnesium release was significantly increased when cells were incubated with histatin 3 (●), compared with incubation with water only (○) (P ≤ 0.0001 [analysis of variance]; values are shown as means ± standard errors of the means).
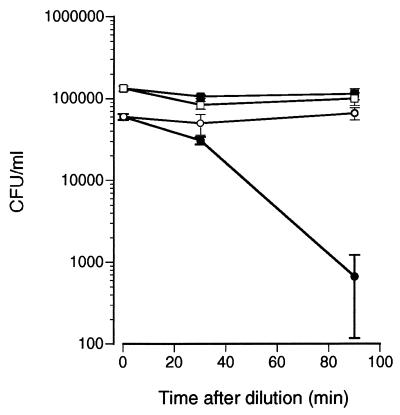
The significance of the extracellular ion concentration on histatin 3-mediated killing of C. albicans was further evaluated in the following experiment. Cells were first incubated in PBS for 60 min with 50 μM histatin 3. This served to preload cells with histatin 3 in conditions that do not result in significant cell death. The cells were then washed and diluted with either PBS or water (final concentration, approximately 15 mM NaCl), after which the incubation was continued for 90 min. Figure 6 shows that only C. albicans cells exposed to histatin 3 and then diluted with water suffered a dramatic loss in viability. These results clearly imply that even though a low extracellular ionic concentration can increase binding of histatin 3 to C. albicans, its significance in cell killing occurs postbinding. In other words, a low-extracellular-salt environment is important for both the binding of histatin 3 and its subsequent action on the cell.
FIG. 6.
Effect of extracellular ion concentration on C. albicans viability after binding of histatin 3. Whole C. albicans cells were first treated with 50 μM histatin 3 for 60 min in PBS. Control cells were incubated without histatin 3. Treated and control cells were washed once in PBS and then diluted approximately 10-fold in either PBS or water. Cell viability was measured immediately after dilution and then measured again 30 and 90 min later. The figure shows the number of CFU ± standard errors of the means of a representative experiment performed in triplicate. ○, histatin 3-treated cells in PBS; ●, histatin 3-treated cells in water; □, control cells in PBS; ■, control cells in water.
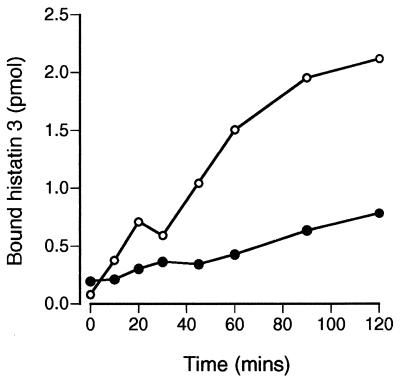
In order to further dissect the mechanism of histatin 3 action on C. albicans, we attempted to inhibit the internalization of histatin 3. Incubation of cells in the presence of sodium azide, 2-deoxyglucose, or bafilomycin was not able to block the uptake of [14C]histatin 3 (data not shown). However, when the binding assay temperature was changed from 30 to 0°C, there was a substantial decrease in [14C]histatin 3 binding to spheroplasts (Fig. 7). The reduction in binding at 0°C was especially notable after 20 min, a point at which the internalization of F-histatin 3 becomes apparent.
FIG. 7.
[14C]histatin 3 binding to C. albicans is temperature dependent. Binding of [14C]histatin 3 to spheroplasts was determined at 30 and 0°C. A total of 2 × 105 cells were incubated in 35 mM PBS containing 2 μM labeled histatin 3 for 0 to 120 min. The specific binding of [14C]histatin 3 was calculated at 0°C (●) and 30°C (○). The data are derived from triplicate measurements in two experiments.
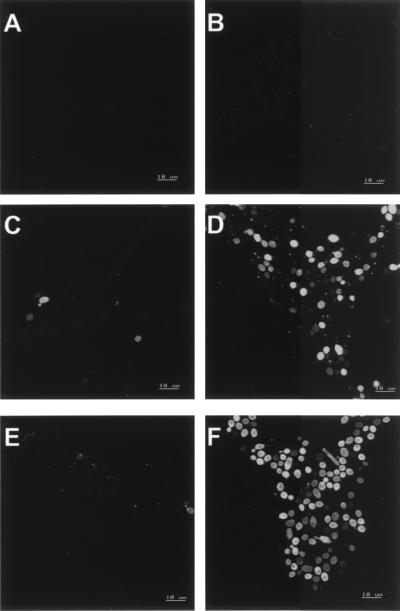
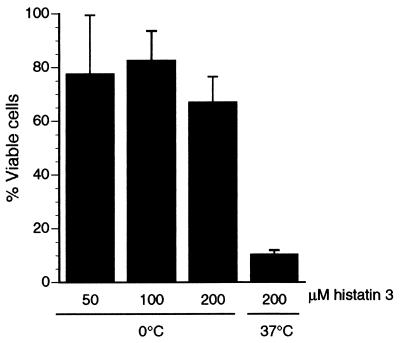
The characteristics of F-histatin 3 binding to C. albicans spheroplasts at 0°C were observed by confocal microscopy (Fig. 8). After 45 min at 0°C in the presence of F-histatin 3, an occasional cell had detectable protein binding (Fig. 8C). By 90 min, several cells demonstrated a small point of F-histatin 3 binding, but very few cells contained histatin intracellularly (Fig. 8E). These results are consistent with our interpretation of the [14C]histatin 3 binding experiment; by reducing the assay temperature, histatin 3 internalization by C. albicans cells is prevented. In contrast, by 45 min at 30°C, many cells clearly showed the punctate pattern of F-histatin 3 binding, while others had substantial protein within them (Fig. 8D). When the process continued to 90 min, almost all of the cells were brightly labeled by F-histatin 3 (Fig. 8F). Optical sections confirmed that the F-histatin 3 was intracellular, typically concentrated around the cell periphery and at one cell pole (Fig. 8). Functional studies of histatin 3 support the significance of temperature in histatin-mediated killing of C. albicans. When cells were incubated with up to 200 nmol of histatin 3 per ml at 0°C, the cell viability remained above 70% of the control (no histatin) level (Fig. 9). However, when cells were treated at 37°C, cell viability was less than 10% of the control. Together, these data imply that it is not sufficient for histatin to associate with the Candida cell membrane for killing to occur. Secondary events, which may include the translocation of histatin to intracellular compartments, are required for cell death.
FIG. 8.
Internalization of histatin 3 by C. albicans is temperature dependent. Whole cells were treated with fluorescently labeled histatin 3 at either 0 or 30°C, in 35 mM PBS. After rinsing and fixation of the cells, 0.2-μm optical sections were generated for each sample. By using a similar instrument gain, the most positive section representing each experimental condition was selected: 10 min at 0°C (A), 10 min at 30°C (B), 45 min at 0°C (C), 45 min at 30°C (D), 90 min at 0°C (E), and 90 min at 30°C (F).
FIG. 9.
C. albicans is resistant to histatin 3-mediated killing at low temperatures. C. albicans cells were treated with up to 200 μM histatin 3 at 0 or 37°C, and their viability was determined by measuring colony formation. Control cells were incubated without histatin. The percentage of viable cells was calculated as (number with histatin/number without histatin) × 100. The data shown are the means from triplicate measurements in two experiments ± standard errors of the means.
DISCUSSION
The role of salivary antifungal proteins in the control of oral Candida populations remains an enigma. Though the histatins have long been demonstrated to have quite potent candidacidal activity in vitro, there are conflicting data with regard to their significance during candidal overgrowth. One reason that it has been difficult to assess the effects of histatins on candidal growth is that the basis of their selective killing of microorganisms remains to be understood. Hence, there is a need to investigate the nature of histatins’ interactions with Candida (and other fungi) and their mechanism of action.
This report demonstrates that histatin 3 binds reversibly to a site on the surface of spheroplasts of C. albicans CA8621. The dissociation constant for the histatin 3 binding site was dependent on the salt concentration: 2.52 μM in 35 mM PBS, compared to 5.1 μM in PBS (Fig. 2). Moreover, the total amount of histatin binding to C. albicans was greater in a low-salt environment, as shown by [14C]histatin 3 and fluorescently labeled histatin 3 (Fig. 1B and 3). These findings are consistent with the reported Kd for 125I-histatin 5 of 0.95 μM in a 10 mM phosphate buffer and the observation that the candidacidal activity of histatin 3 is increased at low ionic strength (7, 31). There was no significant difference between spheroplasts and whole cells in binding [14C]histatin 3 or in histatin-mediated cell death (data not shown), which supports the plasma membrane as the presumptive binding site for histatins. This result concurs with the work of Driscoll and colleagues, who found that spheroplasts and whole cells were equally sensitive to killing by histatin 5, but is different from the work of Edgerton and colleagues, who found that spheroplasts bound histatin 5 poorly and were not susceptible to killing by histatin 5 (6, 7). The disparity between these results is likely due to the method by which the spheroplasts are prepared: lyticase treatment of C. albicans yields spheroplasts that bind histatins, while Zymolyase renders the cells insensitive to histatins. Since these enzymes are prepared from different sources, they can presumably strip different proteins from the cell surface, which may include the histatin binding site. In addition, the interaction of histatin 3 with the hyphal form of C. albicans warrants specific investigation, given the pathogenic significance of this form of the organism and the lack of histatin binding data to date.
Binding of histatin 3 to the C. albicans cell is followed by internalization of the protein, and this internalization is related to cell death (Fig. 3 and 4). In this study, cell death was observed only after histatin 3 had bound to the cell and a hypo-osmotic environment persisted; neither condition alone was sufficient for killing to occur. There was a delay between the initial binding of histatin 3 to the cells and the accumulation of protein inside the cells. The amount of [14C]histatin 3 associated with C. albicans was noticeably greater in 35 mM PBS than in PBS only after 20 min, and the difference was progressive after that. These data indicate that after initial binding to the cell surface, histatin 3 is internalized more rapidly under low-osmolarity conditions. When cells were preloaded with histatin 3 and then switched to either PBS or 35 mM PBS, only those in the hypo-osmotic environment suffered significant death (Fig. 6). Likewise, cells which were not exposed to histatin 3 maintained normal viability in the low-salt environment. Hence, hypo-osmotic conditions appear to favor binding of histatin 3 to Candida, internalization of bound histatin 3, and interactions with the cell that result in cell death.
The above model, which suggests a complex mode of action of histatins on C. albicans, also supports the theory that histatins do not act by simply forming pores in the cell membrane, which cause the cell contents to leak out. It has previously been shown that while histatin binding may cause cells to lose potassium, they do not become permeable to the intracellular dye calcein (molecular weight, 622), and they do not exhibit gross disturbances in intracellular morphology (7, 20, 26). Rather, after binding histatin 3, C. albicans dies by some mechanism which is osmotically dependent and may involve the selective loss of small molecules. Here we have shown that the efflux of potassium from C. albicans begins within 5 min of incubation with histatin 3. Histatin 3-mediated loss of potassium and magnesium is almost maximal by 30 min, which is the same time frame as cell death in a very low osmotic medium. These data are compatible with binding to cell membrane proteins, such as ATPases, which are responsible for regulating potassium and magnesium homeostasis. As histatin 3 is a small protein (32 amino acids) and only weakly amphipathic, the early efflux of potassium and magnesium also does not suggest a mechanism of classical transmembrane channel formation observed with peptides such as cecropin and magainins (5). Since these experiments were performed with synthetic histatin 3, the results do not support the contention that an earlier report of potassium efflux from C. albicans was caused by a contaminant in the histatins purified from human saliva (7, 20).
In support of a specific action of histatin 3 on C. albicans, it is noteworthy that even low levels of histatin binding were able to effect cell death under hypo-osmotic conditions. This was evident when cells were first incubated with histatin 3 in PBS for 60 min—a condition which was shown to result in modest levels of histatin binding (Fig. 1B). When the cells were subsequently washed and incubated in a low-salt environment, almost complete killing of the cells was observed. This result implies that the amount of histatin 3 bound to the cell was sufficient for effective killing and that continuous incubation with histatin was not required. In addition, histatin-mediated killing of C. albicans is known to be pH dependent, which would be difficult to reconcile with a mechanism involving direct permeabilization of the cell membrane (3, 31).
Internalization of histatin 3 by C. albicans was inhibited by lowering the temperature of the cells to 0°C. The initial association of histatin 3 with the cell was not significantly different at the lower temperature, though after approximately 20 min the amount of histatin 3 accumulation in the cell decreased dramatically compared to that in the cells at 30°C (Fig. 7 and 8). Moreover, at 0°C the amount of [14C]histatin 3 binding to spheroplasts plateaus at 60 min, whereas at 30°C the binding increases up to 120 min (Fig. 7). This observation suggests that at 0°C binding of [14C]histatin 3 to the cell membrane becomes saturated, probably because the histatin does not translocate to the cell interior. Notably, cell killing was also attenuated at the lower temperature (Fig. 9). Together, these data support the proposition that the candidacidal activity of the histatins does not result from transmembrane channel formation but rather is due to an indirect action on the cell. Recently, Helmerhorst and colleagues have shown that histatin 5 localizes to mitochondria and that it is capable of dissipating the mitochondrial transmembrane potential (9). Our data are consistent with this view, inasmuch as we have shown that decreasing the internalization of histatin 3 increases cell survival. It has been suggested that other molecules can be cointernalized with histatin 5 into C. albicans, but to date, little is known about the mechanism by which histatin enters cells after binding to its receptor.
Conventional and confocal micrographs indicate clearly that F-histatin 3 binds first to a discrete area of the cell membrane, then concentrates around the cell periphery, and with increased time of incubation accumulates within the cells. We did not attempt to localize the histatin within C. albicans cells, nor is it clear at this point precisely at which stage of histatin penetration the cells die. Given the observation that comparatively small amounts of bound histatin 3 may be lethal to C. albicans, it is possible that the accumulation of histatin may be a late event that occurs even after cells have died. Thus, the mechanisms of histatin 3 lethality for C. albicans may consist of three phases dependent upon temperature and ionic strength: initial binding to the fungal cell membrane, increased permeability of the fungal cell membrane, and intracellular uptake with possible targeting of organelle structures. Future studies will focus on defining the target for histatin 3 binding on the plasma membrane, the nature of the initial increase in cell permeability, and the intracellular role of histatin during candidal cell death.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Dental and Craniofacial Research (ZO1-00675).
We thank Beverly Handyman, Joanne Peter, and Tin Sein for technical assistance with these studies.
REFERENCES
- 1.Azen E A, Leutenegger W, Peters E H. Evolutionary and dietary aspects of salivary basic (Pb) and post Pb (PPb) proteins in anthropod primates. Nature. 1978;273:775–778. doi: 10.1038/273775a0. [DOI] [PubMed] [Google Scholar]
- 2.Balekjian A Y, Longton R W. Histones isolated from human parotid fluid. Biochem Biophys Res Commun. 1973;50:676–682. doi: 10.1016/0006-291x(73)91297-7. [DOI] [PubMed] [Google Scholar]
- 3.Brant E C, Santarpia R P, Pollock J J. Role of pH in salivary histidine-rich polypeptide antifungal germ tube inhibitory activity. Oral Microbiol Immunol. 1990;5:336–339. doi: 10.1111/j.1399-302x.1990.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 4.Dawes C. The effects of flow rate and duration of stimulation on the concentrations of protein and the main electrolytes in human submandibular saliva. Arch Oral Biol. 1974;19:887–895. doi: 10.1016/0003-9969(74)90051-x. [DOI] [PubMed] [Google Scholar]
- 5.De Lucca A J, Walsh T J. Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob Agents Chemother. 1999;43:1–11. doi: 10.1128/aac.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driscoll J, Duan C, Zuo Y, Xu T, Troxler R, Oppenheim F G. Candidacidal activity of human salivary histatin recombinant variants produced by site-directed mutagenesis. Gene. 1996;177:29–34. doi: 10.1016/0378-1119(96)00265-x. [DOI] [PubMed] [Google Scholar]
- 7.Edgerton M, Koshlukova S E, Lo T E, Chrzan B G, Straubinger R M, Raj P A. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J Biol Chem. 1998;273:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- 8.Hay D I. Fractionation of human parotid salivary proteins and the isolation of an histidine-rich acidic peptide which shows high affinity for hydroxyapatite surfaces. Arch Oral Biol. 1975;20:553–558. doi: 10.1016/0003-9969(75)90073-4. [DOI] [PubMed] [Google Scholar]
- 9.Helmerhorst E J, Breeuwer P, van’t Hof W, Walgreen-Weterings E, Oomen L C, Veerman E C, Amerongen A V, Abee T. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem. 1999;274:7286–7291. doi: 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- 10.Helmerhorst E J, Reijnders I M, Hof W V, Simoons-Smit I, Veerman E C, Amerongen A V. Amphotericin B- and fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrob Agents Chemother. 1999;43:702–704. doi: 10.1128/aac.43.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmerhorst E J, Van’t Hof W, Veerman E C, Simoons-Smit I, Nieuw Amerongen A V. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem J. 1997;326:39–45. doi: 10.1042/bj3260039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jentoft N, Dearborn D G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979;254:4359–4365. [PubMed] [Google Scholar]
- 13.Kato T, Takahashi N, Kuramitsu H K. Sequence analysis and characterization of the Porphyromonas gingivalis prtC gene, which expresses a novel collagenase activity. J Bacteriol. 1992;174:3889–3895. doi: 10.1128/jb.174.12.3889-3895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacKay B J, Denepitiya L, Iacono V J, Krost S B, Pollock J J. Growth-inhibitory and bactericidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect Immun. 1984;44:695–701. doi: 10.1128/iai.44.3.695-701.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami Y, Nagata H, Amano A, Takagaki M, Shizukuishi S, Tsunemitsu A, Aimoto S. Inhibitory effects of human salivary histatins and lysozyme on coaggregation between Porphyromonas gingivalis and Streptococcus mitis. Infect Immun. 1991;59:3284–3286. doi: 10.1128/iai.59.9.3284-3286.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami Y, Nagata H, Shizukuishi S, Nakashima K, Okawa T, Takigawa M, Tsunemitsu A. Histatin as a synergistic stimulator with epidermal growth factor of rabbit chondrocyte proliferation. Biochem Biophys Res Commun. 1994;198:274–280. doi: 10.1006/bbrc.1994.1038. [DOI] [PubMed] [Google Scholar]
- 17.Murakami Y, Shizukuishi S, Tsunemitsu A, Nakashima K, Kato Y, Aimoto S. Binding of a histidine-rich peptide to Porphyromonas gingivalis. FEMS Microbiol Lett. 1991;66:253–256. doi: 10.1016/0378-1097(91)90269-g. [DOI] [PubMed] [Google Scholar]
- 18.Nishikata M, Kanehira T, Oh H, Tani H, Tazaki M, Kuboki Y. Salivary histatin as an inhibitor of a protease produced by the oral bacterium Bacteroides gingivalis. Biochem Biophys Res Commun. 1991;174:625–630. doi: 10.1016/0006-291x(91)91463-m. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheim F G, Yang Y C, Diamond R D, Hyslop D, Offner G D, Troxler R F. The primary structure and functional characterization of the neutral histidine-rich polypeptide from human parotid secretion. J Biol Chem. 1986;261:1177–1182. [PubMed] [Google Scholar]
- 20.Pollock J J, Denepitiya L, MacKay B J, Iacono V J. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect Immun. 1984;44:702–707. doi: 10.1128/iai.44.3.702-707.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj P A, Edgerton M, Levine M J. Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J Biol Chem. 1990;265:3898–3905. [PubMed] [Google Scholar]
- 22.Raj P A, Marcus E, Sukumaran D K. Structure of human salivary histatin 5 in aqueous and nonaqueous solutions. Biopolymers. 1998;45:51–67. doi: 10.1002/(SICI)1097-0282(199801)45:1<51::AID-BIP5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 23.Raj P A, Soni S D, Levine M J. Membrane-induced helical conformation of an active candidacidal fragment of salivary histatins. J Biol Chem. 1994;269:9610–9619. [PubMed] [Google Scholar]
- 24.Ramalingam K, Gururaja T L, Ramasubbu N, Levine M J. Stabilization of helix by side-chain interactions in histatin-derived peptides: role in candidacidal activity. Biochem Biophys Res Commun. 1996;225:47–53. doi: 10.1006/bbrc.1996.1129. [DOI] [PubMed] [Google Scholar]
- 25.Rayhan R, Xu L, Santarpia R P, Tylenda C A, Pollock J J. Antifungal activities of salivary histidine-rich polypeptides against Candida albicans and other oral yeast isolates. Oral Microbiol Immunol. 1992;7:51–52. doi: 10.1111/j.1399-302x.1992.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 26.Santarpia R P, Cho M I, Pollock J J. Parameters affecting the inhibition of Candida albicans GDH 2023 and GRI 2773 blastospore viability by purified synthetic salivary histidine-rich polypeptides. Oral Microbiol Immunol. 1990;5:226–232. doi: 10.1111/j.1399-302x.1990.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugiyama K, Suzuki Y, Furuta H. Isolation and characterization of histamine-releasing peptides from human parotid saliva. Life Sci. 1985;37:475–480. doi: 10.1016/0024-3205(85)90410-2. [DOI] [PubMed] [Google Scholar]
- 28.Tsai H, Bobek L A. Studies of the mechanism of human salivary histatin-5 candidacidal activity with histatin-5 variants and azole-sensitive and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:2224–2228. doi: 10.1128/aac.41.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh T J, Aoki S, Mechinaud F, Bacher J, Lee J, Rubin M, Pizzo P A. Effects of preventive, early, and late antifungal chemotherapy with fluconazole in different granulocytopenic models of experimental disseminated candidiasis. J Infect Dis. 1990;161:755–760. doi: 10.1093/infdis/161.4.755. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Lal K, Santarpia R P, Pollock J J. Salivary proteolysis of histidine-rich polypeptides and the antifungal activity of peptide degradation products. Arch Oral Biol. 1993;38:277–283. doi: 10.1016/0003-9969(93)90133-7. [DOI] [PubMed] [Google Scholar]
- 31.Xu T, Levitz S M, Diamond R D, Oppenheim F G. Anticandidal activity of major human salivary histatins. Infect Immun. 1991;59:2549–2554. doi: 10.1128/iai.59.8.2549-2554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]